Keywords: autoimmune disorders, IPEX, Lactobacillus reuteri DSM 17938, probiotics, scurfy mouse
Abstract
Treg deficiency causes a lethal, CD4+ T cell-driven autoimmune disease called IPEX syndrome (immunodysregulation, polyendocrinopathy, and enteropathy, with X-linked inheritance) in humans and in the scurfy (SF) mouse, a mouse model of the disease. Feeding Limosilactobacillus reuteri DSM 17938 (LR 17938, LR) to SF mice reprograms the gut microbiota, reduces disease progression, and prolongs lifespan. However, the efficacy and mechanism of LR, compared with other probiotics, in producing these effects is unknown. We compared LR with Lacticaseibacillus rhamnosus GG (LGG), an extensively investigated probiotic. LR was more effective than LGG in prolonging survival. Both probiotics restored the fecal microbial alpha diversity, but they produced distinct fecal bacterial clusters and differentially modulated microbial relative abundance (RA). LR increased the RA of phylum_Firmicutes, genus_Oscillospira whereas LR reduced phylum_Bacteroidetes, genus_Bacteroides and genus_Parabacteroides, reversing changes attributed to the SF phenotype. LGG primarily reduced the RA of genus_Bacteroides. Both LR and LGG reduced the potentially pathogenic taxon class_γ-proteobacteria. Plasma metabolomics revealed substantial differences among 696 metabolites. We observed similar changes of many clusters of metabolites in SF mice associated with treatment with either LR or LGG. However, a unique effect of LR was to increase the abundance of plasma adenosine metabolites such as inosine, which we previously showed had immune modulatory effects. In conclusion: 1) different probiotics produce distinct signatures in the fecal microbial community in mice with Treg deficiency; and 2) when comparing different probiotics, there are strain-specific microbial products with different anti-inflammatory properties, reinforcing the concept that “one size does not fit all” in the treatment of autoimmune disease.
NEW & NOTEWORTHY In the treatment of Treg-deficiency-induced autoimmunity, Limosilactobacillus reuteri DSM 17938 (LR) showed greater efficacy than Lacticaseibacillus rhamnosus GG (LGG). The study demonstrated that two different probiotics produce distinct signatures in the fecal microbial community in mice with Treg deficiency, but with many similarities in global plasma metabolites in general. However, there are strain-specific microbial products with different anti-inflammatory properties, reinforcing the concept that “one size does not fit all” in the treatment of autoimmune disease.
INTRODUCTION
Tregs maintain immune homeostasis and play a pivotal role in immune tolerance (1). Forkhead box protein 3 (Foxp3) is a major transcription factor that is associated with Treg cell development and function (2). Genetic mutations or deletions of the Foxp3 gene result in a primary immunodeficiency (PID) disease known as IPEX syndrome (immunodysregulation, polyendocrinopathy, and enteropathy, with X-linked inheritance) in humans (3, 4). The most common clinical presentation of IPEX syndrome is the classical triad of severe enteropathy, type I diabetes, and eczema of early onset (at 1.5–2 mo) (3–7). The scurfy (SF) mouse, bearing a mutation in the Foxp3 gene, displays a similar clinical phenotype, with early onset dermatitis, progressive multiorgan inflammation, and death within the first month of life caused by a lymphoproliferative syndrome (8, 9). It is currently unclear as to the trigger that initiates these immune responses.
Probiotics have the capacity not only to induce large-scale changes in the host microbiota but also to modulate the global metabolic functions of intestinal microbiomes (10–14). Probiotic Limosilactobacillus reuteri DSM 17938 (LR 17938 or LR) has been shown to have a mutualistic relationship with the human host (15). Commercially available worldwide, LR was derived from Lactobacillus reuteri ATCC 55730, a strain isolated from a Peruvian mother’s breast milk that was cured from two plasmids encoding antibiotic resistance (15, 16). LR inhibits pathogen growth and modulates the immune system (13). In humans, LR reduces the severity of acute infant diarrhea (17–19), may reduce the incidence of necrotizing enterocolitis (NEC) in premature infants (20–22), and decreases crying time in babies with colic (23, 24).
We have tested the effects of LR in several mouse models of human inflammatory and autoimmune diseases. We found this strain prevents experimental NEC in newborn animals by inhibiting the Toll-like receptor (TLR) 4-mediated NF-κB pathway (25), facilitating the induction of Tregs and lowering the number of inflammatory effector T cells (Teffs) in the intestinal mucosa (26, 27), a process requiring TLR2 (28). In addition, LR reduced the severity of experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis mainly driven by TH1- and TH17-induced inflammation (29). Both of these conditions were associated with altered gut microbiota (29–34).
In testing the effect of LR on SF mice, we found that LR prolonged the survival of SF mice and reduced organ inflammation as well as TH1/TH2-associated cytokines. Our studies identified a novel mechanism wherein the A2A expressed predominantly on T cells was required for probiotic protection, inasmuch as LR had no therapeutic effect on SF mice with genetic knockout of receptor A2A (35, 36). We discovered that the adenosine metabolite inosine (formed from adenosine by the action of adenosine deaminase, ADA) was reduced in plasma of SF mice and restored by LR treatment. Oral administration of inosine to SF mice also prolonged the survival of SF mice and reduced inflammation. Inosine similarly controlled inflammation by acting as an A2A agonist (36). We found that the SF mouse demonstrates dynamic changes of gut microbial dysbiosis over the 22 days of lifespan, and early treatment of SF mice with LR modulates gut microbiota and its associated metabolites (36). However, we did not determine if the effect is specific to LR.
In the current study, we compared the effects of probiotic Lacticaseibacillus rhamnosus GG (LGG) (37) and LR with respect to mouse survival, microbiota, and microbial-associated metabolites in Treg deficiency. LGG is one of the most widely used and well-documented probiotic strains, often used to prevent or treat gastrointestinal infections, antibiotic-associated diarrhea, respiratory infections, and allergies (38, 39). Our studies aimed to further enhance our understanding of the specificity of probiotics in modulating autoimmunity, using a well-characterized model of Treg deficiency.
MATERIALS AND METHODS
Animals
Wild-type (WT) C57BL/6J and heterozygous B6.Cg-Foxp3sf/J mice were purchased from Jackson Laboratories and allowed to acclimatize for 2 wk before experimentation. SF mice with hemizygous B6.Cg-Foxp3sf/Y were generated by breeding heterozygous B6.Cg-Foxp3sf/J female (Jackson Lab 004088) to C57BL/6J male (Jackson Lab 000664) mice. Only male mice were used in this study due to the Foxp3 gene existing on the X chromosome. In each litter of breeding pairs, all males were either SF used as the experimental groups or WT littermates used as the control groups. All mice were housed in the animal facility at the UT Health Science Center in Houston. This study was carried out in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals (NIH) and the Institutional Animal Care and Use Committee (IACUC). The study was approved by the IACUC (protocol numbers: AWC-14-056 and AWC-17-0045).
Preparation of Probiotics and Treatment of SF Mice
Probiotic LR and LGG were provided by BioGaia AB (Sweden) and were prepared as described previously (36). Briefly, probiotics were anaerobically cultured in deMan-Rogos-Sharpe (MRS) medium at 37°C for 24 h followed by plating in MRS agar at specific serial dilution and culturing anaerobically at 37°C for 48–72 h. Quantitative analysis of bacteria in culture media was performed by comparing absorbance at 600 nm of cultures at known concentrations, using a standard curve of bacterial colony forming unit (CFU)/mL grown on MRS agar.
Mice were fed probiotics or media (control) by oral gavage starting from the date of postnatal day 15 (d15) once we noted the SF clinical phenotype (crusted skin on tail and ears and/or deformed ears), and probiotics were given according to the protocols indicated in Supplemental Fig. S1A (all Supplemental material is available at https://doi.org/10.6084/m9.figshare.14062001) and Fig. 1A to observe the survivals. We fed 107 CFU daily, in a volume of 100 µL, using a polypropylene feeding tube (22 gauge × 25 mm, Instech Laboratories, Inc., Plymouth Meeting, PA). The experiments include four groups of SF mice: SF controls (SF, n = 17) or SF mice fed with LR for 1 wk (SF + LR 1 wk, n = 10), for 2 wk (SF + LR 2 wk, n = 9), or for 3 wk (SF + LR 3 wk, n = 10). We also studied SF mice fed with control media (MRS) (SF + MRS 1 wk, n = 6; SF + MRS 2 wk, n = 6; and SF + MRS 3 wk, n = 7). To compare LR with LGG, SF mice were fed 107 CFU/day of each probiotic, given by gavage daily in 100 µL, starting from postnatal d15 for 2 wk, with comparison groups denoted as LR (SF + LR, n = 9) and LGG (SF + LGG, n = 10). We observed the mice daily, recording survival after 2 wk of probiotic treatment. In another sets of experiments, groups WT (n = 7), SF (n = 5), SF + LR (n = 5), and SF + LGG (n = 7) were fed with media from postnatal d15 to d21 to collect plasma and cecal content for analysis of microbiota and metabolomics. Cecum and rectum were opened, and the contents were removed into sterilized microcentrifuge tubes and immediately frozen in liquid nitrogen and stored at −80°C freezer. We euthanized mice at d22 of age (before weaning) to investigate gut microbiota and metabolomics while avoiding microbial variations due to weaning (Fig. 1A).
Figure 1.
The effect of Limosilactobacillus reuteri DSM 17938 (LR) or Lacticaseibacillus rhamnosus GG (LGG) on the survival rate of scurfy (SF) mice. A: scheme for LR or LGG treatment on SF mice for sample analysis and survival observation. B: survival curves of SF, wild-type (WT), SF + LR, and SF + LGG mice (Kaplan–Meier survival curves and log-rank test; n = 9–17 mice per group). CFU, colony-forming unit; MRS, deMan-Rogos-Sharpe.
Determining the Population Structure of the Microbiota
Feces from cecum to rectum of mice were collected. The Louisiana State University School of Medicine Microbial Genomics Resource Group (http://metagenomics.lsuhsc.edu) performed sequencing and bioinformatics. Genomic DNA extraction was performed using QIAamp Fast DNA Stool Mini Kit (Qiagen, Germantown, MD), and bacterial profiling by amplification and sequencing of the V4 region of the 16S rRNA gene was performed as previously described (40). The relative abundance (RA) of each operational taxonomic unit (OTU) was examined at phylum, class, order, family, genus, and species levels. Bacterial α- and β-diversity metrics, as well as taxonomic community assessments, were produced using QIIME 1.8 (open source, http://qiime.org/) (41).
Plasma Global Metabolomics Analysis
We stored plasma samples at −80°C, and when individual studies were completed, samples were sent to Metabolon (https://www.metabolon.com/) for processing and measuring metabolomics profile in plasma from mice of WT, SF, SF + LR, and SF + LGG (36). Sixty hundred and ninety-six compounds of known identity in plasma were detected by a nontargeted metabolomics analysis platform including ultra-performance liquid chromatography-tandem mass spectrometer and gas chromatography-mass spectrometry. Global metabolomics profile data included fold changes of biomolecules from SF/WT, SF + LR/SF, and SF + LGG/SF mice, as well as information derived from the Interactive Pathways Explorer tool from Metabolon.
Statistical Analysis
For data with a Gaussian distribution, results were presented as means ± SE. Significance was determined using one-way ANOVA corrected for multiple comparisons with Tukey and Dunnett’s post-tests, or two-way ANOVA for multiple comparisons with a Bonferroni test. For data with Gaussian-approximate distribution, Kruskal–Wallis analysis was used for comparisons. Kaplan–Meier survival curves were graphed, and the comparison was analyzed using Logrank with χ2 test. The statistical analysis was performed using Prism version 4.0 (GraphPad Software). Differences were reported as significant when P < 0.05.
RESULTS
Feeding Probiotic Prolongs the Survival of Treg-Deficient Scurfy Mice
We previously showed that oral feeding of LR 17938 either as an early treatment (starting at d8 of age) or later (starting on d15 of age) once daily prolongs the survival of SF mice from < 1 mo to > 4 mo of age (36). Initially, we aimed to optimize the treatment duration for different probiotic comparisons. We orally fed SF mice by gavage, 107 CFU/day, daily starting on postnatal d15 once we observed the SF clinical phenotype, for 1, 2, or 3 wk. Subsequently, we stopped feeding LR to observe survival of the SF mice (Supplemental Fig. S1A). We found that oral feeding of LR to SF mice, with any studied duration of LR feeding, significantly increased the survival rate. The survival rate of SF mice with 2- or 3-wk treatments was significantly higher than that with 1-wk treatment; however, there were no significant differences between 2-wk and 3-wk treatment durations (Supplemental Fig. S1B). This finding indicated that 2-wk treatment could be used to compare the effects of LR and LGG on survival of SF mice (Fig. 1A). Results showed that SF mice died before 30 days of age without probiotic treatment, whereas both LGG and LR significantly prolonged the survival of SF mice (SF + LR vs. SF, P < 0.0001; SF + LGG vs. SF, P = 0.0149). However, LR exerted a greater effect on lifespan than LGG: the median lifespan of mice with LR was d73 versus LGG d35.5, and the longest lifespan of mice with LR was d151 versus LGG d66 (SF + LR vs. SF + LGG, P = 0.0053; Fig. 1B).
Probiotic Treatment Reprograms Gut Microbiota in SF Mice
We have previously reported that LR 17938 significantly changes the gut microbial dysbiosis identified in SF mice (36). To compare LR with LGG, we found that 1-wk feeding of probiotic (LR or LGG) led to important changes in the gut microbiota. We observed an increase in α diversity determined by the Shannon diversity index comparing both LR and LGG with SF mice (SF vs. WT, P = 0.008; SF + LR vs. SF, P = 0.014; SF + LGG vs. SF, P = 0.023; and SF + LGG vs. SF + LR, P = 0.088, Fig. 2A). Only LR increased microbial evenness (SF vs. WT, P = 0.0008; SF + LR vs. SF, P = 0.027; SF + LGG vs. SF, P = 0.088; and SF + LGG vs. SF + LR, P = 0.061, Fig. 2B). Either LR or LGG feeding shifted the microbial community composition, as shown by Jaccard_emperor β diversity, when comparing either SF + LR with SF (P < 0.001); SF + LGG with SF (P < 0.001); or SF with WT (P < 0.001). However, the two probiotics yielded populations of fecal microbiota which were comparable with that of WT mice, without a significant difference between them (Fig. 2C).
Figure 2.

Gut microbial alpha- and beta diversity affected by Limosilactobacillus reuteri DSM 17938 (LR) or Lacticaseibacillus rhamnosus GG (LGG). A: gut microbial Shannon diversity analysis and B: gut microbial evenness comparing scurfy (SF) + LR vs. SF; SF + LGG vs. SF; SF + LGG vs. SF + LR, and SF vs. wild-type (WT) (one-way ANOVA; n = 4–7 mice per group). C: Jaccard_emperor β diversity comparing SF + LR vs. SF; SF + LGG vs. SF; or SF vs. WT (n = 4–7 mice per group).
Taxa belonging to the phylum Firmicutes (∼60%) and Bacteroidetes (∼25%) dominated the core microbiota of WT mice (Fig. 3, A–C). The relative abundance of specific bacteria was calculated based on the OTUs of the bacteria normalized to total identified OTU counts by 16S rRNA gene sequencing in the sample. Even though we found comparable changes attributable to different probiotics in gut bacterial clusters, LR and LGG differentially modified some bacteria in the feces of SF mice. We found that at the phylum level, the relative abundance of Firmicutes was reduced in SF mice; however, Firmicutes were increased by LR (Fig. 3B). The relative abundance of Bacteroidetes was increased in SF mice but was reduced by LR (Fig. 3C). In contrast, LGG feeding did not reverse these bacterial changes in SF mice (Fig. 3, B and C).
Figure 3.
Relative abundance of predominant bacteria (>1% in any sample) at the phylum level. The relative abundance of specific bacteria was calculated based on the operational taxonomic units (OTUs) of the bacteria normalized to total identified OTU counts by 16S rRNA gene sequencing of the sample. A: overall relative abundance of bacteria compared among groups. B: the core microbiota of wild-type (WT) mice: the phylum Firmicutes (∼60%); and C: the core microbiota of WT: phylum Bacteroidetes (∼25%). (one-way ANOVA; n = 4–7 mice per group). LR, Limosilactobacillus reuteri DSM 17938; LGG, Lacticaseibacillus rhamnosus GG; SF, scurfy; OD1, Parcubacteria; TM7, Saccharibacteria.
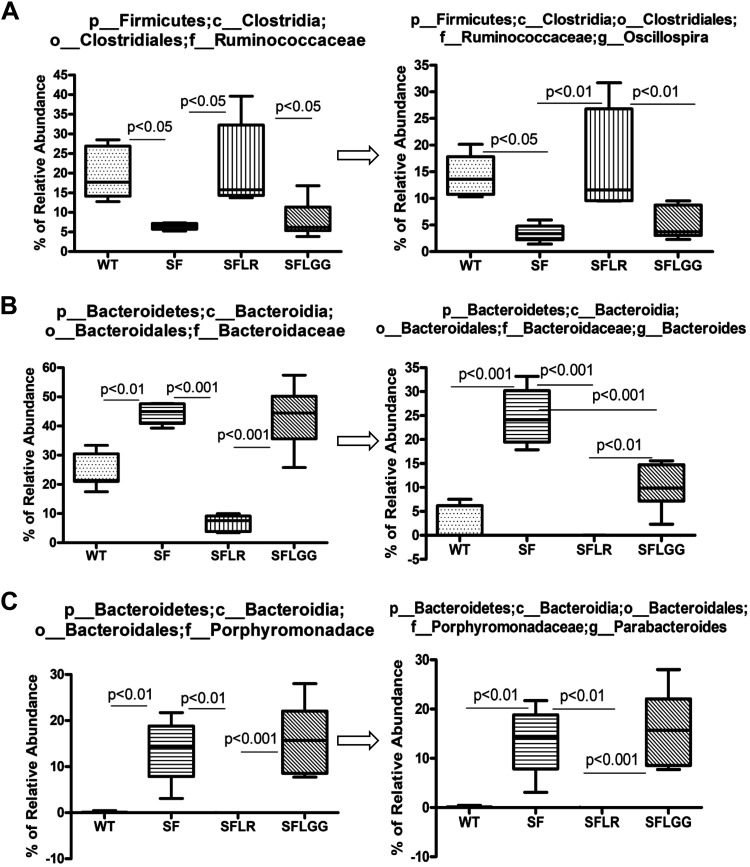
LGG increased the relative abundance of proteobacteria at the phylum level (Fig. 4A). LGG was shown to increase the relative abundance of class level of α-Proteobacteria (Fig. 4B) and β-Proteobacteria (Fig. 4C), but LGG reduced the γ-Proteobacteria (Fig. 4D). Of importance, LGG significantly reduced the relative abundance of Enterobacteriaceae (Fig. 4E) classified as γ-Proteobacteria, as did LR (Fig. 4, D and E). Finally, LR increased the relative abundance of family_Ruminococcaceae, genus_Oscilospira [phylum_Firmicutes] (Fig. 5A) and reduced the relative abundance of family_Bacteroidaceae, genus_Bacteroides (Fig. 5B) and family_Porphyromonadaceae, genus_Parabacteroides (Fig. 5C) [phylum_Bacteroidetes]. LGG but not LR reduced the relative abundance of genus_Bacteroides (Fig. 5, B and C).
Figure 4.
Relative abundance of Proteobacteria affected mainly by Lacticaseibacillus rhamnosus GG (LGG). Phylum Proteobacteria (A); class_α-proteobacteria (B); class_β-proteobacteria (C); class_γ-proteobacteria (D); class_γ-proteobacteria, order_Enterobacteriales; family_Enterobacteriaceae (E) comparing groups wild-type (WT), scurfy (SF), SF + Limosilactobacillus reuteri DSM 17938 (LR), and SF + LGG (one-way ANOVA; n = 4–7 mice per group, data are presented as means ± SE).
Figure 5.
Relative abundance of bacteria at the genus level changed by Limosilactobacillus reuteri DSM 17938 (LR) or Lacticaseibacillus rhamnosus GG (LGG) in scurfy (SF) mice. Family_Ruminococcoceae, genus_Oscillospira (A); family_Bacteroideceae, genus_Bacteroides (B); family_Oirogtrinibadaceael, genus_Parabacteroides (C) comparing groups wild-type (WT), SF, SF + LR, and SF + LGG (one-way ANOVA; n = 4–7 mice per group, data are presented as means ± SE).
Plasma Metabolomic Profiles Are Modulated by Treg Deficiency and Probiotics
Metabolites of commensal bacteria in the lumen and mucosa play a key role in microbe-host interactions (42, 43). We analyzed metabolomic profiles of plasma obtained from WT, SF, and SF with either LR (SF LR) or LGG (SF LGG) after overt symptoms.
Changes in Circulating Metabolites in Response to Probiotic Treatment
There were 696-named biochemicals in plasma that were identified and analyzed. When comparing SF and WT mice, 202 recognized metabolites showed significant changes, with 79 metabolites upregulated and 123 metabolites downregulated. When we compared SF + LR with SF without LR, 419 metabolites were significantly changed—with 336 metabolites upregulated and 83 metabolites downregulated by LR. Comparing SF + LGG with SF without LGG, 316 metabolites were significant changed—with 248 metabolites upregulated and 68 metabolites downregulated.
Similar Changes in Metabolites Associated with LR and LGG
Principal component analysis (PCA) was successful in segregating samples by genotype (WT and SF) and by treatment (control vs. SF + LR, or control vs. SF + LGG) on Components 2 and 1, respectively (Fig. 6A). These results are reflective of profound metabolic differences being present among plasma samples driven primarily by mouse genotype. Of importance to the aims of the study, samples from SF mice treated with different probiotics (LR or LGG) tended to segregate close to each other, suggesting a comparable metabolic profile among these groups. Hierarchical cluster analysis (HCA), a stepwise clustering method that groups metabolically similar samples close to one another, illustrated similar results with successful clustering of samples by probiotic treatment with WT and SF groups clustering to the right (Fig. 6B). N-Acetyl-kynurenine, a tryptophan metabolite strongly associated with systemic inflammation when indoleamine 2,3-dioxygenase (IDO) is activated by proinflammatory cytokines, catalyzes the conversion of tryptophan to kynurenine (Fig. 6Ca). N-Acetyl-kynurenine level was increased in the plasma in SF mice compared with that in WT control, an effect which was reduced by both probiotics, LR or LGG (Fig. 6Cb).
Figure 6.
Plasma metabolomics profiles are modified by Limosilactobacillus reuteri DSM 17938 (LR) or Lacticaseibacillus rhamnosus GG (LGG) treatment of scurfy (SF) mice. A: principal component analysis (PCA) clustering from plasma metabolites of wild-type (WT), SF, SF + LR, and SF + LGG (n = 6–8 mice per group). B: heat map of 696 metabolites in plasma of WT, SF, SF + LR, and SF + LGG mice. Each lane represents a different mouse. C: metabolite N-acetyl-kynurenine associated with systemic inflammation (a) and changed by LR and LGG in SF mice (b) comparing groups WT, SF, SF + LR, and SF + LGG (one-way ANOVA; n = 4–7 mice per group, data are presented as means ± SE); **P < 0.01, SF + LR or SF + LGG compared with SF.
Unique Changes in Metabolites by LR or LGG
Comparative analysis of significantly altered biochemicals in groups SF + LR or SF + LGG compared with SF without probiotic treatment revealed that 47 (of 419 with significant changes) and 30 (of 316 with significant changes) metabolites were uniquely altered by LR and LGG treatment, respectively. Among these unique changes by LR or LGG treatment, we identified several differentially significant changes linked to LR but not to LGG.
Altered Inosine Metabolites Linked to LR
We found a significant decrease in inosine and its associated metabolites, xanthine and xanthosine, in SF-control in relation to WT-control. Notably, treatment of SF mice with LR resulted in significantly increased levels of inosine, xanthine, and xanthosine (Fig. 7, A and B), confirming the results from our previous study showing that the interaction of microbiota and inosine-A2A receptor inhibits TH1 and TH2 cell-associated autoimmune disease (35, 36). In contrast, any changes of inosine metabolites by LGG treatment were not statistically significant (Fig. 7, A and B).
Figure 7.
Altered inosine metabolites by Limosilactobacillus reuteri DSM 17938 (LR) compared with Lacticaseibacillus rhamnosus GG (LGG). A: plasma intensity scale of inosine levels among the groups of wild-type (WT), scurfy (SF), SF + LR, and SF + LGG (n = 6–8 mice per group). B: the Interactive Pathway Analysis of nucleotides. The up- (red circle) or down- (blue circle), or no- (white circle) regulation of metabolites represent the group comparison of SF vs. WT, SF + LR vs. SF, or SF + LGG vs. SF. Each (red or blue or white) circle represents one metabolite. Each numbered circle represents the each nucleotide metabolism indicated in the figure.
Changes in Central Energy Metabolism—the Glycolysis-Tricarboxylic Acid Cycle
Glycolysis represents one of several pathways by which bacteria can catabolize glucose. SF mice showed a decline in glucose and the glycolytic intermediate 3-phosphoglycerate (3-PG) compared with WT mice. Interestingly, treatment of SF mice with LR but not LGG significantly increased relative levels of pyruvate and lactate, indicating increased glucose availability to be used in tricarboxylic acid (TCA) cycle. Significant decreases in several TCA cycle metabolites including α-ketoglutarate, succinate, fumarate, and malate were noted in SF control compared with WT control mice. LR treatment ameliorated the reduced levels of succinate, fumarate, and malate along with aconitate, whereas LGG treatment reversed succinate only (Fig. 8A).
Figure 8.
Differentially changed tricarboxylic acid (TCA) cycle and gamma-glutamyl (GG)-peptides by Limosilactobacillus reuteri DSM 17938 (LR) compared with Lacticaseibacillus rhamnosus GG (LGG). The Interactive Pathway Analysis: metabolites in energy (TCA cycle) (A); metabolites in peptide (GG-peptides) (B). The up- (red circle) or down- (blue circle) or no- (white circle) regulation of metabolites represent the group comparison of scurfy (SF) vs. wild-type (WT), SF + LR vs. SF, or SF + LGG vs. SF. Each (red or blue or white) circle represents one metabolite. Each numbered circle represents the each nucleotide metabolism indicated in the figure.
Evidence of Alterations in Redox Homeostasis with Probiotic LR Treatment in SF Mice
The tripeptide glutathione (GSH) is a small molecular weight thiol with antioxidant properties. Cysteine and methionine metabolism supports glutathione production, but there appeared to be minimal changes in cystathionine to cysteine conversion in probiotic-treated SF mice compared with SF controls. However, LR treatment upregulated the levels of 12 different gamma-glutamyl (GG) peptides (GGglutamate, GGhistidine, GGisoleucine, GGleucine, GG-alpha-lysine, GG-epsilon-lysine, GGmethionine, GGphenyalanine, GGthreonine, GGtryptophan, GGtyrosine GGvaline), whereas LGG treatment upregulated only two different GG peptides (GGglutamate and GGtryptophan) in SF mice (Fig. 8B). The addition of GG residues to amino acids plays a key role in the GG cycle, which maintains glutathione homeostasis and redox status (44).
LGG Effects on Bile Acid Metabolism
LGG uniquely upregulated both primary and secondary bile acid metabolism in SF mice compared with SF control mice. The upregulated primary bile acids in the plasma of SF mice which were fed LGG included cholate, with a fold change (FC) of 15, glycocholate (FC: 7.4), and β-muricholate (FC: 4.4). The upregulated secondary bile acids included 3-dehydrocholate (FC: 16.2), deoxycholate (FC: 4.4), and ursodeozycholate (FC: 3.6).
DISCUSSION
The probiotic L. reuteri DSM 17938 (LR) has immune modulatory functions and has been shown to have beneficial effects in infants with acute diarrhea (17, 18), infantile colic (23), and necrotizing enterocolitis (NEC) (21, 22), and in rodent models of NEC (25, 27, 45) and autoimmunity (35,36, 46). In the current study, we compared LR with another well-characterized probiotic, LGG, in the Treg-deficient SF mouse. We demonstrate that LR has more potency to prevent autoimmunity and prolong survival in this model than LGG, although both probiotics were beneficial. We observed that both probiotics restored fecal microbial alpha diversity, but they produced distinct fecal bacterial clusters and differentially modulated relative abundance of specific taxa. However, plasma metabolomics profiles from two different probiotic treatments were comparable, reflecting similar metabolic effects in vivo, even though some unique changes in metabolomics by LR were observed.
A Shift in Microbial Community Composition
LR treatment clearly altered the microbiota in this model, with markedly increased Firmicutes and reduced Bacteroidetes. After LR gavage feeding to SF mice, there were no observable Porphyromonadaceae, Bacteroides, or Parabacteroides, whereas Ruminococcaceae, important short-chain fatty acid (SCFAs) producers (47), were preserved. SCFAs, such as pentanoate (48), have anti-inflammatory mechanisms—such as inducing tissue levels of the anti-inflammatory cytokine IL-10 and suppressing the generation of proinflammatory cytokine IL-17a. We suggest that the preservation of certain genera by LR supplementation may have a role in immunomodulation. On the other hand, LGG’s major impact was to increase α- and β-Proteobacteria, which are mostly classified as commensal bacteria, even though the exact roles or functions of these microbes are unclear. LGG reduced γ-Proteobacteria, which represent a large class of Gram-negative bacteria that includes a number of potential pathogens such as Klebsiella, Enterobacter, Citrobacter, Salmonella, Escherichia coli, Shigella, Proteus, and Serratia (49). These pathogens occasionally spread to the bloodstream, resulting in life-threatening complications. Interestingly, even though LR did not affect the relative abundance of α- and β-Proteobacteria, it clearly reduced Enterobacteriaceae in SF mice. Both LR and LGG reduced Enterobacteriaceae, suggesting their potential applications for controlling or preventing the colonization or infection by pathogenic Enterobacteriaceae. A possible mechanism for Lactobacilli to reduce Enterobacteriaceae would be by reducing intestinal pH by producing lactic acid, acetic acid, formic acid, and other acids. Importantly, Lactobacilli can also secrete certain antimicrobial molecules, such as reuterin, ethanol, fatty acid, hydrogen peroxide, and bacteriocins to exert the antimicrobial activity (50–52). Lactobacilli have demonstrated the ability to inhibit several bacterial pathogens that belong to Enterobacteriaceae family, including Klebsiella oxytoca (53), E. coli (54), Shigella spp. (55), and carbapenem-resistant Enterobacteriaceae (CRE) (56). Recent studies showed a correlation between antimicrobial activity and metabolic profile produced by LR in cell-free supernatants, with the antimicrobial effect likely related to pH-dependent compounds (57).
Adenosine Receptor-Mediated Inflammatory Control
One factor that may result in the additional benefit of LR in this model was its property to act via leukocyte adenosine receptors. It is known that the lethal lymphoproliferative syndrome in SF mice is predominately mediated by TH1 and TH2 cell-induced pathology (58, 59). Activation of the adenosine/inosine/adenosine receptor 2A (A2A) is one of the main mechanisms by which Treg cells control TH1 and TH2-induced autoinflammation (60, 61). In our previous studies, we demonstrated that LR treatment starting at day of life 8 increased plasma inosine levels. Inosine, a purine capable of activating adenosine receptors, was found to suppress differentiation of TH1/TH2 cells in vitro (36). Moreover, other studies reported that CGS21680, an agonist of A2A receptors, inhibited the proliferation of CD4+ T cells that were isolated from WT mice but failed to block the proliferation of cells obtained from A2A−/− mice (60). These findings demonstrated that even in the absence of Tregs, the activated A2A receptor may play a critical role in the suppression of TH1 and TH2 cells. However, LGG may not share this mechanism of action, because in LGG-treated mice inosine, hypoxanthine, and xanthosine were not upregulated.
Markedly Increased Gamma-Glutamyl Peptides
An unmistakable finding of this study was that LR and not LGG increases the levels of 12 different gamma-glutamyl (GG) peptides. GG peptides are reported to exhibit increased transport properties, because the GG moiety promotes translocation across lipid barriers (44). For example, the addition of GG residues to amino acid increases the translocation of amino acid across placental membranes and thereby helps to maintain glutathione homeostasis and redox status. Furthermore, gamma-glutamyl dipeptides are indicative of improved oxidative stress coping mechanisms (62). For example, microbial communities of patients suffering from ulcerative colitis were associated with a lower disease activity if they exhibited a capacity for quenching reactive oxidative species (ROS). Reduced ROS were associated with enhanced gamma-glutamyl transferase (GGT) activity, shown by a correlation between enrichment of GG peptides and preserved glutathione levels (63).
Changes in the gut microbiota in response to LR may play a role in the regulation of GG amino acids. It has been shown that the enrichment of Akkermansia muciniphila and Parabacteroides correlates with reduced fecal GGT activity and levels of colonic and serum GG peptides (64). The mechanisms by which microbes are able to change the levels of these GG peptides warrant further investigation. It will be important to determine how the microbiota-associated gamma glutamylation of AA plays a role in immunomodulation in other inflammatory and immune processes.
Bile Acid Metabolism Regulated by LGG
Bile acids function as hormones or nutrient signaling molecules that help to regulate glucose, lipid, lipoprotein, energy metabolism, and inflammatory responses (65, 66). Bile acids constitute the products of co-metabolism by both host and microbiome (67). Chronic low-grade inflammation associated with insulin resistance and inflammatory bowel disease (IBD) has shown to inhibit bile acid signaling and disrupt lipid metabolism (65). Currently, there are no specific reports on dysregulated bile acid pools in SF mice or IPEX syndrome. However, in our current studies, it showed that LGG upregulated several primary and secondary bile acids in SF mice. Secondary bile acids can act in multiple ways as signaling molecules, through activation of the nuclear Farnesoid X receptor (FXR), vitamin D receptor, and through surface expressed G-protein-coupled receptors (GPCRs) (66). Engagement of these compounds beneficially affect many physiological aspects (66, 68), especially, recently shown bile acids in regulating Treg-TH17 axis by promoting Treg generation and reducing inflammatory T-cell function (69). However, accumulated higher levels of circulating bile acids have cytotoxic effects. The cytotoxicity of bile acids is dependent on their structural formation associated with their hydrophobicity. Increased serum levels of hydrophobic bile acids, such as chenodeoxycholic acid (CDCA) and deoxycholic acid (DCA), have been associated with colon cancer, gallstones, and other gastrointestinal (GI) diseases (70, 71). Recent studies in the mouse model of liver fibrosis showed that LGG prevents liver fibrosis through inhibiting hepatic bile acid synthesis and enhancing bile acid excretion (72).
The aim of this study was to determine the specificity of LR in Treg-deficiency-induced autoimmunity. From a clinical standpoint, comparative efficacy research for different probiotics in various diseases is very unlikely to take place. We believe that there is clear evidence that specific probiotics may be reliable in benefitting specific inflammatory conditions (12), but there are too many probiotics and too vast a number of diseases to allow comparison of the numerous probiotics; and the appropriate doses have also not been determined. It will also be impossible to conduct in vitro high-throughput studies of the different microbial species using intestinal epithelia or bioreactors, because these models may not be applicable to human diseases. However, in our opinion, a sound approach is to investigate well-established animal models to test a limited number of probiotics with proven physiological effects. Our study is one-step in this direction, and although IPEX syndrome is rare, LR may be beneficial in other Treg-deficiency-related monogenic immune disorders and possibly in other autoimmune conditions (73, 74), early-onset inflammatory bowel disease (IBD) (75), or graft-versus-host disease (GVHD) (76, 77).
SUPPLEMENTAL MATERIAL
Supplemental material is available at https://doi.org/10.6084/m9.figshare.14062001.
GRANTS
This work was supported by National Institutes of Health/National Center for Complementary and Integrative Health (NIH/NCCIH) Grant R01AT007083 (to J. M. Rhoads and Y. Liu) and by National Institute of Allergy and Infectious Diseases (NIH/NIAID) Grant R03AI117442 (Y. Liu). The authors acknowledge support of BioGaia AB in other research projects, but BioGaia did not fund this project.
DISCLOSURES
The authors acknowledge support of BioGaia AB in other research projects, but BioGaia did not fund this project. Stefan Roos has part-time employment by the company BioGaia AB, Stockholm, Sweden. Other authors declare no conflict of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
Y.L. and J.M.R. conceived and designed research; Y.L., T.K.H., E.S.P., J.F., and M.L. performed experiments; Y.L., T.K.H., C.M.T., E.S.P., J.F., and M.L. analyzed data; Y.L., T.K.H., C.M.T., S.R., and J.M.R. interpreted results of experiments; Y.L. and T.K.H. prepared figures; Y.L. drafted manuscript; Y.L., T.K.H., C.M.T., E.S.P., J.F., M.L., S.R., and J.M.R. edited and revised manuscript; Y.L., T.K.H., C.M.T., E.S.P., J.F., M.L., S.R., and J.M.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Guoyao Wu (Department of Animal Science, Texas A&M University, College Station, Texas) for assistance of metabolomics profile interpretation and Dr. Robert Britton (Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas) for interpretation of gut microbiota and metabolomics data. We thank Dr. Eamonn Connolly and Dr. Stefan Roos at BioGaia AB (Stockholm, Sweden) for providing probiotic Limosilactobacillus reuteri DSM 17938 and Lacticaseiobacillus rhamnosus GG.
REFERENCES
- 1.Shevach EM. Special regulatory T cell review: how I became a T suppressor/regulatory cell maven. Immunology 123: 3–5, 2008. doi: 10.1111/j.1365-2567.2007.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shevach EM, Tran DQ, Davidson TS, Andersson J. The critical contribution of TGF-β to the induction of Foxp3 expression and regulatory T cell function. Eur J Immunol 38: 915–917, 2008. doi: 10.1002/eji.200738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett CL, Brunkow ME, Ramsdell F, O'Briant KC, Zhu Q, Fuleihan RL, Shigeoka AO, Ochs HD, Chance PF. A rare polyadenylation signal mutation of the FOXP3 gene (AAUAAA–>AAUGAA) leads to the IPEX syndrome. Immunogenetics 53: 435–439, 2001. doi: 10.1007/s002510100358. [DOI] [PubMed] [Google Scholar]
- 4.Tan QKG, Louie RJ, Sleasman JW. IPEX syndrome. In: GeneReviews [Internet], edited by Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A.. Seattle, WA: University of Washington, Seattle, 2019, p. 1993–2019 [updated 2018 Jul 19]. [PubMed] [Google Scholar]
- 5.Barzaghi F, Amaya Hernandez LC, Neven B, Ricci S, Kucuk ZY, Bleesing JJ , et al.; Primary Immune Deficiency Treatment Consortium (PIDTC) and the Inborn Errors Working Party (IEWP) of the European Society for Blood and Marrow Transplantation (EBMT). Long-term follow-up of IPEX syndrome patients after different therapeutic strategies: an international multicenter retrospective study. J Allergy Clin Immunol 141: 1036–1049, 2018. doi: 10.1016/j.jaci.2017.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett CL, Ochs HD. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr Opin Pediatr 13: 533–538, 2001. doi: 10.1097/00008480-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Duclaux-Loras R, Charbit-Henrion F, Neven B, Nowak J, Collardeau-Frachon S, Malcus C, Ray PF, Moshous D, Beltrand J, Goulet O, Cerf-Bensussan N, Lachaux A, Rieux-Laucat F, Ruemmele FM. Clinical heterogeneity of immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome: a French Multicenter Retrospective Study. Clin Transl Gastroenterol 9: 201, 2018. doi: 10.1038/s41424-018-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfrey VL, Wilkinson JE, Russell LB. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J Pathol 138: 1379–1387, 1991. [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma R, Deshmukh US, Zheng L, Fu SM, Ju ST. X-linked Foxp3 (Scurfy) mutation dominantly inhibits submandibular gland development and inflammation respectively through adaptive and innate immune mechanisms. J Immunol 183: 3212–3218, 2009. doi: 10.4049/jimmunol.0804355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung HJ, Sim JH, Min TS, Choi HK. Metabolomics and lipidomics approaches in the science of probiotics: a review. J Med Food 21: 1086–1095, 2018. doi: 10.1089/jmf.2017.4175. [DOI] [PubMed] [Google Scholar]
- 11.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol 6: 39–51, 2013. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Alookaran JJ, Rhoads JM. Probiotics in autoimmune and inflammatory disorders. Nutrients 10: 1537, 2018. doi: 10.3390/nu10101537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Tran DQ, Rhoads JM. Probiotics in disease prevention and treatment. J Clin Pharmacol 58, Suppl 10: S164–S179, 2018. doi: 10.1002/jcph.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders ME, Guarner F, Guerrant R, Holt PR, Quigley EM, Sartor RB, Sherman PM, Mayer EA. An update on the use and investigation of probiotics in health and disease. Gut 62: 787–796, 2013. doi: 10.1136/gutjnl-2012-302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter J, Britton RA, Roos S. Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc Natl Acad Sci USA 108, Suppl 1: 4645–4652, 2011. doi: 10.1073/pnas.1000099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walter J, Ley R. The human gut microbiome: ecology and recent evolutionary changes. Annu Rev Microbiol 65: 411–429, 2011. doi: 10.1146/annurev-micro-090110-102830. [DOI] [PubMed] [Google Scholar]
- 17.Francavilla R, Lionetti E, Castellaneta S, Ciruzzi F, Indrio F, Masciale A, Fontana C, La Rosa MM, Cavallo L, Francavilla A. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea—a double-blind study. Aliment Pharmacol Ther 36: 363–369, 2012. doi: 10.1111/j.1365-2036.2012.05180.x. [DOI] [PubMed] [Google Scholar]
- 18.Shornikova AV, Casas IA, Isolauri E, Mykkanen H, Vesikari T. Lactobacillus reuteri as a therapeutic agent in acute diarrhea in young children. J Pediatr Gastroenterol Nutr 24: 399–404, 1997. doi: 10.1097/00005176-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Urbańska M, Gieruszczak-Białek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther 43: 1025–1034, 2016. doi: 10.1111/apt.13590. [DOI] [PubMed] [Google Scholar]
- 20.Athalye-Jape G, Rao S, Patole S. Lactobacillus reuteri DSM 17938 as a probiotic for preterm neonates: a strain-specific systematic review. JPEN J Parenter Enteral Nutr 40: 783–794, 2016. doi: 10.1177/0148607115588113. [DOI] [PubMed] [Google Scholar]
- 21.Oncel MY, Sari FN, Arayici S, Guzoglu N, Erdeve O, Uras N, Oguz SS, Dilmen U. Lactobacillus reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 99: F110–F115, 2014. doi: 10.1136/archdischild-2013-304745. [DOI] [PubMed] [Google Scholar]
- 22.Rojas MA, Lozano JM, Rojas MX, Rodriguez VA, Rondon MA, Bastidas JA, Perez LA, Rojas C, Ovalle O, Garcia-Harker JE, Tamayo ME, Ruiz GC, Ballesteros A, Archila MM, Arevalo M. Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics 130: e1113–e1120, 2012. doi: 10.1542/peds.2011-3584. [DOI] [PubMed] [Google Scholar]
- 23.Sung V, Collett S, de GT, Hiscock H, Tang M, Wake M. Probiotics to prevent or treat excessive infant crying: systematic review and meta-analysis. JAMA Pediatr 167: 1150–1157, 2013. doi: 10.1001/jamapediatrics.2013.2572. [DOI] [PubMed] [Google Scholar]
- 24.Urbańska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr 173: 1327–1337, 2014. doi: 10.1007/s00431-014-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Fatheree NY, Mangalat N, Rhoads JM. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-κB signaling in the intestine. Am J Physiol Gastrointest Liver Physiol 302: G608–G617, 2012. doi: 10.1152/ajpgi.00266.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Fatheree NY, Dingle BM, Tran DQ, Rhoads M. Lactobacillus reuteri DSM 17938 changes the frequency of Foxp3+ regulatory T cells in the intestine and mesenteric lymph node in experimental necrotizing enterocolitis. PLoS One 8: e56547, 2013. doi: 10.1371/journal.pone.0056547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Tran DQ, Fatheree NY, Marc RJ. Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3+ regulatory T cells in a mouse model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 307: G177–G186, 2014. doi: 10.1152/ajpgi.00038.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoang TK, He B, Wang T, Tran DQ, Rhoads JM, Liu Y. Protective effect of Lactobacillus reuteri DSM 17938 against experimental necrotizing enterocolitis is mediated by Toll-like receptor 2. Am J Physiol Gastrointest Liver Physiol 315: G231–G240, 2018. doi: 10.1152/ajpgi.00084.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He B, Hoang TK, Tian X, Taylor CM, Blanchard E, Luo M, Bhattacharjee MB, Lindsey JM, Tran DQ, Marc Rhoads J, Liu Y. Lactobacillus reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota. Front Immunol 10:385, 2018. doi: 10.3389/fimmu.2019.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, Theriaque D, Li N, Sharma R, Hudak M, Neu J. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One 6: e20647, 2011. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel RM, Denning PW. Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatr Res 78: 232–238, 2015. doi: 10.1038/pr.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhoads JM, Collins J, Fatheree NY, Hashmi SS, Taylor CM, Luo M, Hoang TK, Gleason WA, Van Arsdall MR, Navarro F, Liu Y. Infant colic represents gut inflammation and dysbiosis. J Pediatr 203: 55–61.e3, 2018. doi: 10.1016/j.jpeds.2018.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhoads JM, Fatheree NY, Norori J, Liu Y, Lucke JF, Tyson JE, Ferris MJ. Altered fecal microflora and increased fecal calprotectin in infants with colic. J Pediatr 155: 823–828, 2009. doi: 10.1016/j.jpeds.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Torrazza RM, Neu J. The altered gut microbiome and necrotizing enterocolitis. Clin Perinatol 40: 93–108, 2013. doi: 10.1016/j.clp.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He B, Hoang TK, Tran DQ, Rhoads JM, Liu Y. Adenosine A2A receptor deletion blocks the beneficial effects of Lactobacillus reuteri in regulatory T-deficient Scurfy mice. Front Immunol 8: 1680, 2017. doi: 10.3389/fimmu.2017.01680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He B, Hoang TK, Wang T, Ferris M, Taylor CM, Tian X, Luo M, Tran DQ, Zhou J, Tatevian N, Luo F, Molina JG, Blackburn MR, Gomez TH, Roos S, Rhoads JM, Liu Y. Resetting microbiota by Lactobacillus reuteri inhibits T reg deficiency-induced autoimmunity via adenosine A2A receptors. J Exp Med 214: 107–123, 2017. doi: 10.1084/jem.20160961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li N, Russell WM, Douglas-Escobar M, Hauser N, Lopez M, Neu J. Live and heat-killed Lactobacillus rhamnosus GG: effects on proinflammatory and anti-inflammatory cytokines/chemokines in gastrostomy-fed infant rats. Pediatr Res 66: 203–207, 2009. doi: 10.1203/pdr.0b013e3181aabd4f. [DOI] [PubMed] [Google Scholar]
- 38.Capurso L. Thirty years of Lactobacillus rhamnosus GG: a review. J Clin Gastroenterol 53, Suppl 1: S1–S41, 2019. doi: 10.1097/MCG.0000000000001170. [DOI] [PubMed] [Google Scholar]
- 39.Segers ME, Lebeer S. Towards a better understanding of Lactobacillus rhamnosus GG–host interactions. Microb Cell Fact 13, Suppl 1: S7, 2014. doi: 10.1186/1475-2859-13-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard E, Taylor CM, Welsh DA, Berthoud HR. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry 77: 607–615, 2015. doi: 10.1016/j.biopsych.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336, 2010. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maslowski KM. Metabolism at the centre of the host-microbe relationship. Clin Exp Immunol 197: 193–204, 2019. doi: 10.1111/cei.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science 336: 1262–1267, 2012. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 44.Griffith OW, Bridges RJ, Meister A. Transport of gamma-glutamyl amino acids: role of glutathione and gamma-glutamyl transpeptidase. Proc Natl Acad Sci USA 76: 6319–6322, 1979. doi: 10.1073/pnas.76.12.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoang TK, He B, Wang T, Tran DQ, Rhoads JM, Liu Y. Protective effect of Lactobacillus reuteri DSM 17938 against experimental necrotizing enterocolitis is mediated by Toll-like receptor 2. Am J Physiol Gastrointest Liver Physiol 315: G231–G240, 2018. doi: 10.1152/ajpgi.00084.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He B, Hoang TK, Tian X, Taylor CM, Blanchard E, Luo M, Bhattacharjee MB, Freeborn J, Park S, Couturier J, Lindsey JW, Tran DQ, Rhoads JM, Liu Y. Lactobacillus reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota. Front Immunol 10: 385, 2019. doi: 10.3389/fimmu.2019.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Martinez I, Walter J, Keshavarzian A, Rose DJ. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 23: 74–81, 2013. doi: 10.1016/j.anaerobe.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 48.Luu M, Pautz S, Kohl V, Singh R, Romero R, Lucas S, Hofmann J, Raifer H, Vachharajani N, Carrascosa LC, Lamp B, Nist A, Stiewe T, Shaul Y, Adhikary T, Zaiss MM, Lauth M, Steinhoff U, Visekruna A. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun 10: 760, 2019. doi: 10.1038/s41467-019-08711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bublitz DC, Wright PC, Bodager JR, Rasambainarivo FT, Bliska JB, Gillespie TR. Epidemiology of pathogenic enterobacteria in humans, livestock, and peridomestic rodents in rural Madagascar. PLoS One 9: e101456, 2014. doi: 10.1371/journal.pone.0101456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Georgieva R, Yocheva L, Tserovska L, Zhelezova G, Stefanova N, Atanasova A, Danguleva A, Ivanova G, Karapetkov N, Rumyan N, Karaivanova E. Antimicrobial activity and antibiotic susceptibility of Lactobacillus and Bifidobacterium spp. intended for use as starter and probiotic cultures. Biotechnol Biotechnol Equip 29: 84–91, 2015. doi: 10.1080/13102818.2014.987450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inglin RC, Stevens MJ, Meile L, Lacroix C, Meile L. High-throughput screening assays for antibacterial and antifungal activities of Lactobacillus species. J Microbiol Methods 114: 26–29, 2015. doi: 10.1016/j.mimet.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Ortiz-Rivera Y, Sanchez-Vega R, Gutierrez-Mendez N, Leon-Felix J, Acosta-Muniz C, Sepulveda DR. Production of reuterin in a fermented milk product by Lactobacillus reuteri: Inhibition of pathogens, spoilage microorganisms, and lactic acid bacteria. J Dairy Sci 100: 4258–4268, 2017. doi: 10.3168/jds.2016-11534. [DOI] [PubMed] [Google Scholar]
- 53.Higashi B, Mariano TB, de Abreu Filho BA, Goncalves RAC, de Oliveira AJB. Effects of fructans and probiotics on the inhibition of Klebsiella oxytoca and the production of short-chain fatty acids assessed by NMR spectroscopy. Carbohydr Polym 248: 116832, 2020. [Erratum in Carbohydr Polym 260: 117568, 2021]. doi: 10.1016/j.carbpol.2020.116832. [DOI] [PubMed] [Google Scholar]
- 54.Kumar M, Dhaka P, Vijay D, Vergis J, Mohan V, Kumar A, Kurkure NV, Barbuddhe SB, Malik SV, Rawool DB. Antimicrobial effects of Lactobacillus plantarum and Lactobacillus acidophilus against multidrug-resistant enteroaggregative Escherichia coli. Int J Antimicrob Agents 48: 265–270, 2016. doi: 10.1016/j.ijantimicag.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 55.Mirnejad R, Vahdati AR, Rashidiani J, Erfani M, Piranfar V. The antimicrobial effect of Lactobacillus casei culture supernatant against multiple drug resistant clinical isolates of Shigella sonnei and Shigella flexneri in vitro. Iran Red Crescent Med J 15: 122–126, 2013. doi: 10.5812/ircmj.7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen CC, Lai CC, Huang HL, Huang WY, Toh HS, Weng TC, Chuang YC, Lu YC, Tang HJ. Antimicrobial activity of Lactobacillus species against carbapenem-resistant enterobacteriaceae. Front Microbiol 10: 789, 2019. doi: 10.3389/fmicb.2019.00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maccelli A, Carradori S, Puca V, Sisto F, Lanuti P, Crestoni ME, Lasalvia A, Muraro R, Bysell H, Di SA, Roos S, Grande R. Correlation between the antimicrobial activity and metabolic profiles of cell free supernatants and membrane vesicles produced by Lactobacillus reuteri DSM 17938. Microorganisms 8: 1653, 2020. doi: 10.3390/microorganisms8111653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma R, Sharma PR, Kim YC, Leitinger N, Lee JK, Fu SM, Ju ST. IL-2-controlled expression of multiple T cell trafficking genes and Th2 cytokines in the regulatory T cell-deficient scurfy mice: implication to multiorgan inflammation and control of skin and lung inflammation. J Immunol 186: 1268–1278, 2011. doi: 10.4049/jimmunol.1002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suscovich TJ, Perdue NR, Campbell DJ. Type-1 immunity drives early lethality in scurfy mice. Eur J Immunol 42: 2305–2310, 2012. doi: 10.1002/eji.201242391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Csoka B, Himer L, Selmeczy Z, Vizi ES, Pacher P, Ledent C, Deitch EA, Spolarics Z, Nemeth ZH, Hasko G. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB J 22: 3491–3499, 2008. doi: 10.1096/fj.08-107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204: 1257–1265, 2007. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mistry D, Stockley RA. Gamma-glutamyl transferase: the silent partner? COPD 7: 285–290, 2010. doi: 10.3109/15412555.2010.496819. [DOI] [PubMed] [Google Scholar]
- 63.Mar JS, LaMere BJ, Lin DL, Levan S, Nazareth M, Mahadevan U, Lynch SV. Disease severity and immune activity relate to distinct interkingdom gut microbiome states in ethnically distinct ulcerative colitis patients. mBio 7: e01072–16, 2016. doi: 10.1128/mBio.01072-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 174: 497, 2018. doi: 10.1016/j.cell.2018.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen ML, Takeda K, Sundrud MS. Emerging roles of bile acids in mucosal immunity and inflammation. Mucosal Immunol 12: 851–861, 2019. doi: 10.1038/s41385-019-0162-4. [DOI] [PubMed] [Google Scholar]
- 66.Zhou H, Hylemon PB. Bile acids are nutrient signaling hormones. Steroids 86: 62–68, 2014. doi: 10.1016/j.steroids.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Funabashi M, Grove TL, Wang M, Varma Y, McFadden ME, Brown LC, Guo C, Higginbottom S, Almo SC, Fischbach MA. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature 582: 566–570, 2020. doi: 10.1038/s41586-020-2396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di CA, Garruti G, Lunardi BR, Molina-Molina E, Bonfrate L, Wang DQ, Portincasa P. Bile acid physiology. Ann Hepatol 16, Suppl 1: S4–S14, 2017. doi: 10.5604/01.3001.0010.5493. [DOI] [PubMed] [Google Scholar]
- 69.Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, Mai C, Jin WB, Guo CJ, Violante S, Ramos RJ, Cross JR, Kadaveru K, Hambor J, Rudensky AY. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581: 475–479, 2020. doi: 10.1038/s41586-020-2193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hanafi NI, Mohamed AS, Sheikh Abdul Kadir SH, Othman MHD. Overview of bile acids signaling and perspective on the signal of ursodeoxycholic acid, the most hydrophilic bile acid, in the heart. Biomolecules 8: 159, 2018. doi: 10.3390/biom8040159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47: 241–259, 2006. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y, Chen K, Li F, Gu Z, Liu Q, He L, Shao T, Song Q, Zhu F, Zhang L, Jiang M, Zhou Y, Barve S, Zhang X, McClain CJ, Feng W. Probiotic Lactobacillus rhamnosus GG prevents liver fibrosis through inhibiting hepatic bile acid synthesis and enhancing bile acid excretion in mice. Hepatology 71: 2050–2066, 2020. doi: 10.1002/hep.30975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Azizi G, Yazdani R, Rae W, Abolhassani H, Rojas M, Aghamohammadi A, Anaya JM. Monogenic polyautoimmunity in primary immunodeficiency diseases. Autoimmun Rev 17: 1028–1039, 2018. doi: 10.1016/j.autrev.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 74.Cheng MH, Anderson MS. Monogenic autoimmunity. Annu Rev Immunol 30: 393–427, 2012. doi: 10.1146/annurev-immunol-020711-074953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pazmandi J, Kalinichenko A, Ardy RC, Boztug K. Early-onset inflammatory bowel disease as a model disease to identify key regulators of immune homeostasis mechanisms. Immunol Rev 287: 162–185, 2019. doi: 10.1111/imr.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fredricks DN. The gut microbiota and graft-versus-host disease. J Clin Invest 129: 1808–1817, 2019. doi: 10.1172/JCI125797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hippen KL, Aguilar EG, Rhee SY, Bolivar-Wagers S, Blazar BR. Distinct regulatory and effector T cell metabolic demands during graft-versus-host disease. Trends Immunol 41: 77–91, 2020. doi: 10.1016/j.it.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]