Abstract
Aims
The association of hyperglycemia and duration of diabetes with intracranial atherosclerotic stenosis (ICAS) in the general population is not well documented. We examined whether elevated glucose and longer diabetes duration is independently associated with ICAS in a community-based sample.
Methods
We cross-sectionally analyzed 1,644 participants (age 67-90 years) of the Atherosclerosis Risk in Communities Study who underwent cerebrovascular magnetic resonance angiography in 2011-13. We applied multivariable ordinal logistic regression to evaluate the association of ICAS category (“no stenosis”, “stenosis <50%”, or “stenosis ≥50%”) with glucose or diabetes duration (<10, 10 to 20, and ≥20 years). We also obtained the corresponding odds ratios applying inverse-probability weighting to account for potential selection bias due to attrition.
Results
Compared to non-diabetic participants in the lowest glucose quartile, the weighted odds ratios (95% confidence interval) of higher ICAS category were 1.88 (1.18, 3.00) and 2.01 (1.08, 3.72) for non-diabetic and diabetic participants in the corresponding highest glucose quartile, respectively. We observed significant positive trends of ICAS across diabetes duration categories in unweighted, but not in weighted, analyses.
Conclusions
Hyperglycemia and longer duration of diabetes were independently associated with ICAS, suggesting the importance of maintaining glycemic control to prevent stroke.
Keywords: glucose, glycated hemoglobin, HbA1c, intracranial atherosclerotic stenosis, diabetes, risk factor
1. Introduction
Intracranial atherosclerotic stenosis (ICAS) is a common cause of stroke, responsible for up to 8% of ischemic strokes in the United States.1 Determinants of ICAS include traditional stroke risk factors such as hypertension and dyslipidemia.2 However, the independent association of measures of hyperglycemia, the presence and duration of diabetes mellitus with ICAS is less well characterized. Most reports documenting positive associations of diabetes with ICAS relied on transcranial Doppler or similar ultrasonography-based devices.3–5 Such devices, however, are limited in the ability of identifying ICAS, inferior to magnetic resonance angiography (MRA).6 We previously reported the prevalence of ICAS in a sub-sample of Atherosclerosis Risk in Communities (ARIC) Study participants using 3-Tesla MRA.7 In that report, we found that midlife diabetes was associated positively with ICAS in black individuals, but not in white participants.7 Community-based Japanese studies using 1.5-Tesla MRA reported mixed results with regard to independent relationship between prevalent diabetes and ICAS.8,9 Thus, the role of hyperglycemia, presence and duration diabetes on ICAS warrants further investigation because it would provide mechanistic explanation for the studies reporting an independent risk of ischemic stroke in diabetic individuals as compared to non-diabetic individuals,10 and the non-diabetic people with higher glycemic marker levels at baseline.11 We hypothesized that higher levels of glucose and HbA1c, a marker of chronic hyperglycemia, in persons with or without diabetes, and longer duration of diagnosed diabetes would be associated with greater prevalence of ICAS.
2. Participants and methods
2.1. Study population
We conducted a cross-sectional study in a subsample of participants of the ARIC Study who participated in the ARIC-Neurocognitive Study (ARIC-NCS) and underwent cerebrovascular MRA. The sampling methods were reported elsewhere.12 In brief, the ARIC cohort initially comprised 15,792 participants aged 45 - 64 years selected from four communities in 1987-1989: Forsyth County, NC (white and black participants), Jackson, MS (black participants), suburban Minneapolis, MN (mostly white participants), and Washington County, MD (mostly white participants). From 2011 to 2013, the ARIC study conducted the fifth examination. Among 10,036 participants who were still alive at the time of study, 6,538 (65%) participated in the visit 5 exam. From those with no known contraindications to MRA, we offered scans to the following participants with the goal of obtaining ≈2,000 subjects: (1) those who had received an ARIC brain magnetic resonance scan in 2004 to 2006 (offered to 573, completed in 433), (2) those with low-current cognitive test scores or large declines on the longitudinally administered tests (offered to 1,047, completed in 664), and (3) an age-stratified random sample of the remaining individuals (offered to 1,202, completed in 861), resulting in a total of 1,958 participants completed a standard MRA protocol.13 We excluded those participants with poor MRA image quality or poor protocol adherence (n=193). MRA images for the remaining participants had adequate or excellent quality for ICAS identification in the vessels of interest, thus were included in the current analysis.12 For the present study, we restricted our analyses to white and African American participants, and excluded 3 participants categorized in other races. We also excluded those with history of stroke (n=60) and those with missing pertinent variables (n=58), leaving 1,644 participants for the final analyses.
2.2. Measurements
2.2.1. MRA protocol and image analysis
Details of the magnetic resonance imaging (MRI) protocol, image analysis, quality control and reliability have been published previously.14 In brief, all MRI scans were performed on 3.0-Tesla Siemens scanners. High-resolution vascular sequences were acquired at the end of a standardized brain MRI protocol and included a 3-dimentional time-of-flight (TOF) MRA. All MRI images were analyzed by seven certified readers at the MRI reading center without knowledge of the participant characteristics. Vessel segments analyzed included the intracranial segment of the internal carotid artery, middle cerebral artery, anterior cerebral artery, intracranial segment of the vertebral artery, basilar artery, and posterior cerebral artery. For each territory, the ordinal degree of narrowing (i.e., no detectable stenosis, <50%, 51-70%, 71-99%, and occlusion) was recorded for the most stenotic plaque using criteria established in the Warfarin-Aspirin Symptomatic Intracranial Disease trial.15 For the present analyses, we grouped the participants into 3 categories based on the highest ICAS category among any of the intracranial arteries we assessed: “no (detectable) ICAS”, “ICAS <50%”, or “ICAS ≥50%”.
2.2.2. Measurements of glycated hemoglobin
HbA1c was measured in whole-blood samples collected at ARIC Visit 5 using high-performance liquid chromatography with Tosoh A1c 2.2 Plus Glycohemoglobin and Tosoh G7 Analyzers (Tosoh Bioscience, South San Francisco, CA), methods standardized to the Diabetes Control and Complications Trial assay.
2.2.3. Ascertainment of diabetes status
Diagnosed diabetes was defined on the basis of self-reported physician diagnosis or current use of diabetes mellitus drugs.16 We calculated duration of diagnosed diabetes at the time of Visit 5 based on information obtained from annual telephone follow-up calls during the prior two decades in ARIC asking the participants about when their diabetes was diagnosed.
2.2.4. Demographic and stroke risk factors
Stroke risk factors were assessed at the time of Visit 5 examination and included body mass index (kg/m2), history of smoking (current, past or never), systolic blood pressure, use of antihypertensive medication, plasma high density lipoprotein cholesterol (HDL), plasma triglycerides, use of cholesterol lowering medications, prevalent myocardial infarction and prevalent stroke. We defined hypertension as systolic/diastolic blood pressure ≥ 140/90 mmHg or use of antihypertensive medications. Non-HDL-cholesterol was calculated by subtracting concentration of HDL-cholesterol from that of total cholesterol.
2.3. Statistical analysis
We tested proportional odds assumption and applied ordinal logistic regression to obtain adjusted odds ratio (ORs) of being in each successive higher category of ICAS (“no ICAS”, “ICAS <50%”, or “ICAS ≥50%”). In the models, we adjusted for age, sex, race-center (five categories: Minnesota whites, Maryland whites, North Carolina whites, Jackson blacks, and the black participants from the remaining three centers combined), education, medications for hypertension and cholesterol (yes/no), systolic blood pressure (mmHg), non-HDL-cholesterol (mg/dL), and smoking (current/non-current), all at Visit 5. Duration of diabetes at Visit 5 was categorized as never (i.e. no diabetes), <10 years, 10 to 19 years, or ≥ 20 years because of the uncertainty of exact duration in those reported their duration longer than 20 years. To characterize the continuous associations of glucose and HbA1c levels with ICAS, we generated restricted cubic splines separately by diabetes status with 5 knots of diabetes-specific percentiles (10th, 25th, 50th, 75th, and 90th) using otherwise same models used in the main analyses. We also examined distribution of age and diabetes duration at Visit 5 across the glycemic ranges divided by the knots to explore if age and/or diabetes duration differ by those ranges.
In main analyses, we categorized the participants into 6 groups according to diagnosed diabetes status-specific quartiles of either glucose or HbA1c, combining the two middle quartiles (i.e. Q1, Q2-3, Q4 of each glycemic marker for participants with diagnosed diabetes and the corresponding categories for those without diagnosed diabetes). To assess diabetes-status combined relationship, we computed overall p-values for trend by modeling the category median of each of the group as a continuous variable, consistent with a previous ARIC study.17 There is no statistical evidence against proportional odds assumption by Score test in the models. Interaction by sex or by race was tested inserting product terms in to the models. In post-hoc analyses, we explored for interaction either by prevalent chronic kidney disease (CKD) or peripheral artery disease (PAD), both assessed at Visit 5, on the association between duration of diabetes and ICAS, because prevalent diabetic complications such as CKD and/or PAD may explain coexisting ICAS. Prevalent CKD was defined as estimated glomerular filtration rate <60 ml/min/1.73 m2, based on the Chronic Kidney Disease Epidemiology Collaboration equation using both serum creatinine and cystatin-C (CKD-EPI creatinine-cystatin C equation.18 PAD was defined as ankle-brachial index ≤ 0.9. (Ninety individuals had PAD status missing.) To account for potential selection bias related to informative censoring and the stratified sampling design19 in the ARIC-NCS study, we conducted inverse-probability weighted ordinal logistic regression as sensitivity analyses.12 All reported p-values are 2-sided, with P<0.05 indicating statistical significance. Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and Stata/SE version 14.2 (StataCorp, College Station, TX).
3. Results
Of the 1,644 ARIC participants included (age range: 67-90 years, women 58%, Blacks 28%, Table 1), 509 individuals (31%) had diagnosed diabetes and approximately 10% of them (51/509) had diabetes for ≥ 20 years. Three hundred forty-six (21.0%) had ICAS of <50%, and 160 (9.7 %) participants had ICAS ≥50 % in at least one of the intracranial arteries. Those who had more advanced stenosis were more likely to be older, black, male, less well educated, and to have higher systolic blood pressure, higher non-HDL-cholesterol concentration, and diagnosed diabetes ≥ 20-years. Those with more advanced stenosis were also likely to have higher glucose and HbA1c (all p-values for trend <0.01). There were no statistical differences in age distributions across the ranges of glucose or HbA1c divided by the knots of the spline (10th, 25th, 50th, 75th) both in non-diabetes and diabetes (p-value>0.099, data not shown). In contrast, diabetes duration differed significantly across the ranges of glucose and HbA1c: specifically, the prevalence of diabetes with duration of ≥20 years was more common at both the lowest (i.e. 10th or less) and higher values of glucose, and at higher values of HbA1c (Supplemental Tables S1, S2). When modeled as restricted cubic splines according to diagnosed diabetes strata, elevated glucose and HbA1c were associated with higher odds of having ICAS both in non-diabetes and diabetes groups (Figure). There was also some evidence for higher odds at very low values of glycemic markers, most evidently at low glucose in participants with diagnosed diabetes (Panel C, in Figure). However, the confidence intervals at low glycemia values in the diabetic participants were wide and overlapped the null value of 1. Multivariable ordinal logistic regression analysis combining diabetes and non-diabetes (Table 2) showed an overall positive relationship between fasting glucose and ICAS in both unweighted and weighted models (overall p-value for trend=0.04 and 0.02, respectively). For example, compared to non-diabetes participants in the lowest glucose quartile, the odds ratio (OR) of higher category of ICAS was 1.88 (95% confidence interval 1.18, 3.00) among the non-diabetes participants in the highest glucose quartile in the weighted model after adjustment for risk factors. The corresponding OR in the diabetes participants in the highest glucose quartile was 2.01 (1.08, 3.72). The association of HbA1c was similar to glucose, but the trend was statistically significant only in the unweighted model (Table 2). There was no statistical evidence of interaction on the association between glycemic markers (glucose, HbA1c) and ICAS either by sex or race.
Table 1.
Characteristics of the participants according to degree of intracranial atherosclerotic stenosis (N=1,644), ARIC visit 5, 2011-2013
| Total (N=1,644) | Intracranial atherosclerotic stenosis in any arteries |
p-value | |||
|---|---|---|---|---|---|
| no stenosis (n=1,138) | <50% (n=346) | ≥50% (n=160) | |||
| Age, years (sd) | 76.3 (5.3) | 75.9 (5.2) | 76.6 (5.5) | 78.4 (4.9) | <0.001 |
| Men, % | 687 (41.8) | 443 (38.9) | 173 (50.0) | 71 (44.4) | 0.006 |
| Race/ethnicity, n (%) | 0.011 | ||||
| Black | 460 (28.0) | 296 (26.0) | 111 (32.1) | 53 (33.1) | |
| Advanced educationa, n (%) | 764 (46.5) | 549 (48.2) | 147 (42.5) | 68 (42.5) | 0.047 |
| Current smoker, n (%) | 79 (4.8) | 52 (4.6) | 20 (5.8) | 7 (4.4) | 0.727 |
| Systolic blood pressure, mmHg (sd) | 131 (18.1) | 130 (17) | 132 (20) | 135 (18) | <0.001 |
| Non HDL-cholesterol, mmol/L (sd) | 3.37 (0.97) | 3.33 (0.94) | 3.42 (0.95) | 3.58 (1.13) | 0.002 |
| Antihypertensive medication, n (%) | 1207 (73.4) | 819 (72.0) | 265 (76.6) | 123 (76.9) | 0.063 |
| Lipid-lowering medication, n (%) | 882 (53.6) | 625 (54.9) | 175 (50.6) | 82 (51.3) | 0.170 |
| Diagnosed diabetesb, n (%) | 509 (31.0) | 351 (30.8) | 105 (30.4) | 53 (33.1) | 0.698 |
| Duration of diagnosed diabetes ≥ 20yrs, n (%) | 51 (3.1) | 25 (2.2) | 15 (4.3) | 11 (6.9) | <0.001 |
| Glucose, mmol/L (sd) | 6.28 (1.60) | 6.20 (1.47) | 6.45 (1.83) | 6.45 (1.89) | 0.007 |
| HbA1c, mmol/mol (sd) | 41 (9) | 41 (9) | 42 (10) | 43 (12) | 0.005 |
| % | 5.9 (0.9) | 5.9 (0.8) | 6.0 (0.9) | 6.1 (1.1) | - |
Values were means (standard deviations) unless otherwise specified. P-values were calculated by linearly regressing on the intracranial atherosclerotic stenosis categories treated as ordinal for continuous variables, and by Mantel-Haenszel Chi-Square test for categorical variables. Unit conversion: Non high-density lipoprotein (HDL)-cholesterol, 1mmol/L=38.6mg/dL; Glucose, 1.0 mmol/L=18.0 mg/dL.
Advanced education was defined as the highest grade completed in school was ≥17 years.
Diagnosed diabetes was defined on the basis of self-reported physician diagnosis or current use of anti-diabetic drugs at Visit 5.
Figure.
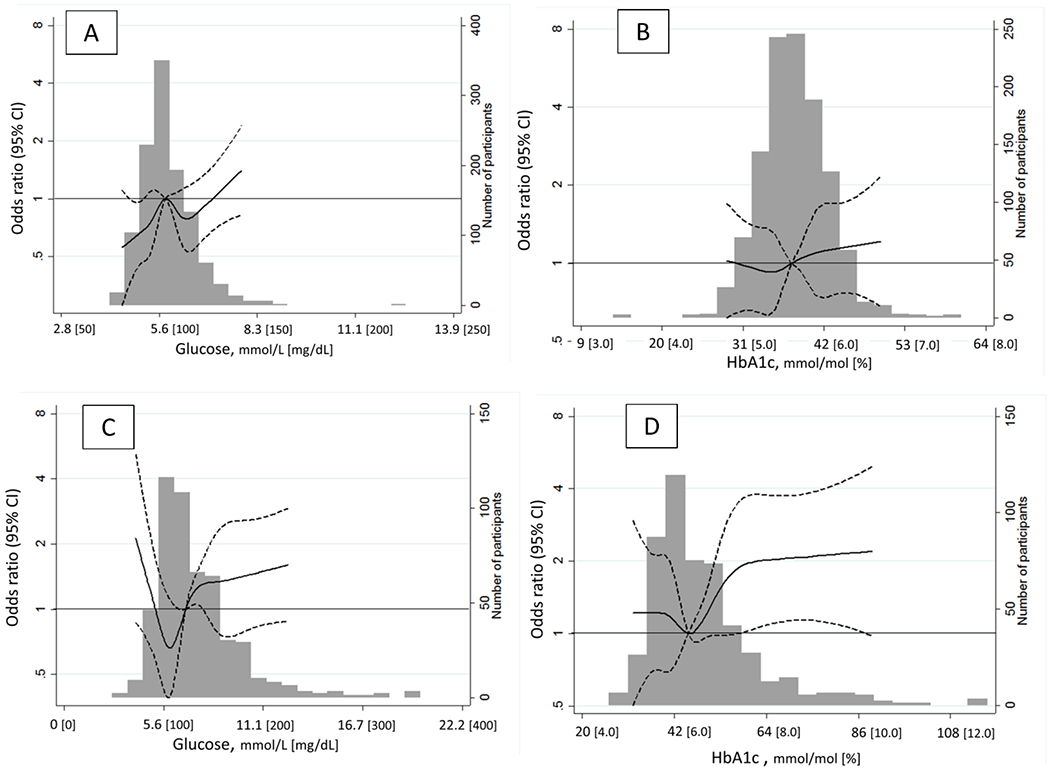
Adjusted odds ratios were obtained from ordinal logistic regression modeling of intracranial atherosclerotic stenosis (ICAS) on glucose or HbA1c for participants without diagnosed diabetes (N=1139, Panels A and B), with diagnosed diabetes (n=509, Panels C and D) at Visit 5 using restricted cubic splines. Knots were placed at diabetes status-specific 10th, 25th, 50th, 75th, and 90th percentiles of glucose (5.0, 5.3, 5.7, 6.2, and 6.7 for non-diabetes; 5.2, 5.9, 6.8, 8.3, and 9.9 for diabetes, respectively [mmol/L]) or HbA1c (33, 36, 38, 41, and 43 mmol/mol [5.2, 5.4, 5.6, 5.9, and 6.1 %] for non-diabetes; (38, 41, 45, 53, and 64 mmol/mol [5.6, 5.9, 6.3, 7.0, and 8.0 %] for diabetes). Solid lines and dotted lines respectively show odds ratios and the corresponding 95% confidence intervals of being in higher categories of ICAS. The median value was set as reference (OR=1.0, depicted in a solid black line) within each group. The cubic lines were truncated at values of 1st and 99th percentile of glucose or HbA1c. Odds ratios were adjusted for age, sex, race-center (5 categories), education, medications for hypertension and cholesterol (yes/no), systolic blood pressure (mmHg), non-HDL-cholesterol (mg/dL), smoking (current/non-current) at Visit 5. The histogram represents the frequency distribution of glucose or HbA1c.
Table 2.
Odds ratios (OR) of intracranial atherosclerotic stenosis according to diagnosed diabetes-specific quantile of glucose and HbA1c measured at visit 5 (N=1,644)
| Diabetes status-specific quantiles | No. | Median | Range (min-max) | Unweighted model |
Weighted model |
||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | ||||||
| Glucose (mmol/L) | |||||||||
| Non-diabetes, Q1 | 262 | 2.36 | (1.94- 2.46) | 1 (ref) | - | - | 1 (ref) | - | - |
| Non-diabetes, Q2-3 | 584 | 2.64 | (2.49- 2.85) | 1.28 | 0.92 | , 1.79 | 1.37 | 0.92 | , 2.06 |
| Non-diabetes, Q4 | 289 | 3.06 | (2.88- 5.85) | 1.28 | 0.88 | , 1.87 | 1.88 | 1.18 | , 3.00 |
| Diabetes, Q1 | 124 | 2.50 | (1.27- 2.72) | 1.13 | 0.69 | , 1.85 | 1.03 | 0.58 | , 1.83 |
| Diabetes, Q2-3 | 255 | 3.13 | (2.75- 3.86) | 1.21 | 0.81 | , 1.81 | 1.53 | 0.92 | , 2.52 |
| Diabetes, Q4 | 130 | 4.51 | (3.89- 9.27) | 1.71 | 1.08 | , 2.70 | 2.01 | 1.08 | , 3.72 |
| Overall p-value for trend | 0.04 | 0.02 | |||||||
| HbA1c (mmol/L in first rows, % in second rows) | |||||||||
| Non-diabetes, Q1 | 345 | 34 | (14- 36) | 1 (ref) | - | - | 1 (ref) | - | - |
| 5.3 | (3.4- 5.4) | ||||||||
| Non-diabetes, Q2-3 | 575 | 39 | (37- 41) | 1.16 | 0.86 | , 1.57 | 1.11 | 0.77 | , 1.59 |
| 5.7 | (5.5- 5.9) | ||||||||
| Non-diabetes, Q4 | 215 | 43 | (42- 61) | 1.21 | 0.83 | , 1.77 | 1.06 | 0.67 | , 1.69 |
| 6.1 | (6.0- 7.7) | ||||||||
| Diabetes, Q1 | 146 | 39 | (27- 41) | 1.04 | 0.67 | , 1.61 | 0.97 | 0.58 | , 1.63 |
| 5.7 | (4.6- 5.9) | ||||||||
| Diabetes, Q2-3 | 240 | 46 | (42- 53) | 1.10 | 0.75 | , 1.62 | 1.13 | 0.68 | , 1.88 |
| 6.4 | (6.0- 7.0) | ||||||||
| Diabetes, Q4 | 123 | 62 | (54- 116) | 1.72 | 1.10 | , 2.68 | 1.38 | 0.81 | , 2.33 |
| 7.8 | (7.1- 12.8) | ||||||||
| Overall p-value for trend | 0.03 | 0.24 | |||||||
Odds ratios were adjusted for age, sex, race-left (five categories), education, medications for hypertension and cholesterol (yes/no), systolic blood pressure (mmHg), non-HDL-cholesterol (mg/dL), and smoking (current/non-current). Those adjusting covariates were the ones assessed at Visit 5. Overall p-value for trend was obtained by modelling the category median (for all six categories) as a continuous variable. 95%CI, 95% confidence interval.
Individuals with longer duration of diagnosed diabetes, particularly those with diagnosed diabetes for ≥ 20 years, were more likely to have advanced ICAS compared to the participants without diagnosed diabetes (Table 3). In the unweighted logistic regression model, the ORs (95% confidence interval) of greater ICAS were 1.32 (0.87, 2.01), and 2.52 (1.45, 4.39) for those with diagnosed diabetes duration of 10-19 years and ≥20 years, respectively (p-value for trend=0.02). In the weighted model, the point estimate for diagnosed diabetes duration of ≥20 years was attenuated to 2.04 and the overall positive trend became non-significant (p-value for trend=0.13). In the post-hoc analyses, CKD and PAD were more prevalent in participants with longer diabetes duration (Supplemental Table S3). Although the association between diabetes duration and ICAS tended to be stronger in those with prevalent CKD in the unweighted logistic regression model parallel to the main one, we observed no statistical evidence for the presence of interaction either by prevalent CKD or prevalent PAD (Supplemental Tables S4, S5).
Table 3.
Prevalence and odds ratios (OR) of intracranial atherosclerotic stenosis according to duration of diagnosed diabetes
| Duration of diagnosed diabetes |
Intracranial atherosclerotic stenosis, n (%) |
Unweighted model |
Weighted model |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | none | stenosis <50% | stenosis ≥50% | OR | (95%CI) | p-value | OR | (95%CI) | p-value | |
| Never (no diabetes) | 1135 | 787 (69.3) | 241 (21.2) | 107 (9.4) | 1 (ref) | - | - | 1 (ref) | - | - |
| Less than 10 years | 345 | 253 (73.3) | 64 (18.6) | 28 (8.1) | 0.88 | (0.67, 1.16) | 0.37 | 0.87 | (0.62, 1.23) | 0.42 |
| 10 to 19 years | 113 | 73 (64.6) | 26 (23.0) | 14 (12.4) | 1.32 | (0.87, 2.01) | 0.19 | 1.35 | (0.82, 2.20) | 0.23 |
| 20years or longer | 51 | 25 (49.0) | 15 (29.4) | 11 (21.6) | 2.52 | (1.45, 4.39) | <0.01 | 2.04 | (0.90, 4.62) | 0.09 |
| Total, n | 1644 | 1138 | 346 | 160 | P-value for trend=0.02 | P-value for trend=0.13 | ||||
Odds ratios (ORs) were adjusted for age, sex, race-left (five categories), education, medications for hypertension and cholesterol (yes/no), systolic blood pressure (mmHg), non-HDL-cholesterol (mg/dL), and smoking (current/non-current). Those adjusting covariates were the ones assessed at Visit 5.
4. Discussion
Key findings of this study are as follows: First, elevated fasting glucose was associated with higher ICAS category in participants with or without diagnosed diabetes, and the associations were independent of other stroke risk factors. The association of HbA1c with ICAS was similar to that of fasting glucose, although statistically significant only in the unweighted model. Second, longer duration of diagnosed diabetes was associated with higher ICAS category in a graded fashion. This is the first study, to our knowledge, that showed the associations of ICAS assessed by high-resolution MRI with elevated glycemic markers and longer duration of diabetes in population-based sample. The findings suggest that elevated glucose and longer duration of diabetes are independent determinant of ICAS, providing mechanistic link from chronic hyperglycemia, diabetes to increased risk of ischemic stroke.
ICAS is a known cause of ischemic stroke, and a significant risk factor for dementia.1,8,13,20 While advanced age and hypertension have been well documented as determinants of ICAS, an independent association of ICAS with diabetes and/or glycemic markers has not been consistently observed.21,22 Most previous studies relied on transcranial Doppler to assess ICAS. For example, Zhang and colleagues reported increased odds of ICAS in individuals with diabetes as compared to those without among 5,440 asymptomatic Chinese adults aged ≥ 40 years. Their assessment for ICAS was based on transcranial Doppler.23 Limitations of transcranial Doppler in diagnosing ICAS are well-established, including its heavy reliance on the operator’s skill and difficulty for assessing vessels other than the middle cerebral artery. Use of 3.0-Tesla MRA in our study is likely to provide better assessment of ICAS as compared to transcranial Doppler or even to 1.5-Tesla MRA.6,24 In addition, our MRA scans were acquired using a relatively high-resolution technique (0.5 mm ×0.5 mm ×0.55 mm), minimizing the effects of dephasing artifacts that would exaggerate stenosis.12 To further improve the accuracy of our image analysis, we excluded all MRAs for which quality was considered inadequate.
Community-based studies in Japan using 1.5-Tesla MRA reported conflicting results with regard to an independent association between prevalent diabetes and ICAS. One study reported no independent association,8 whereas the other reported an independent association only with ICAS of >50% stenosis.9 The ARIC research group previously reported a positive independent association between midlife diabetes and ICAS only in black individuals, but not in white participants.9 Moreover, we assessed neither elevated glycemic values by themselves nor duration of diabetes in our previous report.12 Therefore, the current study provides additional evidence suggesting a harmful effect of hyperglycemia and longer duration of diabetes on ICAS.
Microvascular complications such as nephropathy and neuropathy are well-known consequences of poorly controlled diabetes particularly of its long duration. Accumulating evidence suggests adverse effect of chronic hyperglycemia and diabetes on macrovascular pathologies including ICAS and intracranial plaques,25 similar to coronary atherosclerosis.26 Two meta-analyses reported that higher glycated hemoglobin levels were associated with increased risk of stroke in patients with type 2 diabetes,27,28 although the difference in stroke etiologies was not addressed in the meta-analyses.
As expected, longer duration of diabetes was associated with more diabetic complications such as CKD and PAD. However, our post-hoc analyses did not support an interaction by either CKD or PAD on the association between diabetes duration and ICAS.
Our study also indicates that higher glucose levels in non-diabetes may lead to increased risk of ICAS, providing a mechanistic explanation of a previous ARIC study result: higher glycemic markers among non-diabetes participants independently predicted higher 15-year risk of ischemic stroke.11 Those with higher glycemic markers at baseline having greater arterial exposure to toxic hyperglycemia, are likely to develop diabetes11 earlier, suffer diabetes for a longer duration, all of which may increase the risk of developing ICAS leading to ischemic stroke. In addition, the atherogenic effect of insulin resistance might be a potential explanation.29 In fact, a previous study reported an association between insulin resistance and ICAS in individuals independent of diabetes.30
A higher odds ratio of ICAS at low levels of glucose in participants with diabetes, depicted in the restricted cubic spline figures, was somewhat unexpected. We found that the prevalence of diabetes of long duration was more common at low glucose values (Supplemental Table S1). Thus, confounding by diabetes duration may in part explain the observed J-shape relation of glucose with ICAS in the participants with diagnosed diabetes.
Multiple mechanisms linking hyperglycemia and atherosclerosis have been proposed. In the hyperglycemic state, advanced glycation endproducts (AGEs) are formed as nonenzymatic reactions between the aldehydic group of reducing sugars with proteins, lipids, or nucleic acid. AGEs promote vascular damage and accelerate atherosclerotic plaque progression by altering the functional properties of vessel wall extracellular matrix molecules, or by activating cell receptor-dependent signaling 31.
Our study had some limitations. First, although participants in this study were selected using a stratified random sampling from a population-based cardiovascular cohort, informative censoring due to attrition from the original ARIC sample may have biased our odds ratio estimates. To address this potential problem, we applied an inverse-probability weighting method. There remains a possibility, however, of residual and unmeasured confounding or selection bias if all determinants of selection were not fully adjusted for with the inverse-probability weighting. For example, those with longer diabetes duration may have been exposed to other risk factors longer as well, but we did not account for duration of other risk factors. It is difficult to infer the direction of such bias, if exists. Second, our sample size is relatively limited relative to the entire ARIC participants, which limits our ability to rigorously investigate detailed dose-response relationships between ICAS and glycemic markers according to diabetes strata. Third, we utilized stenotic regions as our outcome of interest, assuming that more advanced stenosis is related to more significant clinical consequences. However, substenotic intracranial plaques may cause cerebral infarcts as well as stenotic ones. Symptomatic intracranial atherosclerotic plaques are characterized not only by a higher degree of luminal stenosis but also by a richer content in lipid, intra-plaque hemorrhage, and inflammatory cell infiltration.32 We are unable to provide information about histopathologic composition of the ICAS in this analysis.
5. Conclusions
A higher degree of ICAS, assessed with high-resolution MRA, was associated with higher fasting glucose and longer duration of diabetes, independent of stroke risk factors in a population-based sample of US adults. Our results provide a mechanistic explanation regarding why higher glycemic markers predict future ischemic stroke, underscoring the importance of maintaining glycemic control to prevent stroke.
Supplementary Material
Acknowledgments
COI statement: Ownership interest by Dr. Wasserman: 3-dimensional black blood MRI technique used (patent pending No. 13/822,111). There has been no royalties or licensing derived from this pending application. Otherwise, all the authors have no conflict-of-interest relevant to this work.
We thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding
This research was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grants R01 DK089174 and K24 DK106414 to Dr Selvin.
Supported by National Heart, Lung, and Blood Institute (NHLBI) grants under award number R01HL105626 and R01HL105930. Dr. Fujiyoshi was supported by Fulbright Program (Fulbright Japan, RS2014-2015).
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, HHSN268201700005I). Neurocognitive data is collected by U01 HL096812, HL096814, HL096899, HL096902, HL096917 with previous brain MRI examinations funded by R01-HL70825. The authors thank the staff and participants of the ARIC study for their important contributions.
REFERENCES
- 1.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26(1):14–20. [DOI] [PubMed] [Google Scholar]
- 2.Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2013;12(11):1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang HW, Guo MH, Lin RJ, et al. Prevalence and risk factors of middle cerebral artery stenosis in asymptomatic residents in Rongqi County, Guangdong. Cerebrovasc Dis. 2007;24(1):111–115. [DOI] [PubMed] [Google Scholar]
- 4.Wong KS, Huang YN, Yang HB, et al. A door-to-door survey of intracranial atherosclerosis in Liangbei County, China. Neurology. 2007;68(23):2031–2034. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Cancio E, Dorado L, Millan M, et al. The Barcelona-Asymptomatic Intracranial Atherosclerosis (AsIA) study: prevalence and risk factors. Atherosclerosis. 2012;221(1):221–225. [DOI] [PubMed] [Google Scholar]
- 6.Feldmann E, Wilterdink JL, Kosinski A, et al. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial. Neurology. 2007;68(24):2099–2106. [DOI] [PubMed] [Google Scholar]
- 7.Qiao Y, Suri FK, Zhang Y, et al. Racial Differences in Prevalence and Risk for Intracranial Atherosclerosis in a US Community-Based Population. JAMA Cardiol. 2017;2(12):1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsui R, Nakagawa T, Takayoshi H, et al. A Prospective Study of Asymptomatic Intracranial Atherosclerotic Stenosis in Neurologically Normal Volunteers in a Japanese Cohort. Front Neurol. 2016;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shitara S, Fujiyoshi A, Hisamatsu T, et al. Intracranial Artery Stenosis and Its Association With Conventional Risk Factors in a General Population of Japanese Men. Stroke. 2019;50(10):2967–2969. [DOI] [PubMed] [Google Scholar]
- 10.Folsom AR, Rasmussen ML, Chambless LE, et al. Prospective associations of fasting insulin, body fat distribution, and diabetes with risk of ischemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Diabetes Care. 1999;22(7):1077–1083. [DOI] [PubMed] [Google Scholar]
- 11.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362(9):800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suri MF, Qiao Y, Ma X, et al. Prevalence of Intracranial Atherosclerotic Stenosis Using High-Resolution Magnetic Resonance Angiography in the General Population: The Atherosclerosis Risk in Communities Study. Stroke. 2016;47(5):1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suri MFK, Zhou J, Qiao Y, et al. Cognitive impairment and intracranial atherosclerotic stenosis in general population. Neurology. 2018;90(14):e1240–e1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiao Y, Guallar E, Suri FK, et al. MR Imaging Measures of Intracranial Atherosclerosis in a Population-based Study. Radiology. 2016;280(3):860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21(4):643–646. [PMC free article] [PubMed] [Google Scholar]
- 16.Selvin E, Rawlings AM, Lutsey PL, et al. Fructosamine and Glycated Albumin and the Risk of Cardiovascular Outcomes and Death. Circulation. 2015;132(4):269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selvin E, Rawlings AM, Grams M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014;2(4):279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23(1):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort. Ann Neurol. 2010;68(2):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suri MF, Johnston SC. Epidemiology of intracranial stenosis. J Neuroimaging. 2009;19 Suppl 1:11S–16S. [DOI] [PubMed] [Google Scholar]
- 22.Ritz K, Denswil NP, Stam OC, van Lieshout JJ, Daemen MJ. Cause and mechanisms of intracranial atherosclerosis. Circulation. 2014;130(16):1407–1414. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Zhou Y, Zhang Y, et al. Prevalence and risk factors of asymptomatic intracranial arterial stenosis in a community-based population of Chinese adults. Eur J Neurol. 2013;20(11):1479–1485. [DOI] [PubMed] [Google Scholar]
- 24.Choi CG, Lee DH, Lee JH, et al. Detection of intracranial atherosclerotic steno-occlusive disease with 3D time-of-flight magnetic resonance angiography with sensitivity encoding at 3T. AJNR Am J Neuroradiol. 2007;28(3):439–446. [PMC free article] [PubMed] [Google Scholar]
- 25.Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008;39(4):1142–1147. [DOI] [PubMed] [Google Scholar]
- 26.Reis JP, Allen NB, Bancks MP, et al. Duration of Diabetes and Prediabetes During Adulthood and Subclinical Atherosclerosis and Cardiac Dysfunction in Middle Age: The CARDIA Study. Diabetes Care. 2018;41(4):731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141(6):421–431. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Hu G, Yuan Z, Chen L. Glycosylated hemoglobin in relationship to cardiovascular outcomes and death in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2012;7(8):e42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Meng R, Liu G, et al. Intracranial atherosclerotic disease. Neurobiol Dis. 2019;124:118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez-Cancio E, Galan A, Dorado L, et al. Biological signatures of asymptomatic extra- and intracranial atherosclerosis: the Barcelona-AsIA (Asymptomatic Intracranial Atherosclerosis) study. Stroke. 2012;43(10):2712–2719. [DOI] [PubMed] [Google Scholar]
- 31.Del Turco S, Basta G. An update on advanced glycation endproducts and atherosclerosis. Biofactors. 2012;38(4):266–274. [DOI] [PubMed] [Google Scholar]
- 32.Arenillas JF. Intracranial atherosclerosis: current concepts. Stroke. 2011;42(1 Suppl):S20–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


