Keywords: female, insulin signaling, liver, testosterone, white adipose tissue
Abstract
Transgender men undergoing hormone therapy are at risk for insulin resistance. However, how virilizing testosterone therapy affects serum insulin and peripheral insulin sensitivity in transgender men is unknown. This study assessed the effect of acute, virilizing testosterone on serum insulin concentrations and insulin signaling in liver, skeletal muscle, and white adipose tissue (WAT) of female pigs as a translational model for transgender men. Females received three doses of intramuscular testosterone cypionate (TEST females; 50 mg/day/pig) or corn oil (control) spaced 6 days apart starting on the day of estrus (D0). Fasting blood was collected on D0, D3, D5, D11, and D13, and females were euthanized on D13. On D13, TEST females had virilizing concentrations of serum testosterone with normal concentrations of serum estradiol. Virilizing serum testosterone concentrations (D13) were associated with decreased serum insulin and C-peptide concentrations. Blood glucose and serum glycerol concentrations were not altered by testosterone. Virilizing concentrations of testosterone downregulated AR and ESR1 in subcutaneous (sc) WAT and upregulated transcript levels of insulin-signaling pathway components in WAT and liver. At the protein level, virilizing testosterone concentrations were associated with increased PI3K 110α in liver and increased insulin receptor (INSR) and phospho(Ser256)-FOXO1 in visceral (v) WAT but decreased phospho(Ser473)-AKT in vWAT and scWAT. These results suggest that acute exposure to virilizing concentrations of testosterone suppresses circulating insulin levels and results in increased abundance of proteins in the insulin-signaling pathway in liver and altered phosphorylation of key proteins in control of insulin sensitivity in WAT.
NEW & NOTEWORTHY Acute virilizing doses of testosterone administered to females suppress circulating insulin levels, upregulate components of the insulin-signaling pathway in liver, and suppress insulin signaling in white adipose tissue. These results suggest that insulin resistance in transgender men may be due to suppression of the insulin-signaling pathway and decreased insulin sensitivity in white adipose tissue.
INTRODUCTION
Nearly 1.5 million Americans identify with a gender other than their birth-assigned gender (1). Masculinizing therapies such as intramuscular injections, subcutaneous implants, and transdermal patches of testosterone cause the development of secondary sex characteristics in females transitioning to males (trans-men) (2). Over a period of 6–9 months after the initiation of testosterone therapy, trans-men experience: cessation of menstruation, increased facial and body hair, a redistribution of body fat, and increased muscle mass (3). However, masculinizing therapy in trans-men has adverse side effects such as acne, androgenic alopecia, and increased risk of cardiometabolic disease (4, 5). Trans-men receiving testosterone therapy are not only at long-term risk for cardiovascular disease, but also experience an increased risk for insulin resistance and type II diabetes mellitus (T2DM) (6). Yet, definitive data on the effects of testosterone therapy on insulin sensitivity in trans-men are inconclusive due to the heterogeneity of the patient population, short-term patient follow-up, and small cohort sizes (7, 8). In addition, there has been little research in females regarding the acute effects of virilizing doses of testosterone on circulating insulin concentrations and the insulin-signaling pathway in tissues that control peripheral insulin sensitivity.
Although liver and skeletal muscles are the principal tissues in control of peripheral insulin sensitivity, white adipose tissue (WAT) is also a significant regulator of peripheral insulin-mediated glucose uptake (9, 10). When delivered at low dosages for several weeks, potent androgens like dihydrotestosterone (DHT) have been shown to downregulate protein and transcript levels in the insulin-signaling pathway in liver and WAT, but not skeletal muscle, of female mice (11), and this effect is caused by direct activity at the androgen receptor (12). DHT has also been shown to block lipogenesis and enhance forskolin-stimulated lipolysis in both visceral (v) WAT (13, 14) and subcutaneous (sc) WAT of men (13). With respect to aromatizable androgens, testosterone causes decreased insulin-mediated glucose uptake in vitro in subcutaneous adipocytes from women but flutamide minimally reverses this effect, indicating that the effect may not be androgen mediated (15). Given that virilizing doses of testosterone could be aromatized locally in WAT as well as other locations in the body, some effects of testosterone in trans-men may not be androgen-mediated. Furthermore, WAT is a heterogenous tissue made up of many cell types including immune cells, stromovascular cells, preadipocytes, and adipocytes (16), and, as such, interpretations of adipocyte function using in vitro monocellular cultures must be made carefully.
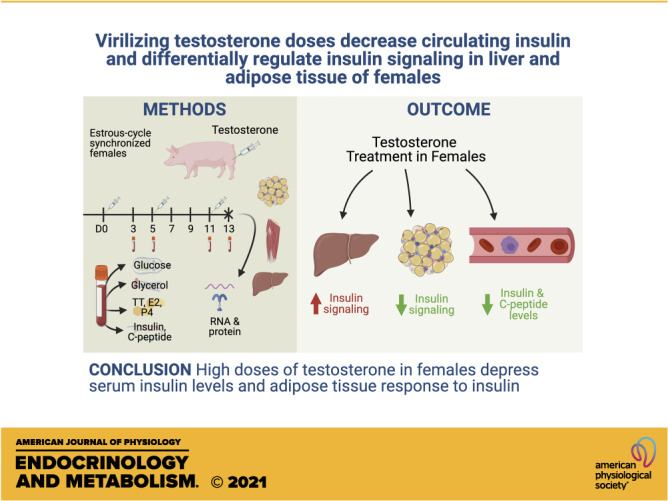
Most studies on the effects of aromatizable (i.e., testosterone) and nonaromatizable (i.e., DHT) androgens on insulin signaling have been conducted in rodents (17, 18), in vitro (19), with low or moderate dosages of androgens (18, 20), or over months or years (21). Therefore, a study of the acute effects of virilizing doses of testosterone in females using a translational animal model would provide an improved knowledge upon which protocols for initial dosing of testosterone in trans men patients could be based. Pigs are an ideal translational model frequently used in cardiometabolic research, which appropriately mimic the development of metabolic syndrome and recapitulate the WAT compartments of humans (22, 23). We hypothesized that acute, virilizing doses of intramuscular testosterone would increase serum insulin concentrations and decrease the expression of insulin-signaling pathway components in liver, vWAT, and scWAT in female pigs.
MATERIALS AND METHODS
Animal Experimental Design
All experimental procedures were performed in compliance with University of Illinois Urbana-Champaign (UIUC) Institutional Animal Care and Use Committee (IACUC) regulation and followed the guidelines of the Guide for the Care and Use of Laboratory Animals (24). The experiments conducted herein were approved by the UIUC IACUC (IACUC Number: 11114).
Eleven landrace-cross female pigs (Sus scrofa) [source: Imported Swine Research Laboratory (ISRL)-UIUC, Champaign, IL] weighing 160–170 kg each were housed individually in pens. Females were fed 2,200 kcal/day of a diet of corn (57.5%) and soy (40%), which provided 16.6% kcal of protein, 80.1% kcal of carbohydrates, and 3.3% kcal of fat (Rund Diet, Urbana, IL). The diet was formulated to meet all requirements for all vitamins and trace minerals were met. Females had ad libitum water and were on a 12:12 h light/dark cycle. At the onset of the study, pigs were 7 mo old. The pigs were housed at ISRL for an acclimation of four estrous cycles (∼3 mo) before beginning their respective treatments. Pigs were age- and sire-matched across treatment groups. A boar was used to detect estrus in each female on a daily basis; vaginal cytology for estimation of estrus in this species is not accurate or typically used. On the morning of estrus (D0) of the fifth cycle when serum estradiol (E2) was at its peak, each female had a 24-h fasting blood drawn followed by an intramuscular injection of either testosterone cypionate (Depo-testosterone, Pfizer, New York City; 50 mg/day/pig or 0.5 mL/day/pig; n = 5) or sterile, filtered corn oil (placebo, 0.5 mL/day/pig; n = 6). A pilot study was undertaken with four female pigs to determine the dose and frequency of testosterone cypionate necessary to achieve masculine levels of serum testosterone (Supplemental Fig. S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.14373545.v1). Based on that preliminary study, pigs were injected intramuscularly with the same volume of their respective treatments also on days 5 (D5) and 11 (D11) postestrus (i.e., every 6 days). Fasting blood was collected from the jugular vein with a vacutainer system on D0, D3, D5, D11, and D13. Whole blood was measured for glucose using a Precision Xtra glucometer (Abbott Laboratories, Bedford, MA) previously validated for use in swine (25). Blood was centrifuged and serum was stored at −20°C until analysis for total testosterone (TT), estradiol (E2), progesterone (P4), insulin, C-peptide, and glycerol. On the 13th day posttreatment onset, animals were sedated with 1 mL/23 kg body wt of Telazol (Fort Dodge Animal Health, Fort Dodge), ketamine hydrochloride (Ketaset, Fort Dodge Animal Health), and xylazine (Ben Venue Laboratories, Inc., Bedford) given intramuscularly. The Telazol, ketamine, and xylazine (TKX) combination was composed of tiletamine (50 mg/mL), zolazepam (50 mg/mL), ketamine (50 mg/mL), and xylazine (50 mg/mL). After sedation with TKX, pigs received a lethal dose (100 mg/kg) of intravenous sodium pentobarbital. The following tissues were collected for the assessment of RNA transcript and protein abundance: liver, skeletal muscle, scWAT, and vWAT.
Serum Analysis
The following serum parameters were assessed during the study period: E2, P4, TT, insulin, C-peptide, and glycerol. Assays for E2, P4, TT, and insulin were validated for use with either swine serum or extracted serum samples as previously described (26). To remove lipids from serum samples and steroids from binding proteins before radioimmunoassay (RIA), all steroid hormones were extracted with a single extraction with diethyl ether (27). Extraction efficiency was monitored using tritiated hormones (∼1,000 cpm/1 mL serum) and was >80% for all samples. All steroid hormones were assessed using Coat-A-Count kits (Siemens Medical Solutions Diagnostics, Terrytown, NY). Although RIA sensitivity is not as great as liquid chromatography-tandem mass spectrometry, the limit of detection of our RIAs was sufficient to detect these steroid hormones in circulation in our model animal. Insulin was analyzed in serum by RIA (Porcine Insulin RIA, Millipore, Burlington, MA). C-peptide was analyzed in serum by an enzyme-linked immunosorbent assay (ELISA; Mercodia Porcine C-peptide ELISA, Mercodia Inc., Winston Salem, NC). Glycerol was analyzed in serum by a Free Glycerol Determination Kit (Millipore Sigma, St. Louis, MO).
Quantitative Real-Time PCR
Total RNA was extracted from liver, skeletal muscle, omental fat (vWAT), and scWAT with TRIzol (TRIZol Reagent, Thermo Fisher Scientific, Waltham, MA). In brief, 100 mg of tissue was homogenized in 1 mL of TRIzol, followed by incubation at room temperature and subsequently on ice. Two hundred microliters of chloroform were added to the TRIzol, and the extraction tubes were shaken followed by centrifugation at 4°C at 12,000 g for 15 min. The upper aqueous phase was removed, and the RNA was precipitated by adding 500 μL of isopropyl alcohol followed by centrifugation at 4°C at 12,000 g for 10 min. The RNA pellet was rinsed several times with RNase-free 75% ethanol and then brought up in RNase-free water. To determine relative fold differences in transcript levels, 400 ng of total RNA from each pig was reverse transcribed to cDNA (High Capacity cDNA Reverse Transcription Kit, Applied Biosystems Inc., Carlsbad, CA), diluted 1:5 with RNAse-free water, followed by quantitative real-time PCR (qRT-PCR; TaqMan Gene Expression Assay, Applied Biosystems Inc.) on a Bio-Rad CFX384 Touch Real-Time PCR Detection System (Bio-Rad, Hurcules, CA). The following target genes were assessed in all aforementioned tissues: insulin receptor (INSR), insulin receptor substrate 1 (IRS1), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), phosphoinositide-dependent kinase 1 (PDK1), protein kinase B (AKT2) forkhead box O1 (FOXO1), peroxisome proliferator activated receptor gamma (PPARG), glucose transporter type 4 (GLUT4), estrogen receptor alpha (ESR1), and androgen receptor (AR). Our endogenous gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), was tested in tissues obtained from control and testosterone-treated pigs and demonstrated similar replication among the treatment groups. We used the following TaqMan probe/primer sets from Applied Biosystems to perform qRT-PCR: INSR: Hs00961561_m1; IRS1: Ss04327584_m1; PI3K: Ss03392908_u1; PDK1: Ss04246164_m1; AKT2: Ss04953562_g1; FOXO1: Ss03388140_s1; PPARG: Ss03394829_m1; GLUT4: Ss03373325_g1; ESR1: Ss03383398_u1; and AR: Ss03822350_s1.
Protein Immunoblotting
Protein was extracted from tissue with radioimmunoprecipitation assay buffer (RIPA) (Cell Signaling Technology, Danvers, MA) containing EDTA-protease inhibitor (Millipore Sigma). Protein was quantified with the Pierce bicinchoninic acid (BCA) Protein Assay Kit (Thermo Fisher Scientific). Forty micrograms of protein was resolved in SDS-PAGE and transferred to PVDF membrane for Western blots in skeletal muscle and liver. For WAT, 60 µg of protein was utilized to allow detection of proteins of low abundance in this tissue. Blots were blocked with 5% milk in Tris-buffered saline-Tween 20 (TBST). Antibodies for AKT (Cat. No. 9227S), phosphorylated AKT at Ser473 (pAKT) (Cat. No. 4060S), FOXO1 (Cat. No. 2880S), phosphorylated FOXO1 at Ser256 (pFOXO1) (Cat. No. 9461S), PI3K 110α (Cat. No. 4249S), INSR-β subunit (Cat. No. 3025S), and GAPDH (Cat. No. 2118S) came from Cell Signaling Technology and were diluted at 1:1,000 in 5% bovine serum albumin (BSA) in TBST. The antibody for β ACTIN (Cat. No. sc-47778 HRP) came from Santa Cruz Biotechnology, Inc. (Dallas, TX) and was diluted 1:1,000 in 5% BSA in TBST, 1:1,000 in 5% BSA in TBST. The aromatase antibody is a monoclonal mouse antibody (clone 677/H7) that was provided by Dean Edwards of the Baylor College of Medicine (28) and was also diluted at 1:1,000 in 5% BSA in TBST. Protein densitometry was performed and analyzed using Multi Gauge software (Fuji Film, Valhalla, NY).
Statistical Analysis
The normality of all data was assessed using a Levene’s test of homogeneity and Shapiro–Wilk test. Transformation of non-normal data was done logarithmically before running either a repeated measures ANOVA (hormones), two-way ANOVA (PCR), or unpaired t test (Western blot) with SAS statistical software (SAS 9.2, SAS, Inc., Cary, NC). The covariance matrix structure utilized for the repeated measures ANOVA was AH(1). Two-way ANOVA and unpaired t test analysis were performed with PROC MIXED. For ANOVA, we used type 3 sums of squares with the PDIFF command to compare treatments (SAS 9.2). For all transformed data, results from PROC MIXED were back transformed, and those data are shown herein. All data presented are least square means ± SEM. The mRNA abundance of target genes was normalized to the endogenous control gene, GAPDH. The relative fold induction of each gene was then compared between treatment groups using the ΔCT method. The protein abundance from Western blot was normalized to either GAPDH or β ACTIN as indicated in the figures. In all statistical tests, P < 0.05 was the criterion for statistical significance.
RESULTS
Serum Steroid Hormone Concentrations
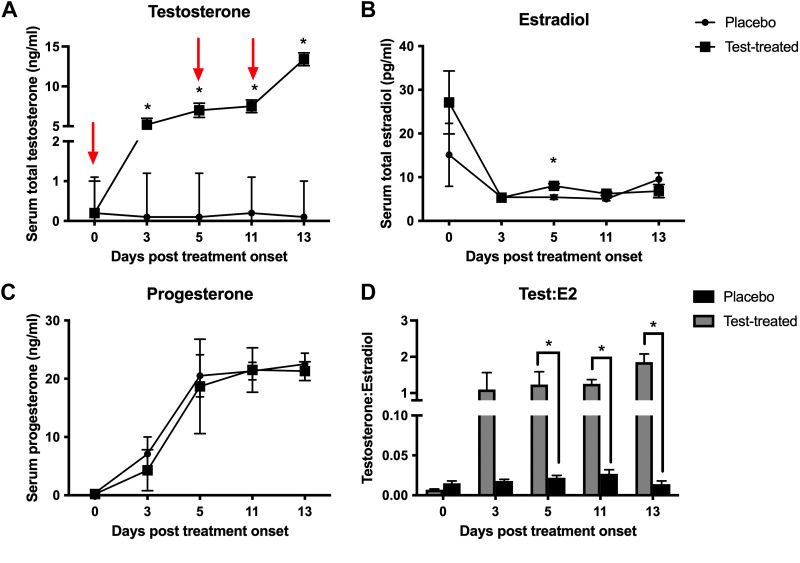
Testosterone-treated females had higher serum total testosterone than control females on D3, D5, D11, and D13 posttreatment onset, reaching a maximum level of 13.4 ng/mL on D13 (Fig. 1A). Testosterone-treated females had similar serum progesterone to control females throughout the treatment period (Fig. 1C) and slightly higher serum estradiol (8.0 ± 0.5 pg/mL) than control females (5.4 ± 0.5 pg/mL) on D5 posttreatment onset (Fig. 1B). The ratio of total testosterone:estradiol, a proxy for systemic aromatization, was greater in the testosterone-treated females on D5, D11, and D13 posttreatment onset (Fig. 1D), which suggests minimal aromatization of testosterone.
Figure 1.
Serum steroid hormone concentrations in placebo and testosterone-treated (test-treated) female pigs. Serum total testosterone (A), serum estradiol (E2) (B), serum progesterone (P4) (C), and testosterone:estradiol (D) in placebo (circles or black bars) and testosterone-treated (squares or gray bars) females over the course of the study. Red arrows indicate the days on which placebo or testosterone was administered intramuscularly. Statistical test used was a repeated-measures ANOVA. We used n = 5 for testosterone-treated females and n = 6 for placebo females for all hormone assessments. *P ≤ 0.05.
Peripheral Insulin Sensitivity and Insulin Secretion
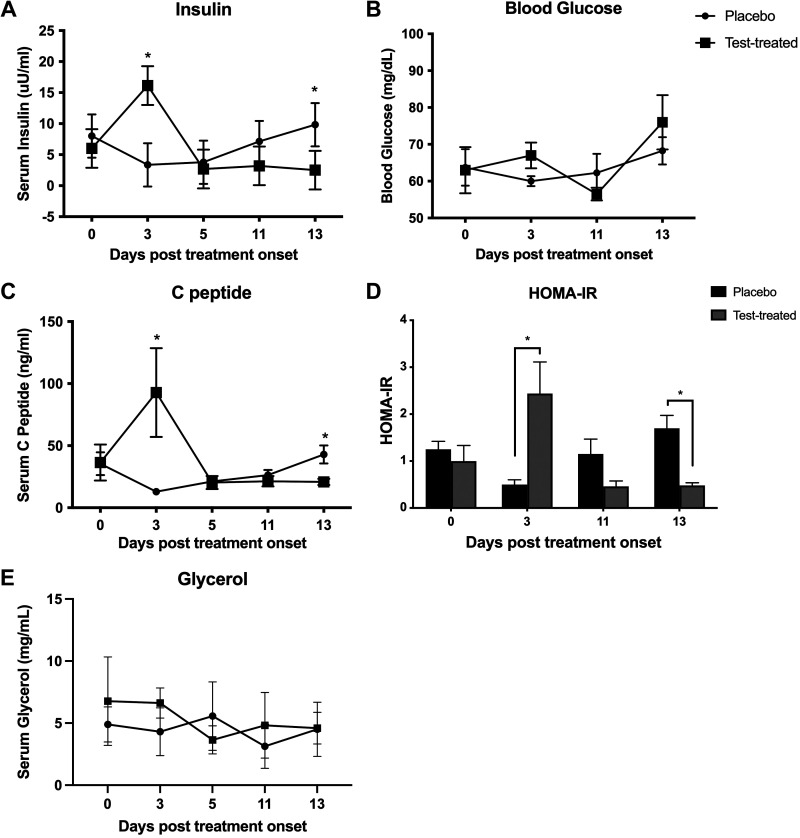
Insulin and C-peptide rose sharply on D3 posttreatment onset in testosterone-treated pigs and fell thereafter (Fig. 2, A and C). Testosterone-treated females had higher insulin and C-peptide serum concentrations than control females on D3 and lower insulin and C-peptide serum concentrations than control females on D13. Whole blood glucose was similar between treatment groups and remained between 55 and 70 mg/dL throughout the treatment period (Fig. 2B). The homeostatic method of assessment for insulin resistance (HOMA-IR), which was calculated by (fasting glucose × fasting insulin)/405 (29), was larger in the testosterone-treated group compared with the control group on D3 but was smaller in the testosterone-treated group compared with the control group on D13 (Fig. 2D). Serum glycerol was similar between treatment groups and was between 3 and 7 mg/mL throughout the treatment period (Fig. 2E).
Figure 2.
Glucose homeostasis in placebo and testosterone-treated (Test-treated) female pigs. Serum insulin (A), whole blood glucose (B), serum C-peptide (C), homeostatic method of assessment for insulin resistance (HOMA-IR; D), and glycerol (E) in control (circles or black bars) and testosterone-treated (squares or gray bars) females over the course of the study. Statistical test used was a repeated-measures ANOVA. We used n = 5 for testosterone-treated females and n = 6 for placebo females for all parameters assessed in this figure. *P ≤ 0.05.
Transcript Levels of Proteins in Insulin-Signaling Pathway in Liver, Skeletal Muscle, and WAT
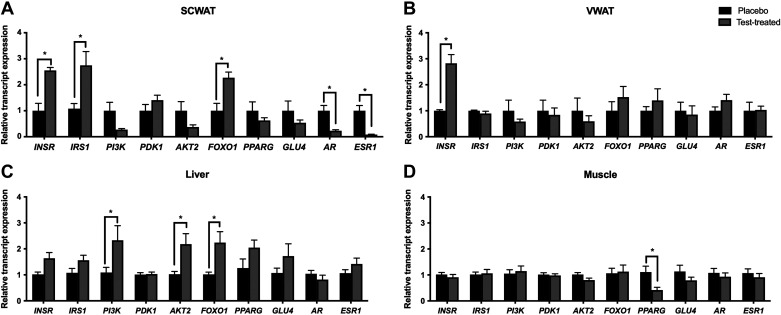
Virilizing serum testosterone concentrations upregulated INSR transcript levels in both vWAT and scWAT (Fig. 3, A and B) but had no effect on INSR transcript levels in liver and skeletal muscle (Fig. 3, C and D). In addition, IRS1 and FOXO1 transcript levels were upregulated in scWAT and ESR1 and AR transcript levels were downregulated in scWAT of testosterone-treated pigs. FOXO1 as well as AKT2 transcript levels were upregulated in the liver of testosterone-treated pigs. The skeletal muscle of testosterone-treated pigs had minimal transcriptional changes with the exception that PPARG was downregulated as compared with placebo pigs.
Figure 3.
Relative transcript expression of proteins related to the insulin signaling cascade, glucose uptake, and steroid hormone receptors in white adipose tissue, liver, and skeletal muscle of placebo and testosterone-treated female pigs. Relative transcript expression in subcutaneous white adipose tissue (scWAT; A), visceral WAT (vWAT; B), liver (C), and skeletal muscle (D) in placebo (black bars) and testosterone-treated (gray bars). qPCR was conducted on tissues collected at D13 of treatment. A two-way ANOVA was used for statistical analysis. We used n = 5 for testosterone-treated females and n = 6 for placebo females for all parameters assessed in this figure. *P ≤ 0.05. AKT2: protein kinase B isoform 2; AR: androgen receptor; ESR1: estrogen receptor α; FOXO1: forkhead box ortholog 1; GLUT4: glucose transporter 4; INSR: insulin receptor; IRS1: insulin receptor substrate 1; PDK1: phosphoinositide-dependent kinase 1; PI3K: phosphoinositide 3 kinase; PPARG: peroxisome proliferator-activated receptor gamma; test-treated: testosterone-treated.
Protein Abundance of Proteins in Insulin-Signaling Pathway in Liver, Skeletal Muscle, and WAT
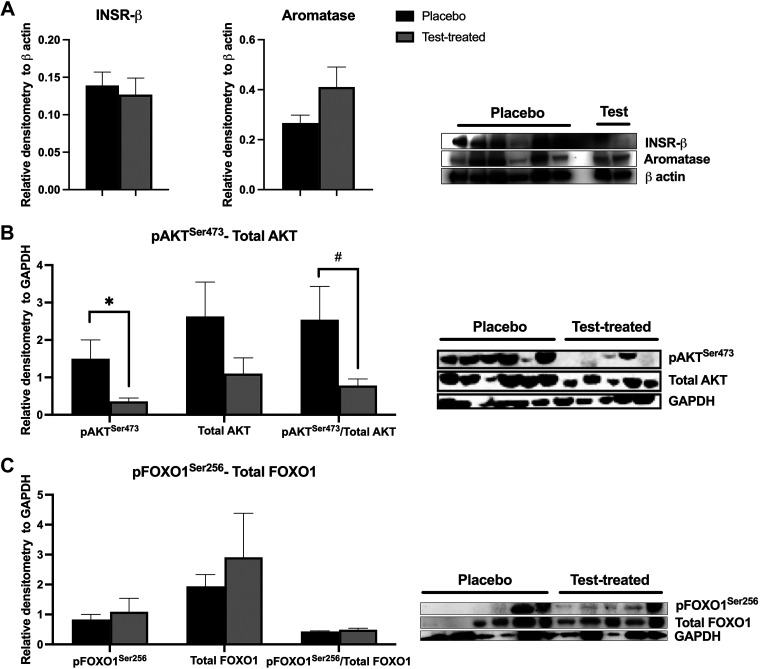
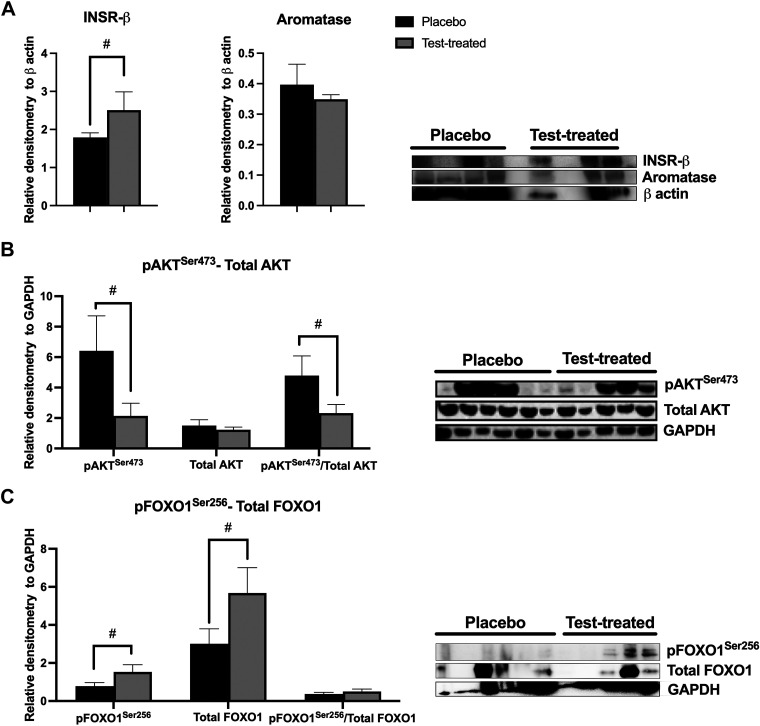
Virilizing serum testosterone concentrations caused decreased abundance of pAKTSer473 in scWAT (Fig. 4B). In addition, there was a trend for a decreased pAKTSer473/total AKT in scWAT of testosterone-treated females. There was no difference between placebo and testosterone-treated females in the abundance of INSR-β subunit, aromatase (Fig. 4A), total AKT (Fig. 4B), pFOXO1Ser256, total FOXO1 proteins, or the pFOXO1Ser256/total FOXO1 (Fig. 4C) in scWAT. There was a trend for increased INSR-β subunit (Fig. 5A), pFOXO1Ser256, and total FOXO1 (Fig. 5C) abundance in vWAT of testosterone-treated females. Yet, there was a trend for decreased pAKTSer473 abundance and a decreased pAKTSer473/total AKT in vWAT of testosterone-treated females (Fig. 5B). There was no difference in aromatase or total AKT protein abundance nor the pFOXO1Ser256/total FOXO1 between the treatment groups.
Figure 4.
Relative protein abundance of insulin receptor β subunit (INSR-β), aromatase, protein kinase B (AKT), and forkhead box ortholog 1 (FOXO1) in subcutaneous white adipose tissue of placebo and testosterone-treated females. INSR-β and aromatase (A); phosphorylated(Ser473) AKT (pAKTSer473) and total AKT (B); phosphorylated(Ser256) FOXO1 (pFOXO1Ser256) and total FOXO1 (C). An unpaired t test was used for statistical analysis between treatment groups for each protein of interest. For INSR-β and aromatase, n = 2 for testosterone-treated (Test-treated) females and n = 6 for placebo females. For AKT and FOXO1, n = 5 for testosterone-treated females and n = 6 for placebo females. Western blotting was conducted on tissues collected at D13 of treatment. *P ≤ 0.05; #0.05 < P ≤ 0.10. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Figure 5.
Relative protein abundance of insulin receptor β subunit (INSR-, aromatase, protein kinase B (AKT), and forkhead box ortholog 1 (FOXO1) in visceral (v) white adipose tissue (WAT) of placebo and testosterone-treated females (Test-treated). INSR-β subunit and aromatase (A); phosphorylated(Ser473) AKT (pAKTSer473) and total AKT (B); phosphorylated(Ser256) FOXO1 (pFOXO1Ser256) and total FOXO1 (C). An unpaired t test was used for statistical analysis between treatment groups for each protein of interest. For INSR-β subunit and aromatase, n = 3 for testosterone-treated females and n = 4 for placebo females. For AKT and FOXO1, n = 5 for testosterone-treated females and n = 6 for placebo females. Western blotting was conducted on tissues collected at D13 of treatment. #0.05 < P ≤ 0.10.
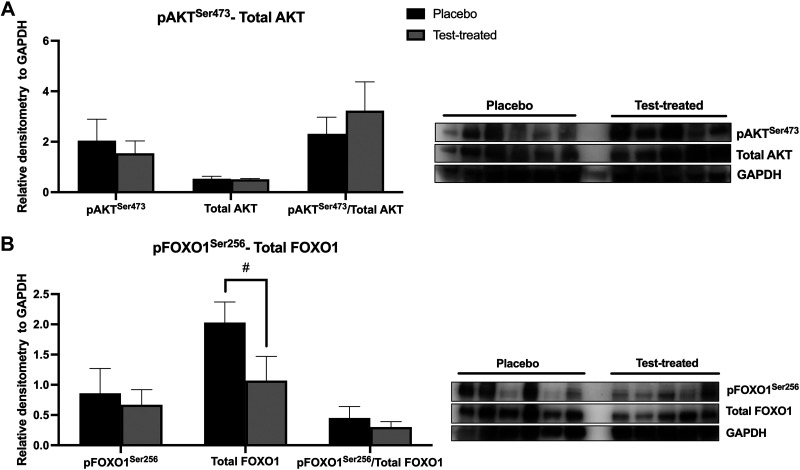
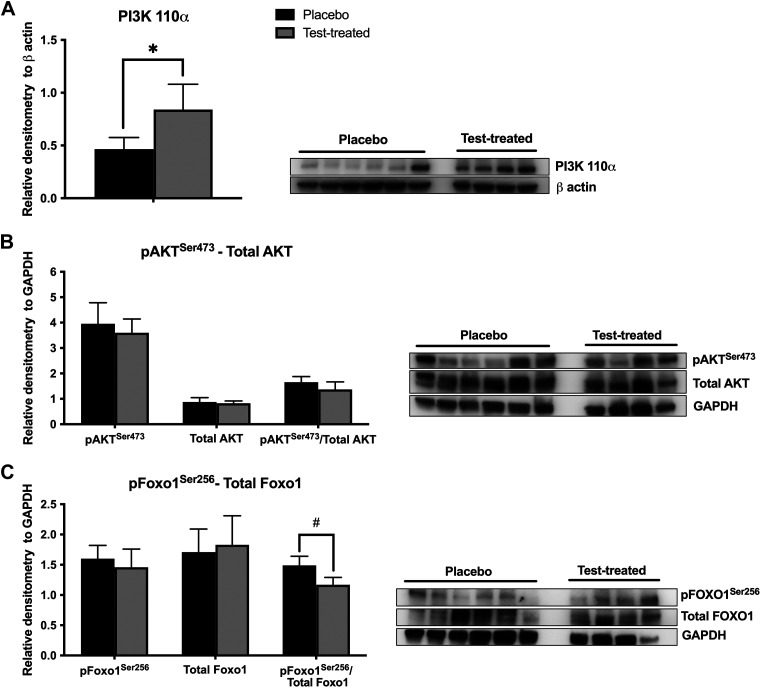
There was no effect of testosterone treatment on the abundance of pAKTSer473, total AKT, or pFOXO1Ser256 in skeletal muscle (Fig. 6). There was also no effect of testosterone treatment on the pAKTSer473/total AKT or the pFOXO1Ser256/total FOXO1 in skeletal muscle. There was a trend for testosterone treatment to decrease total FOXO1 abundance. Virilizing serum testosterone concentrations caused increased abundance of PI3K 110α (Fig. 7A) and a trend for a decreased pFOXO1Ser256/total FOXO1 (Fig. 7C). There was no difference between placebo and testosterone-treated females in the abundance of pAKTSer473, total AKT, pFOXO1Ser256, or total FOXO1 proteins, or in the pAKTSer473/total AKT.
Figure 6.
Relative protein abundance of protein kinase B (AKT) and forkhead box ortholog 1 (FOXO1) in skeletal muscle of placebo and testosterone-treated (Test-treated) females. Phosphorylated(Ser473) AKT (pAKTSer473) and total AKT (A); phosphorylated(Ser256) FOXO1 (pFOXO1Ser256) and total FOXO1 (B). An unpaired t test was utilized for statistical analysis between treatment groups for each protein of interest. For AKT and FOXO1, n = 5 for testosterone-treated females and n = 6 for placebo females. Western blotting was conducted on tissues collected at D13 of treatment. #0.05 < P ≤ 0.10. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Figure 7.
Relative protein abundance of phosphoinositide 3 kinase 110 α subunit (PI3K 110 α), protein kinase B (protein kinase B), and forkhead box ortholog 1 (FOXO1) in liver of placebo and testosterone-treated (Test-treated) females. PI3K 110 α (A); phosphorylated(Ser473) AKT (pAKTSer473) and total AKT (B); phosphorylated(Ser256) FOXO1 (pFOXO1Ser256) and total FOXO1 (C). An unpaired t test was used for statistical analysis between treatment groups for each protein of interest. For all proteins, n = 4 for testosterone-treated females and n = 6 for placebo females. Western blotting was conducted on tissues collected at D13 of treatment. *P ≤ 0.05. #0.05 < P ≤ 0.10.
DISCUSSION
The correlation between hyperandrogenemia and the development of insulin resistance and type II diabetes is well established in patients with moderately elevated testosterone such as women with polycystic ovary syndrome (30, 31) and in patients with severely elevated testosterone such as trans men (32). Yet, how androgens control peripheral insulin sensitivity and signaling is still being uncovered (15, 33). In addition, there is a lack of knowledge about the acute effects of virilizing testosterone doses on systemic insulin concentrations and tissues in control of peripheral insulin sensitivity such as the liver, skeletal muscle, and fat. The major findings of this research are that acute administration of exogenous testosterone causes the following effects in female pigs: 1) decreased fasting serum insulin at virilizing serum testosterone concentrations (12–14 ng/mL); 2) upregulation of transcript levels for proteins in the insulin-signaling pathway in WAT and liver; 3) downregulation of transcript levels for sex steroid receptors in the scWAT; 4) increased protein abundance PI3K in liver and INSR in the vWAT; and 5) changes in protein phosphorylation of AKT and FOXO1 in WAT that are indicative of depression of the insulin signaling cascade.
We found that moderate serum testosterone concentrations resulted in increased fasting serum insulin concentrations but had no effect on whole blood glucose or serum glycerol levels in fasting female pigs. This finding means that we cannot rule out a toxic effect of acute, high exogenous doses of testosterone on pancreatic β-islet cells, causing them to secrete a massive amount of insulin. However, the dose (50 mg/pig), route of delivery (im), and frequency (every 6 days) of testosterone in our model animal, the pig, was developed based on the dose (50–200 mg/person), route (im, sc, transdermal), and frequency (7–14 days) used in humans transitioning from female to male (2) and on our pilot study data in pigs (see Supplemental Fig. S1). Therefore, we did our utmost to mimic the human medical experience in response to the initiation of testosterone therapy in trans-men. Potent androgens have been shown to increase glucose-stimulated insulin secretion via binding to extranuclear androgen receptor which causes increased cAMP production, protein kinase A activation, and increased vesicular release of insulin from pancreatic β-islet cells in female mouse models and cultured pancreatic β-islet cells from women (34). In addition, DHT, via androgen receptor, increases mitochondrial respiration in cultured pancreatic β-islet cells from female mice which results in oxidative damage and secondary pancreatic β-islet cell failure (35). Given that the length of time over which testosterone (13 days) was administered and the increasing of serum testosterone concentrations during this study, we cannot state definitively whether the length of time or the serum testosterone concentration caused the initial increase and/or the subsequent decrease in fasting serum insulin concentration. However, increased serum C-peptide, which has a slower turnover rate than insulin, in testosterone-treated female pigs on D3 hints that exogenous testosterone may acutely stimulate insulin secretion from the pancreas. C-peptide has previously been shown to be associated with serum testosterone concentrations in women (36) but not men (37). It has been previously found that potent androgens like DHT cause increased insulin secretion with subsequent pancreatic β-islet cell exhaustion and failure in mice (34). Our C-peptide and insulin data demonstrate that even aromatizable androgens, such as testosterone, may stimulate secretion of insulin acutely but cause pancreatic β-islet cell exhaustion and failure over time. This finding is likely due to androgenic actions at the androgen receptor in pancreatic β-islet cells because estrogenic effects at estrogen receptor alpha in pancreatic β-islet cells result in protection of pancreatic β-cells from apoptosis through preservation of mitochondrial function and suppression of endoplasmic reticulum stress (38). Although the fasting whole blood glucose levels did not change much over time in testosterone-treated female pigs, an acute increase in fasting HOMA-IR at moderate serum testosterone concentrations was followed by a drop in fasting HOMA-IR at virilizing serum testosterone concentrations. This suggests that moderate concentrations of serum testosterone in female pigs may cause insulin resistance, whereas virilizing concentrations of serum testosterone in female pigs may increase insulin sensitivity as less insulin is needed to maintain blood glucose in a normal physiological range. HOMA-IR is calculated value which approximates insulin resistance in humans (39) and is also routinely calculated in studies with rodents and pigs (40). However, our molecular findings at the protein level refute the suggestion that virilizing testosterone concentrations improve peripheral insulin sensitivity. In addition, as HOMA-IR is a calculation, it is not as accurate a measure of insulin sensitivity as an euglycemic hyperinsulinemic clamp procedure is (41). Furthermore, as this research generated no mechanistic data on the secretion of insulin by the pancreas nor the metabolism and removal of insulin by the liver, we are unable to definitively ascertain what drove the serum insulin levels to decrease by D13 in testosterone-treated pigs.
Our data demonstrate that acute virilizing doses of testosterone cause upregulation of INSR, IRS1, and FOXO1 transcript levels in scWAT and upregulation of AKT2, PI3K, FOXO1 transcript levels in liver. Interestingly, vWAT and skeletal muscle had minimal changes in transcript levels. There has been increasing interest in the sexually dimorphic role of androgens on insulin signaling in insulin-sensitive tissues like liver, skeletal muscle, and WAT. When insulin binds to its receptor, it promotes phosphorylation of AKT via PI3K and PDK1, ultimately resulting in the translocation of GLUT4 to the cell surface to take up glucose into the cell (42, 43). FOXO1 controls glucose and free fatty acid uptake and cellular insulin sensitivity through control of gene transcription of PPARG (44). To date, examination of the effects of DHT on insulin signaling has focused on liver (mouse) (45), scWAT (human) (15), or immortalized human cell lines like the 3T3-L1 line (13). Interestingly, DHT-treated primary hepatocytes from female mice and humans show reduced insulin-mediated phosphorylation of AKT via a mechanism directly at cytoplasmic androgen receptor (33). Contrary to these findings, exogenous testosterone in the female pigs in our study increased the abundance of PI3K 110α but did not decrease phosphorylation of AKT in the liver. In addition, although the transcript and protein data correlated for PI3K in the liver, they did not correlate for AKT or for FOXO1. Our data demonstrate a disconnect between transcript response and protein abundance, which is not uncommon in the examination of factors related to the insulin signaling pathway. Given that our study gave testosterone that can be converted in white adipose tissue into DHT, androstenedione, and androstenedione as well as estradiol (E2) and estrone (46, 47) and it was given in vivo in a pig model, it is not surprising that our findings differ from previously published results in vitro with DHT and hepatocytes from other species. Future research with DHT in vitro in pig hepatocytes is warranted to determine the suitability of this animal model for the study of the effects of androgens on the liver in humans. Overall, our molecular findings suggest that virilizing exogenous doses of testosterone in females increase insulin signaling and, possibly insulin sensitivity, in the liver.
In contrast to our data from the liver, there was minimal effect of virilizing doses of testosterone on the skeletal muscle in female pigs. Although androgen receptors are present in skeletal muscle, androgens have been primarily shown to affect muscles through stimulation of the differentiation of mesenchymal precursor cells (48). Therefore, the lack of effect of testosterone on the insulin-signaling pathway in the skeletal muscle is not surprising. In addition, it is well-known that estrogen receptor alpha (ERα) is intimately in control of glucose transport and fatty acid oxidation in skeletal muscle in mice and humans (49). Given that we found no differences in the protein abundance of aromatase in the WAT and no major increases in serum estradiol in response to virilizing concentrations of circulating testosterone and there were minimal effects of testosterone in the muscle, suggests that testosterone likely exerted its effects in this tissue at the androgen receptor.
The molecular results in both scWAT and vWAT suggest that virilizing concentrations of testosterone suppress the insulin-signaling pathway in these tissues. Similar to our findings in the liver, while transcripts for proteins in the insulin-signaling pathway were upregulated for both scWAT and vWAT, only the INSR protein abundance in vWAT was increased to match the increased INSR transcript levels in this tissue. Interestingly, phosphorylated (Ser473) AKT was decreased in both the scWAT and the vWAT. Following downstream of this, phosphorylated (Ser256) FOXO1 was increased in the vWAT. As phosphorylation of AKT inhibits phosphorylation of FOXO1, this finding provides mechanistic support for the suppression of the insulin pathway in vWAT by virilizing serum levels of testosterone in females. Moreover, as aromatase protein abundance was similar between placebo and testosterone-treated females in both scWAT and vWAT, it indicates that it is testosterone itself or another androgen metabolite that is causing the effects in the WAT. Future studies that track the actual turnover or conversion of testosterone into other metabolites in WAT would be warranted to support this conclusion. It would also be prudent for future studies on the effects of virilizing testosterone on insulin signaling to assess whether phosphorylation of AKT at T308 or phosphorylation of FOXO1 at S253 in WAT would be affected by virilizing doses of testosterone in females. In addition, although transcript levels and phosphorylation of proteins in the insulin-signaling pathway can be affected by serum insulin, they can also be affected by other growth factors (i.e., insulin-like growth factor 1, growth hormone), which could be altered by testosterone treatment (50). Additional mechanistic studies are warranted to verify that the effects of virilizing testosterone on the liver and WAT are due to the changes in the serum insulin concentrations or other factors in testosterone-treated females.
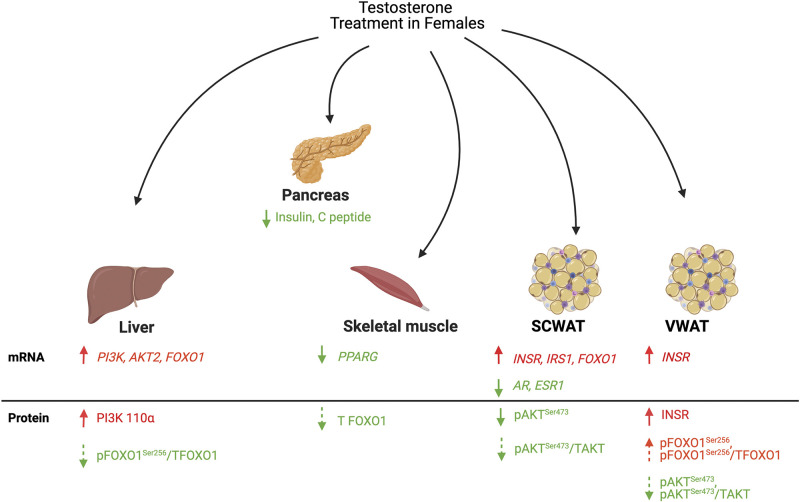
In summary, our study uses a translational animal model to demonstrate that acute virilizing doses of testosterone administered intramuscularly to female pigs cause a decrease in serum insulin and C-peptide concentrations. Virilizing doses of testosterone in female pigs cause upregulation of transcript levels of proteins in the insulin-signaling pathway in liver and scWAT. However, virilizing testosterone concentrations in female pigs increase abundance of proteins in the insulin-signaling pathway in the liver and primarily downregulate the insulin-signaling pathway via AKT and FOXO1 in both the scWAT and the vWAT (Fig. 8). Serum steroid hormone levels and aromatase protein abundance in WAT are similar between treatment groups, indicating that effects of virilizing levels of testosterone on liver and WAT are due to native testosterone or androgen metabolites, rather than estrogens. Future research in this translational animal model should focus on the identification of the mechanisms by which testosterone suppresses serum insulin concentrations and alters protein and transcript availability and function in liver and WAT. Such studies will further build upon the current foundation of knowledge in the transgender medicine field to inform testosterone hormone therapy protocols in trans-men.
Figure 8.
Model diagram of the effects of acute virilizing testosterone on the insulin pathway in tissues responsible for systemic insulin sensitivity. Components in red are upregulated. Components in green are downregulated. Solid arrows are significantly different. Dashed arrows reflect a trend in significance. AR, androgen receptor; ESR1, estrogen receptor α; FOXO1, forkhead box ortholog 1; INSR, insulin receptor; IRS1, insulin receptor substrate 1; mRNA: messenger RNA; pAKTSer473, phosphorylated(Ser473) AKT; pFOXO1Ser256, phosphorylated(Ser256) FOXO1; PI3K, phosphoinositide 3 kinase; PI3K 110 α, phosphoinositide 3 kinase 110 α subunit; PPARG, peroxisome proliferator activated receptor gamma; scWAT, subcutaneous white adipose tissue; TAKT, total AKT; T FOXO1, total FOXO1; VWAT, visceral white adipose tissue.
GRANTS
This project was funded by NIH Grant K01OD011177 (A. E. Newell-Fugate).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.E.N-F. conceived and designed research; E.L., C.B., M.G., M.J., A.G.B-F., and A.E.N-F. performed experiments; K.H.K., V.J., and A.E.N-F. analyzed data; A.E.N-F. interpreted results of experiments; K.H.K. prepared figures; K.H.K. and A.E.N-F. drafted manuscript; K.H.K., V.J., E.L., C.B., M.J., A.G.B-F., J.M.B., R.A.N., and A.E.N-F. edited and revised manuscript; K.H.K., R.A.N., and A.E.N-F. approved final version of manuscript.
DATA AVAILABILITY
All data sets generated or analyzed during this study can be found at TAMU OAK repository.
SUPPLEMENTAL DATA
Supplemental Fig. 1 is available at https://doi.org/10.6084/m9.figshare.14373545.v1.
ACKNOWLEDGMENTS
We thank the many undergraduate researchers and Robert V. Knox who assisted with the collection of samples for this project. We also thank Drs. Vickie Jarrell and Clifford Shipley for support of the project in their capacities with the Agricultural Animal Care and Use Program at University of Illinois. We thank Dean Edwards (Baylor College of Medicine) for generous donation of the clone 667/H7 aromatase antibody.
REFERENCES
- 1.Flores AR, Herman JL, Gates GJ, Brown TNT. How Many Adults Identify as Transgender in the United States? Los Angeles, CA: The Williams Institute, 2016. [Google Scholar]
- 2.Unger CA. Hormone therapy for transgender patients. Transl Androl Urol 5: 877–884, 2016. doi: 10.21037/tau.2016.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoffers IE, de Vries MC, Hannema SE. Physical changes, laboratory parameters, and bone mineral density during testosterone treatment in adolescents with gender dysphoria. J Sex Med 16: 1459–1468, 2019. doi: 10.1016/j.jsxm.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Wierckx K, Van de Peer F, Verhaeghe E, Dedecker D, Van Caenegem E, Toye K, Kaufman JM, T’Sjoen G. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med 11: 222–229, 2014. doi: 10.1111/jsm.12366. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez JD, Tannock LR. Metabolic effects of hormone therapy in transgender patients. Endocr Pract 22: 383–388, 2016. doi: 10.4158/EP15950.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wierckx K, Elaut E, Declercq E, Heylens G, De Cuypere G, Taes Y, Kaufman JM, T'Sjoen G. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: a case-control study. Eur J Endocrinol 169: 471–478, 2013. doi: 10.1530/EJE-13-0493. [DOI] [PubMed] [Google Scholar]
- 7.Defreyne J, Van de Bruaene LDL, Rietzschel E, Van Schuylenbergh J, T’Sjoen GGR. Effects of gender-affirming hormones on lipid, metabolic, and cardiac surrogate blood markers in transgender persons. Clin Chem 65: 119–134, 2019. doi: 10.1373/clinchem.2018.288241. [DOI] [PubMed] [Google Scholar]
- 8.Getahun D, Nash R, Flanders WD, Baird TC, Becerra-Culqui TA, Cromwell L, Hunkeler E, Lash TL, Millman A, Quinn VP, Robinson B, Roblin D, Silverberg MJ, Safer J, Slovis J, Tangpricha V, Goodman M. Cross-sex hormones and acute cardiovascular events in transgender persons: a cohort study. Ann Intern Med 169: 205–213, 2018. doi: 10.7326/M17-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32, Suppl 2: S157–S163, 2009. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malin SK, Kashyap SR, Hammel J, Miyazaki Y, DeFronzo RA, Kirwan JP. Adjusting glucose-stimulated insulin secretion for adipose insulin resistance: an index of beta-cell function in obese adults. Diabetes Care 37: 2940–2946, 2014. doi: 10.2337/dc13-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrisse S, Billings K, Xue P, Wu S. Insulin signaling displayed a differential tissue-specific response to low-dose dihydrotestosterone in female mice. Am J Physiol Endocrinol Metab 314: E353–E365, 2018. doi: 10.1152/ajpendo.00195.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrisse S, Childress S, Ma Y, Billings K, Chen Y, Xue P, Stewart A, Sonko ML, Wolfe A, Wu S. Low-dose dihydrotestosterone drives metabolic dysfunction via cytosolic and nuclear hepatic androgen receptor mechanisms. Endocrinology 158: 531–544, 2017. doi: 10.1210/en.2016-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta V, Bhasin S, Guo W, Singh R, Miki R, Chauhan P, Choong K, Tchkonia T, Lebrasseur NK, Flanagan JN, Hamilton JA, Viereck JC, Narula NS, Kirkland JL, Jasuja R. Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Mol Cell Endocrinol 296: 32–40, 2008. doi: 10.1016/j.mce.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebuffe-Scrive M, Marin P, Bjorntorp P. Effect of testosterone on abdominal adipose tissue in men. Int J Obes 15: 791–795, 1991. [PubMed] [Google Scholar]
- 15.Corbould A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J Endocrinol 192: 585–594, 2007. doi: 10.1677/joe.1.07070. [DOI] [PubMed] [Google Scholar]
- 16.Esteve Rafols M. Adipose tissue: cell heterogeneity and functional diversity. Endocrinol Nutr 61: 100–112, 2014. doi: 10.1016/j.endonu.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Osuka S, Nakanishi N, Murase T, Nakamura T, Goto M, Iwase A, Kikkawa F. Animal models of polycystic ovary syndrome: a review of hormone-induced rodent models focused on hypothalamus-pituitary-ovary axis and neuropeptides. Reprod Med Biol 18: 151–160, 2019. doi: 10.1002/rmb2.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paixão L, Ramos RB, Lavarda A, Morsh DM, Spritzer PM. Animal models of hyperandrogenism and ovarian morphology changes as features of polycystic ovary syndrome: a systematic review. Reprod Biol Endocrinol 15: 12, 2017. doi: 10.1186/s12958-017-0231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varlamov O, White AE, Carroll JM, Bethea CL, Reddy A, Slayden O, O’Rourke RW, Roberts CT Jr.. Androgen effects on adipose tissue architecture and function in nonhuman primates. Endocrinology 153: 3100–3110, 2012. doi: 10.1210/en.2011-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varlamov O, Bishop CV, Handu M, Takahashi D, Srinivasan S, White A, Roberts CT Jr.. Combined androgen excess and Western-style diet accelerates adipose tissue dysfunction in young adult, female nonhuman primates. Hum Reprod 32: 1892–1902, 2017. doi: 10.1093/humrep/dex244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varlamov O, Chu MP, McGee WK, Cameron JL, O'Rourke RW, Meyer KA, Bishop CV, Stouffer RL, Roberts CT Jr.. Ovarian cycle-specific regulation of adipose tissue lipid storage by testosterone in female nonhuman primates. Endocrinology 154: 4126–4135, 2013. doi: 10.1210/en.2013-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Lerman L. Investigating the metabolic syndrome: contributions of swine models. Toxicol Pathol 44: 358–366, 2016. doi: 10.1177/0192623316630835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spurlock ME, Gabler NK. The development of porcine models of obesity and the metabolic syndrome. J Nutr 138: 397–402, 2008. doi: 10.1093/jn/138.2.397. [DOI] [PubMed] [Google Scholar]
- 24. Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. Washington, D.C.: National Academy Press, 2010. [Google Scholar]
- 25.Newell-Fugate AE, Taibl JN, Alloosh M, Sturek M, Bahr JM, Nowak RA, Krisher RL. Effects of obesity and metabolic syndrome on steroidogenesis and folliculogenesis in the female Ossabaw mini-pig. PLoS One 10: e0128749, 2015. doi: 10.1371/journal.pone.0128749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newell-Fugate AE, Taibl JN, Clark SG, Alloosh M, Sturek M, Krisher RL. The effect of diet induced obesity on metabolic parameters and reproductive function in the female Ossabaw mini-pig. Comp Med 61: 44–49, 2014. [PMC free article] [PubMed] [Google Scholar]
- 27.Bahr JM, Wang SC, Huang MY, Calvo FO. Steroid concentrations in isolated theca and granulosa layers of preovulatory follicles during the ovulatory cycle of the domestic hen. Biol Reprod 29: 326–334, 1983. doi: 10.1095/biolreprod29.2.326. [DOI] [PubMed] [Google Scholar]
- 28.Sasano H, Edwards DP, Anderson TJ, Silverberg SG, Evans DB, Santen RJ, Ramage P, Simpson ER, Bhatnagar AS, Miller WR. Validation of new aromatase monoclonal antibodies for immunohistochemistry: progress report. J Steroid Biochem Mol Biol 86: 239–244, 2003. doi: 10.1016/S0960-0760(03)00363-7. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 30.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 22: 141–146, 1999. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 31.Rubin KH, Glintborg D, Nybo M, Abrahamsen B, Andersen M. Development and risk factors of type 2 diabetes in a nationwide population of women with polycystic ovary syndrome. J Clin Endocrinol Metab 102: 3848–3857, 2017. doi: 10.1210/jc.2017-01354. [DOI] [PubMed] [Google Scholar]
- 32.de Souza Santos R, Frank A, Nelson MD, Garcia MM, Palmer BF, Clegg D. Sex, gender, and transgender: metabolic impact of cross hormone therapy. In: Sex and Gender Factors Affecting Metabolic Homeostasis, Diabetes and Obesity, edited by Mauvais-Jarvis F. Cham: Springer International Publishing, 2017, p. 611–627. doi: 10.1007/978-3-319-70178-3_27. [DOI] [PubMed] [Google Scholar]
- 33.Wu S, Andrisse S, Wolfe A, Feng M, Wang Z, Xue P. OR05-4 androgen induced metabolic dysfunction in female mice is prevented by deletion of liver androgen receptor. J Endocr Soc 3: OR05-4, 2019. doi: 10.1210/js.2019-OR05-4. [DOI] [Google Scholar]
- 34.Navarro G, Allard C, Morford JJ, Xu W, Liu S, Molinas AJR, Butcher SM, Fine NH, Blandino-Rosano M, Sure VN, Yu S, Zhang R, Münzberg H, Jacobson DA, Katakam PV, Hodson DJ, Bernal-Mizrachi E, Zsombok A, Mauvais-Jarvis F. Androgen excess in pancreatic β cells and neurons predisposes female mice to type 2 diabetes. JCI Insight 3: e98607, 2018. doi: 10.1172/jci.insight.98607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu W, Morford J, Mauvais-Jarvis F. Emerging role of testosterone in pancreatic β cell function and insulin secretion. J Endocrinol 240: R97–R105, 2019. doi: 10.1530/JOE-18-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zietz B, Cuk A, Hügl S, Büttner R, Straub RH, Bauer B, Daffner P, Schölmerich J, Palitzsch K. Association of increased C-peptide serum levels and testosterone in type 2 diabetes. Eur J Intern Med 11: 322–328, 2000. doi: 10.1016/S0953-6205(00)00122-9. [DOI] [PubMed] [Google Scholar]
- 37.Basu R, Dalla Man C, Campioni M, Basu A, Nair KS, Jensen MD, Khosla S, Klee G, Toffolo G, Cobelli C, Rizza RA. Effect of 2 years of testosterone replacement on insulin secretion, insulin action, glucose effectiveness, hepatic insulin clearance, and postprandial glucose turnover in elderly men. Diabetes Care 30: 1972–1978, 2007. doi: 10.2337/dc07-0359. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Z, Ribas V, Rajbhandari P, Drew BG, Moore TM, Fluitt AH, Whitney KA, Georgia S, Vergnes L, Reue K, Liesa M, Shirihai O, van der Bliek AM, Chi NW, Mahata SK, Tiano JP, Hewitt SC, Tontonoz P, Korach KS, Mauvais-Jarvis F, Hevener AL. Estrogen receptor alpha protects pancreatic beta-cells from apoptosis by preserving mitochondrial function and suppressing endoplasmic reticulum stress. J Biol Chem 293: 4735–4751, 2018. doi: 10.1074/jbc.M117.805069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 27: 1487–1495, 2004. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 40.Mather K. Surrogate measures of insulin resistance: of rats, mice and men. Am J Physiol Endocrinol Metab 296: E398–E399, 2009. doi: 10.1152/ajpendo.90889.2008. [DOI] [PubMed] [Google Scholar]
- 41.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 237: E214–E223, 1979. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 42.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 33: 981–1030, 2012. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puttabyatappa M, Padmanabhan V. Prenatal testosterone programming of insulin resistance in the female sheep. Adv Exp Med Biol 1043: 575–596, 2017. doi: 10.1007/978-3-319-70178-3_25. [DOI] [PubMed] [Google Scholar]
- 44.Gross DN, van den Heuvel APJ, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene 27: 2320–2336, 2008. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 45.Lin H-Y, Yu I-C, Wang R-S, Chen Y-T, Liu N-C, Altuwaijri S, Hsu CL, Ma WL, Jokinen J, Sparks JD, Yeh S, Chang C. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology 47: 1924–1935, 2008. doi: 10.1002/hep.22252. [DOI] [PubMed] [Google Scholar]
- 46.Tchernof A, Mansour MF, Pelletier M, Boulet M-M, Nadeau M, Luu-The V. Updated survey of the steroid-converting enzymes in human adipose tissues. J Steroid Biochem Mol Biol 147: 56–69, 2015. doi: 10.1016/j.jsbmb.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 47.Blouin K, Veilleux A, Luu-The V, Tchernof A. Androgen metabolism in adipose tissue: recent advances. Mol Cell Endocrinol 301: 97–103, 2009. doi: 10.1016/j.mce.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 48.Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab 89: 5245–5255, 2004. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- 49.Hevener AL, Zhou Z, Moore TM, Drew BG, Ribas V. The impact of ERα action on muscle metabolism and insulin sensitivity—strong enough for a man, made for a woman. Mol Metab 15: 20–34, 2018. doi: 10.1016/j.molmet.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris CA, Morris L, Jiang L, LeBrasseur N, Jasuja R, Flanagan J, Bhasin S. Mediation of testosterone action in muscle by growth hormone/IGF-1 signaling. FASEB J 21: A947–A947, 2007. doi: 10.1096/fasebj.21.6.A947. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data sets generated or analyzed during this study can be found at TAMU OAK repository.