Abstract
Purpose
The efficacy and safety of regorafenib have been demonstrated in phase 3 trials for multiple tumor types, including metastatic colorectal cancer (mCRC) (CORRECT [NCT01103323]; CONCUR [NCT01584830]), advanced gastrointestinal stromal tumor (GIST) (GRID [NCT01271712]), and hepatocellular carcinoma (HCC) (RESORCE [NCT01774344]). The objective of this post hoc exploratory analysis was to explore the impact of regorafenib on delaying health-related quality of life (HRQOL) deterioration across these tumor types.
Patients and Methods
HRQOL data (assessed with EORTC QLQ-C30 and EQ-5D questionnaires) were pooled for all trials to determine time until definitive deterioration (TUDD), defined as the patient’s first minimal clinically important deterioration in HRQOL score from baseline that does not resolve, using stratified Kaplan–Meier estimators and Cox proportional hazards models adjusted for relevant trial, cancer type, and baseline covariates. Additional analyses based on cancer type were conducted by pooling mCRC trials (CORRECT and CONCUR) and pooling the two mCRC trials with the HCC trial (RESORCE).
Results
A total of 1699 patients with HRQOL data were pooled across the four trials. The results showed that regorafenib significantly delayed TUDD compared with placebo across all three tumor types. Median time to deterioration across the five scales ranged from 16.3 to 24.1 weeks for regorafenib and 8.6 to 12.1 weeks for placebo. The results from the individual studies, the pooled mCRC trials, and the pooled mCRC and HCC trials were similar to the overall pooled results.
Conclusion
A pooled analysis of four phase 3 trials demonstrated that regorafenib delayed a clinically relevant exploratory endpoint, defined as TUDD, compared with placebo across three different tumor types (mCRC, GIST, and HCC), which supports a novel benefit of the impact of regorafenib with respect to patients with these three types of cancers by allowing initial declines in HRQOL to resolve and patients the opportunity to continue treatment.
Keywords: gastrointestinal cancer, metastatic colorectal cancer, hepatocellular cancer, quality of life, regorafenib
Introduction
Mortality and patient burden from digestive system cancers, including colon, rectal, stomach, and liver, remain a significant problem, as these cancers account for approximately 20% of new cancer cases and 25% of annual cancer deaths worldwide.1,2 Several genetic and environmental factors, including family history, geographical locations, diet, and other lifestyle habits, all contribute to the high incidence rates of digestive system cancers.3 These various risk factors can also contribute to the heterogeneous nature of digestive system cancers and make early diagnosis more difficult. Therefore, many individuals may not receive a diagnosis until the cancer has advanced into later stages. Given the progressive and metastatic nature of these cancers, it is important to explore cancer management and treatment options that have potential for improving quality of life in individuals with later stages of cancer.
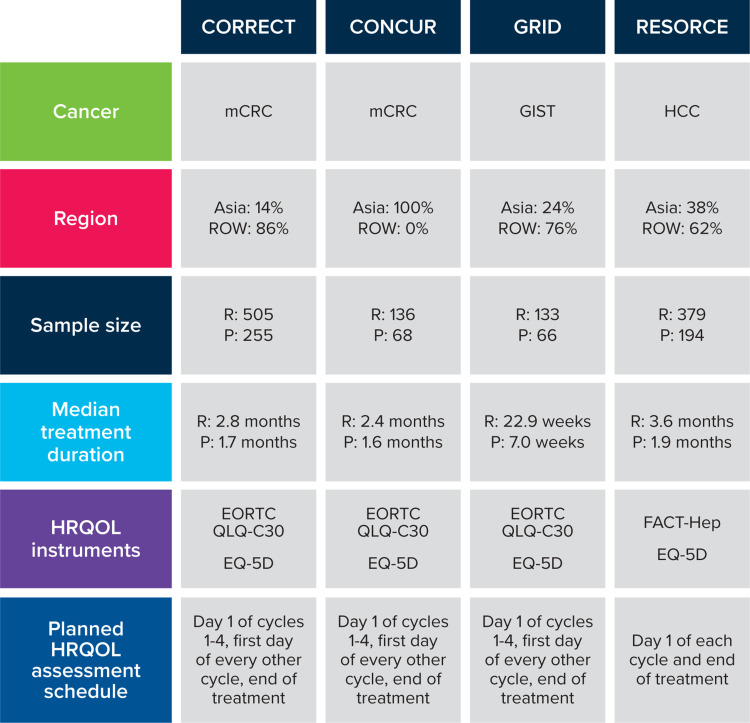
One such treatment is the oral multikinase inhibitor regorafenib, which has shown overall or progression-free survival benefit for patients with metastatic and/or advanced gastrointestinal stromal tumor (GIST), metastatic colorectal cancer (mCRC), and hepatocellular carcinoma (HCC).4 Regorafenib inhibits angiogenesis by targeting angiogenic-related receptors and oncogenic kinases.5 Four phase 3 trials, CORRECT,6 CONCUR,7 GRID,8 and RESORCE,9 have individually demonstrated the efficacy and safety of regorafenib for use in treating these types of cancer. CORRECT6 (NCT01103323) evaluated the efficacy of regorafenib in patients with mCRC (n=505) compared with placebo (n=255) at 114 trial sites in 16 countries across Asia, Australia, Europe, and North America using a double-blind, randomized controlled trial (RCT) design. CONCUR7 (NCT01584830) also used a double-blind RCT design to assess the efficacy of regorafenib in patients with mCRC (n=136) compared with placebo (n=68) during a trial conducted in 25 hospitals located across mainland China, South Korea, Taiwan, Hong Kong, and Vietnam. GRID8 (NCT01271712), a double-blind RCT, assessed the efficacy of regorafenib in patients with GIST stromal tumors (n=133) compared with placebo (n=66) after treatment failure of imatinib and sunitinib at 57 hospitals in 17 countries located across Asia, Europe, North America, and the Middle East. RESORCE9 (NCT01774344), a double-blind RCT, evaluated the efficacy of regorafenib (n=379) compared with placebo (n=194) in patients with HCC who had disease progression with sorafenib treatment at 152 sites across 21 countries.
A pooled analysis using these trials showed that the adverse event (AE) profile for regorafenib was generally consistent across the three types of tumors, with the most patients reporting at least one treatment-emergent AE. The most commonly reported treatment-emergent AEs were asthenia and/or fatigue, hypertension, hand-foot syndrome, decreased appetite, and diarrhea.10 Treatment-related AEs were reported as the primary cause for the frequent dose modifications in each of the trials. The rates of treatment discontinuation due to AEs were generally low. In CORRECT,6 greater than 70% of regorafenib patients required a dose reduction or interruption, with 8% discontinuing regorafenib due to AEs not associated with disease progression. In CONCUR,7 regorafenib treatment was modified in 75% of the patients, with 10% discontinuing treatment due to an AE not associated with disease progression. A similar pattern was observed in GRID8 and RESORCE,9 where 72% and 84% (RESORCE study data on file) of regorafenib patients, respectively, required dose modifications and only 2% and 13% of regorafenib patients, respectively, reported AEs not associated with disease progression leading to permanent discontinuation of treatment.
In this context, we wanted to understand the impact of regorafenib on patient-reported outcomes such as health-related quality of life (HRQOL) (ie, how a patient’s wellbeing is impacted by disease and treatment).11 These four double-blind phase 3 trials have individually shown that overall HRQOL was generally high at baseline. These gastrointestinal cancers have a number of common symptoms, such as fatigue and weakness, abdominal pain or discomfort, and weight loss, all of which may impact a patient’s overall quality of life (QOL) and, more specifically, physical functioning, which has been shown to be a clinically relevant scale for patients.12 Individual regorafenib studies that examined time to first deterioration (TTD) in HRQOL using only patient-reported outcome (PRO) data found no difference between regorafenib and placebo groups.13,14 However, since regorafenib treatment-related AEs typically occur in the first few cycles and can potentially be managed with dose modifications, our goal was to reexamine deterioration in QOL using a measure that accounts for the pattern of early AE occurrence. Furthermore, while TTD is recommended for an adjuvant setting (where reversibility is important), recent research suggests that time until definitive deterioration (TUDD) in HRQOL, defined as the patient’s first clinically meaningful deterioration in HRQOL from baseline that does not subsequently resolve, may be more appropriate for studies in a metastatic setting.15,16 Patients with late-stage disease may have an initial decline in HRQOL due to drug toxicity, which could be alleviated by using clinical interventions that allow patients to continue treatment. Therefore, it is important to understand the long-term impact of the treatment on patient QOL and symptoms in the context of the efficacy and safety profile.
In this study, we conducted post hoc exploratory analyses of PRO data to extend previous research on the capability of regorafenib to delay HRQOL deterioration. The similarities among metastatic gastrointestinal cancers allow for pooling of the available regorafenib trials, which allowed a more in-depth analysis due to the larger sample size and increased power. The objectives were to provide further understanding of the impact and consistency of regorafenib on delaying HRQOL sustained deterioration across GIST, mCRC, and HCC.
Materials and Methods
Selection of Clinical Trials for Evaluation
Patient-level data on HRQOL were pooled across four placebo-controlled, phase 3 trials that evaluated the efficacy and safety of regorafenib: CORRECT (mCRC), CONCUR (mCRC), GRID (GIST), and RESORCE (HCC). The RTI International Institutional Review Board determined this study was not human research (STUDY00020118). HRQOL was measured using the European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire (EORTC QLQ-C30) for three of these trials and EQ-5D for all four of these trials. The Functional Assessment of Cancer Therapy-Hepatobiliary (FACT-Hep) was only collected in RESORCE and therefore was not included in the pooled analysis. Since GIST responds somewhat differently than HCC and mCRC to treatment with regorafenib, subanalyses to isolate further based on cancer type were conducted by pooling the two mCRC trials (CORRECT and CONCUR) with the HCC trial (RESORCE) and pooling only the mCRC trials.
Study Design Elements
The key design elements of each trial, including the patient-reported HRQOL instruments and assessment schedule, are described in Figure 1. The EORTC QLQ-C30 summary score, global health status/QOL, physical functioning scales, and the EQ-5D Index and visual analog scale (VAS) were selected a priori for the TUDD analysis based on clinical relevance and availability across the trials. The EQ-5D was collected in all four trials. The EORTC QLQ-C30 was collected in all trials except RESORCE because of a concern about patient burden since the FACT-Hep was also used to assess patient HRQOL. The baseline and demographics characteristics were reviewed to confirm the appropriateness of pooling data from these studies.
Figure 1.
Placebo-controlled, phase 3 regorafenib trials.
Abbreviations: EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Core Quality-of-Life Questionnaire; FACT-Hep, Functional Assessment of Cancer Therapy-Hepatobiliary; GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; HRQOL, health-related quality of life; mCRC, metastatic colorectal cancer; P, placebo; R, regorafenib; ROW, rest of the world
The EORTC QLQ-C30 was used to assess QOL in patients with cancer on five functional scales (physical, role, cognitive, emotional, and social), three symptom scales (fatigue, pain, and nausea and vomiting), a global health status/QOL scale, and a number of single items assessing additional symptoms commonly reported by patients with cancer (dyspnea, loss of appetite, insomnia, constipation, and diarrhea) and perceived financial impact of the disease. Scores range from 0 to 100, with higher scores indicating better QOL/functioning for the functioning and global health status. Conversely, higher scores signify worsening symptoms for the symptom and financial scales.
The EQ-5D is a general, self-rated health assessment that measures utility values and HRQOL. One component, the EQ-5D Index, measures utility across five dimensions: mobility, self-care, usual activity, pain/discomfort, and anxiety/depression. The summarized utility score can range from −0.594 to 1, with 0 representing the worst imaginable health state or death and 1 being perfect health. Scores less than 0 represent states worse than death. Another component, the EQ-5D VAS, allows patients to self-rate their health status using a scale ranging from 0 (worst imaginable health state) to 100 (best imaginable health state).
Definitive deterioration was defined as the patient’s first deterioration in HRQOL score from baseline that was greater than or equal to the minimal clinically important differences (MCIDs) (used to define the within-person deterioration threshold), with no subsequent improvement greater than or equal to the MCID, or the patient dropped out of the study after the deterioration timepoint. If a patient did not meet this definitive deterioration criterion (ie, patient never recorded an MCID decline or patients had an MCID decline that subsequently improved), they were censored at their last HRQOL assessment. Although there is no general consensus on the most appropriate MCID for these gastrointestinal cancers, the MCIDs used were 10 points for the EORTC QLQ-C30 summary and scale scores based on the general guidelines from Osoba et al17 and on similarly developed small and/or medium difference guidelines found in Cocks et al18, 0.08 points for the EQ-5D Index,19 and 7 points for the EQ-5D VAS.19
Statistical Methods
The pooled analysis was conducted using Kaplan–Meier estimators stratified by trial and Cox proportional hazards models adjusted for cancer and baseline covariates (region, baseline Eastern Cooperative Oncology Group [ECOG] performance status, sex, and baseline PRO score). Sensitivity analyses were performed to compare the results from the pooled analysis of all trials with those of the individual studies since TUDD analyses had not been conducted previously in the individual trials. Subanalyses, to isolate the results further based on cancer type, were conducted by pooling the two mCRC trials (CORRECT and CONCUR) with the HCC trial (RESORCE) and pooling only the mCRC trials. Patients who had completed a baseline assessment and at least one postbaseline HRQOL assessment were included in the TUDD analysis. To avoid any potential bias from the selective placebo crossover in the GRID study, only PRO data collected during the double-blind treatment period were used. The analysis was conducted on observed data with no imputation of missing data. As this was an exploratory, post hoc analysis, all statistical comparisons are presented at the 2-sided alpha=0.05 level without adjustment for multiplicity, and P values should be viewed as descriptive. Analyses were conducted using SAS statistical software, version 9.4.
Results
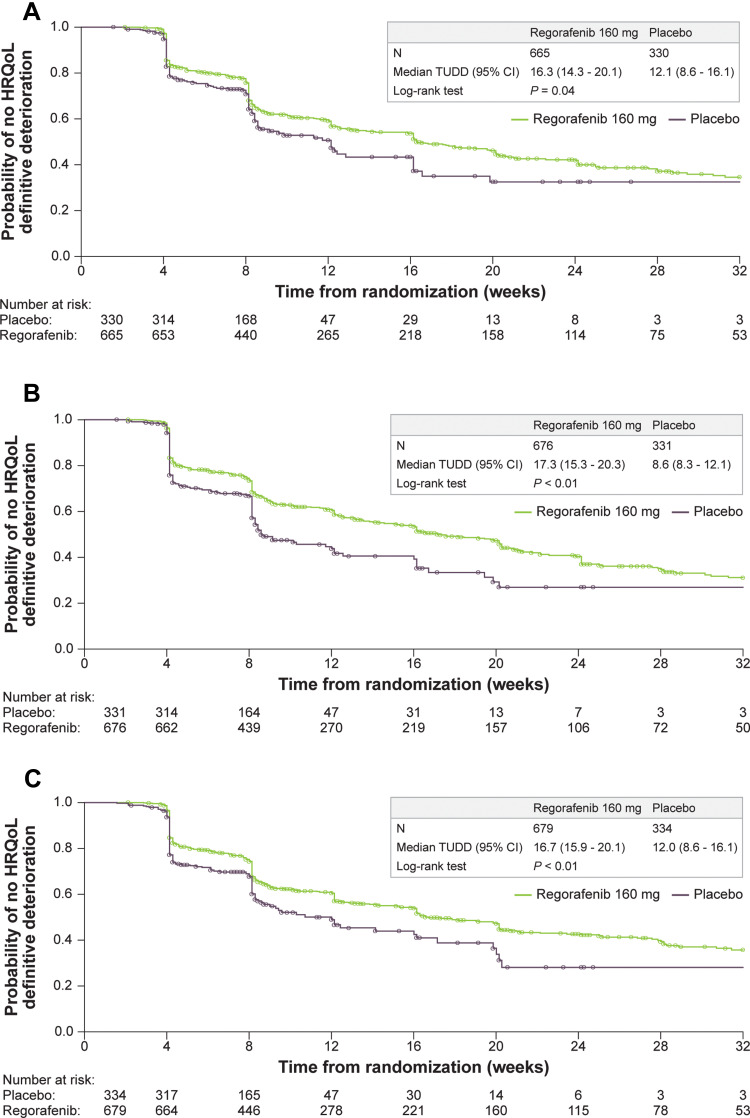
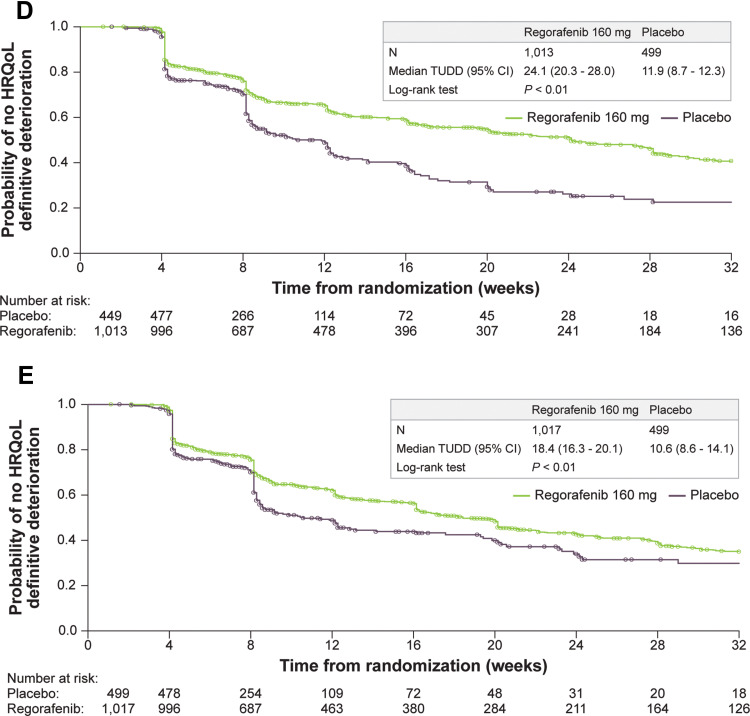
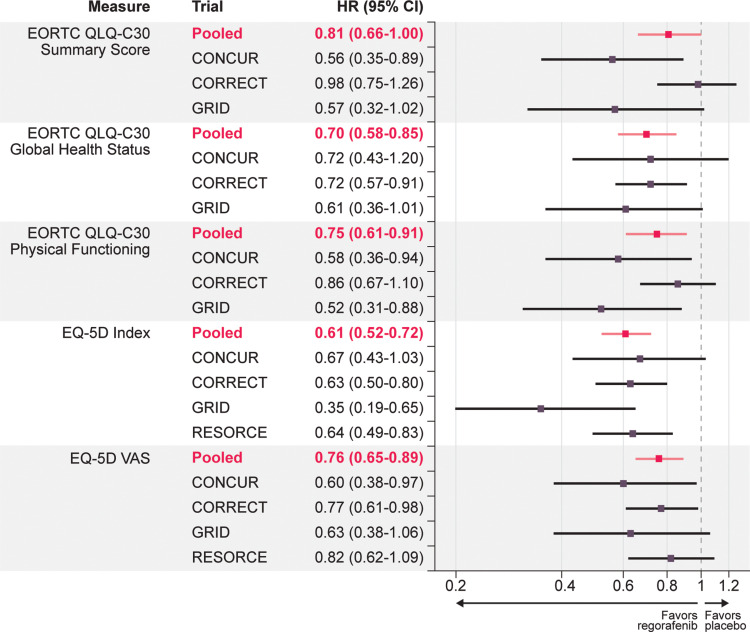
A total of 1699 patients with HRQOL data were pooled across the four trials, with 1516 contributing to the TUDD analyses. Key demographics by trial were examined to determine the appropriateness of the pooled data (Table 1). Geographic region and race were among the study characteristics with the most marked differences across trials, with other differences mainly due to trial design and country of origin. Subsequent analyses were adjusted to account for demographic differences across the trials. The pooled analysis of the four trials showed that regorafenib delays TUDD compared with placebo across all three tumor types (GIST, mCRC, HCC). Based on the results from Kaplan–Meier analysis, regorafenib had a significantly longer TUDD than placebo (all P values from Log rank test ≤ 0.04) (Figure 2). The median TUDD range across the five scales was 16.3 to 24.1 weeks for regorafenib and 8.6 to 12.1 weeks for placebo. The median TUDD for the regorafenib group using the EORTC QLQ-C30 summary score was 16.3 weeks versus 12.1 weeks for the placebo group. The median TUDD for the regorafenib group using the global health status scale was 17.3 weeks versus 8.6 weeks for the placebo group. The median TUDD for the regorafenib group using the physical functioning scale was 16.7 weeks versus 12.0 weeks for the placebo group. The median TUDD for the regorafenib group using the EQ-5D Index was 24.1 weeks versus 11.9 weeks for the placebo group. The median TUDD for the regorafenib group using the EQ-5D VAS components was 18.4 weeks versus 10.6 weeks for the placebo group. The Cox regression results further suggested that regorafenib prolonged TUDD, with hazard ratios (HRs) that ranged from 0.61 to 0.81 in favor of regorafenib across the five scales for TUDD analysis (Figure 3). The HRs were 0.81 (95% confidence interval [CI], 0.66–1.00) for the EORTC QLQ-C30 summary score, 0.70 (95% CI, 0.58–0.85) for global health status, 0.75 (95% CI, 0.61–09.71) for physical functioning, 0.61 (95% CI, 0.52–0.72) for the EQ-5D Index, and 0.76 (95% CI, 0.65–0.89) for the EQ-5D VAS component. Cancer type was a significant covariate in the model, with GIST and HCC demonstrating a longer TUDD than mCRC (Table 2).
Table 1.
Summary of Select Demographics by Trial
| Category | CORRECT6 (N=746) | CONCUR7 (N=204) | GRID8 (N=185) | RESORCE9 (N=564) | ||||
|---|---|---|---|---|---|---|---|---|
| Gender, % | ||||||||
| Female | 39.0 | 42.2 | 36.8 | 12.2 | ||||
| Male | 61.0 | 57.8 | 63.2 | 87.8 | ||||
| Race, % | ||||||||
| White | 78.0 | 0.0 | 68.1 | 35.6 | ||||
| Asian | 14.4 | 100.0 | 25.4 | 41.1 | ||||
| Other | 7.6 | 0.0 | 6.5 | 23.3 | ||||
| Age, %, y | ||||||||
| <65 | 62.7 | 75.0 | 69.2 | 54.8 | ||||
| ≥ 65 | 37.3 | 25.0 | 30.8 | 45.2 | ||||
| ECOG, % | ||||||||
| 0 | 54.4 | 24.5 | 56.2 | 66.7 | ||||
| 1 | 45.6 | 75.5 | 43.8 | 33.3 | ||||
| Regorafenib (n = 493) | Placebo (n = 253) | Regorafenib (n = 136) | Placebo (n = 68) | Regorafenib (n = 123) | Placebo (n = 62) | Regorafenib (n = 375) | Placebo (n = 189) | |
| EORTC QLQ-C30 at baseline, mean (SD)a | ||||||||
| Summary score | 79.2 (15.6) | 80.6 (15.2) | 84.0 (12.7) | 81.2 (13.1) | 81.8 (15.1) | 79.0 (15.6) | NA | NA |
| GHS | 62.6 (21.7) | 64.7 (22.4) | 66.7 (18.4) | 58.0 (23.0) | 67.3 (22.4) | 66.5 (20.0) | NA | NA |
| PF | 78.0 (19.7) | 79.7 (19.6) | 82.2 (17.9) | 80.3 (17.4) | 83.4 (16.7) | 82.4 (17.2) | NA | NA |
| EQ-5D at baseline, mean (SD) | ||||||||
| Index | 0.7 (0.3) | 0.7 (0.3) | 0.8 (0.2) | 0.8 (0.2) | 0.8 (0.2) | 0.75 (0.2) | 0.8 (0.2) | 0.8 (0.2) |
| VAS | 67.4 (47.2) | 65.8 (20.5) | 73.4 (17.3) | 71.4 (17.4) | 69.5 (20.8) | 67.4 (20.3) | 74.4 (17.8) | 73.5 (18.9) |
Notes: CORRECT and RESORCE had 20 and 7 patients, respectively, without a cycle 1 visit, including some whose only visit was an end of treatment visit. These patients were still included in this table. aEORTC QLQ-C30 was not collected in the RESORCE trial and will not be considered in the corresponding pooled calculations.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Core Quality-of-Life Questionnaire; GHS, Global Health Status; NA, not applicable; PF, physical functioning; SD, standard deviation; VAS, visual analog scale.
Figure 2.
Continue.
Figure 2.
Kaplan–Meier curves for time until definitive deterioration by HRQOL score and treatment group (A-E). (A) EORTC QLQ-C30 summary score. (B) EORTC QLQ-C30 global health status. (C) EORTC QLQ-C30 physical functioning. (D) EQ-5D index. (E) EQ-5D VAS.
Abbreviations: EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Core Quality-of-Life Questionnaire; HRQOL, health-related quality of life; TUDD, time until definitive deterioration; VAS, visual analog scale.
Figure 3.
Forest plot of HRs for time until definitive deterioration by pooled and individual trials and HRQOL score.
Note: Hazard ratios less than 1 favor regorafenib.
Abbreviations: CI, confidence interval; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Core Quality-of-Life Questionnaire; HR, hazard ratio; HRQOL, health-related quality of life.
Table 2.
Cox Regression for Time Until Definitive Deterioration by HRQOL Score and Treatment Group
| Model for Each HRQOL Score | Cancer Type Covariate, HR (95% CI) | |
|---|---|---|
| GIST | HCC | |
| EORTC summary | 0.58 (0.44–0.75) | – |
| Global health status | 0.68 (0.53–0.87) | – |
| Physical functioning | 0.69 (0.54–0.89) | – |
| EQ-5D index | 0.48 (0.36–0.64) | 0.63 (0.53–0.75) |
| EQ-5D VAS | 0.66 (0.52–0.83) | 0.54 (0.46–0.64) |
Note: Hazard ratios less than 1 denote an increase in time until definitive deterioration compared with mCRC.
Abbreviations: CI, confidence interval; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Core Quality-of-Life Questionnaire; GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; HR, hazard ratio; HRQOL, health-related quality of life; mCRC, metastatic colorectal cancer; VAS, visual analog score.
The HRs for each scale in the individual studies were similar to those of the pooled results, but not all results were statistically significant (Figure 3). The pooled mCRC results were generally similar to the results seen in the overall pooled analysis for the EORTC QLQ-C30 assessments, with the exception of the summary score. The TUDD for the EORTC QLQ-C30 summary score was significant for the overall pooled analysis (log-rank P = 0.04), but this score was insignificant when only the mCRC trials were pooled (log-rank P = 0.14); however, the direction of the HR was similar to that in the main pooled analysis. For the EQ-5D results, the overall pooled results were similar to those in both the pooled mCRC and pooled mCRC and HCC analyses, with all results having significant log-rank tests and significant HRs in favor of regorafenib. Across the sensitivity analyses, all HRs were less than 1, suggesting a trend toward a reduced risk of definitive deterioration with regorafenib.
Discussion
To the best of our knowledge, the results presented here are the first in the literature to assess the effect of regorafenib on HRQOL across multiple tumor types. This approach demonstrated that regorafenib consistently provided a significantly longer TUDD in HRQOL over placebo for all three different types of tumors. The pooled mCRC results were very similar to the results seen in the overall pooled analysis for the EORTC QLQ-C30 assessments (summary, global health status, and physical functioning). This was not surprising since CORRECT had the largest patient population (n = 760) compared with CONCUR (n = 204) and GRID (n = 199) and therefore had a disproportionate impact on the results. The biggest difference between the studies was the summary score, which resulted in a significant log-rank test for the overall pooled analysis (P = 0.04) but was insignificant when only the mCRC trials were pooled (P = 0.14). However, the direction of the HR was similar to that of the main pooled analysis. For the EQ-5D results, the overall pooled results were very similar for both the pooled mCRC and pooled mCRC and HCC analyses, all of which had significant log-rank tests and significant HRs in favor of regorafenib. As a generic measure, the EQ-5D lacks some sensitivity in the cancer setting. Nevertheless, EQ-5D data were included in these analyses to evaluate consistency in HRQOL results across measures.
Existing clinical evidence has demonstrated the efficacy and manageable tolerability of treating mCRC, GIST, and HCC with regorafenib. In CORRECT6 (mCRC), patients treated with regorafenib had a median overall survival of 6.4 months compared with 5.0 months for patients in the placebo group (HR: 0.77; 95% CI, 0.64–0.94). The median overall survival for the regorafenib group in CONCUR7 (mCRC) was 8.8 months versus 6.3 months for the placebo group (HR: 0.55; 95% CI, 0.40–0.77). Patients who received regorafenib in RESORCE9 (HCC) had an median overall survival of 10.6 months compared with 7.8 months for patients who received a placebo (HR: 0.63; 95% CI, 0.50–0.79). In GRID8 (GIST), the regorafenib group demonstrated a median progression-free survival of 4.8 months compared with 0.9 months for the placebo group (HR: 0.27; 95% CI, 0.19–0.39). Progression-free survival was used as the primary endpoint in GRID,8 whereas overall survival was the primary endpoint for CORRECT,6 CONCUR,7 and RESORCE.9 As the majority of regorafenib patients experience some level of AE, regorafenib dosages may need to be decreased by 40-mg increments to the lowest recommended daily dose of 80 mg.20 The ReDOS trial,21 aphase 2 dose-optimization study in 123 patients with refractory mCRC, demonstrated that weekly dose escalation of regorafenib during the first cycle of treatment allowed more participants to reach the third cycle of treatment. The CONSIGN trial,22 a large single-arm study in mCRC examining 2864 patients treated with regorafenib, allowed for dosages to be increased back to a maximum daily dose of 160 mg. This study supported that treatment-emergent AEs can be managed with regorafenib dose modifications, which allowed patients to remain on therapy with a 9% treatment discontinuation rate.22
The designs of included studies were similar in terms of HRQOL assessments, but there were differences across the studies including in the assessment schedules, global populations, and treatment durations, although the analysis attempted to account for these differences via modeling. While clinical decision making typically concentrates on efficacy (balanced with the safety profile), the pooled analysis presented here provides additional QOL data to complement existing clinical efficacy and safety data. The QOL measures included in this study are not able to capture the full range of patient experiences, particularly those of asymptomatic or minimally symptomatic patients, but these measures do characterize patient experiences across several fundamental domains common to the three cancer types (eg, functional domain, symptom domain). Future research could explore patients’ experiences with treatment for mCRC, GIST, and HCC using disease-specific PRO measures. Another area of interest would be to investigate initial HRQOL MCID declines that resolve and the association of these resolutions with reduction or resolution of AEs through dose modifications.
The chosen endpoint, TUDD, has recently been viewed as a more appropriate endpoint for metastatic settings than TTD.15,16 By focusing on the time to which symptoms do not resolve, the analyses are able to account for dosing variability that allows for patients to continue modified treatment and experience resolution of treatment-related symptoms.
Conclusion
A pooled analysis of four phase 3 trials demonstrated that regorafenib delayed TUDD compared with placebo across three different tumor types (mCRC, GIST, and HCC), which showed the long-term impact of regorafenib with respect to patients’ HRQOL. Although analysis of individual trials did not consistently demonstrate significant improvement in TTD with regorafenib, this TUDD analysis differs from previous analyses by allowing initial declines in HRQOL to resolve. The TUDD analysis may be more appropriate and a better measure of HRQOL with regorafenib in the metastatic setting, where dose adjustments are important for optimizing tolerability and allowing patients to continue treatment.
Acknowledgments
The authors thank Brian Samsell of RTI Health Solutions for medical writing assistance. The abstract of this paper was presented at the World Congress of Gastrointestinal Cancer; 3-6 July 2019; Barcelona, Spain as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstract” in Annals of Oncology 2019;30(4):IV37: https://doi.org/10.1093/annonc/mdz155.135.
Funding Statement
The financial support for the study was provided by Bayer AG. The CORRECT, CONCUR, GRID, and RESORCE trials were sponsored by Bayer. RTI Health Solutions received funding under a research contract with Bayer AG to conduct this study and provide editorial support in the form of manuscript writing, styling, and submission.
Abbreviations
AE, adverse event; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; EORTC, European Organisation for Research and Treatment of Cancer; FACT-Hep, Functional Assessment of Cancer Therapy-Hepatobiliary; GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; HR, hazard ratio; HRQOL, health-related quality of life; MCID, minimal clinically important difference; mCRC, metastatic colorectal cancer; NCT, National Clinical Trial number; PRO, patient-reported outcome; QLQ-C30, Core Quality-of-Life Questionnaire; QOL, quality of life; RCT, randomized controlled trial; ROW, rest of the world; TTD, time to first deterioration; TUDD, time until definitive deterioration; VAS, visual analog scale.
Data Sharing Statement
Data are available from Marisca Marian (marisca.marian@bayer.com) upon reasonable request.
Ethics Approval and Informed Consent
The RTI International Institutional Review Board determined this study was not human research (STUDY00020118).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; have drafted or written, or substantially revised or critically reviewed the article; have agreed on the journal to which the article will be submitted; reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage; and agree to take responsibility and be accountable for the contents of the article.
Disclosure
MM is an employee of Bayer AG. JB and DO are full-time employees of RTI Health Solutions, an independent nonprofit research organization, which received funding pursuant to a contract from Bayer AG to conduct the study, which is the subject of this manuscript. RDH has served in a consulting or advisory role for Amgen, Roche, Merck, Sanofi, Bayer, Ipsen, BMS, MSD; Honoraria: Amgen, Astra Zeneca, Bayer, BMS, Boehringer, Ipsen, Lilly, Medac, Merck, MSD, Roche, Saladax Servier, Pierre Fabre, and Sanofi and reports research grants from Amgen, Medac, Merck, Roche, Saladax, and Sanofi. AG reports grants, personal fees, and non-financial support from Genentech/ Roche; grants, personal fees, and non-financial support from Bayer; grants from Boston Biomedicals; grants from OBI Pharmaceuticals; grants, personal fees, and non-financial support from Array/ Pfizer; and grants from Caris. JB reports grant support from Instituto de Salud Carlos III (PI18/00768) (which funds CIBERehd), AECC (PI044031), and WCR (AICR) 16-0026; consulting honoraria from Arqule, Bayer-Schering Pharma, Novartis, BMS, BTG- Biocompatibles, Eisai, Kowa, Terumo, Gilead, Bio-Alliance, Roche, AbbVie, MSD, Sirtex, Ipsen, Astra-Medimmune, Incyte, Quirem, Adaptimmune, Lilly, Basilea, Nerviano, and Sanofi; and research grants from Bayer and BTG. GDD reports a scientific consultancy with sponsored research to Dana-Farber from Bayer, Pfizer, Novartis, Roche/Genentech, Epizyme, LOXO Oncology, AbbVie, GlaxoSmithKline, Janssen, PharmaMar, ZioPharm, Daiichi-Sankyo, AdaptImmune, Ignyta, and Mirati. GDD reports a scientific consultancy for EMD-Serono, Sanofi, ICON plc, WCG/Arsenal Capital, Polaris Pharmaceuticals, MJ Hennessey/OncLive, and MEDSCAPE. GDD reports being a consultant/SAB member with minor equity holding for G1 Therapeutics, Caris Life Sciences, Champions Biotechnology, Bessor Pharmaceuticals, Erasca Pharmaceuticals, RELAY Therapeutics, and Caprion/HistoGeneX. GDD reports being a Board of Directors member and Scientific Advisory Board Consultant with minor equity holding for Blueprint Medicines, Merrimack Pharmaceuticals (ended October 2019), and Translate BIO. GDD reports a Novartis royalty to Dana-Farber for patent use of imatinib in GIST. GDD reports non-financial interests with McCann Health, Alexandria Summit, and as the AACR Science Policy and Government Affairs Committee Chair. GDD also reports personal fees from C4 Therapeutics, Synlogic, CellCarta, Ikena Oncologu, and Kojin Therapeutics. The authors report no other conflicts of interest in this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–164. [DOI] [PubMed] [Google Scholar]
- 4.Ettrich TJ, Seufferlein T. Regorafenib. Recent Results Cancer Res. 2018;211:45–56. [DOI] [PubMed] [Google Scholar]
- 5.Krishnamoorthy SK, Relias V, Sebastian S, Jayaraman V, Saif MW. Management of regorafenib-related toxicities: a review. Therap Adv Gastroenterol. 2015;8(5):285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grothey A, Cutsem EV, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16(6):619–629. [DOI] [PubMed] [Google Scholar]
- 8.Demetri GD, Reichardt P, Kang Y-K, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. [DOI] [PubMed] [Google Scholar]
- 10.Han G, Meinhardt G, Schlief S, Fiala-Buskies S, Rutstein M, Wuchter-Czerwony C Integrated safety analysis from four phase 3 trials of regorafenib. Paper presented at: International Liver Cancer Association’s (ICLA) 11th Annual Conference; September 15–17, 2017; Seoul, South Korea. [Google Scholar]
- 11.Sitlinger A, Zafar SY. Health-related quality of life: the impact on morbidity and mortality. Surg Oncol Clin N Am. 2018;27(4):675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kluetz PG, Slagle A, Papadopoulos EJ, et al. Focusing on core patient-reported outcomes in cancer clinical trials: symptomatic adverse events, physical function, and disease-related symptoms. Clin Cancer Res. 2016;22(7):1553–1558. [DOI] [PubMed] [Google Scholar]
- 13.Chang J, Casali PG, Reichardt P, et al. Health-related quality of life (HRQoL) of patients with advanced gastrointestinal stromal tumors (GIST) treated with regorafenib (REG) vs placebo (P) in the Phase III GRID trial. Paper presented at: The European Cancer Congress 2013; September 27-October 01, 2013; Amsterdam, The Netherlands. [Google Scholar]
- 14.Chang J, Odom D, Radder C, et al. A post-hoc health-related quality of life (HRQoL) analysis of patients with metastatic colorectal cancer (mCRC) in the phase III CONCUR trial. J Clin Oncol. 2015;33(3_suppl):667.25605839 [Google Scholar]
- 15.Charton E, Cuer B, Cottone F, et al. Time to deterioration in cancer randomized clinical trials for patient-reported outcomes data: a systematic review. Qual Life Res. 2019. [DOI] [PubMed] [Google Scholar]
- 16.Anota A, Hamidou Z, Paget-Bailly S, et al. Time to health-related quality of life score deterioration as a modality of longitudinal analysis for health-related quality of life studies in oncology: do we need RECIST for quality of life to achieve standardization? Qual Life Res. Jan. 2015;24(1):5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. [DOI] [PubMed] [Google Scholar]
- 18.Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol. 2011;29(1):89–96. [DOI] [PubMed] [Google Scholar]
- 19.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Food and Drug Administration. Regorafenib Full Prescribing Information. 2012. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203085lbl.pdf. Accessed January11, 2021.
- 21.Bekaii-Saab TS, Ou FS, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20(8):1070–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van cutsem E, Martinelli E, Cascinu S, et al. Regorafenib for patients with metastatic colorectal cancer who progressed after standard therapy: results of the large, single-arm, open-label phase IIIb CONSIGN study. Oncologist. 2019;24(2):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]