Abstract
Hemorrhagic transformation (HT) is a common serious complication of stroke after thrombolysis treatment, which limits the clinical use of tissue plasminogen activator (t-PA). Since early diagnosis and treatment for HT is important to improve the prognosis of stroke patients, it is urgent to discover the potential biomarkers and therapeutic drugs. Recent evidence shows that pinocembrin, a natural flavonoid compound, exerts anti-cerebral ischemia effect and expands the time window of t-PA. In this study, we investigated the effect of pinocembrin on t-PA-induced HT and the potential biomarkers for HT after t-PA thrombolysis, thereby improving the prognosis of stroke. Electrocoagulation-induced thrombotic focal ischemic rats received intravenous infusion of t-PA (10 mg/kg) 6 h after ischemia. Administration of pinocembrin (10 mg/kg, iv) prior t-PA infusion significantly decreased the infarct volume, ameliorated t-PA-induced HT, and protected blood–brain barrier. Metabolomics analysis revealed that 5 differential metabolites in the cerebral cortex and 16 differential metabolites in serum involved in amino acid metabolism and energy metabolism were significantly changed after t-PA thrombolysis, whereas pinocembrin administration exerted significant intervention effects on these metabolites. Linear regression analysis showed that lactic acid was highly correlated to the occurrence of HT. Further experiments confirmed that t-PA treatment significantly increased the content of lactic acid and the activity of lactate dehydrogenase in the cerebral cortex and serum, and the expression of monocarboxylate transporter 1 (MCT 1) in the cerebral cortex; pinocembrin reversed these changes, which was consistent with the result of metabolomics. These results demonstrate that pinocembrin attenuates HT after t-PA thrombolysis, which may be associated with the regulation of endogenous metabolites. Lactic acid may be a potential biomarker for HT prediction and treatment.
Keywords: stroke, hemorrhagic transformation, tissue plasminogen activator, pinocembrin, metabolomics, biomarker, lactic acid
Introduction
Stroke is a disease that seriously threatens human health and is the leading cause of death and disability worldwide. Stroke can be classified as ischemic stroke or hemorrhagic stroke, and approximately 10%–20% of stroke patients experience hemorrhagic stroke [1]. Hemorrhagic transformation (HT), which is intracerebral hemorrhage that occurs after acute cerebral ischemia and significantly increases the mortality and disability of stroke, is a common serious complication of stroke. Restoration of blood supply to the ischemic area is widely used as a treatment for stroke. Tissue plasminogen activator (t-PA) is the only drug approved by the FDA for clinical thrombolytic therapy, but the narrow therapeutic time window and high occurrence of HT limit the clinical application of t-PA. Clinical studies have shown that t-PA thrombolysis increases the risk of HT by ten times and mortality by up to 60% [2]. The therapeutic time window of t-PA has been extended from 3 to 4.5 h, but this window still does not meet clinical needs, as only 3.4%–5.2% of patients can receive t-PA therapy for ischemic stroke [3]. Therefore, methods to extend the therapeutic window of t-PA, decrease the risk of HT, and ultimately increase the overall efficacy of t-PA therapy, are urgently needed.
Considering that thrombolytic therapy is widely applied to treat acute ischemic stroke, it would be helpful to be able to predict the occurrence of HT. In addition, due to differences in pathogenesis, treatment, and prognosis, it is crucial to distinguish HT from acute ischemic stroke. Once HT occurs, the decision to treat ischemic stroke is complex. Early prediction of HT is very helpful in guiding the precise treatment of ischemic stroke, thereby preventing adverse consequences and poor prognosis. At present, the diagnosis of HT is not straightforward and is challenging. In the clinic, image-based strategies are used to diagnose HT and provide relatively accurate results, such as computed tomography and magnetic resonance imaging [4, 5]. However, these strategies are costly, time-consuming, and not widely available and can only be used to diagnose certain subtypes of HT. Therefore, it is necessary to find a novel method that can be used to accurately predict HT. Biomarkers seem to be useful tools for predicting HT after t-PA thrombolysis, and there are many studies on HT biomarkers. Previous studies have suggested that some biomarkers are related to HT occurrence, such as MMP-9, VEGF, and neutrophils [6]. However, these biomarkers have not been successfully used for clinical diagnosis or treatment, and new biomarkers need to be discovered.
Metabolic disorders play an important role in ischemic brain injury, and understanding the underlying mechanisms is critical to the development of effective treatments. Metabolomics is used to analyze and compare changes in the metabolic profiles of endogenous metabolites in tissues and body fluids to determine the overall metabolic status under physiological or pathological conditions, and help identify potential disease-related biomarkers [7, 8]. When stroke occurs, metabolic dysfunction in the brain leads to changes in endogenous metabolites, including those related to energy metabolism, carbohydrate metabolism, and lipid metabolism, and aggravates brain injury [9]. By comparing the change in endogenous metabolites under healthy conditions and after stroke, effective strategies for treating and improving the prognosis of stroke can be identified. However, the changes in metabolomics that occur after t-PA thrombolysis are not clear.
Pinocembrin (5,7-dihydroxyflavanone) (Fig. 1) is a natural flavonoid compound extracted from propolis that was obtained through high-throughput screening technology by our laboratory. It is a novel drug with effects against cerebral ischemia drug, and it is now in Phase II clinical trials (https://clinicaltrials.gov/ct2/show/NCT02059785). Pinocembrin protects against cerebral ischemia by improving cerebral blood flow, decreasing oxidative stress, and protecting the neurovascular unit [10]. Recent studies have found that pinocembrin alleviates cerebral hemorrhage injury by inhibiting the inflammatory response [11]. Our previous results also indicated that pinocembrin extended the therapeutic window of t-PA thrombolysis [12]. However, whether pinocembrin attenuates HT after t-PA treatment by interfering with endogenous metabolites is unknown.
Fig. 1.
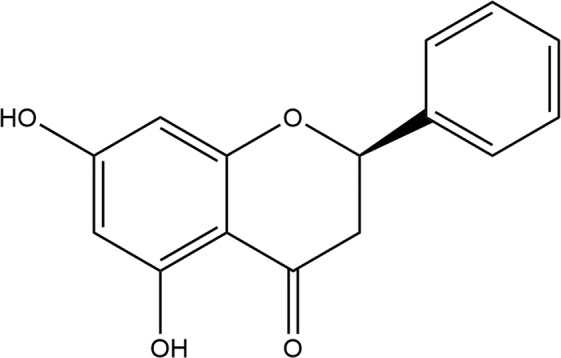
The chemical structure of pinocembrin.
In the present study, the neuroprotective roles of pinocembrin in t-PA-induced HT were confirmed, and the changes in endogenous metabolites and metabolic pathways after t-PA thrombolysis and pinocembrin treatment were investigated by metabolomics. The findings may contribute to the discovery of potential biomarkers for predicting HT and expanding the clinical indication of pinocembrin.
Materials and methods
Animals
Male Sprague-Dawley rats (weight 260–280 g) were supplied by Beijing Vital River Laboratory Animal Technology Co. Ltd and housed in a temperature (22–24 °C) and humidity (<40%) controlled room with free access to food and water. All procedures were approved by the Animal Care and Use Committee of the Peking Union Medical College and the Chinese Academy of Medical Sciences.
Rat model of electrocoagulation-induced thrombotic focal ischemia
A rat model of thrombotic focal ischemia induced by electrocoagulation was established as previously described [12, 13]. After anesthesia with 10% chloral hydrate (4 mL/kg), the common carotid artery (CCA) was placed in the clip of the YLS-14B thrombosis tester (Jinan Yiyan Technology Development Co., Ltd., Jinan, China), and electrical stimulation (1.00 mA) was delivered for 240 s to form a thrombus. The thrombus was crushed and flushed into the ICA (internal carotid artery). The CCA was clamped for an additional 15 min, and then the animal was returned to the cage.
Experimental groups and drugs
Rats were randomly assigned to four groups: the sham group, which included rats that underwent the same surgical procedure as those in the other groups except stimulation of the CCA; the vehicle group, which included rats that underwent surgery and received saline; the t-PA group, which included rats that underwent surgery and received t-PA; and the t-PA + pinocembrin (t-PA + pino) group, which included rats that underwent surgery and received t-PA and pinocembrin. Six hours after ischemia, t-PA (10 mg/kg, Boehringer Ingelheim, Germany) was given, 10% as a bolus and 90% as a continuous infusion for 20 min through the tail vein. Pinocembrin (10 mg/kg, purity > 99%, Institute of Materia Medica) dissolved in saline was given 5 min before t-PA injection via the tail vein. Previous studies have shown that 10 mg/kg is the most effective dose of t-PA in rat stroke models [12]. All rats were sacrificed 24 h after ischemia. In all, 128 SD rats were used; 15 of the rats were excluded due to death, 18 were excluded due to a lack of neurological impairment, and 33 rats were recruited for the experiments.
Animal behavioral tests
The forelimb function test, rotating rod test, and inclined plane test were performed, and neurological scores were evaluated 24 h after ischemia. Longa’s five-point scale was used to measure neurological function. Animals without neurological symptoms and those that died after surgery were excluded, and other rats were recruited. The forelimb function test, rotating rod test and inclined plane test were used to evaluate motor coordination and hemiparesis of the forelimbs and hindlimbs as previously reported [13]. (1) Forelimb function test: the rats were hung from a thick rope with a diameter of 0.5 mm simultaneously from both fore paws. Performance was scored as follows: 1 point, both paws gripped the rope; 2 points, one paw gripped the rope; and 3 points, neither paw gripped the rope. (2) Rotating rod test: each rat was placed on an elevated rod rotating at a speed of 22 rpm per 60 s. Performance was scored as follows: 0 points, the animal walked on the rod for more than 60 s; 1 point, the animal walked on the rod for less than 60 s; 2 points, the animal fell off right after rotation started; and 3 points, the animal fell off the rod before rotation started. (3) Inclined plane test: each rat was placed on an inclined plane with an angle of 80° three times for habituation. In each trial, the duration for which the rat held its position in the middle of the plane was determined. Rats that were able to hold their position for 120 s or longer were given a score of 120 s.
Infarct volume analysis
The animals were anesthetized 24 h after ischemia, and then the brains were excised and cut into 2-mm-thick slices. The slices were immersed in 1% 2,3,5-triphenyltetrazolium chloride (TTC) (T8877, Sigma-Aldrich Chemical Co., St. Louis, MO, USA) in PBS for 30 min at 37 °C and fixed in 4% formalin. Images were obtained with a computer and scanner. The infarct area, the area of the corresponding structure on the contralateral side, and the area of the corresponding structure on the ipsilateral side were calculated with ImageJ software (NIH, Bethesda, MD, USA). The following formula was used to calculate the infarct volume while correcting for edema and atrophy: percentage of infarct volume = [total infarct volume − (ipsilateral hemisphere volume − contralateral hemisphere volume)]/contralateral hemisphere volume × 100%.
Brain water content
The wet/dry method was used to determine the water content in the brain. Twenty-four hours after ischemia, brains were quickly removed and dried in an oven at 110 °C for 24 h. The brain water content was calculated as (wet weight − dry weight)/wet weight × 100%.
Spectrophotometric assay of hemoglobin content
Rats were anesthetized by an overdose of chloral hydrate and transcardially perfused 24 h after ischemia. The brains were immediately excised and cut into the ipsilateral and contralateral hemispheres. After adding 10 mL/g saline to each sample, the tissues were homogenized and centrifuged (13,000 rpm for 30 min). 200 μL of reagent (QuantiChrom Hemoglobin Assay Kit, DIHB-250, BioAssay Systems, NC, USA) was then added to 50 μL of supernatant. After 15 min, the optical density was measured at 400 nm with a spectrophotometer (SpectraMax M5, Molecular Devices, CA, USA).
Quantitative measurement of BBB permeability
Evans blue (E2129, Sigma-Aldrich Chemical Co., St. Louis, MO, USA) (4% solution, 2 mL/kg) was injected through the lingual vein 2 h before sacrifice. The rats were perfused with PBS to remove intravascular dye. For quantitative measurement, the ipsilateral hemisphere was homogenized in 50% trichloroacetic acid solution to precipitate proteins and centrifuged at 12,000 × g for 5 min. The supernatant was diluted with ethanol (1:3). The concentration of dye in the supernatant was measured colorimetrically with a spectrophotometer at a wavelength of 620 nm. EB standards were used to determine the amount of dye in the brain, which was quantified as nanograms per gram of brain tissue.
Transmission electron microscopic examination
Twenty-four hours after ischemia, the rats were anesthetized with 10% chloral hydrate, and 50 mL of saline, 100 mL of 4% paraformaldehyde, and 2.5% glutaraldehyde in 0.1 mol/L cacodylic acid buffer were injected into the left ventricle successively. The fixed brains were dehydrated in a series of ethanol solutions, embedded in epoxy resin, and cut into ultrathin sections. The slices were mounted on copper grids, stained with uranyl acetate and citric acid-lead, and then observed under a transmission electron microscope (H-7650; Hitachi Ltd, Tokyo, Japan).
Metabolomics study
Cerebral cortex and serum samples were prepared according to the literature [14, 15]. Finally, 600 μL of supernatant was transferred into 5-mm NMR tubes for 1H-NMR analysis.
1H-NMR spectra of the serum and cerebral cortex were acquired on a Bruker 600-MHz AVANCE III NMR spectrometer (Bruker, Germany). Serum samples were analyzed using 1D Carr-Purcell-Meibom-Gill spin-echo pulse sequences, and cerebral cortex samples were analyzed using the noesygppr1d pulse sequence. The 1H-NMR spectra were processed using MestReNova software (version 8.0.1, Mestrelab Research, Santiago de Compostella, Spain). All spectra were manually phased, and baselines were corrected. The serum spectra were referenced internally to the chemical shift of creatine at δ 3.04 ppm, and the cerebral cortex spectra were referenced internally to the chemical shift of TSP at δ 0.00 ppm. To avoid the influence of water, the resonance region (δ 4.7–5.0) containing residual water was excluded. The normalized integral values were then subjected to multivariate data analysis using Simca-P 13.0 software (Umetrics, Sweden), and partial least squares discriminant analysis (PLS-DA) and orthogonal partial least squares discriminant analysis (orthogonal PLS-DA, OPLS-DA) were used to identify the differential metabolites. MetaboAnalyst 3.0 was used to perform metabolic pathway enrichment analysis. The crucial metabolic pathways were identified according to the Holm P value (<0.05), false discovery rate (FDR < 0.05), and impact value (>0.2).
Western blotting
Proteins were separated by electrophoresis on a 10% polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane. The membrane was blocked with 5% BSA and then incubated with primary antibody (MCT 1, 1:500, Proteintech, Wuhan, China). After incubation with HRP-conjugated secondary antibody, an enhanced chemiluminescence plus detection system (Tanon 5200 Gel Imaging System, Tanon Science & Technology Co., Ltd, Shanghai, China) was used. The density of each band was quantified by using ImageJ.
Quantitative measurement of lactic acid and lactate dehydrogenase (LDH)
The levels of lactic acid and LDH in cerebral cortex and serum samples were measured with a lactic acid assay kit and LDH assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Measurements were carried out in accordance with the manufacturer’s instructions. Lactic acid and LDH standards were used to determine the amount of lactic acid and LDH in the cerebral cortex and serum.
Statistical analysis
The results are expressed as the mean ± SEM. Data were compared between two groups using unpaired Student’s t-test. For more than two groups, statistical analysis was performed using one-way ANOVA with Bonferroni’s multiple comparison post hoc test. Statistical significance was accepted at P < 0.05.
Results
Pinocembrin improved neurologic function and decreased the infarct volume
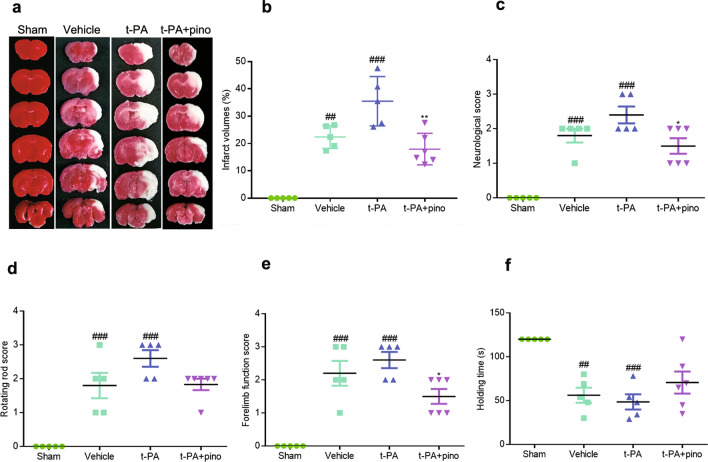
Neurological scores and the infarct volume were used to investigate the effect of pinocembrin in t-PA-induced HT. As shown in Fig. 2, neurological function was impaired, and the infarct volume was increased 24 h after ischemia. Injection of t-PA further aggravated neurological function impairment and the infarct volume compared with vehicle group, while pinocembrin significantly improved neurological function and reduced the infarct volume.
Fig. 2. The effect of pinocembrin on infarct volume and neurological function.
Treatment with t-PA further aggravated the infarct volume and neurological deficits, while pinocembrin reversed these effects. a Representative TTC-stained images. b Infarct volume data. c Neurological scores. d Performance on the rotating rod test. e Performance on the forelimb function test. f Performance on the inclined plane test. The data are presented as the mean ± SEM. ##P < 0.01 and ###P < 0.001 compared with the sham group; *P < 0.05 and **P < 0.01 compared with the t-PA group; n = 5–6.
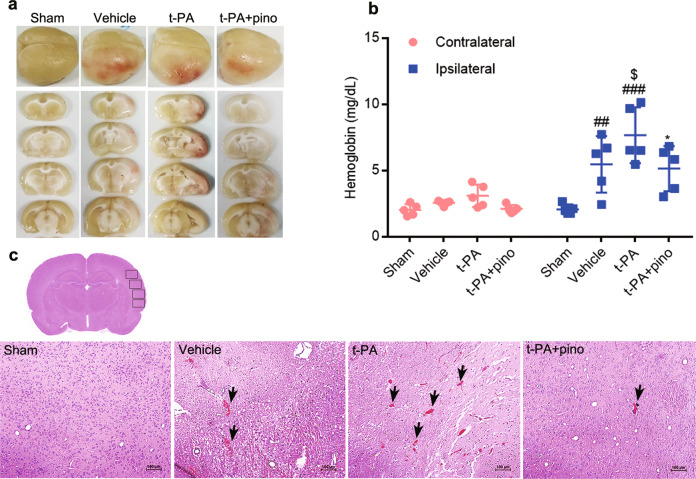
Pinocembrin attenuated t-PA induced HT
The occurrence and severity of HT were evaluated by measurement of hemoglobin content in the brain and H&E staining. As shown in Fig. 3a, b, t-PA induced HT, and hemoglobin content in ischemic tissue was obviously increased. Compared with t-PA group, pinocembrin decreased hemoglobin content and attenuated HT. H&E staining indicated a similar result (Fig. 3c). Slight bleeding was observed in the vehicle group, and serious hemorrhage was observed in the t-PA group. Pinocembrin reduced the degree of bleeding. All the above results showed that pinocembrin could attenuate t-PA-induced HT.
Fig. 3. The effect of pinocembrin on t-PA-induced hemorrhagic transformation.
Delayed t-PA treatment increased the incidence and severity of hemorrhage. Pinocembrin markedly decreased hemorrhage and the amount of hemoglobin. a Representative images of hemorrhagic transformation. b Hemoglobin content. c Representative images of H&E staining. The arrow indicates bleeding. The data are presented as the mean ± SEM. ##P < 0.01 and ###P < 0.001 compared with sham group; $P < 0.05 compared with the vehicle group; *P < 0.05 compared with the t-PA group; n = 5; bar = 100 µm.
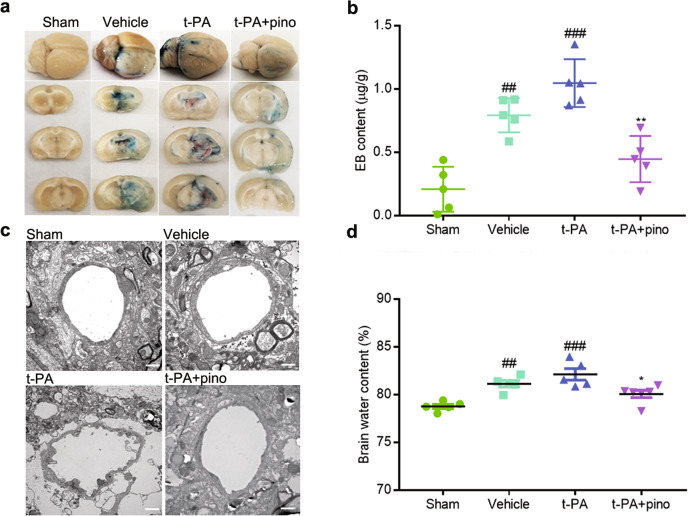
Pinocembrin alleviated BBB disruption
To assess the permeability and structure of the BBB, EB leakage and brain water content were measured, and transmission electron microscopy was performed. Twenty-four hours after ischemia, BBB permeability was increased, and BBB structure was disrupted, leading to an increase in EB levels and water content in ischemic brain tissue. t-PA treatment further disrupted BBB structure and promoted EB leakage and brain edema (Fig. 4). Pinocembrin significantly reduced EB levels and brain water content and protected BBB structure. The above results indicated that pinocembrin inhibited BBB disruption and maintained BBB structure and permeability.
Fig. 4. The effect of pinocembrin on BBB structure and permeability.
Delayed t-PA treatment significantly increased EB leakage, brain water content, and BBB disruption. Pinocembrin dramatically reversed these effects. a Representative images of EB leakage. b EB content. c Representative images of BBB structure. d Brain water content. The data are presented as the mean ± SEM. ##P < 0.01 and ###P < 0.001 compared with the sham group; *P < 0.05 and **P < 0.01 compared with the t-PA group; n = 5; bar = 500 nm.
Metabolic profiles of the cerebral cortex and serum
According to the literature [14, 15], combined with the public databases HMDB (https://hmdb.ca/), BMRB (https://bmrb.io/), and the Chenomx NMR Suite (ChenomxInc, Canada), the primary metabolites in the spectra were identified. A total of 23 and 33 endogenous metabolites were identified in the cerebral cortex (Supplementary Fig. S1 and Table S1) and serum (Supplementary Fig. S2 and Table S2) of rats, respectively.
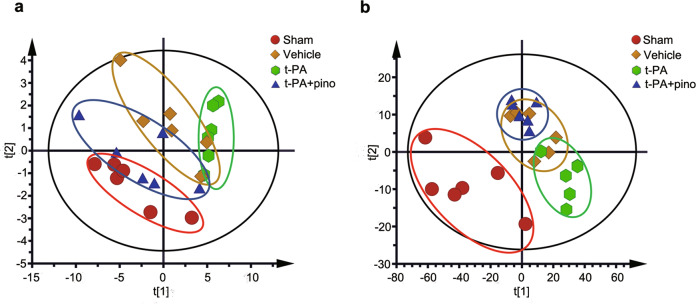
Multivariate data analysis
To analyze the metabolic differences among groups, multivariate statistical analysis was carried out. As shown in Fig. 5a, b, the metabolic profiles of the cerebral cortex and serum in the vehicle group were obviously separated from those in the sham group, and those in the t-PA group were clearly separated from those in the sham group and vehicle group, which indicated that the cerebral ischemia model and t-PA-induced HT model were successfully established. Furthermore, pinocembrin had an obvious regulatory effect on the metabolic profile of the cerebral cortex and serum in t-PA-treated rats.
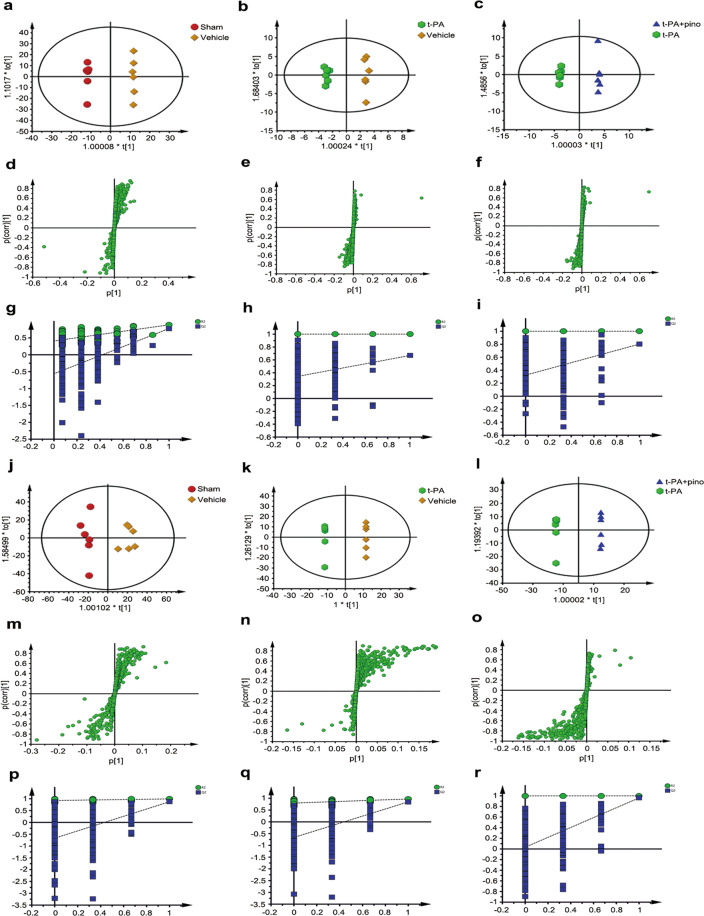
Fig. 5. PLS-DA score plot derived from 1H-NMR spectra of the cerebral cortex and serum of rats.
a Cerebral cortex, R2X = 0.865 and Q2 = 0.663. b Serum, R2X = 0.824 and Q2 = 0.636.
Screening of differential metabolites in the cerebral cortex and serum
Differential metabolites in the cerebral cortex and serum among the different groups were identified. OPLS-DA score plots and S-plots (Fig. 6) were obtained by comparing the vehicle group and sham group (model parameters: cerebral cortex, R2X = 0.885 and Q2 = 0.533; serum, R2X = 0.631 and Q2 = 0.819), t-PA group and vehicle group (model parameters: cerebral cortex, R2X = 0.86 and Q2 = 0.503; serum, R2X = 0.906 and Q2 = 0.875), and t-PA + pino group and t-PA group (model parameters: cerebral cortex, R2X = 0.865 and Q2 = 0.556; serum, R2X = 0.623 and Q2 = 0.851). The model validation results showed that R2 and Q2 values from the permutation model were lower than the original ones or that the Q2 line intercepted a negative value on the Y axis, indicating excellent predictive ability. For multivariate analysis, CV-ANOVA was used to validate the model (cerebral cortex: P = 0.0115047; serum: P = 0.00229137). To investigate the changes of metabolites after t-PA treatment, the t-PA group and vehicle group were compared. Five metabolites in the cerebral cortex and 16 metabolites in serum were identified by comparing the t-PA group and vehicle group by using an S-plot combined with VIP values (>1) and an independent t-test (P < 0.05).
Fig. 6. OPLS score plot, corresponding S-plot, and PLS-DA corresponding validation plot derived from the cerebral cortex and serum.
a–i Cerebral cortex. j–r Serum. a, j OPLS score plot of the vehicle group vs sham group. b, k OPLS score plot of the t-PA group vs vehicle group. c, l OPLS score plot of the t-PA + pino group vs t-PA group. d, m Corresponding S-plot of the vehicle group vs sham group. e, n Corresponding S-plot of the t-PA group vs vehicle group. f, o Corresponding S-plot of the t-PA + pino group vs t-PA group. g, p Corresponding PLS-DA validation plot of the vehicle group vs sham group. h, q Corresponding PLS-DA validation plot of the t-PA group vs vehicle group. i, r Corresponding PLS-DA validation plot of the t-PA + pino group vs t-PA group.
The levels of glycine, glutamate, lactic acid, inositol, and taurine in the cerebral cortex increased in the t-PA group compared with the vehicle group, while pinocembrin significantly decreased the level of these five metabolites (Table 1). The levels of leucine, valine, alanine, acetic acid, glutamate, o-acetyl glycoprotein, phosphocholine, trimethylamine oxide, tyrosine, glycerin, inositol, dimethylamine, arginine, α-glucose, and formic acid in the serum decreased in the t-PA group compared with the vehicle group, while the level of lactic acid increased. Pinocembrin could significantly regulate 15 out of the 16 metabolites except formic acid (Table 1). The above results indicated that pinocembrin had significant effects on t-PA-induced changes of metabolites in the cerebral cortex and serum.
Table. 1.
Differential metabolites in the cerebral cortex and serum after t-PA and pinocembrin treatment.
| No. | Metabolites | Sham | Vehicle | t-PA | t-PA + pino | Sample |
|---|---|---|---|---|---|---|
| 1 | Glycine | 0.20 ± 0.05 | 0.36 ± 0.07 | 0.48 ± 0.06# | 0.30 ± 0.08** | Cortex |
| 2 | Glutamate | 0.07 ± 0.04 | 0.18 ± 0.06 | 0.29 ± 0.08# | 0.14 ± 0.06** | Cortex |
| 3 | Inositol | 0.16 ± 0.04 | 0.17 ± 0.05 | 0.23 ± 0.02# | 0.17 ± 0.05* | Cortex |
| 4 | Taurine | 0.35 ± 0.05 | 0.45 ± 0.07 | 0.54 ± 0.04# | 0.36 ± 0.07*** | Cortex |
| 5 | Lactic acid | 0.85 ± 0.14 | 0.90 ± 0.07 | 1.07 ± 0.06## | 0.79 ± 0.11*** | Cortex |
| 6 | Leucine | 7.36 ± 2.42 | 7.61 ± 1.22 | 5.86 ± 1.29# | 8.70 ± 0.70*** | Serum |
| 7 | Valine | 2.34 ± 1.11 | 3.10 ± 0.67 | 1.84 ± 0.59## | 3.40 ± 0.55*** | Serum |
| 8 | Alanine | 9.21 ± 2.61 | 6.80 ± 1.74 | 4.27 ± 1.07# | 7.13 ± 0.79*** | Serum |
| 9 | Acetic acid | 3.61 ± 0.93 | 3.49 ± 0.68 | 2.21 ± 0.67## | 3.51 ± 0.50** | Serum |
| 10 | Glutamate | 7.82 ± 2.04 | 7.19 ± 1.30 | 4.63 ± 1.42## | 8.38 ± 0.59*** | Serum |
| 11 | O-acetyl glycoprotein | 20.84 ± 5.38 | 21.67 ± 4.63 | 15.17 ± 4.54# | 25.84 ± 2.29*** | Serum |
| 12 | Phosphocholine | 19.89 ± 5.80 | 10.79 ± 2.95 | 7.12 ± 2.15# | 13.56 ± 1.14*** | Serum |
| 13 | Trimethylamine oxide | 41.77 ± 15.67 | 22.23 ± 6.51 | 9.21 ± 2.74## | 25.01 ± 3.24*** | Serum |
| 14 | Tyrosine | 0.71 ± 0.20 | 0.48 ± 0.13 | 0.31 ± 0.07# | 0.58 ± 0.09*** | Serum |
| 15 | Glycerin | 23.78 ± 8.27 | 10.02 ± 3.08 | 4.90 ± 2.25## | 11.55 ± 1.87*** | Serum |
| 16 | Inositol | 4.39 ± 2.76 | 4.15 ± 1.50 | 1.90 ± 0.49## | 4.27 ± 2.00* | Serum |
| 17 | Dimethylamine | 57.67 ± 18.09 | 30.55 ± 8.17 | 11.43 ± 5.73### | 33.21 ± 6.49*** | Serum |
| 18 | Arginine | 22.61 ± 8.37 | 10.77 ± 2.88 | 5.30 ± 1.81## | 12.02 ± 1.58*** | Serum |
| 19 | α-Glucose | 52.21 ± 21.16 | 29.74 ± 8.42 | 8.48 ± 3.00### | 31.41 ± 5.87*** | Serum |
| 20 | Formic acid | 0.61 ± 0.26 | 0.38 ± 0.06 | 0.18 ± 0.11## | 0.31 ± 0.09 | Serum |
| 21 | Lactic acid | 11.30 ± 2.49 | 11.35 ± 1.31 | 15.65 ± 2.57## | 10.94 ± 1.26** | Serum |
Data were shown as mean ± SEM. n = 6.
#P < 0.05, ##P < 0.01 and ###P < 0.001 compared with vehicle group; *P < 0.05, **P < 0.01 and ***P < 0.001 compared with t-PA group.
Metabolic pathways regulated by pinocembrin
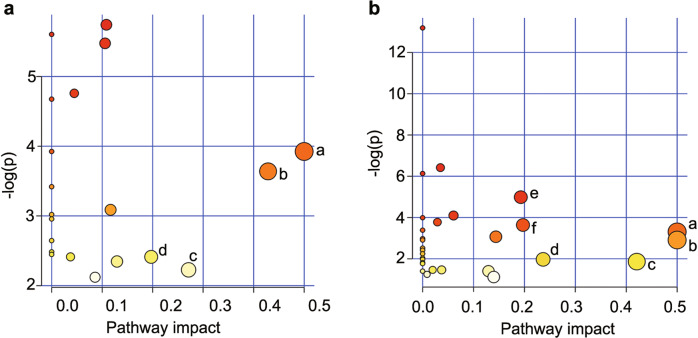
Pathway enrichment analysis of the differential metabolites that were significantly regulated by pinocembrin in t-PA-induced HT was performed. The results showed that pinocembrin significantly regulated four pathways in cerebral cortex, including D-glutamine and D-glutamate metabolism; taurine and hypotaurine metabolism; glycine, serine, and threonine metabolism; and alanine, aspartate, and glutamate metabolism (Fig. 7a). Additionally, pinocembrin significantly regulated six pathways in the serum, including phenylalanine, tyrosine, and tryptophan biosynthesis; D-glutamine and D-glutamate metabolism; starch and sucrose metabolism; glycerolipid metabolism; arginine biosynthesis; and alanine, aspartate, and glutamate metabolism (Fig. 7b).
Fig. 7. Metabolic pathways that are significantly regulated by pinocembrin in the cerebral cortex and serum.
a Cerebral cortex. b Serum. Cerebral cortex: a D-glutamine and D-glutamate metabolism; b taurine and hypotaurine metabolism; c glycine, serine, and threonine metabolism; and d alanine, aspartate, and glutamate metabolism. Serum: a phenylalanine, tyrosine, and tryptophan biosynthesis; b D-glutamine and D-glutamate metabolism; c starch and sucrose metabolism; d glycerolipid metabolism; e arginine biosynthesis; and f alanine, aspartate, and glutamate metabolism.
Correlation analysis between differential metabolites and HT
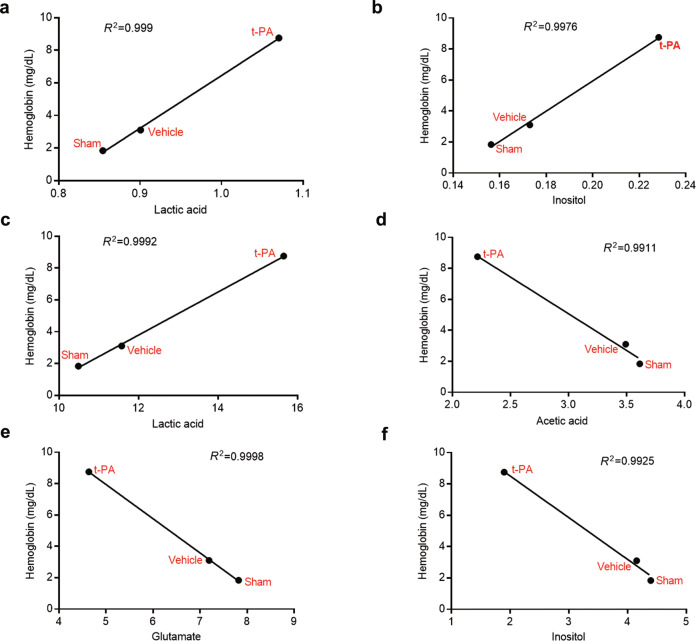
To analyze the correlation between endogenous metabolites and HT, correlation analysis between the levels of differential metabolites in the cerebral cortex or serum and hemoglobin content in the sham group, vehicle group, and t-PA group was performed (Fig. 8 and Supplementary Fig. S3). As illustrated in Fig. 8, there was a good linear relationship between lactic acid and inositol levels in the cerebral cortex and hemoglobin content and between lactic acid, acetic acid, glutamate, and inositol levels in the serum and hemoglobin content in the sham group, vehicle group, and t-PA group (R2 > 0.99). Moreover, treatment with t-PA resulted in the highest lactic acid levels in the cortex and serum, corresponding to more severe hemorrhage, which indicated that the level of lactic acid was most closely related to HT and may be a potential biomarker for HT prediction.
Fig. 8. Correlation analysis between differential metabolites and HT.
a Lactic acid level in the cerebral cortex. b Inositol level in the cerebral cortex. c Lactic acid level in the serum. d Acetic acid level in the serum. e Glutamate level in the serum. f Inositol level in the serum.
Pinocembrin inhibited the production and transport of lactic acid in the cerebral cortex and serum
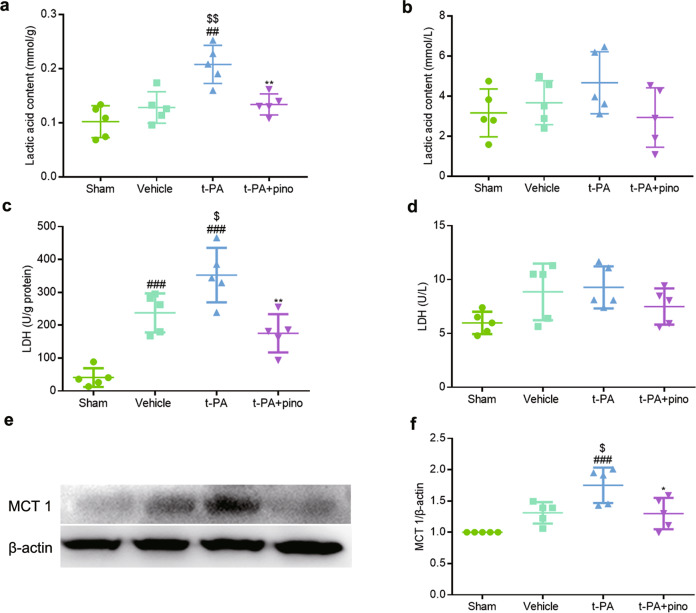
Since lactic acid is highly correlated with HT in the cerebral cortex and serum, the content of lactic acid was quantitatively determined to verify the change of lactic acid. As shown in Fig. 9a, b, the content of lactic acid in both the cerebral cortex and serum increased after t-PA treatment, especially in the cerebral cortex, and pinocembrin significantly decreased the content of lactic acid. In addition, the activity of LDH and the expression of MCT 1 were also measured. The activity of LDH in the cerebral cortex and serum (Fig. 9c, d) and the expression of MCT 1 in the cerebral cortex (Fig. 9, f) were both induced after t-PA treatment, and pinocembrin significantly inhibited LDH activity and MCT 1 expression. The above results suggested that the metabolism of lactic acid was changed by t-PA and that pinocembrin restored the changes of lactic acid by regulating the production and transport of lactic acid.
Fig. 9. The effect of pinocembrin on the production and transport of lactic acid in the cerebral cortex and serum.
Delayed t-PA treatment induced the release of lactic acid. Pinocembrin markedly decreased the content of lactic acid. a The content of lactic acid in the cerebral cortex. b The content of lactic acid in the serum. c The activity of LDH in the cerebral cortex. d The activity of LDH in the serum. e Representative images of MCT 1 expression in the cerebral cortex. f The change in MCT 1 expression. The data are presented as the mean ± SEM. ##P < 0.01 and ###P < 0.001 compared with the sham group; $P < 0.05 and $$P < 0.01 compared with the vehicle group; *P < 0.05 and **P < 0.01 compared with the t-PA group; n = 5.
Discussion
In the present study, we found that pinocembrin alleviated t-PA-induced HT and improved neurological function. The metabolomics results indicated that the expression of a variety of endogenous metabolites involving energy metabolism and amino acid metabolism was induced in the cerebral cortex and serum by t-PA thrombolytic therapy. Pinocembrin could regulate the levels of most abnormally expressed metabolites, possibly due to its effect against HT. In addition, the metabolic change of lactic acid had a good correlation with the occurrence of HT and may be a potential biomarker for HT.
Stroke is a complex pathological process that includes excitatory amino acid release, intracellular calcium instability, free radical generation, and apoptosis, which lead to disorders of energy metabolism, amino acid metabolism, and lipid metabolism and aggravate brain injury [16]. There are no clinically effective drugs for the prevention and treatment of HT after t-PA thrombolysis, which is a serious complication of stroke. Therefore, finding effective drugs and biomarkers is of great value for the treatment of HT.
At present, there are limited ideal HT animal models. The embolic MCAO model is widely used to evaluate the effect of thrombolytic therapy, but it fails to mimic thrombosis and thrombus aging. An electrocoagulation-induced thrombotic focal ischemia model involving the development of a fresh thrombus has been established; in this model, occlusion of the MCA region with a thrombus formed in the CCA is similar to what is observed in thromboembolic stroke in humans, and thus, this model is suitable for the evaluation of thrombolytic therapy. A reproducible and predictable infarct within the territory supplied by the MCA is also formed in this model [13]. Therefore, rats with electrocoagulation-induced thrombotic focal ischemia were used to investigate the role of pinocembrin in t-PA-induced HT. The results further confirmed that pinocembrin decreased the infarct volume and the occurrence of HT and improved BBB structure and permeability, which suggested that pinocembrin may be a potential drug for treating HT. However, the mechanism of pinocembrin in t-PA-induced HT is not clear.
Brain damage caused by stroke is closely related to metabolic disorders, and the prognosis of stroke can be assessed by comparing and analyzing metabolite differences between the health state and after stroke. Metabolomics can reveal overall metabolic changes associated with disease and thus show great potential in identifying disease biomarkers. In recent years, this technique has been used in clinical studies of stroke [17–19], but it has rarely been used to assess changes that occur following t-PA thrombolytic therapy. Therefore, the levels of endogenous metabolites were evaluated after t-PA and pinocembrin treatment by metabolomics to identify potential biomarkers and explore the neuroprotective mechanisms of pinocembrin in HT.
Due to its accuracy in qualitative analysis, simple pretreatment operation, and nondestructive multi-parameter and dynamic analysis, 1H-NMR metabolomics was used to analyze changes of endogenous metabolites after t-PA and pinocembrin treatment. Multivariate statistical analysis indicated that the cerebral ischemia model and t-PA-induced HT model were successfully established. To identify potential biomarkers and evaluate the effect of pinocembrin on endogenous metabolites, OPLS-DA score plots were obtained by comparing the vehicle group and sham group, t-PA group and vehicle group, and t-PA + pino group and t-PA group. Five metabolites in the cerebral cortex and 16 metabolites in serum were identified by comparing the t-PA group and vehicle group, indicating that changes of specific metabolites could be induced after t-PA treatment, which may be associated with HT occurrence.
As a substrate for protein synthesis, amino acids are essential for human function. There are a large number of free amino acids in the nervous system, including γ-aminobutyric acid, aspartic acid, glutamate, taurine, and glycine, which are representative excitatory and inhibitory neurotransmitters and are closely related to the outcome of nervous system diseases. Metabolomics studies have shown abnormal amino acid metabolism in stroke patients [20–22]. In this study, changes of leucine, valine, alanine, glutamate, glycine, tyrosine, taurine, and arginine levels in the cerebral cortex and serum after t-PA treatment were observed. The levels of glutamate, glycine, and taurine increased in the cortex, but the levels of glutamate, glycine, tyrosine, leucine, valine, alanine, and arginine decreased in the serum, which might be due to an inability to excrete metabolites from the brain into the blood after stroke, leading to accumulation in the brain. Other studies have also found differences in the changes of metabolites in the brain and serum and proposed that the changes in the brain may better reflect brain damage [23]. In addition, the levels of glutamate, glycine, and taurine in the cortex were further increased, and the levels of tyrosine and arginine in the serum were further reduced in the t-PA group compared with the vehicle group, which indicated that these amino acids may be closely related to HT occurrence after t-PA treatment. Pinocembrin could interfere with the metabolism of these amino acids and normalize the levels of these amino acids.
Among the affected amino acids, glutamate is the main excitatory neurotransmitter in the central nervous system. Under physiological conditions, there is a dynamic balance between glutamate release, uptake, and reabsorption. After cerebral ischemia, glutamate release from cells increases, and the extracellular concentration of glutamate increases rapidly, leading to the activation of NMDARs and AMPARs, extracellular calcium influx, excitotoxicity, and eventually neuronal death and brain damage [24, 25]. Excitotoxicity caused by glutamate seriously affects the prognosis of stroke patients [26]. In this study, the increased glutamate level in the cortex after t-PA treatment indicated the occurrence of excitotoxicity, which is consistent with a previous study on t-PA-induced excitotoxicity. However, pinocembrin decreased the level of glutamate, suggesting that the effect of pinocembrin against HT may be associated with the inhibition of excitotoxicity induced by t-PA.
Glycine and taurine are inhibitory neurotransmitters in the brain and have neuroprotective effects [27–30]. Glycine inhibits the release of glutamate, which improves the prognosis of patients with ischemic stroke [31]. Taurine regulates the release of neurotransmitters (such as acetylcholine and GABA) and neuronal apoptosis induced by ischemia/hypoxia or glutamate through inhibiting endoplasmic reticulum stress and maintaining intracellular Ca2+ homeostasis [32, 33]. Some studies have found that the levels of glycine and taurine increase after stroke through the body’s stress response [34–36]. In this study, we also observed this phenomenon after t-PA treatment and found that the inhibition of pinocembrin may be related to the regulation of amino acid balance.
In addition to amino acid metabolism, energy metabolism is also affected by stroke. After stroke, glycolysis is enhanced, the tricarboxylic acid cycle is inhibited, and the level of lactic acid increases rapidly. Lactic acid is an important substance that links brain energy metabolism and functional activity [37]. Lactic acid is produced by astrocytes and is transported to the intercellular space by monocarboxylate transporters (MCTs) for use by neurons [38–40]. The accumulation of lactic acid is considered to be one of the main causes of brain tissue damage under cerebral ischemia conditions and is related to nerve cell death and the prognosis of stroke [41]. Abnormally elevated lactic acid levels are observed in the cerebrospinal fluid and serum in stroke patients [41, 42]. This study found an increase of lactic acid levels in the cortex and serum induced by t-PA, which was further confirmed by a lactic acid assay kit.
The level of lactic acid is related to the production and transportation of lactic acid, which is mediated by LDH and MCT 1, respectively. Under hypoxic conditions, glutamate in neurons is released into the intercellular space and is taken up by astrocytes to activate glycolysis-related enzymes and promote glycolysis [43]. LDH catalyzes the conversion of glucose into lactic acid, and the lactic acid, the level of which is increased, is transported to the intercellular space by MCT 1. The activity of LDH and the expression of MCT 1 are regulated by excessive lactic acid and hypoxia [44]. In this study, LDH activity and MCT 1 expression were induced by t-PA and inhibited by pinocembrin, which was consistent with the changes of lactic acid. These results indicated that pinocembrin interfered with the production and transportation of lactic acid, suggesting that pinocembrin had a neuroprotective effect through the regulation of energy metabolism. Furthermore, lactic acid may be a valuable indicator for detecting HT.
Identifying early biomarkers of HT is very important for finding new treatments and predicting the clinical outcome of patients. However, few biomarkers are currently used for the diagnosis of HT. Some studies have reported that hyperacute MMP-9 plasma levels and serum levels of calcium-binding protein (S100B) may be associated with HT after t-PA thrombolysis [44, 45]. In this study, through correlation analysis of endogenous metabolites and HT, we found that lactic acid levels in the serum and cortex and inositol levels in the cortex were positively correlated with hemoglobin content and that acetic acid, glutamate, and inositol levels in the serum were negatively correlated with hemoglobin content. Among them, the change of lactic acid in the serum was consistent with that in the cortex, which was further confirmed by a lactic acid assay kit, indicating that lactic acid may be a potential biomarker for HT prediction. However, the level of lactic acid that indicates the occurrence of HT requires further clinical research. In addition, due to individual differences and health conditions in the clinic, the specificity and sensitivity of lactic acid as a potential biomarker for HT should be further considered. Therefore, the combination of lactic acid measurements and other detection methods, such as imaging, may be able to better predict the occurrence and prognosis of HT.
In conclusion, our study provides the first evidence that t-PA thrombolytic therapy causes changes in the levels of endogenous metabolites, including amino acids and energy metabolism. The effect of pinocembrin against HT is associated with the regulation of endogenous metabolites. Lactic acid may be a potential biomarker for HT prediction and treatment.
Supplementary information
Acknowledgements
This work was supported by the National Key Research and Development Plan of China (2016YFC1000905); the Beijing Municipal Natural Science Foundation (7182113); the CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-3-007); and The Drug Innovation Major Project of China (2018ZX09711001-009-009).
Author contributions
LLK and GHD conceived and designed the experiments. LLK performed the experiments, drafted the paper, and analyzed the data. LG and KXW performed the metabolomics study. NNL, CDL, GDM, and HGY performed the animal experiments. XMQ and GHD reviewed and edited the paper. All authors read and approved the final paper.
Conflict of interest
The authors declare no competing interests.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-021-00664-x.
References
- 1.An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. 2017;19:3–10. doi: 10.5853/jos.2016.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jickling GC, Liu D, Stamova B, Ander BP, Zhan X, Lu A, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34:185–99. doi: 10.1038/jcbfm.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanazawa M, Takahashi T, Nishizawa M, Shimohata T. Therapeutic strategies to attenuate hemorrhagic transformation after tissue plasminogen activator treatment for acute ischemic stroke. J Atheroscler Thromb. 2017;24:240–53. doi: 10.5551/jat.RV16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone JA, Willey JZ, Keyrouz S, Butera J, McTaggart RA, Cutting S, et al. Therapies for hemorrhagic transformation in acute ischemic stroke. Curr Treat Options Neurol. 2017;19:1. doi: 10.1007/s11940-017-0438-5. [DOI] [PubMed] [Google Scholar]
- 5.El Nawar R, Yeung J, Labreuche J, Chadenat ML, Duong DL, De Malherbe M, et al. MRI-based predictors of hemorrhagic transformation in patients with stroke treated by intravenous thrombolysis. Front Neurol. 2019;10:897. doi: 10.3389/fneur.2019.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Li MC, Chen QX, Wang J. Hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke: mechanisms, models, and biomarkers. Mol Neurobiol. 2015;52:1572–9. doi: 10.1007/s12035-014-8952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. 2016;17:451–9. doi: 10.1038/nrm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu PF, Li RT, Antonov AA, Wang LH, Li W, Hua YF, et al. Discovery of metabolite biomarkers for acute ischemic stroke progression. J Proteome Res. 2017;16:773–9. doi: 10.1021/acs.jproteome.6b00779. [DOI] [PubMed] [Google Scholar]
- 9.Ke CF, Pan CW, Zhang YX, Zhu XH, Zhang YH. Metabolomics facilitates the discovery of metabolic biomarkers and pathways for ischemic stroke: a systematic review. Metabolomics. 2019;15:152. doi: 10.1007/s11306-019-1615-1. [DOI] [PubMed] [Google Scholar]
- 10.Lan X, Wang WZ, Li Q, Wang J. The natural flavonoid pinocembrin: molecular targets and potential therapeutic applications. Mol Neurobiol. 2016;53:1794–801. doi: 10.1007/s12035-015-9125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan X, Han XN, Li Q, Li Q, Gao YF, Cheng T, et al. Pinocembrin protects hemorrhagic brain primarily by inhibiting toll-like receptor 4 and reducing M1 phenotype microglia. Brain Behav Immun. 2017;61:326–39. doi: 10.1016/j.bbi.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma YZ, Li L, Kong LL, Zhu ZM, Zhang W, Song JK, et al. Pinocembrin protects blood-brain barrier function and expands the therapeutic time window for tissue-type plasminogen activator treatment in a rat thromboembolic stroke model. Biomed Res Int. 2018;2018:8943210. doi: 10.1155/2018/8943210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma YZ, Li L, Song JK, Niu ZR, Liu HF, Zhou XS, et al. A novel embolic middle cerebral artery occlusion model induced by thrombus formed in common carotid artery in rat. J Neurol Sci. 2015;359:275–9. doi: 10.1016/j.jns.2015.09.362. [DOI] [PubMed] [Google Scholar]
- 14.Zhao FF, Chang YF, Gao L, Qin XM, Du GH, Zhang X, et al. Protective effects of Scutellaria baicalensis Georgi extract on D-galactose induced aging rats. Metab Brain Dis. 2018;33:1401–12. doi: 10.1007/s11011-018-0229-z. [DOI] [PubMed] [Google Scholar]
- 15.Duan DD, Wang KX, Zhou YZ, Qin XM, Gao L, Du GH. Baicalein exerts beneficial effects in D-galactose induced aging rats through attenuation of inflammation and metabolic dysfunction. Rejuvenation Res. 2017;20:506–16. doi: 10.1089/rej.2017.1919. [DOI] [PubMed] [Google Scholar]
- 16.Wesley UV, Bhute VJ, Hatcher JF, Palecek SP, Dempsey RJ. Local and systemic metabolic alterations in brain, plasma, and liver of rats in response to aging and ischemic stroke, as detected by nuclear magnetic resonance (NMR) spectroscopy. Neurochem Int. 2019;127:113–24. doi: 10.1016/j.neuint.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Ruan L, Wang Y, Chen SC, Zhao T, Huang Q, Hu ZL, et al. Metabolite changes in the ipsilateral and contralateral cerebral hemispheres in rats with middle cerebral artery occlusion. Neural Regen Res. 2017;12:931–7. doi: 10.4103/1673-5374.208575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu MT, Tang LY, Liu X, Fang J, Zhan H, Wu HW, et al. An evidence-based review of related metabolites and metabolic network research on cerebral ischemia. Oxid Med Cell Longev. 2016;2016:9162074. doi: 10.1155/2016/9162074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung JY, Lee HS, Kang DG, Kim NS, Cha MH, Bang OS, et al. 1H-NMR-based metabolomics study of cerebral infarction. Stroke. 2011;42:1282–8. doi: 10.1161/STROKEAHA.110.598789. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Liu T, Song HX, Cui SY, Liu G, Christoforou A, et al. Targeted metabolomic profiling reveals association between altered amino acids and poor functional recovery after stroke. Front Neurol. 2020;10:1425. doi: 10.3389/fneur.2019.01425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D, Kong J, Wu JY, Wang XX, Lai MC. GC-MS-based metabolomics identifies an amino acid signature of acute ischemic stroke. Neurosci Lett. 2017;642:7–13. doi: 10.1016/j.neulet.2017.01.039. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y, Khan A, Hong S, Jee SH, Park YH. A metabolomic study on high-risk stroke patients determines low levels of serum lysine metabolites: a retrospective cohort study. Mol Biosyst. 2017;13:1109–20. doi: 10.1039/C6MB00732E. [DOI] [PubMed] [Google Scholar]
- 23.Luo L, Zhen LF, Xu YT, Yang YX, Feng SX, Wang SM, et al. 1H NMR-based metabonomics revealed protective effect of Naodesheng bioactive extract on ischemic stroke rats. J Ethnopharmacol. 2016;186:257–69. doi: 10.1016/j.jep.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 24.Sidorov E, Sanghera DK, Vanamala JKP. Biomarker for ischemic stroke using metabolome: a clinician perspective. J Stroke. 2019;21:31–41. doi: 10.5853/jos.2018.03454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amantea D, Bagetta G. Excitatory and inhibitory amino acid neurotransmitters in stroke: from neurotoxicity to ischemic tolerance. Curr Opin Pharmacol. 2017;35:111–9. doi: 10.1016/j.coph.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Bai W, Li W, Ning YL, Li P, Zhao Y, Yang N, et al. Blood glutamate levels are closely related to acute lung injury and prognosis after stroke. Front Neurol. 2018;8:755. doi: 10.3389/fneur.2017.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu R, Liao XY, Pan MX, Tang JC, Chen SF, Zhang Y, et al. Glycine exhibits neuroprotective effects in ischemic stroke in rats through the inhibition of M1 microglial polarization via the NF-κB p65/Hif-1α signaling pathway. J Immunol. 2019;202:1704–14. doi: 10.4049/jimmunol.1801166. [DOI] [PubMed] [Google Scholar]
- 28.Cai CC, Zhu JH, Ye LX, Dai YY, Fang MC, Hu YY, et al. Glycine protects against hypoxic-ischemic brain injury by regulating mitochondria-mediated autophagy via the AMPK pathway. Oxid Med Cell Longev. 2019;2019:4248529. doi: 10.1155/2019/4248529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin R, Xiao AY, Liu S, Wang M, Li GH. Taurine reduces tPA (tissue-type plasminogen activator)-induced hemorrhage and microvascular thrombosis after embolic stroke in rat. Stroke. 2018;49:1708–18. doi: 10.1161/STROKEAHA.118.020747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu XY, Ma PS, Wu W, Zhou R, Hao YJ, Niu Y, et al. Neuroprotective actions of taurine on hypoxic-ischemic brain damage in neonatal rats. Brain Res Bull. 2016;124:295–305. doi: 10.1016/j.brainresbull.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Gusev EI, Skvortsova VI, Dambinova SA, Raevskiy KS, Alekseev AA, Bashkatova VG, et al. Neuroprotective effects of glycine for therapy of acute ischaemic stroke. Cerebrovasc Dis. 2000;10:49–60. doi: 10.1159/000016025. [DOI] [PubMed] [Google Scholar]
- 32.Prentice H, Gharibani PM, Ma ZY, Alexandrescu A, Genova R, Chen PC, et al. Neuroprotective functions through inhibition of ER stress by taurine or taurine combination treatments in a rat stroke model. Adv Exp Med Biol. 2017;975:193–205. doi: 10.1007/978-94-024-1079-2_17. [DOI] [PubMed] [Google Scholar]
- 33.Prentice H, Pan CL, Gharibani PM, Ma ZY, Price AL, Giraldo GS, et al. Analysis of neuroprotection by taurine and taurine combinations in primary. neuronal cultures and in neuronal cell lines exposed to glutamate excitotoxicity and to hypoxia/re-oxygenation. Adv Exp Med Biol. 2017;975:207–16. doi: 10.1007/978-94-024-1079-2_18. [DOI] [PubMed] [Google Scholar]
- 34.Jirak D, Ziolkowska N, Turnovcova K, Karova K, Sykova E, Jendelova P, et al. Metabolic changes in focal brain ischemia in rats treated with human induced pluripotent stem cell-derived neural precursors confirm the beneficial effect of transplanted cells. Front Neurol. 2019;10:1074. doi: 10.3389/fneur.2019.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghandforoush-Sattari M, Mashayekhi SO, Nemati M, Ayromlou H. Changes in plasma concentration of taurine in stroke. Neurosci Lett. 2011;496:172–5. doi: 10.1016/j.neulet.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Kofler M, Schiefecker A, Ferger B, Beer R, Sohm F, Broessner G, et al. Cerebral taurine levels are associated with brain edema and delayed cerebral infarction in patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2015;23:321–9. doi: 10.1007/s12028-015-0140-y. [DOI] [PubMed] [Google Scholar]
- 37.Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2012;32:1107–38. doi: 10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16:877–85. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosafio K, Castillo X, Hirt L, Pellerin L. Cell-specific modulation of monocarboxylate transporter expression contributes to the metabolic reprograming taking place following cerebral ischemia. Neuroscience. 2016;317:108–20. doi: 10.1016/j.neuroscience.2015.12.052. [DOI] [PubMed] [Google Scholar]
- 40.Lu Y, Zhao HJ, Wang Y, Han BB, Wang T, Zhao H, et al. Electro-acupuncture up-regulates astrocytic MCT1 expression to improve neurological deficit in middle cerebral artery occlusion rats. Life Sci. 2015;134:68–72. doi: 10.1016/j.lfs.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Xie D, Hu D, Zhang Q, Sun YF, Li JM, Zhang YB. Increased high-sensitivity C-reactive protein, erythrocyte sedimentation rate and lactic acid in stroke patients with internal carotid artery occlusion. Arch Med Sci. 2016;12:546–51. doi: 10.5114/aoms.2014.47879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brouns R, Sheorajpanday R, Wauters A, De Surgeloose D, Mariën P, De, et al. Evaluation of lactate as a marker of metabolic stress and cause of secondary damage in acute ischemic stroke or TIA. Clin Chim Acta. 2008;397:27–31. doi: 10.1016/j.cca.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Loaiza A, Porras OH, Barros LF. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J Neurosci. 2003;23:7337–42. doi: 10.1523/JNEUROSCI.23-19-07337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lakhan SE, Kirchgessner A, Tepper D, Leonard A. Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol. 2013;4:32. doi: 10.3389/fneur.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foerch C, Wunderlich MT, Dvorak F, Humpich M, Kahles T, Goertler M, et al. Elevated serum S100B levels indicate a higher risk of hemorrhagic transformation after thrombolytic therapy in acute stroke. Stroke. 2007;38:2491–5. doi: 10.1161/STROKEAHA.106.480111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.