Abstract
Healthy and pathological aging influence brain microstructure via complex processes. Discerning these processes require measurements that are sensitive to specific biological properties of brain tissue. We integrated a novel quantitative R1 measure with multi-shell diffusion weighted imaging to map age-associated changes in macromolecular tissue volume (MTV) along major white matter tracts in healthy older adults and patients with amnestic Mild Cognitive Impairment (aMCI). Reduced MTV in association tracts was associated with older age in healthy aging, was correlated with memory performance, and distinguished aMCI from controls. We also mapped changes in gray matter tissue properties using quantitative R1 measurements. We documented a widespread decrease in R1 with advancing age across cortex and decreased R1 in aMCI compared with controls in regions implicated in episodic memory. Our data are the first to characterize MTV loss along major white matter tracts in aMCI and suggest that qMRI is a sensitive measure for detecting subtle degeneration of white and gray matter tissue that cannot be detected by conventional MRI and diffusion measures.
Keywords: Quantitative MRI, R1, macromolecular, healthy aging, mild cognitive impairment, diffusion
Introduction
The biology of white matter microstructure is complex and plays a critical role in multiple aspects of healthy cognitive function, and it is associated with cognitive decline in aging. Many neurodegenerative disorders – from amnestic mild cognitive impairment (aMCI) to Alzheimer’s disease (AD) – are associated with white-matter abnormalities that may partly be caused by the microstructural decline of the brain’s connective pathways through axonal demyelination (Mito et al., 2018; Yu et al., 2017). The cognitive neuroscience of human aging relies heavily on neuroimaging techniques to describe how cognitive changes map to their neural substrates in white matter tracts. The distinction between healthy aging and pathological age-related brain changes is intricate and heretofore remains ill-defined.
Diffusion weighted magnetic resonance imaging (dMRI) is the primary neuroimaging technique used to measure and model white matter microstructure. To date, there is a substantial amount of dMRI research documenting changes in white matter tissue properties in health aging, aMCI and AD (Bennett et al., 2010; Cox et al., 2016; Strain et al., 2018; Gozdas et al., 2020). dMRI is sensitive to self-diffusion of water molecules in tissue and is affected by alterations in local tissue microstructure. dMRI provides a series of measurements describing the amount of water diffusion along a particular direction in space and is open to many biological interpretations. Variability in these measurements reflect changes in the profile of water diffusion and have been therefore associated with alterations in the underlying tissue microstructure. The diffusion of water is highly dependent on tract orientation as water molecules travel more easily along the white matter fiber bundles than across them due to physical barriers such as myelin and cell membranes. Therefore, conventional measures of diffusion such as fractional anisotropy (FA) are sensitive but nonspecific markers of white matter integrity (Cox et al., 2016; Pierpaoli and Basser, 1996; Metzler-Baddeley et al., 2012).
Healthy and pathological aging influence white matter microstructure via complex processes involving myelination, axonal packing, axonal density, and macromolecular content (Mito et al., 2018; Colgan et al., 2016; Yeatman et al., 2014). Although conventional measures can interrogate white matter integrity, diffusion metrics cannot entirely describe the biological processes associated with healthy and pathological aging. Recent advances in quantitative MRI (qMRI) provided the opportunity to characterize specific biological properties of white matter tissue in vivo. The unique feature of qMRI measurements is that they are designed to be particularly robust to a range of scan parameters and field strengths and therefore have great potential for clinical applications (Mezer et al., 2013). Here we integrate a novel qMRI technique with advanced multi-shell dMRI and tractography to map age-associated changes in white matter tissue composition in healthy aging and how they are affected in aMCI, a prodromal stage for AD. We utilized a qMRI procedure that provides a robust, fast and reliable measure of the longitudinal relaxation rate (quantitative R1) of hydrogen protons in a magnetic field. The quantitative R1 measurements reflect with great accuracy the variation in myelin content (Yeatman et al., 2014; Stüber et al., 2014). Moreover, these measurements can be used to quantify macromolecular tissue volume (MTV) in white matter (Yeatman et al., 2014; Mezer et al., 2013). MTV measurements particularly reflect the quantity of macromolecules and lipid membranes in white matter. A voxel with higher concentration of myelinated axons contains more macromolecules and would result in higher MTV and longer R1 values compared with a voxel filled with cellular tissue that contains higher concentration of water molecules. The association between myelin content and macromolecular tissue volume has been reported in previous studies as well (Rooney et al., 2007; Schyboll et al., 2019).
Previous research suggests that various biomarkers of aging are highly correlated, and therefore distinguishing the biological changes associated with healthy aging from those associated with pathological aging remains challenging (Yeatman et al., 2014; Filo et al., 2019). By combining different measurements with known biophysical properties of different tissue types, it is possible to fully describe the changes in the biological composition of the brain tissue in older age. One theory of aging suggests that several mechanisms have a heterogeneous effect throughout the brain (Cole et al., 2019). So, combining different measurements of brain tissue is crucial to quantify the state of the brain aging. To demonstrate these, it is critical to measure distinct biological aspects of white matter brain tissue.
Further, quantitative R1 measurements can be indicative of changes associated with normal aging (Cowley et al., 2012; Callaghan et al., 2014) and disease related tissue microstructural changes in the cortex. Recent studies have reported that in vivo R1 can characterize microstructural abnormalities that are not obvious upon visual assessments of conventional MRI images in AD and dementia (Su et al., 2015; Su et al., 2016) and can also be used to investigate relationships between pathology and cognition. However, the studies have used mostly conventional methods to map R1 because the gold-standard inversion recovery (IR) pulse sequence (Mezer et al., 2013; Barral et al., 2010) for accurate measurement of quantitative R1 is too long and not applicable to clinical populations. In this study, we have used a slice-shuffling simultaneous multi-slice (SS-SMS) sequence that can produce robust, very fast and unbiased quantitative R1 estimates well suited for clinical application (Wu et al., 2015).
Here we first investigated tract specific differences according to age and hemisphere to characterize which biomarker and which tract was most age sensitive. We provide evidence to support age-related decline in macromolecular tissue content in older age with more pronounced effects in association tracts. Next, we examined quantitative differences in biological properties of white matter tissue between patients with aMCI and healthy older controls that support the assertion that multiple independent processes are governing white matter tissue parameters and that MTV is very sensitive to these processes. Further, we modeled the processes of abnormal white matter degeneration by examining the association between the biological and cognitive decline. Finally, we examined age correlations with quantitative R1 measurements and their alterations in aMCI in the cortex to understand how changes in myelin affect cortical computations and resulting behaviors.
Materials and Methods
Participants
Forty-five healthy controls (HC) (age range 65–88 years, 32/13 females/males) and 19 age- and education-matched patients with aMCI (age range 65–85 years, 8/11 females/males) were included in the study (Supplementary Table S1). All participants were screened for current status and history of medical and psychiatric conditions via an electronic screening form (see Supplementary Methods). The inclusion criteria were the following: no Axis I disorder based on the mini international neuropsychiatric interview (M.I.N.I), a structured clinical interview screening for primary psychiatric conditions; score >=7 on the geriatric depression scale (GDS); score >=24 on the Mini-Mental State Examination (MMSE), a measure of global cognition; intact score on the instrumental activities of daily living (IADL) scale which assesses functional ability on eight independent activities of daily living.
The Clinical Dementia Rating (CDR) was used to categorize participants as aMCI (CDR=0.5) or HC (CDR=0). Further, the cognitive measures were administered using the NIH Toolbox Cognition Battery (www.nihtoolbox.org) (for detailed procedures see Supplementary Methods). The Stanford University Institutional Review Board approved all the data collection procedures and all experiments were performed in accordance with relevant guidelines and regulations. The informed consent was obtained from all participants.
dMRI acquisition and processing
All MRI data were collected on a 3T GE system (General Electric Healthcare, Milwaukee, WI, USA) equipped with a 32-channel head coil (Nova Medical, Wilmington, MA, USA). dMRI data were acquired using a simultaneous multi-slice (SMS) echo-planar imaging (EPI) acquisition scheme at the Center for Cognitive and Neurobiological Imaging at Stanford University (http://www.cni.stanford.edu/). More details are provided in Supplementary Methods for dMRI acquisition, processing and white matter tract identification.
Quantitative MRI mapping protocol
The quantitative T1 sequence is an inversion-recovery (IR) gradient-echo EPI sequence. It uses an interleaved acquisition and a special ordering of the slice acquisition, such that the whole imaging volume can be acquired at multiple different inversion times in a short period of scan time. The sequence also utilizes the SMS to further accelerate the acquisition (Wu et al., 2015). The scan gives us quantitative T1 measurement at 2 mm3 spatial resolution in a minimum time (less than 3 minutes). An additional short scan with reversed phase encoding direction was acquired for the EPI distortion correction. We used an open-source python code (https://github.com/cni/t1fit/blob/master/t1fit_unwarp.py) to process both NIFTI files to get quantitative T1 map after EPI distortion correction using FSL’S TOPUP. The details of scan parameters are as follows: flip angle=77, repetition time (TR)=3s, FOV=24cm, matrix size=120×120, slice thickness=2mm and TI=1. The quantitative R1 measurement obtained with this method have been previously validated against the standard IR spin-echo EPI sequence (Mezer et al., 2013; Barral et al., 2010) (for details see Supplementary Methods).
Mapping MTV along white matter tracts
Tissue properties were calculated along the trajectory of the fiber group by first resampling each fiber to 30 equally spaced nodes and then interpolating the values from a co-registered qMRI image at each node along each fiber (see Supplementary Methods). Each subject’s quantitative T1 map was co-registered to their dMRI data using the ANTS software package to warp the quantitative T1 map to the non-diffusion weighted b0 image. We used the equation validated in previous study (Mezer et al., 2013) to quantify changes in MTV along each fascicle using the quantitative R1 (1/qT1) measurements:
Cortical R1 mapping
The T1-weighted images were used to generate individual cortical surfaces using FreeSurfer (version 6.0.0 available at http://surfer.nmr.mgh.harvard.edu). The automated pipeline for cortical reconstruction steps included skull stripping, gray and white matter segmentation, manual editing for inaccuracies in the automated steps if necessary as well as reconstruction and inflation of the cortical surface. The overall cortical and white matter volumes were estimated by the automated brain segmentation and then we utilized the Human Connectome Project’s multi-modal parcellation (HCPMMP) (Glasser et al., 2016). This parcellation scheme includes 180 cortical brain regions per hemisphere and is based on the cortical architecture, function, connectivity, and topography from 210 healthy individuals. The original annotation files provided by the HCP were converted to the standard cortical surface in FreeSurfer called fsaverage. This fsaverage parcellation was transformed to each participant’s individual cortical surface and converted to volumetric masks. Finally, the 360 masks representing single cortical brain regions yielded by the HCPMMP were linearly transformed into each subject’s native space of the diffusion-weighted images. The transformation was manually checked in ITK-SNAP, and manual orientation was applied if necessary. The R1 values were subsequently extracted from transformed brain regions served as anatomical landmarks across the cortex.
Statistical analysis
All statistical analyses were carried out using scripts written in R (version 3.5.3). Initially, associations between white matter measures along 19 tracts, quantitative R1 in 360 cortical ROIs, and age were modeled by controlling for sex and intracranial brain volume (ICV) using multiple regression. To eliminate the influence of crossing fibers near cortical terminations and to avoid partial volume effects at gray-white matter boundaries, diffusion properties were first averaged over the middle sections (nodes 7–24) of each tract to generate a single mean value of diffusion parameters for each tract. To further investigate whether white matter measures and cortical R1 provide unique microstructural information, we ran ANCOVA models to test the main effect of the group by including age, sex, and ICV as covariates and their interactions. Benjamini-Hochberg false discovery rate (FDR) correction was used to account for multiple comparisons across the white matter tracts and cortical ROIs (pFDR<0.05). The multiple comparisons were applied after including all the diffusion parameters (MTV, MD, RD, AD, and FA) along all white matter tracts for the age association and the most age-sensitive diffusion parameters (MTV and MD) for group comparison. Also, R1 values in 360 cortical ROIs for age association and memory-related cortical ROIs for group difference were included for multiple comparisons.
Results
White matter tissue properties association with age and hemisphere
Each qMRI parameter is sensitive to different white matter tissue properties and we use these measurements for detailed investigation of biological changes associated with healthy aging. We modeled age in association with MTV, MD, axial diffusivity (λax), radial diffusivity (λrad) and FA for 19 fascicles utilizing cross-sectional dMRI and qMRI measurements in our healthy older adult sample. Older age was significantly associated with a lower macromolecular tissue volume (lower MTV; β ≤ −0.6, and lower R1; β ≤ −0.59), a higher magnitude of water diffusion (higher MD, λa,x and λrad; β ≤ 0.7) and lower tract integrity (lower FA β ≤ −0.62) across the majority of tracts (p < 0.05, FDR corrected). Fascicles varied substantially in terms of their amount of white matter change with age. Associations between MTV and MD with age were especially marked in association tracts including cingulum cingulate, cingulum hippocampus, inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus (SLF), Arcuate and Uncinate fibers (Figure 1, Table S2) as well as in the forceps minor fibers. Age associated changes in FA only marked in the left cingulum hippocampus, bilateral IFOF, bilateral ILF, right SLF and right uncinate (Figure 1, Table S2). In addition to tract associations with age, evidently healthier white matter microstructure was found for some measures in the right versus left hemisphere such as higher mean MTV and lower mean MD (Figure S1, Table S2).
Figure 1.
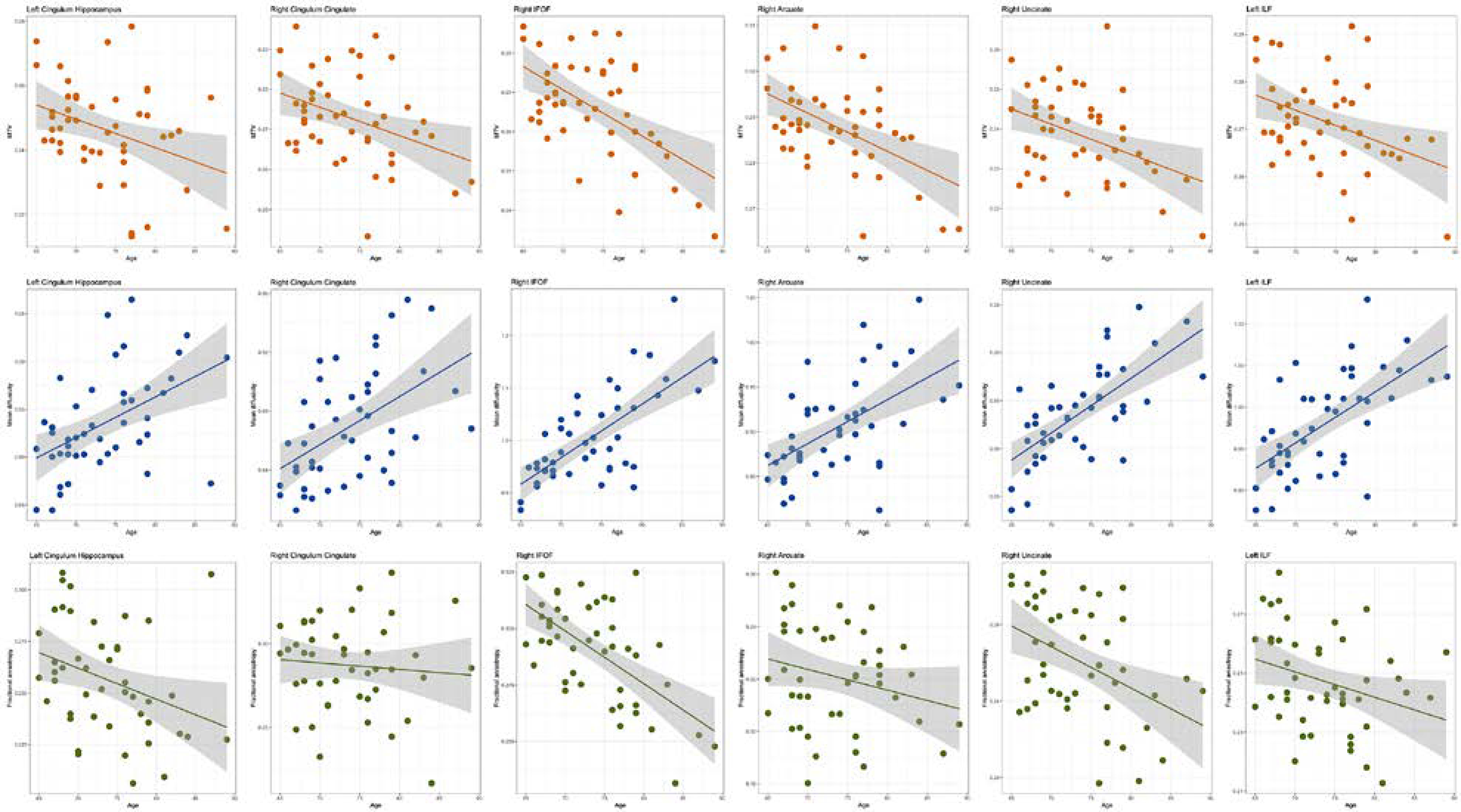
Age associations with the microstructural characteristics of white matter fibers. The width of the line denotes the 95% confidence interval around the linear model fit. Older age was associated with significantly lower MTV and FA and significantly higher MD across the majority of the tracts.
We also contextualized the utility of aforementioned white matter properties by examining how much age variation they could explain beyond that explained by conventional brain volumetric measures (total brain, white matter, gray matter and hippocampal volumes; corrected for intracranial brain volume) in the same group. Increasing age was associated with lower total brain, gray matter and hippocampal volumes (Figure S2). However, age related variance in specific aspects of white matter microstructure was independent of hippocampal, total brain and gray matter volume (p>0.05, FDR corrected).
Changes in white matter microstructure across brain
We next examined whether a given microstructural measure was positively correlated among all tracts across the brain. Within all white matter biomarkers (with the exception of FA), the measurements from across all tracts are correlated positively; for instance, those with lower MTV in one tract tended to have lower MTV in all other tracts (except for callosum forceps minor for MTV) (p<0.001) (Figure S1). Between quantitative white matter measures, MTV and R1 from across all tracts, and between diffusion white matter measures MD, AD and RD from across all tracts are highly positively correlated (p<0.001). To understand the extent of the relationship between MTV and diffusion measures we used a linear model to predict diffusion measures for each tract. On average MTV predicted 26% of the variance in diffusivity values and 18% of the variance in FA values. Most importantly, age predicted a significant amount of MTV values even after removing the effect of FA across the majority of the tracts. These results highlight that qMRI and diffusion measures are weighted to be sensitive to different properties of tissue and that different tissue properties evolve independently with age.
Multiple biological white matter processes in aMCI
MTV measurements can be applied to clinical research to identify and monitor brain tissue change. Specifically, it could serve as a much-needed reliable MRI approach for quantification of brain changes (Mezer et al., 2013) that can be used to detect and quantify tissue loss in neurodegenerative diseases such as AD. We examined changes in the microstructural properties most sensitive to aging, i.e., MTV and MD, in individuals with aMCI compared with healthy older controls (see Table S1 for participant characteristics). We observed significant differences in MTV and MD between groups but in different sets of white matter tracts that support the assertion that multiple distinct processes are governing white matter tissue properties and that MTV is very sensitive to these processes (Figure 2). Particularly, aMCI subjects had lower MTV values in the right cingulum hippocampus and callosum forceps minor which suggests demyelination and macromolecular tissue reduction in those tracts. However, aMCI group had higher MD values in the left cingulum hippocampus (p<0.05, FDR-corrected) (Figure 2). We noted that the magnitude and location of the observed between-group differences in MTV and MD significantly varied across the fascicles and parameters. This highlights that MTV is weighted to different biological processes that drive changes in white matter in aMCI when compared with conventional diffusion measures.
Figure 2.
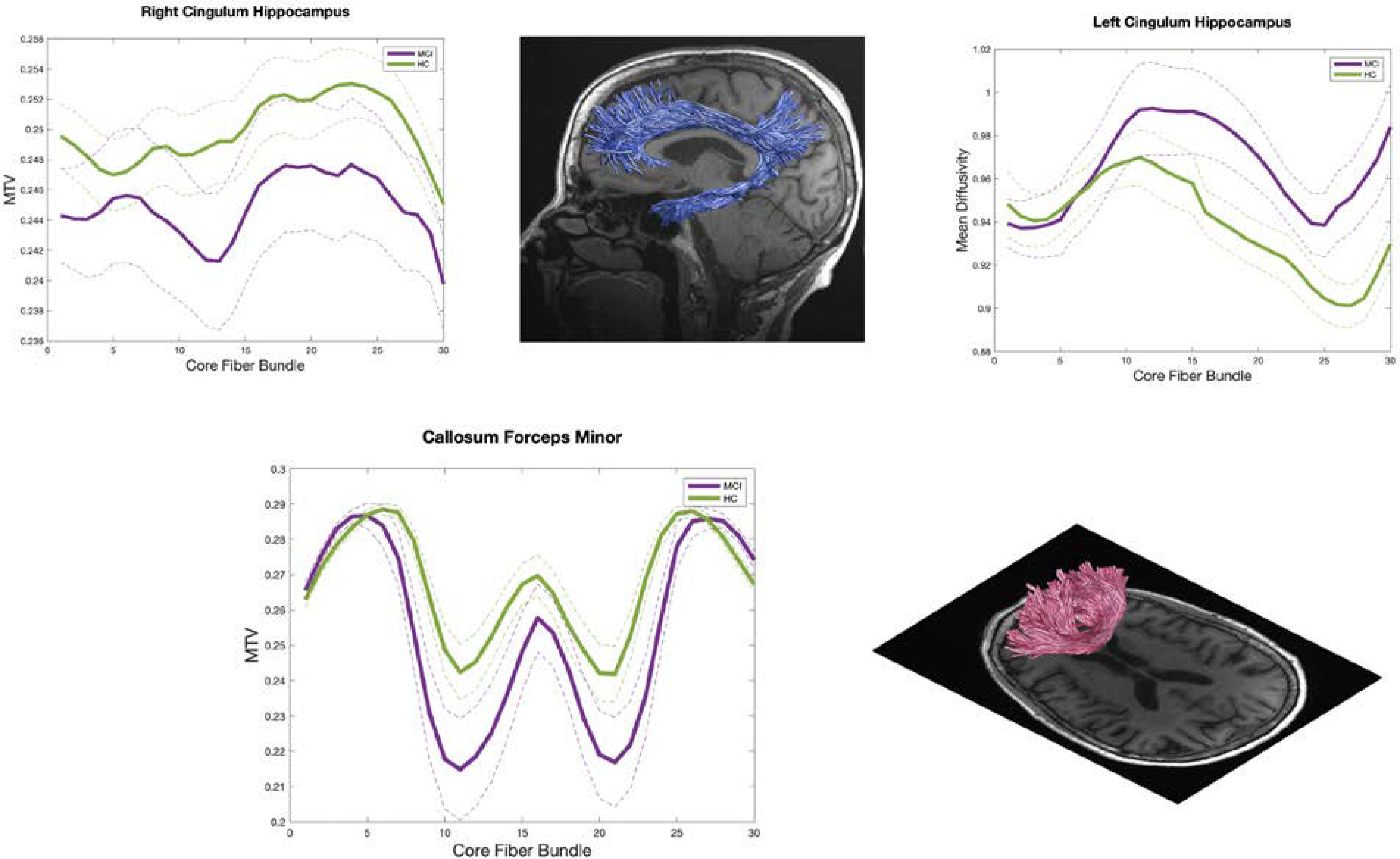
Macromolecular tissue volume (MTV) and Mean diffusivity (MD) in aMCI and healthy control (HC) in the right cingulum hippocampus, left cingulum hippocampus and callosum forceps minor fasciculus. Each line represents the group average MTV and MD across subjects and dotted lines indicate standard error of the mean.
Relationship between white matter microstructure and cognitive decline
aMCI is associated with significant decline in memory function. Therefore, we tested if the observed changes in white matter tissue properties between aMCI and controls would explain group differences in memory performance. We examined changes in white matter microstructures as a function of Rey, Symbol Digit Modalities Test (SDMT) and Picture Sequence Memory Test (PSMT) scores that were significantly impaired in aMCI compared to HC (Table S1). Multiple regression analysis revealed that the SDMT and PSMT scores were positively correlated with MTV in the right cingulum hippocampus and the right corpus callosum (CC) (β ≤ −0.52), and negatively correlated with MD in the left cingulum hippocampus across the groups (β ≤ −0.53). SDMT scores were positively correlated with MTV in the right CC (β = 0.55) and negatively correlated with MD in the left cingulum hippocampus (β = 0.56) in HC while there were no significant correlations in aMCI group. These associations and the association between Rey Score and MTV in the right CC were significantly different between groups with analysis of covariance (p<0.05, FDR-corrected) (Figure 3).
Figure 3.
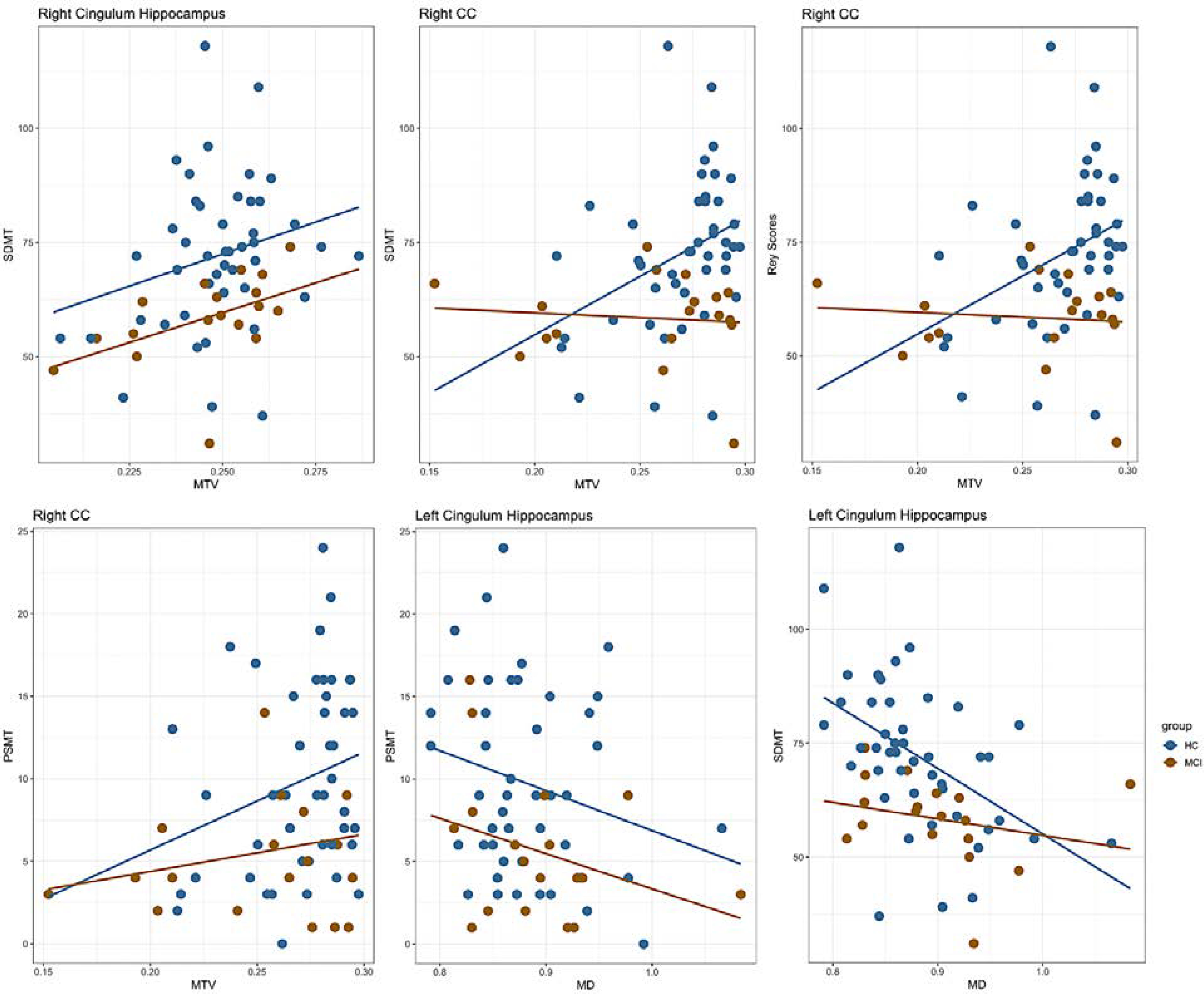
Correlations between white matter measures and cognitive performance in aMCI and HC. As described in the main text, SDMT and PSMT scores were positively correlated with MTV in the right cingulum hippocampus and the right corpus callosum (CC), and negatively correlated with MD in the left cingulum hippocampus across the groups. SDMT scores were positively correlated with MTV in the right CC and negatively correlated with MD in the left cingulum hippocampus in HC while there were no significant correlations in aMCI group. These associations and the association between Rey Score and MTV in the right CC were significantly different between groups with analysis of covariance (p<0.05, FDR-corrected).
Aging and aMCI patterns of Cortical distribution of quantitative R1
In order to investigate microstructural properties within cortical regions, we performed region of interest (ROI) analysis that involved extracting quantitative R1 in native space from 360 cortical ROIs using HCP atlas (Glasser et al., 2016). In the sample of 45 healthy older adults, we found that 135 ROIs showed decreased R1 (p<0.05, FDR-corrected) with age spanning dorsolateral prefrontal, inferior frontal, inferior parietal, lateral and superior temporal, and sensory cortices. (Figure 4a).
Figure 4.
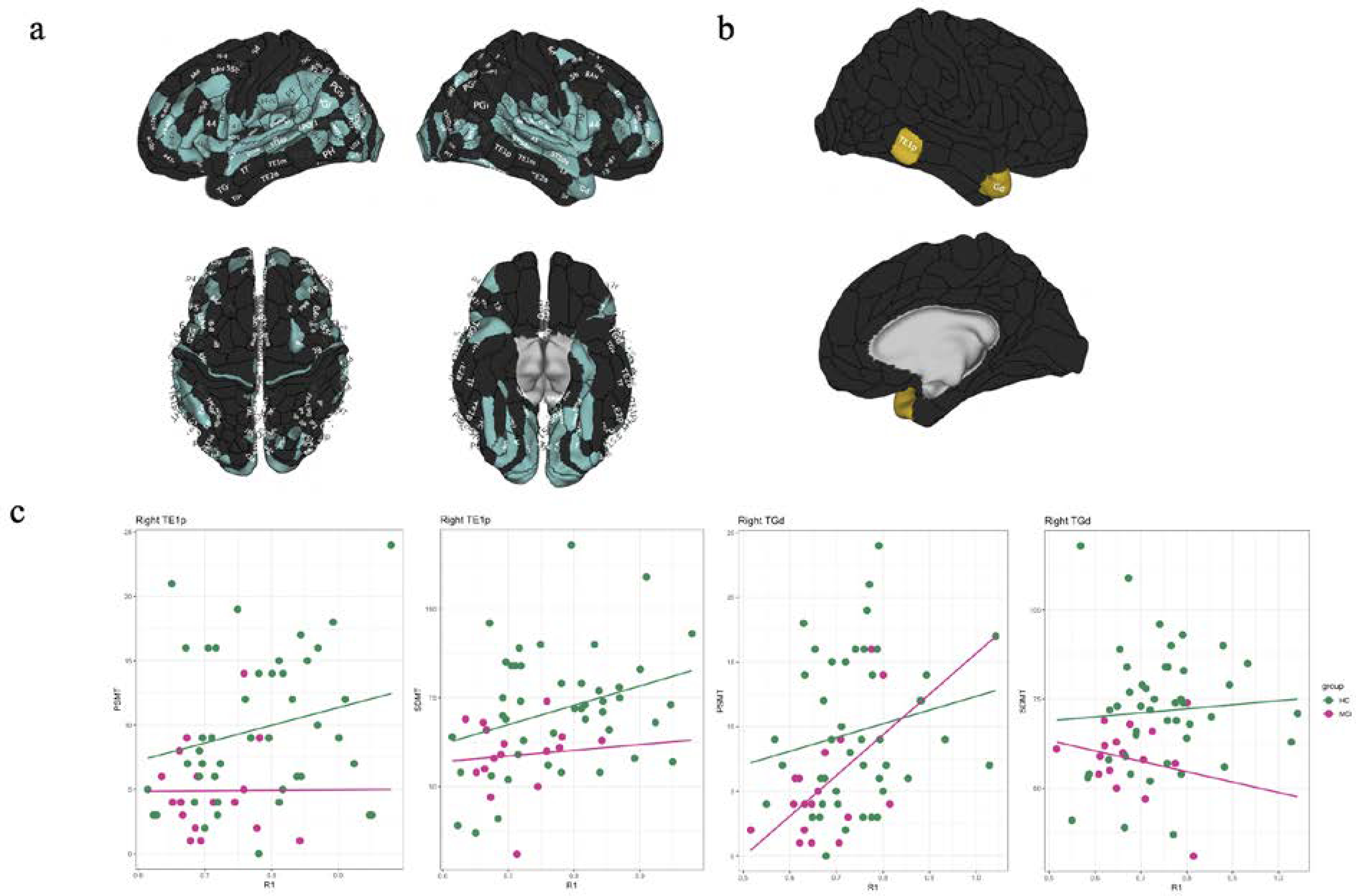
Effects of (a) healthy aging and (b) aMCI on quantitative R1 across the cortex. R1 in cortical gray matter showed a significant age-associated decline in HC (turquoise) and was significantly affected in aMCI (yellow) participants when compared to HC. (c) Correlations between cortical R1 and cognitive performance in aMCI and HC. SDMT scores were positively correlated with R1 in the right TE1p across the groups and within the control group while PCMT scores were positively correlated with R1 in the right TE1p and TGd across the groups. These associations were significantly different between groups (p<0.05, FDR-corrected).
We next examined alterations in quantitative R1 in aMCI in cortical regions known to be related to memory function (Olson et al., 2007). These regions included the inferior medial temporal (hippocampus (H), presubiculum (PreS), entorhinal cortex (EC), the perientorhinal and ectorhinaal complex (PeEc) and pre-hippocampal areas 1, 2, 3 (PHA1, PHA2, PHA3), and the lateral temporal cortex and temporal pole (TE1p, TE1m, TE1a, TGd, TE2p, TF). We observed significantly decreased R1 in aMCI group in the right TE1p and TGd (Figure 4b) while there was no significant group difference in cortical thickness for these ROIs between aMCI and healthy controls (p<0.05, FDR corrected). Finally, SDMT scores were positively correlated with R1 in the right TE1p across the groups (p<0.004, FDR-corrected) and within the control group (p<0.032, FDR-corrected) while PCMT scores were positively correlated with R1 in the right TE1p and TGd across the groups (p<0.05, FDR-corrected). These associations were significantly different between groups (p<0.05, FDR-corrected) (Figure 4c).
Discussion
This study expands our understanding of white matter and cortical microstructure of the brain in healthy aging and aMCI. Older age was associated with less healthy white matter (decreased MTV and increased MD) predominantly in the association fasciculi (Cingulum bundle, ILF, IFOF, SLF, Arcuate and Uncinate) and decreased R1 values indicating diminished tissue quantities in cortical brain regions. Moreover, we found microstructural white and gray matter tissue differences between individuals with aMCI and controls, indicating that quantitative R1 is sensitive to disease pathology and can be used as a robust marker for disease states in addition to its utility in quantifying changes associated with healthy aging. Finally, we combined quantitative R1 measurements with cognitive performance to explain the coupling between biological and cognitive decline in older adults and patients with aMCI.
While the number of neurons remains relatively constant, axons in white matter begin to degenerate due to aging processes (Peters, 2002) and its degeneration appears to be coupled with degeneration of their myelin sheaths (Sandell and Peters, 2001). We found a substantial change in MTV and diffusivity (MD, λax, λrad) in association with aging for the majority of white matter tracts which is consistent with previous reports (Cox et al., 2016; Yeatman et al., 2014). An underlying reason for these results may be that axons increase in diameter and oligodendrocytes wrap myelin around these axons during development and until the period of relative stability before the white matter begins to degenerate. This additional wrap of the myelin provides a barrier to water diffusion and reduces overall water content of the tract. These have a particularly large impact on decreased MTV, and increased diffusivity in older adults. We further found evidence to support age-related reduction in white matter connectivity in older adults especially in association tracts. These findings provide a foundation for further investigation of risk factors and mechanisms of brain and cognitive aging.
Quantitative MTV measures and diffusivity changed substantially in aMCI patients in white matter tracts associated with cognitive and memory skills. First, we found decreased MTV in the right cingulum hippocampus, callosum forceps minor and bilateral CST. MTV (and R1) measurements are truly quantitative and do not depend on specific hardware, pulse sequence and image resolution. They are also less sensitive to moderate subject motion compared with conventional diffusion imaging (Mezer et al., 2013; Barral et al., 2010). Thus, the normative model of healthy MTV aging can potentially be used to detect and quantify early white matter tissue loss in prodromal stage of AD. Our results are the first to use MTV to quantify white matter tissue loss in aMCI participants. Combining MTV with conventional and state-of-the-art diffusion metrics, we found age-related difference in white matter microstructural differences within individuals across tracts and between aMCI and healthy controls. These results lend understanding to the multiple biological processes that distinguish aMCI and healthy aging.
R1 has been used to characterize microstructural tissue abnormalities in specific brain disorders including traumatic brain injury (Saito et al., 2012), multiple sclerosis (Gracien et al., 2016), AD, and dementia (Su et al., 2015; Tang et al., 2018). However, no study has focused on the early stage of AD including aMCI. A few studies have begun to reveal how brain microstructures change with aging in cortical brain regions and along white matter tracts using qMRI measures of R1 (Yeatman et al., 2014; Filo et al., 2019; Erramuzpe et al., 2021). Because R1 is a quantitative measure, it may improve to manifest the underlying biological process of healthy aging and age-related neurological disorders. In the present study, we also focused on cortical microstructures both in healthy aging and the early stage of AD, aMCI. We revealed a widespread age-associated decrease in R1 within the dorsolateral prefrontal, inferior frontal, inferior parietal, lateral and superior temporal, and sensory cortices. Age-associated changes in local biophysical microstructure and biochemical properties of the cortex along with myelin-loss in white matter may contribute to these findings. Moreover, the aMCI group had significantly decreased R1 in the right TE1 and TGd compared with healthy controls. These regions are located in the right lateral temporal cortex and temporal pole, related to episodic memories, being more closely associated with emotion and socially relevant memory (Smith and Kosslyn, 2007). Conversely, there were no significant between-group differences in cortical thickness for any of the ROIs we investigated. While we speculate that our aMCI sample was not big enough to be able to detect differences in cortical thickness between groups, these data suggest that quantitative R1 measurements are more sensitive in detecting potential tissue pathology in aMCI than the conventional brain volumetric measures.
Our data also highlighted substantial white matter degeneration and cortical microstructural changes specific to the right hemisphere in aMCI patients. To date, there is ample neuroimaging research documenting atrophy and white matter changes in the right hippocampus and cingulum bundle in aMCI and AD participants (Nedelska et al., 2012; Spiers et al., 2001; DeIpolyi et al., 2007). Decreased right hippocampal volume was associated with poorer spatial navigation, and decreased integrity (lower FA, higher MD, and lower fiber density) in the right cingulum bundle was associated with lower processing speed reported consistently in aMCI and AD (Metzler-Baddeley et al., 2012; Nedelska et al., 2012). Thus, our results support the critical role of the right hemisphere, particularly when cognitive impairment is present. These results can also be used, along with additional validation, as a basis for identifying patients in the preclinical stage of AD.
One of the major goals of neuroscience is to understand the cellular processes in the living human brain that drive changes in cognitive function in aging. There is an extensive literature linking white matter microstructure with learning and cognitive performance, with correlations typically localized to pathways that are functionally relevant to the interrogated cognitive skill (Cremers et al., 2016). For example, working memory training is associated with increased FA in parietal fibers (Takeuchi et al., 2010) while visual perceptual learning is associated with increased FA in white matter tracts underlying the early visual cortex in older adults (Yotsumoto et al., 22014). Most studies have reported that higher cognitive scores are associated with increased FA or decreased MD, a pattern typically associated with increased myelin or increased density of a fiber bundle in older adults and aMCI patients. However, changes in the opposite direction have also been reported (Taubert et al., 2010). This highlights a challenge in interpreting measures such as FA, which do not show a direct relationship with underlying tissue properties. Here, our results offer a different interpretation of biological mechanisms in white matter tracts. We found that lower MTV and increased MD in white matter tracts is associated with decreased cognitive and memory skills in aMCI patients and older adults. These cognitive and memory skills were also significantly different between groups. While white matter diffusion properties have been consistently linked to cognition and behavior in health and disease, the underlying biological mechanisms have remained a mystery. Combining conventional diffusion measures with qMRI provided us valuable information regarding the potential biological mechanisms linking white matter tissue changes to behavior in aging and aMCI.
This multimodal brain imaging study has provided clear insights into microstructural and biological changes in brain connectivity in older age and aMCI. In this study, we located and quantified the age-related differences in the white matter tracts and cortical brain regions across the brain that provided robust information about which diffusion and quantitative biomarkers were especially sensitive in healthy aging. We also demonstrated the utility of these biomarkers for detection and quantification of abnormal white matter changes in aMCI and how these biological changes linked to behavior. Together, these findings support the goal of using qMRI to quantitatively monitor healthy aging and disease progression within an individual. While the sample size reported here was relatively small to characterize the white matter and cortical tissue properties variation in clinical populations, we hope that data sharing across institutions will assists in obtaining larger, more diverse samples. MTV and R1 are quantitative metrics that provide complementary information to measures of diffusion. Their quantitative nature makes MTV and R1 ideal for scientific and clinical applications. Particularly, because these measures are insensitive to site and scanner differences (Mezer et al., 2013; Weiskopf et al., 2013), they can be used for comparing patients’ data to a control population over time. Our findings provide a basis for further exploration of risk factors and mechanisms of brain and cognitive aging in aMCI. Knowledge of these critical factors will facilitate early identification and treatment of white matter abnormalities in aging and aMCI brain.
Supplementary Material
Acknowledgements
We thank the participants for their involvement in the study as well as the researchers involved in coordinating data collection for this project. We also thank Dr. Michael Greicius for his input on the study design and sample characteristics.
Funding
The study was partly funded by a Career Development Award from the National Institute on Aging (NIA) to SMH (K25AG050759). EG’s effort was partly supported by Stanford Maternal and Child Health Research Institute (MCHRI). SMH’s effort was supported in part by NIA (K25AG050759 and R21AG064263) and National Institute of Mental Health (NIMH) (R61MH119289 and R21MH123873).
Footnotes
Conflict of Interest
The authors declare that they have no competing interests.
References
- Barral JK, Gudmundson E, Stikov N, Etezadi-Amoli M, Stoica P, Nishimura DG, 2010. A robust methodology for in vivo T1 mapping. Magnetic resonance in medicine 64, 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH Jr, 2010. Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Human brain mapping 31, 378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan MF, Freund P, Draganski B, Anderson E, Cappelletti M, Chowdhury R, Diedrichsen J, FitzGerald TH, Smittenaar P, Helms G, 2014. Widespread age-related differences in the human brain microstructure revealed by quantitative magnetic resonance imaging. Neurobiology of aging 35, 1862–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH, Marioni RE, Harris SE, Deary IJ, 2019. Brain age and other bodily ‘ages’: implications for neuropsychiatry. Molecular psychiatry 24, 266–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan N, Siow B, O’Callaghan JM, Harrison IF, Wells JA, Holmes HE, Ismail O, Richardson S, Alexander DC, Collins EC, 2016. Application of neurite orientation dispersion and density imaging (NODDI) to a tau pathology model of Alzheimer’s disease. Neuroimage 125, 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley TR, O’Sullivan J, Blau C, Deighan BF, Jones R, Kerskens C, Richardson JC, Virley D, Upton N, Lynch MA, 2012. Rosiglitazone attenuates the age-related changes in astrocytosis and the deficit in LTP. Neurobiology of aging 33, 162–175. [DOI] [PubMed] [Google Scholar]
- Cox SR, Ritchie SJ, Tucker-Drob EM, Liewald DC, Hagenaars SP, Davies G, Wardlaw JM, Gale CR, Bastin ME, Deary IJ, 2016. Ageing and brain white matter structure in 3,513 UK Biobank participants. Nature communications 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers LG, De Groot M, Hofman A, Krestin GP, Van Der Lugt A, Niessen WJ, Vernooij MW, Ikram MA, 2016. Altered tract-specific white matter microstructure is related to poorer cognitive performance: the Rotterdam Study. Neurobiology of aging 39, 108–117. [DOI] [PubMed] [Google Scholar]
- DeIpolyi A, Rankin K, Mucke L, Miller B, Gorno-Tempini M, 2007. Spatial cognition and the human navigation network in AD and MCI. Neurology 69, 986–997. [DOI] [PubMed] [Google Scholar]
- Erramuzpe A, Schurr R, Yeatman J, Gotlib I, Sacchet M, Travis K, Feldman H, Mezer A, 2021. A Comparison of Quantitative R1 and Cortical Thickness in Identifying Age, Lifespan Dynamics, and Disease States of the Human Cortex. Cerebral Cortex 31, 1211–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filo S, Shtangel O, Salamon N, Kol A, Weisinger B, Shifman S, Mezer AA, 2019. Disentangling molecular alterations from water-content changes in the aging human brain using quantitative MRI. Nature communications 10, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, 2016. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozdas E, Fingerhut H, Chromik LC, O’Hara R, Reiss AL, Hosseini SH, 2020. Focal white matter disruptions along the cingulum tract explain cognitive decline in amnestic mild cognitive impairment (aMCI). Scientific reports 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracien RM, Reitz SC, Hof SM, Fleischer V, Zimmermann H, Droby A, Steinmetz H, Zipp F, Deichmann R, Klein JC, 2016. Assessment of cortical damage in early multiple sclerosis with quantitative T2 relaxometry. NMR in Biomedicine 29, 444–450. [DOI] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Jones DK, Steventon J, Westacott L, Aggleton JP, O’Sullivan MJ, 2012. Cingulum microstructure predicts cognitive control in older age and mild cognitive impairment. Journal of Neuroscience 32, 17612–17619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezer A, Yeatman JD, Stikov N, Kay KN, Cho N-J, Dougherty RF, Perry ML, Parvizi J, Hua LH, Butts-Pauly K, 2013. Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Nature medicine 19, 1667–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mito R, Raffelt D, Dhollander T, Vaughan DN, Tournier J-D, Salvado O, Brodtmann A, Rowe CC, Villemagne VL, Connelly A, 2018. Fibre-specific white matter reductions in Alzheimer’s disease and mild cognitive impairment. Brain 141, 888–902. [DOI] [PubMed] [Google Scholar]
- Nedelska Z, Andel R, Laczó J, Vlcek K, Horinek D, Lisy J, Sheardova K, Bureš J, Hort J, 2012. Spatial navigation impairment is proportional to right hippocampal volume. Proceedings of the National Academy of Sciences 109, 2590–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y, 2007. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain 130, 1718–1731. [DOI] [PubMed] [Google Scholar]
- Peters A, 2002. The effects of normal aging on myelin and nerve fibers: a review. Journal of neurocytology 31, 581–593. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ, 1996. Toward a quantitative assessment of diffusion anisotropy. Magnetic resonance in Medicine 36, 893–906. [DOI] [PubMed] [Google Scholar]
- Rooney WD, Johnson G, Li X, Cohen ER, Kim SG, Ugurbil K, Springer CS Jr, 2007. Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 57, 308–318. [DOI] [PubMed] [Google Scholar]
- Saito S, Aoki I, Sawada K, Suhara T, 2012. Quantitative assessment of central nervous system disorder induced by prenatal X-ray exposure using diffusion and manganese-enhanced MRI. NMR in Biomedicine 25, 75–83. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Peters A, 2001. Effects of age on nerve fibers in the rhesus monkey optic nerve. Journal of Comparative Neurology 429, 541–553. [DOI] [PubMed] [Google Scholar]
- Schyboll F, Jaekel U, Petruccione F, Neeb H, 2019. Dipolar induced spin-lattice relaxation in the myelin sheath: A molecular dynamics study. Scientific reports 9, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Kosslyn S, 2007. Cognitive Psychology: Mind and Brain (International Edition). Upper Saddle River, NJ: Pearson. [Google Scholar]
- Spiers HJ, Burgess N, Maguire EA, Baxendale SA, Hartley T, Thompson PJ, O’Keefe J, 2001. Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain 124, 2476–2489. [DOI] [PubMed] [Google Scholar]
- Strain JF, Smith RX, Beaumont H, Roe CM, Gordon BA, Mishra S, Adeyemo B, Christensen JJ, Su Y, Morris JC, 2018. Loss of white matter integrity reflects tau accumulation in Alzheimer disease defined regions. Neurology 91, e313–e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber C, Morawski M, Schäfer A, Labadie C, Wähnert M, Leuze C, Streicher M, Barapatre N, Reimann K, Geyer S, 2014. Myelin and iron concentration in the human brain: a quantitative study of MRI contrast. Neuroimage 93, 95–106. [DOI] [PubMed] [Google Scholar]
- Su L, Blamire AM, Watson R, He J, Aribisala B, O’Brien JT, 2015. Tissue microstructural changes in dementia with Lewy bodies revealed by quantitative MRI. Journal of neurology 262, 165–172. [DOI] [PubMed] [Google Scholar]
- Su L, M Blamire A, Watson R, He J, Aribisala B, Brien J, 2016. Cortical and subcortical changes in Alzheimer’s disease: a longitudinal and quantitative MRI study. Current Alzheimer Research 13, 534–544. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Yamanouchi T, Suzuki S, Kawashima R, 2010. Training of working memory impacts structural connectivity. Journal of Neuroscience 30, 3297–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Cai F, Ding D-X, Zhang L-L, Cai X-Y, Fang Q, 2018. Magnetic resonance imaging relaxation time in Alzheimer’s disease. Brain research bulletin 140, 176–189. [DOI] [PubMed] [Google Scholar]
- Taubert M, Draganski B, Anwander A, Müller K, Horstmann A, Villringer A, Ragert P, 2010. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. Journal of Neuroscience 30, 11670–11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf N, Suckling J, Williams G, Correia MM, Inkster B, Tait R, Ooi C, Bullmore ET, Lutti A, 2013. Quantitative multi-parameter mapping of R1, PD*, MT, and R2* at 3T: a multi-center validation. Frontiers in neuroscience 7, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Dougherty R, Kerr A, Zhu K, Middione M, Mezer A, 2015. Fast T1 mapping using slice-shuffled Simultaneous Multi-Slice inversion recovery EPI. 21st Annual Meeting of the Organization for Human Brain Mapping, p. 1592. [Google Scholar]
- Yeatman JD, Wandell BA, Mezer AA, 2014. Lifespan maturation and degeneration of human brain white matter. Nature communications 5, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto Y, Chang L-H, Ni R, Pierce R, Andersen GJ, Watanabe T, Sasaki Y, 2014. White matter in the older brain is more plastic than in the younger brain. Nature Communications 5, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Lam CL, Lee TM, 2017. White matter microstructural abnormalities in amnestic mild cognitive impairment: A meta-analysis of whole-brain and ROI-based studies. Neuroscience & Biobehavioral Reviews 83, 405–416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


