Keywords: adipocyte precursor, adipogenesis, beige, differentiation, IL-4, neonatatal, Ucp1
Abstract
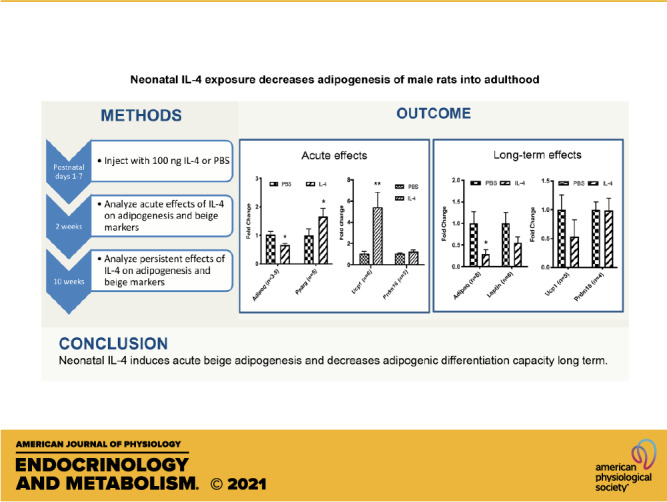
The cytokine interleukin 4 (IL-4) can increase beige adipogenesis in adult rodents. However, neonatal animals use a distinct adipocyte precursor compartment for adipogenesis as compared with adults. In this study, we address whether IL-4 can induce persistent effects on adipose tissue when administered subcutaneously in the interscapular region during the neonatal period in Sprague-Dawley rats. We injected IL-4 into neonatal male rats during postnatal days 1–6, followed by analysis of adipose tissue and adipocyte precursors at 2 wk and 10 wk of age. Adipocyte precursors were cultured and subjected to differentiation in vitro. We found that a short and transient IL-4 exposure in neonates upregulated uncoupling protein 1 (Ucp1) mRNA expression and decreased fat cell size in subcutaneous white adipose tissue (WAT). Adipocyte precursors from mature rats that had been treated with IL-4 as neonates displayed a decrease in adiponectin (Adipoq) but no change in Ucp1 expression, as compared with controls. Thus, neonatal IL-4 induces acute beige adipogenesis and decreases adipogenic differentiation capacity long term. Overall, these findings indicate that the neonatal period is critical for adipocyte development and may influence the later onset of obesity.
NEW & NOTEWORTHY We used neonatal injections in rat to show that IL-4 decreases adipogenesis and increases browning of white fat. In adulthood, adipocyte precursors show persistently decreased adipogenesis but not increased browning. These studies in the neonate are the first, to our knowledge, to show that IL-4 can have long-lasting effects.
INTRODUCTION
Obesity is a major health problem, affecting nearly a third of the world’s population (1). It is tightly linked to type 2 diabetes, heart disease, metabolic syndrome, and certain cancers (2). A chronic imbalance between energy intake and energy expenditure results in the expansion of adipose tissue, which characterizes obesity. Once viewed as an inert organ of energy storage, adipose tissue is now understood to play a central role in regulating systemic metabolism and energy homeostasis. Adipose tissue comprises energy-storing white adipose tissue (WAT), thermogenic brown adipose tissue (BAT), and thermogenic beige adipocytes interspersed within WAT (3). All three types of adipose tissue communicate extensively with other organs in the body to maintain energy homeostasis. WAT adipocytes are characterized by the presence of a large unilocular lipid droplet and a low number of mitochondria. WAT is localized in several discrete and functionally distinct depots. The subcutaneous WAT [represented by inguinal WAT (iWAT) in rodents] and BAT develop first in the fetus, whereas visceral WAT [represented by the epididymal WAT (eWAT) in male mice] appears later, at approximately postnatal day 4 or 5 (4). Brown and beige adipocytes are characterized by multiple small lipid droplets and a high density of mitochondria containing the uncoupling protein uncoupling protein 1 (UCP1). Brown and beige adipocytes have received much attention for their therapeutic potential to augment energy expenditure and thereby combat metabolic disease (5).
Thermogenically competent beige adipocytes develop in WAT depots in response to various stimuli, including cold exposure. Beige adipocytes arise in WAT either from de novo differentiation of adipocyte precursor cells or through the direct conversion of mature adipocytes. The β-adrenergic signaling system plays a key role in cold-induced beige fat biogenesis. Several studies have also implicated a role for regulators of type 2 innate immune signaling, such as interleukin 4 (IL-4) and interleukin 13 (IL-13), in beige fat development in adult animals (6–8). However, it is unknown if type 2 innate immune signaling regulates adipocyte formation during the prenatal and early postnatal period, when there are high levels of adipogenesis and type 2 immunity (9, 10).
IL-4 and IL-13 are type 2 immune cytokines that are present at higher levels in neonates as compared with adults (11). The IL-4 receptor (IL-4R), which can be activated by both IL-4 and IL-13, is highly expressed in adipocyte precursors but not in mature adipocytes (12). The numbers and proliferative activity of adipocyte precursors are decreased in young Il4/Il13DKO mice (6). Conversely, IL-4 treatment of adipocyte precursors from adult mice increases proliferation. With 3T3-L1 cells, IL-4 decreases adipogenesis only when added during the multiclonal expansion phase of differentiation (13). These data led us to consider whether IL-4 regulates adipose tissue growth during the early postnatal period.
In the current study, we made the novel observation that a short and transient IL-4 exposure in neonatal rats decreased adiposity into adulthood. This process was associated with an acute induction of beige adipogenesis, including the upregulation of Ucp1 in young animals and a loss of adipogenic differentiation capacity in precursor cells from adult rats.
METHODS
Animals
Sprague-Dawley 2-mon-old timed pregnant rats were obtained from Charles River Laboratories and all transported by day 17 of pregnancy. They were housed at 22°C (unless otherwise stated) in the vivarium under a 12-h light and dark cycle. Litters were randomly adjusted to an n of 8, and only males were used in these studies. Litters were randomly divided into two treatment groups: vehicle (PBS) or IL-4 (Peprotech). Then, 1× PBS or 50 ng IL-4 or 100 ng IL-4 was made up to a volume of 100 µL for daily injections delivered subcutaneously at the nape during the first 6 days of life. To measure IL-4 localization to serum, IL-4 was injected subcutaneously at the nape at postnatal day 1 (P1), and serum was isolated ∼5 min later. No rat pups were cannibalized. Single pups in litters counted as an n of 1, but each litter counted as a biological replicate and each experiment had a minimum of three biological replicates. Pups were either euthanized at P14 for downstream analyses or weaned at 3 wk of age and then fed normal chow until 10 wk of age. Euthanasia of pups was performed by decapitation before weaning. After weaning, rats were weighed weekly until euthanasia at 10 wk of age using a CO2 chamber followed by bilateral thoracotomy. The animal work was conducted with the approval of the University of Pennsylvania and the Children’s Hospital of Philadelphia Institutional Animal Care and Use Committees. The animals were treated humanely with due consideration to the alleviation of distress and discomfort.
Isolation of Fat Depots
Interscapular BAT, inguinal WAT, and epididymal WAT were dissected from animals and weighed. Samples were then flash frozen in liquid nitrogen for qRT-PCR analysis or digested for isolation of stromal vascular cells.
Processing of Stromal Vascular Cells
Adipose tissues from P3 and P7 controls as well as P7 IL-4 treated animals were manually minced and digested with collagenase D (1.5 U/mL; Roche) and dispase II (2.4 U/mL; Roche) in DMEM/F12 containing 0.8% fatty acid-free bovine serum albumin (Sigma) at 37°C for 45 min and vortexed for 15 s at 15-min intervals. The digestion was quenched with DMEM/F12 containing 10% FBS, and the cells were passed through a 230-µM steel mesh filter followed by centrifugation at 400 g for 5 min. The supernatant containing mature adipocytes was then aspirated, and the pellet containing stromal vascular cells (SVCs) was resuspended in ammonium-chloride-potassium (ACK) lysis buffer (0.15 M NH4Cl, 0.01 M KHCO3, and 0.0001 M Na2EDTA) for 2 min at room temperature and then quenched in DMEM/F12 containing 10% FBS. Cells were then collected by centrifugation at 400 g for 5 min and resuspended in FACS buffer (HBSS containing 1% FBS and 5 mM EDTA) for cell counting, cell culture, and flow cytometry.
Cell Culture and Differentiation
SVCs from P3 (control) rat pups were plated at 2 × 105 cells/well in 48-well plates. The cells were cultured in DMEM/F12 containing 10% FBS and Primocin (50 ng/mL) (InvivoGen ant-pm-1) for 24 h to encourage attachment. Cells were treated for another 48 h with either 10 nM IL-4 or PBS (vehicle) and then counted and analyzed for beige lineage genes using qRT-PCR.
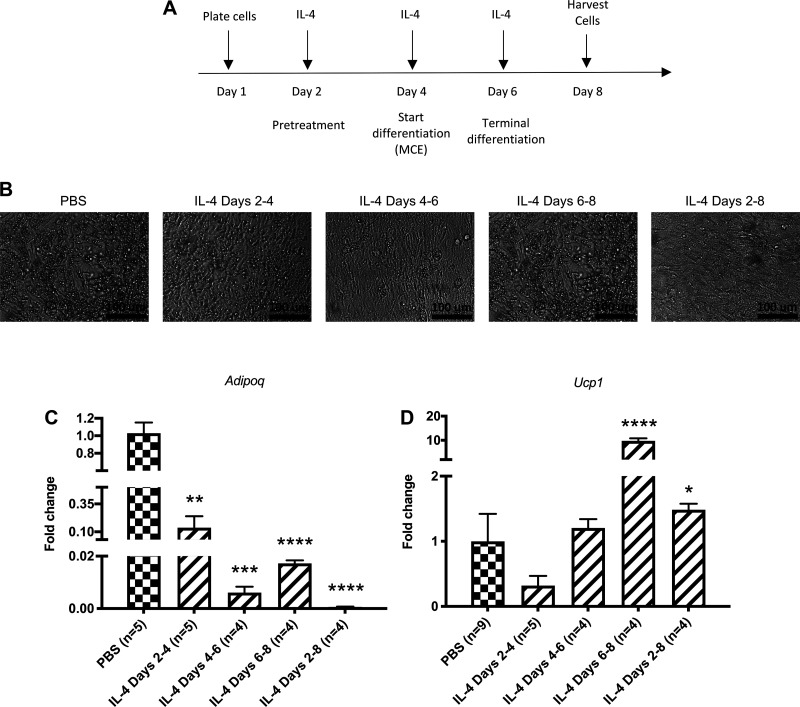
SVCs from either P3 (control) or 10-wk-old (control and IL-4 injected) rats were also plated at 2 × 105 cells/well in 48-well plates for differentiation. On day 1, the cells were cultured in DMEM/F12 containing 10% FBS and Primocin (50 ng/mL) (InvivoGen ant-pm-1) for 24 h to encourage attachment. On days 2–4, cells received a pretreatment. On days 4–6 for adipogenic differentiation, cells were cultured in DMEM/F12 containing 10% FBS and a full adipogenic cocktail of 3.3 mU/mL insulin, 1 nM T3, 1 µM dexamethasone (Sigma D4902), 0.5 µM isobutylmethylxanthine (Sigma 17018), 125 nM indomethacin (Sigma D8280), and 1 µM rosiglitazone (Sigma R2408). For days 6–8, cells were cultured in adipocyte maintenance medium of DMEM/F12 containing 10% FBS, 3.3 mU/mL insulin, 1 nM T3, and 1 µM rosiglitazone. Medium was changed every 2 days and cells harvested between days 8 and 12 of culture. IL-4 or PBS (vehicle) was added for different exposure periods during differentiation [pretreatment, IL-4 treatment from day 2 to 4; multiclonal expansion (MCE), IL-4 treatment from day 4 to 6; terminal differentiation, IL-4 treatment from day 6 to 8; throughout, IL-4 treatment from day 2 to 8].
Flow Cytometry
SVCs from P7 IL-4-treated and PBS control pups were resuspended in FACS buffer for incubation with anti-mouse ICAM1-PE/Cy7 (Biolegend Cat. No. 116122; 1:100) and anti-mouse CD45-APC/Cy7 (Biolegend Cat. No. 103116; 1:1000) for 30 min at 4°C. DAPI (Roche Cat. No. 10236276001; 1:10,000) was added for the final 5 min. The cells were washed three times with FACS buffer to remove unbound antibodies. The cells were analyzed with an LSR Fortessa (BD Biosciences). All compensation was performed at the time of analysis in Diva software by using compensation beads (Invitrogen Cat. No. 01111141) for single color staining and SVCs for negative staining and fluorescence (DAPI).
Histology
Tissues were fixed with 4% paraformaldehyde for 48 h. The tissues were dehydrated through a series of ethanol washes and then embedded in paraffin for thin sectioning. Slides were stained either with hematoxylin and eosin or with the following antibodies: Ki67 (Abcam),
UCP1 (Abcam), CD68 (Abcam), and CD3 (BD PharMingen). For antibody staining, samples underwent antigen retrieval and peroxide wash followed by blocking for 2 h in 10% goat serum with 1% BSA. Images were captured on a Leica DMRBE microscope with ×20 magnification and QImaging Micropublisher 5.0 RTV camera. iVision software version 4.0.16 was used for acquisition, and Aperio software was used for immunohistochemistry (IHC) quantification. ImageJ version 1.52 was used for cell size quantification and cells were excluded if they bordered the image edge. For Ki67 staining, three fields of view from three different sections for each cohort were used, and ∼500 cells were counted in each section. For calculating the average cell size, one field of view from three different sections for each cohort were used and ∼50 cells were measured in each section.
Dual X-Ray Absorbance
At P14, pups were anesthetized using pentobarbital injected intraperitoneally at 50 mg/kg body weight. Body composition was measured in animals from each treatment group using the Lunar PIXImus2 Dual X-ray Absorbance (DEXA) from the neck down, excluding the head. This machine includes software suitable for small animals. The percentage of fat and lean tissue per animal was calculated per gram of total body weight.
Quantitative Reverse Transcription PCR
Total RNA was isolated from fat depots of 2-wk-old and 10-wk-old rats as well as cell culture using the RNeasy Mini Kit (Qiagen). RNA was bioanalyzed for RNA integrity and quantity (Agilent). One microgram of RNA was reverse-transcribed using the iScript cDNA synthesis kit, and then, real-time polymerase chain reaction (14) was performed using Taqman universal master mix on a Via machine. Taqman probes used include Tmem26 (Rn01428021_m1), Tnfrsf9 (Rn01519016_m1), Klhl13 (Rn01475504_m1), Pparγ (Rn00440945_m1), Lep (Rn00565158_m1), Ucp1 (Rn00562126_m1), IL-4r (Rn01507024_m1), Adipoq (Rn00595250_m1), and Retn (Rn00595224_m1). Tata-binding protein (Tbp, Rn01455646_m1) was used as a normalization control. Statistical analyses were carried out using ΔΔCt values, and data were graphed using 2−ΔΔCt (fold change of IL-4-treated samples as compared with PBS controls).
Statistical Methods
All data were analyzed using Prism (GraphPad). Data points were analyzed for normal distribution using the Shapiro–Wilk test and log transformed if necessary. Power analysis was used to calculate sample size, and we approximated a 25% change with a standard deviation of 20%, 80% power, and a significance level α of 0.05. Nested one-way ANOVA was used to exclude litter as a significant variable, and data were analyzed using unpaired two-tailed Student’s t tests. All P values are reported with adjustment for multiple comparisons if necessary (such as when multiple genes were compared), and a P value of < 0.05 was considered to be statistically significant and is presented as * (P < 0.05), ** (P < 0.01), *** (P < 0.001), or **** (P < 0.0001) (15). Error bars represent the standard error of the mean (SE).
RESULTS
Acute IL-4 Treatment of Neonatal Rats Decreases iWAT Expansion
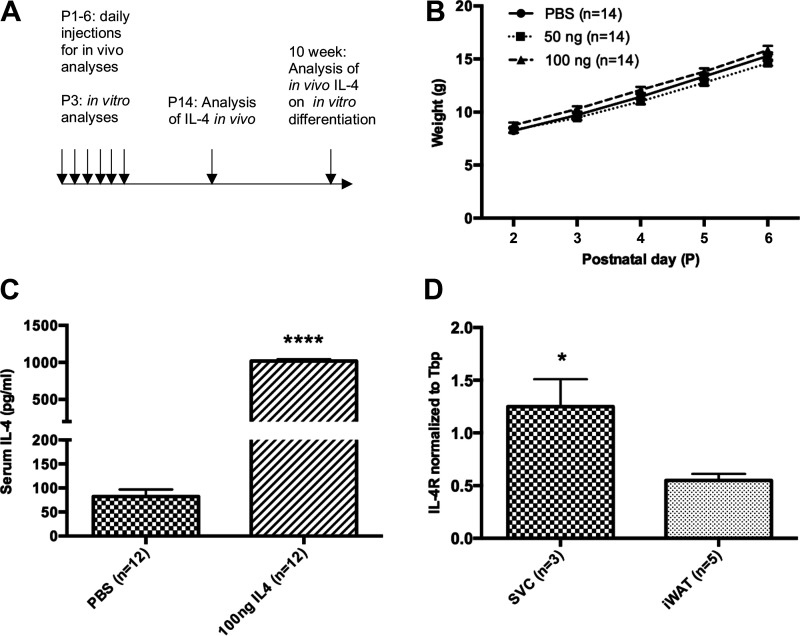
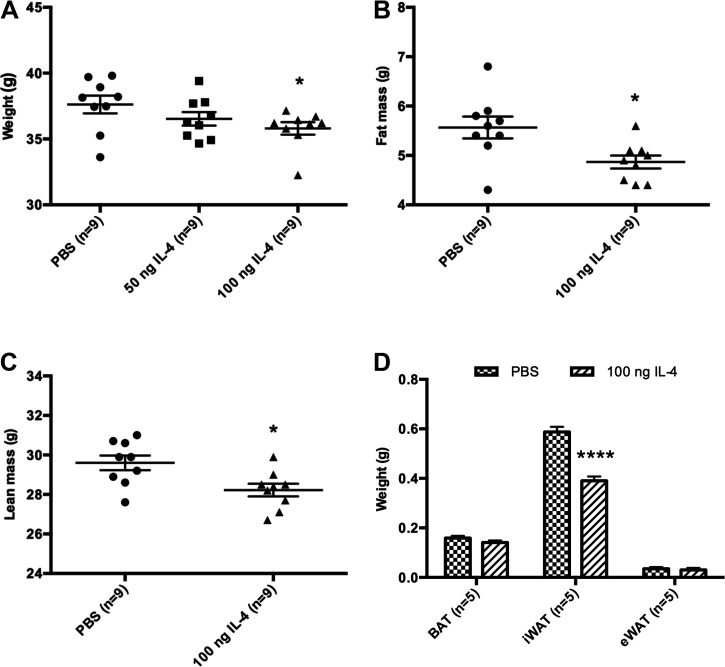
We treated rats with IL-4 daily from postnatal day 1 through 6 (16) to investigate the effects on adipose tissue (Fig. 1A). Our previous studies demonstrate that perturbations during this early postnatal period cause long-lasting changes in adult metabolic health (17–20). We used the two lowest doses of IL-4 that were previously shown to exert effects on adipogenesis in adult mice: 50 ng and 100 ng (21). Pups treated with both doses of IL-4 and PBS control gained the same amount of weight during the treatment period (Fig. 1B), demonstrating that IL-4 had no overt effects on feeding or growth. Approximately 5 min after the first injection of IL-4, there was a dramatic rise in serum IL-4 levels (Fig. 1C). Because IL-4 has a short half-life in circulation (minutes), we did not assess additional time points. Gene expression analysis showed that Il4r expression was enriched in the stromal vascular cell (SVC) fraction, containing adipocyte precursor cells, as compared with whole iWAT (Fig. 1D). By P14, animals treated with 100 ng of IL-4 weighed significantly less than control animals (Fig. 2A). Animals treated with 50 ng of IL-4 weighed the same as controls but did show decreased fat mass (Fig. 2A and Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.13224623.v1). A nested ANOVA of fat mass data from DEXA scans showed that there is significant variation among treatments and control [F(2,6)=6.865, P = 0.0281] but not among litters (P = 0.3165). Therefore, litter was excluded as a variable. DEXA scans showed that 100 ng IL-4 decreased both fat (∼0.6 g) and lean mass (Fig. 2, B and C). Further dissection of the adipose tissue depots revealed that IL-4 significantly decreased the weight of iWAT (∼0.2 g) but not BAT or eWAT (Fig. 2D). This decrease in iWAT does not account for the entire decrease in fat as quantified by DEXA. The rest of the fat decrease is likely spread throughout the entire animal, including eWAT and BAT, but we do not have the power needed to detect these small changes. Thus, we focused further experiments only on iWAT.
Figure 1.
Exogenous IL-4 had no overt effects on neonatal feeding or growth. A: experimental design: injection during P1–P6 of PBS, 50 ng IL-4, or 100 ng IL-4. Analyses performed at P14 and 10 wk of age. B: weights for individual rat pups from 3 different litters throughout injection period and before tissue collection (n = 14). C: IL-4 concentration in serum on day 1 of injections from individual rat pups in 3 different litters (n = 12). D: IL-4 receptor mRNA levels in whole inguinal white adipose tissue (iWAT) as compared with iWAT-isolated stromal vascular cells (SVCs) from individual P1 rat pups in 3 different litters (n = 3–5). *P < 0.05; ****P < 0.0001.
Figure 2.
IL-4 decreases iWAT in neonates. A: 100 ng IL-4, but not 50 ng IL-4, decreases weight as compared with PBS at P14 in 9 pups from 3 different litters (n = 9). One-way ANOVA was carried out and excluded litter as a variable. B: DEXA scan shows IL-4-injected cohort has decreased fat mass as compared with PBS control at P14 (n = 9). C: IL-4-injected cohort also shows decreased lean mass as compared with PBS control at P14 (n = 9). D: weight is decreased in iWAT and not in other depots at P14 (n = 5). BAT, brown adipose tissue; DEXA, dual X-ray absorbance; eWAT, epididymal white adipose tissue; iWAT, inguinal white adipose tissue. *P < 0.05; ****P < 0.0001.
IL-4 Increases Beige Adipogenesis Acutely in Neonatal iWAT
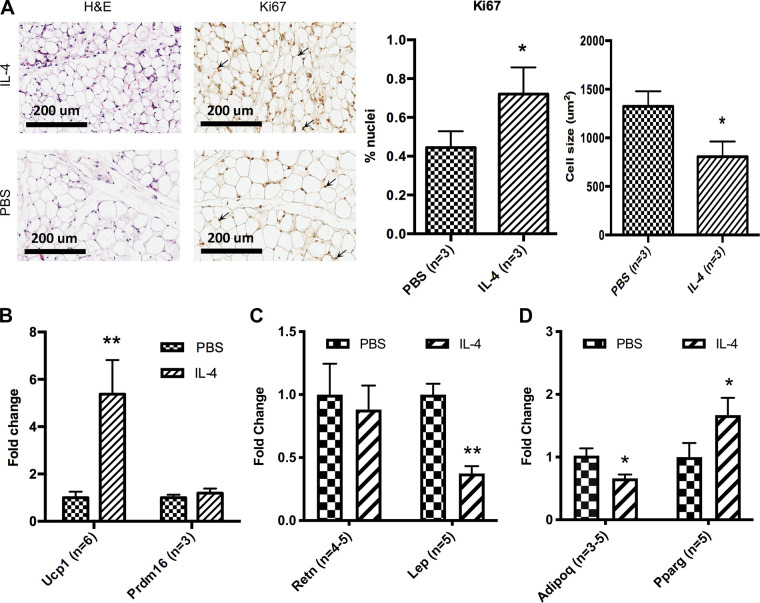
Beige adipose tissue can dissipate energy and suppress weight gain. We thus considered whether IL-4 decreased iWAT mass by promoting beige adipogenesis. Histological examination of iWAT in control P14 animals revealed only unilocular lipid droplets (Fig. 3A). BAT adipocytes displayed a multilocular morphology, and eWAT adipocytes displayed a unilocular lipid morphology (Supplemental Fig. S2; see https://doi.org/10.6084/m9.figshare.12991058.v3). In contrast, analysis of iWAT sections from the IL-4-injected cohort revealed smaller adipocyte size as compared with controls (Fig. 3A). Ki67 staining showed that neonatal IL-4 increased the percentage of proliferating cells in iWAT at P14 (Fig. 3A). Next, we looked at gene expression using qRT-PCR and verified the efficiency of each primer (Supplemental Fig. S3; see https://doi.org/10.6084/m9.figshare.14077346.v2). Ucp1 mRNA levels were markedly increased in the iWAT of IL-4-treated animals, whereas Prdm16 showed no changes (Fig. 3B). IL-4 treatment also increased the expression levels of the adipogenic regulator Pparγ, an early marker of adipogenesis, and decreased the expression levels of white versus beige fat-enriched marker genes Adipoq and Lep in iWAT (Fig. 3, C and D). Retn, another white fat-enriched gene showed no change (Fig. 3C). Altogether, these results suggest that IL-4 exposure in the early postnatal period directs adipocyte precursor differentiation toward the beige versus white fat fate. Although early IL-4 does not increase Prdm16 mRNA expression, even beige adipogenesis induced by cold is not associated with a higher level of Prdm16 (22). Although Prdm16 is required for beige differentiation, it is not increased Prdm16 expression that determines beige adipogenesis.
Figure 3.
IL-4 increases beige adipogenesis in neonatal iWAT. A: representative images of hematoxylin and eosin and Ki67 staining of P14 iWAT 5-µm histology sections. These images were taken at ×20 magnification and show the morphology of iWAT at P14 in PBS- and IL-4-injected cohorts and BAT at P14 in PBS-injected cohort. Ki67-stained nuclei are identified with arrows. Percentage of nuclei with Ki67 and average cell size were also quantified. qRT-PCR analysis from individual pups in 3 different litters shows the relative expression levels of beige adipocyte genes (Ucp1 and Prdm16) in P14 iWAT (n = 3–6, B), white adipocyte genes (Retn and Lep) in P14 iWAT (n = 4 or 5, C), and adipogenesis genes (Adipoq and Pparg) in P14 iWAT (n = 5 or 6, D). qRT-PCR analyses were carried out using ΔΔCt values, and data were graphed using 2−ΔΔCt (fold change of IL-4-treated samples as compared with PBS controls). iWAT, inguinal white adipose tissue. *P < 0.05; **P < 0.01.
IL-4 Increases Proliferation of ICAM1 Population
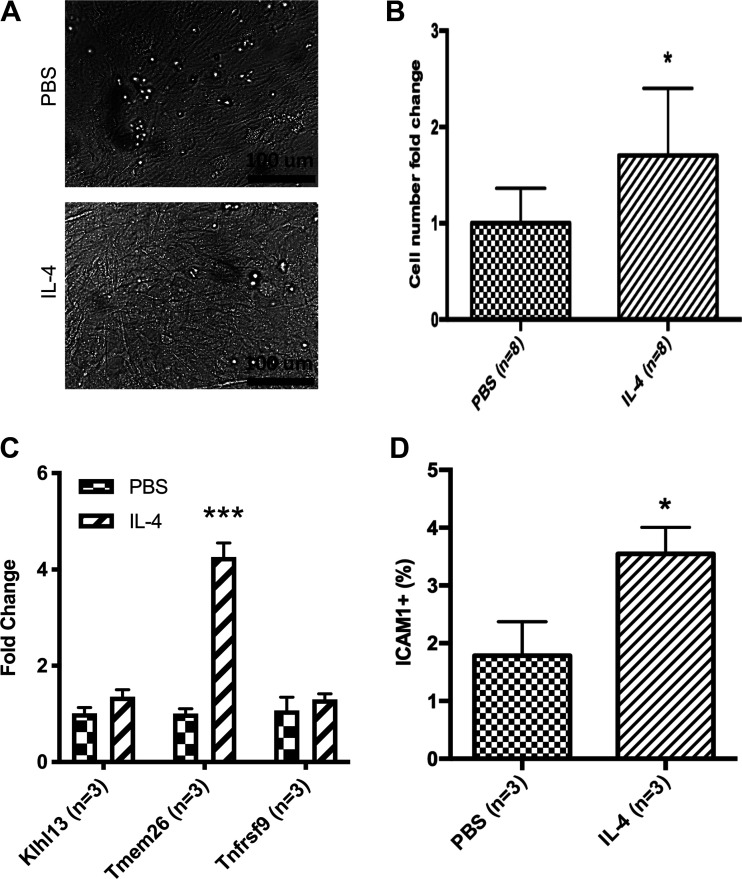
We next sought to separate the effects of IL-4 directly on the neonatal adipocyte precursor and the effects of IL-4 on the differentiation process of adipogenesis. To test the former, SVCs were isolated from the iWAT of control P3 animals, plated in culture to enrich for adipocyte precursors, and incubated with either 10 nM IL-4 or PBS (as control) for 48 h. Images taken after 48 h of IL-4 or PBS incubation show similar adipocyte precursor morphology (Fig. 4A). IL-4 treatment significantly increased cell numbers as compared with PBS treatment (Fig. 4B). The expression of select beige fat marker genes was quantified in these cultures, revealing that IL-4 augmented the levels of Tmem26 mRNA but did not affect Klhl13 or Tnfrsf9 levels (Fig. 4C). Inguinal WAT SVCs were also isolated from P7 neonates that had been injected with either PBS or IL-4 for 6 days for flow cytometry analysis. ICAM1 was used as an adipocyte precursor maker because it is the only one that has been shown to define the preadipocyte population in neonatal rodents (23). IL-4-injected animals had a significant increase in ICAM1+ cells as compared with PBS-injected controls (Fig. 4D) (Supplemental Fig. S4; see https://doi.org/10.6084/m9.figshare.12993794.v5). Therefore, these data suggest that IL-4 increases the proliferation of the ICAM1+ adipocyte precursors.
Figure 4.
IL-4 increases proliferation of ICAM1 population. A: representative images of adipocyte precursors after exposure to IL-4 for 48 h. B: cells were plated equally in pairs, counted after incubation using a hemocytometer, and fold change was calculated (n = 8). C: qRT-PCR analysis shows the expression level of beige adipocyte lineage genes (Klhl13, Tmem26, and Tnfrsf9) from individual pups in 3 different litters (n = 3). qRT-PCR analyses were carried out using ΔΔCt values, and data were graphed using 2−ΔΔCt (fold change of IL-4-treated samples as compared with PBS controls). D: percentage of ICAM1+ preadipocytes by flow cytometry after IL-4 injections (n = 3). *P < 0.05; ***P < 0.001.
IL-4 Treatment of Mature Adipocytes Activates Ucp1 Expression
We then tested in vitro whether IL-4 affects the adipocyte differentiation process. Our goal was to determine the following: 1) the effect of IL-4 pretreatment on adipocyte differentiation, 2) the effect of IL-4 regulation during either phases of adipocyte differentiation [multiclonal expansion (MCE) and terminal differentiation], and 3) the effect of IL-4 throughout pretreatment and differentiation. SVCs from P3 rats were plated at confluency and pretreated for 2 days before incubation with a complete adipocyte differentiation cocktail for an additional 6 days. IL-4 was added in the following treatment conditions: 1) pretreatment for 2 days before inducing adipocyte differentiation, 2) during MCE for 2 days, and 3) during terminal differentiation for 2 days (Fig. 5A) (Supplemental Fig. S5; see https://doi.org/10.6084/m9.figshare.12993800.v5). IL-4 exposure at various time points led to a decrease in the development of lipid droplets containing adipocytes (Fig. 5B). The expression levels of the mature adipocyte marker Adipoq were also dramatically reduced in IL-4-treated cultures (Fig. 5C). Chronic IL-4 treatment throughout differentiation caused the most significant decrease in Adipoq expression. Notably, IL-4 increased Ucp1 levels only when added during terminal differentiation (Fig. 5D). When IL-4 was continually added during days 2–6 (IL-4 throughout), there was still a significant increase in Ucp1 mRNA expression as compared with PBS control, but it is likely driven by the IL-4 added during terminal differentiation. Collectively, these data indicate that IL-4 is sufficient to decrease overall adipogenesis, as well as induce beige fat programming of adipogenic precursor cells under in vitro conditions.
Figure 5.
IL-4 decreases adipocyte maturation in vitro. A: scheme for experimental method shows the different IL-4 exposure periods of cells during differentiation [pretreatment, IL-4 treatment from day 2 to 4; multiclonal expansion (MCE), IL-4 treatment from day 4 to 6; terminal differentiation, IL-4 treatment from day 6 to 8; throughout, IL-4 treatment from day 2 to 8]. Cultures used SVCs from individual pups in 3 different litters. B: representative images of adipocyte precursors from all treatment conditions on day 8. C: qRT-PCR analysis of Adipoq mRNA expression from all treatment conditions on day 8: IL-4 added during pretreatment (n = 5), MCE (n = 4), terminal differentiation (n = 4), and throughout pretreatment and differentiation (n = 4). D: qRT-PCR analysis of Ucp1 mRNA expression from all treatment conditions on day 8: IL-4 added during pretreatment (n = 5), MCE (n = 4), terminal differentiation, (n = 4) and throughout pretreatment and differentiation (n = 4). qRT-PCR analyses were carried out using ΔΔCt values, and data were graphed using 2−ΔΔCt (fold change of IL-4-treated samples as compared with PBS controls). SVC, stromal vascular cell. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Capacity for Adipogenesis Is Persistently Decreased in Adipocyte Precursors after Neonatal IL-4 Exposure
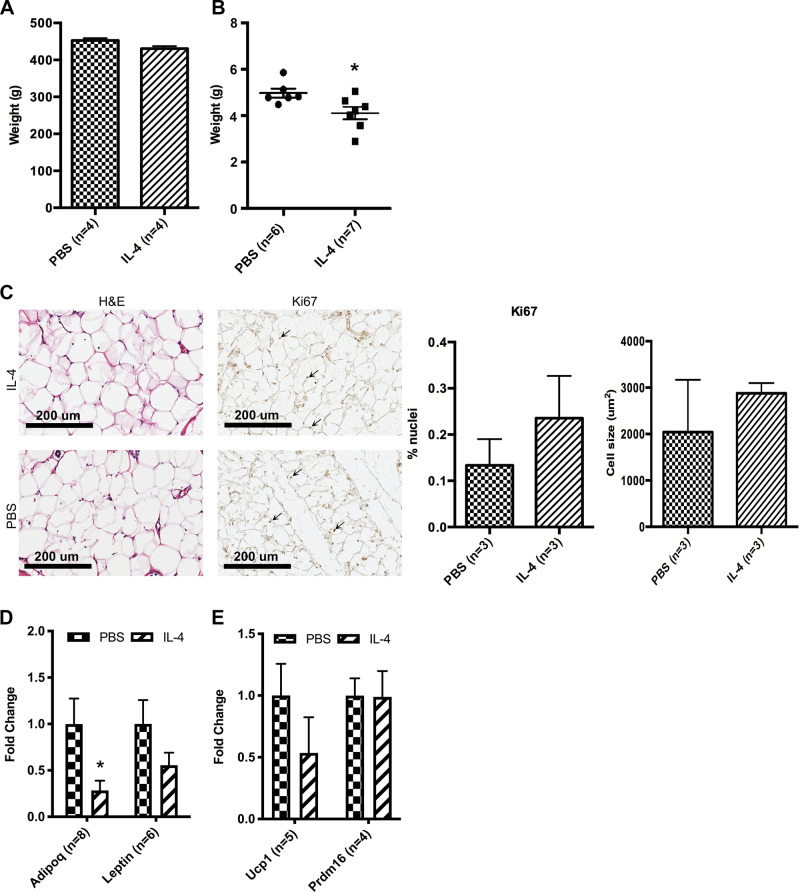
To determine if IL-4 treatment of neonates induces long-lasting effects on adipose tissue, we followed a subset of IL4-treated and control animals for 10 wk. Although IL-4-treated animals had decreased body weight as compared with controls at 2 wk of age, body weight was normalized by 10 wk (Fig. 6A). Fasting glucose levels of IL-4-treated animals were also decreased at 10 wk of age as compared with those of PBS controls (Supplemental Fig. S6; see https://doi.org/10.6084/m9.figshare.13224647.v3). Dissection of individual fat depots revealed that the iWAT depot weighed significantly less in treated adult animals as compared with controls (Fig. 6B). Histological staining of the iWAT depot shows that 10-wk control adipocytes are similar to those of IL-4-treated animals in size, proliferation, and beige adipogenesis (Fig. 6C). Analysis of pancreatic islets using perifusion also showed no change in basal and glucose-stimulated insulin secretion in IL-4-treated cohort as compared with PBS controls (Supplemental Fig. S7; see https://doi.org/10.6084/m9.figshare.13224665.v3).
Figure 6.
Capacity for adipogenesis is persistently decreased in adipocyte precursors after neonatal IL-4 exposure. A: whole body weight was measured at 10 wk of age for individual rats from 3 different litters (n = 4). B: iWAT was weighed for individual rats from 3 different litters. iWAT has decreased weight at 10 wk of age in IL-4-injected cohort as compared with PBS control (n = 6 or 7). C: representative images of hematoxylin-and-eosin- and Ki67-stained iWAT from treatment and control rats at 10 wk of age. Ki67-stained nuclei are identified with arrows. Percentage of nuclei with Ki67 and average cell size were quantified. D: qRT-PCR of genes from differentiated SVCs originating in individual adult controls that came from 3 different litters or individual treatment rats that came from 3 different litters. Adipogenesis genes Adipoq and Pparg (n = 7) and beige adipocyte genes Ucp1 and Prdm16 were analyzed (n = 6, E). qRT-PCR analyses were carried out using ΔΔCt values, and data were graphed using 2−ΔΔCt (fold change of IL-4-treated samples as compared with PBS controls). iWAT, inguinal white adipose tissue; SVC, stromal vascular cell. *P < 0.05.
Because there was a significant decrease in iWAT depot weight between control and IL-4-treated adult animals that did not correlate with adipocyte morphology, we analyzed SVCs from the adult animals. SVCs were isolated from 10-wk-old rats previously treated with IL-4 and control (as newborns). SVCs from IL-4-treated rats displayed a reduced capacity for adipocyte differentiation as reflected by diminished expression of Adipoq mRNA expression in day 8 cultures (Fig. 6D). There was no difference in Ucp1, Prdm16, or Lep expression between differentiated cultures from control and IL-4-treated rats. (Fig. 6, D and E). These results suggest that beige adipocytes induced by neonatal IL-4 exposure were not maintained into adulthood.
DISCUSSION
In this study, we aimed to determine the immediate and long-term effects of the cytokine IL-4 in neonates. First, we made the novel observation that low-dose exposure of IL-4 during the first week of life significantly decreases body fat and lean mass by P14. We also observed increased adipocyte proliferation and decreased adipocyte size in juvenile animals. Transgenic expression of IL-4 induced a similar adipose tissue phenotype in older mice (24). Several studies have reported that small adipocytes are especially important for counteracting obesity-associated metabolic decline and have shown that small adipocytes are often correlated with decreased susceptibility to developing diabetes (15, 25). But these studies were done in adult animals, and it remains uncertain if adipocyte size in very young animals also correlates with improved glucose homeostasis. Interestingly, the effects of IL-4 on adipocyte size and cell proliferation in adipose tissue did not persist into adulthood.
Previous studies demonstrated that type 2 cytokines, including IL-4, promote beige fat development in adult rodents (15). However, neonates utilize a different adipocyte precursor population than adults (26–28). This is supported by Hong et al. (29), who first demonstrated that fetal and neonatal adipose precursors are functionally different from adult adipose precursors. In addition, Wang et al. (30) reported that C/EBPα is not required for the development of WAT in the perinatal period or the development of beige adipose tissue in the adult but is required for the maturation of WAT in adulthood. In this study, we show that in vivo IL-4 treatment decreases iWAT weight. IL-4 did not affect eWAT weight, potentially explained by its later development at P5 and thus only having 2 days of exposure to IL-4. In addition, epididymal adipose tissue is much less prone to beige adipocyte development. We also discovered that IL-4 increased Ucp1 mRNA expression in neonatal adipose tissue in vivo. In tandem, IL-4 significantly decreased the expression of the white fat-enriched markers Adipoq and Lep in IL-4-treated animals. These results suggest that IL-4 increases the proportion of beige versus white adipocytes in young rats.
In vitro we added IL-4 for different periods during differentiation, according to a scheme similar to Tsao et al. (13). They found that IL-4 added during the first 2 days of 3T3-L1 adipogenic differentiation led to decreased adipogenesis. Strikingly, we see that IL-4 added at any point during differentiation decreases adipogenesis and that this effect is additive. Lee et al. (6) reported that IL-4 pretreatment enhanced capacity for beige adipogenesis in differentiated adipocyte precursors from adult mice. Consistent with our in vivo findings, IL-4 induced Ucp1 expression in our differentiated primary adipocyte cultures. Interestingly, IL-4 added for periods before terminal differentiation or continuously throughout differentiation did not induce Ucp1 expression. This result suggests that IL-4 exerts possible opposing effects in precursor cells and during different phases of adipogenesis, the basis of which requires further study.
A limitation of our study is the use of only male neonates as there could be sex-specific differences (31). In addition, although we found no IL-4-induced change in insulin levels (basal or glucose stimulated) from pancreas in this study, further analysis of neonatal IL-4 in other metabolically active tissues, such as liver and muscle, will be required to understand how IL-4 regulates metabolism during development.
In humans, maternal IL-4 does not cross the placenta, but a deficiency in IL-4 and/or other anti-inflammatory cytokines contributes to infertility, spontaneous abortions, and other pregnancy disorders (32). In utero, high levels of IL-4 are produced at the fetal-maternal interface to mediate active maternal tolerance (10). These high IL-4 levels persist after birth in newborns and decline with age (33). Significant associations between IL-4 promoter single-nucleotide polymorphisms (SNPs) and type 2 diabetes have also been found in large human cohort studies (34, 35). In rodents, Il-4 was previously shown to decrease adult fat in mice, and now we have shown that adipocyte precursors in the newborn rat respond to IL-4 by acutely increasing beige adipogenesis and persistently reducing fat mass (6). These insights into developmental adipogenesis may help in developing future therapies for obesity and related diseases.
GRANTS
This work was supported by the National Institutes of Health Grant No. R01-DK-114054 to R.A.S. The University of Pennsylvania Mouse Phenotyping, Physiology and Metabolism Core receive support from the Institute for Diabetes, Obesity and Metabolism National Institutes of Health Research Center Grant No. P30-DK-19525. T. Golden was supported by the NIH training Grant ES019851.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
T.Y., P.S., and R.A.S. conceived and designed research; T.Y., T.N.G., L.C. and J.I. performed experiments; T.Y., T.N.G., L.C. and J.I. analyzed data; T.Y., P.S., and R.A.S. interpreted results of experiments; T.Y. prepared figures; T.Y. drafted manuscript; T.Y., T.N.G., P.S., and R.A.S. edited and revised manuscript; T.Y., T.N.G., L.C., J.I., P.S., and R.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the University of Pennsylvania Mouse Phenotyping, Physiology and Metabolism Core for assistance with DEXA scanning and the Children’s Hospital of Philadelphia Pathology Core for assistance with histology profiling.
REFERENCES
- 1.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism 92: 6–10, 2019. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 9: 88, 2009. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikeda K, Maretich P, Kajimura S. The common and distinct features of Brown and Beige adipocytes. Trends Endocrinol Metab 29: 191–200, 2018. doi: 10.1016/j.tem.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenwood MR, Hirsch J. Postnatal development of adipocyte cellularity in the normal rat. J Lipid Res 15: 474–483, 1974. doi: 10.1016/S0022-2275(20)36767-5. [DOI] [PubMed] [Google Scholar]
- 5.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest 125: 478–486, 2015. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160: 74–87, 2015. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157: 1279–1291, 2014. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lizcano F, Vargas D, Gómez Á, Torrado A. Human ADMC-derived adipocyte thermogenic capacity is regulated by IL-4 receptor. Stem Cells Int 2017: 2767916, 2017. doi: 10.1155/2017/2767916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hepler C, Vishvanath L, Gupta RK. Sorting out adipocyte precursors and their role in physiology and disease. Genes Dev 31: 127–140, 2017. doi: 10.1101/gad.293704.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohr E, Siegrist CA. Vaccination in early life: standing up to the challenges. Curr Opin Immunol 41: 1–8, 2016. doi: 10.1016/j.coi.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157: 1292–1308, 2014. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng J, Hua K, Lesser SS, Greiner AH, Walter AW, Marrero MB, Harp JB. Interleukin-4 mediates STAT6 activation in 3T3-L1 preadipocytes but not adipocytes. Biochem Biophys Res Commun 267: 516–520, 2000. doi: 10.1006/bbrc.1999.1993. [DOI] [PubMed] [Google Scholar]
- 13.Tsao CH, Shiau MY, Chuang PH, Chang YH, Hwang J. Interleukin-4 regulates lipid metabolism by inhibiting adipogenesis and promoting lipolysis. J Lipid Res 55: 385–397, 2014. doi: 10.1194/jlr.M041392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozak LP, Koza RA, Anunciado-Koza R, Mendoza T, Newman S. Inherent plasticity of brown adipogenesis in white fat of mice allows for recovery from effects of post-natal malnutrition. PLoS One 7: e30396, 2012. doi: 10.1371/journal.pone.0030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lönn M, Mehlig K, Bengtsson C, Lissner L. Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J 24: 326–331, 2010. doi: 10.1096/fj.09-133058. [DOI] [PubMed] [Google Scholar]
- 16.Han J, Lee JE, Jin J, Lim JS, Oh N, Kim K, Chang SI, Shibuya M, Kim H, Koh GY. The spatiotemporal development of adipose tissue. Development 138: 5027–5037, 2011. doi: 10.1242/dev.067686. [DOI] [PubMed] [Google Scholar]
- 17.Stoffers DA, Desai BM, DeLeon DD, Simmons RA. Neonatal exendin-4 prevents the development of diabetes in the intrauterine growth retarded rat. Diabetes 52: 734–740, 2003. doi: 10.2337/diabetes.52.3.73. [DOI] [PubMed] [Google Scholar]
- 18.Pinney SE, Jaeckle Santos LJ, Han Y, Stoffers DA, Simmons RA. Exendin-4 increases histone acetylase activity and reverses epigenetic modifications that silence Pdx1 in the intrauterine growth retarded rat. Diabetologia 54: 2606–2614, 2011. doi: 10.1007/s00125-011-2250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaeckle Santos LJ, Li C, Doulias PT, Ischiropoulos H, Worthen GS, Simmons RA. Neutralizing Th2 inflammation in neonatal islets prevents β-cell failure in adult IUGR rats. Diabetes 63: 1672–1684, 2014. doi: 10.2337/db13-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, Mukundan L, Wu D, Locksley RM, Chawla A. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci USA 107: 22617–22622, 2010. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mj C, Ga A, P Z, Sw C, Rm S, S C, Tl D. IL-4 prevents insulitis and insulin dependent diabetes mellitus in nonobese diabetic mice by potentiation of regulatory T helper2 cell function. J Immunol 159: 4686–4692, 1997. [PubMed] [Google Scholar]
- 22.Waldén TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab 302: E19–E31, 2012. doi: 10.1152/ajpendo.00249.2011. [DOI] [PubMed] [Google Scholar]
- 23.Merrick D, Sakers A, Irgebay Z, Okada C, Calvert C, Morley MP, Persec I, Seale P. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 364: eaav2501, 2019. doi: 10.1126/science.aav2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elbe-Bürger A, Egyed A, Olt S, Klubal R, Mann U, Rappersberger K, Rot A, Stingl G. Overexpression of IL-4 alters the homeostasis in the skin. J Invest Dermatol 118: 767–778, 2002. doi: 10.1046/j.1523-1747.2002.01753.x. [DOI] [PubMed] [Google Scholar]
- 25.Lundgren M, Svensson M, Lindmark S, Renström F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and 'hyperleptinaemia'. Diabetologia 50: 625–633, 2007. doi: 10.1007/s00125-006-0572-1. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, Berry DC, Tang W, Graff JM. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep 9: 1007–1022, 2014. doi: 10.1016/j.celrep.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwalie PC, Dong H, Zachara M, Russeil J, Alpern D, Akchiche N, Caprara C, Sun W, Schlaudraff KU, Soldati G, Wolfrum C, Deplancke B. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 559: 103–108, 2018. doi: 10.1038/s41586-018-0226-8. [DOI] [PubMed] [Google Scholar]
- 28.Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol 17: 376–385, 2015. doi: 10.1038/ncb3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong KY, Bae H, Park I, Park DY, Kim KH, Kubota Y, Cho ES, Kim H, Adams RH, Yoo OJ, Koh GY. Perilipin+ embryonic preadipocytes actively proliferate along growing vasculatures for adipose expansion. Development 142: 2623–2632, 2015. doi: 10.1242/dev.125336. [DOI] [PubMed] [Google Scholar]
- 30.Wang QA, Tao C, Jiang L, Shao M, Ye R, Zhu Y, Gordillo R, Ali A, Lian Y, Holland WL, Gupta RK, Scherer PE. Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat Cell Biol 17: 1099–1111, 2015. doi: 10.1038/ncb3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dearden L, Bouret SG, Ozanne SE. Sex and gender differences in developmental programming of metabolism. Mol Metab 15: 8–19, 2018. doi: 10.1016/j.molmet.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol 5: 253, 2014. doi: 10.3389/fimmu.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim RH, Kobzik L. Transplacental passage of interleukins 4 and 13? PLoS One 4: e4660, 2009.doi: 10.1371/journal.pone.0004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho KT, Shiau MY, Chang YH, Chen CM, Yang SC, Huang CN. Association of interleukin-4 promoter polymorphisms in Taiwanese patients with type 2 diabetes mellitus. Metabolism 59: 1717–1722, 2010. doi: 10.1016/j.metabol.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Bid HK, Konwar R, Agrawal CG, Banerjee M. Association of IL-4 and IL-1RN (receptor antagonist) gene variants and the risk of type 2 diabetes mellitus: a study in the north Indian population. Indian J Med Sci 62: 259–266, 2008. doi: 10.4103/0019-5359.42021. [DOI] [PubMed] [Google Scholar]