Abstract
The Coronary Artery Risk Development in Young Adults study (CARDIA) began in 1985-86 with enrollment of 5,115 Black or White men and women ages 18 to 30 years from four U.S. communities. Over 35 years, CARDIA has contributed fundamentally to our understanding of the contemporary epidemiology and life course of cardiovascular health and disease, as well as pulmonary, renal, neurologic, and other manifestations of aging. CARDIA has established associations between the neighborhood environment and the evolution of lifestyle behaviors with biological risk factors, subclinical disease, and early clinical events. CARDIA has also identified the nature and major determinants of Black-White differences in the development of cardiovascular risk. CARDIA will continue to be a unique resource for understanding determinants, mechanisms, and outcomes of cardiovascular health and disease across the life course, leveraging ongoing pan-omics work from genomics to metabolomics that will define mechanistic pathways involved in cardiometabolic aging.
Keywords: Cardiovascular risk factors, cardiovascular disease, cardiovascular health, race, social determinants of health
CONDENSED ABSTRACT
The Coronary Artery Risk Development in Young Adults study (CARDIA) began in 1985-86 with enrollment of 5,115 Black or White men and women ages 18 to 30 years from four U.S. communities. Over 35 years, CARDIA has contributed fundamentally to our understanding of the contemporary epidemiology and life course of cardiovascular health and disease, as well as pulmonary, renal, and neurologic aging. CARDIA has also identified the nature and major determinants of Black-White differences in the development of cardiovascular risk. CARDIA continues to be a unique resource for understanding determinants, mechanisms, and outcomes of cardiovascular health from young adulthood onwards.
INTRODUCTION
The Coronary Artery Risk Development in Young Adults (CARDIA) study(1) was begun in 1985-86. The study enrolled 5,115 Black and White men and women ages 18-30 years old at four centers in Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA. Originally conceived as a study to understand the contributing factors (behavioral, environmental, and race- and sex-associated) underlying the transition from healthy young adulthood to the development of cardiovascular disease (CVD) risk factors (RFs), CARDIA has now become one of the premier studies of aging, cardiovascular health (CVH), and CVD across the life course. It has also contributed substantially to our understanding of the effects of the United States obesity epidemic (which had its inflection point in 1985) on cardiovascular and overall health. This monograph describes the original goals and most important contributions of the CARDIA study to date, as well as future research directions.
ORIGINAL GOALS, STUDY DESIGN, AND FOLLOW UP
The original aims of the CARDIA study were to examine the distribution of CVD RFs in young adults; to identify associated lifestyle, psychosocial, and other factors; and to assess longitudinal RF evolution in early adulthood. Recruitment strategies were designed to achieve a representative sample of the underlying US Black and White populations at the time from diverse geographic regions, and involved random-digit dialing or in-person contact with households in selected areas and census tracts in Birmingham, Chicago, and Minneapolis. Participants at the Oakland center were randomly selected from among subscribers of the Kaiser Permanente Medical Care Program living in specific residential areas around Oakland. Within each field center, attempts were made to achieve approximately equal numbers balanced on age (over/under age 25 years), sex (male/female), self-reported race (Black/White), and education status (high school or less/more than high school). Self-reported Hispanic (or other) ethnicity was not initially gathered but the proportion of Hispanic individuals is low. The final cohort included 5,115 Black or White men and women, whose baseline and follow up characteristics at each exam are shown in Table 1.
TABLE 1.
Characteristics of the CARDIA cohort at each examination cycle.
| Variable | YEAR 0 1985-86 |
YEAR 2 1987-88 |
YEAR 5 1990-91 |
YEAR 7 1992-93 |
YEAR 10 1995-96 |
YEAR 15 2000-01 |
YEAR 20 2005-06 |
YEAR 25 2010-11 |
YEAR 30 2015-16 |
|---|---|---|---|---|---|---|---|---|---|
| No. examined | 5115 | 4624 | 4352 | 4086 | 3950 | 3672 | 3549 | 3499 | 3358 |
| Black race,n (%) | 2637 (51.6%) | 2285 (49.4%) | 2116 (48.7%) | 1973 (48.3%) | 1922 (48.8%) | 1730 (47.1%) | 1651 (46.5%) | 1640 (46.9%) | 1605 (47.8%) |
| Male, n (%) | 2327 (45.5%) | 2089 (45.2%) | 1958 (45.0%) | 1836 (45.0%) | 1754 (44.5%) | 1619 (44.1%) | 1535 (43.3%) | 1517 (43.4%) | 1444 (43.0%) |
| Mean age, years (SD) | 24.8 (3.7) | 26.9 (3.6) | 30.0 (3.6) | 32.0 (3.6) | 35.0 (3.7) | 40.2 (3.6) | 45.2 (3.6) | 50.2 (3.6) | 55.1 (3.6) |
| Mean education, years (SD) | 13.8 (2.3) | 14.1 (2.9) | 14.4 (2.4) | 14.6 (2.5) | 14.7 (3.2) | 14.9 (2.5) | 15.0 (2.6) | 15.1 (2.7) | 15.1 (2.6) |
| <High School, n (%) | 510 (10.0%) | 347 (7.6%) | 267 (6.2%) | 233 (5.8%) | 247 (6.3%) | 183 (5.0%) | 150 (4.3%) | 160 (4.6%) | 143 .3%) |
| High School, n (%) | 1519 (29.7%) | 1209 (26.4%) | 1112 (25.6%) | 947 (23.4%) | 907 (23.1%) | 659 (18.0%) | 702 (19.9%) | 628 (18.0%) | 632 9.0%) |
| >High School, n (%) | 3080 (60.3%) | 3024 (66.0%) | 2958 (68.2%) | 2871 (70.9%) | 2766 (70.6%) | 2817 (77.0%) | 2676 (75.9%) | 2694 (77.4%) | 2557 (76.7%) |
| Alcohol, ml/day (SD) | 12.1 (22.0) | 14.2 (24.0) | 11.2 (25.6) | 11.2 (23.4) | 10.9 (22.1) | 11.0 (24.9) | 10.8 (22.2) | 11.7 (23.4) | 11.2 (19.8) |
| AHA ideal diet, n (%) | 615 (12.0%) | 565 (14.3%) | 325 (10.3%) | ||||||
| AHA intermediate diet, n (%) | 2817 (55.1%) | 2330 (59.1%) | 2135 (68.0%) | ||||||
| AHA poor diet, n (%) | 1676 (32.8%) | 1046 (26.5%) | 682 (21.7%) | ||||||
| Mean physical activity intensity score, units (SD) | 420.1 (300.8) | 382.1 (288.8) | 379.3 (292.5) | 338.1 (274.0) | 330.7 (274.8) | 347.2 (283.6) | 335.9 (274.1) | 337.8 (275.6) | 321.3 (271.5) |
| Body mass index, kg/m2 (SD) | 24.5 (5.0) | 25.2 (5.4) | 26.1 (5.9) | 26.8 (6.1) | 27.5 (6.5) | 28.8 (6.8) | 29.4 (7.0) | 30.2 (7.2) | 30.5 (7.2) |
| Mean systolic blood pressure, mm Hg (SD) | 110.4 (11.0) | 107.9 (10.8) | 107.8 (11.6) | 108.7 (12.4) | 110.0 (12.8) | 113.2 (14.9) | 115.7 (14.7) | 119.7 (16.2) | 120.8 (16.7) |
| Mean diastolic blood pressure, mm Hg (SD) | 68.6 (9.6) | 67.4 (9.7) | 69.2 (10.2) | 69.3 (10.3) | 72.4 (10.2) | 74.5 (11.6) | 72.2 (11.2) | 74.9 (11.3) | 74.1 (11.1) |
| Mean fasting glucose, mg/dL (SD) | 82.6 (16.3) | 90.1 (19.4) | 88.2 (20.4) | 86.7 (21.0) | 98.0 (26.5) | 99.5 (28.6) | 102.6 (31.8) | ||
| Current smoker, n (%) | 1544 (30.4%) | 1358 (29.6%) | 1241 (28.6%) | 1096 (27.0%) | 1002 (25.6%) | 807 (22.0%) | 683 (19.4%) | 589 (17.1%) | 463 (14.0%) |
| Lipid-lowering treatment, n (%) | 11 (0.3%) | 10 (0.2%) | 19 (0.5%) | 87 (2.4%) | 313 (8.8%) | 544 (15.6%) | 671 (20.0%) | ||
| Blood pressure-lowering treatment, n (%) | 115 (2.2%) | 123 (2.7%) | 69 (1.6%) | 80 (2.0%) | 135 (3.4%) | 292 (8.0%) | 619 (17.5%) | 942 (27.1%) | 1114 (33.2%) |
| Mean total cholesterol, mg/dL (SD) | 176.8 (33.5) | 177.0 (34.1) | 178.1 (34.4) | 177.0 (34.3) | 178.0 (34.6) | 184.7 (35.8) | 185.7 (35.0) | 192.3 (36.9) | 191.3 (38.1) |
| Mean HDL-cholesterol, mg/dL (SD) | 53.2 (13.2) | 53.1 (13.7) | 53.3 (14.2) | 52.1 (14.2) | 50.3 (14.0) | 50.7 (14.6) | 54.2 (16.7) | 58.0 (18.0) | 59.8 (18.9) |
| Mean LDL-cholesterol, mg/dL (SD) | 109.1 (31.2) | 112.5 (33.4) | 108.5 (32.1) | 107.6 (31.6) | 109.2 (32.1) | 113.0 (32.3) | 110.0 (32.1) | 111.9 (32.8) | 110.3 (33.2) |
| Mean triglycerides, mg/dL (SD) | 72.9 (48.5) | 78.9 (53.4) | 80.8 (72.3) | 86.4 (75.7) | 92.1 (74.7) | 105.5 (92.8) | 109.4 (79.7) | 114.2 (87.2) | 107.9 (97.8) |
| Diabetes, n (%) | 32 (0.6%) | 33 (0.7%) | 37 (0.9%) | 70 (1.8%) | 101 (2.7%) | 159 (4.4%) | 272 (7.8%) | 381 (11.0%) | 492 (14.9%) |
| Mean AHA cardiovascular health CVH score, out of 14 points (SD) | 10.2 (1.9) | 9.9 (2.1) | 9.1 (2.2) | ||||||
| Mean AHA cardiovascular health CVH score excluding diet, out of 12 points (SD) | 9.42 (1.7) | 9.45 (1.7) | 9.3 (1.7) | 9.1 (1.9) | 9.0 (1.9) | 8.6 (2.0) | 8.2 (2.1) | 7.7 (2.2) | 7.6 (2.2) |
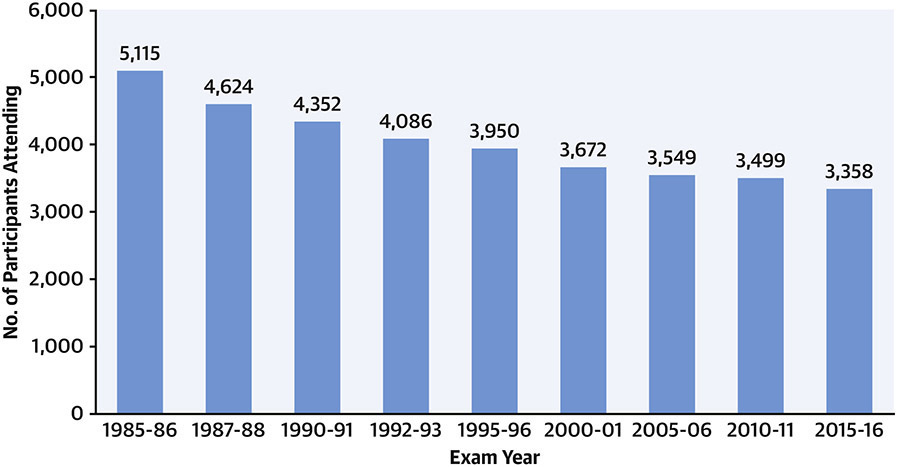
Follow-up in-person examinations have been completed at Years 2 (1987-88), 5 (1990-91), 7 (1992-93), 10 (1995-96), 15 (2000-01), 20 (2005-06), 25 (2010-11), and 30 (2015-16). Participation in the follow-up examinations is displayed in Figure 1; >71% of surviving participants attended the Year 30 examination. The Year 35 exam is ongoing. Contact is maintained with participants every 6 months, with annual interim medical history ascertainment. Over the last 5 years, >90% of the surviving cohort members have been directly contacted, and follow up for vital status is virtually complete.
Figure 1. Numbers of participants attending each CARDIA examination cycle.
CARDIA participants have undergone in-person examinations at baseline (Year 0) and follow up Years 2, 5, 7, 10, 15, 20, 25, and 30. Retention rates among surviving participants at each in-person examination were 91%, 86%, 81%, 79%, 74%, 72%, 72%, and 71%, respectively.
Beyond standard anthropometric and laboratory measures, and collection of biospecimen samples for storage, phenotypic assessments of attending CARDIA participants have included: physical fitness at Years 0, 7, and 20; diet at Years 0, 7, and 20; pulmonary function at Years 0, 2, 5, 10, 20, and 30; coronary artery calcium (CAC) at Years 10 (subset), 15, 20, and 25; carotid intima-media thickness at Year 20; echocardiography at Years 5, 10 (subset), 25, and 30; cognitive function at Years 25 and 30; brain magnetic resonance imaging in a subset at Years 25 and 30; body composition including dual-energy X-ray absorptiometry at Year 20 and abdominal computed tomography at Year 25; and repeated assessment of genetic, psychosocial, neighborhood, environmental, lifestyle and behavioral factors; prescription, recreational, and illicit drug use; and more. Hundreds of ancillary studies have also been undertaken to collect additional data or leverage existing data. A full list of data elements collected at each examination cycle is available at: https://www.cardia.dopm.uab.edu/images/more/2021/CARDIA_Exam_Components---AllYears2021-04-01.pdf.
Use of novel phenotyping methods (as they have developed over the course of the study) has allowed observation of the evolution from high early life health status to development of CVD RFs, to manifestations of subclinical and clinical CVD and other chronic diseases of aging. The unique design of CARDIA with regard to its balance on race and sex has also allowed for important potential insights into aging in demographic subgroups, and associated upstream social determinants of health, including structural racism and psychosocial and behavioral factors.
CARDIA has repeatedly measured biomarkers related to oxidative stress, inflammation, endothelial function, and many other pathways over time. Extensive use of the biorepository has enabled an increasing focus on systems biology, with studies of genetic, epigenetic, transcriptomic, proteomic, and metabolomic patterns at multiple time points. As a biracial cohort that follows diverse individuals from young adulthood to middle age, CARDIA is also a critical linchpin in data sharing and harmonization across cohorts to define the life course of CVH and CVD.
TOP TEN MOST IMPORTANT CONTRIBUTIONS
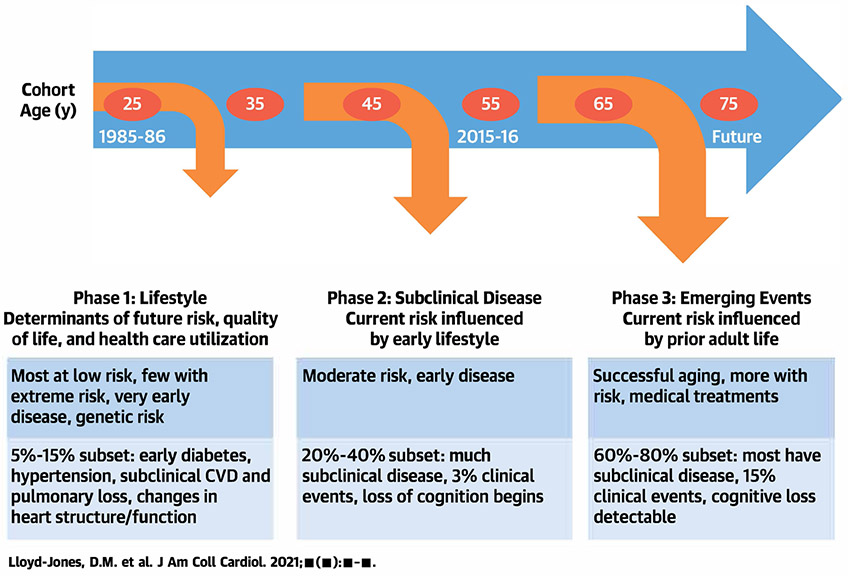
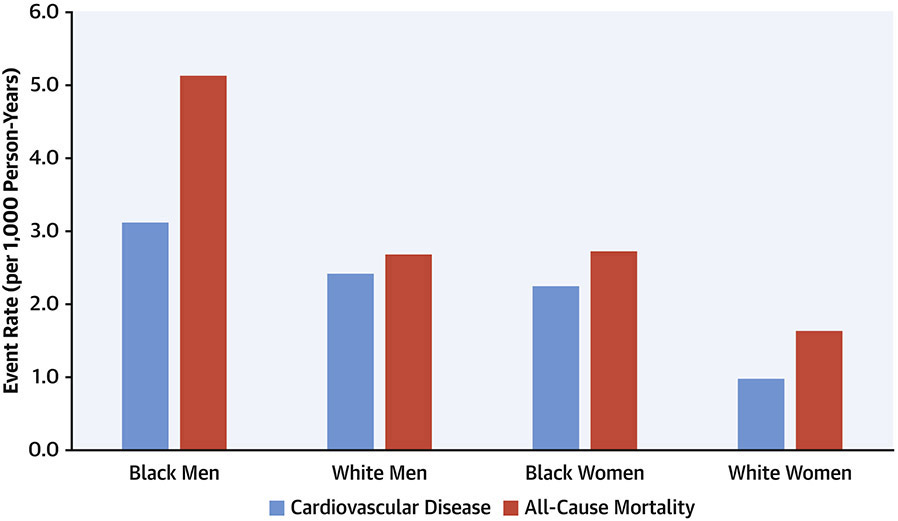
CARDIA has defined key processes in the progression from healthy young adulthood to middle age across diverse states of health and preclinical and clinical disease (Central Illustration). As the cohort has entered middle age, the number of clinical events has started to increase. Through 35 years of follow up, CARDIA participants have experienced 545 total deaths (106.5 deaths per 1000 participants since 1985), of which 17.3% were due to CVD, 20.9% to cancer, 18.6% to homicide, suicide, or trauma, and 10.9% to human immunodeficiency virus/acquired immunodeficiency syndrome. In addition, there have been 478 participants with adjudicated definite and probable incident CVD events, including 107 incident myocardial infarctions, 108 strokes, and 86 heart failure events. As shown in Figure 2, there are notable differences in incidence of death and CVD events across race/sex subgroups.
Central Illustration. CARDIA and the Life Course of Cardiovascular Health.
Schematic of the Coronary Artery Risk Development in Young Adults study (CARDIA) and its contributions to understanding the life course of cardiovascular health and disease. CVD = cardiovascular disease.
Figure 2. Incidence rates of cardiovascular disease and all-cause mortality in the CARDIA cohort.
Contact is maintained with CARDIA participants via telephone, mail, or email every 6 months, with annual interim medical history ascertainment. Over the last 5 years, >90% of the surviving cohort members have been directly contacted, and follow up for vital status is virtually complete through related contacts and intermittent National Death Index searches. The figure shows incident event rates from 1985 through February, 2021 for cardiovascular disease (light blue columns) and total mortality (dark blue columns) by sex and race groups.
The following sections summarize the top ten most important contributions to date from the CARDIA study, as judged by consensus of longstanding study leadership.
1. Transition from Healthy Young Adulthood to Development of CVD Risk Factors
CARDIA’s age range, long-term follow-up, and serial deep phenotyping make it uniquely suited to answer important questions regarding the natural history of CVD RF development before the onset of extensive comorbidities or use of drug therapies. CARDIA investigators have been at the forefront of developing and applying novel techniques to exploit the longitudinal, repeated measures data structure. These pioneering studies have provided critical insights on disentangling aging from secular trends,(2) analyses of RF trajectories and cumulative exposures,(3) elucidation of critical exposure periods and “points of no return,”(4) analyses of highly correlated data, and missing data patterns.
Blood pressure (BP).
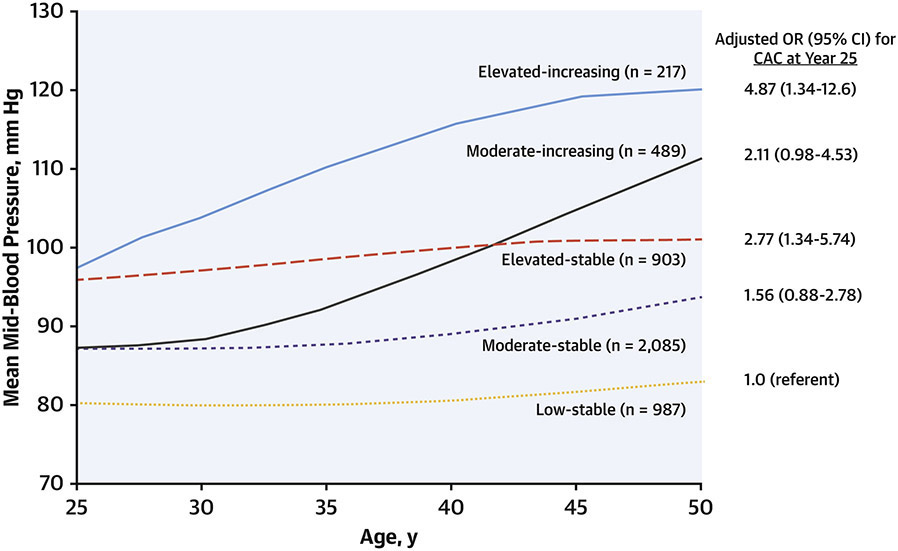
BP has been known to be a major RF for the development of atherosclerosis for decades. However, most prospective analyses have examined a single baseline BP measurement and its association with subsequent outcomes. In a novel analysis of BP exposure patterns over the life course, Allen(3) examined repeated measures from CARDIA participants collected from Years 0 to 25. Latent mixture modeling was used to identify groups of participants with distinct BP trajectories, describing both the levels and shape of change in BP over time. There were 5 distinct trajectories of BP (Figure 3), which were described by their baseline levels and the shape of change over time as: “low-stable” (representing 21.8% of participants), “moderate-stable” (42.3%), “moderate-increasing” (12.2%), “elevated-stable” (19.0%), and “elevated-increasing” (4.8%).(3)
Figure 3. Blood pressure trajectories across young adulthood and coronary artery calcification.
Mid-blood pressure (mean of systolic and diastolic blood pressure) was recorded at each CARDIA exam and participants were clustered using latent mixture modeling. Five unique groups were identified based on trajectory patterns across 25 years. Groups with blood pressure trajectories that were elevated at baseline with subsequent stable or increasing values, and those with moderate levels at baseline and increasing trajectory, had significantly higher odds of presence of coronary artery calcification at the Year 25 examination. Adapted with permission from reference 3.
Groups with elevated BP levels at baseline, and those with increasing trajectories over time, had greater odds of having a CAC score of 100 AU or greater at Year 25 (Figure 3). Of note, associations with CAC were not altered after adjustment for baseline and year 25 BP levels, or by cumulative BP exposure, indicating a unique contribution of the trajectory of BP rise over time.(3) BP trajectory data, that may be increasingly available through electronic health records, may thus assist in more accurate identification of individuals at risk for atherosclerosis.
Cholesterol concentrations.
In addition to trajectory analysis, RF exposure can also be described by considering the cumulative exposure and the slope of exposure over time, or by examining early age cumulative versus later age cumulative exposure, to understand whether there may be critical age periods of exposure. Domanski(5) recently used novel analytic methods to understand early exposure patterns to low-density lipoprotein cholesterol, and their ultimate associations with CVD events. Using repeated measures, each participant’s cumulative exposure prior to age 40 years was quantified in mg/dL times years; in addition, the slope of change from baseline to age 40, as well as the cumulative exposure from ages 18 to 30 versus 30 to 40 years, was quantified.(5)
Low-density lipoprotein cholesterol exposure prior to age 40 was associated with occurrence of CVD events after age 40 years. Both cumulative exposure prior to age 40 (hazard ratio [HR] 1.05 per 100 mg/dL x years; P<0.001) and the slope of exposure (HR 0.80 per mg/dL/year; P=0.045) were significantly associated with CVD risk after age 40. The inverse association for the slope indicates that higher earlier exposure was associated with greater risk than was later exposure. Exclusion of participants taking lipid lowering medications did not alter these findings, and they were confirmed with models showing that cumulative low-density lipoprotein cholesterol exposure from age 18 to 30 was significantly associated with CVD risk after age 40, whereas cumulative exposure from age 30 to 40 was not, when both variables were in the model. These findings indicate that both cumulative exposure and exposure that happens earlier in young adulthood are more important contributors to mid-life CVD events than later concentrations, with potential implications for early life primordial prevention strategies.(5)
Loss of CVH.
In 2010, the American Heart Association defined a novel construct of CVH that relies on levels of seven health behaviors and health factors: diet, physical activity, smoking status, body weight, BP, blood cholesterol, and blood glucose concentrations. Each of the component metrics is classified as ideal (optimal levels), intermediate (treated and controlled, or untreated/elevated), or poor (uncontrolled) using clinical thresholds. A composite CVH score (range 0 to 14 points) can be created for an individual by assigning 2 points for each ideal metric, 1 point for each intermediate metric, and 0 points for each poor metric.
CARDIA has produced key data on the evolution of CVH through young adulthood, and antecedent health behaviors that are associated with maintenance of higher CVH. Among CARDIA participants who attended the Year 0, 7, and 20 examinations, Liu(6) examined pursuit of five healthy lifestyle indicators (lean BMI, low or no alcohol intake, healthy diet score, higher levels of physical activity, and non-smoking). For participants who followed 0 or 1, 2, 3, 4, or all 5 healthy lifestyle factors at baseline, the age-, sex-, and race-adjusted prevalences of the ideal CVH profile at Year 25 were 3.0%, 14.6%, 29.5%, 39.2%, and 60.7%, respectively (Ptrend <0.001). Similar associations were observed for each race/sex group. Whereas longer duration of healthy lifestyles was associated with the highest prevalence of ideal mid-life CVH, those whose healthy lifestyle factors improved over time were more likely to maintain ideal CVH than those whose lifestyles did not, indicating that healthy change was an important predictor of mid-life ideal CVH, and the earlier that change occurred, the better.(6)
Women’s reproductive health and RF changes.
CARDIA has contributed substantially to our understanding of the associations between women’s reproductive health and CVD RFs with measurement of RF levels before and after pregnancy, a unique design feature. For example, among overweight women, 26.7% with 1 or more CVD RFs developed gestational diabetes mellitus versus 7.4% with none.(7) Impaired fasting glucose and low levels of high-density lipoprotein cholesterol before pregnancy were particularly strong RFs. CARDIA investigators have also shown significant associations between longer duration of post-partum lactation and lower incidence of diabetes or metabolic syndrome,(8,9) as well as lower burden of subclinical atherosclerosis.(10)
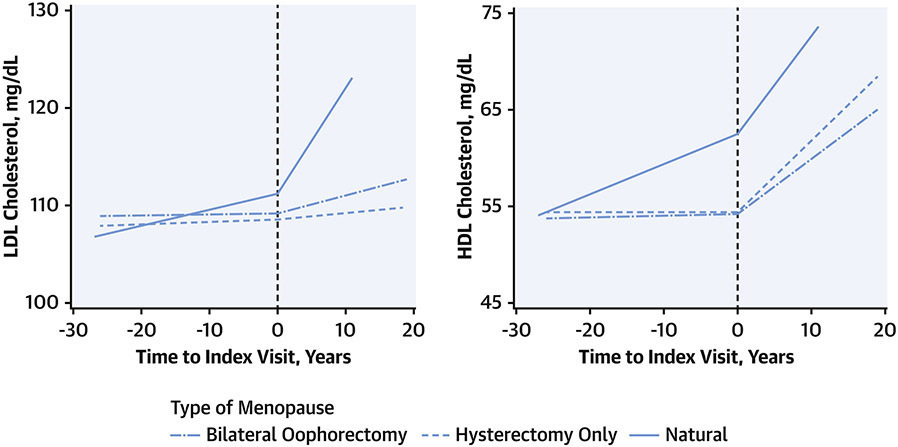
CARDIA affords the opportunity to study landmark life events (e.g., menopause, which may occur at diverse ages) and subsequent changes in RFs. Appiah(11) examined changes in CVD RF levels before and after menopause (Figure 4). They observed significantly steeper increases in low-density lipoprotein cholesterol after menopause in women with natural menopause compared with surgical menopause with hysterectomy only or with bilateral oophorectomy. Women with natural menopause also had a steeper slope of high-density lipoprotein cholesterol increase compared with the other two groups prior to menopause, and all three groups had similar rates of increase after menopause.(11)
Figure 4. Changes in cholesterol levels by time and type of menopause.
Concentrations of high-density (HDL) and low-density (LDL) lipoprotein cholesterol were measured over time in women in the CARDIA cohort and compared before and after onset of menopause (Time 0), according to the type of menopause: surgical hysterectomy with bilateral oophorectomy (dash-dot line), surgical hysterectomy only (dashed line), or natural (solid line). Women with natural menopause had greater increases in LDL-cholesterol after menopause, and in HDL-cholesterol before menopause. Adapted with permission from reference 12.
2. Transitions from CVD RFs to Subclinical CVD and Clinical Events
Development of CAC and progression to clinical CVD events.
CARDIA was one of the first population-based cohort studies to implement CAC measurement systematically, and CARDIA data have been instrumental in shaping clinical practice prevention guideline recommendations. CAC measurement was performed in the vast majority of participants attending the Year 15, 20, and 25 examinations, spanning the age range of 32 to 56 years, including large numbers of repeat measurements within individual participants.
At Year 15 (mean age 40 years), 10% of CARDIA participants had any CAC, with a greater prevalence among men than women (15.0% vs. 5.1%) and among White than Black men (17.6% vs. 11.3%). CAC prevalence was also higher for those aged 40 to 45 years than 33 to 39 years (13.3% vs. 5.5%). RF levels measured at Year 0 (mean age 25 years) discriminated CAC presence equally as well as average RF levels measured over exam Years 0, 2, 5, 7, 10, and 15, and better than concurrent levels at Year 15. Multivariable-adjusted odds ratios (ORs) of having CAC by ages 33 to 45 years were 1.5 (95% confidence interval, 1.3 - 1.7) per 10 cigarettes, 1.5 (1.3 - 1.8) per 30 mg/dL low-density lipoprotein cholesterol, 1.3 (1.1 - 1.5) per 10 mm Hg systolic BP, and 1.2 (1.1 - 1.4) per 15 mg/dL glucose at baseline.(12)
The 5-year incidence of CAC was 11.9% between Years 15 and 20 (mean ages 40 and 45 years) and 14.4% between Years 20 and 25 (45 and 50 years). As expected, incidence of CAC was highly associated with prior RF levels.(13)
After 12.5 years’ follow up, those with any CAC at Year 15 had a multivariable-adjusted HR of 5.0 (2.8-8.7) for coronary heart disease events and 3.0 (1.9-4.7) for CVD events, even after adjustment for demographics and RFs. Within CAC score strata of 1-19, 20-99, and ≥100 AU, the HRs for coronary events were 2.6 (1.0-5.7), 5.8 (2.6-12.1), and 9.8 (4.5-20.5), respectively. Addition of CAC score information added significant value to the Framingham risk score, particularly when 10-year predicted coronary risk was 5%-11%.(13)
Progression from early adult RFs to left ventricular (LV) structural and functional abnormalities and clinical events.
CARDIA participants have undergone echocardiographic examinations at Years 5, 25, and 30, with recent examinations including tissue Doppler, speckle tracking, strain measurements, and 3D imaging. Consequently, CARDIA is one of the few studies that has been able to document reference ranges and normative findings(14-17) and changes throughout young adulthood, as well as prevalence of abnormal findings and their antecedent RFs, in a non-referral setting.
Numerous investigations have documented antecedent RFs and risk markers for development of adverse LV structure and function. Among those factors found to be associated with echocardiographic outcomes in CARDIA are: self-identified race;(18) obesity/adiposity,(19-21) duration of obesity and patterns of obesity and weight gain over time; insulin resistance and glycemia patterns as well as diabetes and duration of diabetes;(22,23) non-alcoholic fatty liver disease;(24) baseline and cumulative BP exposures,(25) as well as long-term visit-to-visit BP variability;(26) renal function;(27) menopause;(28) alcohol intake;(29) and level of composite CVH.(30,31)
Using echocardiographic data from Years 5, 25 and 30, CARDIA investigators recently defined normative age-related changes.(32,33) With increasing age, LV relative wall thickness, LV mass index, and indexed left atrial diameter all increased significantly and monotonically over time, whereas the ratio of mitral early to late diastolic velocities (E/A) decreased substantially and monotonically. LV ejection fraction tended to increase until the mid-40s, followed by a decrease. The prevalence of any Stage B heart failure abnormality (asymptomatic abnormal LV geometry such as concentric remodeling, concentric hypertrophy, and eccentric hypertrophy; LV ejection fraction <50%; and/or presence of diastolic dysfunction) rose from a mean of 10.5% (9.4 – 11.8%) at age 25 years to 45.0% (42.0 – 48.1%) at age 60. Black participants had far higher prevalences than White participants of all adverse LV outcomes. The most significant predictors of incident Stage B abnormalities with aging were cumulative RF values from Year 5 to Year 30, rather than baseline or change values. In turn, both Year 5 echo parameters and the cumulative RF levels also were significantly associated with incident clinical heart failure.(32,33)
Using data from the Year 5 examination (mean age 23 to 35 years), severe diastolic dysfunction was present in 1.1% and abnormal relaxation in 9.3% of CARDIA participants. After multivariable adjustment, those with severe diastolic dysfunction and abnormal relaxation had greater risk of a composite outcome of death, myocardial infarction, heart failure, or stroke, with HRs of 4.3 (2.0-9.3) and 1.6 (1.1, 2.5), respectively, over 20 years.(34)
3. Incidence and Predictors of Premature Cardiovascular Events
Premature heart failure and stroke events.
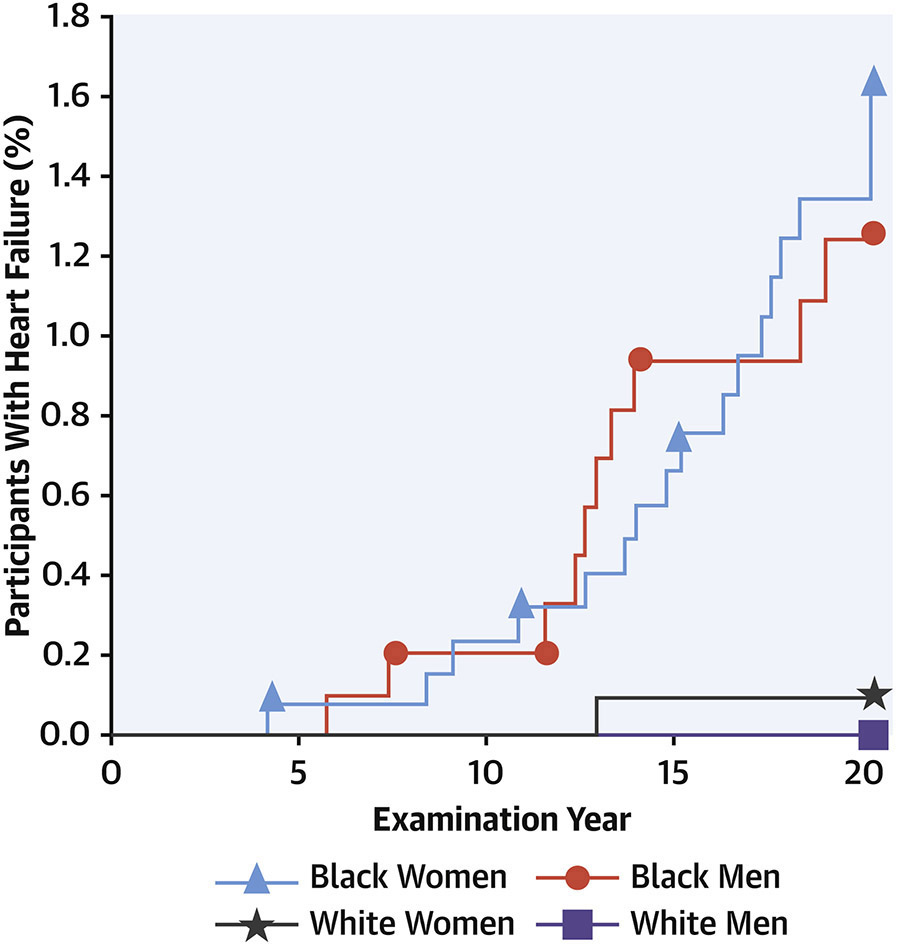
Whereas overall event incidence has been relatively low to date due to the young cohort age at inception, nonetheless CARDIA has provided key data on the incidence of premature CVD events. In one seminal analysis of CARDIA participants up to the age of 50 years, Bibbins-Domingo(35) observed that incident heart failure developed in 27 participants with mean age at onset of 39±6 years. Strikingly, 26 of the 27 participants with early-onset heart failure self-identified as being Black (Figure 5). Cumulative incidence rates of heart failure before the age of 50 years were 1.1% (0.6 - 1.7%) in Black women, 0.9% (0.5 - 1.4%) in Black men, 0.08% (0.0 - 0.5%) in White women, and 0% (0 - 0.4%) in White men (P=0.001 for Black versus White participants). Among Black participants, baseline variables that were significantly associated with incident heart failure included higher diastolic BP, higher BMI, lower high-density lipoprotein cholesterol, and presence of kidney disease. Three-fourths of those in whom heart failure developed had hypertension by the time they were 40 years of age, compared with a hypertension prevalence of 12% among those who did not develop heart failure. Depressed LV systolic function measured at CARDIA Year 5 was also independently associated with incident heart failure. Thus, hypertension, obesity, and systolic dysfunction that are present before 35 years of age, particularly in Black American individuals, appear to be especially important antecedents that may be targets for the prevention of clinical heart failure.(35)
Figure 5. Heart failure incidence by race and sex groups.
By 20 years’ follow up in the CARDIA cohort (ages 18-30 at baseline), there were 27 cases of incident heart failure. Strikingly, of the 27 participants affected, 26 self-identified as being Black. Among Black participants, baseline variables significantly associated with incident heart failure included higher diastolic blood pressure and body mass index, lower high-density lipoprotein cholesterol, and presence of kidney disease. Three-fourths of those in whom heart failure developed had hypertension by the time they were 40 years of age. From reference 35 with permission.
Gerber also noted marked disparities in premature stroke events between Black and White participants over 30 years’ follow up. Stroke incidence was four times higher in Black (120 per 100,000 person-years; 95–149) versus White (29; 18–46) participants. Blood pressure was a key driver of stroke risk, even within levels below the threshold for diagnosing hypertension. These findings further highlight the importance of primordial prevention strategies to reduce population BP levels, particularly in young Black adults.(36)
Premature CVD events.
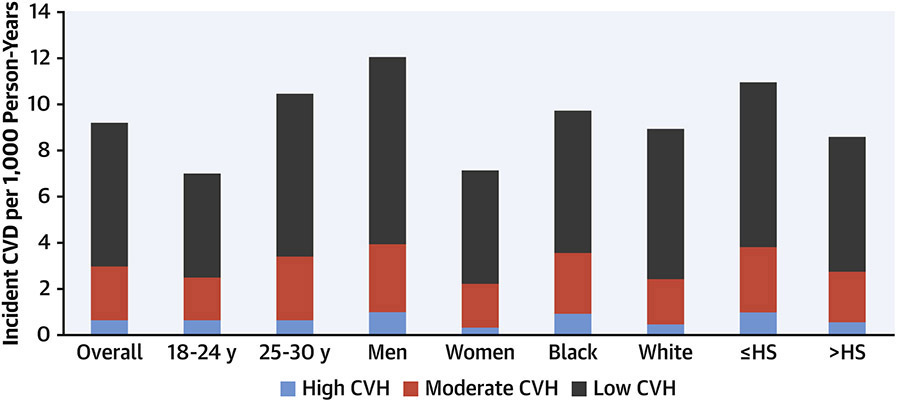
In a recent analysis, Perak(37) examined the incidence of all premature CVD events (including CVD death and incident non-fatal myocardial infarction, coronary revascularization, stroke, and heart failure) prior to approximately age 60 years among CARDIA participants. They examined the association of CVH status measured at ages 18 to 30 years with incident events and estimated population attributable fractions for CVD events. Year 0 CVH was high in 28.8%, moderate in 65.0%, and low in 6.3%. There were modest differences in the prevalence of high CVH status across subgroups by age and sex, and notable differences by self-reported race (20.7% in Black participants compared with 37.0% in White participants) and maximal educational attainment (13.6% among participants with less than or equal to high school completion compared with 32.6% among participants with more than high school education). The adjusted HR for high (vs. low) CVH was 0.14 (0.99 – 0.22) for CVD events (Figure 6). The population attributable fractions for combined moderate or low (versus high) CVH were 0.63 (0.47 to 0.74) for CVD, 0.81 (0.55 to 0.92) for CVD mortality, and 0.42 (0.26 – 0.54) for all-cause mortality. Among individuals with high CVH, rates of premature events were very low in all sociodemographic subgroups. Thus, maintenance of high CVH into late adolescence or early adulthood is associated with markedly lower risk for premature onset CVD, and these data suggest that maintenance of high CVH into young adulthood could prevent as much as 60% - 80% of premature CVD events and mortality.(37)
Figure 6. Cardiovascular disease incidence by cardiovascular health (CVH) status at baseline.
Cardiovascular health status at ages 18-30 years was assessed and defined as high (12-14 points), moderate (8-11 points), or low (0-7 points) based on levels of 7 health behaviors and factors. After 30 years’ follow up, cardiovascular event rates were very low in those with high CVH at baseline, and significantly higher for those with moderate or poor CVH, overall and by demographic subgroups. From reference 38.
4. Health Disparities Associated with Self-identified Race and Discrimination
The CARDIA cohort was designed explicitly to understand differences in CVD RF development in young adulthood by self-identified race and sex, and has matured to the point of observing major disparities in premature CVD events (Figures 5 and 6), particularly heart failure and stroke. Early CARDIA results documented disparities in RF levels, similar to those seen in national data, among young Black or White men and women, but CARDIA also provided critical insights into the reasons for those differences. For example, Greenlund documented significant differences in prevalence of RFs (BP, obesity) and health behaviors (such as smoking) across race and sex groups overall, with Black participants having more unfavorable levels at the baseline examination. However, they also noted striking differences in prevalence of RFs and health behaviors within individual race/sex strata (e.g., smoking prevalence in Black women) depending on geography and education status. Race differences in smoking rates were particularly apparent among those with less than or up to a high school education. Wagenknecht provided further insight, noting that after adjustment for age and education, race and sex differences in prevalence of smoking were no longer evident. Smoking prevalence was lower with greater educational attainment, from 54% among participants with less than a high school education to 12% among those with graduate degrees (P < 0.001). These data were early indications of the strong underlying influence of social determinants of health on apparent racial differences in cardiovascular risk.(38,39)
CARDIA has assessed participants’ self-reported lived experiences with racial/ethnic discrimination using a validated instrument at Years 7, 15, 25, and 30. As early as Year 7, associations of discriminatory experiences with RF levels were noted to be significant. For example, at Year 7, 48% of Black women and 57% of Black men reported experiencing three or more episodes of racial discrimination in life situations, compared with 5% and 4% of White men and women, respectively. Observed Black-White differences in BP were substantially reduced by taking into account reported experiences of racial discrimination and responses to unfair treatment. These early data highlighted the complex nature of lived experiences in association with physiologic CVD RFs.(40,41)
Subsequent analyses have examined self-reported discrimination and its associations with Black-White differences in birth outcomes,(42) weight change,(43) and self-rated physical and mental health,(44,45) among other outcomes. In one study using data from Year 7 and 15, an increase in self-reported experiences of racial/ethnic discrimination over time was significantly associated with an increase in waist circumference and BMI among Black women.(43) In another study, discrimination was statistically significantly associated with worse physical and mental health in both men and women, even after adjustment for age, education, income, and skin color.(44)
Weathering and psychosocial stress.
A number of recent CARDIA publications have examined “weathering,” or the difference between chronological age and composite measures of biological aging in Black and White participants.(46,47) In one study using seven age-related biomarkers (including lipid levels, glucose, C-reactive protein, BMI, pulmonary function and mean arterial pressure) to gauge accelerated biological vs. chronological aging, Black participants on average had a biological age that was 2.6±11.8 years older than their chronological age, whereas the average biological age among White participants was 3.5±10.0 years younger than their chronological age. In other words, Black individuals weathered a mean of 6.1 years faster than White individuals. Belonging to more social groups was associated with less weathering in Black but not White participants, and lower socioeconomic position and more depressive symptoms were associated with more weathering among Black than White individuals.(47) In a follow-up analysis examining outcomes, a one-year greater difference in weathering (i.e., one year greater difference in biological minus chronological age) was associated with greater odds of developing CVD (OR 1.04 per year; 1.02 - 1.06), stroke (OR 1.12; 1.07 - 1.17), and all-cause mortality (OR 1.05; 1.02 - 1.08). There were no significant overall racial differences in associations, but given the greater burden of weathering in Black participants, the implication is that these measures of accelerated aging may indicate an important marker of risk for Black individuals.(46)
Other measures, such as leukocyte telomere length and epigenetic age acceleration, also provide evidence of accelerated biological aging in Black compared with White individuals. For example, burden of racial discrimination reported at Year 15 was associated with more rapid shortening of telomere length over the ensuing 10 years, adding to evidence that racial discrimination contributes to accelerated physiologic weathering and health declines among Black Americans through its impact on biological systems.(48)
5. Social Determinants of Health as Mediators of Racial Disparities
CARDIA has measured numerous metrics of social determinants and socioeconomic position multiple times through young adulthood. Early reports identified racial disparities in RF levels and health disparities; however, associations of more adverse RFs with Black race were typically attenuated to non-significance with simple adjustment for socioeconomic factors.(49)
Socioeconomic position, even at young ages, has been associated with adverse health outcomes. Matthews used data from CARDIA Year 20, at which 19% of participants had CAC, in association with reported early life socioeconomic position. They found that lower paternal education in Black participants and lower maternal occupational status in all participants were associated with higher risk of having CAC, independent of adult socioeconomic position. Lower average adult education, occupation, and income were also associated with higher risk of having CAC, with associations seen primarily in Black individuals.(50) Elfassy examined income volatility in young adulthood and associations with subsequent CVD events. Income status was assessed at exams from 1990 to 2005, and CVD events were assessed from 2005 to 2015. Black participants were far more likely than White ones to experience income volatility and income drops from mean ages 30 to 45 years. After adjustment for demographic, behavioral, and other CVD RFs, higher income volatility (HR 2.07; 1.10 – 3.90) and more income drops (HR 2.54 for ≥2 versus 0 income drops; 1.24 - 5.19) were associated with risk for CVD.(51)
Education has been associated consistently with health outcomes, and the differences in education attainment between Black and White participants may account for some disparities in health outcomes. For example, to date cancer and cardiovascular diseases have been the most common causes of death in this younger cohort, but homicide and AIDS, which disproportionately affected Black participants, were associated with the most years of potential life lost. In multivariable models, each higher level of education achieved (from high school or less to some college to college degree or more) was associated with 1.37 fewer years of potential life lost (−2.37 - −0.37) whereas race was no longer independently associated.(52)
Both White and Black participants in CARDIA have had worsening of health behaviors and RF levels through young adulthood, although deterioration for Black participants has been more significant.(53) Whitaker(54) examined whether socioeconomic, psychosocial, and neighborhood environmental factors may mediate racial differences in CVH behaviors (as represented by smoking, physical activity, and diet patterns). They found that Black participants had significantly lower CVH behavior scores than White participants consistently across 30 years of follow up. Individual socioeconomic factors mediated 49 to 70% of the association between race and health behavior score, psychosocial factors 20 to 30%, and neighborhood factors 22 to 41% (p<0.01 for all). Thus, racial disparities in health behavior scores appeared to be mediated predominantly by large race-related differences in socioeconomic factors, highlighting the profound impact of factors that are mostly not under an individual’s control.(54)
The availability of geocoding has allowed for additional insights into social determinants of health and the potential influence of neighborhood racial segregation patterns on CVD risk and outcomes. For example, Kershaw(56) found that 81.6% of CARDIA participants were living in a high-segregation, 12.2% in a medium-segregation, and 6.2% in a low-segregation neighborhood. Systolic BP increased by a mean of 0.16 (0.06-0.26) mm Hg with each 1-SD increase in segregation score after adjusting for interactions of time with age, sex, and field center. Among those who lived in high-segregation neighborhoods at baseline, reductions in exposure to segregation were associated with reductions in systolic BP.(55) Similarly, data from the first 25 years of CARDIA follow up indicated that Black women living in highly segregated neighborhoods were 30% more likely to become obese between exam periods compared with women living in neighborhoods with low levels of segregation.(56)
6. Obesity Incidence as a Function of Young Adult Behaviors
Given the CARDIA study’s inception in 1985, the year in which US obesity prevalence started to climb, investigators have been uniquely positioned to assess the upstream determinants and correlates of weight gain in the CARDIA cohort.
Weight Gain in All Race/Sex Groups.
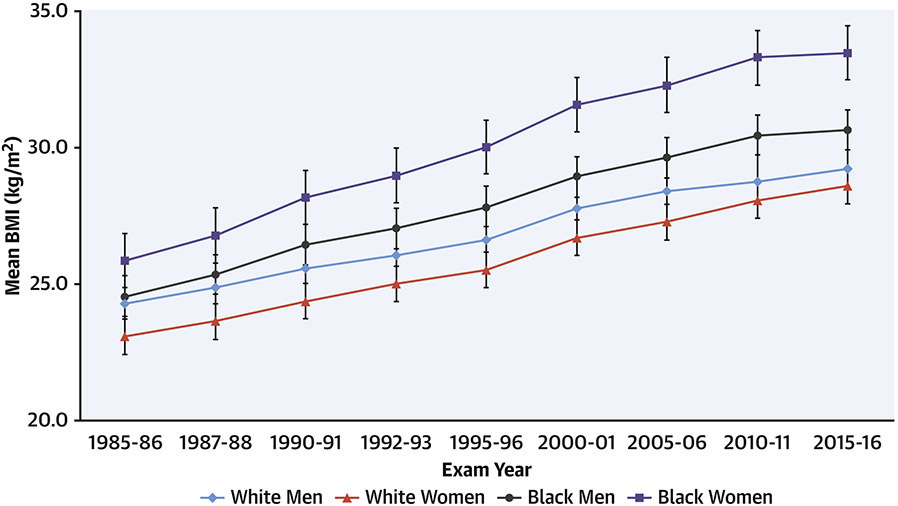
From Year 0 to Year 30 (mean ages 25 to 50 years), there have been significant mean weight gains in the cohort overall and in all 4 race/sex groups in CARDIA (Figure 7). There were larger gains noted early in CARDIA, with some flattening of increases in weight in later years, but overall consistent patterns across groups. The slope for time- related weight gain was consistent for those with normal weight, overweight, and obesity at baseline in young adulthood. Thus, among groups defined by baseline BMI in young adulthood, BMI tended to track and to increase consistently across all groups.(57)
Figure 7. Trends in body mass index (BMI) by race and sex over 30 years.
BMI (weight in kilograms divided by the square of height in meters) has increased steadily from baseline (Year 0; 1985-86) to the most recent exams in CARDIA. Rates of increase in BMI have been similar across race/sex groups.
Dietary Quality, Fast Food Availability, and Weight Change.
CARDIA has administered a detailed dietary history at exam Years 0, 7, and 20 (and is doing so again at Year 35). The diet history method obtains much more in depth information than a food frequency questionnaire, obtaining all food items eaten in the past 28 days, named by the participant, by brand name where possible, in response to over 100 open ended questions, such as “Do you eat meat?” with in-depth follow up of answers. Diet quality was assessed as a function of the proportion of plant-based whole foods. Diet quality was associated differentially with weight gain in Black and White participants. Among White participants, high vs. low diet quality was associated with less mean 20-year weight gain (+11.2 kg in high vs. +13.9 kg in low-quality diet groups), whereas among Black participants high diet quality was associated with greater weight gain (+19.4 kg vs. +17.8 kg).(58) Another analysis assessed change in plant-centered diet quality. In multivariable analysis, each 1-SD increment in plant-based diet quality over 20 years was associated with lower gains in BMI (−0.39±0.14 kg/m2; P = 0.004), waist circumference (−0.90±0.27 cm; P < 0.001) and weight (−1.14±0.33 kg; P < 0.001) during the same period, as well as with lower risk for subsequent diabetes.(59)
Geocoding of the CARDIA participants’ addresses at diverse exam cycles has also allowed assessment of neighborhood food availability. In general, higher numbers of neighborhood fast food restaurants and lower numbers of sit-down restaurants have been associated with higher consumption of an obesogenic fast food-type diet and greater BMI gains.(60) Also, there were inverse associations noted between fast food price and overall consumption, as well as with BMI, with stronger inverse associations in more (vs. less) deprived neighborhoods,(61) indicating that fast food availability and cheaper pricing in economically vulnerable neighborhoods appear to be strong correlates of greater obesity prevalence in participants living in those neighborhoods.
In CARDIA participants, frequency of fast-food dining was lowest for White women (1.3 times per week) compared with other race/sex groups (about twice weekly). Compared with the average 15-year weight gain in participants with infrequent (less than once a week) fast-food restaurant use at baseline and follow-up, those with frequent (more than twice weekly) visits to fast-food restaurants at baseline and follow-up gained an extra 4.5 kg of bodyweight and had a two-fold greater increase in insulin resistance.(62)
Physical and Sedentary Activity.
CARDIA has collected self-reported physical activity at all examinations, as well as intermittent measures of sedentary activities (e.g., television viewing), and objective measures of physical activity and sedentary behavior via accelerometry and cardiorespiratory fitness based on graded treadmill tests. Using self-reported activity level over 20 years, Hankinson et al, noted that CARDIA participants at all levels of physical activity gained weight. However, maintaining high levels of activity was associated with smaller gains in BMI and waist circumference compared with low activity levels. Men and women maintaining high activity (compared with those maintaining the lowest activity) gained 2.6 and 6.1 fewer kilograms, and 3.1 and 3.8 fewer centimeters in waist circumference, respectively.(63)
With regard to sedentary activity, television viewing was directly associated with size of abdominal fat depots on CT imaging. For each 1 SD increment in television viewing (1.5 hours/day), visceral, subcutaneous, and intra-muscular adipose tissue depots were greater by 3.5, 3.4, and 3.9 cm, respectively. In the same study, for each 1 SD increment in physical activity, visceral, subcutaneous and intra-muscular adipose tissue were lower by 7.6, 6.7, and 8.1 cm, respectively.(64) In further analyses, statistical replacement of sedentary time with light-intensity physical activity or moderate-to-vigorous intensity physical activity was associated with improved indices of adiposity and cardiometabolic health 10 years later.(65)
7. Metabolic Health through Young Adulthood – Metabolic Syndrome, Diabetes, and Non-Alcoholic Fatty Liver Disease
Influence of Weight Gain on RF Levels and Development of Metabolic Syndrome.
Through 15 years of follow up, 16.3% of CARDIA participants maintained a stable BMI, 73.9% had an increasing BMI, and 9.8% had a fluctuating BMI. Participants with stable BMI had essentially unchanged levels of cardiometabolic RFs, regardless of baseline BMI, whereas those with increasing BMI had progressively worsening levels. For example, among men with a baseline normal BMI, those who also had stable BMI during follow-up had a mean increase of only 15 mg/dL in fasting triglycerides over 15 years, compared with 65 mg/dL (P<0.001) in those whose BMI increased over time. Incidence of metabolic syndrome at year 15 was lower in the stable BMI group (2.2%) compared with the increased BMI group (18.8%; P<0.001). These data suggest that adverse progression of cardiometabolic RFs with advancing age may not be inevitable. Young adults who maintained stable BMI over time had minimal progression of RFs and lower incidence of metabolic syndrome regardless of baseline BMI, indicating the importance of weight stabilization as a clinical and public health strategy.(66,67)
Weight and Incident Diabetes.
Obesity and weight gain are significant RFs for incident diabetes. Reis examined years of exposure to abdominal obesity (defined as waist circumference >102 cm in men and >88 cm in women) over 25 years. HRs for 0, 1-5, 6-10, 11-15, 16-20, and >20 years of abdominal obesity were 1.00 (referent), 2.06 (1.43-2.98), 3.45 (2.28-5.22), 3.43 (2.28-5.22), 2.80 (1.73-4.54), and 2.91 (1.60-5.29), respectively (P<0.001).(68)
8. Longitudinal Pulmonary Function and Lung and Cardiovascular Outcomes
Pulmonary function testing with spirometry has been obtained at Years 0, 2, 5, 10, 20, and 30. Evidence for airflow obstruction was present in 6.9% and 7.8% of participants at Years 0 and 20, respectively. Lung function declined in all groups with age, but the effect of cigarette smoking on lung function decline was most evident in young adults with preexisting airflow obstruction. The forced expiratory volume in 1 second/forced vital capacity ratio at Year 0 was highly predictive of airflow obstruction 20 years later.(69)
Trajectories of change in lung function in CARDIA participants over 30 years were associated with emphysema identified on CT imaging. There were 5 distinct trajectories describing peak and change in forced expiratory volume, denoted as "Preserved Ideal," "Preserved Good," "Preserved Impaired," "Worsening," and "Persistently Poor." Ever smokers comprised part of all 5 trajectory groups. The prevalence of emphysema at mean ages of 40, 45, and 50 years was 1.7%, 2.5%, and 7.1%, respectively. Baseline poor and worsening lung health trajectories were associated with 5-fold higher odds of future emphysema independent of chronic tobacco smoke exposure. Thus, lower peak and accelerated decline in lung function were found to be significant RFs for future emphysema independent of smoking status.(70)
Following the observation in CARDIA that decline in forced vital capacity from average age at peak (29 years) to 35 years old predicted incident hypertension between average ages 35 and 45 years,(71) Cuttica examined 20-year changes in lung function and observed significant associations with left-heart structure and function. Specifically, decline in forced vital capacity from peak was associated with larger LV mass and greater cardiac output. Decline in airflow was associated with smaller left atrial internal dimension and lower cardiac output. Finally, decline in forced vital capacity was associated with diastolic dysfunction. They concluded that decline in airflow is associated with underfilling of the left heart and low cardiac output whereas decline in forced vital capacity with preserved airflow is associated with LV hypertrophy and diastolic dysfunction. These cardiopulmonary interactions appeared to evolve concurrently from early adulthood forward, but the precise mechanisms remain obscure.(72)
Pulmonary function at baseline (mean age 25 years) was also found to be independently associated with CVD events over 29 years’ follow up. Baseline forced expiratory volume in 1 second (HR 1.18 per 10- unit lower; 1.06–1.3) and forced vital capacity (HR 1.19 per 10- unit lower; 1.06–1.33) were both associated with incident CVD events independent of traditional RFs, including BMI. These associations were observed for outcomes of heart failure and cerebrovascular events but not coronary events. These findings add to a growing body of evidence that peak lung function in young adulthood has important implications for future respiratory and overall health.(73)
9. Cognitive Function and Brain Health in Mid-Life
Cognitive function was first measured at Year 25 (mean age 50 years), and was repeated at Year 30. State-of-the-art brain magnetic resonance imaging was obtained in a subset of participants at Year 25 and repeated in the same participants at Year 30.
Higher quality dietary patterns at Year 0 and 20 were associated with better cognitive function at Year 25 among apparently healthy middle-aged participants.(74) Specifically, greater adherence to Mediterranean or more plant-based dietary patterns during young adulthood were associated with better midlife cognitive performance.(75) Likewise, greater cardiorespiratory fitness at baseline (Year 0) and maintenance of fitness from Year 0 to 20 were also associated with higher performance on diverse domains of cognitive function at Year 25.(76)
Cumulative CVD RF exposures have also proven to be significantly associated with midlife cognitive function. Higher cumulative systolic and diastolic BP and fasting blood glucose across 25 years were significantly associated with worse cognition on all domains of testing. Fewer significant associations were observed for cholesterol exposure.(77) Similarly, a greater number of ideal CVH metrics in young adulthood and middle age were independently associated with better cognitive function in midlife. Participants who had ≥5 ideal metrics at a greater number of examinations over the 25-year period exhibited better performance on each cognitive test in middle age.(78) Between Years 25 and 30 (mean ages 50 and 55, respectively), 5% of participants had accelerated cognitive decline. Midlife smoking, hypertension, and diabetes mellitus were associated with greater likelihood of accelerated decline in both Black and White participants.(79)
In parallel with cognitive function testing, longitudinal CVD RFs have been associated with structural and functional findings on brain imaging. Current smoking, hypertension, and higher BMI were significantly associated with lower gray matter cerebral blood flow; current smoking, hypertension, and diabetes with lower total brain volume; higher BMI and hypertension with more abnormal white matter lesions; and hypertension with white matter fractional anisotropy. These findings suggest that worsening mid-life CVD RFs should be considered for early intervention and as markers of future risk for cerebrovascular disease and dementia.(80)
10. Effects of Chronic Marijuana Exposure on Health
CARDIA has ascertained drug and alcohol use patterns at all examinations through young adulthood, allowing assessment of cumulative lifetime exposure. Over 84% of CARDIA participants have reported a history of marijuana use at any point in time, although fewer than 12% persisted with use in middle age.(81)
In an analysis after Year 15, although marijuana use was not independently associated with presence of any of the traditional CVD RFs, it was associated with other unhealthy behaviors, such as high energy intake, smoking, and other non-prescription drug use. With regard to CVD events, compared with no marijuana use, cumulative lifetime and recent marijuana use showed no association with incident CVD overall or in any subgroups.(82) However, when analyzing cognitive function at Year 25, current daily use of marijuana was associated with worse verbal memory and processing speed, and cumulative lifetime exposure was associated with worse performance in all domains of cognitive function. After excluding current users and adjusting for potential confounders, cumulative lifetime exposure to marijuana remained significantly associated with worse verbal memory, corresponding to participants remembering 1 word fewer from a list of 15 words for every 5 years of use.(81)
FUTURE RESEARCH DIRECTIONS
Future CARDIA research directions will incorporate data from the ongoing Year 35 examination in 2020-22, and leverage the expanding resource of multi-omics data across young adulthood. For example, whole-genome sequencing and genome-wide DNA methylation data (from 4 exams from Years 15 to 30) are available in approximately 4,000 CARDIA participants. Initial studies indicate the potential power and limitations of polygenic risk scores,(83,84) and of epigenetic age acceleration,(85,86) for young adult cardiometabolic phenotypes. In addition, RNA has been collected and stored at two exams. Proteomic and metabolomic analyses are ongoing and are delineating the correlates and predictive utility of these platforms for cardiometabolic and other health outcomes. At Year 35, data collection will include first-time assessments of physical functioning (e.g., chair stand, grip strength), hearing and vestibular dysfunction, and dedicated lung and abdominal imaging, as well as repeat measures of RFs, sleep and accelerometry, cognitive function and brain imaging, and dietary quality, among other measures.
As the CARDIA cohort ages toward retirement and later life, it will continue to be a unique resource for understanding the determinants, mechanisms, and outcomes of CVH and CVD across the life course from young adulthood on. Future exam cycles will continue to provide opportunities for insight into the processes of aging.
CONCLUSIONS
In summary, CARDIA has become one of the premier US population-based cohort studies, and has contributed fundamentally to our understanding of the life course of CVH and CVD, as well as pulmonary, renal, neurologic, and other manifestations of aging (Central Illustration; Table 2). Due to its inception in early adulthood, CARDIA has defined the contemporary epidemiology of CVH/CVD in the first half of adult life in a cohort that has experienced the obesity and diabetes epidemics. CARDIA has established associations between the neighborhood environment and the life course evolution of lifestyle behaviors with biological RFs, subclinical disease, and early clinical CVD events from early adulthood on. CARDIA has also identified the nature and major determinants of Black-White differences in the loss of CVH and development of CVD risk, studying the progression of subclinical disease to clinical events during early to middle adulthood. Leveraging ongoing pan-omics work from genomic to metabolics and the microbiome will allow CARDIA investigators to make unique insights into the systems biology of cardiometabolic aging. CARDIA investigators are eager to collaborate with new, and particularly early-stage, investigators, and can be contacted through https://www.cardia.dopm.uab.edu/.
TABLE 2.
Summary of key findings from the CARDIA study, 1985-2021.
| Key Findings of the CARDIA Study | |
|---|---|
Transition from Healthy Young Adulthood to Development of CVD Risk Factors
|
Obesity Incidence as a Function of Young Adult Behaviors
|
Transitions from CVD RFs to Subclinical CVD and Clinical Events
|
Metabolic Health through Young Adulthood – Metabolic Syndrome, Diabetes, and Non-Alcoholic Fatty Liver Disease
|
Incidence and Predictors of Premature Cardiovascular Events
|
Longitudinal Pulmonary Function and Lung and Cardiovascular Outcomes
|
Health Disparities Associated with Self-identified Race and Discrimination
|
Cognitive Function and Brain Health in Mid-Life
|
Social Determinants of Health as Mediators of Racial Disparities
|
Effects of Chronic Marijuana Exposure on Health
|
HIGHLIGHTS.
Cardiovascular health is lost steadily through young adulthood, with implications for long-term CVD risk
Racial disparities in CVD risk arise largely from social determinants of health and lived experiences
The obesity epidemic has had major consequences on cardiac structure/function and cardiometabolic risk
The CARDIA Study continues to define the life course and mechanisms of CVD and aging
ACKNOWLEDGMENTS
The authors would like to thank all of the CARDIA participants for their dedication to the study, and the numerous CARDIA investigators that have led the innovative science of the study over the years. The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201800003I, HHSN268201800004I, HHSN268201800005I, HHSN268201800006I, and HHSN268201800007I from the National Heart, Lung, and Blood Institute (NHLBI).
LIST OF ABBREVIATIONS
- BMI
body mass index
- BP
blood pressure
- CAC
coronary artery calcium
- CARDIA
Coronary Artery Risk Development in Young Adults Study
- CVH
cardiovascular health
- CVD
cardiovascular disease
- HR
hazard ratio
- LV
left ventricular
- OR
odds ratio
- RF
risk factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures. None of the authors has any relevant disclosures/relationships with industry. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Contributor Information
Donald M. Lloyd-Jones, Department of Preventive Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL.
Cora E. Lewis, Department of Epidemiology, University of Alabama at Birmingham School of Public Health, Birmingham, AL.
Pamela J. Schreiner, Division of Epidemiology and Community Health, University of Minnesota School of Public Health.
James M. Shikany, Division of Preventive Medicine, University of Alabama at Birmingham School of Medicine.
Stephen Sidney, Kaiser Permanente Northern California Division of Research, Oakland CA.
Jared P. Reis, National Heart, Lung, and Blood Institute, Bethesda, MD.
REFERENCES
- 1.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–16. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs DR Jr., Hannan PJ, Wallace D, Liu K, Williams OD, Lewis CE. Interpreting age, period and cohort effects in plasma lipids and serum insulin using repeated measures regression analysis: the CARDIA Study. Stat Med 1999;18:655–79. [DOI] [PubMed] [Google Scholar]
- 3.Allen NB, Siddique J, Wilkins JT, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA 2014;311:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu K, Colangelo LA, Daviglus ML, et al. Can Antihypertensive Treatment Restore the Risk of Cardiovascular Disease to Ideal Levels?. J Am Heart Assoc 2015;4:e002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domanski MJ, Tian X, Wu CO, et al. Time Course of LDL Cholesterol Exposure and Cardiovascular Disease Event Risk. J Am Coll Cardiol 2020;76:1507–1516. [DOI] [PubMed] [Google Scholar]
- 6.Liu K, Daviglus ML, Loria CM, et al. Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age. Circulation 2012;125:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunderson EP, Quesenberry CP Jr., Jacobs DR Jr., Feng J, Lewis CE, Sidney S. Longitudinal study of prepregnancy cardiometabolic risk factors and subsequent risk of gestational diabetes mellitus. Am J Epidemiol 2010;172:1131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunderson EP, Jacobs DR Jr., Chiang V, et al. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status. Diabetes 2010;59:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunderson EP, Lewis CE, Lin Y, et al. Lactation Duration and Progression to Diabetes in Women Across the Childbearing Years. JAMA Intern Med 2018;178:328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunderson EP, Quesenberry CP Jr., Ning X, et al. Lactation Duration and Midlife Atherosclerosis. Obstet Gynecol 2015;126:381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appiah D, Schreiner PJ, Bower JK, Sternfeld B, Lewis CE, Wellons MF. Is Surgical Menopause Associated With Future Levels of Cardiovascular Risk Factor Independent of Antecedent Levels? Am J Epidemiol 2015;182:991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loria CM, Liu K, Lewis CE, et al. Early adult risk factor levels and subsequent coronary artery calcification. J Am Coll Cardiol 2007;49:2013–20. [DOI] [PubMed] [Google Scholar]
- 13.Carr JJ, Jacobs DR Jr., Terry JG, et al. Association of Coronary Artery Calcium in Adults Aged 32 to 46 Years With Incident Coronary Heart Disease and Death. JAMA Cardiol 2017;2:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreira HT, Nwabuo CC, Armstrong AC, et al. Reference Ranges and Regional Patterns of Left Ventricular Strain and Strain Rate Using Two-Dimensional Speckle-Tracking Echocardiography in a Healthy Middle-Aged Black and White Population. J Am Soc Echocardiogr 2017;30:647–658 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogunyankin KO, Liu K, Lloyd-Jones DM, Colangelo LA, Gardin JM. Reference values of right ventricular end-diastolic area defined by ethnicity and gender in a young adult population. Echocardiography 2011;28:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmussen-Torvik LJ, Colangelo LA, Lima JAC, et al. Prevalence and Predictors of Diastolic Dysfunction According to Different Classification Criteria. Am J Epidemiol 2017;185:1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teixido-Tura G, Almeida AL, Choi EY, et al. Determinants of Aortic Root Dilatation and Reference Values Among Young Adults Over a 20-Year Period. Hypertension 2015;66:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishi S, Reis JP, Venkatesh BA, et al. Race-ethnic and sex differences in left ventricular structure and function. J Am Heart Assoc 2015;4:e001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan SS, Shah SJ, Colangelo LA, et al. Association of Patterns of Change in Adiposity With Diastolic Function and Systolic Myocardial Mechanics From Early Adulthood to Middle Age. J Am Soc Echocardiogr 2018;31:1261–1269 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kishi S, Armstrong AC, Gidding SS, et al. Association of obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling. JACC Heart Fail 2014;2:500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reis JP, Allen N, Gibbs BB, et al. Association of the degree of adiposity and duration of obesity with measures of cardiac structure and function. Obesity (Silver Spring) 2014;22:2434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishi S, Gidding SS, Reis JP, et al. Association of Insulin Resistance and Glycemic Metabolic Abnormalities With LV Structure and Function in Middle Age. JACC Cardiovasc Imaging 2017;10:105–114. [DOI] [PubMed] [Google Scholar]
- 23.Reis JP, Allen NB, Bancks MP, et al. Duration of Diabetes and Prediabetes During Adulthood and Subclinical Atherosclerosis and Cardiac Dysfunction in Middle Age. Diabetes Care 2018;41:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.VanWagner LB, Wilcox JE, Colangelo LA, et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction. Hepatology 2015;62:773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishi S, Teixido-Tura G, Ning H, et al. Cumulative Blood Pressure in Early Adulthood and Cardiac Dysfunction in Middle Age. J Am Coll Cardiol 2015;65:2679–87. [DOI] [PubMed] [Google Scholar]
- 26.Nwabuo CC, Yano Y, Moreira HT, et al. Association Between Visit-to-Visit Blood Pressure Variability in Early Adulthood and Myocardial Structure and Function in Later Life. JAMA Cardiol 2020;5:795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bansal N, Lin F, Vittinghoff E, et al. Estimated GFR and Subsequent Higher Left Ventricular Mass in Young and Middle-Aged Adults With Normal Kidney Function. Am J Kidney Dis 2016;67:227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Appiah D, Schreiner PJ, Nwabuo CC, Wellons MF, Lewis CE, Lima JA. The association of surgical versus natural menopause with future left ventricular structure and function. Menopause 2017;24:1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodrigues P, Santos-Ribeiro S, Teodoro T, et al. Association Between Alcohol Intake and Cardiac Remodeling. J Am Coll Cardiol 2018;72:1452–1462. [DOI] [PubMed] [Google Scholar]
- 30.Desai CS, Ning H, Liu K, et al. Cardiovascular Health in Young Adulthood and Association with Left Ventricular Structure and Function Later in Life. J Am Soc Echocardiogr 2015;28:1452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gidding SS, Carnethon MR, Daniels S, et al. Low cardiovascular risk is associated with favorable left ventricular mass, left ventricular relative wall thickness, and left atrial size. J Am Soc Echocardiogr 2010;23:816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gidding SS, Lloyd-Jones D, Lima J, et al. Prevalence of American Heart Association Heart Failure Stages in Black and White Young and Middle-Aged Adults. Circ Heart Fail 2019;12:e005730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perak AM, Khan SS, Colangelo LA, et al. Age-Related Development of Cardiac Remodeling and Dysfunction in Young Black and White Adults. J Am Soc Echocardiogr 2021;34:388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desai CS, Colangelo LA, Liu K, et al. Prevalence, prospective risk markers, and prognosis associated with the presence of left ventricular diastolic dysfunction in young adults. Am J Epidemiol 2013;177:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med 2009;360:1179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerber Y, Rana JS, Jacobs DR Jr., et al. Blood Pressure Levels in Young Adulthood and Midlife Stroke Incidence in a Diverse Cohort. Hypertension 2021;77:1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perak AM, Ning H, Khan SS, et al. Associations of Late Adolescent or Young Adult Cardiovascular Health With Premature Cardiovascular Disease and Mortality. J Am Coll Cardiol 2020;76:2695–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenlund KJ, Kiefe CI, Gidding SS, et al. Differences in cardiovascular disease risk factors in black and white young adults. Ann Epidemiol 1998;8:22–30. [DOI] [PubMed] [Google Scholar]
- 39.Wagenknecht LE, Cutter GR, Haley NJ, et al. Racial differences in serum cotinine levels among smokers. Am J Public Health 1990;80:1053–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krieger N, Sidney S. Racial discrimination and blood pressure. Am J Public Health 1996;86:1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med 2005;61:1576–96. [DOI] [PubMed] [Google Scholar]
- 42.Mustillo S, Krieger N, Gunderson EP, Sidney S, McCreath H, Kiefe CI. Self-reported experiences of racial discrimination and Black-White differences in preterm and low-birthweight deliveries. Am J Public Health 2004;94:2125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunningham TJ, Berkman LF, Kawachi I, et al. Changes in waist circumference and body mass index in the US CARDIA cohort. Journal of biosocial science 2013;45:267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borrell LN, Kiefe CI, Williams DR, Diez-Roux AV, Gordon-Larsen P. Self-reported health, perceived racial discrimination, and skin color in African Americans in the CARDIA study. Soc Sci Med 2006;63:1415–27. [DOI] [PubMed] [Google Scholar]
- 45.Hudson DL, Puterman E, Bibbins-Domingo K, Matthews KA, Adler NE. Race, life course socioeconomic position, racial discrimination, depressive symptoms and self-rated health. Soc Sci Med 2013;97:7–14. [DOI] [PubMed] [Google Scholar]
- 46.Forrester SN, Zmora R, Schreiner PJ, et al. Racial differences in the association of accelerated aging with future cardiovascular events and all-cause mortality. Ethn Health 2020:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forrester SN, Zmora R, Schreiner PJ, et al. Accelerated aging: A marker for social factors resulting in cardiovascular events? SSM Popul Health 2021;13:100733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chae DH, Wang Y, Martz CD, et al. Racial discrimination and telomere shortening among African Americans. Health Psychol 2020;39:209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiefe CI, Williams OD, Lewis CE, Allison JJ, Sekar P, Wagenknecht LE. Ten-year changes in smoking among young adults. Am J Public Health 2001;91:213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matthews KA, Schwartz JE, Cohen S. Indices of socioeconomic position across the life course as predictors of coronary calcification in black and white men and women. Soc Sci Med 2011;73:768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elfassy T, Swift SL, Glymour MM, et al. Associations of Income Volatility With Incident Cardiovascular Disease and All-Cause Mortality in a US Cohort. Circulation 2019;139:850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roy B, Kiefe CI, Jacobs DR, et al. Education, Race/Ethnicity, and Causes of Premature Mortality Among Middle-Aged Adults in 4 US Urban Communities. Am J Public Health 2020;110:530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Booth JN 3rd, Allen NB, Calhoun D, et al. Racial Differences in Maintaining Optimal Health Behaviors Into Middle Age. Am J Prev Med 2019;56:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitaker KM, Jacobs DR Jr., Kershaw KN, et al. Racial Disparities in Cardiovascular Health Behaviors. Am J Prev Med 2018;55:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kershaw KN, Robinson WR, Gordon-Larsen P, et al. Association of Changes in Neighborhood-Level Racial Residential Segregation With Changes in Blood Pressure Among Black Adults. JAMA Intern Med 2017;177:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pool LR, Carnethon MR, Goff DC Jr., Gordon-Larsen P, Robinson WR, Kershaw KN. Longitudinal Associations of Neighborhood-level Racial Residential Segregation with Obesity Among Blacks. Epidemiology 2018;29:207–214. [DOI] [PubMed] [Google Scholar]
- 57.Dutton GR, Kim Y, Jacobs DR Jr., et al. 25-year weight gain in a racially balanced sample of U.S. adults. Obesity (Silver Spring) 2016;24:1962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zamora D, Gordon-Larsen P, Jacobs DR Jr., Popkin BM. Diet quality and weight gain among black and white young adults. Am J Clin Nutr 2010;92:784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi Y, Larson N, Gallaher DD, et al. A Shift Toward a Plant-Centered Diet From Young to Middle Adulthood and Subsequent Risk of Type 2 Diabetes and Weight Gain. Diabetes Care 2020;43:2796–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richardson AS, Meyer KA, Howard AG, et al. Multiple pathways from the neighborhood food environment to increased body mass index through dietary behaviors. Health Place 2015;36:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rummo PE, Meyer KA, Green Howard A, Shikany JM, Guilkey DK, Gordon-Larsen P. Fast food price, diet behavior, and cardiometabolic health. Health Place 2015;35:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pereira MA, Kartashov AI, Ebbeling CB, et al. Fast-food habits, weight gain, and insulin resistance. Lancet 2005;365:36–42. [DOI] [PubMed] [Google Scholar]
- 63.Hankinson AL, Daviglus ML, Bouchard C, et al. Maintaining a high physical activity level over 20 years and weight gain. JAMA 2010;304:2603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitaker KM, Pereira MA, Jacobs DR Jr., Sidney S, Odegaard AO. Sedentary Behavior, Physical Activity, and Abdominal Adipose Tissue Deposition. Med Sci Sports Exerc 2017;49:450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whitaker KM, Pettee Gabriel K, Buman MP, et al. Associations of Accelerometer-Measured Sedentary Time and Physical Activity With Prospectively Assessed Cardiometabolic Risk Factors. J Am Heart Assoc 2019;8:e010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lloyd-Jones DM, Liu K, Colangelo LA, et al. Consistently stable or decreased body mass index in young adulthood and longitudinal changes in metabolic syndrome components. Circulation 2007; 115:1004–11. [DOI] [PubMed] [Google Scholar]
- 67.Truesdale KP, Stevens J, Lewis CE, Schreiner PJ, Loria CM, Cai J. Changes in risk factors for cardiovascular disease by baseline weight status in young adults who maintain or gain weight over 15 years. Int J Obes (Lond) 2006;30:1397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reis JP, Hankinson AL, Loria CM, et al. Duration of abdominal obesity beginning in young adulthood and incident diabetes through middle age. Diabetes Care 2013;36:1241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalhan R, Arynchyn A, Colangelo LA, Dransfield MT, Gerald LB, Smith LJ. Lung function in young adults predicts airflow obstruction 20 years later. Am J Med 2010;123:468 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Washko GR, Colangelo LA, Estepar RSJ, et al. Adult Life-Course Trajectories of Lung Function and the Development of Emphysema. Am J Med 2020;133:222–230 ell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacobs DR Jr., Yatsuya H, Hearst MO, et al. Rate of decline of forced vital capacity predicts future arterial hypertension. Hypertension 2012;59:219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cuttica MJ, Colangelo LA, Shah SJ, et al. Loss of Lung Health from Young Adulthood and Cardiac Phenotypes in Middle Age. Am J Respir Crit Care Med 2015;192:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cuttica MJ, Colangelo LA, Dransfield MT, et al. Lung Function in Young Adults and Risk of Cardiovascular Events Over 29 Years. J Am Heart Assoc 2018;7:e010672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu N, Jacobs DR, Meyer KA, et al. Cognitive function in a middle aged cohort is related to higher quality dietary pattern 5 and 25 years earlier. J Nutr Health Aging 2015;19:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McEvoy CT, Hoang T, Sidney S, et al. Dietary patterns during adulthood and cognitive performance in midlife. Neurology 2019;92:e1589–e1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu N, Jacobs DR Jr., Schreiner PJ, et al. Cardiorespiratory fitness and cognitive function in middle age. Neurology 2014;82:1339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yaffe K, Vittinghoff E, Pletcher MJ, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 2014;129:1560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reis JP, Loria CM, Launer LJ, et al. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol 2013;73:170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yaffe K, Bahorik AL, Hoang TD, et al. Cardiovascular risk factors and accelerated cognitive decline in midlife. Neurology 2020;95:e839–e846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Launer LJ, Lewis CE, Schreiner PJ, et al. Vascular factors and multiple measures of early brain health. PLoS One 2015;10:e0122138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Auer R, Vittinghoff E, Yaffe K, et al. Association Between Lifetime Marijuana Use and Cognitive Function in Middle Age. JAMA Intern Med 2016;176:352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reis JP, Auer R, Bancks MP, et al. Cumulative Lifetime Marijuana Use and Incident Cardiovascular Disease in Middle Age. Am J Public Health 2017;107:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murthy VL, Xia R, Baldridge AS, et al. Polygenic Risk, Fitness, and Obesity. JAMA Cardiol 2020;5:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Natarajan P, Young R, Stitziel NO, et al. Polygenic Risk Score Identifies Subgroup With Higher Burden of Atherosclerosis and Greater Relative Benefit From Statin Therapy in the Primary Prevention Setting. Circulation 2017;135:2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim K, Joyce B, Zheng Y, et al. DNA methylation GrimAge and Incident Diabetes. Diabetes 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nannini DR, Joyce BT, Zheng Y, et al. Epigenetic age acceleration and metabolic syndrome. Clin Epigenetics 2019;11:160. [DOI] [PMC free article] [PubMed] [Google Scholar]