Abstract
Installation of fluorine into pharmaceutically relevant molecules plays a vital role in their properties of biology or medicinal chemistry. Direct difunctionalization of alkenes and 1,3-dienes to achieve fluorinated compounds through transition-metal catalysis is challenging, due to the facile β-H elimination from the Csp3‒[M] intermediate. Here we report a cobalt-catalyzed regioselective difluoroalkylarylation of both activated and unactivated alkenes with solid arylzinc pivalates and difluoroalkyl bromides through a cascade Csp3‒Csp3/Csp3‒Csp2 bond formation under mild reaction conditions. Indeed, a wide range of functional groups on difluoroalkyl bromides, olefins, 1,3-dienes as well as (hetero)arylzinc pivalates are well tolerated by the cobalt-catalyst, thus furnishing three-component coupling products in good yields and with high regio- and diastereoselectivity. Kinetic experiments comparing arylzinc pivalates and conventional arylzinc halides highlight the unique reactivity of these organozinc pivalates. Mechanistic studies strongly support that the reaction involves direct halogen atom abstraction via single electron transfer to difluoroalkyl bromides from the in situ formed cobalt(I) species, thus realizing a Co(I)/Co(II)/Co(III) catalytic cycle.
Subject terms: Homogeneous catalysis, Synthetic chemistry methodology
Transition metal-catalyzed regioselective difunctionalizations of alkenes with two different functional groups are useful for preparing organic compounds, but the construction of two new C–C bonds is challenging. Here, the authors report cobalt-catalyzed regioselective difluoroalkylarylation of alkenes with solid arylzinc pivalates and difluoroalkyl bromides, through a cascade Csp3 ‒Csp3/Csp3 ‒Csp2 bond formation.
Introduction
Organometallic reagents, due to their versatile reactivity and high functional group compatibility, have been broadly used for the synthesis of pharmaceuticals and agrochemicals. Especially, transition metal-catalyzed cross-coupling strategy, is of great importance for the development of modern organic chemistry1–6. Among them, boron7–10 and zinc organometallics11–15 have found extensive applications in such coupling reactions for the preparation of a wide range of highly complex molecules. As compared with the bench-stable, commercially available boronic derivatives, conventional organozinc reagents (RZnX, X = Cl, Br, I) displayed even more exquisite reactivity under mild reaction conditions in many coupling reactions; however, highly air- and moisture-sensitive still represent drawbacks for their synthetic applications. Remarkably, Knochel and coworkers recently developed a solid organozinc pivalate (RZnX‧Mg(OPiv)2‧LiCl, which is abbreviated henceforth as RZnOPiv for the sake of clarity)16, which shows greatly enhanced air and moisture stability after solvent evaporation17. These organozinc reagents, including (hetero)aryl17–22, alkynyl23,24, and alkyl zinc pivalates25, exhibited good reactivity in Pd-catalyzed cross-coupling reactions with unsaturated halides, which further proved to be broadly applicable for the late-stage functionalizations of biologically active molecules26. Although palladium catalysts are very useful, the 3d transition metals27–34, especially cobalt35–39, have recently found numerous applications due to its low toxicity, low cost, and natural abundance. Recently, (hetero)arylzinc pivalates have been successfully used for cobalt-catalyzed cross-couplings with unsaturated halides40, alkenyl acetates41, N-hydroxylamine benzoates42, anthranils43, N-hydroxyphthalimide44, as well as thiopyridyl ester derivatives45. Therefore, the ease of preparation, stability, and exquisite reactivity of these solid zinc reagents have attracted considerable attention of synthetic chemists.
Transition-metal-catalyzed regioselective difunctionalizations of olefins with two different functional groups have been recognized as an increasingly viable tool for preparing complex organic compounds from readily available starting materials46–48. However, due to the facile β–H elimination from the Csp3‒[M] intermediate49–52, it still remained challenging to construct two C‒C bonds through transition-metal catalyzed multicomponent dicarbofunctionalization of alkenes (Fig. 1a)53–55. Importantly, highly regioselective Ni-catalyzed alkylarylation of vinylarenes with alkyl halides and arylzinc iodides has been recently developed by Giri and coworkers56, they further extended the substrate scope to α-halocarbonyl derivatives (Fig. 1a)57. Besides, the installation of fluorine into bioactive molecules uniquely plays a vital role in their properties of relevance to biology or medicinal chemistry58–63, although major advances in transition-metal-catalyzed fluoroalkylation have been achieved in recent years64–68. It is worth noting that the elegant Ni-catalyzed tandem difluoroalkylation–(alkyl)arylation of enamides to the synthesis of difluoroalkylated amides was illustrated by Zhang and coworkers69–71. To the best of our knowledge, organozinc reagents for transition-metal-catalyzed difunctionalization of alkenes and 1,3-dienes to achieve fluorinated compounds were rather rare and limited to the use of nickel catalysis with activated alkenes71. In particular, the much less toxic and industrial-friendly cobalt catalysts have unfortunately thus far proven elusive for the aforementioned three-component cascade coupling reactions72,73.
Fig. 1. Background and our objective.
a Organometallic reagents for difunctionalization of olefins. b Organozinc pivalates for cobalt-catalyzed difluoroalkylarylation of olefins.
Recently, our laboratory reported the fluorine installation through cobalt-74 and copper-catalyzed75 alkyne and alkene difunctionalization strategies. In this work, we report a versatile cobalt-catalyzed regioselective difluoroalkylarylation of (un)activated alkenes and 1,3-dienes with polyfunctionalized arylzinc pivalates and difluoroalkyl bromides (Fig. 1b), which provides an expedient method to install fluorine into complex compounds. Of special interest in this cobalt catalysis is that the arylzinc pivalates seem very crucial for promoting the overall catalytic efficacy.
Results
We initiated our studies by optimizing reaction conditions for the envisioned cobalt-catalyzed regioselective three-component coupling of alkenylarene (2a) with bromodifluoroacetate (1a) and phenylzinc pivalate (3a, PhZnOPiv). A cascade cross-coupling reaction was observed in the presence of 10.0 mol% CoBr2 under ligand-free conditions, thus affording the desired aryl-difluoroalkylated product 4 in 83% yield with high regioselectivity (Fig. 2a, entry 1). Among a number of representative chelating ligands, bipyridines have given negative effects, and only a trace amount of product was observed (entries 2‒3); tridentate 2,6-bis(N-pyrazolyl)pyridine, 1,10-phenanthrolines, diimine, TMEDA, ME4DACH, as well as dppbz ligands gave poor-to-high yields, whereas the neocuproine (L5) afforded 4 in 92% (entries 4‒10). Further, testing reactions with different solvents verified the crucial importance of MeCN as the reaction medium (entry 11; see SI). Switching from CoBr2 to other representative cobalt salts, such as CoCl2 and CoCl2(PPh3)2, led to significant reduced yields (entry 12‒13). In sharp contrast, replacement of CoBr2 by using NiBr2, FeCl2, CrCl2, or CuBr failed to furnish the desired product 4 (entry 14).
Fig. 2. Reaction optimization and reactivity of different arylzinc reagents.
a Optimization for cobalt-catalyzed difluoroalkylarylation. b Kinetic experiments with different phenylzinc reagents of 3a‒X.
Lei76–78 demonstrated that arylzinc reagents prepared by different methods possess very different kinetics in palladium- and nickel-catalyzed oxidative couplings, and further X-ray absorption spectroscopy studies show that changing the halide anion from Cl to Br or I will result in an increase of the Zn‒C bond distance and thereby improve the trans-metalation rate79. In order to preliminarily reveal the different kinetics between this solid zinc reagent and conventional zinc reagents, a series of control experiments with six different phenylzinc reagents, which prepared by transmetallation of the corresponding phenylmagnesium halides and zinc halides (Note: PhZnOPiv was prepared from PhMgCl and Zn(OPiv)2 with 1:1.2 ratio; Ph2Zn‧2Mg(OPiv)Cl was prepared from PhMgCl and Zn(OPiv)2 with 2:1 ratio; PhZnX was prepared from PhMgX and ZnX2 with 1:1.2 ratio; Ph2Zn‧2MgCl2 was prepared from PhMgCl and ZnCl2 with 2:1 ratio), were also performed under the ligand-free cobalt catalysis (Fig. 2b). Interestingly, all of these reactions were almost finished within remarkably short reaction times of only 15 min. It is worth noting that significantly reduced conversions of 4 were observed when using PhZnX (X = Cl, Br, or I), Ph2Zn‧2MgCl2, or Ph2Zn‧2Mg(OPiv)Cl instead of PhZnOPiv. Moreover, the results of comparison experiments between Ph2Zn‧2Mg(OPiv)Cl and Ph2Zn‧2MgCl2 show the superiority of the former as well. Hence, these observations highlighted that the presence of M(OPiv)2 (M = Mg or Zn) has made these organozinc pivalates stand out among salt-supported organometallics, thus displaying the distinct advantage of reacting well in our regioselective cobalt-catalyzed difluoroalkylarylation of olefins.
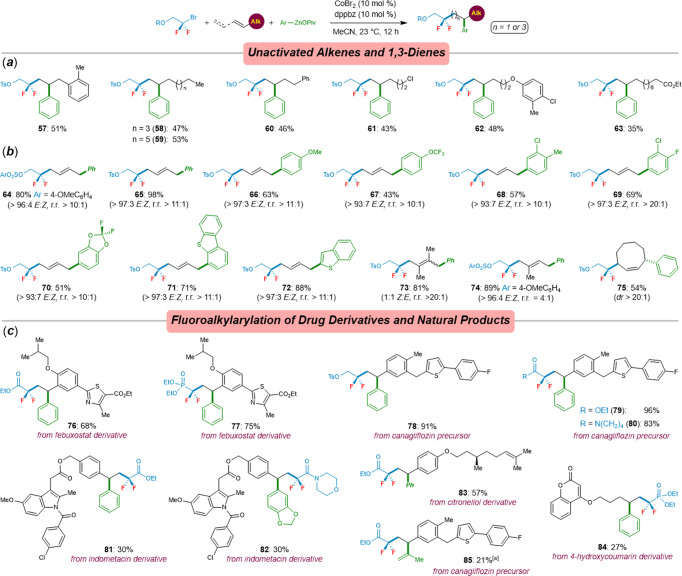
Subsequently, the versatility of this optimized cobalt(II) catalyst was examined in a range of difluoroalkylarylation reactions with various polyfunctionalized arylzinc pivalates 3 (Fig. 3a). All arylzinc pivalates were prepared from the corresponding aryl halides by Mg insertion in the presence of LiCl80. Although the neocuproine (L5) gave the optimal results in the model reaction, in our efforts to extend the substrate scope of this domino reaction, ligand-free CoBr2 proved to be superior (see the results of products 7, 9, 11). A variety of para- and/or metal-substituted arylzinc pivalates were identified as viable nucleophiles for difluoroalkylarylation with bromodifluoroacetate (1a) and 4-methoxystyrene (2a) to afford the desired products 4‒16 in moderate yields. More sterically hindered 4-chloro-2-methylphenylzinc pivalate was successfully employed, leading to the desired difluoroalkylarylated product 17 in 62% yield. Notably, ferrocenylzinc pivalate, as well as 3-thienylzinc pivalate also smoothly underwent the cobalt-catalyzed cascade cross-coupling, albeit yielding the products 18‒19 in relatively lower yields. Thus far, electron-withdrawing groups substituted arylzinc pivalates proven to be unsuitable nucleophiles.
Fig. 3. Scope of cobalt-catalyzed cascade cross-coupling reaction.
a Substrate scope of arylzinc pivalates. b Substrate scope of alkenylarenes and c fluoroalkyl bromides. Reaction conditions: alkyl bromides (0.50 mmol, 2.0 equiv), alkenes (0.25 mmol, 1.0 equiv), arylzinc pivalates (0.50 mmol, 2.0 equiv), CoBr2 (10 mol %), and MeCN (2.0 mL), 23 °C, 3‒6 h. [a] About 11 mol% of L5 was used. [b] CoBr2 (20 mmol%) was used. [c] Ar2Zn‧2Mg (OPiv)Cl (2.0 equiv) was used.
Thereafter, we have explored the substrate scope of the difluoroalkylarylation reaction with a wide range of vinylarenes and bromodifluoroacetate/amides (Fig. 3b). Remarkably, alkenylarenes bearing various valuable electrophilic functional groups, such as ether (22), fluoro (23), chloro (24), bromo (25, 35, 37), trifluoromethyl (26), methyloxy (27‒28, 31‒32, 38‒39), cyano (30), acetate (33), esters (36), and isobutyl (34) substituents, as well as vinylnaphthalene (29) and unsubstituted styrenes (20‒21), were well tolerated under the reaction conditions and converted to the corresponding difluorinated 1,1-diarylalkanes in moderate-to-excellent yields (40‒98%), as were also observed when using different bromodifluoroacetamides as the fluorinating reagents. Also, internal alkene with (E)-β-methylstyrene was examined under our cobalt catalysis, but only a trace amount of the desired product was detected by GC analysis (see SI). In sharp contrast, coupling of arylzinc pivalate, bromodifluoroacetate with indene gave the desired difluoroalkylarylated product 40 in 55% yield, with high diastereoselctivity (dr > 20:1). In addition, we further investigated the cross-coupling of various fluoroalkyl bromides with olefins and arylzinc pivalates. In contrast to bromodifluoroacetate, the bromomonofluoroacetate only gave 34% yield under the standard reaction conditions, and with a poor diastereoselectivity (dr = 1:1) (Fig. 3c; 41). We were also pleased to find that bromodifluoromethylphosphonate smoothly underwent the envisioned cobalt-catalyzed difluoromethylarylation to afford the desired 1,1-diarylalkylphosphonates 42‒45 in 51‒97% yields, and compound 45 was obtained with high diastereoselectivity (dr > 20:1). Besides, the unactivated alkene furnished the desired difluoromethylarylated phosphonate 46 as well, albeit in a modest yield. Additionally, using as substrate of α-bromodifluoromethyl-substituted benzoxazole proved to be viable with versatile cobalt catalyst and, thereby, provided 47‒48 as the products in 51‒55% yields. Remarkably, this cobalt-catalyzed regioselective difluoroalkylarylation reaction was further extended to the decorated difluoroalkyl bromide. Functional groups, such as arylsulfonate, ester, were well tolerated under the standard reaction conditions, thus delivering the desired products 49‒52 in good yields and with high diastereoselectivity of 51 (dr > 20:1). To our delight, the secondary and tertiary alkyl iodides were also successfully performed for the cobalt-catalyzed alkylarylation, albeit delivering the desired products 53‒56 in 24‒59% yields.
Transformations of unactivated alkenes are acknowledged widely as a challenge in transition-metal-catalyzed difunctionalization of alkenes49–56,69–71. The reaction conditions previously optimized for the alkenylarenes led to an unsatisfactorily low yield of 57, because significant amounts of a Heck-type coupling product were formed as well. However, we were delighted to find that the transformation of the unactivated alkene difluoroalkylarylation process was significantly improved when using dppbz (L9, 10 mol%) as the ligand, leading to 57 in 54% yield. A number of unactivated alkenes were readily converted into the desired difluoroalkylarylated products 58‒63 in moderate yields (See more details from the Supporting Information). Moreover, various synthetically valuable functional groups, including chloro, ether, and ester, remained intact by the cobalt catalyst (Fig. 4a). Beyond that, the possibility of cobalt-catalyzed difluoroalkylation to form an allyl radical, which subsequently underwent 1,3-shift and Csp3‒Csp2 cross-couplings with arylzinc pivalates, was also investigated (Fig. 4b). Indeed, difluoroalkyl bromide 1k and a quite range of functionalized (hetero)aryl-zinc reagents were realized: 1,4-difunctionalization of 1,3-dienes with good regioselectivity and diastereoselectivity, thus furnishing 64‒74 in 43‒98% yields, albeit products 73 and 74 were obtained with 1:1 E:Z selectivity and 4:1 regioselectivity, respectively. To our delight, 1,3-octadiene was proven to be a suitable substrate as well, giving the product 75 with high diastereoselectivity.
Fig. 4. Difluoroalkylarylation of alkenes.
a Scope of unactivated alkenes. b Scope of 1,3-dienes. c Late-stage difluoroalkylarylation of drug derivatives and natural products. Reaction conditions: 1k (0.25 mmol), unactivated alkenes or 1,3-dienes (0.5 mmol), arylzinc pivalates (0.5 mmol), CoBr2 (10 mol%), dppbz (10 mol%), and MeCN, 23 °C, 12 h. [a] A second portion of 1a and isopropenylzinc pivalate was added.
To further illustrate the potential applications of this cobalt-catalyzed regioselective difluoroalkylarylation in late-stage functionalizations of pharmaceutically active molecules, alkenylarenes derivatized from (pre-)drug molecules, such as febuxostat, canagliflozin, as well as indomethacin, were well difluoroalkylarylated with arylzinc pivalates and α-bromodifluorocarbonyl compounds or bromodifluoromethylphosphonate, leading to the corresponding products 76‒82 in 30‒96% yields. These results show the potential utility of this protocol for the discovery of bioactive drugs. Importantly, citronellol derivative was readily incorporated into the product 83 with remarkably high regioselectivity and chemoselectivity. Moreover, an unactivated alkene bearing a 4-hydroxycoumarin proved to be a viable substrate as well, albeit delivering the phosphonate 84 in a rather modest yield. Finally, we showed that isopropenylzinc pivalate is well suited for the cobalt-catalyzed difluoroalkylalkenylation, although the reaction proceeded with lower yield (Fig. 4c).
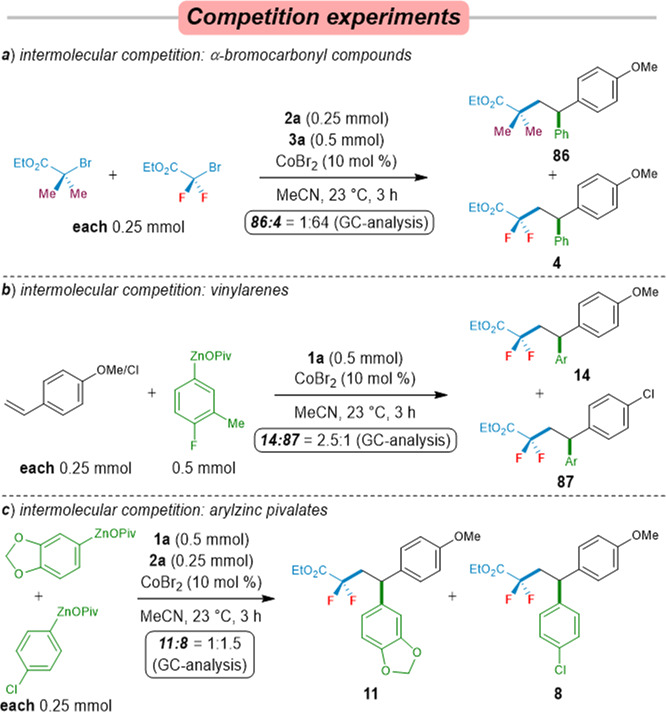
Intrigued by the high regioselectivity and efficacy of our cobalt-catalyzed difluoroalkylarylation, a series of intermolecular competition experiments were performed (Fig. 5). A competition experiment between bromodifluoroacetate (1a) and 2-bromo-2-methylpropanoate showed that BrCF2CO2Et reacted much faster than these α-bromocarbonyl compounds. These findings can be rationalized in terms of a prioritized direct halogen atom abstraction from difluoroalkyl bromides via single-electron transfer from a cobalt catalyst (Fig. 5a)81. Intermolecular competition experiments with different alkenylarenes and arylzinc pivalates revealed electron-rich styrenes and electron-deficient arylzinc pivalates to be slightly reactive substrates (Fig. 5b, c). These results suggested that vinylarenes and arylzinc reagents might not be involved in the rate-determined step56.
Fig. 5. Control experiments.
a Competition experiments between different alkyl bromides. b Competition experiments between different vinylarenes. c Competition experiments between different arylzinc pivalates.
Beyond that, the radical-clock experiment with substrate 88 bearing a radical clock cyclopropane moiety, the ring-opened difluoroalkylarylated product 89, was generated in 11% yield. Similarly, both three- and two-component coupling products were observed when using N,N-diallyl-2-bromo-2,2-difluoroacetamide (90) as a radical probe under the standard reaction conditions, the cyclized products 91 (dr = 2:1) and 92 were generated in 17% and 34% yields, respectively. Moreover, a difluoroalkylated benzylic radical homocoupling dimer 93 was detected by GC as well. With these findings, we propose that this cobalt-catalyzed difluoroalkylarylation involves a single-electron-transfer (SET) process (Fig. 6a).
Fig. 6. Mechanistic studies for cobalt-catalyzed cascade difluoroalkylarylation.
a Radical evidences. b Reduction of CoBr2 by arylzinc pivalates. c Reactivity of well-defined Co(I), Co(0) catalysts. d Catalytic activity investigation of the in situ low-valent cobalt species. e EPR experiments with DMPO (DMPO = 5,5-dimethyl-1-pyrroline-1-oxide).
According to the earlier mechanistic studies for cobalt-catalyzed cross-coupling reactions with using organomagnesium reagents, an in situ low-valent Co(0) was proposed as the catalytically active species52,72,73,82,83. On the other hand, a mechanism involving Co(I)/(III) couple was also proposed for many cobalt-catalyzed cross-couplings37–39,81. Therefore, we performed experiments of CoBr2 (1.0 equiv) with excess of ArZnOPiv under typical reaction conditions for 30 min. These reactions furnished the corresponding homoproducts of 94a and 94b in near-0.5 equiv ratio to that of CoBr2, respectively. These findings support the formation of a Co(I) species based on the stoichiometry shown in Fig. 6b. In this context, the well-defined Co(I) complex, such as CoCl(PPh3)3 was proved to be active for the desired difluoroalkylarylated process, yielding product 4 in 66%, while Co2(CO)8 gave a poor yield (Fig. 6c). Further experiments to examine the catalytic activity of the in situ-generated low-valent cobalt(I) species were performed. A mixture of vinylarene 2a (0.25 mmol) and CoBr2 (0.025 mmol) was treated with 2.0 equiv of 3,4-(methylenedioxy)phenylzinc pivalate (0.05 mmol) at 23 °C for 30 min to generate the proposed Co(I) species, followed by addition of bromodifluoroacetate 1a (0.3 mmol) and another 0.5 mmol of phenylzinc pivalate. The difluoroalkylarylated product 4 was isolated in 57% yield as the sole product, while the product 11 was obtained in 79% yield when exchanging the order of the two arylzinc reagents (Fig. 6d). These findings are consistent with the in situ-generated low-valent cobalt(I) species that might be the active catalyst for the current three-component cross-coupling reaction. A series of EPR spin-trapping experiments show the existence of C-centered radicals trapped by DMPO (g = 2.0066, AN = 13.9 G, AH = 19.3 G), which was considered to be •CF2R84. These results strongly supported that the single-electron-transfer process for the activation of BrCF2R was only promoted by the in situ-formed Co(I) species (Fig. 6e).
Based on the above experimental findings, along with previous mechanistic insights37–39,74,81, a mechanism for this regioselective cobalt-catalyzed difluoroalkylarylation of alkenes has been proposed as shown in Fig. 6f. The reduction of the precatalyst CoBr2 with arylzinc pivalates forms the catalytically active Co(I) species (A), which reduces difluoroalkyl bromides (1) by SET and generates difluoroalkyl radical B, then followed by a facile radical addition of B into olefins (2) to afford a secondary alkyl radical species, along with subsequent rapid trapping with LnCo(II)XBr (X = Br) into intermediate C, which undergoes trans-metalation with ArZnOPiv (3) to lead to the organocobalt(III) species D. Subsequent reductive elimination finally delivers the difluoroalkylarylated product and regenerates the active cobalt(I) catalyst (path a). In addition, another possible pathway is that transmetallation of arylzinc pivalates could also occur after the initial reduction step, thus in situ forming the LnCo(I)X (X = Ar) species as the catalyst to promote the SET process. Radical addition and reductive elimination give rise to the desired products and regenerate the active Co(I) species (path b).
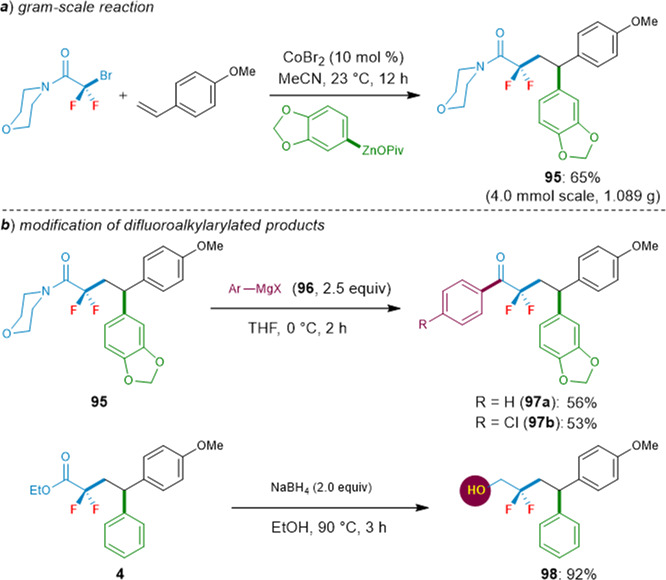
We were also pleased to find that this cobalt-catalyzed difluoroalkylarylation can be easily scaled up to gram level. Under the optimized reaction conditions, the difluoroalkylarylated product 95 was afforded with high efficacy (65% yield, Fig. 7a). Finally, we further demonstrated the synthetic potential of this cobalt-catalyzed difluoroalkylarylation strategy through the late-stage modification of the obtained difluoroalkylarylated products. For example, the resulting N-morpholino amide 95 can be readily converted into various ketones by treating with Grignard reagents, thus furnishing the products 97a‒b in moderate yields. Moreover, the reduction of the ester group of substrate 4 by using NaBH4 provides the corresponding alcohol 98, which readily undergoes various derivatizations (Fig. 7b).
Fig. 7. Applications.
a Gram-scale reaction. b Modification of difluoroalkylarylated products.
Discussion
In conclusion, we have reported the practical cobalt catalysis for regioselective difluoroalkylarylation of alkenes or 1,3-dienes with functionalized arylzinc pivalates and difluoroalkyl bromides. This simple cobalt catalyst enables three-component cross-couplings through cascade Csp3‒Csp3/Csp3‒Csp2 bond formation in one-pot fashion, thus generating difluoroalkylarylated products with predictable regioselectivity and high diastereoselectivity. The reaction proceeds under remarkable mild conditions with high efficacy, excellent functional group tolerance, as well as a broad substrate scope. Notable features of this approach are the use of less toxic and low-cost cobalt catalyst, as well as user-friendly solid zinc reagents. Straightforward late-stage functionalizations of pharmaceutically active molecules show the potential applications of this protocol for the discovery of bioactive drugs. Beyond that, among a series of kinetic experiments with six types of phenylzinc reagents, these solid arylzinc pivalates displayed the distinct advantage of reactivity for the current reaction. Detailed mechanistic studies demonstrated that the reaction undergoes a direct halogen atom abstraction via single-electron transfer from the in situ-formed cobalt(I) species to difluoroalkyl bromides.
Methods
Cobalt-catalyzed difluoroalkylarylation of alkenes
A suspension of CoBr2 (10 mol%), olefin (0.25 mmol, 1.0 equiv), difluoroalkyl bromide (0.5 mmol, 2.0 equiv), and aryl zinc pivalates (0.5 mmol, 2.0 equiv) in degas MeCN (1.0 mL) was stirred at 23 °C for 3 h under an atmosphere of Ar. At ambient temperature, the solvent was evaporated under reduced pressure and the remaining residue was purified by column chromatography on silica gel to yield the desired products.
Supplementary information
Acknowledgements
We thank the National Natural Science Foundation of China (21602083) and Start-up Grant of Soochow University (GJ10900220) for financial support. We are grateful to Prof. Yi-hung Chen for their kind discussions, and Jianlin Yao, and Chen Zhu for their kind support and accessing to the laboratory.
Author contributions
X. C., X. L., and S. W. contributed equally to this work and they planned, conducted, and analyzed the experiments. X. C., X. L., S. W., Y. H., and B. H. performed the experiments. J. L., and A. L. designed and directed the project and wrote the paper with contributions from all coworkers.
Data availability
The authors declare that all other data supporting the findings of this study, including experimental procedures and compound characterization, are available within the article and its Supplementary Information files.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xinyi Cheng, Xingchen Liu, Shengchun Wang.
Contributor Information
Aiwen Lei, Email: aiwenlei@whu.edu.cn.
Jie Li, Email: jjackli@suda.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-24596-6.
References
- 1.Knochel, P. (ed.) Handbook of Functionalized Organometallics: Applications in Synthesis (Wiley, 2005).
- 2.Miyaura, N. (ed.) Cross-Coupling reactions. A Practical Guide (Springer, 2002).
- 3.de Meijere, A. & Diederich, F. (eds) Metal-Catalyzed Cross-Coupling Reactions (Wiley, 2004).
- 4.Hartwig, J. F. (ed.) Organotransition Metal Chemistry (University Science Books, 2010).
- 5.Li, J. J. & Johnson, D. S. (eds) Modern Drug Synthesis (Wiley, 2010).
- 6.Boudier A, Bromm LO, Lotz M, Knochel P. New applications of polyfunctional organometallics in organic synthesis. Angew. Chem. Int. Ed. 2000;39:4414–4435. doi: 10.1002/1521-3773(20001215)39:24<4414::AID-ANIE4414>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Hall, D. G. (ed.) Boronic acids. Preparation, Applications in Organic Synthesis and Medicine 2nd edn (Wiley, 2005).
- 8.Suzuki A. Cross-coupling reactions of organoboranes: an easy way to construct C–C bonds (Nobel Lecture) Angew. Chem. Int. Ed. 2011;50:6722–6737. doi: 10.1002/anie.201101379. [DOI] [PubMed] [Google Scholar]
- 9.Molander GA, Canturk B. Organotrifluoroborates and monocoordinated Palladium complexes as catalysts—a perfect combination for Suzuki–Miyaura coupling. Angew. Chem. Int. Ed. 2009;48:9240–9261. doi: 10.1002/anie.200904306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyaura N, Suzuki A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995;95:2457–2483. doi: 10.1021/cr00039a007. [DOI] [Google Scholar]
- 11.de Meijere, A., Bräse, S. & Oestreich, M. (eds) Metal-Catalyzed Cross-Coupling Reactions and More 3rd edn (Wiley, 2014).
- 12.Haas D, Hammann JM, Greiner R, Knochel P. Recent developments in negishi cross-coupling reactions. ACS Catal. 2016;6:1540–1552. doi: 10.1021/acscatal.5b02718. [DOI] [Google Scholar]
- 13.Negishi E. Magical power of transition metals: past, present, and future (Nobel Lecture) Angew. Chem. Int. Ed. 2011;50:6738–6764. doi: 10.1002/anie.201101380. [DOI] [PubMed] [Google Scholar]
- 14.Phapale VB, Cárdenas DJ. Nickel-catalysed negishi cross-coupling reactions: scope and mechanisms. Chem. Soc. Rev. 2009;38:1598–1607. doi: 10.1039/b805648j. [DOI] [PubMed] [Google Scholar]
- 15.Knochel P, Singer RD. Preparation and reactions of polyfunctional organozinc reagents in organic synthesis. Chem. Rev. 1993;93:2117–2188. doi: 10.1021/cr00022a008. [DOI] [Google Scholar]
- 16.Hernán-Gómez A, et al. Organozinc pivalate reagents: segregation, solubility, stabilization, and structural insights. Angew. Chem. Int. Ed. 2014;53:2706–2710. doi: 10.1002/anie.201309841. [DOI] [PubMed] [Google Scholar]
- 17.Bernhardt S, Manolikakes G, Kunz T, Knochel P. Preparation of solid salt-stabilized functionalized organozinc compounds and their application to cross-coupling and carbonyl addition reactions. Angew. Chem. Int. Ed. 2011;50:9205–9209. doi: 10.1002/anie.201104291. [DOI] [PubMed] [Google Scholar]
- 18.Manolikakes SM, Ellwart M, Stathakis CI, Knochel P. Air-stable solid aryl and heteroaryl organozinc pivalates: syntheses and applications in organic synthesis. Chem. Eur. J. 2014;20:12289–12297. doi: 10.1002/chem.201403015. [DOI] [PubMed] [Google Scholar]
- 19.Colombe JR, Bernhardt S, Stathakis C, Buchwald SL, Knochel P. Synthesis of solid 2-pyridylzinc reagents and their application in negishi reactions. Org. Lett. 2013;15:5754–5757. doi: 10.1021/ol402798z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stathakis CI, Manolikakes SM, Knochel P. TMPZnOPiv•LiCl: a new base for the preparation of air-stable solid zinc pivalates of sensitive aromatics and heteroaromatics. Org. Lett. 2013;15:1302–1305. doi: 10.1021/ol400242r. [DOI] [PubMed] [Google Scholar]
- 21.Stathakis CI, Bernhardt S, Quint V, Knochel P. Improved air-stable solid aromatic and heterocyclic zinc reagents by highly selective metalations for negishi cross-couplings. Angew. Chem. Int. Ed. 2012;51:9428–9432. doi: 10.1002/anie.201204526. [DOI] [PubMed] [Google Scholar]
- 22.Hofmayer MS, Lutter FH, Grokenberger L, Hammann JM, Knochel P. Practical Ni-catalyzed cross-coupling of unsaturated zinc pivalates with unsaturated nonaflates and triflates. Org. Lett. 2019;21:36–39. doi: 10.1021/acs.orglett.8b03417. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y-H, Tüllmann CP, Ellwart M, Knochel P. Preparation of solid polyfunctional alkynylzinc pivalates with enhanced air and moisture stability for organic synthesis. Angew. Chem. Int. Ed. 2017;56:9236–9239. doi: 10.1002/anie.201704400. [DOI] [PubMed] [Google Scholar]
- 24.Tüllmann CP, Chen Y-H, Schuster RJ, Knochel P. Preparation and reactions of mono- and bis-pivaloyloxyzinc acetylides. Org. Lett. 2018;20:4601–4605. doi: 10.1021/acs.orglett.8b01892. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y-H, Ellwart M, Toupalas G, Ebe Y, Knochel P. Preparation and application of solid, salt-stabilized zinc amide enolates with enhanced air and moisture stability. Angew. Chem. Int. Ed. 2017;56:4612–4616. doi: 10.1002/anie.201700216. [DOI] [PubMed] [Google Scholar]
- 26.Greshock TJ, et al. Synthesis of complex druglike molecules by the use of highly functionalized bench-stable organozinc reagents. Angew. Chem. Int. Ed. 2016;55:13714–13718. doi: 10.1002/anie.201604652. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Knochel P. Chromiun-catalyzed cross-couplings and related reactions. Synthesis. 2019;51:2100–2106. doi: 10.1055/s-0037-1611700. [DOI] [Google Scholar]
- 28.Piontek A, Bisz E, Szostak M. Iron-catalyzed cross-couplings in the synthesis of pharmaceuticals: in pursuit of sustainability. Angew. Chem. Int. Ed. 2018;57:11116–11128. doi: 10.1002/anie.201800364. [DOI] [PubMed] [Google Scholar]
- 29.Guérinot A, Cossy J. Iron-catalyzed C‒C cross-couplings using organometallics. Top. Curr. Chem. 2016;374:1–74. doi: 10.1007/s41061-015-0002-2. [DOI] [PubMed] [Google Scholar]
- 30.Tobisu M, Chatani N. Cross-couplings using aryl ethers via C–O bond activation enabled by nickel catalysts. Acc. Chem. Res. 2015;48:1717–1726. doi: 10.1021/acs.accounts.5b00051. [DOI] [PubMed] [Google Scholar]
- 31.Bauer I, Knölker H-J. Iron catalysis in organic synthesis. Chem. Rev. 2015;115:3170–3387. doi: 10.1021/cr500425u. [DOI] [PubMed] [Google Scholar]
- 32.Bedforf RB. How low does iron go? Chasing the active species in Fe-catalyzed cross-coupling reactions. Acc. Chem. Res. 2015;48:1485–1493. doi: 10.1021/acs.accounts.5b00042. [DOI] [PubMed] [Google Scholar]
- 33.Rosen BM, et al. Nickel-catalyzed cross-couplings involving carbon−oxygen bonds. Chem. Rev. 2011;111:1346–1416. doi: 10.1021/cr100259t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherry BD, Fürstner A. The promise and challenge of iron-catalyzed cross coupling. Acc. Chem. Res. 2008;41:1500–1511. doi: 10.1021/ar800039x. [DOI] [PubMed] [Google Scholar]
- 35.Lutter FH, et al. Cobalt-catalyzed cross-couplings and electrophilic aminations using organozinc pivalates. ChemCatChem. 2019;11:5188–5197. doi: 10.1002/cctc.201900070. [DOI] [Google Scholar]
- 36.Hammann JM, Hofmayer MS, Lutter FH, Thomas L, Knochel P. Recent advances in cobalt-catalyzed Csp2 and Csp3 cross-couplings. Synthesis. 2017;49:3887–3894. doi: 10.1055/s-0036-1588430. [DOI] [Google Scholar]
- 37.Knappke CEI, et al. Reductive cross-coupling reactions between two electrophiles. Chemistry. 2014;20:6828–6842. doi: 10.1002/chem.201402302. [DOI] [PubMed] [Google Scholar]
- 38.Cahiez G, Moyeux A. Cobalt-catalyzed cross-coupling reactions. Chem. Rev. 2010;110:1435–1462. doi: 10.1021/cr9000786. [DOI] [PubMed] [Google Scholar]
- 39.Gosmini, C., Begouin, J.-M. & Moncomble, A. Cobalt-catalyzed cross-coupling reactions. Chem. Commun. 44, 3221–3233 (2008). [DOI] [PubMed]
- 40.Hammann JM, Lutter FH, Haas D, Knochel P. A robust and broadly applicable cobalt-catalyzed cross-coupling of functionalized bench-stable organozinc pivalates with unsaturated halides. Angew. Chem. Int. Ed. 2017;56:1082–1086. doi: 10.1002/anie.201610324. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Knochel P. Cobalt-catalyzed cross-couplings between alkenyl acetates and aryl or alkenyl zinc pivalates. Angew. Chem. Int. Ed. 2018;57:11436–11440. doi: 10.1002/anie.201805486. [DOI] [PubMed] [Google Scholar]
- 42.Chen YH, Grassl S, Knochel P. Cobalt-catalyzed electrophilic amination of aryl- and heteroarylzinc pivalates with N-hydroxylamine benzoates. Angew. Chem. Int. Ed. 2018;57:1108–1111. doi: 10.1002/anie.201710931. [DOI] [PubMed] [Google Scholar]
- 43.Li J, et al. Cobalt-catalyzed electrophilic aminations with anthranils: an expedient route to condensed quinolines. J. Am. Chem. Soc. 2019;141:98–103. doi: 10.1021/jacs.8b11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X-G, et al. Decarboxylative negishi coupling of redox-active aliphatic esters by cobalt catalysis. Angew. Chem. Int. Ed. 2018;57:13096–13100. doi: 10.1002/anie.201806799. [DOI] [PubMed] [Google Scholar]
- 45.Lutter FH, Grokenberger L, Hofmayer MS, Knochel P. Cobalt-catalyzed acylation-reactions of (hetero)arylzinc pivalates with thiopyridyl ester derivatives. Chem. Sci. 2019;10:8241–8245. doi: 10.1039/C9SC01817D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin G, Mu X, Liu G. Palladium (II)-catalyzed oxidative difunctionalization of alkenes: bond forming at a high-valent palladium center. Acc. Chem. Res. 2016;49:2413–2423. doi: 10.1021/acs.accounts.6b00328. [DOI] [PubMed] [Google Scholar]
- 47.McDonald RI, Liu G, Stahl SS. Palladium(II)-catalyzed alkene functionalization via nucleopalladation: stereochemical pathways and enantioselective catalytic applications. Chem. Rev. 2011;111:2981–3019. doi: 10.1021/cr100371y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beller M, Seayad J, Tillack A, Jiao H. Catalytic markovnikov and antimarkovnikov functionalization of alkenes and alkynes: recent developments and trends. Angew. Chem. Int. Ed. 2004;43:3368–3398. doi: 10.1002/anie.200300616. [DOI] [PubMed] [Google Scholar]
- 49.Orlandi M, Hilton MJ, Yamamoto E, Toste FD, Sigman MS. Mechanistic investigations of the Pd(0)-catalyzed enantioselective 1,1-diarylation of benzyl acrylates. J. Am. Chem. Soc. 2017;139:12688–12695. doi: 10.1021/jacs.7b06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saini V, Sigman MS. Palladium-catalyzed 1,1-difunctionalization of ethylene. J. Am. Chem. Soc. 2012;134:11372–11375. doi: 10.1021/ja304344h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Werner EW, Urkalan KB, Sigman MS. PdII-catalyzed oxidative 1,1-diarylation of terminal olefins. Org. Lett. 2010;12:2848–2851. doi: 10.1021/ol1009575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikeda Y, Nakamura T, Yorimitsu H, Oshima K. Cobalt-catalyzed Heck-type reaction of alkyl halides with styrene. J. Am. Chem. Soc. 2002;124:6514–6515. doi: 10.1021/ja026296l. [DOI] [PubMed] [Google Scholar]
- 53.Qin T, et al. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science. 2016;352:801–805. doi: 10.1126/science.aaf6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L, et al. Catalytic conjunctive cross-couplingenabled by metal-inducedmetallate rearrangement. Science. 2016;351:70–74. doi: 10.1126/science.aad6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kischkewitz M, Okamoto K, Mück-Lichtenfeld C, Studer A. Radical-polar crossover reactions of vinylboron ate complexes. Science. 2017;355:936–938. doi: 10.1126/science.aal3803. [DOI] [PubMed] [Google Scholar]
- 56.Kc S, et al. Ni-catalyzed regioselective alkylarylation of vinylarenes via C(sp3)−C(sp3)/C(sp3)−C(sp2) bond formation and mechanistic studies. J. Am. Chem. Soc. 2018;140:9801–9805. doi: 10.1021/jacs.8b05374. [DOI] [PubMed] [Google Scholar]
- 57.Kc S, Dhungana RK, Khanal N, Giri R. Nickel-catalyzed α‐carbonylalkylarylation of vinylarenes: expedient access to γ,γ‐diarylcarbonyl and aryltetralone derivatives. Angew. Chem. Int. Ed. 2020;59:8047–8051. doi: 10.1002/anie.201913435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Link JO, et al. Discovery of ledipasvir (GS-5885): a potent, once-daily oral NS5A inhibitor for the treatment of hepatitis C virus infection. J. Med. Chem. 2014;57:2033–2046. doi: 10.1021/jm401499g. [DOI] [PubMed] [Google Scholar]
- 59.Begue, J. P. & Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine (Wiley, 2008).
- 60.Purser S, Moore PR, Swallow S, Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008;37:320–330. doi: 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]
- 61.O’Hagan D. Understanding organofluorine chemistry. introduction C.−F. bond. Chem. Soc. Rev. 2008;37:308–319. doi: 10.1039/B711844A. [DOI] [PubMed] [Google Scholar]
- 62.Muller K, Faeh C, Diederich F. Fluorine in pharmaceuticals: looking beyond intuition. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 63.Mikami K, Itoh Y, Yamanaka M. Fluorinated carbonyl and olefinic compounds: basic character and asymmetric catalytic reactions. Chem. Rev. 2004;104:1–16. doi: 10.1021/cr030685w. [DOI] [PubMed] [Google Scholar]
- 64.Feng Z, Xiao Y-L, Zhang X. Transition-metal (Cu, Pd, Ni)-catalyzed difluoroalkylation via cross-coupling with difluoroalkyl halides. Acc. Chem. Res. 2018;51:2264–2278. doi: 10.1021/acs.accounts.8b00230. [DOI] [PubMed] [Google Scholar]
- 65.Li C, et al. Cobalt-catalyzed difluoroalkylation of tertiary aryl ketones for facile synthesis of quaternary alkyl difluorides. Nat. Commun. 2018;9:4951–4957. doi: 10.1038/s41467-018-07525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belhomme M-C, Besset T, Poisson T, Pannecoucke X. Recent progress toward the introduction of functionalized difluoromethylated building blocks onto C(sp2) and C(sp) centers. Chemistry. 2015;21:12836–12865. doi: 10.1002/chem.201501475. [DOI] [PubMed] [Google Scholar]
- 67.Yang X, Wu T, Phipps RJ, Toste FD. Advances in catalytic enantioselective fluorination, mono-, di-, and trifluoromethylation, and trifluoromethylthiolation reactions. Chem. Rev. 2015;115:826–870. doi: 10.1021/cr500277b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liang T, Neumann CN, Ritter T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 2013;52:8214–8264. doi: 10.1002/anie.201206566. [DOI] [PubMed] [Google Scholar]
- 69.Gu J-W, Min Q-Q, Yu L-C, Zhang Z. Tandem difluoroalkylationarylation of enamides catalyzed by nickel. Angew. Chem. Int. Ed. 2016;55:12270–12274. doi: 10.1002/anie.201606458. [DOI] [PubMed] [Google Scholar]
- 70.Xu C, Yang Z-F, An L, Zhang X. Nickel-catalyzed difluoroalkylation-alkylation of enamides. ACS Catal. 2019;9:8224–8229. doi: 10.1021/acscatal.9b02488. [DOI] [Google Scholar]
- 71.Xu C, Cheng R, Luo Y-C, Wang M-K, Zhang X. trans-Selective aryldifluoroalkylation of endocyclic enecarbamates and enamides via nickel catalysis. Angew. Chem. Int. Ed. 2020;59:18741–18747. doi: 10.1002/anie.202008498. [DOI] [PubMed] [Google Scholar]
- 72.Mizutani K, Shinokubo H, Oshima K. Cobalt-catalyzed three-component coupling reaction of alkyl halides, 1,3-dienes, and trimethylsilylmethylmagnesium chloride. Org. Lett. 2003;5:3959–3961. doi: 10.1021/ol0356643. [DOI] [PubMed] [Google Scholar]
- 73.Lv X-L, Wang C, Wang Q-L, Shu W. Rapid synthesis of γ-arylated carbonyls enabled by the merge of copper- and photocatalytic radical relay alkylarylation of alkenes. Org. Lett. 2019;21:56–59. doi: 10.1021/acs.orglett.8b03485. [DOI] [PubMed] [Google Scholar]
- 74.Han S, Liu S, Liu L, Ackermann L, Li J. Cobalt-catalyzed diastereoselective difluoroalkylation/Giese addition domino reactions. Org. Lett. 2019;21:5387–5391. doi: 10.1021/acs.orglett.9b01400. [DOI] [PubMed] [Google Scholar]
- 75.Da Y, et al. Copper(I)-catalyzed difluoroalkylation of alkenes: a route to functionalization of lactones. Org. Lett. 2018;20:5149–5152. doi: 10.1021/acs.orglett.8b02069. [DOI] [PubMed] [Google Scholar]
- 76.Li J, Jin L, Liu C, Lei A. Quantitative kinetic investigation on transmetalation of ArZnX in a Pd-catalysed oxidative coupling. Chem. Commun. 2013;49:9615–9617. doi: 10.1039/c3cc44894k. [DOI] [PubMed] [Google Scholar]
- 77.Jin L, Xin J, Huang Z, He J, Lei A. Transmetalation is the rate-limiting step: quantitative kinetic investigation of nickel-catalyzed oxidative coupling of arylzinc reagents. J. Am. Chem. Soc. 2010;132:9607–9609. doi: 10.1021/ja1045296. [DOI] [PubMed] [Google Scholar]
- 78.Jin L, et al. Revelation of the difference between arylzinc reagents prepared from aryl Grignard and aryllithium reagents respectively: Kinetic and structural features. J. Am. Chem. Soc. 2009;131:16656–16657. doi: 10.1021/ja908198d. [DOI] [PubMed] [Google Scholar]
- 79.Zhang G, et al. Structure–kinetic relationship study of organozinc reagents. Chem. Commun. 2014;50:8709–8711. doi: 10.1039/C4CC01135J. [DOI] [PubMed] [Google Scholar]
- 80.Piller FM, Appukkuttan P, Gavryushin A, Helm M, Knochel P. Convenient preparation of polyfunctional aryl magnesium reagents by a direct magnesium insertion in the presence of LiCl. Angew. Chem. Int. Ed. 2008;47:6802–6806. doi: 10.1002/anie.200801968. [DOI] [PubMed] [Google Scholar]
- 81.Wu G, von Wangelin AJ. Stereoselective cobalt catalyzed halofluoroalkylation of alkynes. Chem. Sci. 2018;9:1795–1802. doi: 10.1039/C7SC04916A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohmiya H, Yorimitsu H, Oshima K. Co-mediated synthesis of alkenyl- and alkynyl silanes from alkyl halides. Org. Lett. 2006;8:3093–3096. doi: 10.1021/ol0611144. [DOI] [PubMed] [Google Scholar]
- 83.Ikeda Y, Yorimitsu H, Shinokubo H, Oshima K. Cobalt-Mediated Mizoroki–Heck‐type reaction of epoxide with styrene. Adv. Synth. Catal. 2004;346:1631–1634. doi: 10.1002/adsc.200404074. [DOI] [Google Scholar]
- 84.Fan W-T, Li Y, Wang D, Ji S-J, Zhao Y. Iron-catalyzed highly para-selective difluoromethylation of arenes. J. Am. Chem. Soc. 2020;142:20524–20530. doi: 10.1021/jacs.0c09545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all other data supporting the findings of this study, including experimental procedures and compound characterization, are available within the article and its Supplementary Information files.