Abstract
Purpose
To evaluate polymyxin-resistant Klebsiella pneumoniae and Escherichia coli prevalence and characteristics in the Henan province, China.
Materials and Methods
A total of 2301 bacterial isolates collected at six hospitals were assessed. Their response to polymyxin was evaluated by minimum inhibitory concentration (MIC) analysis, and the mobilized colistin resistance (mcr) and carbapenemase gene were explored. Mutations on mgrB, phoPQ, pmrAB, and crrAB in polymyxin-resistant K. pneumoniae were detected by PCR. phoP, phoQ, pmrK, pmrA, pmrB, and pmrC transcriptional levels were quantified by RT-qPCR. Pulsed-field gel electrophoresis (PFGE) and multi-locus sequence typing were performed to determine the phylogenetic relationship between the polymyxin-resistant isolates.
Results
Of the E. coli and K. pneumoniae isolates identified, 0.3% and 1.4% were polymyxin-resistant, respectively, with MICs of 4–64 μg/mL. All polymyxin-resistant isolates were susceptible to tigecycline. Four E. coli isolates were mcr-1-positive and one was carbapenem-resistant, carrying blaNDM-5 and mcr-1. One K. pneumoniae isolate was mcr-1-positive and nine were carbapenem-resistant (PRCRKP), carrying blaKPC-2 but not mcr-1. The five E. coli isolates belonged to four sequence types (ST2, ST132, ST632, and ST983). All PRCRKP isolates belonged to ST11. However, all 16 isolates belonged to different PFGE types with <95% genetic similarity. Insertion sequences in mgrB were detected in nine (81.8%) polymyxin-resistant K. pneumoniae samples. Colistin resistance was linked with pmrHFIJKLM operon upregulation, with phoP, phoQ, and pmrK being overexpressed in all but one of the polymyxin-resistant K. pneumoniae samples. Furthermore, 33.3% of patients carrying polymyxin-resistant isolates had previously used polymyxin, and 66.7% patients displayed good clinical outcomes.
Conclusion
The K. pneumoniae polymyxin resistance rate was slightly higher than that of E. coli and mcr-1 was more common in E. coli than in K. pneumoniae. Moreover, the insertion of ISkpn14 into mgrB may be the main contributor to polymyxin-resistance in K. pneumoniae in Henan.
Keywords: polymyxin, Escherichia coli, Klebsiella pneumoniae, mcr, Henan province
Introduction
In recent years, antibiotic resistance has become a global public health priority. Colistin, also known as polymyxin, is one of the few therapeutic options available for the treatment of infectious diseases caused by multidrug-resistant gram-negative bacteria.1 In China, polymyxin was approved in January 2017 as an injectable drug for the treatment of bacterial infections. However, because of the increased usage of polymyxin in the clinical setting, polymyxin-resistant strains, especially those carrying the plasmid-borne mobilized colistin resistance (mcr) gene, have appeared in China and various countries worldwide.2 Moreover, intraspecies transmission of resistant isolates has already been reported.1,3
Since its discovery in southern China in late 2015,4 mcr-1 has spread to over 40 countries and regions, implying that it plays a prevalent role in the transferability of polymyxin resistance. Mcr-1-positive strains have also appeared in the Henan province, and it has been reported that mcr exists in pig-derived Escherichia coli isolates.5 Clinical E. coli isolates coproducing blaNDM and mcr-1 were previously reported by our laboratory.6 A novel conjugative mcr-8.2-bearing plasmid was identified in an almost pan-resistant hypermucoviscous Klebsiella pneumoniae ST11 isolate in Henan.7 However, overall, the report about mcr in human-derived E. coli and K. pneumoniae isolates was primarily focused outside Henan.
Except for mcr, colistin resistance in K. pneumoniae can be mediated by chromosomal mutations in genes involved in lipopolysaccharide synthesis, namely phoPQ, pmrAB, and crrA/crrB, as well as the regulatory mgrB.8–10
To better understand the epidemiological trends and characteristics of polymyxin-resistant clinical strains, these strains were screened using isolates collected at six hospitals located in the Henan province between 2018 and 2019. A total of 16 polymyxin-resistant strains were collected and their molecular resistance characteristics were analyzed. To the best of our knowledge, this is the first multicenter study to screen and investigate the molecular mechanisms of polymyxin resistance among E. coli and K. pneumoniae strains in the Henan province.
Materials and Methods
Sample Collection
Non-duplicated E. coli and K. pneumoniae strains were obtained from routine microbiological cultures of clinical samples from patients, including blood, urine, sputum, bronchoalveolar lavage fluid (BAL), bile, hydrothorax, ascites, and various other specimens. A total of 2301 strains were collected from six hospitals in the Henan province. Identification at the species level was performed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker Daltonik GmbH, Bremen, Germany).
Susceptibility Testing and Minimum Inhibitory Concentrations (MICs) Determination
Susceptibility to polymyxin was screened using polymyxin B susceptibility test Strip (Antu, Zhengzhou, China). MICs higher than 2 µg/mL were confirmed by the microbroth dilution method based on the clinical break points defined by the European Committee on Antimicrobial Susceptibility Testing.11
Susceptibility of polymyxin-resistant strains to ampicillin (AMP), meropenem (MEM), imipenem (IPM), ceftazidime (CAZ), cefotaxime (CTX), cefazolin (KZ), ampicillin/sulbactam (SAM), aztreonam (ATM), cefepime (FEP), piperacillin/tazobactam (TZP), levofloxacin (LEV), amikacin (AK), gentamicin (GN), trimethoprim/sulfamethoxazole (SXT), ceftazidime/avibactam (CZA), and tigecycline (TGC) were determined using the microbroth dilution method and interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines.12 Escherichia coli ATCC25922 and Pseudomonas aeruginosa ATCC27853 were used as quality controls.
Multi-Locus Sequence Typing (MLST)
Polymyxin-resistant K. pneumonia and E. coli isolates were typed using MLST following the scheme established by the Pasteur Institute (https://bigsdb.pasteur.fr/klebsiella/klebsiella.html and https://bigsdb.pasteur.fr/ecoli/ecoli.html, respectively).
Characterization of Mcr, mgrB, phoPQ, pmrAB, crrAB, and Carbapenem Resistance Genes
The modified carbapenem inactivation method (mCIM) and ethylenediaminetetraacetic acid-modified carbapenem inactivation method (eCIM), which are recommended by the CLSI, were used for phenotypic detection to confirm carbapenemase production. The presence of carbapenem resistance genes (blaVIM, blaIMP, blaKPC, blaNDM, and blaoxa-48-like) and the polymyxin resistance gene mcr1–8 in polymyxin-resistant isolates were screened by polymerase chain reaction (PCR) using the methods described previously.13–15 The full-length mgrB, pmrA, pmrB, phoP, phoQ, crrA, and crrB genes in polymyxin-resistant K. pneumoniae were amplified and sequenced. Next, the translated amino acid sequences were analyzed and compared with those of polymyxin-susceptible K. pneumoniae.
Transcriptional Analysis Real-Time Quantitative PCR
RNA extraction and transcription were carried out as previously described.16 Real-time quantitative PCR (RT-qPCR) was used to measure the expression of the phoQ, phoP, pmrK, pmrA, pmrB, and pmrC genes using the primers as previously described.16–18 Normalization was performed against the rpsL gene using the ΔΔCT method (relative) with the rpsL gene as internal control. The obtained values were then normalized against those obtained with polymyxin-susceptible strains.
Pulsed-Field Gel Electrophoresis (PFGE)
The molecular epidemiology of all polymyxin-resistant strains was determined by PFGE after total chromosomal DNA digestion with XbaI, in accordance with a previous report.19 The PFGE patterns were analyzed with the BIONUMERICS software (Applied Maths NV, Sint-Martens-Latem, Belgium) using the dice similarity coefficient. Isolates were considered as the same strain (PFGE type) if they possessed a genetic similarity of ≥ 95%.
Results
Overall Prevalence of Polymyxin-Resistant Strains
Over the course of the study, 16 (0.7%) isolates were found to be polymyxin-resistant among the 2301 identified E. coli and K. pneumoniae isolates, of which 5 and 11 were E. coli and K. pneumoniae isolates, respectively. The prevalence of polymyxin resistance in E. coli and K. pneumoniae was of 0.3% and 1.4%, respectively (Table 1).
Table 1.
Prevalence of Polymyxin-Resistant Isolates in the Six Participating Hospitals
| Isolates | Hospitals | No. of Isolates | No. of Polymyxin-Resistant Isolates (%) |
|---|---|---|---|
| Escherichia coli | |||
| Hospital 1 | 326 | 3 (0.9) | |
| Hospital 2 | 231 | 1 (0.4) | |
| Hospital 6 | 942 | 1 (0.1) | |
| Total | 1499 | 5 (0.3) | |
| Klebsiella pneumoniae | |||
| Hospital 3 | 141 | 1 (0.7) | |
| Hospital 4 | 133 | 1 (0.8) | |
| Hospital 5 | 78 | 2 (2.6) | |
| Hospital 6 | 450 | 7 (1.6) | |
| Total | 802 | 11 (1.4) | |
| Overall total | 2,301 | 16 (0.7) |
Antimicrobial Susceptibility Testing for Polymyxin-Resistant Isolates
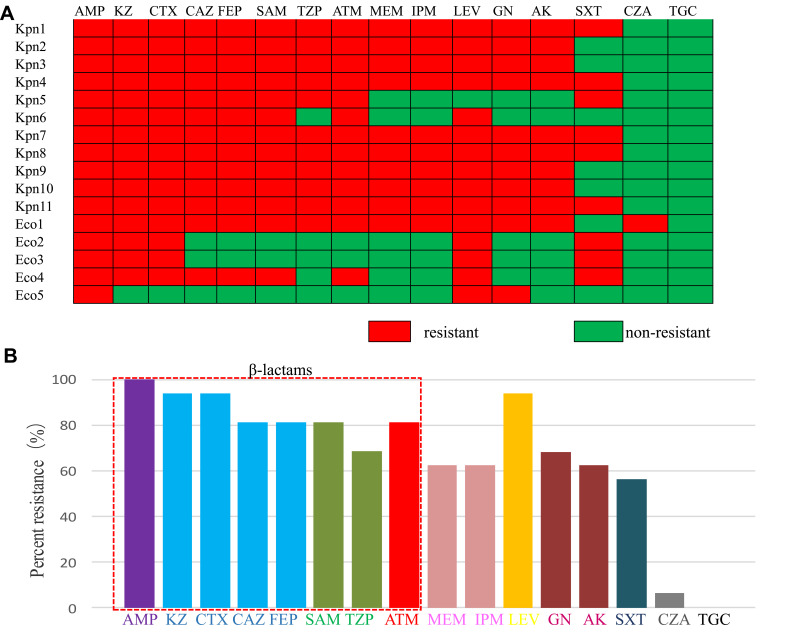
Antimicrobial susceptibility testing for the 16 polymyxin-resistant isolates showed that all of the isolates were resistant to AMP, KZ, and CTX; 93.3% were resistant to LEV; 86.6% were resistant to CAZ, FEP, and ATM; 80% were resistant to SAM and TZP; 66% were resistant to GN and AK; 62.5% were resistant to IPM and MEM; and 60% were resistant to SXT. Only one isolate was resistant to CZA (6.3%), and all of them were susceptible to TGC (Figure 1).
Figure 1.
Antimicrobial susceptibility profiles of the 16 polymyxin-resistant isolates. (A) Heatmap showing the resistance phenotypes of each of the isolates. (B) Percentage of strains resistant to the tested antibiotics.
Abbreviations: AK, amikacin; AMP, ampicillin; ATM, aztreonam; CAZ, ceftazidime; CTX, cefotaxime; CZA, ceftazidime Avibactam; FEP, cefepime; GN, gentamicin; IPM, imipenem; KZ, cefazolin; LEV, levofloxacin; MEM, meropenem; SAM, ampicillin/sulbactam; SXT, trimethoprim/sulfamethoxazole; TGC, tigecycline; TZP, piperacillin/tazobactam.
The MICs of these 16 strains to polymyxin ranged from 4–64 μg/mL. K. pneumoniae isolates had MICs in the range of 4–64 μg/mL (median: 64 μg/mL), whereas the MICs for polymyxin-resistant E. coli isolates were all of 4 μg/mL (Table 2).
Table 2.
Phenotypic and Genotypic Characteristics of the Polymyxin-Resistant Strains
| Site | Isolate | Polymyxin MIC (μg/mL) |
mCIM | eCIM | KPC | NDM | mcr–1 |
|---|---|---|---|---|---|---|---|
| Hospital 5 | Kpn1 | 4 | + | – | KPC-2 | – | – |
| Hospital 4 | Kpn2 | 32 | + | – | KPC-2 | – | – |
| Hospital 6 | Kpn3 | 64 | + | – | KPC-2 | – | – |
| Hospital 5 | Kpn4 | 8 | + | – | KPC-2 | – | – |
| Hospital 6 | Kpn5 | 64 | ND | ND | – | – | + |
| Hospital 3 | Kpn6 | 64 | ND | ND | – | – | – |
| Hospital 6 | Kpn7 | 16 | + | – | KPC-2 | – | – |
| Hospital 6 | Kpn8 | 32 | + | – | KPC-2 | – | – |
| Hospital 6 | Kpn9 | 64 | + | – | KPC-2 | – | – |
| Hospital 6 | Kpn10 | 64 | + | – | KPC-2 | – | – |
| Hospital 6 | Kpn11 | 64 | + | – | KPC-2 | – | – |
| Hospital 1 | Eco1 | 4 | + | + | – | NDM–5 | + |
| Hospital 1 | Eco2 | 4 | ND | ND | – | – | + |
| Hospital 1 | Eco3 | 4 | ND | ND | – | – | + |
| Hospital 2 | Eco4 | 4 | ND | ND | – | – | + |
| Hospital 6 | Eco5 | 4 | ND | ND | – | – | – |
Abbreviations: eCIM, ethylenediaminetetraacetic acid-modified carbapenem inactivation method; Eco, Escherichia coli; KPC-2, K. pneumoniae carbapenemase-2; Kpn, Klebsiella pneumoniae; mCIM, modified carbapenem inactivation method; mcr-1, mobilized colistin resistance-1; MIC, minimal inhibitory concentration; ND, data were not collected; NDM-5, New Delhi metallo-enzyme-5.
Detection of Antimicrobial Resistance Genes
Among the 16 polymyxin-resistant isolates, five carried mcr-1, including 1 K. pneumoniae and four E. coli isolates. No other mcr genes were detected in the 16 polymyxin-resistant isolates. In addition, nine K. pneumoniae and one E. coli isolates were carbapenemase-positive. The mCIM and eCIM results showed that nine K. pneumoniae isolates were serine carbapenemase-positive and one E. coli isolate was metallo-carbapenemase-positive. The PCR results showed that nine K. pneumoniae isolates were blaKPC-2-positive, but none of them carried mcr-1. Furthermore, one E. coli isolate was both blaNDM-5- and mcr-1-positive. No other carbapenemase genes, such as blaIMP, blaVIM, or blaOXA48-like, were detected (Table 2).
Among the 11 polymyxin-resistant K. pneumoniae samples, crrA was detected in 72.7% (8/11) isolates, and crrB was detected in 54.5% (6/11) isolates. Nine (81.8%) strains carried sequence insertions at the coding region for the mgrB gene. Due to the generation of amplicons larger than the normal size of the mgrB gene, one strain carried the normal mgrB gene and one strain had a W47R mutation in mgrB. ISKpn14 was the most common insertion sequence found in mgrB and was identified in seven (63.6%) isolates, followed by IS5 (two, 18.2%). No mutations were identified in phoP, phoQ, crrA, or pmrB. Only one strain carried M66I in the pmrA gene and another carried a frameshift in the crrB gene (Table 3).
Table 3.
Chromosomal Mutations and Insertion Sequences (IS) in Polymyxin-Resistant K. pneumoniae Strains
| Site | Isolate | mgrB | phoP | PhoQ | pmrA | pmrB | crrA | crrB |
|---|---|---|---|---|---|---|---|---|
| Hospital 5 | Kpn1 | ISkpn14 | + | + | + | + | + | Fr at16L |
| Hospital 4 | Kpn2 | ISkpn14 | + | + | + | + | + | – |
| Hospital 6 | Kpn3 | ISkpn14 | + | + | + | + | – | – |
| Hospital 5 | Kpn4 | ISkpn14 | + | + | + | + | + | + |
| Hospital 6 | Kpn5 | + | + | + | M66I | + | + | + |
| Hospital 3 | Kpn6 | W47R | + | + | + | + | + | + |
| Hospital 6 | Kpn7 | ISkpn14 | + | + | + | + | + | + |
| Hospital 6 | Kpn8 | ISkpn5-like | + | + | + | + | - | - |
| Hospital 6 | Kpn9 | ISkpn5-like | + | + | + | + | + | - |
| Hospital 6 | Kpn10 | ISkpn14 | + | + | + | + | - | - |
| Hospital 6 | Kpn11 | ISkpn14 | + | + | + | + | + | + |
Abbreviations: Fr, frameshift, +, presence of PCR product and no change in the nucleotide/amino acid sequences; −, absence of PCR product.
Overexpression of phoPQ and pmrK Contribute to Polymyxin-Resistance in K. pneumoniae
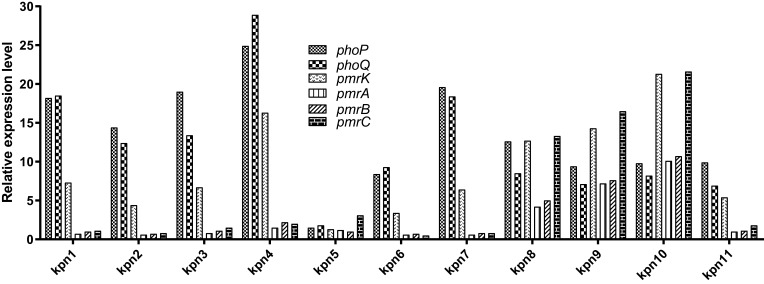
In general, transcriptional upregulation of the phoQ, phoP, and pmrK genes was observed in all strains with mgrB alterations, except for KPN5 with a normal mgrB gene. Compared to the levels obtained for our laboratory polymyxin-susceptible wild-type strains, the transcription level of phoP was elevated from 8.4- to 24.9-fold, that of phoQ was elevated from 6.9- to 28.9-fold, and that of pmrK was elevated from 3.4- to 21.3-fold. Moreover, three polymyxin-resistant K. pneumoniae isolates were found to have increased expression of the pmrA gene from 4.2- to 10.1-fold, of the pmrB gene from 5.0- to 10.7-fold, and of the pmrC gene from 13.3- to 21.6-fold (Figure 2).
Figure 2.
Transcriptional levels of phoP, phoQ, pmrK, pmrA, pmrB, and pmrC in polymyxin-resistant K. pneumoniae.
Epidemiological Characterization
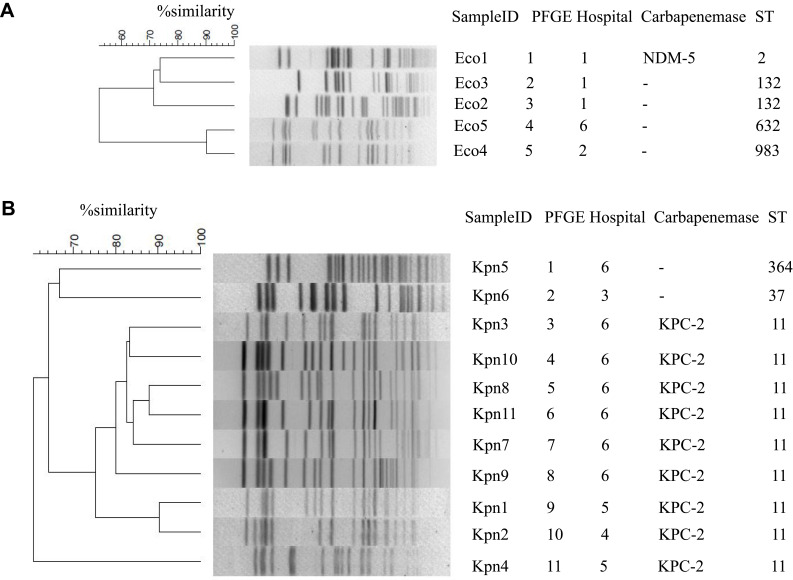
MLST analysis showed that nine K. pneumoniae isolates belonged to the sequence type (ST) 11, and the other two isolates belonged to ST37 and ST364, respectively. Among the five E. coli isolates, two belonged to ST132, and the other three isolates belonged to ST2, ST983, and ST632 (Figure 3).
Figure 3.
Pulsed-field gel electrophoresis (PFGE)-based dendrogram of polymyxin-resistant Escherichia coli (A) and Klebsiella pneumoniae (B) strains.
Abbreviations: Eco, E coli; KPC, K, pneumoniae carbapenemase; Kpn, K, pneumoniae; NDM-5, New Delhi metallo-enzyme-5; ST, sequence type.
Dendrogram analysis of PFGE at ≥ 95% similarity revealed that homology among the five E. coli and 11 K. pneumoniae isolates was low and sporadic, suggesting a very low likelihood of clonal spread (Figure 3).
Clinical Characteristics of Patients Harboring Polymyxin-Resistant Isolates
The 16 polymyxin-resistant isolates were collected within 1 year from 15 patients aged 2 months to 93 years. One patient had two isolates collected separately from blood and urine, whereas from all other 14 patients each isolate had been collected from urine (n = 4), BAL (n = 3), blood (n = 3), secretion (n = 2), peritoneal puncture fluid (n = 1), and sputum (n = 1) samples. The diagnosed diseases for these patients included cerebrovascular disease (n = 3), urinary tract disease (n = 3), pneumonia (n = 2), sepsis (n = 1), fever (n = 1), acute coronary syndrome (n=1), pregnancy-induced hypertension (n = 1), premature baby (n = 1), infection around the prosthesis (n = 1), and Guillain-Barre syndrome (n = 1). Five patients received polymyxin treatment before the isolation of polymyxin-resistant strains. Ten patients displayed positive clinical outcomes (Table 4).
Table 4.
Clinical Characteristics of Patients Carrying Polymyxin-Resistant Bacterial Strains
| Pt | Sex/Age (Years) | Isolate | Source | Clinical Diagnosis | Underlying Disease | Indwelling Devices | Antimicrobial Use Prior to Culture Within 30 Days | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | M/87 | Kpn | Blood | Cerebral infarction | Diabetes | Tracheal cannula | TGC, Carbapenems | Discharge |
| 2 | F/33 | Kpn | Secretion | Pneumonia, AFE | No | No | Clindamycin, Quinolones | Discharge |
| 3 | M/38 | Kpn | Sputum | Septic shock | No | CVC, Tracheal cannula | β-lactam, Quinolones | Death |
| 4 | F/69 | Eco/Eco | Blood/urine | Acute coronary syndrome | Diabetes, CHD | No | β-lactam, Quinolones | Discharge |
| 5 | M/81 | Eco | Blood | Fever | Hemodialysis | No | β-lactam, Quinolones, | Discharge |
| 6 | M/93 | Eco | BAL | Cerebral hemorrhage | Diabetes | CVC, Tracheal cannula | Carbapenems | Discharge |
| 7 | F/69 | Kpn | Urine | Urinary retention | No | CVC | β-lactam | Worsening |
| 8 | M/62 | Kpn | BAL | Cerebral hemorrhage | No | Tracheotomy | TGC, Polymyxin | Worsening |
| 9 | F/89 | Eco | Urethral secretions | cUTI | Hypertension, CHD | Urethral catheter | β-lactam, Quinolones | Death |
| 10 | M/56 | Kpn | Urine | Guillain-Barre syndrome | Hypertension | Tracheal cannula | TGC, Polymyxin, Carbapenems | Discharge |
| 11 | M/67 | Kpn | Urine | Urethral injury | Hypertension | Urethral catheter | β-lactam | Discharge |
| 12 | F/2 months | Kpn | BAL | Premature baby | No | Tracheal cannula | Polymyxin, Fosfomycin, fluconazole | Discharge |
| 13 | F/28 | Kpn | Peritoneal puncture fluid | Pregnancy-induced hypertension | SLE | Peritoneal drainage tube | TGC, Polymyxin, Carbapenems | Discharge |
| 14 | M/89 | Kpn | Blood | Severe pneumonia | No | Tracheal cannula | Polymyxin, Carbapenems, fluconazole | Death |
| 15 | F/54 | Kpn | Secretion | Infection around the prosthesis | No | No | Carbapenems, Quinolones | Discharge |
Abbreviations: AFE, amniotic fluid embolism; BAL, bronchoalveolar lavage fluid; Kpn, Klebsiella pneumoniae; CVC, central venous catheter; CHD, coronary heart disease; cUTI, complicated urinary tract infection; Eco, Escherichia coli; SLE, systemic lupus erythematosus; TGC, tigecycline.
Discussion
Polymyxin has been used against aggressive infections caused by multidrug-resistant bacteria, but its use has been severely compromised by the emergence of plasmid-mediated polymyxin resistance in Enterobacteriaceae. Hence, we surveyed polymyxin resistance rates and mechanisms in E. coli and K. pneumoniae isolates from hospitalized patients at six local hospitals in the Henan province.
Among the total 2301 E. coli and K. pneumoniae isolates, 16 (0.7%) were polymyxin-resistant, five of which carried mcr-1. In addition, 0.88% (34/3854) of the E. coli isolates and 0.21% (5/2410) of the K. pneumoniae isolates carrying mcr-1 were previously reported in the China Antimicrobial Resistance Surveillance Trial.20 Of the 1499 E. coli isolates in this study, 5 (0.3%) were polymyxin-resistant and 4 were mcr-1-positive. Previous research found that 1% (20/1495) of the E. coli isolates and 0.18% (1/571) of the K. pneumoniae isolates recovered from bloodstream infections in China were mcr-1-positive.21 Herein, of the 802 K. pneumoniae isolates, 11 (1.4%) were polymyxin-resistant, one of which carried mcr-1. Although mcr-1 was more common in E. coli than in K. pneumoniae isolates, the polymyxin resistance rate of K. pneumoniae was slightly higher than that of E. coli in our study, which is possibly due to antibiotic selection driven by the high detection rate (32.8%) of carbapenem-resistant K. pneumoniae (PRCRKP) in Henan among all Chinese provinces in 2019.22
Compared to polymyxin-resistant E. coli, polymyxin-resistant K. pneumoniae had 8–64 times higher MICs, and insertion sequences in the mgrB were detected in 81.8% of polymyxin-resistant K. pneumoniae isolates, which suggested that chromosomal mutations by insertional inactivation mediated by mobile insertion sequences may play important roles in polymyxin resistance in K. pneumoniae in the Henan province. Many studies found that mgrB alterations, including insertion elements, non-silent point mutations, and small intragenic deletions, mediate colistin resistance.16,18,23 ISkpn14 was the most common insertion observed in our study. However, an ISKpn26-like element was predominant in Taiwan23 and Greece.10 One strain carried a W47R mutation in the mgrB gene, which has been previously reported.24 M66I mutation in the pmrA gene and a frameshift at 16L in the crrB gene were newly found in this study, but further research is needed to clarify the mechanism.
The mgrB gene was a negative feedback regulator of the PhoQ-PhoP signaling system; therefore, phoP, phoQ, and pmrK were all upregulated in polymyxin-resistant K. pneumoniae with mgrB alterations in this study, as previously reported.16,25 pmrA, pmrB, and pmrC overexpression was found only in three polymyxin-resistant K. pneumoniae isolates, which implies that mgrB alterations might be the main reason for polymyxin resistance in K. pneumoniae.
Two carbapenemase genes, blaKPC and blaNDM, are responsible for the phenotypic resistance of 90% of carbapenem-resistant Enterobacteriaceae strains in China.26 The co-existence of mcr and carbapenemase genes, such as blaNDM-5,27 blaNDM-4,28 blaKPC,29 and blaOXA,30 has been sporadically reported in different countries. In the national monitoring data from China, one report showed that the mcr-1 was detected in 4.6% (13/282) of carbapenem-resistant E. coli isolates and coexisted with New Delhi metallo-enzyme (NDM)-5 in one strain.31 In another study, mcr-1 prevalence among carbapenem-resistant E. coli and PRCRKP isolates was 3.7% (14/376) and 0% (0/1134), respectively, and 14 carbapenem-resistant E. coli isolates coproduced blaNDM4/5/9 with mcr-1.32 In this study, only one E. coli isolate coproduced mcr-1 and blaNDM-5.
An E. coli isolate belonging to ST167 that coproduced blaNDM and mcr-1 was previously reported in Henan,6,33 but in our study, the aforementioned coproducing E. coli isolate belonged to ST2. The other E. coli strains in this study belonged to ST132, ST983, and ST632. Nine PRCRKP isolates belonged to ST11, but PFGE showed that they belonged to different types and were not clonal, even when isolated from the same hospital. These results demonstrated that polymyxin-resistant isolates were non-clonal and had different resistance potentials. It was reported that ST11 KPC-Kp was clonally heterogeneous and could be further classified into eleven mobile genetic element (MGE) types and fourteen PFGE subtypes.34
The patients carrying polymyxin-resistant isolates had varying severities of illness, and 33.3% of them had a history of polymyxin use. Moreover, 66.7% of them were cured, which could be explained by the retained susceptibility to other antimicrobials, such as CZA, SXT, and TGC, that most polymyxin-resistant isolates showed.
The limitation of this study is the use of the polymyxin B susceptibility test strip to screen polymyxin-resistant isolates, which is not the gold standard for identifying resistance to polymyxin. Indeed, it has been reported that results obtained by this method has high very major errors (VMEs), leading to an underestimation of the resistance.35–37
Conclusions
In conclusion, the polymyxin resistance rate of K. pneumoniae was slightly higher than that of E. coli, and mcr-1 occurrence was lower in polymyxin-resistant K. pneumoniae than in polymyxin-resistant E. coli in the Henan province of China. Further molecular investigations showed that insertion sequences in the mgrB gene might be the main mechanism contributing to polymyxin resistance in K. pneumoniae in Henan. In addition, continuous and close monitoring is required to prevent the dissemination of polymyxin-resistant K. pneumoniae and E. coli strains.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Funding Statement
This work was supported by the Science and Technology Research Project of Henan Province [grant number SBGJ2018084, SBGJ 201903018 and 202102310355], the Joint Program of Medical Science and Technology Research of Henan Province (LHGJ20190611), and the Collaborative Innovation Major Project of Zhengzhou [grant number 20XTZX05015]. The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of Henan Provincial People’s Hospital, Henan, China (20210055). No personally identifiable information was collected in this study. The requirement for informed consent from patients was also waived.
Author Contributions
All authors made a significant contribution to the work reported, including the conception, study design, execution, acquisition of data, analysis and interpretation. All authors took part in drafting, revising and critically reviewing the article, and approved the final manuscript. In addition, the authors have agreed on the journal to which this manuscript has been submitted and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sun J, Zhang H, Liu YH, Feng Y. Towards understanding MCR-like colistin resistance. Trends Microbiol. 2018;26(9):794–808. [DOI] [PubMed] [Google Scholar]
- 2.Giamarellou H. Epidemiology of infections caused by polymyxin-resistant pathogens. Int J Antimicrob Agents. 2016;48(6):614–621. [DOI] [PubMed] [Google Scholar]
- 3.Nordmann P, Poirel L. Plasmid-mediated colistin resistance: an additional antibiotic resistance menace. Clin Microbiol Infect. 2016;22(5):398–400. [DOI] [PubMed] [Google Scholar]
- 4.Liu YY, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–168. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Liu B, Dong P, Li F, Yuan L, Hu G. The prevalence of mcr-1 and resistance characteristics of Escherichia coli isolates from diseased and healthy pigs. Diagn Microbiol Infect Dis. 2018;91(1):63–65. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Sun Q, Shen Y, et al. Rapid Increase in prevalence of carbapenem-resistant Enterobacteriaceae (CRE) and emergence of colistin resistance gene mcr-1 in CRE in a hospital in Henan, China. J Clin Microbiol. 2018;56(4):e01932–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin S, Zhang C, Schwarz S, et al. Identification of a novel conjugative mcr-8.2-bearing plasmid in an almost pan-resistant hypermucoviscous Klebsiella pneumoniae ST11 isolate with enhanced virulence. J Antimicrob Chemother. 2020;75(9):2696–2699. [DOI] [PubMed] [Google Scholar]
- 8.Pitt ME, Elliott A, Cao MD, et al. Multifactorial chromosomal variants regulate polymyxin resistance in extensively drug-resistant Klebsiella pneumoniae. Microb Genom. 2018;4(3):e000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poirel L, Jayol A, Bontron S, et al. The mgrB gene as a key target for acquired resistance to colistin in Klebsiella pneumoniae. J Antimicrob Chemother. 2015;70(1):75–80. [DOI] [PubMed] [Google Scholar]
- 10.Hamel M, Chatzipanagiotou S, Hadjadj L, et al. Inactivation of mgrB gene regulator and resistance to colistin is becoming endemic in carbapenem-resistant Klebsiella pneumoniae in Greece: a nationwide study from 2014 to 2017. Int J Antimicrob Agents. 2020;55(4):105930. [DOI] [PubMed] [Google Scholar]
- 11.European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, Version 9.0; 2019. Available from: https://www.eucast.org/clinical_breakpoints/. Accessed July2, 2021.
- 12.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 29th ed. Wayne (PA): CLSI supplement M100 Clinical and Laboratory Standards Institute; 2019. [Google Scholar]
- 13.Dallenne C, Da Costa A, Decré D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010;65(3):490–495. [DOI] [PubMed] [Google Scholar]
- 14.Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20(3):440–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Wang Y, Zhou Y, et al. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg Microbes Infect. 2018;7(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haeili M, Javani A, Moradi J, Jafari Z, Feizabadi MM, Babaei E. MgrB alterations mediate colistin resistance in Klebsiella pneumoniae isolates from Iran. Front Microbiol. 2017;8:2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cannatelli A, Di Pilato V, Giani T, et al. In vivo evolution to colistin resistance by PmrB sensor kinase mutation in KPC-producing Klebsiella pneumoniae is associated with low-dosage colistin treatment. Antimicrob Agents Chemother. 2014;58(8):4399–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannatelli A, D’Andrea MM, Giani T, et al. In vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator. Antimicrob Agents Chemother. 2013;57(11):5521–5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He J, Jia X, Yang S, et al. Heteroresistance to carbapenems in invasive Pseudomonas aeruginosa infections. Int J Antimicrob Agents. 2018;51(3):413–421. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Wang Y, Cui L, et al. A retrospective study on mcr-1 in clinical Escherichia coli and Klebsiella pneumoniae isolates in China from 2007 to 2016. J Antimicrob Chemother. 2018;73(7):1786–1790. [DOI] [PubMed] [Google Scholar]
- 21.Quan J, Li X, Chen Y, et al. Prevalence of mcr-1 in Escherichia coli and Klebsiella pneumoniae recovered from bloodstream infections in China: a multicentre longitudinal study. Lancet Infect Dis. 2017;17(4):400–410. [DOI] [PubMed] [Google Scholar]
- 22.China Antimicrobial Resistance Surveillance System. National Bacterial Resistance Surveillance Report. Short Version (in Chinese); 2019. Available from: http://www.carss.cn/Report/Details?aId=770. Accessed November19, 2020.
- 23.Yang TY, Wang SF, Lin JE, et al. Contributions of insertion sequences conferring colistin resistance in Klebsiella pneumoniae. Int J Antimicrob Agents. 2020;55(3):105894. [DOI] [PubMed] [Google Scholar]
- 24.Nordmann P, Jayol A, Poirel L. Rapid detection of polymyxin resistance in Enterobacteriaceae. Emerg Infect Dis. 2016;22(6):1038–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cannatelli A, Giani T, D’Andrea MM, et al. MgrB inactivation is a common mechanism of colistin resistance in KPC-producing Klebsiella pneumoniae of clinical origin. Antimicrob Agents Chemother. 2014;58(10):5696–5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang R, Liu L, Zhou H, et al. Nationwide surveillance of clinical carbapenem-resistant Enterobacteriaceae (CRE) strains in China. EBioMedicine. 2017;19:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen Z, Hu Y, Sun Q, et al. Emerging carriage of NDM-5 and MCR-1 in Escherichia coli from healthy people in multiple regions in China: a cross sectional observational study. EClinicalMedicine. 2018;6:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le L, Tran LK, Le-ha TD, et al. Coexistence of plasmid-mediated mcr-1 and blaNDM-4 genes in a clinical strain in a Klebsiella pneumoniae clinical strain in Vietnam. Infect Drug Resist. 2019;12:3703–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JY, Heo ST, Kwon KT, Song DY, Lee KJ, Choi JA. MCR1 and KPC2 co-producing Klebsiella pneumoniae bacteremia: first case in Korea. Infect Chemother. 2019;51(4):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nukui Y, Ayibieke A, Taniguchik M, et al. Whole-genome analysis of EC129, an NDM-5-, CTX-M-14-, OXA-10- and MCR-1-co-producing Escherichia coli ST167 strain isolated from Japan. J Glob Antimicrob Resist. 2019;18:148–150. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Wang X, Wang J, et al. Phenotypic and genotypic characterization of carbapenem-resistant Enterobacteriaceae: data from a longitudinal large-scale CRE study in China (2012–2016). Clin Infect Dis. 2018;67(Suppl 2):S196–S205. [DOI] [PubMed] [Google Scholar]
- 32.Huang H, Dong N, Shu L, et al. Colistin-resistance gene mcr in clinical carbapenem-resistant Enterobacteriaceae strains in China, 2014–2019. Emerg Microbes Infect. 2020;9(1):237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu L, Wang P, Cheng J, Qin S, Xie W. Characterization of a novel blaNDM-5-harboring IncFII plasmid and an mcr-1-bearing IncI2 plasmid in a single Escherichia coli ST167 clinical isolate. Infect Drug Resist. 2019;12:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu P, Tang Y, Li G, et al. Pandemic spread of blaKPC-2 among Klebsiella pneumoniae ST11 in China is associated with horizontal transfer mediated by IncFII-like plasmids. Int J Antimicrob Agents. 2019;54:117–124. [DOI] [PubMed] [Google Scholar]
- 35.Shamina OV, Kryzhanovskaya OA, Lazareva AV, et al. The comparison of methods for determination of colistin susceptibility in carbapenem-resistant Klebsiella pneumoniae. Klin Lab Diagn. 2018;63(10):646–650. [DOI] [PubMed] [Google Scholar]
- 36.Chew KL, La MV, Lin RTP, et al. Colistin and Polymyxin B Susceptibility testing for carbapenem-resistant and mcr-Positive Enterobacteriaceae: comparison of Sensititre, MicroScan, Vitek 2, and Etest with Broth microdilution. J Clin Microbiol. 2017;55(9):2609–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Berglund B, Zhu Y, et al. Performance of different methods for testing polymyxin B: comparison of broth microdilution, agar dilution and MIC test strip in mcr-1 positive and negative Escherichia coli. Lett Appl Microbiol. 2021. [DOI] [PubMed] [Google Scholar]