Abstract
Background
The coronavirus disease 2019 (COVID-19) global pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), began in late 2019. Researchers around the world are aggressively working to develop a vaccine. One of the vaccines approved against COVID-19 is Oxford–AstraZeneca chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19.
Case report
We described a patient who developed four limb distal paraesthesia, postural instability, and facial diplegia, ten days after vaccination with ChAdOx1 nCoV-19 (ABW1277). The electrophysiological findings were compatible with acute demyelinating motor polyneuropathy (Guillain-Barrè syndrome).
Discussion
We therefore want to describe a temporal correlation between administration of ChAdOx1 nCoV-19 (ABW1277) vaccine and GBS without evidence of other predisposing infectious or autoimmune factors. This paper aims to highlight the importance of pharmacovigilance and subsequent reports will be needed to evaluate the possible correlation between these two events.
Keywords: COVID-19, Pandemic, Vaccine, Guillain-Barrè syndrome
Background
The coronavirus disease 2019 (COVID-19) global pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), began in late 2019. Researchers around the world are aggressively working to develop a vaccine [1] and this way is considered crucial to establishing herd immunity in the COVID-19 pandemic. One of the vaccines approved against COVID-19 is Oxford–AstraZeneca chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19 [2], which is safe and effective in reducing the risk of serious infection to almost 100%. However, for the first time, the French National Agency for Medicines and Health Products Safety (ANSM) has reported in April 14th five new cases of facial paralysis and three of acute polyradiculoneuropathy (including Guillain-Barré syndrome) after ChAdOx1 nCoV-19 vaccine (https://ansm.sante.fr/actualites/point-de-situation-sur-la-surveillance-des-vaccins-contre-la-covid-19-periode-du-02-04-2021-au-08-04-2021, consulted on date 17/04/21), alerting clinicians to this possibility. Shortly after, two case series from the UK and India [3, 4] reported similar findings. These events, albeit rare, require post-vaccination surveillance programs, and a special monitoring and follow-up.
Case report
We described a case of a 59-year-old Caucasian male who acutely developed four limb distal paraesthesia and postural instability ten days after vaccination with ChAdOx1 nCoV-19 (ABW1277). Because of the symptoms’ persistence on the fifteenth day after vaccination, he underwent a neurological examination. Past medical history was positive only for hypertension and hyperuricemia.
The physical examination showed gait ataxia, global areflexia, and distal paraesthesia both at the lower and upper limbs; pallesthesia was normal. Segmental strength was diffusely preserved (MRC: 5/5). No cranial nerve, vegetative, or sphincter involvement was observed. He had no spine sensory level.
He underwent electromyography (EMG), which revealed motor polyradiculoneuropathy with temporal dispersion of the tibial nerve Compound Muscle Action Potential (cMAP) bilaterally, with F reflex absent in all districts. There was no sensory involvement, particularly no temporal dispersion of the sural nerve Sensory Action Potential (SAP) bilaterally. The electrophysiological findings were compatible with demyelinating motor polyneuropathy.
Based on these findings, the patient was hospitalized for further investigations. The molecular oropharyngeal swab was negative for COVID-19 infection.
Routine laboratory results were clinically non-significant (including thyroid function). Serological test for HBV, HCV, Mycoplasma, Zika, Chikungunya, west Nile virus, Borrelia Burgdorferi, and Cytomegalovirus was negative for active infection. The fecal investigation for Campylobacter jejuni was negative. The autoimmune hematological screening was unremarkable.
In the following 24 h, the patient showed clinical worsening, due to cranial nerves involvement with bilateral facial palsy (House-Brackmann grade V), without dysphagia or dysphonia, ocular motility limitations, or respiratory distress.
Lumbar puncture was performed, and CSF analysis showed elevated proteins: 140 mg/dl (normal values: 20.00–40.00 mg/dl), with normal white blood cell count and glycorrhachia. The liquor and serum antibodies antiGM1 IgG-IgM, GQIb IgG-IgM, GM2 IgG-IgM, anti MAG, anti-sulfatide, and anti-GAD were negative. Brain and cervical MRI with gadolinium infusion were unremarkable, without pathological enhancement.
After acquiring informed consent, patients started IV immunoglobulin (IVIg) 0.4 mg/kg for 5 days. He started physiotherapy. On the fifth day, the patient slowly improved first for the gait, and then for the forehead motility with moderate movement; eyes have complete closure with effort; the mouth was slightly weak with maximum effort (House-Brackmann grade IV).
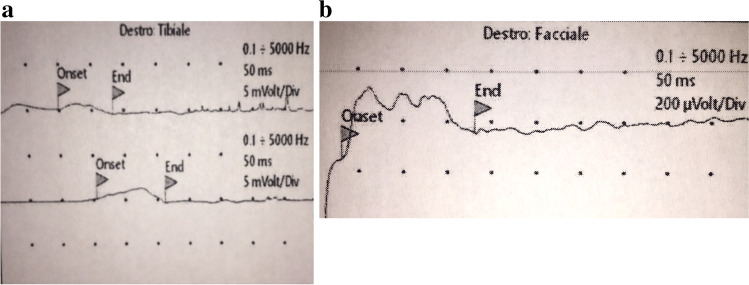
After 10 days, we repeated the EMG. Not surprisingly, the exam showed worsening of the electrophysiological findings, with diffuse and evident signs of motor nerve demyelination (upper and lower limbs, and cranial district) (Table 1; Fig. 1a and b).
Table 1.
Electromyography findings after 10 days
| Nerve stimulated | Stimulation site | *Amplitude | Latency (ms) | Conduction velocity (m/s) | F wave | ||||
|---|---|---|---|---|---|---|---|---|---|
| R | L | R | L | R | L | R | L | ||
| Superficial peroneal nerve (s) | Calf | 21 | 18 | 3 | 2.7 | 44 | 51 | ||
| Ulnar (s) | Wrist | 4.2 | 2 | 50 | |||||
| Sural (s) | Calf | 28 | 25 | 2.3 | 2.9 | 52 | 48 | ||
| Radial (s) | Wrist | 10 | 1.9 | 47 | |||||
| Facial (m) | Jaw | 0.2 | 3.1 | ||||||
| Ulnar (m) | Wrist | 9 | 3.8 | 50 | Absent | Absent | |||
| Below elbow | 7 | 8.6 | 43 | ||||||
| Above elbow | 7 | 10.9 | |||||||
| Tibial (m) | Ankle | 1.1 | 5.2 | 9.9 | 7.6 | 58 | 48 | Absent | Absent |
| Popliteal Fossa | 1.7 | 3 | 15.4 | 15.8 | |||||
| Peroneal (m) | Ankle | 2.3 | 2.4 | 12.6 | 9.3 | 43 | 60 | Absent | Absent |
| Below fibula | 2.3 | 2.1 | 19.8 | 14.4 | 42 | 54 | |||
| Above fibula | 2.2 | 2.1 | 22.4 | 16.6 | |||||
*Amplitude motor = mV, sensory = µV; m, motor study; s, sensory study; R, right; L, left
Fig. 1.
Tibial (a) and facial (b) nerve compound muscle action potential (cMAP), showing potential temporal dispersion
Discussion
This paper reported a case of Guillain–Barre (GBS) syndrome in a patient who recently received the ChAdOx1 nCoV-19 vaccine. GBS is an acute generalized inflammatory polyradiculoneuropathy associated with several viral infections (e.g., Campylobacter jejuni, Epstein-Barr virus, cytomegalovirus, influenza). The etiopathogenesis of GBS seems to be immune-mediated, in which antibodies respond to antigens and cross-react with nerve-ending antigens, with ascending weakness, areflexia, and eventually respiratory failure [5].
The neurological manifestations in SARS-Cov-2 patients are also well-known, due to a possible aberrant immune response, including GBS [6, 7] but, to date, the apparent risk factors for GBS (both during the COVID-19 infection and after the vaccine for it) have not been consistently defined in the literature.
We, therefore, want to describe a temporal correlation between administration of ChAdOx1 nCoV-19 (ABW1277) vaccine and GBS, in a patient without evidence of other predisposing infectious or autoimmune factors.
This paper aims to highlight the importance of pharmacovigilance and post-marketing surveillance to evaluate the possible correlation between these two events. In addition, most GBS cases are commonly treated with IVIg (typically given at 0.4 g/kg body weight daily for 5 days or at 2 g/kg body weight in 2 days), which can be associated with thromboembolic adverse events. Because of the thromboembolic risk associated with ChAdOx1 nCoV-19, these cases should be strongly monitored during the treatment [8]. However, the clinical course was benign with clinical improvement after immunoglobulin and physiotherapy confirm a risk/benefit ratio in favor of the vaccine administration in the population, as already reported also for the influenza vaccines, where the event rarity would not justify the influenza vaccine discontinuation [9]. As clinical practice, we currently want to point out a precaution for revaccination (i.e., second dose) only in patients with GBS history after a COVID-19 vaccine.
Acknowledgements
We want to thank Dr. Savoini G., an experienced neurologist, who presented the case to us in the month of his retirement.
Declarations
Ethical approval
We obtained the patient informed consent for the publication of this report.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sharma O, Sultan AA, Ding H, Triggle CR. A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol. 2020;14(11):585354. doi: 10.3389/fimmu.2020.585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397(10269):72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maramattom BV, Krishnan P, Paul R, Padmanabhan S, Cherukudal Vishnu Nampoothiri S, Syed AA, Mangat HS. Guillain-Barré Syndrome following ChAdOx1-S/nCoV-19 Vaccine. Ann Neurol. 2021 doi: 10.1002/ana.26143. [DOI] [PubMed] [Google Scholar]
- 4.Allen CM, Ramsamy S, Tarr AW, Tighe PJ, Irving WL, Tanasescu R, Evans JR. Guillain-Barré syndrome variant occurring after SARS-CoV-2 vaccination. Ann Neurol. 2021 doi: 10.1002/ana.26144. [DOI] [PubMed] [Google Scholar]
- 5.Donofrio PD. Guillain-Barré Syndrome. Continuum (Minneap Minn) 2017;23(5, Peripheral Nerve and Motor Neuron Disorders):1295–1309. doi: 10.1212/CON.0000000000000513. [DOI] [PubMed] [Google Scholar]
- 6.Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95(5):e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 7.Whittaker A, Anson M, Harky A. Neurological Manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand. 2020;142(1):14–22. doi: 10.1111/ane.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo Y, Tian X, Wang X, Xiao Z. Adverse effects of immunoglobulin therapy. Front Immunol. 2018;8(9):1299. doi: 10.3389/fimmu.2018.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodd CN, Romio SA, Black S, et al. Global H1N1 GBS Consortium. International collaboration to assess the risk of Guillain Barré Syndrome following Influenza A (H1N1) 2009 monovalent vaccines. Vaccine. 2013;31(40):4448–58. doi: 10.1016/j.vaccine.2013.06.032. [DOI] [PubMed] [Google Scholar]