Keywords: constipation, fecal retention, mechanical stress, narcotic bowel syndrome, visceral sensitivity
Abstract
Constipation and abdominal pain are commonly encountered in opioid-induced bowel dysfunction (OBD). The underlying mechanisms are incompletely understood, and treatments are not satisfactory. As patients with OBD often have fecal retention, we aimed to determine whether fecal retention plays a pathogenic role in the development of constipation and abdominal pain in OBD, and if so to investigate the mechanisms. A rodent model of OBD was established by daily morphine treatment at 10 mg/kg for 7 days. Bowel movements, colonic muscle contractility, visceromotor response to colorectal distention, and cell excitability of colon-projecting dorsal root ganglion neurons were determined in rats fed with normal pellet food, or with clear liquid diet. Morphine treatment (Mor) reduced fecal outputs starting on day 1, and caused fecal retention afterward. Compared with controls, Mor rats demonstrated suppressed muscle contractility, increased neuronal excitability, and visceral hypersensitivity. Expression of cyclooxygenase-2 (COX-2) and nerve growth factor (NGF) was upregulated in the smooth muscle of the distended colon in Mor rats. However, prevention of fecal retention by feeding rats with clear liquid diet blocked upregulation of COX-2 and NGF, restored muscle contractility, and attenuated visceral hypersensitivity in Mor rats. Moreover, inhibition of COX-2 improved smooth muscle function and fecal outputs, whereas anti-NGF antibody administration attenuated visceral hypersensitivity in Mor rats. Morphine-induced fecal retention is an independent pathogenic factor for motility dysfunction and visceral hypersensitivity in rats with OBD. Liquid diet may have therapeutic potential for OBD by preventing fecal retention-induced mechanotranscription of COX-2 and NGF.
NEW & NOTEWORTHY Our preclinical study shows that fecal retention is a pathogenic factor in opioid-induced bowel dysfunction, as prevention of fecal retention with liquid diet improved motility and attenuated visceral hyperalgesia in morphine-treated animals by blocking expression of cyclooxygenase-2 and nerve growth factor in the colon.
INTRODUCTION
Opioid analgesics are the mainstay for treating moderate-to-severe pain, especially cancer pain (1–4); their use has escalated over the past 2 decades (5–8). The number of prescriptions for opioids has increased from 76 million in 1991 to 207 million in 2013 in the United States alone (1, 4). The United States consumes more than 80% of the world’s total opioid supplies (3, 4). Unfortunately, the use of opioids is commonly associated with opioid-induced bowel dysfunction (OBD), including constipation, nausea, bloating, and abdominal pain (9–11). Among these, opioid-induced constipation (OIC) is the most common; it is present in up to 90% of opioid users (7, 8). Moreover, repeated use or large doses of opioid analgesics may result in opioid-induced abdominal pain (OAP) or hyperalgesia. Tuteja et al. (11) reported that chronic abdominal pain is experienced in 58% of patients who took opioids for 10 days or longer. When abdominal pain becomes severe, and worsens with continued or escalating dosages of narcotics, it is diagnosed as narcotic bowel syndrome (NBS) (11–13). Current treatments such as laxatives, secretogogues, antidepressants, and anxiolytics for OIC and OAP are not very satisfactory (9–11, 13–15). Although peripheral opioid antagonists were highly anticipated for their utility in OBD, recent studies showed that a majority of patients remain constipated despite treatment with methylnaltrexone or naloxegol (16, 17). Because of intolerable OBD, one-third of patients eventually choose to discontinue or decrease the use of opioid analgesics for pain management (1, 7, 10–12). To develop effective or alternative therapies for OBD, further investigation into the underlying mechanisms of constipation and abdominal pain in OBD is needed.
Current theories for mechanisms of OIC focus on opioid receptors [i.e., μ-opioid receptor (MOR)] in the enteric nervous system (ENS) and the enteric neuronal-mediated blockade of secretomotor function in the gut (9, 15, 18). Activation of MOR was shown to inhibit enteric neurotransmitter release to slow intestinal transit and decrease mucosa secretion (15, 18). These changes may account for the initiation of constipation in OIC. However, constipation persists long after tolerance to opioid-associated analgesia and other effects have been developed (5, 7, 15). What accounts for the sustained effect on constipation is not clear. On the other hand, central nervous system is the focus of putative mechanisms for opioid-induced hyperalgesia and abdominal pain (3, 13, 14). Grunkemeier et al. (13) proposed that activation of excitatory antianalgesic pathways within a bimodal opioid regulation system, descending facilitation of pain at the rostral ventral medulla, and glia cell activation in the dorsal horn may account for the enhanced pain perception in chronic opioid users. However, preclinical studies found that sensitization of peripheral sensory neurons is associated with opioid-induced visceral hyperalgesia (19, 20). The mechanisms underlying peripheral sensitization in opioid-induced visceral hyperalgesia are not well understood.
It is noteworthy that OBD is a functional condition, as it is not associated with any well-recognized physical abnormalities. However, fecal retention with bowel distention is an obvious symptom in OIC (5, 7). Clinical evidence showed that bowel distention associated with fecal retention, partial obstruction, and pseudo-obstruction is also very common in OAP and NBS (12, 13). In the original description of NBS, Sandgren et al. (12) reported that all five patients with NBS demonstrated features of intestinal pseudo-obstruction with prolonged use of opioids. In the clinical observation by Grunkemeier et al. (13), three out of five NBS cases had objective evidence (radiographic) or clinical symptoms of bowel distention or pseudo-obstruction. Importantly, chronic lumen distention, i.e., fecal retention, partial obstruction, or pseudo-obstruction, represents a circumferential mechanical stress to the gut wall (21–23), and has been proposed as a common cause in functional bowel disorders (24). Previous studies, in vitro and in vivo, demonstrated that mechanical stress is a potent stimulus to induce expression of proinflammatory and pain mediators such as cyclo-oxygenase-2 (COX-2) and nerve growth factor (NGF) in gut smooth muscle cells (SMC) (22, 25). We hypothesize that fecal retention (mechanical stress)-induced upregulation of COX-2 and NGF may play a critical role in neuromuscular dysfunctions in the colon, contributing to constipation and abdominal pain in OBD. We found that morphine treatment led to marked fecal retention in a rodent model of OBD, when rats were fed with regular pellet food. However, fecal retention was prevented if rats were fed exclusively with clear liquid diet. We determined colonic motor function, visceral sensitivity, and colon-projecting sensory neuron excitability in vehicle-treated and morphine-treated rats fed with either pellet food or liquid diet. We also sought to determine the pathogenic role of fecal retention-induced COX-2 and NGF in the development of motility dysfunction and visceral hypersensitivity in the OBD model.
METHODS
Rodent Model of OBD and Experimental Protocols
Sprague-Dawley male rats aged 8–10 wk (Harlan Sprague-Dawley, Indianapolis, IN) were used for the study. The rats were housed in a controlled environment (22°C, 12-h light-dark cycle) and always allowed food and water ad libitum unless stated otherwise. The Institutional Animal Care and Use Committee at the University of Texas Medical Branch approved all procedures performed on the animals.
A rat model of OBD was established as previously described by daily subcutaneous injection (sc) of morphine sulfate (Mor, Hikma, Eatontown, NJ) in slow-release emulsion at 10 mg/kg for 7 days (19) (Fig. 1). Vehicle control rats (Veh) were treated only with the emulsion (50% liquid paraffin and 50% Arlacel A, Sigma, St. Louis, MO) (19). Rodent pellet food LM-485 (Harlan, Indianapolis, IN) was used as a regular food. For the groups fed a clear liquid diet, the regular pellet food was removed 1 day before Veh or Mor treatment, and rats were given bowel cleanser PEG 3350 (GoLYTELY, Braintree, MA) for overnight, and then were fed ad lib with liquid diet Ensure Clear (Abbott Nutrition, Lake Forest, IL), and kept in wire-bottomed cages. All rats had free access to water at all time. In some experiments involving in vivo inhibition of COX-2 activity, Veh or Mor rats were treated with COX-2 inhibitor NS-398 (Cayman Chemical, Ann Arbor, MI) at 10 mg/kg intraperitoneally (ip) in 250 µL of 20% DMSO (22, 26). In experiments involving neutralization of NGF, rats were treated with anti-NGF antibody (R&D Systems, Minneapolis, MN) at 20 µg/kg ip daily (25).
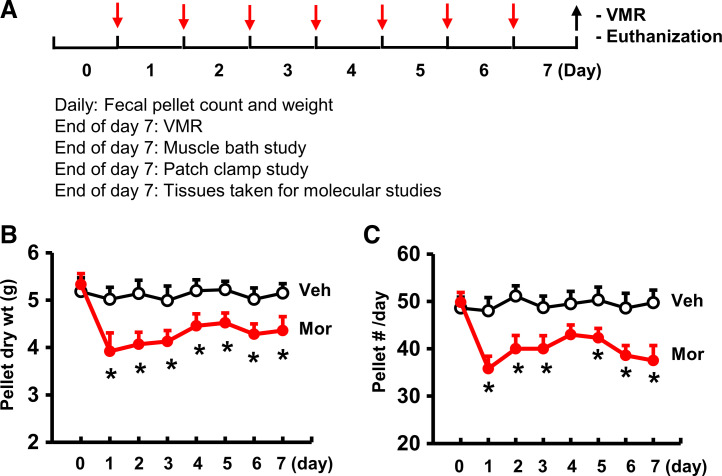
Figure 1.
Rodent model of opioid-induced bowel dysfunction. A: experimental protocol. Red arrows indicate daily vehicle or morphine treatment (10 mg/kg sc daily). Fecal pellet count and weight were measured daily starting 1 day before vehicle or morphine treatment. At the end of day 7 (black arrows), some rats were tested for visceromotor response (VMR), and others were euthanized for muscle bath, patch-clamp, and molecular studies. B and C: opioid-induced constipation. Morphine treatment reduced fecal outputs immediately and led to constipation afterward. Both daily fecal weight (B) and pellet number (C) were decreased in morphine-treated rats (Mor) compared with vehicle control rats (Veh). N = 6 rats in each group. *P < 0.05 vs. Veh.
Tissue Isolation
The 5 cm-long distal colon (starting 2 cm from the anus) was collected and placed immediately in carbogenated Krebs buffer (in mmol/L: 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1 NaH2PO4, 1.2 MgCl2, 11 d-glucose, and 25 NaHCO3). The colonic mucosa/submucosa (M/S) and muscularis externa (ME) layers were separated by microdissection as described previously (22, 27–29). The tissues were snap-frozen in liquid nitrogen and stored at −80°C until further work. In some experiments, the fresh ME tissues were used for the measurement of muscle contractility.
RNA Preparation and Quantitative RT-PCR
Total RNA was extracted from the colon muscularis externae samples using the Qiagen RNeasy kit (Qiagen, Valencia, CA), and reverse transcribed with SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA) (23, 25, 29, 30). Real-time quantitative RT-PCR was performed using the Bio-Rad CFX96 Real-Time PCR system (Hercules, CA), as described previously. The TaqMan probes for detection of rat COX-2 (Rn00568225-m1) and NGF (Rn01533872m) mRNAs were purchased from Invitrogen (23, 25, 29, 30). The fold-change relative to control was calculated with the comparative CT (ΔΔCT) method with endogenous reference 18S rRNA (Part no. 4352930E, Applied Biosystems) as the normalizer.
Protein Extraction and Western Blotting
Whole cell protein was extracted from the colon ME samples. The tissues were homogenized on ice in lysis buffer (Cat. No. 9806S, Cell Signaling Technology, Danvers, MA) supplemented with protease inhibitor cocktails (Sigma Aldrich, St. Louis, MO). After spinning at 12,000 g at 4°C for 15 min, the supernatant proteins were collected and resolved by a standard immunoblotting method (22, 25, 29, 31). In brief, protein samples in equal quantity (20 µg) were run on premade 4%–12% Bis-Tris SDS-PAGE (Invitrogen, Carlsbad, CA). The proteins were transferred from the gel to the membrane. After being blocked with the Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE), the membrane was incubated with primary antibodies to COX-2 (1:1,000, Cayman Chemical, Ann Arbor, MI) (22, 23), or β-actin (loading control, 1:5,000, Sigma, St. Louis, MO) (22, 23) at 4°C overnight. Then, the membrane was incubated with secondary anti-rabbit antibody (1:2,000, Invitrogen, Carlsbad, CA) and anti-mouse antibody (1:10,000, Invitrogen, Carlsbad, CA) for detection of COX-2 and β-actin, respectively, at room temperature for 1 h (22). The imaging detection and analysis were done using Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
Enzyme Immunoassay
Colonic ME tissue was homogenized in cold PBS buffer (in mmol/L: 137 NaCl, 2.7 KCl, 10 Na2HPO4, KH2PO4, pH 7.4) supplemented with protease inhibitors. NGF or prostaglandin E2 (PGE2) in the extraction was measured with the enzyme immunoassay kits purchased from R&D Systems (Minneapolis, MN) and Cayman Chemical (Ann Arbor, MI), respectively, by following the manufacturers’ protocols (22, 25, 32).
Immunohistochemistry Study
Immunohistochemical staining of COX-2 and NGF protein was performed on formalin-fixed, paraffin-embedded colon segments (3 to 4 cm from the anus) isolated from rats with sham and morphine treatment (7 day), as described previously (22, 25). Sections at 4 µm thickness were blocked with 5% normal goat serum in PBS for 20 min at room temperature, and incubated with the rabbit anti-COX-2 antibody (1:200, Cayman Chemical, MI) or anti-NGF antibody (1:200, Santa Cruz Biotech, CA), and a biotin-conjugated anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA) (22, 25). After being incubated with avidin-biotin complex (Vector kit, Vector Laboratories), the sections were stained in diaminobenzidine tetrahydrochloride with 0.03% hydrogen peroxide. As negative controls, sections of the same specimens were processed by the same method but omitting the anti-COX-2 or anti-NGF primary antibody.
Measurement of Visceromotor Response
Visceral sensitivity was measured by electromyographical (EMG) measurements of visceromotor response (VMR) to colorectal distention (CRD) as described previously (23). Briefly, two electrodes were implanted in the external oblique muscle and externalized behind the head. Rats were allowed 1 wk to recover from the surgery. Under mild sedation with 2% isoflurane, a balloon (5 cm) was inserted 7 cm into the distal colon via the anus and held in place by taping the tubing to the tail. Rats were placed in a container and allowed to adapt for 30 min, and then CRD was performed by rapidly inflating the balloon to constant pressure. Pressure was measured via a sphygmomanometer connected to a pressure transducer. The balloon was inflated to various pressures (20, 30, 40, 50, 60, and 80 mmHg) for a 20-s stimulation period followed by a 2-min rest. EMG was recorded continuously during the experiment with a Biopac System EMG 100 C (Biopac Systems, Goleta, CA). EMG signals were amplified (5,000×), filtered with a 1-Hz high-pass filter and a 500-Hz low-pass filter, and digitized by use of Acknowledge (Biopac Systems). The area under the curve (AUC) for the EMG signal during each 20 s of distention was calculated by use of an in-house-written computer program (23). The net value for each distention was calculated by subtracting the baseline value derived from the AUC for the 20 s predistention period.
Labeling of Colon-Specific Sensory Neurons in DRG
Colon-specific neurons in the dorsal root ganglia (DRG) were labeled for patch-clamp recordings by injecting 1,1′-dioleyl-3,3,3′,3-tetramethylindocarbocyanine methane sulfonate (DiI, invitrogen, Carlsbad, CA) into the colon wall as described previously (23, 25, 29, 30). In brief, animals were anesthetized by 2% isoflurane with an E-Z anesthesia vaporizer. After a midline laparotomy, 1 µL of DiI (50 mg/mL in methanol) was injected into 10 sites on the exposed distal colon (∼5 cm in length). Animals were returned to normal housing and were treated for Mor or Veh before euthanasia for patch-clamp recordings ∼7–10 days after DiI injection.
DRG Neuron Dispersion and Patch-Clamp Study
Isolation of DRG neurons from adult rats has been described previously (23, 25, 29, 30). Briefly, rats were euthanized by decapitation. The spinal column was removed and transferred to ice-cold, oxygenated fresh dissecting solution containing (in mmol/L): 130 NaCl, 5 KCl, 2 KH2PO4, 1.5 CaCl2, 6 MgSO4, 10 glucose, and 10 HEPES, pH 7.2 (osmolarity, 305 mosM). Thoracolumbar DRG (T13–L2) were obtained bilaterally. The ganglia were digested in dissecting solution containing collagenase D (∼1.5 mg/mL; Roche, Indianapolis, IN) and trypsin (∼1.2 mg/mL; Sigma, St. Louis, MO) at 34.5°C for 1.5 h. The DRG samples were washed in enzyme-free solution and triturated repetitively with glass pipettes to obtain single-cell suspension. Cells were plated onto acid-cleaned glass coverslips and perfused with normal external solution containing (in mmol/L): 130 NaCl, 5 KCl, 2 KH2PO4, 2.5 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH adjusted to 7.4 (osmolarity, ∼295–300 mosM). DiI-labeled neurons (bright red) were identified by the fluorescence microscope (Olympus, Tokyo, Japan) with a rhodamine filter (excitation 546 mm, barrier filter at 580 mm). Whole cell current and voltage were recorded by a Dagan 3911 patch-clamp amplifier (Dagan, Minneapolis, MN) (23, 25, 29, 30). The currents were filtered at ∼2–5 kHz and sampled at 50 or 100 µs per point. To obtain rheobase values, a series of stimulation currents (300 ms in duration, 5 pA in steps) were injected to induce action potentials. Data were acquired and analyzed by pCLAMP 9.2 (Axon Instruments, Sunnyvale, CA).
Muscle Bath Experiments
Distal colon was opened along the mesenteric border, and pinned flat in a Petri dish with Sylgard base in carbogenated Krebs buffer. The mucosa/submucosa layers were separated and discarded by microdissection. The smooth muscle strips (3 mm × 10 mm) were mounted along the circular muscle orientation in individual muscle baths (Radnoti Glass, Monrovia, CA) filled with 10 mL of carbogenated Krebs solution at 37°C. The contractile activity was recorded as previously described (22, 27, 32, 33) with isometric force transducers and amplifiers (Grass Instruments) connected to Biopac data acquisition system (Biopac Systems, Goleta, CA). The muscle strips were equilibrated in the muscle bath under 1 g tension for 60 min at 37°C before they were tested for contractility. Muscle contractility was tested in response to acetylcholine (ACh; 10−6 to 10−2 M) and to KCl (62.5 mM). The strips were washed after the test of each concentration of ACh and KCl, and left to equilibrate for 15 to 20 min before another addition of different concentration of ACh or KCl. When the contractile response to KCl was tested, the concentration of KCl (62.5 mM) in the Krebs buffer was increased by the equimolar replacement of NaCl. The contractile response was quantified as the increase in area under contractions (AUC) during 4 min after the addition of ACh or KCl over the baseline AUC during 4 min before the addition of ACh or KCl.
Statistical Analysis
Data points are expressed as means ± SE, unless otherwise specified. Statistical analysis was performed by analysis of variance with nonrepeated measures by Student–Newman–Keuls test for comparisons of multiple groups, or by Kruskal–Wallis test followed by Dunn’s for nonparametric multiple comparisons, if data in a group does not follow normal distribution. Student’s t test was used for comparisons of two groups. A P value of ≤ 0.05 was considered statistically significant.
RESULTS
Morphine Treatment Led to Fecal Retention in the Distal Colon
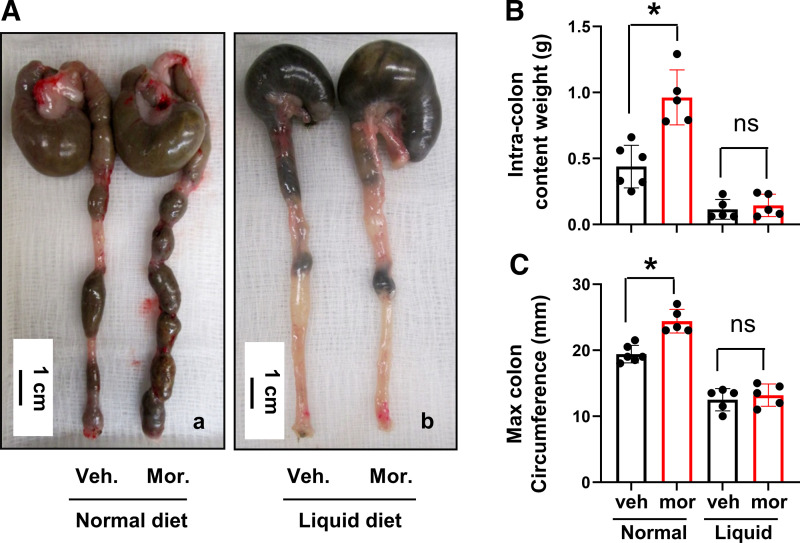
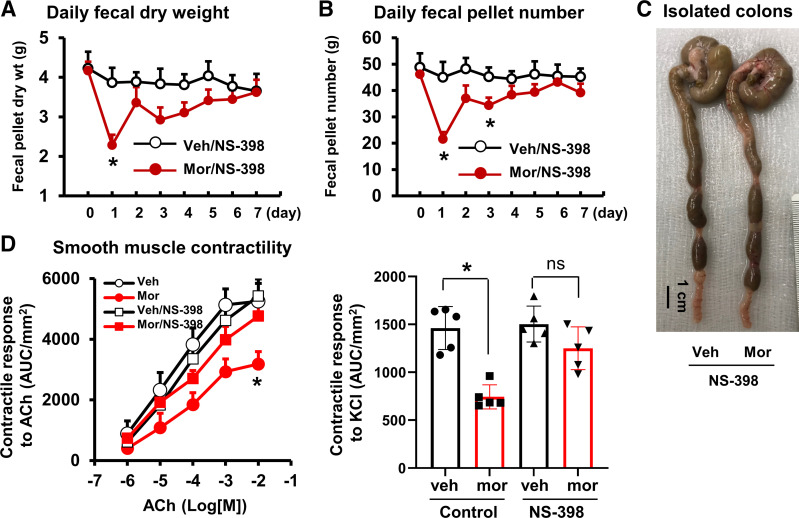
Morphine treatment significantly reduced fecal outputs in rats (Fig. 1), consistent with previous findings in humans (6, 9) and rodents (34, 35). The daily fecal dry weight and pellet number decreased dramatically starting on day 1, and throughout the 7-day period of morphine treatment (Fig. 1, A–C). The reduction of fecal output was associated with apparent fecal retention (Fig. 2A). As measured on day 7, the fecal content in the distal colon (5 cm) was significantly increased and maximal colon circumference was enlarged (Fig. 2, B and C). These changes indicate that the distal colon was subject to significant mechanical stress in the morphine-treated (Mor) rats, as in patients (5, 7, 12).
Figure 2.
Morphine-induced fecal retention in the distal colon: effect of colon cleansing. Although morphine treatment reduced fecal outputs, it increased fecal retention in the colon, leading to colon distention (Aa). The fecal content in the distal colon (5 cm) was significantly increased (B) and maximal colon circumference was enlarged (C). However, when normal pellet food was removed, and rats were treated with colon cleanser on day 0 and fed exclusively a clear liquid diet afterward (liquid diet), fecal retention (Ab) and fecal retention-associated mechanical stress in the colon (B and C) were prevented. Data are represented as means ± SD. N = 5 or 6 rats in each group. *P < 0.05 vs. Veh. ns: P > 0.05. ns, nonsignificant; Veh, vehicle control rats.
Morphine Treatment Led to Impairment of Colon Smooth Muscle Contractility
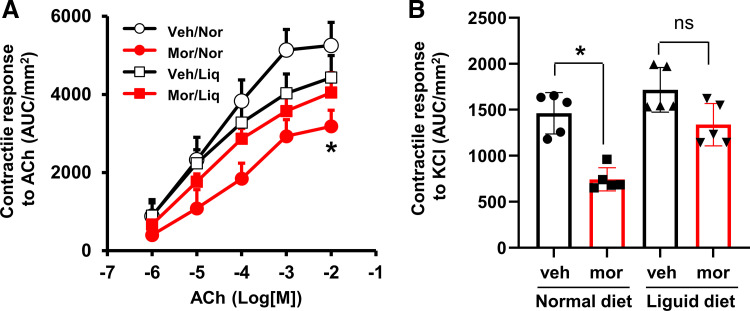
It is known that morphine treatment slows colon transit via opioid receptors on the enteric nervous system (15, 18). Whether gut smooth muscle function is affected by chronic treatment of morphine is not well known. We determined the contractility of circular smooth muscle isolated from the distal colon as previously described (22). The contractile response of colon circular muscle to cholinergic activation by acetylcholine (ACh; 10−7 to 10−2 M) was decreased significantly in Mor rats, with the maximal response of 3,185 ± 408 AUC/mm2 in Mor compared with 5,254 ± 590 AUC/mm2 in vehicle controls (Veh; P < 0.05) (Fig. 3A). It seems that morphine treatment did not change the potency of cholinergic receptors, as the EC50 values were not significantly changed between Veh [2.8 (±1.0) × 10−5 M] and Mor rats [3.8 (±1.3) × 10−5 M, P > 0.05]. The smooth muscle contractile response to cell membrane depolarization with a high concentration of KCl (62.5 mM) was also decreased significantly by nearly 50% in the Mor rats (Fig. 3B). Earlier studies by others and ourselves (36–38) revealed that ACh-induced and KCl-induced contractile response mainly tests gut smooth muscle contractility, as blocking ENS activity with tetrodotoxin (TTX) pretreatment does not significantly change ACh-induced or KCl-induced response. These data suggest that the contractility of colonic smooth muscle is suppressed by chronic treatment of morphine. Impairments of colonic smooth muscle contractile activity may well contribute to motility dysfunction (32, 39) in OIC.
Figure 3.
Effect of morphine treatment on contractile response of colon circular smooth muscle to acetylcholine (A) and to membrane depolarization by KCl (62.5 mM) (B) in rats fed with normal pellet diet and clear liquid diet. When rats were fed normal pellet food, the contractility of colon circular muscle decreased significantly (*P < 0.05 vs. Veh) in Mor rats (day 7). However, when rats were fed a liquid diet and the colon was cleansed, the contractile response was not different between Veh and mor rats. N = 5 rats in each group. Liq, liquid diet; Mor, morphine-treated rats; Nor, normal pellet diet; Veh, vehicle control rats.
Morphine Treatment Increased Visceral Sensitivity
We next assessed the effect of chronic morphine treatment on visceral sensitivity by recording visceromotor response (VMR) of the rats in response to colorectal distention (23). Electromyogram recording of the abdominal muscle contractions found that morphine-treated rats showed a significantly heightened visceromotor response to graded colorectal distensions compared with controls (Fig. 4A).
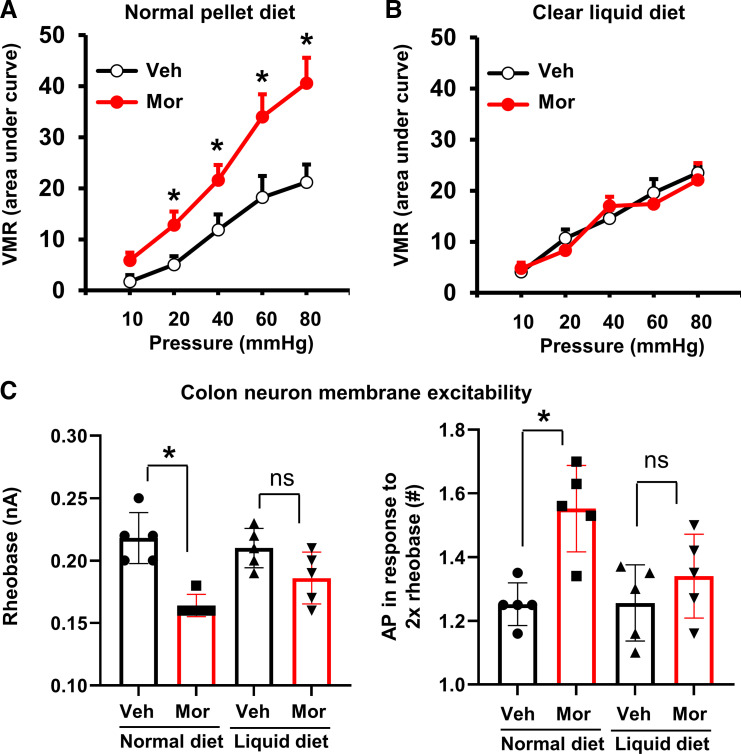
Figure 4.
Opioid-induced visceral hypersensitivity in rats: effect of colon cleansing. The visceromotor response (VMR) was increased significantly by morphine treatment (7 days) in rats when fed a normal pellet food diet (A), but not in rats fed a clear liquid diet (B). Patch-clamp study (C) found that the colon-projecting DRG neurons demonstrated hyperexcitability in Mor rats with decreased rheobase (left) and increased number of action potential in response to 2× rheobase (right), when rats were fed normal pellet food. However, colon cleansing attenuated morphine-induced neuronal hyperexcitability. N = 5 rats in each group, with ∼17–20 neurons recorded for each group in patch-clamp study. *P < 0.05 vs. normal/Veh. P > 0.05. DRG, dorsal root ganglia; Mor, morphine-treated rats; NS, no significant difference; Veh, vehicle control rats.
Furthermore, patch-clamp studies (23, 25) found that the colon-projecting DRG neurons (colon neurons, ∼20–28 µm in diameter) demonstrated an abnormal hyperexcitability in Mor rats with decreased rheobase (0.22 ± 0.02 nA vs. 0.16 ± 0.01 nA, P < 0.01) and increased number of action potential in response to 2× rheobase (1.25 ± 0.07 vs. 1.56 ± 0.09, P < 0.01) (Fig. 4C). The resting membrane potential (RP) changed from 54.9 ± 0.23 mV in Veh to 51.8 ± 0.46 mV in Mor rats (P < 0.05). The cell capacitance (43.4 ± 1.16 pF and 44.1 ± 0.44 pF in Veh and Mor, respectively, P > 0.05) and input resistance (548 ± 22.5 MΩ vs. 488 ± 20.1 MΩ, P > 0.05) were not significantly changed between Veh and Mor groups. These data suggests that visceral hypersensitivity in the OBD model is associated with peripheral sensitization of the colon neurons.
Liquid Diet Prevented Fecal Retention and Mechanical Stress in Morphine-Treated Rats
To determine if morphine-treatment-associated fecal retention plays an independent role in neuromuscular dysfunction in the rodent model of OBD, we developed the following procedure to cleanse the colon and prevent fecal retention in rats. Regular pellet diet (LM-485, Harlan, Indianapolis, IN) was removed 1 day before Veh or Mor treatment, and rats were given bowel cleanser GoLYTELY overnight. Rats were then fed ad lib a liquid diet of Ensure Clear and kept in wire-bottomed cages with free access to water throughout the 7-day experimental period. Liquid diet has been used effectively as exclusive enteral nutrition in the management of Crohn’s disease (40, 41). Indeed, this procedure of colon cleansing left fewer or no residue feces in the colon in both Veh and Mor rats (Fig. 2A). It effectively prevented fecal retention and colon distention in the morphine-treated rats, as both the intracolon content and max colon circumference were decreased to nearly the same level as in the control rats (Fig. 2, B and C).
Liquid Diet Restores Smooth Muscle Contractility and Visceral Sensitivity Affected by Morphine Treatment
We then measured colon smooth muscle contractility in the liquid-diet-treated rats and found that the muscle contractility to ACh or KCl was not significantly different between Mor and Veh rats when the bowel was cleansed (Fig. 3, A and B). The maximal response to ACh (4,432 ± 562 AUC/mm2 and 4,047 ± 510 AUC/mm2 for Veh and Mor, respectively, P > 0.05) and EC50 values [2.2 (±1.1) × 10−5 M and 2.9 (±1.7) × 10−5 M, P > 0.05] were not significantly different in the Veh and Mor groups when rats were fed with liquid diet.
Colon cleansing also attenuated visceral hypersensitivity associated with chronic morphine treatment. As shown in Fig. 4B, morphine treatment did not significantly increase visceromotor response to colon distention when rats were kept in clear liquid diet. Furthermore, when the colon is cleansed, the colon-projecting DRG neurons of the morphine-treated rats demonstrated a nearly similar level of membrane excitability as in the vehicle-treated rats (Fig. 4C).
Effect of Liquid Diet on Expression of COX-2 and NGF in the Colon
COX-2 and NGF are well recognized in suppressing gut smooth muscle contractility and increasing visceral sensitivity, respectively, in inflammatory or obstructive disorders in the gut (22, 25, 42–44). More importantly, expression of COX-2 and NGF in gut smooth muscle are highly sensitive to mechanical stress in vivo and in vitro (22, 25). To determine specifically if mechanotranscription of COX-2 and NGF is involved in mediating neuromuscular dysfunction in chronic use of morphine, we then determined mRNA and protein expression of COX-2 and NGF in the colon of control and Mor rats fed with either regular pellet food or liquid diet.
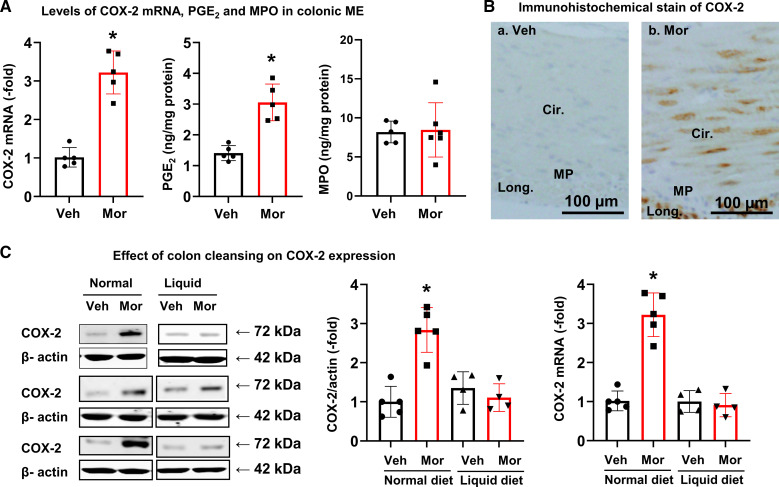
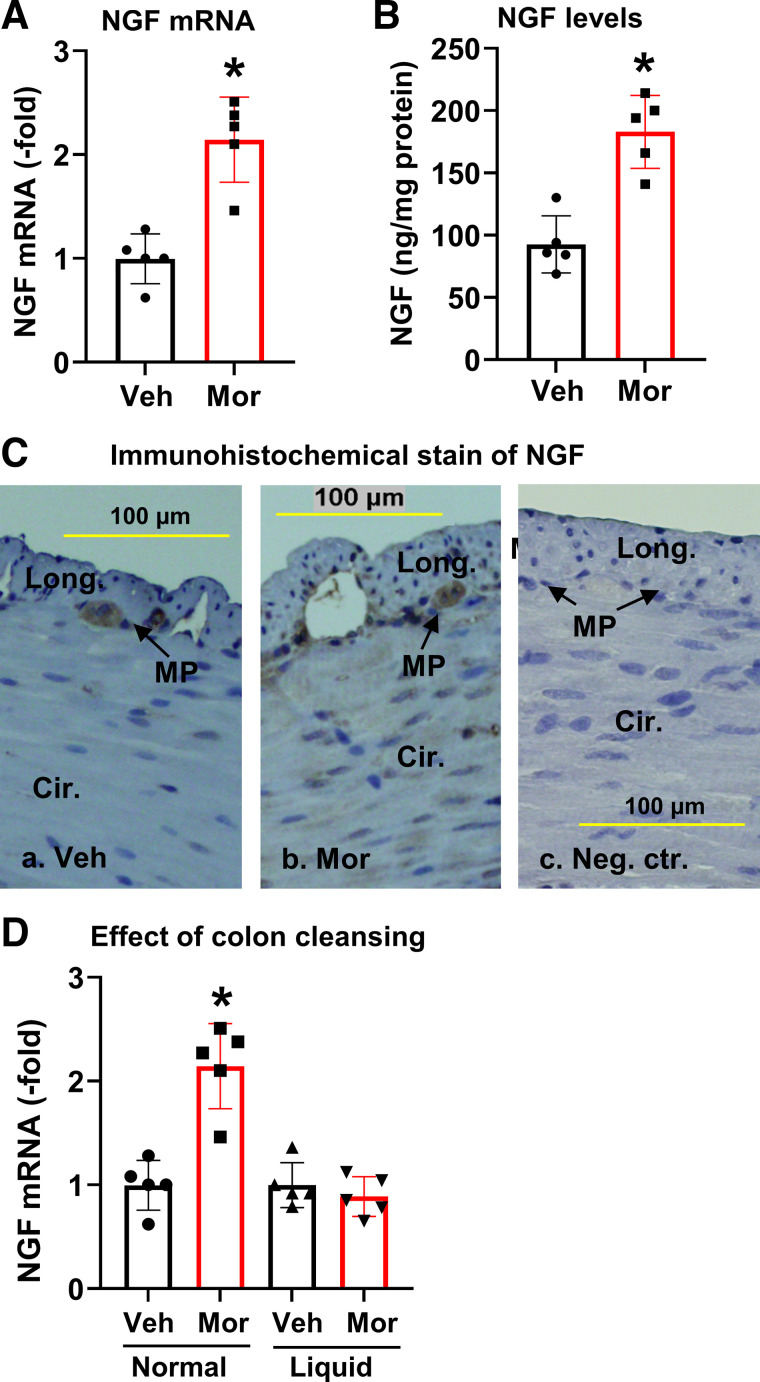
When rats were fed a regular pellet diet, COX-2 mRNA expression was significantly increased in the colonic muscularis externae in Mor rats (Fig. 5A). COX-2 expression was upregulated by 3.2 (± 0.53)-fold (P < 0.05) in the muscularis externae of distended colon in Mor rats compared with Veh controls. This is associated with increased PGE2 in the tissue. However, the myeloperoxidase (MPO) levels in colonic muscularis externae were not changed, indicating that there was no clear inflammation in the Mor tissue (Fig. 5A). Further immunohistochemical study found that COX-2 expression was increased mainly in the smooth muscle cells of the distended colon in Mor tissue (Fig. 5B). Expression of NGF mRNA and protein was also increased in the colonic muscularis externae in the morphine-treated rats (Fig. 6, A and B), with the NGF mRNA level increased by 1.9 (± 0.38)-fold compared with controls. Normally, NGF is detectable only in the myenteric plexus, but not in smooth muscle cells (Fig. 6Ca). However, its expression was increased in the colonic smooth muscle cells in morphine-treated rats (Fig. 6Cb). Negative control without primary antibody shows no immunostaining in either myenteric plexus or smooth muscle cells (Fig. 6Cc).
Figure 5.
Expression of COX-2 in colonic smooth muscle in morphine-treated rats. A: COX-2 mRNA expression and PGE2 production are increased in muscularis externae of the distended distal colon in Mor rats compared with Veh. However, MPO levels are not different between Veh and Mor groups. B: immunohistochemical stain showed that expression of COX-2 (stained in brown) is increased in SMC in Mor rats. C: however, when colon was cleansed (rats on a clear liquid diet), the expression of COX-2 protein and mRNA was not different between Mor and Veh rats. Data are expressed as means ± SD. N = 4 or 5 rats in each group. *P < 0.05 vs. Veh. Cir, circular smooth muscle; COX-2, cyclo-oxygenase-2; Long, longitudinal smooth muscle; Mor, morphine-treated rats; MP, myenteric plexus. MPO, myeloperoxidase; NS, no significant difference; SMC, smooth muscle cells; Veh, vehicle control rats.
Figure 6.
Expression of NGF in colonic smooth muscle in morphine-treated rats. Morphine treatment increased NGF mRNA expression (A) and protein levels (B) in muscularis externae tissue of the distal colon in Mor rats compared with Veh. C: immunohistochemical stain of colonic muscularis externae showed that NGF expression (stained in brown) is detectable in MP in Veh rats (a), and expression of NGF is increased in SMC in Mor rats (b). In the negative control, where no primary antibody was added, no immunoreactivity was detected in either MP or SMC (c). D: colon cleansing with liquid-diet treatment blocked morphine-induced upregulation of NGF expression. Data are expressed as means ± SD. N = 5 rats in each group. *P < 0.05 vs. Veh. Cir, circular smooth muscle; Long, longitudinal smooth muscle; Mor, morphine-treated rats; MP, myenteric plexus; NGF, nerve growth factor; SMC, smooth muscle cells; Veh, vehicle control rats.
When the colon was cleansed with liquid-diet treatment, morphine-induced upregulation of COX-2 protein and mRNA (Fig. 5C) and NGF expression (Fig. 6D) was almost completely blocked. These results suggest that upregulation of COX-2 and NGF in the colon of Mor rats is a mechanosensitive process and is induced by fecal retention-associated mechanical stress.
Inhibition of COX-2 and NGF Attenuated the Impacts of Morphine Treatment on Colonic Motor Function and Visceral Sensitivity
To further determine if fecal retention-associated upregulation of COX-2 contributes to the sustained motility dysfunction in morphine-treated rats, we administered COX-2 inhibitor NS-398 (22, 26) in control and Mor rats fed regular pellet food. Figure 7, A and B, shows the daily fecal output data with the use of COX-2 inhibitor in the vehicle control and morphine-treated rats. NS-398 did not affect morphine-induced decrease of fecal output on day 1. However, it almost completely blocked the fecal output changes with morphine-treatment afterward (Fig. 7, A and B) and improved fecal accumulation in the colon (Fig. 7C). Studies of the colon circular muscle strips in vitro found that the smooth muscle contractility was largely restored with COX-2 inhibition in the morphine-treated rats (day 7) (Fig. 7D). The maximal contractile response was 5,425 ± 562 and 4,767 ± 512 AUC/mm2 (P > 0.05), and the EC50 values were 3.7 (±1.5) × 10−5 M and 2.2 (±1.1) × 10−5 M (P > 0.05) in Veh and Mor rats, respectively, when administered with NS-398.
Figure 7.
Inhibition of COX-2 improves colon motor function in morphine-treated rats. Daily administration of COX-2 inhibitor NS-398 (10 mg/kg ip) did not affect morphine-induced decrease of fecal outputs on day 1 (A and B), but improved fecal outputs afterward (A–C). The colonic smooth muscle contractility in response to ACh (left) and KCl (right) was also improved with NS-398 treatment in the morphine-treated rats (day 7) (D). N = 5 or 6 rats in each group. *P < 0.05 vs. Veh. ACh, acetylcholine; COX-2, cyclo-oxygenase-2; Veh, vehicle control rats.
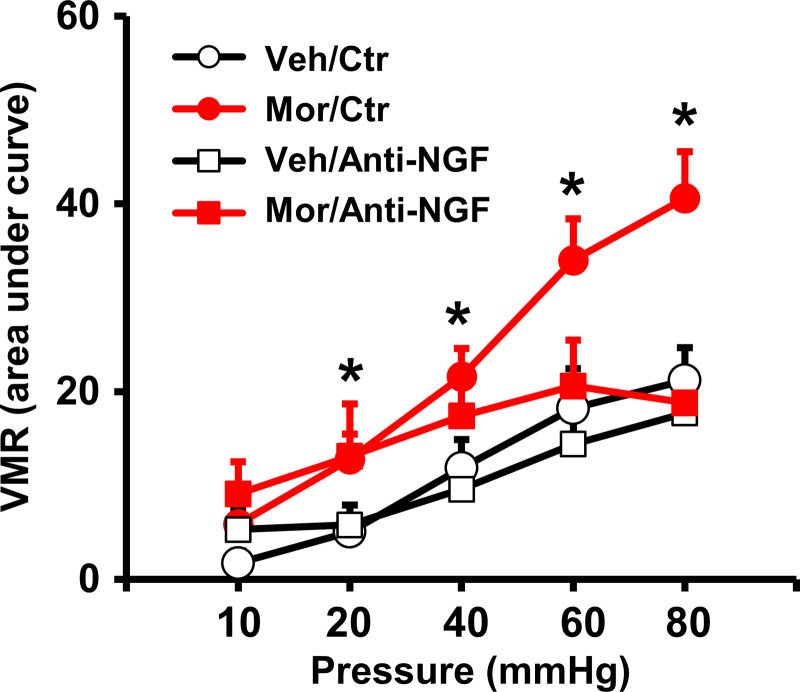
Finally, to determine if fecal retention-associated expression of NGF is involved in visceral hypersensitivity in morphine-treated rats, we administered anti-NGF antibody (25, 43) in Veh and Mor rats. Quantitative measurements of the visceromotor response to colorectal distention showed that anti-NGF treatment significantly attenuated morphine-induced visceral hypersensitivity in the rats (Fig. 8), suggesting that fecal retention-associated induction of pain mediators such as NGF in the colon may contribute to visceral hyperalgesia in OBD.
Figure 8.
Effect of anti-NGF treatment on morphine-induced visceral hypersensitivity. The visceromotor response to colorectal distention was increased by chronic use of morphine (7 days). However, anti-NGF treatment (20 µg/kg ip daily) attenuated morphine-induced visceral hypersensitivity. N = 5 or 6 rats in each group. *P < 0.05 vs. Veh. NGF, nerve growth factor; Veh, vehicle control rats.
DISCUSSION
Bowel dysfunctions are among the most common adverse effects associated with chronic use of opioids (3–7). Although the mechanisms for constipation and abdominal pain in OBD are not clear, research has been focused on the enteric and central nervous systems (6, 9, 13, 15, 18). However, the present study suggests that a peripheral mechanism involving the distal colon may play a critical role in the development of sustained constipation and visceral hyperalgesia. We found that treatment with morphine leads to profound fecal retention in the distal colon. The fecal retention-associated mechanical stress causes upregulation of COX-2 and NGF in the colon smooth muscle, and suppression of muscle contractility and increase of visceral sensitivity. Interestingly, when fecal retention is prevented, morphine treatment does not lead to suppression of muscle contractility or increase of visceral sensitivity. Expression of COX-2 and NGF in the colon smooth muscle is also blocked when fecal retention is prevented. Our study thus reveals a previously unrecognized peripheral mechanism in OBD that fecal retention, a common phenomenon in opioid users, serves as an independent pathogenic factor in motility dysfunction and visceral hyperalgesia in OBD. Intervention studies found that COX-2 inhibitor improves smooth muscle contractility and bowel movement in morphine-treated rats. Anti-NGF treatment attenuated visceral hypersensitivity in the OBD model. Thus, mechanical distention-induced expression of COX-2 and NGF in the colon may play a critical role in the development of opioid-induced constipation and abdominal pain.
Constipation is a sustained adverse effect in chronic opioid users (5–7). Although tolerance to analgesia and other effects are commonly present with chronic use of opioids, constipation still persists. In fact, tolerance to the effects of morphine or opioid receptor stimulation occurs in all gastrointestinal organs, except in the colon (15, 45). In search for the mechanisms underlying OIC, investigators have focused mainly on opioid receptors in the ENS (6, 15, 18, 45). The μ-opioidreceptor (MOR) is known to affect ENS to inhibit enteric neuron excitability, and to reduce release of excitatory neurotransmitters, i.e., acetylcholine (ACh), thus slowing intestinal transit and reducing mucosal secretion (6, 15, 18). These opioid receptor-dependent effects may well be the primary reason for initial stage of constipation (Fig. 9). However, constipation consequently leads to fecal retention in the distal bowel. In fact, we found that constipation and fecal retention start on the very first day of morphine treatment and remain throughout the 7-day treatment of morphine.
Figure 9.
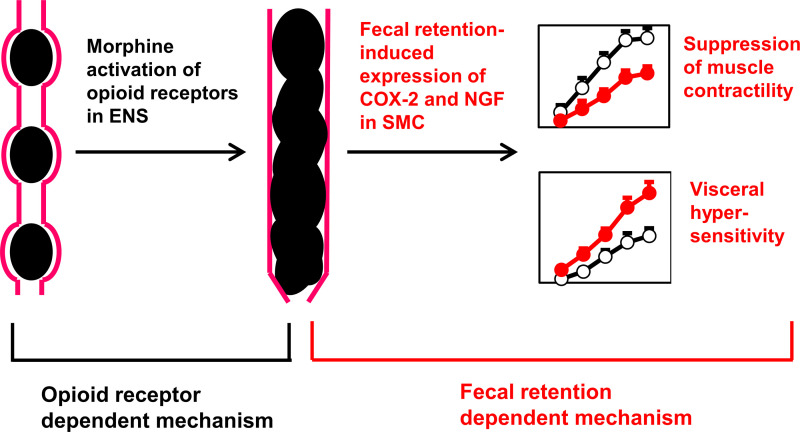
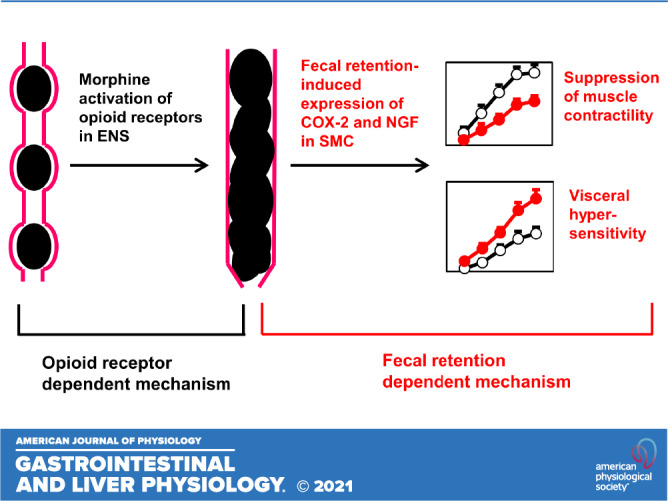
Proposed mechanisms underlying OBD. Opioid receptor-dependent mechanism via enteric nervous system (ENS) leads to fecal retention in the distal colon, which induces expression of COX-2 and NGF in the colonic smooth muscle. The fecal retention-dependent expression of COX-2 and NGF may underlie motility dysfunction and visceral hyperalgesia in prolonged use of opioids. Our study shows that prevention of fecal retention by colon cleansing with clear liquid diet eliminates the effect of opioid receptor-dependent mechanism and blocks the initiation of fecal retention-dependent mechanism. COX-2, cyclo-oxygenase-2; NGF, nerve growth factor; OBD, opioid-induced bowel dysfunction.
Our data suggests that fecal retention itself may play a critical role in contractility changes of colon smooth muscle, which may well contribute to the sustained motility dysfunction and constipation (32, 39), as seen in chronic use of morphine. As summarized in Fig. 9, we propose that an opioid receptor-dependent mechanism to inhibit enteric neural activity may be the initial cause of fecal retention, and that an opioid receptor-independent mechanism, i.e., fecal retention-initiated mechanotranscription (i.e., COX-2), may account for gut smooth muscle dysfunction, contributing to sustained constipation in OBD. This is supported by multiple lines of evidence. First, although analgesic tolerance to opioids is well documented, there is no or least tolerance to opioid-induced constipation with extended use of opioids (15, 18, 45). This indicates that an opioid receptor-independent mechanism may likely be involved. Second, peripheral opioid receptor antagonists are not very effective in releasing OIC, especially after long-term use of opioids (7, 8, 16, 17). More importantly, our results in the present study found that inhibition of COX-2 does not affect opioid-induced constipation on day 1 of morphine treatment, indicating that early phase of constipation may not be due to mechanotranscription of COX-2, but most possibly due to opioid receptor-dependent mechanism as previously proposed (9, 15, 18). However, COX-2 inhibitor effectively blocked morphine-associated reduction of feces production for almost all other days except day 1, and restored smooth muscle function. Given that prevention of fecal retention blocked COX-2 expression and also restored smooth muscle function, these data suggest that mechanotranscription of COX-2 may play a critical role in sustained motility dysfunction and chronic constipation. In fact, COX-2 expression in gut SMC is highly sensitive to mechanical stress, as previously demonstrated in mechanical distention model in vivo (22, 23) and in cultured SMC in vitro (22, 27). Although COX-2 is often considered a proinflammatory mediator, we found no detectable colonic inflammation in our OBD model. Our study thus suggests that peripheral opioid receptor antagonists may be useful to prevent initiation of OIC, and that mechanotranscription-dependent process shall be explored as a novel therapeutic target for the management of sustained OIC.
More than half of chronic opioid users experience abdominal pain in addition to constipation and fecal retention (3, 11). In fact, when abdominal pain becomes a predominant symptom in chronic use of opioids, the condition is defined as NBS, a subset of OBD (3, 11–13). Clinical and preclinical studies found that visceral hyperalgesia or hypersensitivity, as a widely recognized mechanism for abdominal pain (19, 23, 46), is present in chronic use of opioids (3, 11, 19, 20). Mechanisms of visceral hyperalgesia in OAP and NBS are incompletely understood. Current theories are largely based on reports in broader fields of opioid-induced hyperalgesia and addiction, but not on visceral pain (3, 13, 14). However, not any therapeutic agent for OAP or NBS have been developed around these concepts. The current treatment for NBS consists of opioid withdrawal and nonspecific treatments such as antidepressants and other psychosocial interventions (3, 13, 14). This so-called “detoxification management” led to only 35% reduction of abdominal pain after a 3-mo treatment (14). Half of the patients returned to using narcotics with recurrent NBS (14). Nevertheless, bowel distention or fecal retention is a distinctive feature in OAP and NBS (12, 13). In the present study, we found that chronic use of morphine leads to visceral hypersensitivity and sensory neuron hyperexcitability in rats fed with regular pellet food. These rats demonstrated apparent fecal retention, and upregulation of pain-mediator NGF in the muscle tissue of the distended colon. Interestingly, colon cleansing not only prevented fecal retention and NGF upregulation but also attenuated visceral hypersensitivity and sensory neuron hyperexcitability. To determine visceral sensitivity, we measured viscero-motor response to colorectal distention via a balloon inserted into the distal colon. This measurement is a widely used assessment of visceral sensitivity of the colon (19, 23). However, it is not known if morphine-treatment-associated fecal retention affects colon compliance and visceromotor response results. Thus, we also measured cell excitability of isolated colon-specific DRG neurons, and found that these neurons were highly excited in the morphine group, suggesting that peripheral visceral hypersensitivity is present in the OBD model. Taken together, we propose a peripheral mechanism in OAP that opioid-initiated fecal retention triggers mechanotranscription of pain mediators, such as NGF in colonic SMC, which sensitizes primary afferent neurons to contribute to visceral hyperalgesia (Fig. 9). As processes such as mechanotranscription of pain mediators and sensitization of afferent neurons may take days, visceral hyperalgesia may not be present immediately, but after prolonged use of narcotics (11–13). The fecal retention-dependent mechanism of peripheral sensitization represents a novel pathway in the development of visceral hyperalgesia in OBD.
To prevent fecal retention in opioid treatment, we removed normal pellet food from rats and applied bowel cleanser the day before morphine treatment and kept rats exclusively in liquid diet throughout the 7-day period of morphine treatment. This protocol almost completely cleansed the colon in vehicle-treated and morphine-treated rats, and prevented fecal retention-associated mechanotranscription and neuromuscular changes associated with morphine treatment. These results suggest that colon cleansing could be an effective treatment for opioid bowel dysfunction. Notably, colon cleansing has been tried as an alternative treatment for constipation, especially neurogenic and idiopathic constipation (47–51). When first-line constipation treatments failed, colon cleansing (i.e., colonic irrigation) was found to be effective in 65%–90% of the patients (47–50). Unfortunately, these studies did not further investigate the possible mechanisms underlying the efficacy of colon cleansing. Based on our results, we believe that the benefits of colon cleansing in persistent constipation may be a result of inhibition of mechanotranscription of mediators such as COX-2. In fact, Cong et al. (52) reported significant increase of COX-2 expression and prostaglandin production in colonic smooth muscle of patients with chronic constipation.
To the best of our knowledge, the utility of colonic irrigation as a way to cleanse bowel has not been tried for patients with OBD. In fact, colonic irrigation may have safety and compliance concerns (50, 53). Our regimen with liquid diet after bowel cleanser may offer a better choice to achieve colon cleansing, as it is very efficient in keeping the colon from fecal retention for extended time. Exclusive enteral nutrition (EEN), involving oral or nasogastric tube feeding of liquid diet for 6–8 wk, is an effective therapy in the management of Crohn’s disease, especially in pediatric patients (40, 41). Our study suggests that EEN may also be useful for the management of OBD. We found that liquid diet not only physically preempts fecal accumulation in the colon but also attenuates fecal retention-induced gene expression of proinflammatory and pain mediators such as COX-2 and NGF. Consequently, it helps to mitigate COX-2-mediated and NGF-mediated motility dysfunction and visceral hyperalgesia in OBD. EEN may affect gut microbiota composition and diversity (40, 54), though these changes do not account for the benefits of EEN treatment for Crohn’s disease. In an attempt to minimize the effect of possible liquid-diet-associated microbiota changes, we have always included vehicle control along with morphine treatment in each diet group (either normal chow or liquid diet). Liquid-diet treatment left fewer or no residual feces in the colon in both Veh and Mor rats, indicating that the effect of microbiota changes, if any, would be similar in the Veh and Mor rats. It will be interesting to determine in the future if microbiota may play any role in the improvement of bowel function by liquid diet in OBD.
In summary, opioids may initially act on opioid receptors on the enteric nervous system to cause fecal retention. However, we found that fecal retention, as a mechanical stress in the distal bowel, subsequently induces mechanotranscription of COX-2 and NGF in colonic smooth muscle, which contributes to sustained motility dysfunction, constipation, and visceral hypersensitivity in the rodent model of OBD (Fig. 9). Thus, fecal retention, may play an independent pathogenic role in OBD. As liquid diet prevents fecal retention, it may be useful for the management of OBD.
GRANTS
This study was supported in part by the National Institute of Health National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK102811 and R01 DK124611 (to X-Z.S.) and by the US Department of Defense Grant W81XWH2010681 (to X-Z.S.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X-Z.S. conceived and designed research; Y-M.L., Y.T., Y.F., S.H., D.W.S., and X-Z.S. performed experiments; Y-M.L., Y.T., Y.F., S.H., D.W.S., L-Y.M.H., and X-Z.S. analyzed data; Y-M.L., Y.T., Y.F., L-Y.M.H., and X-Z.S. interpreted results of experiments; Y-M.L., Y.F., and X-Z.S. prepared figures; Y-M.L. and X-Z.S. drafted manuscript; Y.T., S.H., D.W.S., L-Y.M.H., and X-Z.S. edited and revised manuscript; Y-M.L., Y.T., Y.F., S.H., D.W.S., L-Y.M.H., and X-Z.S. approved final version of manuscript.
ACKNOWLEDGEMENT
Present addresses: Yanbo T, Dept. of Gastroenterology, Liuzhou People’s Hospital, The Fifth Affiliated Hospital of Guangxi Medical University. Liuzhou, Guanxi, China; Shrilakshmi H, Liverpool School of Tropical Medicine. Pembroke Place, Liverpool, L3 5QA, UK.
REFERENCES
- 1.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 152: 85–92, 2010. doi: 10.7326/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehendale AW, Goldman MP, Mehendale RP. Opioid overuse pain syndrome (OOPS): the story of opioids, prometheus unbound. J Opioid Manag 9: 421–438, 2013. doi: 10.5055/jom.2013.0185. [DOI] [PubMed] [Google Scholar]
- 3.Szigethy E, Schwartz M, Drossman D. Narcotic bowel syndrome and opioid-induced constipation. Curr Gastroenterol Rep 16: 410, 2014. doi: 10.1007/s11894-014-0410-4. [DOI] [PubMed] [Google Scholar]
- 4.Manchikanti L, Helm S, 2nd, Fellows B, Janata JW, Pampati V, Grider JS, Boswell MV. Opioid epidemic in the United States. Pain Physician 15: ES9–S38, 2012. doi: 10.36076/ppj.2012/15/ES9. [DOI] [PubMed] [Google Scholar]
- 5.Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg 182: 11S–18S, 2001. doi: 10.1016/s0002-9610(01)00782-6. [DOI] [PubMed] [Google Scholar]
- 6.Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs 63: 649–671, 2003. doi: 10.2165/00003495-200363070-00003. [DOI] [PubMed] [Google Scholar]
- 7.Bell TJ, Panchal SJ, Miaskowski C, Bolge SC, Milanova T, Williamson R. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 10: 35–42, 2009. doi: 10.1111/j.1526-4637.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- 8.Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care 54: 901–906, 2016. doi: 10.1097/MLR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol 106: 835–842, 2011. doi: 10.1038/ajg.2011.30. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri M, Drossman DA, Becker G, Webster LR, Davies AN, Mawe GM. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil 26: 1386–1395, 2014. doi: 10.1111/nmo.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuteja AK, Biskupiak J, Stoddard GJ, Lipman AG. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil 22: 424–430.e96, 2010. doi: 10.1111/j.1365-2982.2009.01458.x. [DOI] [PubMed] [Google Scholar]
- 12.Sandgren JE, McPhee MS, Greenberger NJ. Narcotic bowel syndrome treated with clonidine: Resolution of abdominal pain and intestinal pseudo-obstruction. Ann Intern Med 101: 331–334, 1984. doi: 10.7326/0003-4819-101-3-331. [DOI] [PubMed] [Google Scholar]
- 13.Grunkemeier DM, Cassara JE, Dalton CB, Drossman DA. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol 5: 1126–1139, 2007. doi: 10.1016/j.cgh.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drossman DA, Morris CB, Edwards H, Wrennall CE, Weinland SR, Aderoju AO, Kulkarni-Kelapure RR, Hu YJ, Dalton C, Bouma MH, Zimmerman J, Rooker C, Leserman J, Bangdiwala SI. Diagnosis, characterization, and 3-month outcome after detoxification of 39 patients with narcotic bowel syndrome. Am J Gastroenterol 107: 1426–1440, 2012. doi: 10.1038/ajg.2012.142. [DOI] [PubMed] [Google Scholar]
- 15.Farmer AD, Holt CB, Downes TJ, Ruggeri E, Del Vecchio S, De Giorgio R. Pathophysiology, diagnosis, and management of opioid-induced constipation. Lancet Gastroenterol Hepatol 3: 203–212, 2018. doi: 10.1016/S2468-1253(18)30008-6. [DOI] [PubMed] [Google Scholar]
- 16.Sonu I, Triadafilopoulos G, Gardner JD. A questionable investment for our patients: challenging the efficacy of new therapies for opioid-induced and chronic idiopathic constipation (Abstract). Gastroenterol 148: S-191, 2015. [Google Scholar]
- 17.Chey WD, Webster L, Sostek M, Lappalainen J, Barker PN, Tack J. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med 370: 2387–2396, 2014. doi: 10.1056/NEJMoa1310246. [DOI] [PubMed] [Google Scholar]
- 18.Galligan JJ, Akbarali HI. Molecular physiology of enteric opioid receptors. Am J Gastroenterol Suppl 2: 17–21, 2014. doi: 10.1038/ajgsup.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agostini S, Eutamene H, Cartier C, Broccardo M, Improta G, Houdeau E, Petrella C, Ferrier L, Theodorou V, Bueno L. Evidence of central and peripheral sensitization in a rat model of narcotic bowel-like syndrome. Gastroenterol 139: 553–563, 2010. doi: 10.1053/j.gastro.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 20.Ross GR, Gade AR, Dewey WL, Akbarali HI. Opioid-induced hypernociception is associated with hyperexcitability and altered tetrodotoxin-resistant Na+ channel function of dorsal root ganglia. Am J Physiol Cell Physiol 302: C1152–C1161, 2012. doi: 10.1152/ajpcell.00171.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi XZ. Mechanical regulation of gene expression in gut smooth muscle cells. Front Physiol 8: 1000, 2017. doi: 10.3389/fphys.2017.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi XZ, Lin YM, Powell DW, Sarna SK. Pathophysiology of motility dysfunction in bowel obstruction: role of stretch-induced COX-2. Am J Physiol Gastrointest Liver Physiol 300: G99–G108, 2011. doi: 10.1152/ajpgi.00379.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin YM, Fu Y, Wu CC, Xu GY, Huang LY, Shi XZ. Colon distention induces persistent visceral hypersensitivity by mechanotranscription of pain mediators in colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 308: G434–G441, 2015. doi: 10.1152/ajpgi.00328.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raahave D. Faecal retention: a common cause in functional bowel disorders, appendicitis and haemorrhoids–with medical and surgical therapy. Dan Med J 62: B–5031., 2015. [PubMed] [Google Scholar]
- 25.Lin YM, Fu Y, Winston J, Radhakrishnan R, Sarna SK, Huang LM, Shi XZ. Pathogenesis of abdominal pain in bowel obstruction: role of mechanical stress-induced upregulation of nerve growth factor in gut smooth muscle cells. Pain 158: 583–592, 2017. doi: 10.1097/j.pain.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin YM, Sarna SK, Shi XZ. Prophylactic and therapeutic benefits of COX-2 inhibitor on motility dysfunction in bowel obstruction: roles of PGE2 and EP receptors. Am J Physiol Gastrointest Liver Physiol 302: G267–G275, 2012. doi: 10.1152/ajpgi.00326.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li F, Lin YM, Sarna SK, Shi XZ. Cellular mechanism of mechanotranscription in colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 303: G670–G679, 2012. doi: 10.1152/ajpgi.00440.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin YM, Li F, Shi XZ. Mechano-transcription of COX-2 is a common response to lumen dilation of the rat gastrointestinal tract. Neurogastroenterol Motil 24: 670–676.e295–296, 2012. doi: 10.1111/j.1365-2982.2012.01918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegde S, Lin YM, Fu Y, Savidge T, Shi XZ. Precision Lactobacillus reuteri therapy attenuates luminal distention-associated visceral hypersensitivity by inducing peripheral opioid receptors in the colon. Pain 161: 2737–2749, 2020. doi: 10.1097/j.pain.0000000000001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu Y, Lin YM, Winston JH, Radhakrishnan R, Huang LM, Shi XZ. Role of brain-derived neurotrophic factor in the pathogenesis of distention-associated abdominal pain in bowel obstruction. Neurogastroenterol Motil 30: e13373, 2018. doi: 10.1111/nmo.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi XZ, Sarna SK. G protein-mediated dysfunction of excitation-contraction coupling in ileal inflammation. Am J Physiol Gastrointest Liver Physiol 286: G899–G905, 2004. doi: 10.1152/ajpgi.00408.2003. [DOI] [PubMed] [Google Scholar]
- 32.Lin YM, Fu Y, Hegde S, Tang Y, Radhakrishnan R, Shi XZ. Microsomal prostaglandin E synthase-1 plays a critical role in long-term motility dysfunction after bowel obstruction. Sci Rep 8: 8831, 2018. doi: 10.1038/s41598-018-27230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi XZ, Pazdrak K, Saada N, Dai B, Palade P, Sarna SK. Negative transcriptional regulation of human colonic smooth muscle Cav1.2 channels by p50 and p65 subunits of nuclear factor-kappaB. Gastroenterol 129: 1518–1532, 2005. doi: 10.1053/j.gastro.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 34.Niwa T, Nakao M, Hoshi S, Yamada K, Inagaki K, Nishida M, Nabeshima T. Effect of dietary fiber on morphine-induced constipation in rats. Biosci Biotechnol Biochem 66: 1233–1240, 2002. doi: 10.1271/bbb.66.1233. [DOI] [PubMed] [Google Scholar]
- 35.Harada Y, Iizuka S, Saegusa Y, Mogami S, Fujitsuka N, Hattori T. Mashiningan improves opioid-induced constipation in rats by activating cystic fibrosis transmembrane conductance regulator chloride channel. J Pharmacol Exp Ther 362: 78–84, 2017. doi: 10.1124/jpet.117.240630. [DOI] [PubMed] [Google Scholar]
- 36.Aubé AC, Blottière HM, Scarpignato C, Cherbut C, Rozé C, Galmiche JP. Inhibition of acetylcholine induced intestinal motility by interleukin 1 beta in the rat. Gut 39: 470–474, 1996. doi: 10.1136/gut.39.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi XZ, Sarna SK. Inflammatory modulation of muscarinic receptor activation in canine ileal circular muscle cells. Gastroenterology 112: 864–874, 1997. doi: 10.1053/gast.1997.v112.pm9041248. [DOI] [PubMed] [Google Scholar]
- 38.Motomura Y, Khan WI, El-Sharkawy RT, Verma-Gandhu M, Grencis RK, Collins SM. Mechanisms underlying gut dysfunction in a murine model of chronic parasitic infection. Am J Physiol Gastrointest Liver Physiol 299: G1354–G1360, 2010. doi: 10.1152/ajpgi.00324.2010. [DOI] [PubMed] [Google Scholar]
- 39.Sarna SK, Shi XZ. Function and regulation of colonic contractions in health and disease. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. Amsterdam: Elsevier Academic Press, 2006, p. 965993. [Google Scholar]
- 40.Ashton JJ, Gavin J, Beattie RM. Exclusive enteral nutrition in Crohn's disease: evidence and practicalities. Clin Nutr 38: 80–89, 2019. doi: 10.1016/j.clnu.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Narula N, Dhillon A, Zhang D, Sherlock ME, Tondeur M, Zachos M. Enteral nutritional therapy for induction of remission in Crohn's disease. Cochrane Database Syst Rev 4: CD000542, 2018. doi: 10.1002/14651858.CD000542.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwarz NT, Kalff JC, Türler A, Engel BM, Watkins SC, Billiar TR, Bauer AJ. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterol 121: 1354–1371, 2001. doi: 10.1053/gast.2001.29605. [DOI] [PubMed] [Google Scholar]
- 43.Winston JH, Xu GY, Sarna SK. Adrenergic stimulation mediates visceral hypersensitivity to colorectal distension following heterotypic chronic stress. Gastroenterology 138: 294–304, 2010. doi: 10.1053/j.gastro.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Y, Colak T, Shenoy M, Liu L, Pai R, Li C, Mehta K, Pasricha PJ. Nerve growth factor modulates TRPV1 expression and function and mediates pain in chronic pancreatitis. Gastroenterology 141: 370–377, 2011. doi: 10.1053/j.gastro.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson AD, Camilleri M. Opioid-induced constipation: advances and clinical guidance. Ther Adv Chronic Dis 7: 121–134, 2016. doi: 10.1177/2040622315627801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi XZ, Lin YM, Hegde S. Novel insights into the mechanisms of abdominal pain in obstructive bowel disorders. Front Integr Neurosci 12: 23, 2018. doi: 10.3389/fnint.2018.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mugie SM, Machado RS, Mousa HM, Punati JB, Hogan M, Benninga MA, Di Lorenzo C. Ten-year experience using antegrade enemas in children. J Pediatr 161: 700–704, 2012. doi: 10.1016/j.jpeds.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 48.Christensen P, Bazzocchi G, Coggrave M, Abel R, Hultling C, Krogh K, Media S, Laurberg S. A randomized, controlled trial of transanal irrigation versus conservative bowel management in spinal cord-injured patients. Gastroenterology 131: 738–747, 2006. doi: 10.1053/j.gastro.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Emmanuel AV, Krogh K, Bazzocchi G, Leroi AM, Bremers A, Leder D, van Kuppevelt D, Mosiello G, Vogel M, Perrouin-Verbe B, Coggrave M, Christensen P; Members of working group on Trans Anal Irrigation from UK, Denmark, Italy, Germany, France and Netherlands. Consensus review of best practice of transanal irrigation in adults. Spinal Cord 51: 732–738, 2013. doi: 10.1038/sc.2013.86. [DOI] [PubMed] [Google Scholar]
- 50.Christensen P, Krogh K. Transanal irrigation for disordered defecation: a systematic review. Scand J Gastroenterol 45: 517–527, 2010. doi: 10.3109/00365520903583855. [DOI] [PubMed] [Google Scholar]
- 51.Sakamoto M, Fukai K, Mine H. Effect of colonic irrigation on the bowel habits of constipated young women. Kawasaki J Med Welfare 9: 9–14, 2003. [Google Scholar]
- 52.Cong P, Pricolo V, Biancani P, Behar J. Abnormalities of prostaglandins and cyclooxygenase enzymes in female patients with slow-transit constipation. Gastroenterology 133: 445–453, 2007. doi: 10.1053/j.gastro.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 53.Wilson M. A review of transanal irrigation in adults. Br J Nurs 26: 846–856, 2017. doi: 10.12968/bjon.2017.26.15.846. [DOI] [PubMed] [Google Scholar]
- 54.MacLellan A, Connors J, Grant S, Cahill L, Langille M, Van Limbergen J. The impact of exclusive enteral nutrition (EEN) on the gut microbiome in Crohn's disease: a review. Nutrients 9: 447, 2017. doi: 10.3390/nu9050447. [DOI] [PMC free article] [PubMed] [Google Scholar]