Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to spread around the world. As of the end of June 2021, there were approximately 181 million confirmed cases and more than 3.9 million deaths across the globe. The colossal impact of coronavirus disease 2019 (COVID-19) is driving the biggest vaccination campaign in human history. All 3 vaccines authorized for emergency use by the US Food and Drug Administration (Pfizer-BioNTech, Moderna, and Janssen/Johnson & Johnson) have been thoroughly studied and found to be safe and effective in preventing severe COVID-19 cases. While short-term side effects of COVID-19 vaccine resemble those of other vaccines, long-term side effects remain unknown. Rare side effects continue to surface as millions of people receive COVID-19 vaccines around the world, as compared with the thousands enrolled in the clinical trials. We report a case of new-onset renal-limited ANCA-associated vasculitis (AAV) in a 78-year-old woman with previously normal kidney function after receiving the Pfizer-BioNTech COVID-19 vaccine. The patient developed acute kidney injury with proteinuria and microscopic hematuria with many dysmorphic red blood cells in the urine. Anti-myeloperoxidase antibody titer was elevated. Kidney biopsy showed pauci-immune crescentic necrotizing glomerulonephritis. Kidney function improved after treatment with steroids and rituximab. Our patient had normal routine laboratory testing before the vaccination. Although this case cannot demonstrate a causal relationship between COVID-19 vaccination and AAV, ongoing surveillance for similar complications would be prudent as worldwide vaccination efforts continue.
Index Words: Coronavirus disease 2019 (COVID-19), vaccine, antineutrophil cytoplasmic antibody (ANCA), ANCA-associated vasculitis, acute kidney injury (AKI), crescentic necrotizing glomerulonephritis, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), case report
Introduction
As of June 2021, a total of 33 million cases of coronavirus disease 2019 (COVID-19), with more than a half-million COVID-19–related deaths, have been reported in the United States alone.1 The US Food and Drug Administration (FDA) issued an emergency use authorization for 2 COVID-19 vaccines (Pfizer-BioNTech and Moderna) in December 2020 and a third (Janssen/Johnson & Johnson) in February 2021. Large clinical trials showed that the vaccines are safe and effective. Common adverse events include mild-to-moderate tenderness at the injection site, fever, fatigue, body aches, and headaches.2 , 3 Reports of anaphylaxis to COVID-19 vaccines also started to surface soon after the COVID-19 vaccination campaign began,4 , 5 but long-term sequalae of the vaccines remain unknown.
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a small vessel vasculitis hallmarked by the presence of antibodies against antigens in cytoplasmic granules of neutrophils.6 While there are many case reports describing a temporal association between influenza vaccination and new onset/relapse of AAV,7, 8, 9, 10, 11 there are few reports of this occurring after receiving the COVID-19 vaccine.12 We report a case of new-onset renal-limited anti-myeloperoxidase (MPO) AAV following COVID-19 vaccination.
Case Report
A 78-year-old woman with a past medical history of type 2 diabetes mellitus, hypertension, and paroxysmal atrial fibrillation received her first dose of the Pfizer-BioNTech COVID-19 vaccine in early February 2021, after which she developed nausea, vomiting, and diarrhea. Routine laboratory assessments obtained 16 days after vaccination were notable for a serum creatinine level (Scr) of 1.31 mg/dL and urinalysis with blood (3+), 99 red blood cells (RBCs) per high-power field, 7 white blood cells (WBCs) per high-power field, and 100 mg/dL protein (Table 1 ). Routine laboratory assessments obtained a few weeks prior to vaccination were notable for an Scr of 0.77 mg/dL and urinalysis with absent hematuria and proteinuria. Her symptoms improved spontaneously, and she received the second dose of the Pfizer-BioNTech COVID-19 vaccine 22 days after the first injection. After the second dose, she once again noted symptoms of nausea, vomiting, and diarrhea, as well as new-onset lethargy. At the time of presentation, 28 days after the first vaccine dose, laboratory assessments were notable for an Scr of 3.54 mg/dL and urinalysis with blood (3+), 56 RBCs per high-power field, 13 WBCs per highpower field, and 100 mg/dL protein (Table 1). The patient had no documented history of COVID-19.
Table 1.
Clinical Laboratory Results
| Days Relative to First Vaccine Dose |
Reference Range | |||
|---|---|---|---|---|
| −21 | +16 | +28 | ||
| Serum sodium, mEq/L | 135 | 136 | 135 | 135-145 |
| Serum potassium, mEq/L | 4.2 | 4.5 | 4.5 | 3.6-5.1 |
| Serum chloride, mEq/L | 97 | 100 | 97 | 98-110 |
| Serum bicarbonate, mEq/L | 29 | 30 | 26 | 22-32 |
| SUN, mg/dL | 18 | 22 | 42 | 6-24 |
| Serum creatinine, mg/dL | 0.77 | 1.31 | 3.54 | 0.4-1 |
| Urinalysis | ||||
| Specific gravity | 1.021 | 1.013 | 1.017 | 1.010-1.030 |
| Blood | Negative | 3+ | 3+ | Negative |
| Glucose | Negative | Negative | Negative | Negative |
| Ketones | Negative | Negative | 2+ | Negative |
| Leukocyte esterase | Negative | Trace | Negative | Negative |
| Nitrite level | Negative | Negative | Negative | Negative |
| Protein, mg/dL | Negative | 100 | 100 | <10 |
| RBCs per HPF | 0 | 99 | 56 | 0-5 |
| WBCs per HPF | 0 | 7 | 13 | 0-6 |
Abbreviations: RBCs, red blood cells; HPF, high-power field; WBCs, white blood cells.
She was referred to the emergency department, where computed tomography of the abdomen and pelvis showed no acute abnormality. She was started on intravenous crystalloid, without improvement in Scr, prompting nephrology consult. A manual urine microscopy revealed 1-2 granular casts per high-power field, few renal tubular epithelial cells, too-numerous-to-count RBCs (>10% dysmorphic), and few WBCs. Random urinary albumin-creatinine ratio was 2.05 g/g.
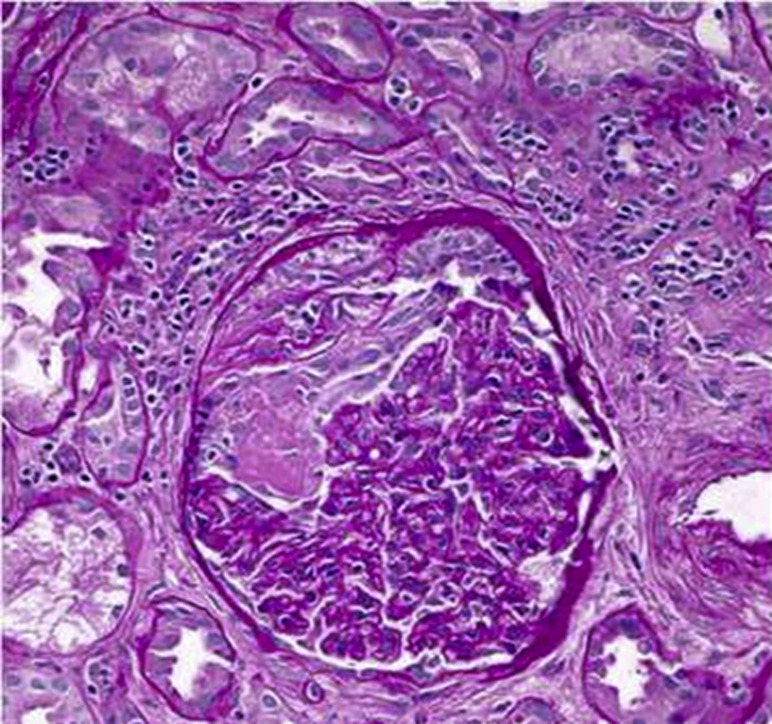
The patient was found to have an elevated titer of anti-MPO antibody (titer: 1.1 AI; normal: <0.2 AI). Complement levels and other serologic tests were unremarkable. She was started on intravenous methylprednisolone for 3 days and prednisone 1 mg/kg daily after that. Kidney biopsy revealed crescentic necrotizing glomerulonephritis with moderate interstitial inflammation on light microscopy (Fig 1 ). Immunofluorescence confirmed pauci-immune glomerulonephritis. She was diagnosed with renal-limited MPO-AAV and she was started on rituximab. Her Scr improved to 2.25 mg/dL at the time of discharge and 1.71 mg/dL at the 1 month follow-up.
Figure 1.
Light microscopy of glomerulus showing segmental fibrinoid necrosis (left side of tuft) and epithelial crescent formation at the top of the glomerulus (periodic acid–Schiff, ×400).
Discussion
Vaccines reduce the risk of many life-threatening diseases by enhancing natural immune responses. As the body builds up the immunity, minor symptoms, including fever, body aches, headaches, and injection site soreness, are expected. Recently, the FDA expeditiously approved 3 different COVID-19 vaccines. Whether autoimmune diseases can be triggered after vaccination remains disputed among experts.13 Here, we describe a case of new-onset renal-limited MPO-AAV after COVID-19 vaccination. Millions of people are being vaccinated around the world,5 and thus it is conceivable that people may develop other diseases temporally associated with vaccination but which are unrelated to the vaccine itself. Our patient had normal routine laboratory parameters before the vaccination, raising the index of suspicion for the observed correlation.
Although there is some dispute on the relationship between vaccination and AAV recurrence in patients with pre-existing autoimmune disease after influenza vaccination, many of the studies might have been underpowered to detect this very rare but significant side effect.14 The temporal relationship could be explained by theoretical mechanisms, like molecular mimicry, polyclonal activation, or a transient systemic proinflammatory cytokine response, which can provoke autoimmune diseases in genetically predisposed individuals.8
Observational studies have suggested a connection between different infections and development of vasculitides, although no direct proof exists.15 Interestingly, Jeffs et al8 noted increased ANCA production in response to viral RNA–based influenza and rabies vaccines. These authors also noted substantially reduced ANCA response to RNA vaccine once it was treated with ribonuclease.8 ANCA-associated vasculitis and autoimmune reactions have been reported with COVID-19 infection and following vaccination,12 , 16, 17, 18, 19, 20 which prompts the question if this response could be a direct reaction to the RNA. It will be interesting to see in future studies if vasculitides are reported more often with COVID-19 vaccines that are messenger RNA–based versus not.
We propose that the immune response to COVID-19 vaccine may have triggered AAV. Although this case cannot conclusively determine if there is a causal relationship between COVID-19 vaccination and AAV, ongoing surveillance for similar complications is prudent as our worldwide vaccination efforts continue.
Article Information
Authors’ Full Names and Academic Degrees
Muhammad Tariq Shakoor, MD, Mark P. Birkenbach, MD, and Matthew Lynch, MD.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Patient Protections
The authors declare that they have obtained consent from the patient reported in this article for publication of the information about her that appears within this Case Report.
Peer Review
Received April 8, 2021. Evaluated by 2 external peer reviewers, with direct editorial input from the Pathology Editor, an Associate Editor, and a Deputy Editor. Accepted in revised form June 22, 2021.
Footnotes
Complete author and article information provided before references.
References
- 1.Johns Hopkins University Coronavirus Resource Center. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. June 1, 2021. https://coronavirus.jhu.edu/map.html
- 2.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimabukuro T.T., Cole M., Su J.R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC COVID-19 Response Team; Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine - United States, December 21, 2020-January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(4):125–129. doi: 10.15585/mmwr.mm7004e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jennette J.C., Nachman P.H. ANCA glomerulonephritis and vasculitis. Clin J Am Soc Nephrol. 2017;12(10):1680–1691. doi: 10.2215/CJN.02500317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birck R., Kaelsch I., Schnuelle P., Flores-Suárez L.F., Nowack R. ANCA-associated vasculitis following influenza vaccination: causal association or mere coincidence? J Clin Rheumatol. 2009;15(6):289–291. doi: 10.1097/RHU.0b013e3181b55fe4. [DOI] [PubMed] [Google Scholar]
- 8.Jeffs L.S., Nitschke J., Tervaert J.W., Peh C.A., Hurtado P.R. Viral RNA in the influenza vaccine may have contributed to the development of ANCA-associated vasculitis in a patient following immunisation. Clin Rheumatol. 2016;35(4):943–951. doi: 10.1007/s10067-015-3073-0. [DOI] [PubMed] [Google Scholar]
- 9.Zafrir Y., Agmon-Levin N., Shoenfeld Y. Post-influenza vaccination vasculitides: a possible new entity. J Clin Rheumatol. 2009;15(6):269–270. doi: 10.1097/RHU.0b013e3181b56177. [DOI] [PubMed] [Google Scholar]
- 10.Duggal T., Segal P., Shah M., Carter-Monroe N., Manoharan P., Geetha D. Antineutrophil cytoplasmic antibody vasculitis associated with influenza vaccination. Am J Nephrol. 2013;38(2):174–178. doi: 10.1159/000354084. [DOI] [PubMed] [Google Scholar]
- 11.Eindhoven S., Levels J., Huisman M., de Winter K.R., Dalm V., Alwani R. MPO-ANCA associated vasculitis with mononeuritis multiplex following influenza vaccination. Allergy Asthma Clin Immunol. 2017;13:49. doi: 10.1186/s13223-017-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekar A., Campbell R., Tabbara J., Rastogi P. ANCA glomerulonephritis after the Moderna COVID-19 vaccination. Kidney Int. 2021;100(2):473–474. doi: 10.1016/j.kint.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe T. Vasculitis following influenza vaccination: a review of the literature. Curr Rheumatol Rev. 2017;13(3):188–196. doi: 10.2174/1573397113666170517155443. [DOI] [PubMed] [Google Scholar]
- 14.Jeffs L.S., Peh C.A., Jose M.D., Lange K., Hurtado P.R. Randomized trial investigating the safety and efficacy of influenza vaccination in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Nephrology (Carlton) 2015;20(5):343–351. doi: 10.1111/nep.12416. [DOI] [PubMed] [Google Scholar]
- 15.van Timmeren M.M., Heeringa P., Kallenberg C.G. Infectious triggers for vasculitis. Curr Opin Rheumatol. 2014;26(4):416–423. doi: 10.1097/BOR.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 16.Hussein A., Al Khalil K., Bawazir Y.M. Anti-neutrophilic cytoplasmic antibody (ANCA) vasculitis presented as pulmonary hemorrhage in a positive COVID-19 patient: a case report. Cureus. 2020;12(8) doi: 10.7759/cureus.9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uppal N.N., Kello N., Shah H.H., et al. De novo ANCA-associated vasculitis with glomerulonephritis in COVID-19. Kidney Int Rep. 2020;5(11):2079–2083. doi: 10.1016/j.ekir.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moeinzadeh F., Dezfouli M., Naimi A., Shahidi S., Moradi H. Newly diagnosed glomerulonephritis during COVID-19 infection undergoing immunosuppression therapy, a case report. Iran J Kidney Dis. 2020;14(3):239–242. [PubMed] [Google Scholar]
- 19.Izci Duran T., Turkmen E., Dilek M., Sayarlioglu H., Arik N. ANCA-associated vasculitis after COVID-19. Rheumatol Int. 2021;41(8):1523–1529. doi: 10.1007/s00296-021-04914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vlachoyiannopoulos P.G., Magira E., Alexopoulos H., et al. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann Rheum Dis. 2020;79:1661–1663. doi: 10.1136/annrheumdis-2020-218009. [DOI] [PubMed] [Google Scholar]