Abstract
Diabetic kidney disease (DKD) is a common complication of diabetes, which frequently leads to end-stage renal failure and increases cardiovascular disease risk. Hyperglycemia promotes renal pathologies such as glomerulosclerosis, tubular hypertrophy, microalbuminuria, and a decline in glomerular filtration rate. Importantly, recent clinical data have demonstrated distinct sexual dimorphism in the pathogenesis of DKD in people with diabetes, which impacts both severity- and age-related risk factors. This study aimed to define sexual dimorphism and renal function in a nonobese type 2 diabetes model with the spontaneous development of advanced diabetic nephropathy (T2DN rats). T2DN rats at 12- and over 48-wk old were used to define disease progression and kidney injury development. We found impaired glucose tolerance and glomerular hyperfiltration in T2DN rats to compare with nondiabetic Wistar control. The T2DN rat displays a significant sexual dimorphism in insulin resistance, plasma cholesterol, renal and glomerular injury, urinary nephrin shedding, and albumin handling. Our results indicate that both male and female T2DN rats developed nonobese type 2 DKD phenotype, where the females had significant protection from the development of severe forms of DKD. Our findings provide further evidence for the T2DN rat strain’s effectiveness for studying the multiple facets of DKD.
Keywords: diabetic kidney disease, diabetic nephropathy, nonobese, sex differences
INTRODUCTION
Approximately 500 million adults have diabetes worldwide that results in over 4 million deaths every year (1–3). One of the major life-threatening outcomes of diabetes is diabetic kidney disease (DKD), which may progress to end-stage kidney disease (ESKD). DKD is characterized by a severe decline in renal function and drastic morphological changes to the kidney (fibrosis, glomerular injury, and hypertrophy). In diabetes, these changes are direct and indirect results of hyperglycemia (3, 4). Beyond this, chronic kidney disease (CKD) and diabetes are frequent comorbidities. Thus, in the last years, almost 25% of all patients with CKD in stages 3 and 4 are diagnosed with diabetes. The vast majority of diabetic cases are type 2 diabetes mellitus (T2DM) and account for up to 90% of adult-onset diabetes cases, and roughly 20% of these patients develop renal injury (3). Given the predominance of T2DM, the research into treatments and understanding the development of pathologies is paramount.
The progression of DKD in T2DM frequently follows two major pathways: proteinuric, which typically manifests as albuminuria, glomerulopathy, and other signs of diabetic nephropathy (DN); and the second pathway is non-proteinuric with predominant vascular and interstitial changes, increased blood pressure, and substantial decline in glomerular filtration rate (GFR) (5–7). The effect of gender and the development of DKD is rarely reported, and current conclusions on sexual dimorphism are somewhat contradictory. Some studies have found that women with diabetes die at higher rates from diabetes-related comorbidities, including DKD and cardiovascular complications (8, 9). Moreover, women with type 2 diabetes have a greater risk of developing fatal coronary heart disease (10). However, another study using a cohort of patients with DN found that despite increased risk for diabetes development, women of all ages were 28% less likely to develop DN than men. Despite the lower prevalence of DN, women in this cohort had a higher incidence of advanced DKD (11). The current literature reports a pronounced range in regard to DKD prevalence and severity in men and women, which can vary from distinct differences between the sexes to none at all (12–15). These conflicting results using different patient cohorts and disease parameters demonstrate the need to better understand sex differences in DKD.
Rodent models of diabetes are widely used in research and are valuable tools to delineate sexual dimorphism in DKD (16, 17). Similar to clinical observations, results in rodent models for the progression of DKD and cardiovascular risks vary. Recent reports show that the well-accepted DN model eNOS−/− db/db mice do not exhibit sex differences in renal structure or functional damage (18). In the obese metabolic syndrome diabetic model Zucker rat, where adipose tissue plays a major role in the regulation of glucose homeostasis and insulin sensitivity, obese females have a slightly higher death rate from renal complications and significantly lower cardiac-related mortality (19). Despite these findings, few experimental studies of sexual dimorphism in type 2 DKD have been performed. Here, we characterized sexual dimorphism, glucose homeostasis, and renal function in a model of nonobese type 2 diabetic rats with the spontaneous development of advanced diabetic nephropathy [Type 2 Diabetic Nephropathy (T2DN) rats]. This rodent model displays similar renal abnormalities and hyperglycemic levels seen in humans with the disease (20–23). Our results indicate that both sexes developed nonobese T2DM phenotypes, where females had significant protection from the development of severe forms of DKD. Using this model, we aim to delineate the sex-linked divergence in the severity and progression of type 2 diabetes-related kidney damage. The discoveries of this research offer an avenue for further understanding of the mechanisms and developing an optimal strategy for glycemic control and kidney protection in nonobese patients with diabetes.
METHODS
Ethical Statement
The research was conducted in full compliance, and strict accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Institutional Animal Care and Use Committees (IACUCs) of the Medical College of Wisconsin (#AUA00001061).
Experimental Protocol and Animals
Wistar (nondiabetic control) and Type 2 Diabetic Nephropathy (T2DN) animals of both sexes at different ages, 12- (young adult), or >48- (aged) wk-old rats were used for experiments. Wistar rats were purchased from Charles River Laboratories (12 wk old). T2DN rats had been inbred for multiple generations at the Medical College of Wisconsin. Animals were fed a normal salt diet (#5001, LabDiet, Purina) with water and food provided ad libitum. The aged groups of rats were retired breeders housed individually for at least 2 wk before all experimental procedures (Wistar and T2DN, male and female, n ≥ 6 per group). For urine collection, rats were placed in metabolic cages (#40615, Laboratory Products) for 24-h urine collection. These urine samples were used to determine electrolytes, microalbumin, creatinine, and nephrin levels. Whole blood and urine electrolytes were measured with a blood gas and electrolyte analyzer (ABL system 800 Flex, Radiometer, Copenhagen, Denmark). Screening for albuminuria (albumin/creatinine ratio) was performed by an assay kit acquired from Active Motif (Carlsbad, CA) and the creatinine assay kit from Cayman Chemical (Ann Arbor, MI). Plasma levels of albumin, globulin, cholesterol, Alanine Aminotransferase, and Alkaline Phosphatase (ALT and Alk.Ptase, correspondingly) were tested at Marshfield Labs (Marshfield, WI).
Glucose Tolerance Test and Insulin Tolerance Test
For both protocols, animals were fasted overnight, and all tests were performed in the 11 AM to 3 PM window (Wistar and T2DN, male and female, >48 (aged)-wk-old rats, n ≥ 3 per group). Glucose monitoring was performed on conscious animals using a Contour strip glucometer (Bayer, Mishawaka, IN). Blood samples were obtained by pricking the lateral tail vein. For both protocols glucose levels were checked at 15, 30, 60, 90, and 120 min post-dosing. Following the baseline glucose measurement, animals received an oral gavage (OGTT) of a 1.11 M glucose solution (5 mL/kg). For the insulin tolerance test, animals received an intraperitoneal injection of Humulin-R (1 unit/kg; Eli Lilly, IN).
Kidney Isolation
Rats were anesthetized (T2DN male and female, 12- (young adult) and >48 (aged)-wk-old rats, n ≥ 4 per group), and kidneys were flushed with PBS via aortic catheterization (6 mL/min/kidney until blanched). One kidney was placed in 10% formalin for histological analysis, and the other kidney was either flash-frozen or used for glomerular isolation.
Western Blot Analysis
Expression levels of sodium-glucose cotransporter 2 (SGLT2; #AGT-032, Alomone) and nephrin (#ab58968, Abcam) were detected by Western blot analysis of kidney cortex lysates as described previously (22), >48 (aged)-wk-old rats. Nephrin was also analyzed in urine samples.
Histological Analysis of Renal Injury
Rat kidneys were formalin-fixed, paraffin-embedded, sectioned, and mounted on slides as previously described (24). Slides were stained with Masson’s trichrome and used for the detection of medullary protein casts, interstitial cortical fibrosis, and glomerular damage. Protein cast analysis was performed with Metamorph (Molecular Devices, Sunnyvale, CA) software. Interstitial fibrosis was measured using manual color deconvolution filters (Fiji; ImageJ v1.51u). A convolutional neural network was used for the localization of glomeruli, and the glomerular injury score was assessed using semiquantitative morphometric analysis based on a scale of 0–4 as previously described (22, 25). Megalin abs were kindly provided by Dr. Franziska Theilig (University of Kiel, Germany) (26).
FITC-Inulin Measurement of Glomerular Filtration Rate
Assessment of GFR in conscious, ambulatory rats was performed using a modified version of a protocol described by Dr. Timo Rieg (27). Briefly, animals (Wistar and T2DN, male and female, >48 (aged)-wk-old rats, n ≥ 4 per group) are sedated and then receive a tail vein injection of 2% FITC-Inulin (TdB Consultancy AB, Uppsala, Sweden). The decay in the fluorescence of freely filtered FITC-Inulin is then tracked using a NanoDrop 3300 Fluorospectrometer (Thermo Fisher Scientific). GFR was calculated from the observed decrease in FITC fluorescence and corresponding inulin clearance from the blood. The decrease in FITC fluorescence was a product of the initial fast decay representing the redistribution of FITC-inulin from the intravascular compartment to the extracellular fluid, and the slower phase reflecting clearance from the blood. The differential rate law was used to evaluate changes in FITC concentration. GFR was estimated from the rate constant of the second-order reaction of FITC fluorescence changes.
Podocin Expression in Freshly Isolated Glomeruli
The detailed protocol was described previously (22). The glomeruli of >48 (aged)-wk-old rats were probed with Podocin primary antibody (1:500; #Ab50339, Abcam) and labeled with Alexa fluorophore secondary antibody (1:500, Thermo Fisher Scientific). For nuclei staining, glomeruli were incubated with 0.5 μg/mL Hoechst in PBS for 10 min at room temperature in the dark. Images obtained by the Nikon A1R inverted confocal microscope using a Plan Apo ×40 DIC M N2 objective with 0.95 numerical aperture controlled by Nikon Elements AR software (Nikon, Japan) and later processed with Fiji software.
Echocardiography
To examine the cardiac condition, transthoracic echocardiography (Vivid 7; GE Healthcare, Waukesha, WI) was used as previously described by Paterson et al. (28). Briefly, isoflurane-anesthetized rats (Wistar and T2DN, male and female, >48 (aged)-wk-old rats, n ≥ 3 per group) are screened for ventricular wall thickness, chamber dimensions, heart rate, and cardiac function (ejection fraction, cardiac output, and percentage of fractional shortening) using pulsed-wave Doppler echocardiography.
Microbiome Analysis
The cecal luminal content was obtained during the kidney isolation experiment described above (T2DN male and female, >48 (aged)-wk-old rats, n = 8 per group). Ceca were opened longitudinally, and contents were collected using a sterile spatula into sterile tubes. All tubes with samples were flash-frozen in liquid nitrogen and stored at −80°C until processing. Microbiome analysis was performed as previously described (29). Briefly, DNA of the cecal luminal content was extracted from each piece using QIAamp PowerFecal DNA Kit (Qiagen) followed by the manufacture’s protocol. For PCR library preparation, the V3 to V4 regions of the 16S rRNA gene were amplified following the Illumina User Guide using T100 thermal cycler (BioRad). The 10 pM denatured and diluted library was mixed with 15% PhiX control and loaded on Illumina MiSeq V3 flow cell kit with 2 × 300 cycles. Raw paired-end reads were merged to create consensus sequences and then quality filtered using USEARCH (30). Chimeric sequences were identified and filtered using Quantitative Insights Into Microbial Ecology (QIIME) software package (v. 1.9.1) (31) combined with the USEARCH algorithm. Operational taxonomic units were subsequently picked using QIIME combined with the USEARCH algorithm, and taxonomy assignment was performed using Greengenes (32) as the reference database. Alpha- and beta-diversity analyses were performed using QIIME. Chao1 was used as the algorithm to calculate the alpha-diversity. The analysis of similarities (ANOSIM) method was used to calculate the statistical significance of the beta-diversity. Taxonomic features with differential abundance were further summarized using linear discriminant analysis effect size (33) for group comparisons.
Statistics
Data are presented as box plots, where all data points are shown; the box denotes SE, error bars are standard deviation, and the horizontal line is the mean value. All data collection and analyses were performed in a blinded manner. Determination or exclusion of outliners within datasets was not applied. Most of the analyzed groups had more than six rats in each group; however, some groups (all numbers are included in figure legends) had a lower number of animals due to limited availability, especially for the aged group. Data were tested for normality (Shapiro–Wilk) and equal variance (Levene’s homogeneity test). Statistical analysis between sex groups consisted of one-way ANOVA. Two-way ANOVA was applied for comparisons between strain and sexes. P values of < 0.05 were considered significant. Tukey or Holm–Sidak multiple-comparisons adjustment were conducted only if the ANOVA F value was significant. SigmaPlot 12.5 or OriginPro 9.0 software was used to perform statistical tests.
RESULTS
Characterization of Glucose Tolerance and Insulin Sensitivity in T2DN Rats
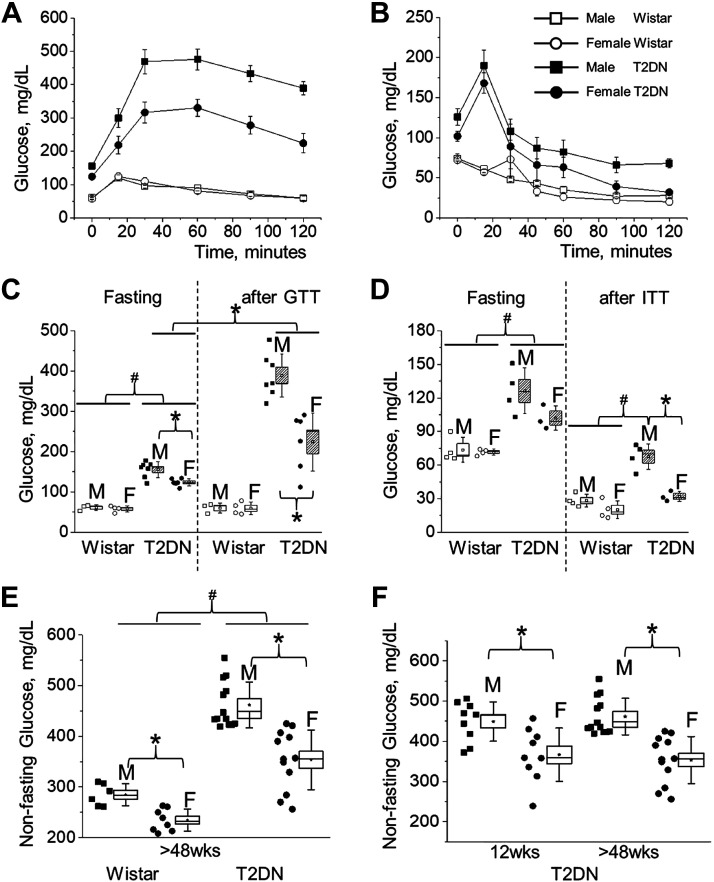
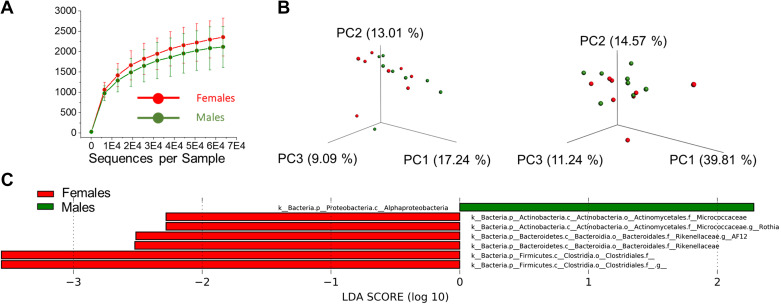
To characterize glucose tolerance of T2DN rats at the stage of fully developed DKD (over 48 wk of age), we performed a standard OGTT protocol. Age-matched male and female Wistar and T2DN rats’ blood glucose levels were checked at specified time points over the course of 2 h post glucose bolus. The T2DN rats had significantly elevated fasting glucose levels compared to Wistar animals. Moreover, fasting glucose in T2DN males was significantly higher than in females (61 ± 4 and 58 ± 4 vs. 156 ± 8 and 124 ± 4 mg/dL for Wistar vs. T2DN, male and female, correspondingly; Fig. 1, A and C). As expected, at 120 min after glucose loading, the blood glucose levels of Wistar rats decreased to the fasting levels. In contrast, levels failed to return to baseline in the T2DN rats. The results of the OGTT indicate the presence of type 2 diabetes (glucose intolerance) in both male and female T2DNs. Furthermore, the data demonstrates significantly impaired glucose clearance in males particularly (224 ± 30 vs. 389 ± 20 mg/dL in female vs. male; Fig. 1, A and C). The insulin tolerance test (ITT) gave a further depiction of the major differences in the diabetic phenotype between sexes. Insulin is synthesized by the pancreas and helps transport glucose into the body’s cells. In addition to hyperglycemia, insulin resistance (insensitivity) is a defining characteristic of type 2 diabetes (34). The injection of insulin produces a normal decline in blood glucose in the nondiabetic Wistar rats. Interestingly, both groups of T2DNs exhibit a delayed response to insulin injection. At the completion of the test, only the male T2DNs display insulin resistance (28 ± 3 and 20 ± 4 vs. 68 ± 6 and 32 ± 3 mg/dL for Wistar vs. T2DN, male and female, correspondingly; Fig. 1, B and D). Moreover, non-fasting glucose levels in Wistar and T2DN rats under similar dietary and housing conditions offered more prospective on type 2 diabetic phenotype in male versus female T2DNs (Fig. 1E). During the development of DKD, non-fasting glucose levels were unchanged in T2DN rats (12 vs. >48 wk old; Fig. 1F). This data suggests that female T2DNs better control their glycemic state as the disease progresses, whereas their male counterpart suffers a greater decline in glycemic control in a fed state. In addition, T2DN female rats can maintain the state of mild glucose intolerance by increasing insulin secretion, whereas male T2DN rats cannot due to severe insulin resistance. Serum analyses also show elevated levels of alkaline phosphatase (ALP) in both sexes, a measurement of liver damage, as well as high blood cholesterol in male subjects, which suggest the presence of insulin-resistant cells releasing a large amount of free fatty acids and possibly triggering cholesterol biosynthesis (35) (Table 1). Several studies have reported gut microbiome dysbiosis as a factor in the rapid progression of insulin resistance in T2DM (36). To further clarify gender-related variations in insulin sensitivity, the microbiome was analyzed to detect a possible contribution of gut microbiota. Even though alpha- and beta-diversities did not show an overall significant difference of gut microbiota between male and female T2DNs at >48 wk of age (Fig. 2, A and B), a few bacterial taxa were identified to be significantly enriched in either male or female group (Fig. 2C). For example, the bacterial class Alphaproteobacteria was more abundant in the male group, whereas the bacterial families, Micrococcaceae and Rikenellaceae, were more abundant in the female group (Fig. 2C). This analysis allows us to conclude that since there were no clear differences in the microbiota, the mechanisms for the observed sex differences are exclusively due to host factors.
Figure 1.
The severity of the diabetic phenotype in male and female T2DN rats. A: glucose tolerance test (GTT) over a 2-h period in Wistar and T2DN rats (oral gavage 1.11 M glucose; 5 mL/kg; >48 wk of age; AUC 100 ± 9 and 99 ± 11 vs. 483 ± 32 and 323 ± 31, male and female Wistar vs. T2DN; Wistar male vs. female n = 3 vs. 4; T2DN male vs. female n = 7 vs. 6). B: insulin tolerance test (ITT) over a 2-hr period in Wistar and T2DN rats (ip insulin; 1 U/kg of Humulin-R; >48 wk of age; AUC 100 ± 8 and 93 ± 16 vs. 238 ± 31 and 179 ± 30, male and female Wistar vs. T2DN; Wistar male vs. female n = 4 vs. 4; T2DN male vs. female n = 4 vs. 3). C and D: statistical summary of GTT and ITT tests. E and F: nonfasting blood glucose levels in males and females. E: Wistar and T2DN rats at >48 wk of age (Wistar male vs. female n = 6 vs. 8; T2DN male vs. female n = 13 vs. 12). F: T2DN animals at 12 and >48 wk of age (12 wk male vs. female n = 9 vs. 9; >48 wk male vs. female n = 13 vs. 12). *,#P < 0.05 between rat sex or strain groups, respectively. AUC, area under curve; T2DN, Type 2 Diabetic Nephropathy.
Table 1.
Serum tests for T2DN rats >48 wk of age
| T2DN >48 wk Group |
|||||
|---|---|---|---|---|---|
| Units | Males |
Females |
|||
| Mean | ± SE | Mean | ± SE | ||
| ALT (GPT) | U/L | 43.17 | ± 6.23 | 41.14 | ± 4.4 |
| Alk.Ptase | U/L | 157.83 | ± 13.07 | 138.00 | ± 5.37 |
| Total Protein | g/dL | 5.68 | ± 0.06 | 6.04* | ± 0.11 |
| Creatinine | mg/dL | 0.47 | ± 0.03 | 0.31* | ± 0.01 |
| Calcium | mmol/L | 9.72 | ± 0.12 | 9.50 | ± 0.10 |
| Sodium | mmol/L | 137.50 | ± 0.56 | 139.29 | ± 0.61 |
| Potassium | mmol/L | 4.18 | ± 0.21 | 3.40** | ± 0.05 |
| Chloride | mmol/L | 99.83 | ± 0.95 | 104.43 | ± 0.90 |
| Globulin | g/dL | 3.15 | ± 0.12 | 2.73** | ± 0.04 |
| Cholesterol | mg/mL | 261 | ± 46.7 | 87* | ± 3 |
| Blood pH | 7.46 | ± 0.02 | 7.43 | ± 0.02 | |
n ≥ 6 for each group. ANOVA *P < 0.05; **P < 0.005; Tukey post hoc P < 0.05. Bold indicates significant difference between values. ALT (GPT), alanine aminotransferase (glutamic-pyruvic transaminase); T2DN, Type 2 Diabetic Nephropathy.
Figure 2.
Summary of 16S rRNA analysis using cecal contents of T2DN females and males (n = 8 rats per group). A: average α-diversity of microbiota in T2DN females and males. All values are expressed as means ± SD. B: unweighted and weighted β-diversity of microbiota in T2DN females and males. C: LEfSe plot showing enriched bacterial taxa in T2DN females and males. Bacterial taxa with negative LDA scores (red) are enriched in the female group, whereas bacterial taxa with positive LDA scores (green) are enriched in the male group. Bacterial taxa, which have a score of linear discriminant analysis (LDA) greater than 2.0, was considered to be significantly differential between male and female groups. T2DN, Type 2 Diabetic Nephropathy.
Sexual Dimorphism in the Basic Renal Pathology
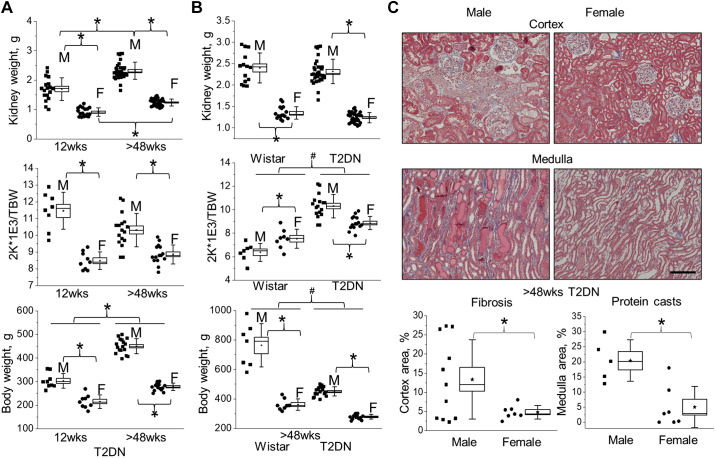
Commonly, obesity is considered the main cause of insulin resistance; however, insulin resistance in patients who are nonobese predicts a twofold greater risk for the development of DKD (37). There are limited data about insulin resistance in nonobese subjects due to the lack of research models. The male T2DN rat spontaneously developed diabetes and severe insulin resistance without any presence of obesity. As shown in Fig. 3B, Wistar rats have significantly higher body weight than the corresponding sex in T2DN. However, there are no significant differences in the kidneys’ weights between the strains. Female T2DN rats have lower kidney weight and corresponding kidney-to-body-weight ratio in comparison to males at the early stage of diabetes, and this trend is consistent with the further development of DKD and aging (Fig. 3A). Further histological assessment demonstrates a significant difference in the presence of tubulointerstitial fibrosis and medullar protein casts between male and female T2DN rats at >48 wk of age. As shown in Fig. 3C, the male T2DN rats have more pronounced fibrosis in the renal cortex and protein cast formation in the medulla. Blood and urine electrolyte analyses also demonstrate elevated levels of plasma creatinine and decreased electrolyte excretion in males (Tables 1 and 2).
Figure 3.
Sexual dimorphism in the progression of kidney injury in T2DN rats. A: the progression of diabetic renal hypertrophy, normalized two kidneys (2 K) per total body weight (TBW) ratio for male and female T2DN rats at 12 and >48 wk of age. B: the comparison in renal hypertrophy, normalized two kidneys (2 K) per total body weight (TBW) for Wistar and T2DN rats at >48 wk of age. Wistar male vs. female n = 7 vs. 8; T2DN male vs. female n = 17 vs. 15. 12 wk male vs. female n = 8 vs. 9; >48 wk male vs. female n = 17 vs. 15. C: representative images of cortical fibrosis/tubular injury and medullary protein cast formation in the kidneys of male and female T2DN rats (Masson’s trichrome staining; >48 wk of age). Scale bar = 100 µm. Below, summary graphs indicating differences in cortical fibrosis and medullary protein cast formation in male and female kidneys of T2DN rats (>48 wk of age). T2DN male vs. female n = 11 vs. 7, fibrosis; n = 5 vs. 7, protein cast. *P < 0.05 between male and female groups. T2DN, Type 2 Diabetic Nephropathy.
Table 2.
Urinary excretion of electrolytes and glucose tests for T2DN rats
| T2DN >48 wk Group |
|||||
|---|---|---|---|---|---|
| ANOVA |
Male |
Female |
|||
| P Value | Mean | ± SE | Mean | ± SE | |
| Potassium/Cre | 0.013 | 27.9 | ± 1.7 | 34.8 | ± 1.7 |
| Sodium/Cre | 0.037 | 11 | ± 1 | 19 | ± 3 |
| Calcium/Cre | NS | 0.16 | ± 0.02 | 0.17 | ± 0.06 |
| Chloride/Cre | 0.001 | 20 | ± 3 | 35 | ± 2 |
| Glucose/Cre | NS | 20 | ± 13 | 4 | ± 1 |
n ≥ 8 for each group; normality Test (Shapiro–Wilk); homogeneity of variance (Levene’s Test); ANOVA, Tukey post hoc P < 0.05. NS, nonsignificant values. Bold indicates significant difference between values. Cre, Creatinine; T2DN, Type 2 Diabetic Nephropathy.
Renal Filtration and Glomerular Pathology in Male and Female T2DN Rats
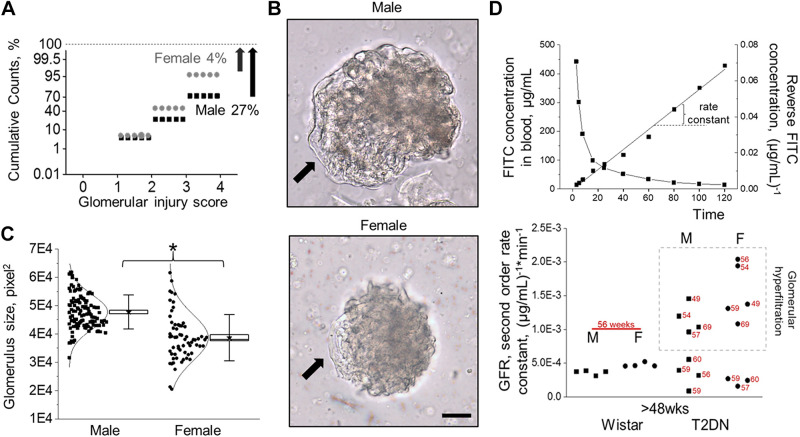
To evaluate sex differences in the DKD, we checked glomerular damage and renal filtration in male and female T2DN rats in late-stage T2DM. Using semiautomatic glomerular scoring, we quantified glomerular injury. Figure 4A shows the distribution of glomerular damage in T2DN rats (scored for injury on a 0 to 4 scale with 4 representing a severely sclerotic glomerulus). Overall, female rats had significantly lower glomerular pathology, with only a 4% probability of detecting severe, nonfunctional glomeruli compared with 27% in the male group. The severity of glomeruli damage in both sexes can be detected in bright-field microscopy. Figure 4B shows examples of male and female glomeruli. Male kidneys exhibit glomerular hypertrophy compared to females, although glomeruli from females have greater variability in size (Fig. 4C). Glomerular hypertrophy in DM is often associated with an increase in GFR. Plasma kinetics of FITC-inulin following a single-dose intravenous injection was used in diabetic and nondiabetic control groups to measure GFR in conscious animals. Wistar rats of both sexes had similar and consistent GFRs. In contrast, the distribution of GFR values shows significant variability in T2DN animals. GFRs in the T2DN group for both sexes appear to shift toward glomerular hyperfiltration, with some rats showing low GFR values compared with nondiabetic controls (Fig. 4D).
Figure 4.
Progression of glomerular injury in male vs. female T2DN rats. A: summary graph of analysis of glomerular damage in T2DN rats at >48 wk of age (>1,600 glomeruli per group; a Kolmogorov–Smirnov test was used to identify significant differences between male and female groups; P < 0.001). Note a significantly higher probability of a glomerulus score of 4 in males (27 vs. 4%; male vs. female). B: representative images (transmitted light) of male (top) and female (bottom) T2DN glomeruli at >48 wk of age. Arrows indicate areas with the loss of podocytes that enwrap the glomerular capillaries. Scale bar = 50 µm. C: the distribution of the glomerular size in T2DN rats at >48 wk of age. *P < 0.05 statistical significance between male and female groups. D: representative curve of FITC-Inulin decay in plasma samples of T2DN rat and corresponding differential rate law graph for the second-order reaction (top). GFR (rate constant) for Wistar and T2DN groups (bottom). The punctate box indicates animals demonstrating glomerular hyperfiltration. Each dot demonstrates an individual rat, and numbers indicate a specific animal’s age (in wk). GFR, glomerular filtration rate; T2DN, Type 2 Diabetic Nephropathy.
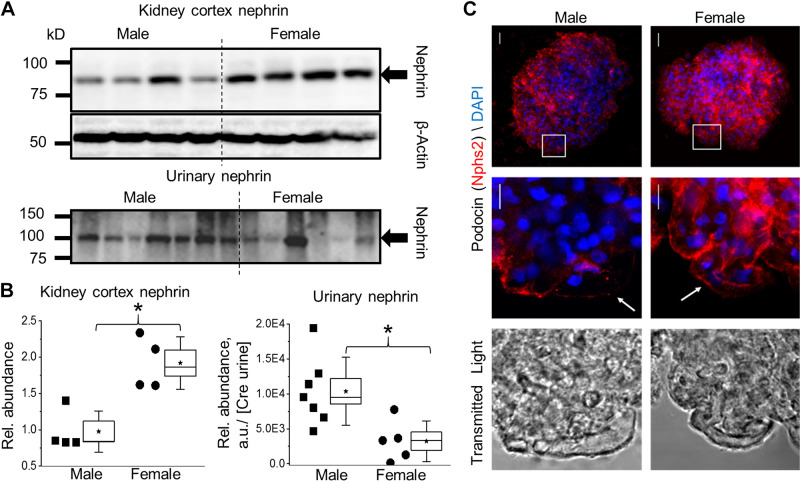
Podocyte function is essential for the integrity of the glomerular filtration barrier (GFB) and is directly involved in the regulation of GFRs. Nephrin (Nphs1) is a podocyte protein and a central component of the slit-diaphragm responsible for filtration. Nephrin abundance in urine (nephrin shedding) and kidney cortex expression was determined via Western blot to establish podocyte damage and structural changes that would impact GFB integrity (Fig. 5A). Assessing both cortical nephrin and urinary nephrin allows estimation of GFB damage; greater cortical nephrin is associated with less damage whereas greater urinary nephrin is related to greater glomerular damage. The expression of cortical nephrin was significantly higher in female T2DN rats, whereas urinary nephrin was elevated in males (Fig. 5B). Additionally, glomeruli from male and female aged T2DN rats were labeled with the podocyte marker podocin. Overall, the integrated fluorescent signal representing the total expression of podocin of the z-projection in males is reduced compared to females (Fig. 5C, top). Furthermore, the higher magnification images show a capillary loop on the glomerular exterior. Note the presence of capillaries, which almost entirely lack integrated foot processes (as demonstrated by loss of fluorescent signal), indicating a focal region with low podocin expression in both male and female T2DN glomerulus (Fig. 5C).
Figure 5.
Renal cortex and urinary markers of podocyte damage in male and female T2DN rats. A: Western blot analysis of nephrin, the major component in formation and maintenance of the podocyte slit diaphragm, in renal cortex and urine (nephrin shedding) in male and female T2DN rats at >48 wk of age. B: summary graph for Western blotting analyses represented in A. *P < 0.05 between male and female groups. C: representative images of isolated glomeruli labeled with the podocyte marker podocin (Nphs2; red). Top: 3-D structure projected into 2-D image showing total podocin in the glomerulus. Middle: single section of a z-stack indicating a focal region with low podocin expression in male and female T2DN glomerulus. Bottom: corresponding transmitted light image for the region of higher magnification. n ≥ 4 for all groups and analysis. T2DN, Type 2 Diabetic Nephropathy.
Albumin Handling and Proximal Tubules Function
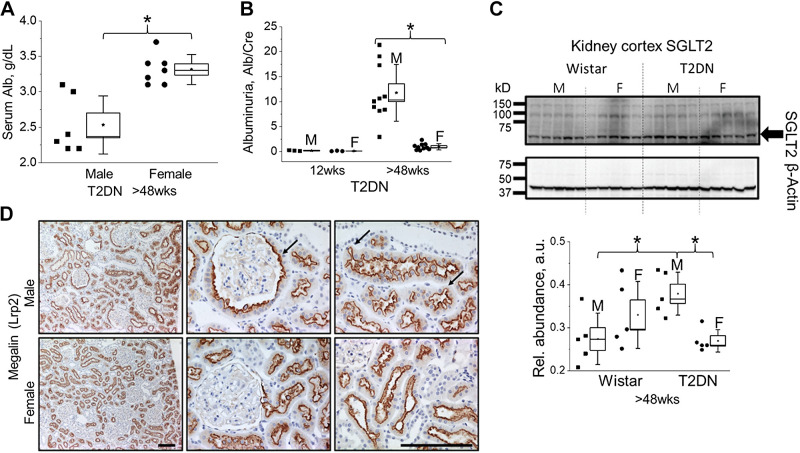
The loss of GFB integrity leads to increased filtered blood proteins and albumin in the urine. At the late stage of the disease, T2DN males exhibit a decline in serum albumin (Fig. 6A) and a significant elevation in albuminuria (Fig. 6B) compared to females (or age-matched Wistar male rats at the age of >48 wk; Alb/Cre ratio 1.8 ± 0.2, P < 0.05). To further test proximal tubule function, we assessed the expression of sodium-glucose cotransporter 2 (SGLT2). Overall, the expression of SGLT2 in T2DN males was significantly elevated compared with age-matched male Wistar (Fig. 6C). Recent studies suggest the higher expression of SGLT1 and SGLT2 in female rats under normal physiological conditions (17). However, as shown in Fig. 6C, SGLT2 expression was significantly lower in female aged T2DN rats, indicating the relation between the severity of hyperglycemia and sex difference in this model. The excessive amount of filtered albumin leads to proximal tubular injury and exacerbates the progression of DKD. Kidney sections labeled for megalin (Lrp2), a key proximal tubule apical transporter, demonstrate distinct pathological remodeling in T2DN rats. In males, there is an active proliferation of proximal tubule cells, resulting in invasion into the Bowman’s capsule and remodeling of the proximal tubule brush border to maximize reabsorptive surface area. In contrast, female T2DN rats showed the absence of distinct pathological changes in proximal tubules (Fig. 6D).
Figure 6.
Albumin handling and proximal tubule remodeling in male and female diabetic kidneys. A: Serum level of albumin in male and female T2DN rats at >48 wk of age. B: urinary albumin excretion shows the development of albuminuria in male at >48 wk of age. *P < 0.05 between male and female groups. C: kidney cortical tissue expression of SGLT2 in male vs. female Wistar and T2DN rats at >48 wk of age. Western blotting representation for probing of sodium-glucose cotransporter 2 (SGLT2) and its relative abundance normalized on β-actin used as a loading control. *P < 0.05 statistical significance between groups. n ≥ 5 for all groups and analysis. D: immunostaining for megalin (Lrp2) expression reveals active proliferation of proximal tubule cells into Bowman’s capsule of diabetic male rats (middle; marked by arrow). Left, megalin staining indicates remodeling of proximal tubule morphology in diabetic male rats (arrows). T2DN, Type 2 Diabetic Nephropathy.
Cardiac Phenotype in T2DN Rats
We further assessed potential cardiac abnormalities in the development of DKD in T2DN rats. Echocardiogram analysis of left ventricle wall thickness in systole, heart rate, cardiac output, ejection fraction, and fibrosis in the heart tissue showed no differences between diabetic and nondiabetic control strains (Table 3). There were no striking differences in cardiac status detected between male and female T2DN rats, which further support the direct impact of diabetes on the developed renal disease.
Table 3.
Assessment of cardiac status in male vs. female Wistar and T2DN rats (>48 wk of age)
| Wistar >48 wk Group |
T2DN >48 wk Group |
|||||||
|---|---|---|---|---|---|---|---|---|
| Male |
Female |
Male |
Female |
|||||
| Means | ± SE | Means | ± SE | Means | ± SE | Means | ± SE | |
| HWT/TBW | 2.4 | 0.1 | 3.5* | 0.1 | 3.5 | 0.1 | 4.2* | 0.1 |
| Heart Fibrosis, % area | 5.6 | 2.5 | 4.7 | 0.6 | 4.0 | 1.6 | 7.1 | 2.2 |
| VWTs, mm | 3.2 | 0.3 | 2.7 | 0.2 | 2.9 | 0.1 | 2.7 | 0.1 |
| HR, beats/min | 338 | 11 | 359 | 14 | 295 | 17 | 349* | 14 |
| CO (Teich) L/min | 0.43 | 0.05 | 0.32 | 0.04 | 0.34 | 0.03 | 0.27 | 0.02 |
| EF(Cube), % | 80.3 | 2.5 | 81.9 | 2.8 | 72.5 | 3.0 | 73.5 | 2.3 |
| Fractional shortening, % | 42.3 | 2.6 | 44.1 | 2.8 | 35.6 | 2.4 | 36.2 | 1.8 |
n ≥ 3 for all groups.*P < 0.05 between male and female groups. CO, cardiac output; EF, ejection fraction; HW/TBW, heart weights normalized to total body weight; HR, heart rate; T2DN, Type 2 Diabetic Nephropathy. VWTs, echocardiogram analysis of left ventricle wall thickness in systole.
DISCUSSION
The mechanisms initiating DKD are similar in men and women; however, marked variations exist at the molecular and tissue levels. These physiological differences between sexes likely contribute to the disease progression, prevalence, outcome, and response to treatment. Biological age, immune system, sex hormones, lifestyle, and environmental factors contribute to the complexity of our understanding and treatment of DKD. Animal research models are an important tool to decrease variability and modulate disease progression in a controlled environment to better understand sex differences in diabetes and associated DKD. The present study details the pathophysiological disparities between the sexes in the progression of the nonobese type 2 diabetic rat model with DN.
In the T2DN strain, hyperglycemia is detected before the presence of severe renal damage (12 wk of age) in both males and females. During late-stage DKD (>48 wk), females move sugar from the blood into tissues more efficiently, potentially due to less severe insulin resistance. The underlying sex difference mechanisms by which hyperinsulinemia, induced by insulin resistance, regulates cholesterol metabolism remains open and could be further explored using the T2DN model.
Men with T2DM have a greater prevalence for irregular fasting glucose levels than women; however, it has been reported that women more frequently have impaired glucose tolerance. Kautzky-Willer et al. in addition reported sex-based differences in insulin sensitivity where women had a greater reduction. Despite these differences in insulin sensitivity, both sexes had above normal insulin secretion (38). In the T2DN model, we found that male T2DN rats compared to nondiabetic Wistars and their female T2DN counterparts had a distinct elevation in their fasting glucose levels resembling the human population. In terms of overall glucose tolerance, female T2DNs had a better outcome compared to male T2DN rats. Interestingly, an Australian study found that at the conclusion of the 2-h OGTT in nondiabetics and diabetics, women had greater endpoint blood glucose levels (39). However, this study had a fixed glucose load that was given irrelevant to body mass (39, 40). In our testing, dosing was fixed for weight at 5 mL/kg to correct for the fact that females were lighter than the male rats for both strains. Another point is that though many have found women to have a greater incidence of glucose intolerance, most are observing changes during early stages of diabetes, combining type 1 and type 2 diabetes data, do not consider DN as a factor, or a combination of these points (39–41). Using the T2DN animals allowed for tighter control of these factors and showed more definitively whether or not females are at a far greater advantage than males for the diabetic phenotype.
We also found that female T2DN rats, although still displaying signs of pronounced hyperglycemia, had reduced non-fasting glucose levels in comparison to males. This data correlated well with the findings of Díaz et al., who showed that the type 2 diabetic Goto–Kakizaki (GK) rat has distinct differences in the glycemic levels between males and females. Female GK rats had remarkably stable blood glucose levels that ranged between 100 and 200 mg/dL from as young as 16 wk to well over 72 wk of age, whereas males began increasing in blood glucose levels at ∼37 wk. Interestingly, the increases in non-fasting blood glucose levels between the T2DN sexes show an age-related divergence, with only male T2DN rats having a sharp increase between 12 and >48 wk. Similarly, male GK rats consistently increased in blood glucose levels from 16 wk of age until euthanasia at 44–60 wk of age (42). Other studies have reported the protective effects of the female sex on pancreatic islets of insulin production/regulation in diabetic mouse and human models (43–45). These noticeable differences in glucose handling prompted a brief analysis of essential glucose handlers in type 2 diabetes, like SGLT2. A study utilizing human primary cultured proximal tubule epithelial cells found that patients with T2DM have significant increases in SGLT2 expression, which we also observe in the male T2DN rats (46). A key function of the kidney is the ability to filter blood. An increased GFR is a typical feature of patients with diabetes and plays a central role in the pathogenesis and progression of renal damage. Meta-analyses studies suggest that hyperfiltration accelerates renal damage and progression of albuminuria (47). We observed hyperfiltration in both sexes in T2DN rats, which was more prevalent in females. It should be noted that some animals of both sexes also had lower GFRs, which can suggest a transition from hyperfiltration to a substantial decline in kidney function that ultimately leads to hypofiltration. Indeed, already established glomerular disease and decline in GFR may reflect the reduced functional reserve in males. On the other hand, the protective phenotype in females may cause a delay in the progression of renal disease resulting in an observed GFR increase. In this case, males may have an increase in GFR earlier in life. The higher risk of renal dysfunction and decline in GFRs in a T2DM female cohort is often associated with cardiovascular problems or poor metabolic control (14, 48). Yet, T2DN rats do not exhibit cardiac abnormalities, and significant metabolic disturbances were only observed in the male group.
T2DN rats show glomerular damage in both sexes as the disease progresses from early to last stage DKD. Nevertheless, our findings show that female T2DN rats have a blunted development of renal damage compared to males, which in part could be explained by disparities in glucose handling and diabetes progression. The male T2DN rat kidneys have typical signs of DN, including drastic tubular atrophy, interstitial inflammation, global glomerulosclerosis, and arteriolar hyalinosis (22). In contrast, female T2DN rats have reduced glomerular damage and glomerular hypertrophy. This reduction in glomerular injury is further demonstrated in the reduced urinary nephrin shedding and increased cortical expression of nephrin in females relative to males. These changes directly reflect the sex differences in albumin handling, fibrosis, and pathological remodeling in proximal tubules. The proximal tubule morphology in male T2DN rats, revealed by the megalin staining, indicates leakage of proteins through injured glomeruli and excessive protein reabsorption, leading to tubular degeneration. Interestingly, that epithelial cells at the urinary pole of Bowman’s capsule undergo a transition to a mesenchymal phenotype and extend into the capsule. Proximal tubule hyper-reabsorption and impaired tubular transport are characteristic symptoms for patients with T2DM triggered by the additive interaction of hyperglycemia and albuminuria (49, 50). Several clinical studies have shown that type 2 diabetic men have higher albuminuria levels than women, correlating with our findings (51, 52). These studies additionally suggest that gender-based differences in albuminuria are age-related in T2DM males. Astoundingly like the human cohorts, female T2DN rats maintain reduced albuminuria at >48 wk of age that resemble their levels at 12 wk, a phenomenon not found in male T2DN rats, which showed an age-stimulated increase in albuminuria. Our observations suggest that females may have renoprotection; however, this may be the result of having a less severe diabetic phenotype.
As stated previously, the scientific literature is scattered with contradicting data regarding sexual dimorphism in DKD-associated pathologies and negative outcomes. Maric–Bilkan recently summarized the existing data reporting either a higher risk of DKD in men or women or no sex differences and described the impact of sex on DKD in type 1 and patients with type 2 DM (8). Much of this confusion can be attributed to the need to consider multiple variables when assessing original human population data sets. The majority of the studies did not consider the hormonal status (pre- or postmenopause) or whether female patients were undergoing hormone replacement (8, 12, 39, 53). Additionally, women with type 2 diabetes are still underrepresented in T2DM/DKD-related CVD studies, despite being at greater risk (54). Another potential complication is that T2DM often overlaps with obesity, which is also different between men and women and is often not considered when analyzing these datasets.
A recent perspectives article published as a follow-up of an NIDDK workshop entitled “Sex and the Kidneys” has stated that one of the critical needs and opportunities for the studies of sex differences in renal disease is leveraging existing animal models and developing novel ones (16). In this paper, we demonstrate that the T2DN rat displays sexual dimorphism and may be a highly useful model to understand more mechanisms underlying these differences in pathology development between sexes. Although no animal model is ideal, the T2DN rat resembles the human population on the severity of renal tissue damage, albuminuria, and hyperglycemia. Moreover, female T2DNs show a stunted disease progression relative to males, which can be exploited to further probe different physiological components underlying differences between males and females in T2DM.
GRANTS
This work was supported by the Department of Veteran Affairs Grant I01 BX004024 (to A.S.), National Institutes of Health Grants R35 HL135749, P01 HL116264 (to A.S.), R01 DK126720 (to O.P.), R01 HL143082 (to B.J.), T32 HL134643 (CVC A.O. Smith Fellowship to C.A.K.), NIDDK Diabetic Complications Consortium (RRID:SCR_001415, www.diacomp.org) Grants DK076169 and DK115255, and the American Heart Association Grant 18PRE34030127 (to D.R.S.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.R.S., O.P., and A.S. conceived and designed research; D.R.S., O.P., V.L., E.I., C.A.K., S.K., A.K., X.C., and J.-Y.Y. performed experiments; D.R.S., O.P., V.L., E.I., C.A.K., O.N., A.K., X.C., and J.-Y.Y. analyzed data; D.R.S., O.P., E.I., A.K., X.C., J.-Y.Y., B.J., and A.S. interpreted results of experiments; D.R.S., O.P., C.A.K., S.K., and O.N. prepared figures; D.R.S., O.P., and A.S. drafted manuscript; D.R.S., O.P., and A.S. edited and revised manuscript; D.R.S., O.P., V.L., E.I., C.A.K., S.K., O.N., A.K., X.C., J.-Y.Y., B.J., and A.S. approved final version of manuscript.
REFERENCES
- 1.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 12: 2032–2045, 2017. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuttle KR. Diabetic kidney disease. Adv Chronic Kidney Dis 25: 119–120, 2018. doi: 10.1053/j.ackd.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Zelnick LR, Weiss NS, Kestenbaum BR, Robinson-Cohen C, Heagerty PJ, Tuttle K, Hall YN, Hirsch IB, de Boer IH. Diabetes and CKD in the United States population, 2009–2014. Clin J Am Soc Nephrol 12: 1984–1990, 2017. doi: 10.2215/CJN.03700417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad I, Zelnick LR, Batacchi Z, Robinson N, Dighe A, Manski-Nankervis JE, Furler J, O'Neal DN, Little R, Trence D, Hirsch IB, Bansal N, de Boer IH. Hypoglycemia in people with type 2 diabetes and CKD. Clin J Am Soc Nephrol 14: 844–853, 2019. doi: 10.2215/CJN.11650918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Lee K, Ni Z, He JC. Diabetic kidney disease: challenges, advances, and opportunities. Kidney Dis (Basel) 6: 215–225, 2020. doi: 10.1159/000506634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopel J, Pena-Hernandez C, Nugent K. Evolving spectrum of diabetic nephropathy. World J Diabetes 10: 269–279, 2019. doi: 10.4239/wjd.v10.i5.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robles NR, Villa J, Gallego RH. Non-proteinuric diabetic nephropathy. J Clin Med 4: 1761–1773, 2015. doi: 10.3390/jcm4091761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maric-Bilkan C. Sex differences in diabetic kidney disease. Mayo Clin Proc 95: 587–599, 2020. doi: 10.1016/j.mayocp.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Maric-Bilkan C. Sex differences in micro- and macro-vascular complications of diabetes mellitus. Clin Sci (Lond) 131: 833–846, 2017. doi: 10.1042/CS20160998. [DOI] [PubMed] [Google Scholar]
- 10.Cobo G, Hecking M, Port FK, Exner I, Lindholm B, Stenvinkel P, Carrero JJ. Sex and gender differences in chronic kidney disease: progression to end-stage renal disease and haemodialysis. Clin Sci (Lond) 130: 1147–1163, 2016. doi: 10.1042/CS20160047. [DOI] [PubMed] [Google Scholar]
- 11.Yu MK, Lyles CR, Bent-Shaw LA, Young BA; Pathways Authors. Risk factor, age and sex differences in chronic kidney disease prevalence in a diabetic cohort: the pathways study. Am J Nephrol 36: 245–251, 2012. doi: 10.1159/000342210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Hauteclocque A, Ragot S, Slaoui Y, Gand E, Miot A, Sosner P, Halimi JM, Zaoui P, Rigalleau V, Roussel R, Saulnier PJ, Hadjadj Samy S; The SURDIAGENE Study group. The influence of sex on renal function decline in people with type 2 diabetes. Diabet Med 31: 1121–1128, 2014. doi: 10.1111/dme.12478. [DOI] [PubMed] [Google Scholar]
- 13.Hanai K, Babazono T, Yoshida N, Nyumura I, Toya K, Hayashi T, Bouchi R, Tanaka N, Ishii A, Iwamoto Y. Gender differences in the association between HDL cholesterol and the progression of diabetic kidney disease in type 2 diabetic patients. Nephrol Dial Transplant 27: 1070–1075, 2012. doi: 10.1093/ndt/gfr417. [DOI] [PubMed] [Google Scholar]
- 14.Kajiwara A, Kita A, Saruwatari J, Miyazaki H, Kawata Y, Morita K, Oniki K, Yoshida A, Jinnouchi H, Nakagawa K. Sex differences in the renal function decline of patients with type 2 diabetes. J Diabetes Res 2016: 4626382, 2016. doi: 10.1155/2016/4626382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA 290: 1884–1890, 2003. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 16.Bairey Merz CN, Dember LM, Ingelfinger JR, Vinson A, Neugarten J, Sandberg KL, Sullivan JC, Maric-Bilkan C, Rankin TL, Kimmel PL, Star RA; participants of the National Institute of D, Digestive, Kidney Diseases Workshop on “Sex and the Kidneys. Sex and the kidneys: current understanding and research opportunities. Nat Rev Nephrol 15: 776–783, 2019. doi: 10.1038/s41581-019-0208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shepard BD. Sex differences in diabetes and kidney disease: mechanisms and consequences. Am J Physiol Renal Physiol 317: F456–F462, 2019. doi: 10.1152/ajprenal.00249.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Y, Li W, Shotorbani PY, Dubansky BH, Huang L, Chaudhari S, Wu P, Wang LA, Ryou M-G, Zhou Z, Ma R. Comparison of diabetic nephropathy between male and female eNOS−/− db/db mice. Am J Physiol Renal Physiol 316: F889–F897, 2019. doi: 10.1152/ajprenal.00023.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson PR, Stern JS, Horwitz BA, Harris RE Jr, Greene SF. Longevity in obese and lean male and female rats of the Zucker strain: prevention of hyperphagia. Am J Clin Nutr 66: 890–903, 1997. doi: 10.1093/ajcn/66.4.890. [DOI] [PubMed] [Google Scholar]
- 20.Kojima N, Williams JM, Takahashi T, Miyata N, Roman RJ. Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Ther 345: 464–472, 2013. doi: 10.1124/jpet.113.203869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nobrega MA, Fleming S, Roman RJ, Shiozawa M, Schlick N, Lazar J, Jacob HJ. Initial characterization of a rat model of diabetic nephropathy. Diabetes 53: 735–742, 2004. doi: 10.2337/diabetes.53.3.735. [DOI] [PubMed] [Google Scholar]
- 22.Palygin O, Spires D, Levchenko V, Bohovyk R, Fedoriuk M, Klemens CA, Sykes O, Bukowy JD, Cowley AW Jr, Lazar J, Ilatovskaya DV, Staruschenko A. Progression of diabetic kidney disease in T2DN rats. Am J Physiol Renal Physiol 317: F1450–F1461, 2019. doi: 10.1152/ajprenal.00246.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spires D, Poudel B, Shields CA, Pennington A, Fizer B, Taylor L, McPherson KC, Cornelius DC, Williams JM. Prevention of the progression of renal injury in diabetic rodent models with preexisting renal disease with chronic endothelin A receptor blockade. Am J Physiol Renal Physiol 315: F977–F985, 2018. doi: 10.1152/ajprenal.00182.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Pochynyuk OM, Jacob HJ, Geurts AM, Hodges MR, Staruschenko A. Essential role of Kir5.1 channels in renal salt handling and blood pressure control. JCI insight 2: e92331, 2017. doi: 10.1172/jci.insight.92331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bukowy JD, Dayton A, Cloutier D, Manis AD, Staruschenko A, Lombard JH, Solberg Woods LC, Beard DA, Cowley AW Jr.. Region-based convolutional neural nets for localization of glomeruli in trichrome-stained whole kidney sections. J Am Soc Nephrol 29: 2081–2088, 2018. doi: 10.1681/ASN.2017111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grahammer F, Ramakrishnan SK, Rinschen MM, Larionov AA, Syed M, Khatib H, Roerden M, Sass JO, Helmstaedter M, Osenberg D, Kühne L, Kretz O, Wanner N, Jouret F, Benzing T, Artunc F, Huber TB, Theilig F. mTOR regulates endocytosis and nutrient transport in proximal tubular cells. J Am Soc Nephrol 28: 230–241, 2017. doi: 10.1681/ASN.2015111224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rieg T. A high-throughput method for measurement of glomerular filtration rate in conscious mice. J Vis Exp 75: e50330, 2013. doi: 10.3791/50330.[23712131] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paterson MR, Geurts AM, Kriegel AJ. miR-146b-5p has a sex-specific role in renal and cardiac pathology in a rat model of chronic kidney disease. Kidney Int 96: 1332–1345, 2019. doi: 10.1016/j.kint.2019.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakraborty S, Mandal J, Cheng X, Galla S, Hindupur A, Saha P, Yeoh BS, Mell B, Yeo JY, Vijay-Kumar M, Yang T, Joe B. Diurnal timing dependent alterations in gut microbial composition are synchronously linked to salt-sensitive hypertension and renal damage. Hypertension 76: 59–72, 2020. doi: 10.1161/HYPERTENSIONAHA.120.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26: 2460–2461, 2010. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 31.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336, 2010. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72: 5069–5072, 2006. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol 12: R60, 2011. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 37, Suppl 1: S81–S90, 2014. doi: 10.2337/dc14-s081. [DOI] [PubMed] [Google Scholar]
- 35.Simonen PP, Gylling HK, Miettinen TA. Diabetes contributes to cholesterol metabolism regardless of obesity. Diabetes Care 25: 1511–1515, 2002. doi: 10.2337/diacare.25.9.1511. [DOI] [PubMed] [Google Scholar]
- 36.Sharma S, Tripathi P. Gut microbiome and type 2 diabetes: where we are and where to go? J Nutr Biochem 63: 101–108, 2019. doi: 10.1016/j.jnutbio.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Owei I, Umekwe N, Provo C, Wan J, Dagogo-Jack S. Insulin-sensitive and insulin-resistant obese and non-obese phenotypes: role in prediction of incident pre-diabetes in a longitudinal biracial cohort. BMJ Open Diabetes Res Care 5: e000415, 2017. doi: 10.1136/bmjdrc-2017-000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev 37: 278–316, 2016. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sicree RA, Zimmet PZ, Dunstan DW, Cameron AJ, Welborn TA, Shaw JE. Differences in height explain gender differences in the response to the oral glucose tolerance test—the AusDiab study. Diabetic Med 25: 296–302, 2008. doi: 10.1111/j.1464-5491.2007.02362.x. [DOI] [PubMed] [Google Scholar]
- 40.Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol Behav 187: 20–23, 2018. doi: 10.1016/j.physbeh.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Genugten RE, Utzschneider KM, Tong J, Gerchman F, Zraika S, Udayasankar J, Boyko EJ, Fujimoto WY, Kahn SE; American Diabetes Association GENNID Study Group. Effects of sex and hormone replacement therapy use on the prevalence of isolated impaired fasting glucose and isolated impaired glucose tolerance in subjects with a family history of type 2 diabetes. Diabetes 55: 3529–3535, 2006. doi: 10.2337/db06-0577. [DOI] [PubMed] [Google Scholar]
- 42.Díaz A, López-Grueso R, Gambini J, Monleón D, Mas-Bargues C, Abdelaziz KM, Viña J, Borrás C. Sex differences in age-associated type 2 diabetes in rats-role of estrogens and oxidative stress. Oxid Med Cell Longev 2019: 6734836, 2019. doi: 10.1155/2019/6734836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. Estrogens protect pancreatic β-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA 103: 9232–9237, 2006. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Louet J-F, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep 6: 180–185, 2004. doi: 10.1007/s11883-004-0030-9. [DOI] [PubMed] [Google Scholar]
- 45.Mauvais-Jarvis F. Are estrogens promoting immune modulation and islet protection in type 1 diabetes? J Diabetes Complications 31: 1563–1564, 2017. doi: 10.1016/j.jdiacomp.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 54: 3427–3434, 2005. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 47.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 52: 691–697, 2009. doi: 10.1007/s00125-009-1268-0. [DOI] [PubMed] [Google Scholar]
- 48.Earle KA, Ng L, White S, Zitouni K. Sex differences in vascular stiffness and relationship to the risk of renal functional decline in patients with type 2 diabetes. Diab Vasc Dis Res 14: 304–309, 2017. doi: 10.1177/1479164116687237. [DOI] [PubMed] [Google Scholar]
- 49.Chevalier RL. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol Renal Physiol 311: F145–F161, 2016. doi: 10.1152/ajprenal.00164.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 300: R1009–R1022, 2011. doi: 10.1152/ajpregu.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clotet S, Riera M, Pascual J, Soler MJ. RAS and sex differences in diabetic nephropathy. Am J Physiol Renal Physiol 310: F945–F957, 2016. doi: 10.1152/ajprenal.00292.2015. [DOI] [PubMed] [Google Scholar]
- 52.Parving HH, Gall MA, Skøtt P, Jørgensen HE, Løkkegaard H, Jørgensen F, Nielsen B, Larsen S. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int 41: 758–762, 1992. doi: 10.1038/ki.1992.118. [DOI] [PubMed] [Google Scholar]
- 53.Maric C, Sullivan S. Estrogens and the diabetic kidney. Gend Med 5, Suppl A: S103–113, 2008. doi: 10.1016/j.genm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Salameh A, Chanson P, Bucher S, Ringa V, Becquemont L. Cardiovascular disease in type 2 diabetes: a review of sex-related differences in predisposition and prevention. Mayo Clin Proc 94: 287–308, 2019. doi: 10.1016/j.mayocp.2018.08.007. [DOI] [PubMed] [Google Scholar]