Abstract
Massive testing to detect SARS-CoV-2 is an imperious need in times of epidemic but also presents challenges in terms of its concretization. The use of saliva as an alternative to nasopharyngeal swabs (NPS) has advantages, being more friendly to the patient and not requiring trained health workers, so much needed in other functions.
This study used a total of 452 dual samples (saliva and NPS) of patients suspected of having COVID-19 to compare results obtained for the different specimens when using RT-PCR of RNA extracted from NPS and saliva, as well as saliva directly without RNA extraction.
SARS-CoV-2 was not detected in 13 saliva (direct) of the 80 positive NPS samples and in 16 saliva (RNA) of a total of 76 NPS positive samples. Sensitivity of detection of viral genes ORF1ab, E and N in saliva is affected differently and detection of these genes in saliva samples presents great variability when NPS samples present Ct-values above approximately 20, with sensitivities ranging from 76.3% to 86.3%. On average an increase in 7.3 Ct-values (average standard deviation of 4.78) is observed in saliva samples when compared to NPS.
The use of this specimen should be carefully considered due to the false negative rate and the system used for detection may be also very relevant since the different viral genes are affected differently in terms of detection sensitivity using saliva.
Keywords: SARS-CoV-2, COVID-19, RT-PCR, Sensitivity, Saliva, False negatives
1. Introduction
Hitherto, and more than a year after the start of the Covid-19 pandemic, nasopharyngeal swabs (NPS) remain the most frequent sampling method, and RT-PCR, the golden standard to test for the presence of SARS-CoV-2 [1]. This is justified mainly due to the high viral load in the upper respiratory tract in comparison to other sampling points [2] and the sensitivity and specificity of RT-PCR. Nonetheless, many other tests have been developed until now but care should be taken in the interpretation of results due to the myriad of factors that can affect the quality and reliability of the results [3]. Pandemic periods lead to the need for mass testing, something that can be challenging mainly since the collection of samples is dependent on trained professionals and specific materials, even as simple as swabs. Collection of NPS samples does not only require specialized resources but also adds costs to the process, risks to healthcare professionals and discomfort to the patient. Alternative matrices have also been tested for the presence of SARS-CoV-2 [4]. Indeed, saliva has proven to be an interesting diagnostic specimen for respiratory virus [5], [6], [7] and particularly for SARS-CoV-2 [5, [8], [9], [10], [11], [12], [13], [14], [15], [16]]. More data concerning the use of alternative testing methods and specimens are needed to support decisions on alternative approaches with maximum safety and certainty, and the work here presented aims at contributing to a better understanding of the use of saliva specimens in RT-PCR to detect SARS-CoV-2.
2. Material and methods
2.1. Sample collection
A total of 452 dual samples (NPS and saliva) were collected for the present work between 14th December 2020 and 27th January 2021. Sample providers for this study were patients oriented to perform the detection of SARS-CoV-2 either because of having experienced high-risk contacts with positive COVID-19 patients or because of showing symptoms potentially indicative of COVID-19. They were previously informed in writing about the purpose and procedure of the study and consented to participate by providing the samples. This work was considered exempt from review by an institutional ethical review board, because it comprises use of completely anonymized specimens obtained voluntarily and informed.
NPS samples were collected by healthcare workers from ACES Cávado III Barcelos/Esposende using a sterile flocked swab placed in a sterile tube containing 1 mL of sample preservation fluid (Biogen, China). The saliva samples were collected under the supervision of a healthcare worker, by just letting drop mouth accumulated saliva into empty sterile sputum containers. All samples were stored at 4 °C until processed. RNA was stored at −80°C.
2.2. Nucleic acids extraction and preparation of samples
The extraction of RNA from samples of nasopharyngeal swabs (NPS) and saliva was performed using MagaBio plus Virus DNA/RNA purification kit II (BioFlux, Bioer, China) in accordance with the manufacturer's instructions and using the Bioer GenePure Pro-Nucleic Acid Purification System NPA-32P NPS samples were preserved in sample preservation fluid (Biogen, China) and 200 μL of fluid were used for the extraction. Saliva samples were diluted 1:1 with sample preservation fluid (Biogen, China) and 200 μL were used for nucleic acid extraction. Elution of RNA was performed using 80 μL of elution solution.
Saliva samples were also prepared to be used directly in the RT-PCR reaction without RNA extraction according generally to a published work [17], using proteinase K (NZYTech, Portugal) and heating at 95°C for 5 min.
2.3. Real-Time reverse transcriptase-polymerase chain reaction assay (RT-PCR) for SARS-CoV-2
Extracted nucleic acid samples were tested for SARS-CoV-2 using Novel Coronavirus (2019-nCoV) RT-PCR Detection Kit (Shanghai Fosun Long March Medical Science) and the CFX96 real-time PCR system (BioRad, Germany) in accordance with the manufacturer's instructions. According to the manufacturer's information the lower limit of detection of the test was 300 copies/mL for both swab and sputum samples. Prepared as previously described, 10 μL of extracted RNA or saliva samples were added into 20 μL of the reaction mixture. Reactions were incubated at 50 °C for 15 min and 95 °C for 3 min, followed by 5 cycles at 95 °C for 5 s and 60 °C for 40 s, and 40 cycles at 95 °C for 5 s and 60 °C for 40 s, targeting SARS-CoV-2 genes N, E and ORF1ab. At the end of each of the last 40 cycles, the signals of FAM, JOE, ROX and Cy5 fluorescence signals were registered. The internal control present in all the reactions was detected using the Cy5 fluorescence signal. For each gene, a cycle threshold value (Ct value) less than or equal to 36 was defined as positive, and more than 36 or no value was considered as negative.
2.4. Statistical analysis
Data were analysed following conventional methodologies [18], mainly as available in the R base package [19], with a special reference to the "binom.test" and the "mcnemar.test" functions to compare proportions and the "wilcox.test" to carry out Wilcoxon signed-rank tests. The "nortest" package was used to test for normality using Anderson-Darling, Cramer von Mises and Shapiro-Wilk tests. All graphs were produced using routines written by the authors using the R language.
3. Results
3.1. Characterization of the participants in the study
In this study, which occurred during one of the most critical periods of the pandemic in Portugal, a total of 226 participants were tested for the presence of SARS-CoV-2, by collecting in parallel NPS and saliva samples. Approximately 39% of the positive NPS samples were from men and 61% from women across several age groups (Table 1 ).
Table 1.
Characterization of the participants.
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Age | N° | % | Positive (NPS) | Negative (NPS) | Positive (NPS) | Negative (NPS) |
| 0 a 10 | 4 | 1.7 | 0 | 3 | 0 | 1 |
| 11 a 20 | 21 | 9.29 | 2 | 7 | 3 | 9 |
| 21 a 30 | 27 | 11.95 | 3 | 12 | 4 | 8 |
| 31 a 40 | 32 | 14.16 | 6 | 13 | 4 | 9 |
| 41 a 50 | 41 | 18.14 | 12 | 16 | 4 | 9 |
| 51 a 60 | 49 | 21.68 | 14 | 17 | 9 | 9 |
| 61 a 70 | 33 | 14.60 | 9 | 11 | 3 | 10 |
| 71 a 80 | 13 | 5.75 | 2 | 6 | 3 | 2 |
| 81 a 90 | 6 | 2.65 | 1 | 1 | 1 | 3 |
| Total | 226 | 100 | 49 | 86 | 31 | 60 |
3.2. Comparison between results obtained for NPS and saliva samples
In the present work, saliva samples from the same patient were obtained and analysed to detect possible differences in the detection of COVID-19 patients, when compared to NPS samples, considered as reference. A total of 452 dual samples were tested, of which 80 (35.4%) patients revealed to be positive for SARS-CoV-2 when NPS was analysed.
Saliva samples were analysed directly without extraction (SD) and its RNA after extraction (SE). As can be observed in Table 2 , all the 142 negative samples obtained when analysing SD samples were also NPS negative. However, four negative NPS samples, were positive in SD. The comparison of the NPS results (detected/not detected) with the SD and SE results, was carried out through a binomial test considering the number of successes as the number of different conclusions between the NPS and SD, NPS and SE, and between SD and SE. The null hypothesis was "H0: there is no difference between the results".
Table 2.
Detection of SARS-CoV-2 in NPS and saliva.
| NPS | ||
|---|---|---|
| SD | Detected | Not detected |
| Detected | 67 (83.8%) | 4 (2.7%) |
| Not detected | 13 (2.7%) | 142 (97.3%) |
| SE | ||
| Detected | 60 (78.9%) | 0 |
| Not detected | 16 (21.1%) | 16 (100%) |
There were 16.25% of cases in which the virus is detected in the NPS and not in the SD, which allows us to estimate, with 95% certainty, that this situation will occur for 9 to 26% of the positive NPS samples. Of the 80 positive NPS samples, 76 were also tested for the presence of SARS-CoV-2 after RNA extraction from saliva (4 samples did not have enough volume for RNA extraction). Approximately 21% of non-concordant results were observed, so it was estimated that the non-agreement rate is, with 95% certainty, between 13 and 32%. Comparing the SD with the SE results, there are again significant differences, albeit minor, with 95% certainty that failures to detect the presence of the virus in SE in comparison with SD will occur in 3–16% of the samples analysed.
As mentioned before the rationale for the analysis presented above is that NPS is the reference type of sample against which all other results are checked. However, it can be argued that there is no reason to expect that any of the positive results obtained in this study, whatever the type of sample used, constitute a false positive and therefore the four negative NPS but positive SD results should probably be faced as positive. It is then reasonable to compute a composite reference standard against which all other results (NP and saliva, with or without extraction) can be compared, hoping that this could avoid any biased estimate for sensitivity.
Following this reasoning, and using Tables 1 and 2, a composite matrix can easily be calculated, with a total of 84 specimens, out of 226, giving a positive result (whatever the method used). As a consequence, NPS will detect 80 out of the 84 positive specimens, SD will detect 71 out of the 84, and SE will detect 60. Therefore, the sensitivities of NPS, SD and SE will be 95.24%, 84.52% and 71.43% respectively. Also, comparing methods in pairs, i.e., NPS vs SD, NPS vs SE, and SD vs SE, using McNemar tests, it will be seen that all methods are non-equivalent, with p-values of 5.075 × 10−7, 3.176 × 10−9 and 1.448 × 10−10 respectively.
Despite a good overall agreement of conclusive results between NPS and SD samples (92.5%, Table 2), this should be analysed carefully because, as it can be seen in Table 3 , the sensitivity to detect each of the 3 genes decreases clearly when using saliva. The gene ORF1ab, is detected in just 76.3% of the NPS positive samples when SD is analysed, while genes E and N are detected in 86.3% of the NPS positive samples.
Table 3.
Detection of viral genes in saliva (SD and SE) and NPS (positive) samples.
| Target gene | NPS | SD | SE |
|---|---|---|---|
| E | 80/80 (100%) | 69/80 (86.3%) | 59/76 (77.6%) |
| N | 80/80 (100%) | 69/80 (86.3%) | 65/76 (85.5%) |
| ORF1ab | 79/80 (98.8%) | 61/80 (76.3%) | 60/76 (78.9%) |
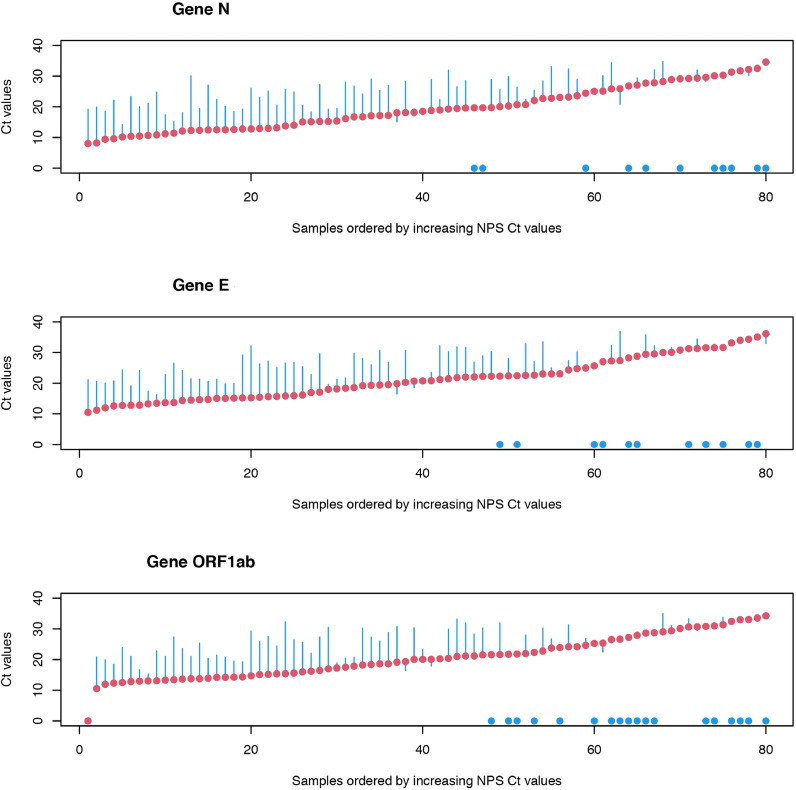
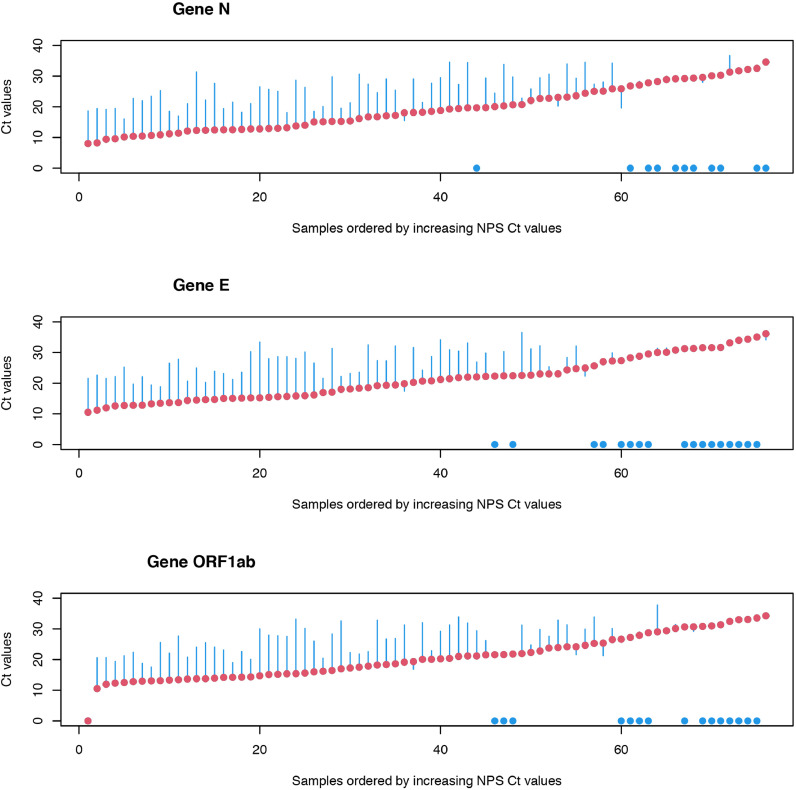
A Wilcoxon signed-rank test was used to compare the Ct values for each gene of NPS samples with the values of the SD and SE samples, with the hypothesis "H0: there is no difference". The results obtained show that the differences between NPS and SD are very significant, with p-values in the range of 10−10 to 10−11. Student's t tests for paired samples showed results similar to Wilcoxon's tests. In view of these results, a study of the observed differences was carried out. For this purpose, the differences between the results obtained (differences in Ct values, SD-NPS and SE-NPS) were calculated and compared with the NPS values. The results thus obtained are shown in Fig. 1, Fig. 2 . In these figures, red points represent the reference values (NPS), organized in ascending order of the respective Ct values, and the blue bars represent the observed differences between the values of reference (NPS) and the values obtained for the SD or SE samples (blue dots represent no amplification of the gene in SD or SE samples). A Ct value close to 20 (NPS samples) can be considered as the point at which, in general, the first situations of non-detection of genes in saliva samples arise. Above this Ct value, the number of situations with non-detection of genes in saliva begins to increase significantly.
Fig. 1.
Title: Ct values (NPS vs SD). Legend: NPS (red) and differences of NPS to SD (blue) Ct values. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Title: Ct values (NPS vs SE). Legend: NPS (red) and differences of NPS to SE (blue) Ct values. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The analysis of these figures also shows that there is non-uniform variation in the differences between the Ct values in saliva and NPS. For the analysis of the differences, all situations where there were results for the SD and SE samples were considered, calculating maximum and minimum values, as well as the quartiles and inter-quartile ranges, means and standard deviations (Table 4 ). Anderson-Darling, Cramer von Mises and Shapiro-Wilk tests for normality were also carried out (Table 5 ). The results obtained show that the hypothesis that the differences between the results in the NPS and in the saliva samples, either SD or SE, follow the laws of a normal distribution cannot be rejected, as indicated by the normality tests, for all cases. This fact seems to indicate that the observed differences can be characterized by an average increase in Ct of approximately 7.3 (higher in saliva samples), with an average standard deviation of 4.78, which corresponds to a high coefficient of variation of about 66%. This fact explains why above Ct≈20 in NPS, as Ct increases in NPS, progressively less genes are detected in saliva.
Table 4.
Statistics for differences between NPS and SD and between NPS and SE Ct values.
| Ct differences | gene | Mean | SD | IQR | MIN | Q1 | Q2 | Q3 | MAX |
|---|---|---|---|---|---|---|---|---|
| SD-NPS | gene N | 6.81 | 4.78 | 6.36 | 17.74 | 10.16 | 7.09 | 3.80 | 4.97 |
| SE-NPS | gene N | 7.94 | 5.10 | 6.57 | 19.08 | 11.52 | 8.40 | 4.95 | 6.25 |
| SD-NPS | gene E | 6.32 | 4.51 | 7.00 | 16.90 | 10.03 | 6.63 | 3.03 | 3.26 |
| SE-NPS | gene E | 8.06 | 4.76 | 6.66 | 18.14 | 11.82 | 8.45 | 5.16 | 2.61 |
| SD-NPS | ORF1ab gene | 6.77 | 4.68 | 7.36 | 16.92 | 10.31 | 7.31 | 2.95 | 2.90 |
| SE-NPS | ORF1ab gene | 7.80 | 4.81 | 6.35 | 17.76 | 11.07 | 8.66 | 4.73 | 4.08 |
Table 5.
Normality tests for differences between NPS and SD and between NPS and SE Ct values.
| Ct differences | gene | AD (A) | AD (p.value) | CVM (W) | CVM (p.value) | SW (W) | SW (p.value) |
|---|---|---|---|---|---|---|
| SD-NPS | gene N | 0.48936 | 0.2148 | 0.076515 | 0.2262 | 0.98128 | 0.3886 |
| SE-NPS | gene N | 0.35488 | 0.4506 | 0.052738 | 0.4660 | 0.98146 | 0.4381 |
| SD-NPS | gene E | 0.65813 | 0.08226 | 0.10271 | 0.1009 | 0.97419 | 0.1635 |
| SE-NPS | gene E | 0.47494 | 0.2319 | 0.060891 | 0.3626 | 0.97134 | 0.1773 |
| SD-NPS | ORF1ab gene | 0.59294 | 0.1190 | 0.10277 | 0.1003 | 0.97336 | 0.2039 |
| SE-NPS | ORF1ab gene | 0.48324 | 0.2213 | 0.080567 | 0.1997 | 0.97582 | 0.2779 |
The analysis of RNA extracted from saliva, instead of its use directly in the reaction, did perform worse in terms of agreement of results. The detection rate in NPS positive samples was 4.9% lower when purified RNA was used instead of saliva directly. In this case, the sensitivity of the method to detect the gene E dropped significantly, as it presented amplification in only 77.6% of the samples when compared to NPS.
4. Discussion
A meta-analysis recently performed by Guillaume Butler-Laporte and colleagues [20] using comparative studies of performance between saliva and NPS samples for the detection of SARS-CoV-2 reports that the diagnostic sensitivity for saliva RT-PCR viral detection is approximately 83.2%, with values ranging from as low as 60.6% [21] to 89.4% [22]. In our study, involving 452 dual samples, an agreement of 83.9% between NPS positive samples and saliva samples used directly in the RT-PCR reaction was observed.
The extraction of RNA from saliva did not show any advantage over its use directly in the reaction. In fact, detection rate was reduced, probably due to losses of RNA during the extraction step and the fact that most of the RNA present in saliva originates from its microbiome, as only 1 out of 900 000 RNA copies is from human origin [23], presenting some challenges in terms of relative concentration. The rate of agreement (78.9%) was affected mainly due to a significant decrease in sensitivity to detect the ORF1ab and E genes. As mentioned by Chantal Vogels and colleagues [17], several works have already shown that the RNA extraction step can be skipped without significant impact on sensitivity.
According to previous works, the ORF1ab gene, the most conserved of the three viral genes tested, presents low sensitivity, and the other target genes N and E, are less conservative but more sensitive [24]. We found a significant decrease in the sensitivity to detect the presence of the ORF1ab gene when saliva is used directly, and also in gene E, mainly when RNA is extracted from saliva. In fact, the use of SD or SE samples started failing to be reliable at Ct values higher than approximately 20 (in NPS), with some genes failing to be detected in saliva.
Just as in the present work, in previous comparative studies, some negative NPS samples have been found to be positive in saliva or sputum [10, 15, 21, 25]. This may result from the existence of false NPS positives, but it must be kept in mind that PCR positivity seems to decrease slower in sputum than in NPS [26].
In this work, performance of saliva as a specimen to detect SARS-CoV-2 was overall good when compared to NPS results (209/226, 92.5%). Nevertheless, one must realize that, around 16% of NPS positive specimens pass undetected in saliva used directly in the reaction and 21,5% in RNA extracted from saliva. Therefore, its use in daily routine analysis should be carefully taken and critically analysed in terms of scope and circumstances. Due to a lower sensitivity of some viral genes to be detected in saliva, its use in diagnosis does not seem to be the right choice. However, in massive screening of populations, it is probably an option that may present a good compromise between convenience and efficacy. The high degree of transmissibility, and the fact that many SARS-CoV-2 carriers are asymptomatic, leads to the need for extensive screening campaigns to identify infected individuals, thus breaking the transmission chain.
The apparent limitation of presenting lower sensitivity to detect SARS-CoV-2 positives should be analysed carefully in terms of its clinical relevance as some authors refer that PCR positivity seems to decrease slower in sputum than in NPS [26]. Culturability of viral particles detected in samples presenting high Ct values is also very low and the probability of isolating infectious SARS-CoV-2 is less than 5% when the viral load is below 6.63 log10 RNA copies/mL [27]. Jared Bullard and colleagues [28] have failed to infect Vero cells when samples presented Ct values higher than 24 for the E gene. Despite being one of the most reliable and used methods to detect the presence of SAR-CoV-2 carriers, its high sensitivity leads to the detection of positives with such a low viral load that the probability of spreading the virus may also be reduced [29, 30]. In fact, even after long periods after infection, it is possible to find viral load in NPS and sputum samples by RT-PCR. Licia Bordi and colleagues [8] have observed viral shedding even up to 100 days from symptoms onset. Saliva may therefore be considered for massive screenings of general population, taking in attention the gene targets due to different loss of sensitivity in saliva. But in preventive screenings, often taken on places with large numbers of vulnerable and high-risk groups, as older people, where the main purpose of the screening is to prevent, in the very beginning, the start of any chain of transmission, its use may present high risks because it will not be able to detect early infected patients presenting low SARS-CoV-2 levels.
Funding
This work was supported by Project 72,545, 02/SAICT/2020, FEDER, Portugal2020.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to nurses at the ACES Cávado III Barcelos/Esposende for the collection of the samples, and to assistance of Luisa Imperadeiro on the laboratory activities.
References
- 1.Oliveira B.A., Oliveira L.C, Sabino E.C., Okay T.S. SARS-CoV-2 and the COVID-19 disease: a mini review on diagnostic methods. 29 JuneRev. Inst. Med. Trop. Sao Paulo. 2020;2020;62(e44):1–8. doi: 10.1590/S1678-9946202062044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva S., Pena L.J. A word of caution in interpreting COVID-19 diagnostics tests. J Med Virol. 2021;93:717–718. doi: 10.1002/jmv.26531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.To K.K.W., Yip C.C.Y., Lai C.Y.W., Wong C.K.H., Ho D.T.Y., Pang P.K.P., et al. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clini. Microbiol. Infect.: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2019;25:372–378. doi: 10.1016/j.cmi.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 6.To K.K.W., Lu L., Yip C.C.Y., Poon R.W.S., Fung A.M.Y., Cheng A., et al. Additional molecular testing of saliva specimens improves the detection of respiratory viruses. Emerg. Microb. Infect. 2017;6:1–7. doi: 10.1038/emi.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim Y-g, Yun S.G., Kim M.Y., Park K., Cho C.H., Yoon S.Y., et al. Comparison between Saliva and Nasopharyngeal Swab Specimens for Detection of Respiratory Viruses by Multiplex Reverse Transcription-PCR. J. Clin. Microbiol. 2017;55:226. doi: 10.1128/JCM.01704-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bordi L., Sberna G., Lalle E., Piselli P., Colavita F., Nicastri E., et al. Frequency and Duration of SARS-CoV-2 Shedding in Oral Fluid Samples Assessed by a Modified Commercial Rapid Molecular Assay. Viruses (1999-4915) 2020;12:1184. doi: 10.3390/v12101184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrne R.L., Kay G.A., Kontogianni K., Aljayyoussi G., Brown L., Collins A.M., et al. Saliva Alternative to Upper Respiratory Swabs for SARS-CoV-2 Diagnosis. Emerg. Infect. Dis. 2020;26:2769–2770. doi: 10.3201/eid2611.203283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan J., Yu F., Wang X., Zou Q., Lou B., Xie G., et al. Hock-a-loogie saliva as a diagnostic specimen for SARS-CoV-2 by a PCR-based assay: a diagnostic validity study. Clin. Chim. Acta. 2020;511:177–180. doi: 10.1016/j.cca.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes L.L., Pacheco V.B., Borges L., Athwal H.K., de Paula, Eduardo F., Bezinelli L., et al. Saliva in the Diagnosis of COVID-19: a Review and New Research Directions. J. Dent. Res. 2020;99:1435–1443. doi: 10.1177/0022034520960070. [DOI] [PubMed] [Google Scholar]
- 12.Medeiros da Silva R.C., Nogueira Marinho L.C., de Araújo Silva D.N., Costa de Lima K., Pirih F.Q., Luz de Aquino Martins A.R. Saliva as a possible tool for the SARS-CoV-2 detection: a review. Travel Medicine and Infectious Disease. 2020:38. doi: 10.1016/j.tmaid.2020.101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasomsub E., Watcharananan S.P., Boonyawat K., Janchompoo P., Wongtabtim G., Suksuwan W., et al. Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: a cross-sectional study. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakanashi D., Asai N., Nakamura A., Miyazaki N., Kawamoto Y., Ohno T., et al. Comparative evaluation of nasopharyngeal swab and saliva specimens for the molecular detection of SARS-CoV-2 RNA in Japanese patients with COVID-19. Journal of Infection & Chemotherapy (Elsevier Inc) 2021;27:126–129. doi: 10.1016/j.jiac.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaz S.N., DSd Santana, Netto E.M., Pedroso C., Wang W.-.K., Santos F.D.A., et al. Saliva is a reliable, non-invasive specimen for SARS-CoV-2 detection. Brazilian Journal of Infectious Diseases. 2020;24:422–427. doi: 10.1016/j.bjid.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyllie A.L., Fournier J., Casanovas-Massana A., Campbell M., Tokuyama M., Vijayakumar P., et al. Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2. N. Engl. J. Med. 2020;383:1283–1286. doi: 10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogels C.B.F., Brackney D.E., Wang J., Kalinich C.C., Ott I.M., Kudo E., et al. SalivaDirect: simple and sensitive molecular diagnostic test for SARS-CoV-2 surveillance. medRxiv. 2020 2020.08.03.20167791. [Google Scholar]
- 18.Kloke J.M., J. CRC Press, Taylor and Francis Group; Boca-Raton: 2015. Non-parametric Statistical Methods Using R. [Google Scholar]
- 19.Team R.C. R Foundation for Statistical Computing; Vienna, Austria: 2017. R: A language and Environment For Statistical Computing. [Google Scholar]
- 20.Butler-Laporte G., Lawandi A., Schiller I., Yao M., Dendukuri N., McDonald E.G., et al. Comparison of Saliva and Nasopharyngeal Swab Nucleic Acid Amplification Testing for Detection of SARS-CoV-2: a Systematic Review and Meta-analysis. JAMA Intern. Med. 2021;181:353–360. doi: 10.1001/jamainternmed.2020.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dogan O.A., Kose B., Agaoglu N.B., Yildiz J., Alkurt G., Demirkol Y.K., et al. Does sampling saliva increase detection of SARS-CoV-2 by RT-PCR? Comparing saliva with oro-nasopharyngeal swabs. J Virol Methods. 2020;290 doi: 10.1016/j.jviromet.2020.114049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teo A.K.J., Choudhury Y., Tan I.B., Cher C.Y., Chew S.H., Wan Z.Y., et al. Validation of Saliva and Self-Administered Nasal Swabs for COVID-19 Testing. medRxiv. 2020 2020.08.13.20173807. [Google Scholar]
- 23.Ostheim P., Tichý A., Sirak I., Davidkova M., Stastna M.M., Kultova G., et al. Overcoming challenges in human saliva gene expression measurements. Sci. Rep. 2020;10:11147. doi: 10.1038/s41598-020-67825-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y., Pei F., Ji M., Wang L., Zhao H., Li H., et al. Sensitivity evaluation of 2019 novel coronavirus (SARS-CoV-2) RT-PCR detection kits and strategy to reduce false negative. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berenger B.M., Conly J.M., Fonseca K., Hu J., Louie T., Schneider A.R., et al. Saliva Collected in Universal Transport Media is an effective, Simple and High-Volume Amenable Method to Detect SARS-CoV-2. Clinical microbiology and infection: the Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2020:S1198-743X(20)30689-3. [DOI] [PMC free article] [PubMed]
- 26.Sheikhzadeh E., Eissa S., Ismail A., Zourob M. Diagnostic techniques for COVID-19 and new developments. Talanta. 2020:220. doi: 10.1016/j.talanta.2020.121392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Kampen J.J.A., van de Vijver D.A.M.C., Fraaij P.L.A., Haagmans B.L., Lamers M.M., Okba N., et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19) Nat. Commun. 2021;12:267. doi: 10.1038/s41467-020-20568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L., et al. Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkar B., Sinha R.N., Sarkar K. Initial Viral Load of a COVID-19-Infected Case Indicated by its Cycle Threshold Value of Polymerase Chain Reaction Could be used as a Predictor of its Transmissibility - An Experience from Gujarat, India. Indian J Community Med. 2020;45:278–282. doi: 10.4103/ijcm.IJCM_593_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tom M.R., Mina M.J. To Interpret the SARS-CoV-2 Test, Consider the Cycle Threshold Value. Clin. Infect. Dis. 2020;71:2252–2254. doi: 10.1093/cid/ciaa619. [DOI] [PMC free article] [PubMed] [Google Scholar]