Abstract
Trauma-induced hemorrhage is a leading cause of disability and death due, in part, to impaired perfusion and oxygenation of the brain. It is unknown if cerebrovascular responses to blood loss are differentiated based on sex. We hypothesized that compared to males, females would have reduced tolerance to simulated hemorrhage induced by maximal lower body negative pressure (LBNP), and this would be associated with an earlier reduction in cerebral blood flow and cerebral oxygenation. Healthy young males (n = 29, 26 ± 4 yr) and females (n = 23, 27 ± 5 yr) completed a step-wise LBNP protocol to presyncope. Mean arterial pressure (MAP), stroke volume (SV), middle cerebral artery velocity (MCAv), end-tidal CO2 (etCO2), and cerebral oxygen saturation (ScO2) were measured continuously. Unexpectedly, tolerance to LBNP was similar between the sexes (males, 1,604 ± 68 s vs. females, 1,453 ± 78 s; P = 0.15). Accordingly, decreases (%Δ) in MAP, SV, MCAv, and ScO2 were similar between males and females throughout LBNP and at presyncope (P ≥ 0.20). Interestingly, although decreases in etCO2 were similar between the sexes throughout LBNP (P = 0.16), at presyncope, the %Δ etCO2 from baseline was greater in males compared to females (−30.8 ± 2.6% vs. −21.3 ± 3.0%; P = 0.02). Contrary to our hypothesis, sex does not influence tolerance, or the central or cerebral hemodynamic responses to simulated hemorrhage. However, the etCO2 responses at presyncope do suggest potential sex differences in cerebral vascular sensitivity to CO2 during central hypovolemia.
NEW & NOTEWORTHY Tolerance and cerebral blood velocity responses to simulated hemorrhage (elicited by lower body negative pressure) were similar between male and female subjects. Interestingly, the change in etCO2 from baseline was greater in males compared to females at presyncope, suggesting potential sex differences in cerebral vascular sensitivity to CO2 during simulated hemorrhage. These findings may facilitate development of individualized therapeutic interventions to improve survival from hemorrhagic injuries in both men and women.
Keywords: cerebral blood velocity, lower body negative pressure, sex differences
INTRODUCTION
Trauma-induced hemorrhage is a leading cause of disability and death in the civilian and military settings (1–4) due, in part, to impaired tissue perfusion and oxygenation of the brain. Tolerance to reduced central blood volume induced by hemorrhage depends upon cardiovascular mediated compensatory mechanisms to maintain adequate blood flow and oxygen delivery to the vital end-organs (5). Impairment or reduced sensitivity of these compensatory responses leads to a reduction in arterial pressure and blood flow, resulting in cardiovascular decompensation, loss of consciousness, and ultimately, death. Elucidating potential sex differences in cerebral vascular responses to hemorrhage may be important for development and implementation of individualized therapeutic interventions to improve survival from these injuries.
In many prior studies, young female human subjects have demonstrated a lower tolerance to experimentally induced acute central hypovolemia via application of lower body negative pressure (LBNP) compared with young males (6–12). To our knowledge, Montgomery et al. (13) was the first to examine the hemodynamic differences between males and females in response to short-duration, submaximal LBNP (5-min bouts of −20, −40, and −60 mmHg). In this small study of six males and four females, these investigators reported that females exhibited a greater reduction in arterial blood pressure and pelvic blood flow, and a greater compensatory increase in heart rate during −60 mmHg LBNP compared to males. Tolerance to LBNP was not specifically assessed in this study, making it difficult to determine sex differences at presyncope; however, most female subjects were not able to complete the −60 mmHg step of LBNP, whereas all of the men completed this step. A study by White et al. (12) was one of the first to report that women have lower tolerance to a progressive step-wise LBNP protocol than men, assessing presyncopal end points in all subjects (defined by at least one of the following criteria: 1) subjective presyncopal symptoms such as dizziness, and/or 2) sustained systolic arterial pressure (SAP) below 90 mmHg, and/or 3) sudden relative bradycardia, and/or 4) voluntary subject termination) (12). Although heart rate was higher in females than males when exposed to the same submaximal stage of LBNP (−50 mmHg LBNP), there were no differences in other central hemodynamic responses (arterial pressure, stroke volume, cardiac output, systemic vascular resistance) throughout LBNP or at presyncope (12).
Lower tolerance to LBNP in females has since been attributed to differences in multiple physiological compensatory mechanisms when compared with males including, reduced cardiac filling (9) and cardiac output (8, 11), reduced coupling between blood pressure and sympathetic activity (6), and a reduced circulating catecholamine response (7–9) with maximal LBNP. Sex differences in cerebral blood flow and oxygenation, which are fundamental components in provoking syncope (14), may also explain the lower tolerance to central hypovolemia observed in females compared with males.
Sex differences in cerebrovascular regulation have been reported; young women have higher total cerebral blood flow and velocity at rest (15, 16) and higher cerebrovascular reactivity to a hypercapnic challenge (17–19). Recently, however, Lewis et al. (11) reported no sex differences in the absolute reduction in velocity in the middle (MCA) and posterior cerebral arteries (PCA) in response to maximal LBNP, despite the finding that female subjects exhibited lower tolerance; there was no assessment of the temporal responses at submaximal LBNP stages. In a separate analysis, these investigators demonstrated that decreases in cerebral blood flow (or velocity) elicited compensatory increases in cerebral oxygen extraction (11), but this analysis was not performed during LBNP or between men and women. In our laboratory, we recently demonstrated that individuals with higher tolerance to central hypovolemia induced by LBNP exhibit prolonged preservation of cerebral blood velocity in the posterior cerebral circulation and sustained cerebral tissue oxygenation of the frontal lobe throughout submaximal LBNP stages (20). However, the role of sex on these responses was also not assessed in this prior work.
Since most studies have previously shown that females have lower tolerance to LBNP compared to males, it is possible that this lower tolerance is related to greater reductions in both posterior cerebral blood flow and frontal lobe cerebral oxygenation in women compared with men. Therefore, we hypothesized that females would have reduced tolerance to central hypovolemia induced by maximal LBNP, and this would be associated with an earlier reduction in blood flow to the posterior cerebral circulation, no differences in anterior cerebral circulation responses, and a greater reduction in frontal lobe cerebral tissue oxygenation.
METHODS
Subjects
Sixty-two healthy young adults completed this cross-sectional study, conducted at the University of North Texas Health Science Center (UNTHSC) in Fort Worth, TX, as part of three independent studies. Data from these subjects have previously been reported in a number of prior publications focusing on independent research questions (20–22). The three experimental protocols were reviewed and approved by the UNTHSC Institutional Review Board (Protocol Numbers: 2012-163, 2014-127, 2018-120). All subjects were nonsmokers and free from diagnosed cardiovascular, respiratory, metabolic, or inflammatory diseases. Each subject attended a familiarization session, where a medical history, and standing and seated blood pressure and ECG measurements were obtained for physician approval before participation in the study. Female subjects underwent a urine pregnancy test during the familiarization session and were excluded if pregnant. Subjects were given a verbal briefing and written description of all the measurements and risks associated with the protocol and were familiarized to the laboratory, personnel, procedures, and monitoring equipment. All subjects provided written informed consent before participation in the study.
Due to potential confounding effects on cardiovascular and autonomic regulation, all subjects were asked to arrive for testing at the laboratory having abstained for the 24 h before each session from: alcohol consumption, stimulants (e.g., caffeine, and cold medications), prescription and nonprescription drugs, herbal medications, and exercise. Subjects were also instructed to remain hydrated (ad libitum water consumption) and maintain routine sleep patterns before familiarization and experimental sessions. All female subjects were tested within days 1–4 of the start of menses, or if taking oral contraceptives, during the blank pill or no pill days; menstrual phase was self-reported. A urine pregnancy test was repeated immediately before the experimental session. Additionally, all subjects were encouraged to empty their bladder before instrumentation to control for potential confounding effects of bladder distension on blood pressure regulation (23). Experimental sessions were performed at the same time of day (morning) for all subjects in a temperature controlled laboratory (22–24°C).
Instrumentation
Subjects were positioned inside the LBNP chamber (VUV Analytics Inc., Austin, TX) in the supine posture, straddling a bicycle seat with their waist (at the iliac crest) in line with the opening of the chamber. A durable plastic sleeve and neoprene band were wrapped around the subject’s waist to form an airtight seal with the LBNP chamber. All subjects were instrumented for continuous measurement of heart rate (HR) via a standard lead II ECG (shielded leads, cable, and amplifier, AD Instruments, Bella Vista, NSW, Australia), and beat-to-beat arterial pressure and stroke volume (SV; via ModelFlow®) measurements were obtained via infrared finger photoplethysmography (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands). Respiration rate and end tidal CO2 (etCO2) were measured on a breath-by-breath basis through either a facemask or an oral nasal cannula via capnography (ML206 Gas Analyzer, AD Instruments, Bella Vista, NSW, Australia). In accordance with standard approaches outlined in the literature (24), cerebral blood velocity was recorded from the right MCA and from the left PCA with transcranial Doppler ultrasound probes (2-MHz probes; ST3, Spencer Technologies, Seattle, WA) held in place over the temporal windows with a cushioned and adjustable headframe (Marc 600, Spencer Technologies, Seattle, WA). Oxy-hemoglobin (HbO2), deoxy-hemoglobin (dHb), and cerebral tissue oxygen saturation {ScO2; [HbO2/(HbO2 + dHb) * 100]} were measured or calculated from the right side of the frontal cortex via a near infrared spectroscopy (NIRS; OxiplexTS, ISS, Champaign-Urbana, IL) probe that was held in place with 4-in self-adhering bandaging tape (3 M Vetrap, St. Paul, MN) underneath the cushioned and adjustable TCD headframe. Efforts were made to ensure both MCA blood velocity (MCAv) and cerebral oxygenation measurements were recorded from the same side of the head in each subject.
Protocol
Each subject completed a LBNP test to the point of maximal tolerance (i.e., presyncope). The protocol consisted of a 5-min baseline followed by application of progressively decreasing LBNP every 5 min to −15, −30, −45, −60, −70, −80, −90, and −100 mmHg, or until the onset of presyncopal symptoms (25). The protocol was terminated when subjects reached one or more of the following termination criteria: 1) instantaneous systolic arterial pressure (SAP) below 80 mmHg; 2) sudden relative bradycardia, and/or 3) voluntary subject termination due to subjective presyncopal symptoms such as gray-out, nausea, sweating, dizziness, blurred vision, or general discomfort (20, 22). The chamber pressure was released immediately at the onset of presyncope, or upon reaching the end of 5 min at −100 mmHg LBNP. Upon release of the chamber pressure, presyncopal symptoms generally resolved within 30–60 s. Following completion of the LBNP protocol, subjects remained in the chamber for a 10-min recovery period.
Data Analysis
All continuous waveform data [e.g., ECG, arterial pressure, SV, MCAv, PCA blood velocity (PCAv), ScO2, etCO2] were collected at 1,000 Hz (LabChart, AD Instruments, Bella Vista, NSW, Australia) and analyzed offline via specialized software (WinCPRS, Absolute Aliens, Turku, Finland). R-waves from the ECG signal were detected to determine the timing of each cardiac cycle. Beat-to-beat SAP and diastolic arterial pressures (DAP) were then detected from the continuous arterial pressure tracings. Systolic and diastolic cerebral blood velocities were also detected and marked from the continuous MCAv and PCAv tracings. Mean arterial pressure (MAP), mean MCAv, and mean PCAv were automatically calculated as the area under the arterial pressure and cerebral blood velocity waveforms via the WinCPRS software. To control for the confounding influence of differences in total body surface area (BSA) between males and females, BSA was calculated for all subjects via the Du Bois method (26); SV was then indexed to BSA (SVi), cardiac output index (Qi) was calculated as the product of HR and SVi, and systemic vascular resistance index (SVRi) was calculated as MAP divided by Qi. Cerebrovascular resistance (CVR) in the MCA and PCA was calculated as MAP divided by MCAv, and MAP divided by PCAv.
Oscillatory patterns of R-to-R intervals (RRI), MAP, SAP, mean MCAv, and mean PCAv were determined via power spectral analysis. Data were made equidistant by interpolating linearly and resampling at 5 Hz, then passed through a low-pass filter with a cutoff frequency of 0.5 Hz. Three-minute data sets were fast Fourier transformed to obtain power spectra (27) and are expressed as the integrated area within the low frequency (LF, 0.04–0.15 Hz) and high frequency (HF, 0.15–0.40 Hz) ranges. Coherence between MAP and mean MCAv in the LF range was calculated by dividing the squared cross-spectral densities of the two signals by the product of the individual autospectra. Absolute and normalized transfer function gain between MAP and mean MCAv were calculated only when coherence values were ≥ 0.5. Following the same procedures, coherence and transfer function gain were also calculated between MAP and mean PCAv, and between SAP and RRI in the LF range.
Statistical Analysis
Physiological responses were compared between male and female subjects at each LBNP stage up to −60 mmHg LBNP, as this was the last common stage for the majority of subjects. All time and frequency domain variables were calculated from the final 3 min of each stage of LBNP. In addition, to compare physiological responses between male and female subjects at presyncope, the final 1 min (PS-1; for time domain variables) and 3 min (PS-3; for frequency domain variables) immediately before presyncope was assessed. Absolute and percentage change from baseline values are reported for the key variables of interest. Sex differences at baseline, and presyncope, were assessed using separate unpaired t tests. Our primary outcome variables were analyzed using two-factor (sex, time) linear mixed model analyses with repeated measures from baseline to −60 mmHg LBNP, followed by Holm-corrected post hoc tests for multiple comparisons (which were run on the least squared means generated by the linear mixed model analysis). JMP was used for all statistical analyses (Pro 12, SAS Institute, Cary, NC). All subject demographic data (Table 1) are reported as mean ± standard deviation (SD) to demonstrate the variability in this sample of subjects. All other data are presented as mean ± standard error (SE), which provides a measure of precision of the estimated population mean, as we are interested in making inferences about data from our sample of young adults to the population. Exact P values are reported for all comparisons. The actual P values are reported instead of selecting an arbitrary threshold in an effort to address and remove the dichotomous use of the term “significant” and allow the reader to make their own judgments about the interpretation of the results (28, 29).
Table 1.
Demographic data for male and female subjects at baseline
| Male | Female | P Value | |
|---|---|---|---|
| N | 29 | 23 | - |
| Age, yr | 26 ± 4 | 27 ± 5 | 0.16 |
| Height, cm | 173 ± 6 | 163 ± 8 | 0.002 |
| Weight, kg | 78 ± 13 | 67 ± 12 | 0.03 |
| BSA, m2 | 1.9 ± 0.2 | 1.7 ± 0.2 | 0.01 |
| BMI, kg/m2 | 25.9 ± 3.5 | 25.2 ± 2.9 | 0.54 |
| Baseline HR, beats/min | 62.3 ± 1.4 | 64.3 ± 1.7 | 0.37 |
| Baseline MAP, mmHg | 93.6 ± 1.5 | 94.7 ± 1.8 | 0.64 |
| Baseline SVi, mL/m2 | 51.1 ± 1.3 | 46.8 ± 1.2 | 0.02 |
| Baseline mean MCAv, cm/s | 60.4 ± 2.2 | 68.5 ± 3.2 | 0.03 |
| Baseline mean PCAv, cm/s | 43.6 ± 2.1 | 46.7 ± 2.4 | 0.35 |
| Baseline mean etCO2, mmHg | 40.3 ± 0.7 | 39.4 ± 0.9 | 0.41 |
| Baseline mean ScO2, % | 69.5 ± 1.3 | 63.3 ± 1.9 | 0.006 |
Data are means ± SD for age, height, weight, BSA, and BMI. Means ± SE are reported for all other data. N = number of subjects. BMI, body mass index; BSA, body surface area; etCO2, end tidal carbon dioxide; HR, heart rate; MAP, mean arterial pressure; MCAv, middle cerebral artery velocity; PCAv, posterior cerebral artery velocity; ScO2, cerebral oxygen saturation; SVi, stroke volume indexed to BSA. Unpaired t tests were used to compare male and female subjects.
RESULTS
LBNP Tolerance
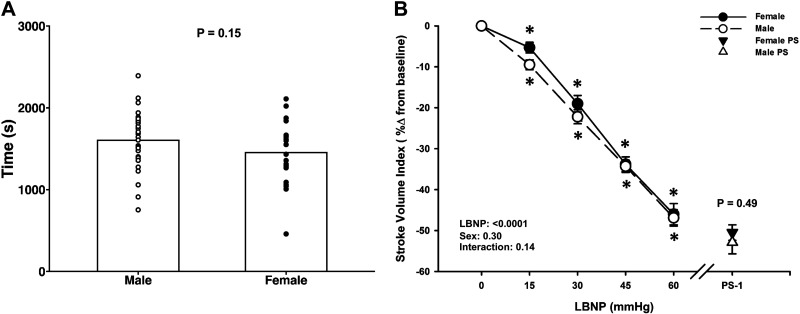
Of the 62 subjects who completed the LBNP protocol, data were only included for 52 subjects who reached true presyncope (defined as mean SAP ≤ 100 mmHg for the 1 min before presyncope, and/or minimum SAP ≤ 90 mmHg within the 1 min before presyncope) as we have previously reported (20, 22). For these 52 subjects, the LBNP protocol was terminated at −15 mmHg for one subject (1 female), at −30 mmHg LBNP for one subject (1 male), at −45 mmHg LBNP for seven subjects (3 male/4 female), at −60 mmHg LBNP for 12 subjects (6 male/6 female), at −70 mmHg LBNP for 19 subjects (10 male/9 female), at −80 mmHg LBNP for eight subjects (6 male/2 female), and at −90 mmHg LBNP for four subjects (3 male/1 female). No subjects reached the −100 mmHg stage of the LBNP protocol. Additionally, at the termination of the LBNP protocol, all but four subjects exhibited at least one subjective presyncopal symptom (i.e., blurred vision, sweating, nausea, dizziness, lightheaded). Of the final 52 subjects, 29 were male and 23 were female; baseline characteristics are compared in Table 1. As expected, females were shorter, weighed less, had a smaller BSA, a lower SVi, and a higher mean MCAv (P ≤ 0.03). Unexpectedly, there was no difference in time to presyncope between males (1,604 ± 68 s) and females (1,453 ± 78 s; P = 0.15, Fig. 1A). The average maximal LBNP at presyncope was 68.1 ± 2.7 mmHg for males and 62.4 ± 3.3 mmHg for females (P = 0.18).
Figure 1.
Tolerance time (in seconds; A) to lower body negative pressure (LBNP) in male and female subjects. Mean (bars) and individual subject data (circles) are presented. P = 0.15, assessed via unpaired t test. Stroke volume index (B) decreased in both males (dashed line, open circle) and females (solid line, closed circle) during lower body negative pressure (LBNP). Over the 1 min prior to presyncope (PS-1), there were no differences in these responses between the sexes. Mean ± SE. *Difference from baseline, P ≤ 0.002. A two-factor (sex, time) linear mixed model analysis with Holm-corrected post hoc tests run on the least squared means (within and between males and females up to −60 mmHg LBNP), and unpaired t tests (between males and females at PS-1) were used for analysis. PS, presyncope.
Central Hemodynamic Responses to LBNP
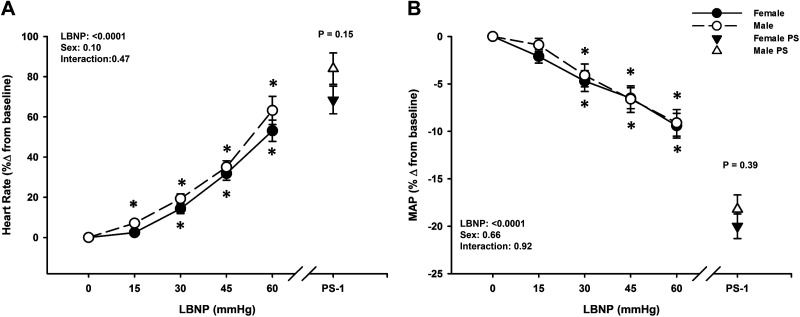
Both sexes experienced a progressive decrease in SVi, MAP, and Qi during LBNP until the point of presyncope (LBNP effect, P < 0.0001; Figs. 1B and 2B; Table 2), with no differences between males and females during LBNP (Sex effect, P ≥ 0.13). Note that all SVi, Qi, and SVRi data were analyzed with only 28 male subjects, due to loss of the SV signal during data collection in one subject. In response to these reductions in central blood volume, heart rate increased in both males and females (LBNP effect, P < 0.0001), with no differences between groups (Sex effect, P = 0.10), including comparable changes from baseline to PS-1 (Male: 84 ± 8% vs. Female: 68 ± 7%; P = 0.15; Fig. 2A). Similarly, SVRi increased with LBNP in both sexes (LBNP effect, P < 0.0001), with no differences between groups (Sex effect, P = 0.66), and comparable changes from baseline to PS-1 (Male: 1.2 ± 3.7% vs. Female: 1.4 ± 4.4%; P = 0.97; Table 2). MAP decreased with LBNP for both males and females (LBNP effect, P < 0.0001) and was similar between groups throughout LBNP (Sex effect, P = 0.66) and at PS-1 (Males: −18.2 ± 1.5% vs. Females: −20.0 ± 1.3%; P = 0.39; Fig. 2B).
Figure 2.
Percent change from baseline for heart rate (A) increased and mean arterial pressure (MAP, B) decreased in both males (dashed line, open circle) and females (solid line, closed circle) during lower body negative pressure (LBNP). Over the 1 min prior to presyncope (PS-1), both heart rate and MAP were not different between sexes. Mean ± SE. *Difference from baseline, P < 0.09. A two-factor (sex, time) linear mixed model analysis with Holm-corrected post hoc tests run on the least squared means (within and between males and females up to −60 mmHg LBNP), and unpaired t tests (between males and females at PS-1) were used for analysis. PS, presyncope.
Table 2.
Central hemodynamic responses during progressive LBNP to presyncope in males and females
| LBNP Stage |
P Values |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | LBNP | Sex | Interaction | PS-1 | P value | |
| HR, beats/min | ||||||||||
| Male | 62.3 ± 1.4 | 66.7 ± 1.6* | 73.9 ± 1.8* | 83.7 ± 2.3* | 97.7 ± 3.5* | <0.0001 | 0.98 | 0.72 | 112.7 ± 3.5 | P = 0.36 |
| Female | 64.3 ± 1.7 | 65.6 ± 2.0 | 73.3 ± 2.8* | 85.8 ± 3.5* | 97.2 ± 4.8* | 107.6 ± 4.5 | ||||
| MAP, mmHg | ||||||||||
| Male | 93.6 ± 1.5 | 92.7 ± 1.6 | 89.9 ± 1.7* | 88.1 ± 1.7* | 86.3 ± 1.5* | <0.0001 | 0.80 | 0.90 | 76.2 ± 1.2 | P = 0.62 |
| Female | 94.7 ± 1.8 | 92.2 ± 1.6 | 89.7 ± 1.7* | 87.4 ± 1.9* | 83.6 ± 2.4* | 75.4 ± 0.9 | ||||
| SVi, mL/m2 | ||||||||||
| Male | 51.1 ± 1.3† | 46.2 ± 1.4* | 40.0 ± 1.4* | 33.6 ± 1.2* | 27.1 ± 1.4* | <0.0001 | 0.13 | 0.21 | 23.7 ± 1.3 | P = 0.79 |
| Female | 46.8 ± 1.2 | 44.2 ± 1.3* | 37.8 ± 1.4* | 30.3 ± 1.1* | 24.9 ± 1.1* | 23.2 ± 1.2 | ||||
| Qi, (L/min)/m2 | ||||||||||
| Male | 3.2 ± 0.1 | 3.1 ± 0.1* | 2.9 ± 0.1* | 2.8 ± 0.1* | 2.6 ± 0.1* | <0.0001 | 0.13 | 0.68 | 2.6 ± 0.1 | P = 0.17 |
| Female | 3.0 ± 0.1 | 2.9 ± 0.1 | 2.7 ± 0.1* | 2.6 ± 0.1* | 2.4 ± 0.1* | 2.4 ± 0.1 | ||||
| Qi, %Δ | ||||||||||
| Male | – | −3.7 ± 0.9* | −8.1 ± 1.2* | −12.0 ± 2.0* | −15.2 ± 2.0* | <0.0001 | 0.48 | 0.53 | −16.5 ± 3.7 | P = 0.74 |
| Female | – | −3.2 ± 1.1 | −8.2 ± 1.4* | −13.3 ± 2.0* | −18.4 ± 2.8* | −18.2 ± 3.3 | ||||
| SVRi, (mmHg/L·min−1)/m2 | ||||||||||
| Male | 30.5 ± 1.2 | 31.4 ± 1.3 | 31.9 ± 1.3 | 32.7 ± 1.5* | 34.5 ± 1.7* | <0.0001 | 0.25 | 0.48 | 30.6 ± 1.5 | P = 0.46 |
| Female | 32.2 ± 1.1 | 32.7 ± 1.0 | 33.6 ± 1.1 | 34.8 ± 1.3* | 35.5 ± 1.1* | 32.0 ± 1.2 | ||||
| SVRi, %Δ | ||||||||||
| Male | – | 3.2 ± 1.2 | 4.9 ± 1.8* | 6.9 ± 2.0* | 8.3 ± 2.9* | <0.0001 | 0.66 | 0.34 | 1.2 ± 3.7 | P = 0.97 |
| Female | – | 1.4 ± 1.3 | 4.3 ± 1.7 | 8.8 ± 2.6* | 12.3 ± 3.8* | 1.4 ± 4.4 | ||||
Data are presented as absolute and relative means ± SE. HR, heart rate; LBNP, lower body negative pressure; MAP, mean arterial pressure; Qi, cardiac output index; SVi, stroke volume index; SVRi, systemic vascular resistance index; Δ, change. PS-1 time point refers to the 1 min prior to presyncope. A two-factor linear mixed model analysis with repeated measures from baseline to −60 mmHg LBNP was performed, followed by Holm-corrected post hoc tests for multiple comparisons (run on the least squared means generated by the linear mixed model analysis).
*P ≤ 0.07 compared to baseline within a group. †P ≤ 0.09 between male and female groups. Sex differences at presyncope were assessed using unpaired t tests. Exact P values are reported for all comparisons.
Cerebral Hemodynamic and Oxygenation Responses to LBNP
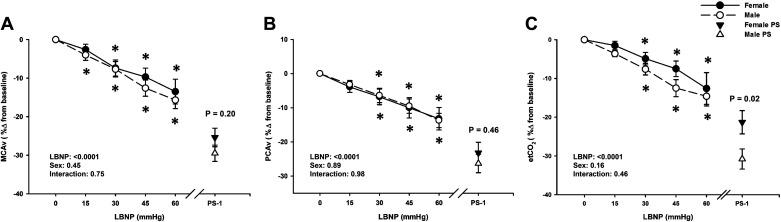
Mean MCAv decreased below baseline during LBNP in both males (from −15 mmHg LBNP) and females (from −30 mmHg LBNP; LBNP effect P < 0.0001), but there was no overall difference in responses between the sexes (Sex effect, P = 0.45; Fig. 3A). By presyncope, mean MCAv decreased by the same magnitude in both sexes (Male: −29.5 ± 2.1% vs. Female: −25.4 ± 2.4%; P = 0.20; Fig. 3A), but to a lower absolute value in males (P = 0.007; Table 3). Similarly, a decrease in mean PCAv from baseline was observed in both males and females by −30 mmHg LBNP (P ≤ 0.0001; Fig. 3B), and there were no differences in responses between the sexes (Sex effect, P = 0.89). By presyncope, mean PCAv decreased by the same magnitude in both sexes (Male: −26.3 ± 2.7% vs. Female: −23.2 ± 3.1%; P = 0.46; Fig. 3B). Note that all PCAv data was analyzed with only 18 male and 20 female subjects, due to the inability to obtain high quality PCAv signals in the remaining subjects during the experiments. MCAv CVR (% change) progressively increased from baseline during LBNP in the male subjects only (P ≤ 0.07 vs. baseline), but was not different between groups at presyncope (P = 0.14; Table 3). Interestingly, PCAv CVR (% change) increased from baseline during LBNP in the female group only (P ≤ 0.02 vs. baseline), with no differences between groups at presyncope (P = 0.81; Table 3). Respiration rate decreased slightly throughout LBNP in both groups (LBNP effect, P = 0.0002) but was no different between groups (Sex effects, P = 0.84), including at presyncope (P = 0.53; Table 3). EtCO2 progressively decreased from baseline in both groups (LBNP effect, P < 0.0001), and a between group difference was only evident at presyncope (Male: −30.8 ± 2.6% vs. Female: −21.3 ± 3.0%; P = 0.02; Fig. 3C).
Figure 3.
Percent change from baseline for middle cerebral artery velocity (MCAv; A), posterior cerebral artery velocity (PCAv; B), and end-tidal CO2 (etCO2; C) decreased in both males (dashed line, open circle) and females (solid line, closed circle) during lower body negative pressure (LBNP). Over the 1 min prior to presyncope (PS-1), there were no differences in MCAv and PCAv responses between the sexes; however, etCO2 was lower in males (open triangle) compared to females (closed triangle). Mean ± SE. *Difference from baseline, P < 0.05. A two-factor (sex, time) linear mixed model analysis with Holm-corrected post hoc tests run on the least squared means (within and between males and females up to −60 mmHg LBNP) and unpaired t tests (between males and females at PS-1) were used for analysis. PS, presyncope.
Table 3.
Cerebral hemodynamic responses during progressive LBNP to presyncope in males and females
|
LBNP Stage |
P Values |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | LBNP | Sex | Interaction | PS-1 | P Value | |
| Mean MCAv, cm/s | ||||||||||
| Male | 60.4 ± 2.2 | 57.8 ± 2.1*† | 55.7 ± 2.4* | 53.4 ± 2.3* | 51.4 ± 1.9*† | <0.0001 | 0.02 | 0.77 | 42.1 ± 1.5 | P = 0.007 |
| Female | 68.5 ± 3.2 | 66.2 ± 3.5 | 62.7 ± 3.3* | 60.2 ± 3.3* | 55.1 ± 4.2* | 51.1 ± 3.0 | ||||
| Mean PCAv, cm/s | ||||||||||
| Male | 43.6 ± 2.1 | 42.1 ± 2.0 | 40.8 ± 2.4* | 40.8 ± 2.4* | 37.4 ± 1.6* | <0.0001 | 0.38 | 0.99 | 32.1 ± 1.6 | P = 0.23 |
| Female | 46.7 ± 2.4 | 44.3 ± 2.9 | 42.5 ± 2.6* | 41.1 ± 3.1* | 40.6 ± 3.1* | 35.8 ± 2.6 | ||||
| Mean MCAv CVR, mmHg/(cm/s) | ||||||||||
| Male | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1* | 1.7 ± 0.1* | <0.0001 | 0.04 | 0.79 | 1.9 ± 0.1 | P = 0.01 |
| Female | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | ||||
| Mean MCAv CVR, %Δ | ||||||||||
| Male | – | 3.8 ± 1.6 | 5.3 ± 2.7* | 8.3 ± 3.0* | 9.1 ± 3.1* | <0.0001 | 0.29 | 0.65 | 18.1 ± 4.2 | P = 0.14 |
| Female | – | 0.8 ± 1.2 | 3.8 ± 2.0 | 4.4 ± 2.3 | 5.9 ± 3.2 | 9.5 ± 3.6 | ||||
| Mean PCAv CVR, mmHg/(cm/s) | ||||||||||
| Male | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.1 | 0.003 | 0.63 | 0.30 | 2.4 ± 0.1 | P = 0.44 |
| Female | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.2* | 2.2 ± 0.2* | 2.3 ± 0.2 | ||||
| Mean PCAv CVR, %Δ | ||||||||||
| Male | – | 1.4 ± 1.2 | 1.0 ± 1.2 | 1.0 ± 1.3 | 3.3 ± 2.5 | 0.006 | 0.23 | 0.39 | 9.8 ± 4.0 | P = 0.81 |
| Female | – | 2.3 ± 1.5 | 3.6 ± 2.5 | 5.9 ± 3.2* | 5.7 ± 3.6* | 8.3 ± 4.5 | ||||
| ScO2, % | ||||||||||
| Male | 69.5 ± 1.3† | 68.5 ± 1.3*† | 67.6 ± 1.3*† | 66.5 ± 1.4*† | 66.1 ± 1.5*† | <0.0001 | 0.01 | 0.08 | 64.2 ± 1.4 | P = 0.06 |
| Female | 63.3 ± 1.9 | 62.4 ± 1.9* | 61.7 ± 2.0* | 60.6 ± 2.4* | 59.3 ± 3.6* | 59.6 ± 2.1 | ||||
| HbO2, µM | ||||||||||
| Male | 27.4 ± 1.5*† | 26.6 ± 1.7*† | 26.6 ± 1.6*† | 26.0 ± 1.9*† | 26.2 ± 2.6*† | <0.0001 | 0.0004 | 0.01 | 25.4 ± 1.6 | P = 0.002 |
| Female | 38.4 ± 2.2* | 37.2 ± 2.1* | 36.1 ± 2.0* | 35.4 ± 2.2* | 34.1 ± 2.6* | 34.0 ± 1.9 | ||||
| dHb, µM | ||||||||||
| Male | 15.7 ± 1.0 | 15.8 ± 1.0 | 16.3 ± 1.0* | 16.7 ± 1.3* | 17.8 ± 1.9* | <0.0001 | 0.46 | 0.01 | 17.0 ± 1.1 | P = 0.30 |
| Female | 16.5 ± 1.0 | 16.8 ± 1.1 | 17.1 ± 1.1 | 17.6 ± 1.2* | 17.4 ± 1.6* | 18.7 ± 1.1 | ||||
| Respiratory Rate, breaths/min | ||||||||||
| Male | 14.6 ± 0.8 | 13.7 ± 0.7 | 13.1 ± 0.8* | 13.1 ± 0.9* | 13.6 ± 1.1 | 0.0002 | 0.84 | 0.46 | 15.1 ± 1.1 | P = 0.53 |
| Female | 14.9 ± 1.0 | 14.0 ± 0.8 | 13.5 ± 0.8 | 12.9 ± 0.8* | 12.1 ± 1.0* | 14.0 ± 1.3 | ||||
| etCO2, mmHg | ||||||||||
| Male | 40.3 ± 0.7 | 38.9 ± 0.8 | 37.2 ± 1.0* | 35.3 ± 1.3* | 34.6 ± 1.4* | <0.0001 | 0.90 | 0.63 | 27.8 ± 1.1 | P = 0.08 |
| Female | 39.4 ± 0.9 | 38.7 ± 0.7 | 37.4 ± 0.8* | 35.3 ± 0.9* | 33.0 ± 1.6* | 30.9 ± 1.3 | ||||
Data are presented as absolute and relative means ± SE. dHb, deoxygenated hemoglobin concentration; etCO2, end tidal carbon dioxide; HbO2, oxygenated hemoglobin concentration; LBNP, lower body negative pressure; MCAv, middle cerebral artery velocity; PCAv, posterior cerebral artery velocity; ScO2, cerebral oxygen saturation. PS-1 time point refers to the 1 min prior to presyncope. A two-factor linear mixed model analysis with repeated measures from baseline to −60 mmHg LBNP was performed, followed by Holm-corrected post hoc tests for multiple comparisons (run on the least squared means generated by the linear mixed model analysis).
*P ≤ 0.07 compared to baseline within a group. †P ≤ 0.09 between male and female groups. Sex differences at presyncope were assessed using unpaired t tests. Exact P values are reported for all comparisons.
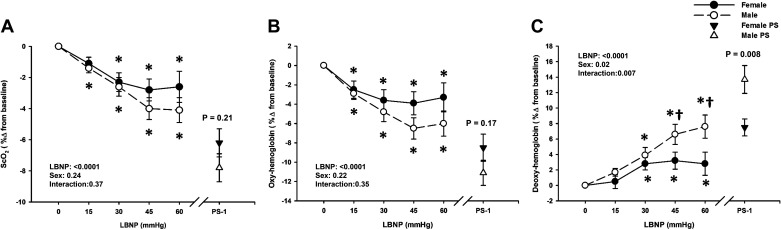
ScO2, HbO2, and dHb data were analyzed with 28 male and 22 female subjects, due to loss of the signal during data collection in two subjects. Overall, males exhibited higher absolute ScO2 and lower absolute HbO2 at baseline, and throughout LBNP (Sex effect, P ≤ 0.01; Table 3). There was a progressive decrease in ScO2 (% change) in the males from −15 mmHg LBNP (P ≤ 0.02), and in the females from −30 mmHg LBNP (P ≤ 0.002; Fig. 4A). At presyncope, ScO2 decreased by the same magnitude from baseline in both sexes (Males: −7.8 ± 0.9% vs. Females: −6.2 ± 0.9%; P = 0.21; Fig. 4A). Despite similar reductions in ScO2 and HbO2 from baseline in both sexes, males exhibited a greater increase in dHb with LBNP as shown in Fig. 4C (Sex effect, P = 0.02). At presyncope, HbO2 decreased by ∼11% in males and 9% in females (P = 0.17; Fig. 4B), but dHb increased by ∼14% in males and 8% in females (P = 0.008; Fig. 4C).
Figure 4.
Percent change from baseline for cerebral oxygen saturation (ScO2; A) and oxygenated hemoglobin (Oxy-hemoglobin; B) decreased in both males (dashed line, open circle) and females (solid line, closed circle) during lower body negative pressure (LBNP). Over the 1 min prior to presyncope (PS-1), there were no differences in ScO2 and Oxy-hemoglobin responses between the sexes. Deoxygenated hemoglobin (Deoxy-hemoglobin; C) progressively increased from −30 mmHg LBNP in males (dashed line, open circle) and females (solid line, closed circle), however, was higher in males than females at −45 mmHg, −60 mmHg LBNP, and at the PS-1 time point. Mean ± SE. *Difference from baseline, P < 0.08. †Sex differences, P range from 0.01 to 0.08. A two-factor (sex, time) linear mixed model analysis with Holm-corrected post hoc tests run on the least squared means (within and between males and females up to −60 mmHg LBNP) and unpaired t tests (between males and females at PS-1) were used for analysis. PS, presyncope.
Frequency Domain Responses to LBNP
The LF and HF power spectral density data are presented in Table 4. RRI HF power decreased with LBNP (LBNP effect, P < 0.0001), with no differences between groups (Sex effect, P = 0.36). MAP LF power increased with LBNP (LBNP effect, P ≤ 0.0001), with no differences between groups (Sex effect, P = 0.58). LF power for mean MCAv and mean PCAv did not change with LBNP (LBNP effect, P ≥ 0.10), with a difference between groups in mean PCAv LF power only (Sex effect, P = 0.05). On average, LF coherence between MAP-mean MCAv, MAP-mean PCAv, and SAP-RRI were stable and ≥0.5 throughout LBNP in both groups (LBNP effects, P ≥ 0.10). However, due to low coherence (<0.5) between signals throughout LBNP for some subjects, absolute and normalized MAP-mean MCAv LF gain data were analyzed with 24 male and 20 female subjects, absolute and normalized MAP-mean PCAv LF gain data were analyzed with 16 male and 17 female subjects, and SAP-RRI LF gain data were analyzed with 21 male and 19 female subjects (see Table 4). LF transfer function gain for absolute MAP-mean MCAv, absolute MAP-mean PCAv, and SAP-RRI decreased from baseline throughout LBNP (LBNP effects, P ≤ 0.02), and LF transfer function gains for absolute MAP-mean MCAv, absolute MAP-mean PCAv, and normalized MAP-mean PCAv were lower in males compared with females (Sex effects, P ≤ 0.07). The only sex differences at presyncope for the frequency domain metrics were absolute MAP-mean MCAv LF gain, and SAP-RRI LF coherence and gain, which were all lower for male subjects versus female subjects (P ≤ 0.09; Table 4).
Table 4.
Frequency domain responses during progressive LBNP to presyncope in males and females
| LBNP Stage |
P Values |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | LBNP | Sex | Interaction | PS 3-Min | P Value | |
| RRI HF, ms2 | ||||||||||
| Male | 856 ± 165 | 567 ± 174 | 319 ± 48* | 267 ± 56* | 79 ± 20* | <0.0001 | 0.36 | 0.73 | 92 ± 33 | P = 0.47 |
| Female | 1070 ± 224 | 830 ± 219 | 451 ± 104* | 187 ± 34* | 132 ± 24* | 135 ± 52 | ||||
| MAP LF, mmHg2 | ||||||||||
| Male | 5.9 ± 0.6 | 6.5 ± 0.9 | 11.3 ± 1.6* | 12.7 ± 1.6* | 14.6 ± 2.4* | <0.0001 | 0.58 | 0.05 | 15.1 ± 2.3 | P = 0.49 |
| Female | 6.0 ± 1.2 | 5.7 ± 0.8 | 6.8 ± 1.1 | 11.1 ± 2.1* | 17.1 ± 4.7* | 12.9 ± 2.0 | ||||
| Mean MCAv LF, (cm/s)2 | ||||||||||
| Male | 3.7 ± 0.5 | 4.0 ± 0.8 | 5.5 ± 1.1 | 4.9 ± 0.9 | 5.5 ± 1.0 | 0.17 | 0.49 | 0.57 | 5.0 ± 0.8 | P = 0.67 |
| Female | 4.6 ± 0.8 | 4.6 ± 1.7 | 4.3 ± 0.6 | 5.1 ± 0.8 | 4.9 ± 0.7 | 5.5 ± 0.8 | ||||
| Mean PCAv LF, (cm/s)2 | ||||||||||
| Male | 2.2 ± 0.4 | 1.7 ± 0.3 | 2.3 ± 0.5 | 2.6 ± 0.4 | 2.1 ± 0.4† | 0.10 | 0.05 | 0.13 | 2.2 ± 0.5 | P = 0.66 |
| Female | 2.0 ± 0.3 | 2.2 ± 0.6 | 2.1 ± 0.4 | 2.6 ± 0.4 | 3.1 ± 0.5* | 2.5 ± 0.5 | ||||
| MAP-mean MCAv LF Coherence | ||||||||||
| Male | 0.65 ± 0.02 | 0.64 ± 0.03 | 0.69 ± 0.02 | 0.69 ± 0.02 | 0.69 ± 0.03 | 0.14 | 0.74 | 0.38 | 0.68 ± 0.03 | P = 0.63 |
| Female | 0.65 ± 0.04 | 0.64 ± 0.03 | 0.67 ± 0.03 | 0.61 ± 0.04 | 0.71 ± 0.04 | 0.70 ± 0.03 | ||||
| MAP-mean MCAv LF Gain, cm/s·mmHg−1 | ||||||||||
| Male | 0.72 ± 0.04† | 0.67 ± 0.04† | 0.65 ± 0.05† | 0.59 ± 0.03*† | 0.56 ± 0.03 | <0.0001 | <0.0001 | 0.37 | 0.56 ± 0.03 | P = 0.09 |
| Female | 0.84 ± 0.09 | 0.80 ± 0.08 | 0.82 ± 0.08 | 0.58 ± 0.04* | 0.59 ± 0.07* | 0.70 ± 0.08 | ||||
| Normalized MAP-mean MCAv LF Gain, %·mmHg−1 | ||||||||||
| Male | 1.19 ± 0.05 | 1.17 ± 0.06 | 1.14 ± 0.06 | 1.12 ± 0.06 | 1.15 ± 0.06 | 0.21 | 0.33 | 0.18 | 1.24 ± 0.05 | P = 0.99 |
| Female | 1.21 ± 0.09 | 1.18 ± 0.08 | 1.28 ± 0.08 | 1.02 ± 0.07 | 1.05 ± 0.09 | 1.24 ± 0.08 | ||||
| MAP-mean PCAv LF Coherence | ||||||||||
| Male | 0.65 ± 0.03 | 0.64 ± 0.03 | 0.67 ± 0.02 | 0.65 ± 0.03 | 0.63 ± 0.04 | 0.85 | 0.84 | 0.81 | 0.65 ± 0.03 | P = 0.62 |
| Female | 0.65 ± 0.04 | 0.63 ± 0.04 | 0.65 ± 0.03 | 0.62 ± 0.04 | 0.69 ± 0.04 | 0.67 ± 0.04 | ||||
| MAP-mean PCAv LF Gain, cm/s·mmHg−1 | ||||||||||
| Male | 0.54 ± 0.05† | 0.50 ± 0.04† | 0.46 ± 0.04† | 0.44 ± 0.04† | 0.42 ± 0.04† | 0.02 | <0.0001 | 0.97 | 0.42 ± 0.03 | P = 0.65 |
| Female | 0.60 ± 0.08 | 0.58 ± 0.10 | 0.54 ± 0.06 | 0.45 ± 0.04 | 0.49 ± 0.05 | 0.44 ± 0.05 | ||||
| Normalized MAP-mean PCAv LF Gain, %·mmHg−1 | ||||||||||
| Male | 1.25 ± 0.10 | 1.17 ± 0.07 | 1.12 ± 0.08 | 1.07 ± 0.10 | 1.11 ± 0.08 | 0.49 | 0.07 | 0.94 | 1.23 ± 0.07 | P = 0.79 |
| Female | 1.22 ± 0.09 | 1.24 ± 0.13 | 1.24 ± 0.08 | 1.12 ± 0.10 | 1.22 ± 0.10 | 1.20 ± 0.09 | ||||
| SAP-RRI LF Coherence | ||||||||||
| Male | 0.60 ± 0.03 | 0.59 ± 0.03 | 0.60 ± 0.03 | 0.68 ± 0.03 | 0.62 ± 0.04 | 0.10 | 0.23 | 0.82 | 0.58 ± 0.03 | P = 0.05 |
| Female | 0.65 ± 0.03 | 0.61 ± 0.04 | 0.66 ± 0.04 | 0.67 ± 0.03 | 0.65 ± 0.05 | 0.66 ± 0.03 | ||||
| SAP-RRI LF Gain, ms/mmHg | ||||||||||
| Male | 14.45 ± 1.32 | 13.26 ± 1.03 | 8.83 ± 0.55* | 7.41 ± 0.64* | 5.38 ± 0.68* | <0.0001 | 0.18 | 0.91 | 3.28 ± 0.51 | P = 0.07 |
| Female | 15.80 ± 2.09 | 13.43 ± 1.79 | 10.73 ± 1.07* | 7.47 ± 0.84* | 6.35 ± 1.00* | 5.60 ± 1.11 | ||||
Data are presented as means ± SE. HF, high frequency; LBNP, lower body negative pressure; LF, low frequency; MAP, mean arterial pressure; MCAv, middle cerebral artery velocity; PCAv, posterior cerebral artery velocity; RRI, R-to-R interval; SAP, systolic arterial pressure. PS 3-min refers to the 3 min prior to presyncope. A two-factor linear mixed model analysis with repeated measures from baseline to −60 mmHg LBNP was performed, followed by Holm-corrected post hoc tests for multiple comparisons (run on the least squared means generated by the linear mixed model analysis).
*P ≤ 0.05 compared to baseline within a group. †P ≤ 0.09 between male and female groups. Sex differences at presyncope were assessed using unpaired t tests. Exact P values are reported for all comparisons.
DISCUSSION
There are several important findings from this study: 1) tolerance to LBNP was not different between males and females; 2) anterior cerebral blood velocity (indexed by mean MCAv), and posterior cerebral blood velocity (indexed by mean PCAv) decreased throughout LBNP for both sexes, with no difference between males and females during LBNP or at presyncope; 3) males demonstrated greater frontal lobe cerebral oxygen extraction in response to LBNP compared to females; and 4) although decreases in end-tidal CO2 were similar between the sexes during LBNP, at presyncope the change in etCO2 from baseline was greater in males compared to females. Contrary to our hypothesis, males and females exhibited similar tolerance and hemodynamic responses to central hypovolemia, but differential etCO2 responses, which suggests potential differences in cerebral vascular sensitivity to CO2 between males and females during acute central hypovolemia.
Many prior studies provide evidence that compared to young females, young males have a higher tolerance to acute presyncopal-limited central hypovolemia induced by LBNP (6–12). Although our findings are contrary to this literature, there are other studies that have demonstrated similar results. For example, Hordinsky et al. (30) assessed cardiovascular responses to LBNP in 12 males and 12 females and reported that tolerance was “slightly less” in female versus male subjects, but statistical comparisons indicated no difference. Additionally, in a much larger study, Lightfoot and Tsintgiras (31) evaluated 86 young males and 33 young females to determine the impact of various physical characteristics on LBNP tolerance. Sex, in addition to age, height, weight, and maximal aerobic capacity were not associated with tolerance to LBNP. At maximal LBNP, males and females demonstrated similar compensatory increases in heart rate and systemic vascular resistance in response to comparable blood pooling in the legs and declines in arterial pressure; sex specific cardiovascular responses were not reported during submaximal levels of LBNP (31).
We report that sex does not influence tolerance to central hypovolemia, or the majority of central hemodynamic responses to this stress, including the magnitude of hypotension (indexed by MAP) or central hypovolemia (indexed by SVi), and the compensatory increases in heart rate and systemic vascular resistance. These maximal hemodynamic responses are consistent with prior studies which report similar cardiovascular responses at presyncope between men and women, although there were differences in tolerance to LBNP in these investigations (8, 10, 12). In contrast, other studies have demonstrated sex differences in both tolerance to LBNP, and in cardiovascular responses at presyncope; females exhibited greater reductions in stroke volume, cardiac output, and arterial pressure (7, 9), and had a smaller stroke volume reserve (6, 11). It would be expected that if stroke volume, cardiac output, and arterial pressure were lower in females with maximal LBNP, that their compensatory increases in heart rate and vascular resistance would be greater compared to males. However, this did not occur in the above mentioned studies [except for a higher heart rate response in the Fu et al. (9) study], suggesting that lower tolerance to LBNP in female subjects in these studies may have been due to their inability to compensate for greater central hypovolemia and hypotension.
In addition, females exhibited lower circulating plasma epinephrine (9) and norepinephrine (7) concentrations, and reduced coherence between arterial blood pressure and muscle sympathetic nerve activity (6) at presyncope, indicating impaired baroreflex responsiveness. Baroreflex-mediated elevations in heart rate, cardiac contractility, and systemic vascular resistance provide a protective mechanism to buffer temporary reductions in arterial blood pressure. In the present study, cardiac baroreflex function (indexed by SAP-RRI transfer function gain) was progressively reduced during LBNP, and there were no differences in this response between males and females, except at presyncope; this reduction in baroreflex gain may contribute to the progressive hypotension induced by maximal LBNP. Although the overall lack of sex differences in cardiac baroreflex gain is expected based on similar LBNP tolerance between males and females, this finding is contrary to previous literature demonstrating that lower tolerance to LBNP was associated with lower carotid-cardiac baroreflex responsiveness in females (7).
The primary hypothesis for this study was that reduced tolerance to central hypovolemia in females would be associated with earlier reductions in both blood flow to the posterior cerebral circulation and frontal lobe cerebral tissue oxygenation, but with no differences in blood flow to the anterior cerebral circulation. Contrary to our hypothesis, males and females exhibited similar tolerance to maximal LBNP, and similar reductions in both anterior (indexed by MCAv) and posterior (indexed by PCAv) cerebral blood flow throughout LBNP. The only study, to our knowledge, that has assessed the influence of sex on cerebrovascular responses to presyncopal LBNP reported similar MCAv and PCAv at baseline and presyncope, despite males exhibiting higher tolerance compared with females (11). The authors concluded that females reached a threshold of cerebral hypoperfusion faster than males due to their lower cardiac reserve (lower cardiac output at rest and presyncope). Since cardiac output was not indexed to BSA, however, determining the role of cardiovascular reserve on LBNP tolerance between males and females is difficult. Interestingly, in separate analyses not focused on sex differences, Lewis et al. also reported that cerebral hypoperfusion at rest (induced via either hypocapnia or indomethacin) or at presyncope does not affect tolerance to maximal LBNP, which may be due to a compensatory increase in cerebral oxygen extraction; cerebral oxygen extraction was only assessed invasively via arterial-jugular venous oxygen differences during resting conditions with hypocapnia and with indomethacin (11). Unfortunately, as sex differences in cerebral oxygen extraction were not assessed in this study, it is unclear whether differences in cerebral oxygen extraction existed between males and females during maximal LBNP; our data suggests this is the case using non-invasive NIRS measurements.
It is important to acknowledge that cerebral NIRS measures a mixed sample volume consisting of ∼75% venous, 20% capillary, and 5% arterial blood, so we interpret decreases in oxygenated hemoglobin and increases in deoxygenated hemoglobin as an increase in extraction of oxygen from the cerebral circulation into the tissues. We know that the brain compensates for decreases in cerebral perfusion by increasing cerebral oxygen extraction (11, 32) and increases in cerebral oxygen extraction have been reported during submaximal and maximal LBNP (20). Our data are the first, to our knowledge, to demonstrate differential oxygen extraction profiles between males and females during LBNP and at presyncope. Our results show that both sexes progressively increase oxygen extraction in response to the decrease in cerebral blood flow (i.e., decreases in MCAv and PCAv), evidenced by progressive decreases in oxygenated hemoglobin and increases in deoxygenated hemoglobin. However, males appear to have a greater oxygen extraction compared to females during LBNP and at presyncope, as demonstrated by a greater increase in deoxygenated hemoglobin (14% in males versus 8% in females at presyncope). Although the precise mechanism underlying these responses cannot be elucidated based on the data collected in the current study (e.g., differences in metabolic demand), these findings are intriguing and deserve further attention in future studies.
Cerebral blood flow is highly sensitive to changes in the arterial partial pressure of CO2, with hypocapnia increasing cerebral vascular resistance and decreasing cerebral blood flow (33). In the current study, end-tidal CO2 (our index of the partial pressure of arterial CO2) decreased throughout LBNP for both sexes, but decreased to a lower minimum at presyncope in males. This end-tidal CO2 response coincides with the progressive increase in MCA vascular resistance only in the males, while PCA vascular resistance increased only in the females, despite no sex differences in mean MCAv or PCAv responses during LBNP. The similar MCAv and PCAv responses with differential etCO2 responses suggest potential differences in cerebral vascular sensitivity to hypocapnia between males and females, which may also exhibit regional variations (i.e., MCA versus PCA). The vast majority of prior studies investigating sex differences in cerebral vascular reactivity to CO2 have explored responses to hypercapnia, not hypocapnia. Previous studies using transcranial Doppler ultrasound demonstrated higher cerebral vascular reactivity to hypercapnia in young females compared to young males (17–19); however, in a recent 4 D flow magnetic resonance imaging study, higher cerebral vascular reactivity to CO2 was observed in young males compared to females (34). Continued assessment of sex differences in cerebral vascular reactivity to both hyper- and hypocapnia under conditions of central hypovolemia is warranted based on the findings of the current study.
Interestingly, high tolerance to LBNP has also been previously associated with increased power in LF oscillations in MAP and MCAv (25, 35, 36). The contribution of these hemodynamic oscillations on LBNP tolerance between the sexes has not been explored, however. In the current study, we observed that MAP LF and MCAv LF oscillations did not differ throughout LBNP or at presyncope between males and females, suggesting that increases in hemodynamic oscillations contributed equally to tolerance in both sexes. In addition to examining MAP and MCAv LF power, transfer function gains between arterial pressure and cerebral blood flow were also assessed. Transfer function gain between MAP and MCAv or PCAv provides an index of the reliance of cerebral blood flow variations on arterial pressure variations, traditionally interpreted as a metric of “cerebral autoregulation” (37). These measurements of gain in the LF decreased for both sexes during LBNP, but were lower for males, including at presyncope for MAP-MCAv LF gain. Despite these sex differences in markers of cerebral autoregulation, however, there were no sex differences in subsequent cerebral blood flow responses. Interestingly, SAP-RRI LF gain (an index of cardiac baroreceptor sensitivity, BRS) was also lower at presyncope for males, indicating lower BRS. These results demonstrate the reciprocal relationship between BRS and cerebral autoregulation as previously reported (38), but in male subjects only. Therefore, the lower absolute gain for cerebral autoregulation (i.e., less reliance of cerebral blood flow on arterial blood pressure) may be compensating for the lower BRS in males during the later stages of LBNP and at presyncope. Interestingly, however, this relationship was no longer present when normalized MAP-MCAv LF gain was used in the analysis; the physiological implication of this discrepancy requires further investigation.
Methodological Considerations
One possible explanation for our unexpected finding of similar LBNP tolerance between men and women is the variable definition of true presyncope between studies. Data included for analysis in the current study was carefully determined based on the primary objective criteria of hypotension, defined as mean SAP ≤ 100 mmHg for the entire 1 min before presyncope, and/or minimum SAP ≤ 90 mmHg within the 1 min before presyncope. Although many studies use similar strict objective criteria, it is possible that other studies may rely on subjective symptoms of presyncope such as lightheadedness, dizziness and nausea, which, while ethically required as termination points, are often unreliable and do not always represent the true physiological status of the subject.
Inferences from transcranial Doppler ultrasound analyses are limited, as changes in cerebral blood velocity indicate changes in cerebral blood flow only if the cross-sectional area of the insonated vessel remains constant. Recent evidence suggests caution when using cerebral blood velocity as a proxy for cerebral blood flow during conditions that alter arterial Pco2 due to the vasoactive effects of CO2 on the cerebral vessels (39, 40). Since progressive maximal LBNP elicited hypocapnia in both sexes (although to greater magnitude in males at presyncope), it is possible that our measures of cerebral blood velocity may be underestimating the actual reductions in cerebral blood flow (41). Future studies assessing cerebral arterial inflow via the internal carotid and vertebral arteries, or global and regional blood flow via advanced imaging approaches, may provide greater insight into differential cerebral blood flow regulation between males and females during central hypovolemia. Additionally, we recognize that TFA conducted on spontaneous fluctuations in arterial blood pressure has lower signal-to-noise ratio and reproducibility compared with TFA performed on forced fluctuations in arterial blood pressure (42). However, as we were primarily focused on exploring sex differences in cerebral vascular regulation during simulated hemorrhage, the design of this study did not facilitate use of forced oscillation techniques.
Blood samples and hydration status were not obtained in this study, which limits our ability to determine whether sex influences hormonal responses (e.g., renin-angiotensin aldosterone system, vasopressin, catecholamines) to progressive LBNP, or the impact that hydration may have had on the primary findings of this study. Additionally, although cardiorespiratory fitness may impact dynamic cerebral autoregulation (43, 44) and cerebrovascular reactivity to CO2 (45, 46), we did not assess cardiorespiratory fitness of our subjects, so we are not able to make conclusions about how this may have impacted our outcomes.
Conclusions
In summary, tolerance and hemodynamic responses to central hypovolemia induced by maximal LBNP were not influenced by sex. Cerebral blood velocity responses to LBNP were similar between the sexes, consistent with similar tolerance, although cerebral oxygen extraction appeared to be greater in male subjects. Interestingly, the magnitude of hypocapnia at presyncope was greater in males compared to females, suggesting potential differences in cerebral vascular sensitivity to CO2 between the sexes. Assessing these sex differences in cerebral vascular function in response to acute central hypovolemia may facilitate development of individualized therapeutic interventions to improve survival from hemorrhagic injuries in men and women.
GRANTS
Funding for this study was provided, in part, by the U.S. Army Medical Research and Materiel Command Combat Casualty Care Research Program (Grant W81XWH-11-2-0137; C.A.R.), the William and Ella Owens Medical Research Foundation (C.A.R.), a contract with Pendar Medical LLC (C.A.R.), and training fellowships awarded to G.K.A. through a National Institutes of Health-supported Neurobiology of Aging Training Grant (T32 AG020494, Principal Investigator: N. Sumien), and an American Heart Association Predoctoral Fellowship (20PRE35210249), to A.J.R. through a Ruth L. Kirchstein National Research Service Award (NRSA) F32 Postdoctoral Fellowship (1F32 HL144082-01A1), and to J.D.S. through a National Institutes of Health-supported Neurobiology of Aging Training Grant (T32 AG020494, Principal Investigator: S. Singh) and a Ruth L. Kirchstein NRSA F31 Predoctoral Fellowship (1 F31 HL134242-01A1).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views the US Department of Defense.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.A.R. conceived and designed research; A.J.R., V.L.K., G.K.A., M-L.L., H.J.B., J.D.S., and C.A.R. performed experiments; A.J.R., V.L.K., M-L.L., and H.J.B. analyzed data; A.J.R., G.K.A., and C.A.R. interpreted results of experiments; A.J.R. and G.K.A. prepared figures; A.J.R. drafted manuscript; A.J.R., V.L.K., G.K.A., M-L.L., H.J.B., J.D.S., and C.A.R. edited and revised manuscript; A.J.R., V.L.K., G.K.A., M-L.L., H.J.B., J.D.S., and C.A.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank our subjects for their time and cheerful cooperation, Hannah Colby for her assistance with data collection on this project, and Drs. Albert Yurvati, Levi Rice, and Sibi Thomas for their assistance with subject medical examinations.
REFERENCES
- 1.Becker LB, Weisfeldt ML, Weil MH, Budinger T, Carrico J, Kern K, Nichol G, Shechter I, Traystman R, Webb C, Wiedemann H, Wise R, Sopko G. The PULSE initiative: scientific priorities and strategic planning for resuscitation research and life saving therapies. Circulation 105: 2562–2570, 2002. doi: 10.1161/01.cir.0000017142.39991.c3. [DOI] [PubMed] [Google Scholar]
- 2.Eastridge BJ, Hardin M, Cantrell J, Oetjen-Gerdes L, Zubko T, Mallak C, Wade CE, Simmons J, Mace J, Mabry R, Bolenbaucher R, Blackbourne LH. Died of wounds on the battlefield: causation and implications for improving combat casualty care. J Trauma 71: S4–S8, 2011. doi: 10.1097/TA.0b013e318221147b. [DOI] [PubMed] [Google Scholar]
- 3.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, Butler FK, Kotwal RS, Holcomb JB, Wade C, Champion H, Lawnick M, Moores L, Blackbourne LH. Death on the battlefield (2001-2011): implications for the future of combat casualty care. J Trauma Acute Care Surg 73: S431–S437, 2012. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 4.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma 60: S3–S11, 2006. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 5.Rickards CA. Cerebral blood-flow regulation during hemorrhage. Compr Physiol 5: 1585–1621, 2015. doi: 10.1002/cphy.c140058. [DOI] [PubMed] [Google Scholar]
- 6.Carter R , 3rd, Hinojosa-Laborde C, Convertino VA. Sex comparisons in muscle sympathetic nerve activity and arterial pressure oscillations during progressive central hypovolemia. Physiol Rep 3: e12420, 2015. doi: 10.14814/phy2.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 275: R1909–R1920, 1998. doi: 10.1152/ajpregu.1998.275.6.R1909. [DOI] [PubMed] [Google Scholar]
- 8.Franke WD, Johnson CP, Steinkamp JA, Wang R, Halliwill JR. Cardiovascular and autonomic responses to lower body negative pressure: do not explain gender differences in orthostatic tolerance. Clin Auton Res 13: 36–44, 2003. doi: 10.1007/s10286-003-0066-x. [DOI] [PubMed] [Google Scholar]
- 9.Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, Levine BD. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol 286: H449–H457, 2004. doi: 10.1152/ajpheart.00735.2002. [DOI] [PubMed] [Google Scholar]
- 10.Gotshall RW. Gender differences in tolerance to lower body negative pressure. Aviat Space Environ Med 71: 1104–1110, 2000. [PubMed] [Google Scholar]
- 11.Lewis NC, Bain AR, MacLeod DB, Wildfong KW, Smith KJ, Willie CK, Sanders ML, Numan T, Morrison SA, Foster GE, Stewart JM, Ainslie PN. Impact of hypocapnia and cerebral perfusion on orthostatic tolerance. J Physiol 592: 5203–5219, 2014. doi: 10.1113/jphysiol.2014.280586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White DD, Gotshall RW, Tucker A. Women have lower tolerance to lower body negative pressure than men. J Appl Physiol (1985) 80: 1138–1143, 1996. doi: 10.1152/jappl.1996.80.4.1138. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery LD, Kirk PJ, Payne PA, Gerber RL, Newton SD, Williams BA. Cardiovascular responses of men and women to lower body negative pressure. Aviat Space Environ Med 48: 138–145, 1977. [PubMed] [Google Scholar]
- 14.Van Lieshout JJ, Wieling W, Karemaker JM, Secher NH. Syncope, cerebral perfusion, and oxygenation. J Appl Physiol (1985) 94: 833–848, 2003. doi: 10.1152/japplphysiol.00260.2002. [DOI] [PubMed] [Google Scholar]
- 15.Aanerud J, Borghammer P, Rodell A, Jonsdottir KY, Gjedde A. Sex differences of human cortical blood flow and energy metabolism. J Cereb Blood Flow Metab 37: 2433–2440, 2017. doi: 10.1177/0271678X16668536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarumi T, Ayaz KM, Liu J, Tseng BY, Parker R, Riley J, Tinajero C, Zhang R. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab 34: 971–978, 2014. doi: 10.1038/jcbfm.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kastrup A, Dichgans J, Niemeier M, Schabet M. Changes of cerebrovascular CO2 reactivity during normal aging. Stroke 29: 1311–1314, 1998. doi: 10.1161/01.STR.29.7.1311. [DOI] [PubMed] [Google Scholar]
- 18.Kastrup A, Happe V, Hartmann C, Schabet M. Gender-related effects of indomethacin on cerebrovascular CO2 reactivity. J Neurol Sci 162: 127–132, 1999. doi: 10.1016/S0022-510X(98)00288-3. [DOI] [PubMed] [Google Scholar]
- 19.Kastrup A, Thomas C, Hartmann C, Schabet M. Sex dependency of cerebrovascular CO2 reactivity in normal subjects. Stroke 28: 2353–2356, 1997. doi: 10.1161/01.STR.28.12.2353. [DOI] [PubMed] [Google Scholar]
- 20.Kay VL, Rickards CA. The role of cerebral oxygenation and regional cerebral blood flow on tolerance to central hypovolemia. Am J Physiol Regul Integr Comp Physiol 310: R375–R383, 2016. doi: 10.1152/ajpregu.00367.2015. [DOI] [PubMed] [Google Scholar]
- 21.Kay VL, Rickards CA. Reproducibility of a continuous ramp lower body negative pressure protocol for simulating hemorrhage. Physiol Rep 3: e12640, 2015. doi: 10.14814/phy2.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kay VL, Sprick JD, Rickards CA. Cerebral oxygenation and regional cerebral perfusion responses with resistance breathing during central hypovolemia. Am J Physiol Regul Integr Comp Physiol 313: R132–R139, 2017. doi: 10.1152/ajpregu.00385.2016. [DOI] [PubMed] [Google Scholar]
- 23.Fagius J, Karhuvaara S. Sympathetic activity and blood pressure increases with bladder distension in humans. Hypertension 14: 511–517, 1989. doi: 10.1161/01.hyp.14.5.511. [DOI] [PubMed] [Google Scholar]
- 24.Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK, Ainslie PN. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 196: 221–237, 2011. doi: 10.1016/j.jneumeth.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Rickards CA, Ryan KL, Cooke WH, Convertino VA. Tolerance to central hypovolemia: the influence of oscillations in arterial pressure and cerebral blood velocity. J Appl Physiol (1985) 111: 1048–1058, 2011. doi: 10.1152/japplphysiol.00231.2011. [DOI] [PubMed] [Google Scholar]
- 26.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med (Chic) XVII: 863–871, 1916. doi: 10.1001/archinte.1916.00080130010002. [DOI] [PubMed] [Google Scholar]
- 27.Mahdi A, Nikolic D, Birch AA, Payne SJ. At what data length do cerebral autoregulation measures stabilise? Physiol Meas 38: 1396–1404, 2017. doi: 10.1088/1361-6579/aa76a9. [DOI] [PubMed] [Google Scholar]
- 28.Curran-Everett D. Evolution in statistics: P values, statistical significance, kayaks, and walking trees. Adv Physiol Educ 44: 221–224, 2020. doi: 10.1152/advan.00054.2020. [DOI] [PubMed] [Google Scholar]
- 29.Curran-Everett D, Benos DJ. Guidelines for reporting statistics in journals published by the American Physiological Society. Am J Physiol Regul Integr Comp Physiol 287: R247–R249, 2004. doi: 10.1152/ajpregu.00346.2004. [DOI] [PubMed] [Google Scholar]
- 30.Hordinsky JR, Gebhardt U, Wegmann HM, Schafer G. Cardiovascular and biochemical response to simulated space flight entry. Aviat Space Environ Med 52: 16–18, 1981. [PubMed] [Google Scholar]
- 31.Lightfoot JT, Tsintgiras KM. Quantification of tolerance to lower body negative pressure in a healthy population. Med Sci Sport Exer 27: 697–706, 1995. [PubMed] [Google Scholar]
- 32.Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol 29: 231–240, 1991. doi: 10.1002/ana.410290302. [DOI] [PubMed] [Google Scholar]
- 33.Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND, Ikeda K, Graham J, Lewis NC, Day TA, Ainslie PN. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 590: 3261–3275, 2012. doi: 10.1113/jphysiol.2012.228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller KB, Howery AJ, Rivera-Rivera LA, Johnson SC, Rowley HA, Wieben O, Barnes JN. Age-related reductions in cerebrovascular reactivity using 4D flow MRI. Front Aging Neurosci 11: 281, 2019. doi: 10.3389/fnagi.2019.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson GK, Sprick JD, Park FS, Rosenberg AJ, Rickards CA. Responses of cerebral blood velocity and tissue oxygenation to low-frequency oscillations during simulated haemorrhagic stress in humans. Exp Physiol 104: 1190–1201, 2019. doi: 10.1113/EP087358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucas SJ, Lewis NC, Sikken EL, Thomas KN, Ainslie PN. Slow breathing as a means to improve orthostatic tolerance: a randomized sham-controlled trial. J Appl Physiol (1985) 115: 202–211, 2013. doi: 10.1152/japplphysiol.00128.2013. [DOI] [PubMed] [Google Scholar]
- 37.Claassen JA, Meel-van den Abeelen AS, Simpson DM, Panerai RB; International Cerebral Autoregulation Research Network (CARNet). Transfer function analysis of dynamic cerebral autoregulation: a white paper from the International Cerebral Autoregulation Research Network. J Cereb Blood Flow Metab 36: 665–680, 2016. doi: 10.1177/0271678X15626425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tzeng YC, Lucas SJ, Atkinson G, Willie CK, Ainslie PN. Fundamental relationships between arterial baroreflex sensitivity and dynamic cerebral autoregulation in humans. J Appl Physiol (1985) 108: 1162–1168, 2010. doi: 10.1152/japplphysiol.01390.2009. [DOI] [PubMed] [Google Scholar]
- 39.Al-Khazraji BK, Shoemaker LN, Gati JS, Szekeres T, Shoemaker JK. Reactivity of larger intracranial arteries using 7 T MRI in young adults. J Cereb Blood Flow Metab 39: 1204–1214, 2019. doi: 10.1177/0271678X18762880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol (1985) 117: 1090–1096, 2014. doi: 10.1152/japplphysiol.00285.2014. [DOI] [PubMed] [Google Scholar]
- 41.Lewis NC, Smith KJ, Bain AR, Wildfong KW, Numan T, Ainslie PN. Impact of transient hypotension on regional cerebral blood flow in humans. Clin Sci (Lond) 129: 169–178, 2015. doi: 10.1042/CS20140751. [DOI] [PubMed] [Google Scholar]
- 42.Smirl JD, Hoffman K, Tzeng YC, Hansen A, Ainslie PN. Methodological comparison of active- and passive-driven oscillations in blood pressure; implications for the assessment of cerebral pressure-flow relationships. J Appl Physiol (1985) 119: 487–501, 2015. doi: 10.1152/japplphysiol.00264.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labrecque L, Rahimaly K, Imhoff S, Paquette M, Le BO, Malenfant S, Lucas SJE, Bailey DM, Smirl JD, Brassard P. Diminished dynamic cerebral autoregulatory capacity with forced oscillations in mean arterial pressure with elevated cardiorespiratory fitness. Physiol Rep 5: e13486, 2017. doi: 10.14814/phy2.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lind-Holst M, Cotter JD, Helge JW, Boushel R, Augustesen H, Van Lieshout JJ, Pott FC. Cerebral autoregulation dynamics in endurance-trained individuals. J Appl Physiol (1985) 110: 1327–1333, 2011. doi: 10.1152/japplphysiol.01497.2010. [DOI] [PubMed] [Google Scholar]
- 45.Bailey DM, Marley CJ, Brugniaux JV, Hodson D, New KJ, Ogoh S, Ainslie PN. Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke 44: 3235–3238, 2013. doi: 10.1161/STROKEAHA.113.002589. [DOI] [PubMed] [Google Scholar]
- 46.Marley CJ, Brugniaux JV, Davis D, Calverley TA, Owens TS, Stacey BS, Tsukamoto H, Ogoh S, Ainslie PN, Bailey DM. Long-term exercise confers equivalent neuroprotection in females despite lower cardiorespiratory fitness. Neuroscience 427: 58–63, 2020. doi: 10.1016/j.neuroscience.2019.12.008. [DOI] [PubMed] [Google Scholar]