Abstract
While it has long been known that contraction robustly stimulates skeletal muscle glucose uptake, the molecular steps regulating this increase remain incompletely defined. The mammalian ortholog of Sir2, sirtuin 1 (SIRT1), is an NAD+-dependent protein deacetylase that is thought to link perturbations in energy flux associated with exercise to subsequent cellular adaptations. Nevertheless, its role in contraction-stimulated glucose uptake has not been described. The objective of this study was to determine the importance of SIRT1 to contraction-stimulated glucose uptake in mouse skeletal muscle. Using a radioactive 2-deoxyglucose uptake (2DOGU) approach, we measured ex vivo glucose uptake in unstimulated (rested) and electrically stimulated (100 Hz contraction every 15 s for 10 min; contracted) extensor digitorum longus (EDL) and soleus from ∼15-wk-old male and female mice with muscle-specific knockout of SIRT1 deacetylase activity and their wild-type littermates. Skeletal muscle force decreased over the contraction protocol, although there were no differences in the rate of fatigue between genotypes. In EDL and soleus, loss of SIRT1 deacetylase activity did not affect contraction-induced increase in glucose uptake in either sex. Interestingly, the absolute rate of contraction-stimulated 2DOGU was ∼1.4-fold higher in female compared with male mice, regardless of muscle type. Taken together, our findings demonstrate that SIRT1 is not required for contraction-stimulated glucose uptake in mouse skeletal muscle. Moreover, to our knowledge, this is the first demonstration of sex-based differences in contraction-stimulated glucose uptake in mouse skeletal muscle.
NEW & NOTEWORTHY Here, we demonstrate that glucose uptake in response to ex vivo contractions is not affected by the loss of sirtuin 1 (SIRT1) deacetylase function in muscle, regardless of sex or muscle type. Interestingly, however, similar to studies on insulin-stimulated glucose uptake, we demonstrate that contraction-stimulated glucose uptake is robustly higher in female compared with the male skeletal muscle. To our knowledge, this is the first demonstration of sex-based differences in contraction-stimulated glucose uptake in skeletal muscle.
Keywords: deacetylase, 2-deoxyglucose, exercise, sex dimorphism
INTRODUCTION
Exercise robustly increases glucose uptake into the contracting muscle independently of insulin, making exercise a cornerstone intervention for the prevention and treatment of type 2 diabetes (1, 2). While this contraction-stimulated glucose uptake by skeletal muscle was first described some 50 yr ago (3), the precise molecular mechanism/s that drive this process remain relatively unknown. 5′-AMP-activated protein kinase (AMPK) was long thought to be the key modulator connecting the metabolic stress of exercise to an increase in muscle glucose uptake (4, 5). However, while activation of AMPK can stimulate glucose uptake into skeletal muscle (6, 7) (independently of insulin), recent studies demonstrate that AMPK is not obligatory for glucose uptake during contraction/exercise (8–10); glucose uptake is sustained in inducible skeletal muscle-specific AMPKα1/2 double knockout (KO) mice during exercise (11). As such, other metabolic-sensing proteins and/or pathways must also be involved in contraction-stimulated glucose uptake.
The sirtuin (SIRT) family of proteins are a highly conserved family of class III NAD+-dependent deacetylases involved in metabolic regulation (12, 13). Of the seven proteins that comprise the SIRT family, SIRT1 is the most extensively studied, including numerous investigations on the contribution of SIRT1 to the adaptive response to exercise (14). Indeed, as part of this work, SIRT1 and AMPK have been proposed as an interdependent energy sensing network that contribute to exercise-induced adaptations (15, 16). Notably, some studies suggest that SIRT1 is regulated by AMPK (via an AMPK-mediated increase in NAD+) (16, 17), while others suggest that AMPK is regulated by SIRT1 (via SIRT1-mediated acetylation of liver kinase B) (18, 19). Additionally, acute exercise increases SIRT1 activity in rodent (16, 20) and human skeletal muscle (21, 22). Considering these points together, here we used a mouse model with muscle-specific knockout of SIRT1 deacetylase activity (referred to as mKO) to determine the importance of SIRT1 to contraction-stimulated glucose uptake in mouse skeletal muscle. We hypothesized that contraction-stimulated glucose uptake would be reduced in mKO mice, regardless of sex or muscle fiber type.
MATERIALS AND METHODS
Animals
Mice with a muscle-specific knockout of SIRT1 (mKO), which we previously generated using Cre-LoxP methodology (23, 24), were used for this study; importantly, as validated in our previous work, deletion of exon 4 of the Sirt1 gene in the mKO mouse is specific to muscle, including the soleus and extensor digitorum longus (EDL), but does not occur in adipose tissue and liver (23, 24). Briefly, floxed mice harboring loxP sites flanking exon 4 of the SIRT1 gene (25) were crossed with mice expressing Cre recombinase under the muscle creatine kinase promoter; deletion of exon 4, which encodes the deacetylase domain, results in a truncated SIRT1 protein that lacks deacetylase functionality (25). Our breeding strategy was to breed a mKO [i.e., flox/flox, Cre-positive (1 allele)] mouse with a “wild-type” (WT; i.e., flox/flox, Cre-negative) mouse. As such, litters produced both WT and mKO littermates and these mice were housed together with a limit of five mice per cage; thus littermate controls were used for all aspects of this work. Mice were housed on a 12:12-h light-dark cycle at standard room temperature (∼21°C) and had ad libitum access to chow (catalog no. 7912, irradiated; Envigo Teklad) and water. All studies were conducted in male and female mKO and WT littermates at ∼15 wk of age. Experiments were carried out with the approval of, and in accordance with, the Animal Care Program and Institutional Animal Care and Use Committee at the University of California, San Diego.
Muscle Collection and Ex Vivo Electrical Stimulation
After a 3-h fast, mice were anesthetized (25 mg/kg ketamine, 1 mg/kg acepromazine, and 2 mg/kg xylazine) via intraperitoneal injection. Once anesthetized, suture (size: 6-0) was tied at the myo-tendinous junction at each end of the EDL and soleus and the muscles from both legs were removed and pre-incubated (20 min, 35°C) in oxygenated (95% O2-5% CO2) Krebs-Henseleit buffer (KHB) containing 2 mM sodium pyruvate and 9 mM mannitol (PreInc-KHB). After the 20-min preincubation period, one soleus and one EDL were mounted in a specialized muscle chamber containing PreInc-KHB (25°C), with continuous oxygenation (room air). The muscle origin was tied to a rigid post, and the insertion was secured to the arm of a dual-mode ergometer (model 300B; Aurora Scientific, ON, Canada). Muscles were stretched to optimal length based on resting tension (2.4 g for soleus and 1.1 g for EDL) in the preincubation buffer. This criterion for setting optimal length was done to prevent additional stimulation of the muscle, which could impact glucose uptake. Specifically, to determine the resting tension at optimal length, in preliminary studies EDL and soleus were gradually lengthened and the corresponding tension (in grams) at which supramaximal stimulation produced maximal isometric tetanic force was calculated: soleus (n = 7): 2.4 ± 0.77 g; EDL (n = 13): 1.1 ± 0.45 g. After resting tension was established, muscles were stimulated (100 Hz, 35 V, 2-s train, 0.2-ms pulse) every 15 s for 10 min (40 total contractions) via an electrical stimulator (model S88; Astro-Med, West Warwick, RI) and parallel platinum plate electrodes that extended the length of the muscle. Tension was recorded throughout the contraction protocol and specific force was calculated by normalizing muscle force to muscle physiological cross-sectional area (26). Accumulated tension is the summation of all the force output during the contraction protocol. Fatigability was calculated as the time to reach 60% and 40% of the force of the initial contraction.
Ex Vivo 2-Deoxyglucose Uptake
Immediately after the last contraction, the contracted muscle and contralateral rested muscle were transferred to flasks containing KHB containing 1 mM 2DG, 8 mM mannitol, 2 mM Na-pyruvate, 0.053 mCi/mmol [14C]-mannitol [American Radiolabeled Chemical (ARC)], and 3 mCi/mmol [3H]-2DG (ARC). After 10 min, the muscles were blotted on filter paper, trimmed, rapidly frozen in liquid nitrogen, and stored (–80°C). The 2-deoxyglucose uptake (2DOGU) rate was calculated as previously described (24).
Muscle Homogenization
Soleus and EDL were homogenized (Bullet Blender Tissue Homogenizer, Next Advance No. BT24M) in 500 µL ice-cold homogenization buffer [50 mm Tris, pH 7.5, 250 mm sucrose, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 50 mm NaF, 1 mm NaVO2 Na2(PO4)2, and 0.1% DTT] containing phosphatase inhibitor cocktail (PIC) 2 (MilliporeSigma No. P5726), PIC 3 (MilliporeSigma No. P0044), Complete (MilliporeSigma No. 11836170001), 1 mM trichostatin A (Cell Signaling No. 9950S), and 1 M nicotinamide (MilliporeSigma No. N0636). After homogenization, muscles were rotated for 2 h at 4°C and the supernatant was collected after centrifugation (12,000 rpm/14,167 g) for 20 min at 4°C and then stored at −80°C for counting for 2DOGU and immunoblotting.
GLUT4 Translocation Assay
GLUT4 exocytosis was measured as previously described (27, 28). L6-G4-myc (Kerafast, Boston, MA) and L6 (American Type Culture Collection, Manassas, VA) myoblasts were grown in low glucose DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Differentiation into myotubes was induced by changing media to low-glucose DMEM supplemented with 2% horse serum for 2–3 days. On the day of experimentation, myotubes were serum starved for 3 h before treatment with nicotinamide (NAM) for 1 h at concentrations indicated; as the NAM stock (1 M) was dissolved in water, the same volume of water was used as the experimental control. Cells were stimulated with the pan-AMPK activator MK8722 (10 µM) for 40 min before being washed with ice-cold PBS, fixed with 3% paraformaldehyde for 10 min, blocked with 3% goat serum, and incubated with polyclonal anti-Myc-Tag antibody (1:200) for 60 min at 4°C. Following primary antibody incubation cells were incubated with horseradish peroxidase-conjugated goat anti-rabbit antibody (1:2,000) for 60 min at 4°C. Cells were then washed with PBS and incubated with o-phenylenediamine dihydrochloride for 20–30 min at room temperature. Incubation was stopped with 3 M HCL, and absorbance of the supernatant was measured at 492 nM using a Thermofisher Multiskan Spectrophotometer (Thermofisher, Waltham, MA). Background myc-tag binding was determined from L6 myoblasts that do not express the myc-tagged GLUT4 and subtracted from appropriate values. Values were normalized to control-basal (i.e., no NAM and no MK8722) for each individual experiment; three individual experiments were conducted with each condition run in triplicate.
Immunoblotting
Protein concentration of the lysates used for 2DOG analysis were quantified via bicinchoninic acid method (Pierce BCA Protein Assay Reagent A No. 23223 and Reagent B No. 23224), and then samples were prepped to the same protein concentration (1 μg/uL) in 1× Laemmli sample buffer. After boiling for 5 min at 100°C, equal amounts of protein (20 μg) were separated on XT criterion precast gels (Bio-Rad Laboratories) under reducing conditions and were then transferred to nitrocellulose (Thermo Fisher Scientific). The nitrocellulose membranes were stained with ponceau S solution [0.1% (wt/vol) ponceau S in 5% acetic acid], imaged (ChemiDoc XRS+ Imaging System, Bio-Rad Laboratories), and then washed with 1× TBS Tween (TBST). Next, the membranes were blocked with 5% milk in TBST for 1 h at room temperature and were then incubated overnight with gentle agitation in primary antibodies at 4°C. The following primary antibodies from Cell Signaling Technology were diluted 1:1,000 in 5% BSA: p38 MAPK (p38; CS 9212), phospho-p38Thr180/Tyr182 MAPK (p-p38T180/Y182; CS 9211), phospho-AMPKαThr172 (pAMPKT172; CS 2531), hexokinase II (HKII; CS 2867), and eukaryotic elongation factor 2 (eEF2; CS 2332). Total AMPKα1 and AMPKα2 primary antibodies were generously provided by Grahame Hardie, University of Dundee. Following overnight primary antibody incubation, membranes were incubated in appropriate secondary antibodies (1:10,000 in 5% milk in TBST) for 1 h. The blots were developed utilizing a horseradish peroxidase chemiluminescent substrate (Bio-Rad Laboratories No. 1705061) and imaged using a ChemiDoc XRS+ Imaging System (Bio-Rad Laboratories). Densitometric analysis of Ponceau S staining and immunoblots was conducted using Image Lab Software 6.1 (Bio-Rad Laboratories). Phosphorylated proteins were normalized to total abundance, and total proteins were normalized to Ponceau S (29, 30). For total protein normalization to Ponceau S, a band at ∼42 kDa was used, as this band corresponds to β-actin, a commonly used internal loading control; we found no effect of rest versus contraction, genotype, or sex on the intensity of this band (data not shown).
Statistics
Statistical analyses were performed using Prism 8 (GraphPad Software Incorporated, La Jolla, CA). All data were analyzed using an unpaired Student’s t test or two-way ANOVA followed by a Tukey's post hoc test for pairwise comparison. To study potential sex differences in basal and contraction-stimulated 2DOGU, muscle mechanics and HKII, within each sex data for mKO and WT, were collapsed together; this was done as there were no genotype differences in these parameters within each sex. Statistical significance for all the data was set at P < 0.05, and values are expressed as means ± SE.
RESULTS
Contractile Function and Fatigability Were Not Different between mKO and WT Mice
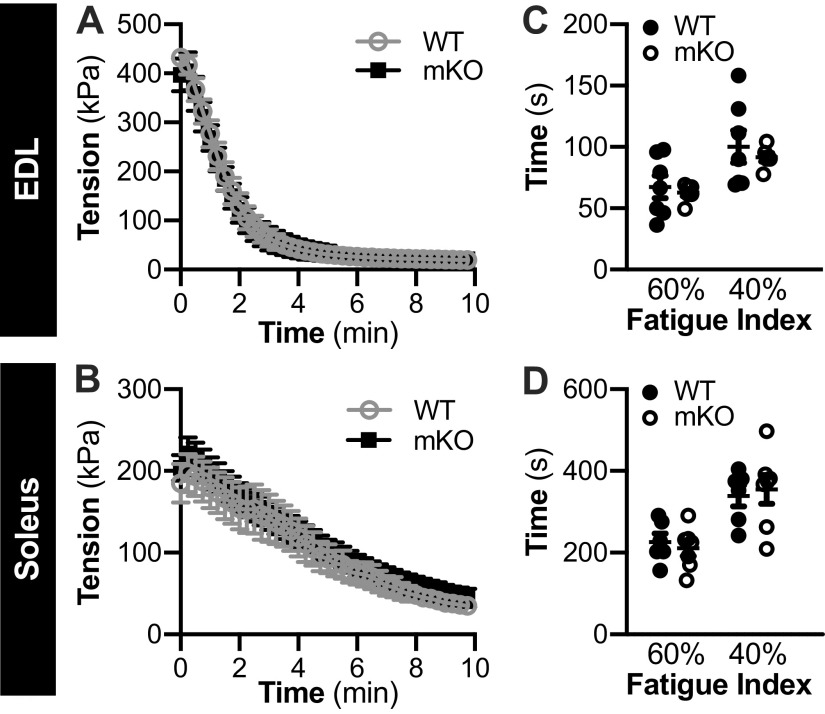
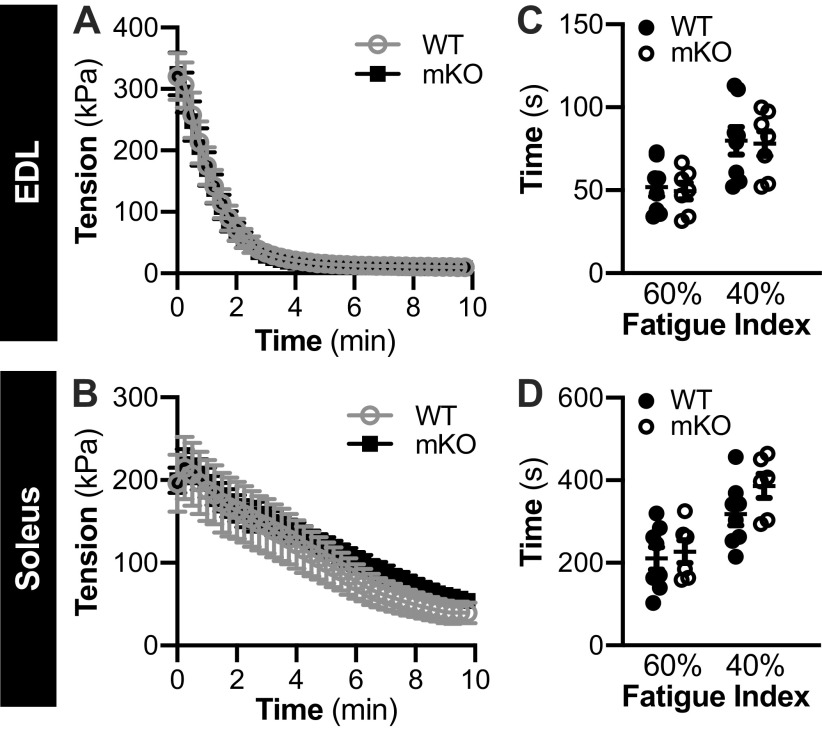
Initial maximal tetanic tension and tetanic tension during the contraction protocol was not different between genotypes in the EDL or soleus from female (Fig. 1, A and B, respectively) or male mice (Fig. 2, A and B, respectively). As calculated from the force-time data, the fatigue index, which represents the time taken for force to decrease to 60% and 40% of initial force, was not different between genotypes, in the EDL or soleus of female (Fig. 1, C and D, respectively) or male (Fig. 2, C and D, respectively) mice.
Figure 1.
Female muscle-specific knockout of sirtuin 1 (mKO) mice show no impairments in contractile function or fatiguability compared with wild type (WT). A and B: in WT and mKO mice, temporal force production recording during a 10-min contraction protocol (40 subsequent contractions) in the extensor digitorum longus (EDL; A) and soleus (B). In both EDL and soleus, there was a main effect of time (i.e., contraction) on force production, but no effect of genotype. P < 0.05. Statistics: data were analyzed by repeated measures two-way ANOVA. C and D: fatigue index (time taken for force to decrease to 60% and 40% of initial force) in the EDL (C) and soleus (D) of WT and mKO female mice. Statistics: data were analyzed by a repeated measures two-way ANOVA. Note: 2-deoxyglucose uptake was not measured in all muscles. Female: WT-soleus, n = 6; WT-EDL, n = 7; mKO-soleus, n = 7; mKO-EDL, n = 7. Data are presented as means ± SE.
Figure 2.
Male muscle-specific knockout of sirtuin 1 (mKO) mice show no impairments in contractile function or fatiguability compared with wild type (WT). A and B: in WT and mKO mice, temporal force production recording during a 10-min contraction protocol (40 subsequent contractions) in the extensor digitorum longus (EDL; A) and soleus (B). In both EDL and soleus muscles, there was a main effect of time (i.e., contraction) on force production but no effect of genotype. Statistics: data were analyzed by repeated measures two-way ANOVA. *P < 0.05. C and D: fatigue index (time taken for force to decrease to 60% and 40%, of initial force) in the EDL (C) and soleus (D) of WT and mKO male mice. Statistics: data were analyzed by a repeated measures two-way ANOVA. Note: 2-deoxyglucose uptake was not measured in all muscles. Male: WT-soleus, n = 8; WT-EDL, n = 8; mKO-soleus, n = 7; mKO-EDL, n = 7. Data are presented as means ± SE.
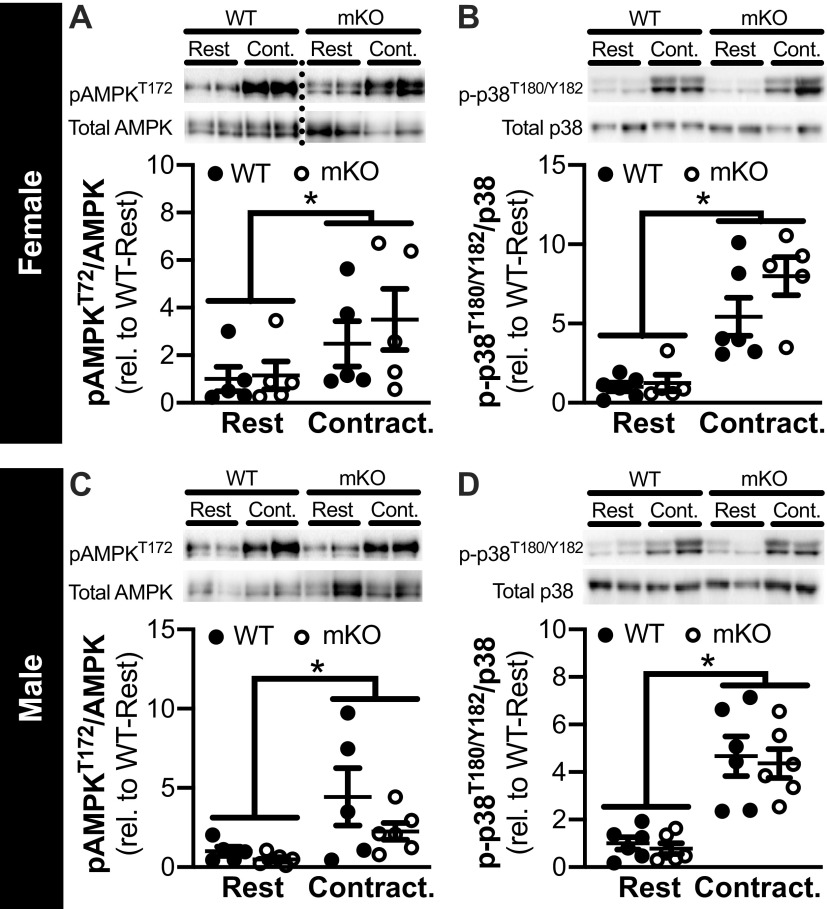
Phosphorylation of p38 and AMPK is Increased Similarly in mKO and WT Mice after Contraction
In the EDL, pAMPKT172 and p-p38T180/Y182 was significantly higher in contraction versus rest in both female (Fig. 3, A and B, respectively) and male (Fig. 3, C and D, respectively), regardless of genotype. Within each sex, total abundance of p38 and AMPK was not different by genotype or between rest/contraction, and basal pAMPKT172 and p-p38T180/Y182 were not different between genotypes.
Figure 3.
Contraction-stimulated increases in the phosphorylation of AMPK and p38 are similar in muscle-specific knockout of sirtuin 1 (mKO) and wild-type (WT) mice. A–D: representative images and quantification of pAMPKT172 (A and C) and p-p38T180/Y182 (B and D) from the rested (Rest) and contracted (Contract.) paired extensor digitorum longus (EDL) of female (A and B) and male (C and D) WT and mKO mice; analysis was conducted in the same muscles used for 2-deoxyglucose uptake analysis. Protein phosphorylation was corrected for total abundance, and the data are presented as relative (rel.) to WT-Rest. Dotted line indicates spliced images from gels that have been exposed together for the same duration of time. Statistics: data were analyzed by a repeated measures two-way ANOVA. *P < 0.05, main effect of contraction. Male: WT-AMPK, n = 5; WT-p38, n = 6; mKO-AMPK, n = 6; mKO-p38, n = 6. Female: WT-AMPK, n = 5; WT-p38, n = 6; mKO-AMPK, n = 5; mKO-p38, n = 5. Data are presented as means ± SE.
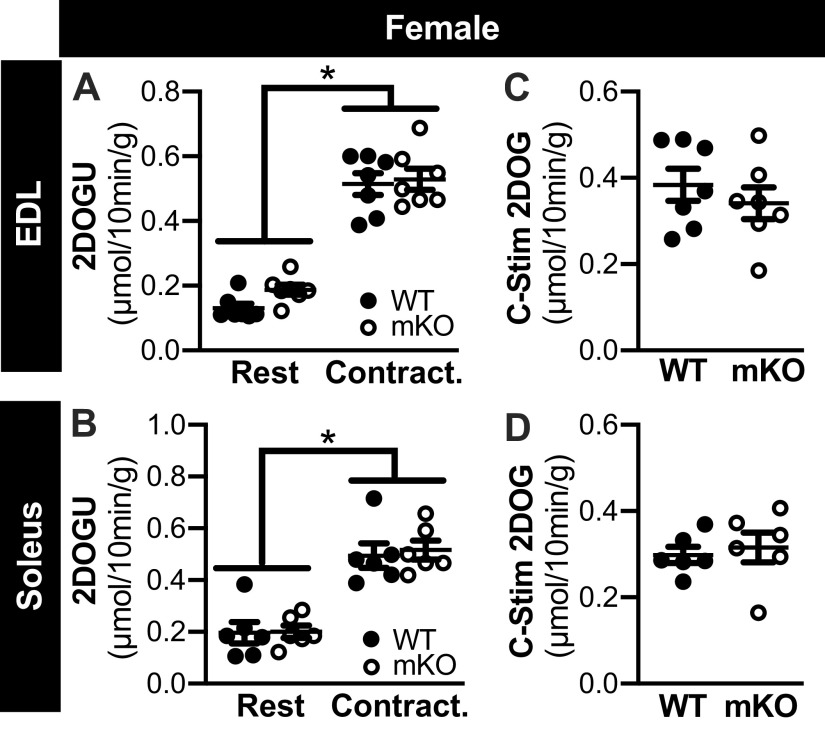
Contraction-Stimulated Glucose Uptake is Comparable in mKO and WT Mice, Regardless of Sex
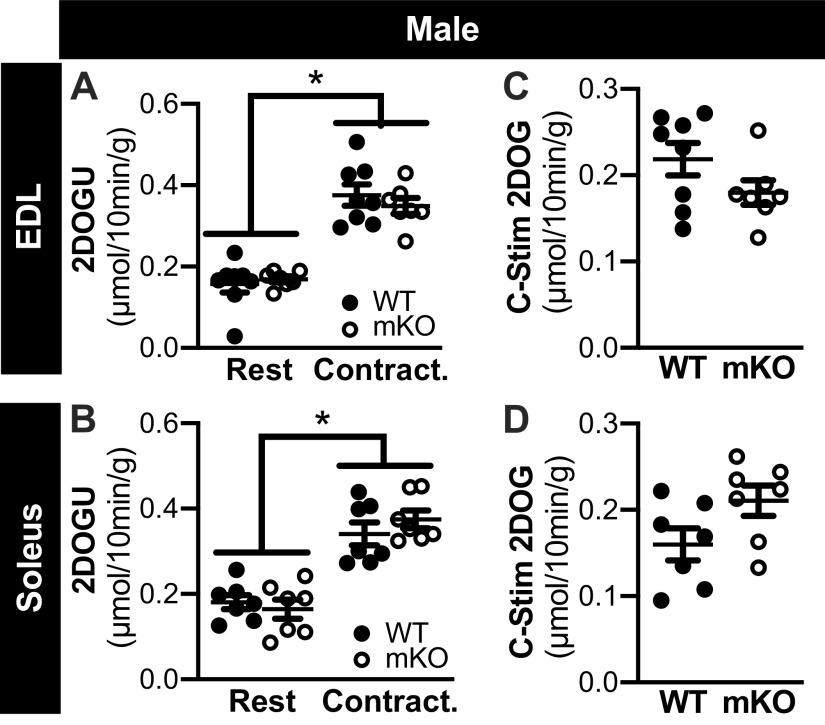
In the EDL and soleus from female WT and mKO mice, 2DOGU uptake was significantly higher in contraction versus rest (Fig. 4, A and B, respectively); no genotype differences in 2DOGU uptake were evident within rest or contraction. In both muscle types, contraction-stimulated (C-Stim) 2DOGU uptake (i.e., C-Stim = Contraction 2DOGU − Rest 2DOGU) was comparable between the WT and mKO female mice (Fig. 4, C and D). Similarly, in the EDL and soleus from male mice, 2DOGU uptake was significantly higher in contraction versus rest (Fig. 5, A and B, respectively); no genotype differences in 2DOGU uptake were evident within rest or contraction, and C-Stim 2DOGU by the soleus and EDL was comparable between genotypes (Fig. 5, C and D, respectively).
Figure 4.
Contraction-stimulated glucose uptake is comparable in muscle-specific knockout of sirtuin 1 (mKO) and wild type (WT) of female mice. A and B: in WT and mKO female mice, 2-deoxyglucose uptake (2DOGU) in paired rested (Rest) and contracted (Contract.) extensor digitorum longus (EDL; A) and soleus (B). There was a main effect of contraction on 2DOGU but no effect of genotype. Statistics: data were analyzed by a repeated measures two-way ANOVA. *P < 0.05, main effect of contraction. C and D: contraction-stimulated (C-Stim) 2DOGU (i.e., Contraction 2DOGU – Rest 2DOGU) in the EDL (C) and soleus (D) from WT and mKO female mice. Statistics: data were analyzed by an unpaired t test. Female: WT-soleus, n = 6; WT-EDL, n = 7; mKO-soleus, n = 6; mKO-EDL, n = 7. Data are presented as means ± SE.
Figure 5.
Contraction-stimulated glucose uptake is comparable in muscle-specific knockout of sirtuin 1 (mKO) and wild type (WT) of male mice. A and B: in WT and mKO male mice, 2-deoxyglucose uptake (2DOGU) in paired rested (Rest) and contracted (Contract.) extensor digitorum longus (EDL; A) and soleus (B). There was a main effect of contraction on 2DOGU but no effect of genotype. Statistics: data were analyzed by a repeated measures two-way ANOVA. *P < 0.05, main effect of contraction. C and D: contraction-stimulated (C-Stim) 2DOGU (i.e., Contraction 2DOGU – Rest 2DOGU) in the EDL (C) and soleus (D) from WT and mKO male mice. Statistics: data were analyzed by an unpaired t test. Male: WT-soleus, n = 8; WT-EDL, n = 8; mKO-soleus, n = 7; mKO-EDL, n = 7. Data are presented as means ± SE.
Pan-SIRT inhibition Does Not Reduce AMPK-Stimulated GLUT4 Translocation to the Plasma Membrane in L6 Myotubes
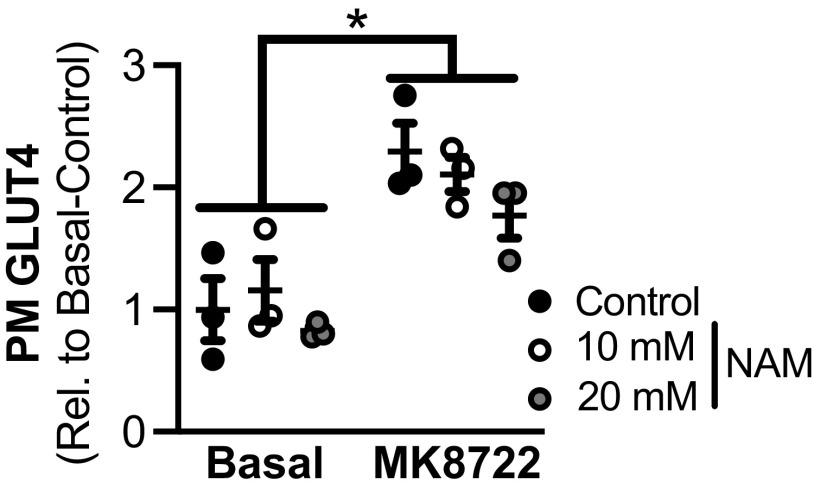
While the data above establish that contraction-stimulated glucose uptake was not impaired by loss of SIRT1, it is possible that other sirtuins are important. To address this possibility, we assessed whether pan-sirtuin inhibition with nicotinamide impacts AMPK-stimulated (using MK8722) GLUT4 translocation, in vitro. As expected, plasma membrane GLUT4 abundance significantly increased in MK8722-stimulated myotubes versus basal (Fig. 6); however, preincubation with NAM did not affect the MK8722-stimulated increase in plasma membrane GLUT4, regardless of concentration.
Figure 6.
Acute pan-inhibition of deacetylases in L6 myotubes does not impair AMPK-stimulated GLUT4 translocation to the plasma membrane. Plasma membrane (PM) GLUT4 abundance in basal and MK8722 (10 µM)-stimulated L6 myotubes treated without and with NAM at 10 mM and 20 mM is shown. There was a main effect of MK8722 but no effect of nicotinamide (NAM) treatment in L6 myotubes. Data are presented as relative to basal-control. Statistics: data were analyzed by a repeated measures two-way ANOVA. *P < 0.05, main effect of MK8722 stimulation. Data represent n = 3 in all groups. Data are presented as means ± SE.
Female Mice Have Higher Contraction-Stimulated Glucose Uptake Compared with Males
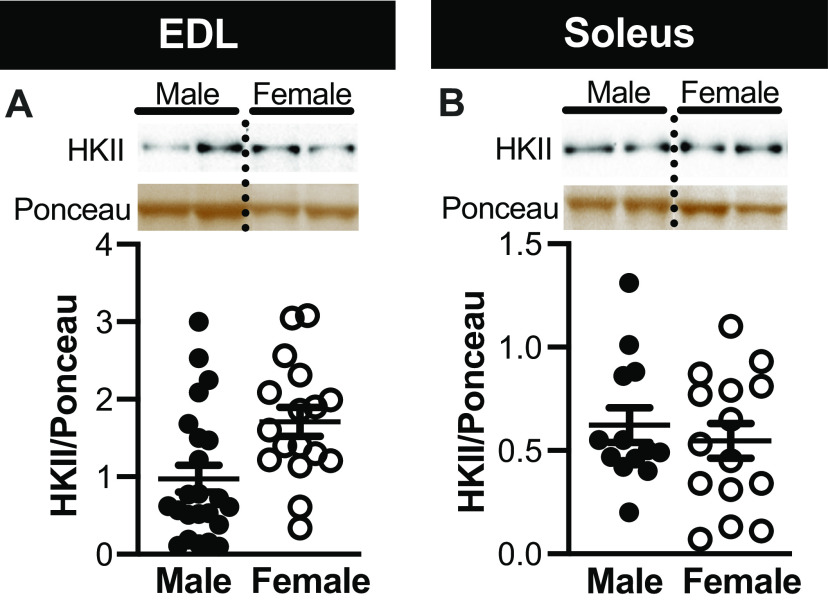
To investigate potential sex differences in contraction-stimulated glucose uptake in the soleus and EDL, we collapsed together the 2DOGU data for WT and mKO mice within each sex; we did this based on the fact that genotype, as described above did not impact 2DOGU. Interestingly, 2DOGU in the contracted EDL and soleus (Fig. 7, A and B, respectively), as well as C-Stim 2DOGU (Fig. 7, C and D, respectively), was significantly higher (∼1.4-fold in absolute fold and ∼1.7-fold in C-Stim) in female compared with male. This difference was not due to dissimilarities in 2DOGU in the rested muscle (Fig. 7, A and B) nor was it due to disparity in HKII abundance in either muscle type (Fig. 8, A and B). Moreover, this difference was not due to accumulated tension (Fig. 7, E and F), fatiguability (Fig. 7, G and H), variances in tension over the course of the stimulation protocol (data not shown) or activation/phosphorylation of pAMPKT172 or p-p38T180/Y182 (data not shown).
Figure 7.
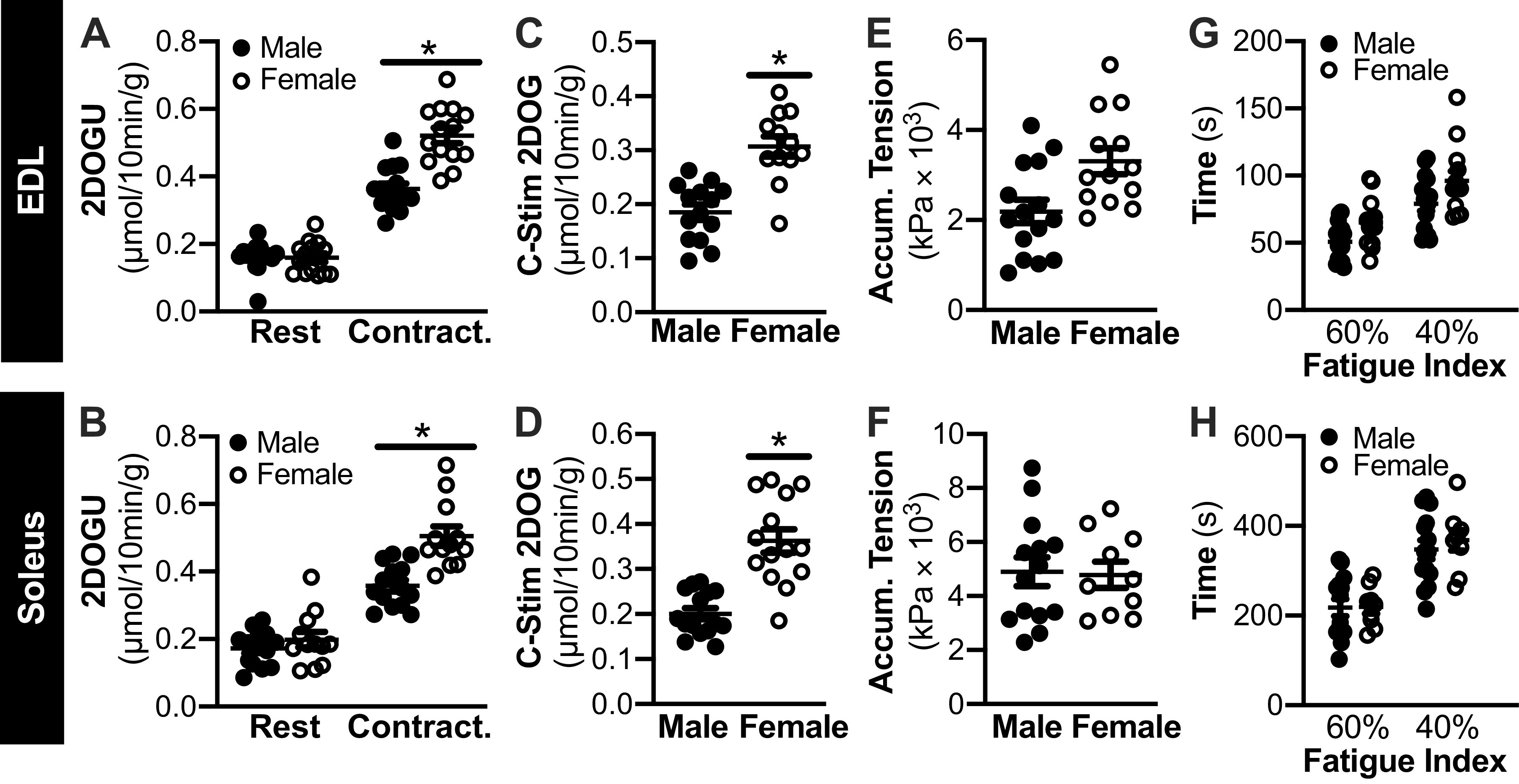
Contraction-stimulated glucose uptake is higher in female vs. male mice. A and B: in female and male mice, rested (Rest) and contraction (Contract.) 2-deoxyglucose uptake (2DOGU) in paired extensor digitorum longus (EDL; A) and paired soleus (B); because there were no genotype differences in contraction-stimulated (C-Stim) 2DOGU, wild-type (WT), and muscle-specific knockout of sirtuin 1 (mKO) data were collapsed together to assess sex differences. Statistics: data were analyzed by a repeated measures two-way ANOVA. C and D: C-Stim 2DOGU in the EDL (C) and soleus (D) from male and female mice. Statistics: data were analyzed by an unpaired t test. E and F: in female and male mice, accumulated tension during the 10-min contraction protocol (summation of temporal force production recordings produced during 40 subsequent contractions) in the EDL (E) and soleus (F). Statistics: data were analyzed by an unpaired t test. G and H: fatigue index (time taken for force to decrease to 60% and 40% initial force) in the EDL (G) and soleus (H) female and male mice. Statistics: data were analyzed by a repeated measures two-way ANOVA. *P < 0.05, main effect of sex. Male: soleus, n = 14; EDL, n = 15. Female: soleus, n = 10-12; EDL, n = 13-14. Data are presented as means ± SE.
Figure 8.
No difference in hexokinase II (HKII) abundance between male and female mice, regardless of muscle type. A and B: representative images and quantification of HKII (normalized to the Ponceau S band at ∼42 kDa) in the extensor digitorum longus (EDL; A) and soleus (B) of male and female mice; because no effect of genotype or contraction was noted in HKII abundance within each sex, data were combined to test for sex differences in HKII abundance. Dotted line indicates spliced images from gels that have been exposed together for the same duration of time. Statistics: data were analyzed by an unpaired t test. Soleus: male, n = 13; female, n = 15. EDL: male, n = 23; female, n = 17. Data are presented as means ± SE.
DISCUSSION
The intracellular signals and signaling steps that link contraction to an increase in glucose uptake remain to be fully defined. Due to its sensitivity to changes in cellular NAD+ concentration and the NAD+/NADH ratio (13, 31), both of which are impacted during exercise, and interregulatory relationship with AMPK (15, 16), SIRT1 has been proposed as a potential integrator and effector of metabolic adaptations to various aspects of muscle physiology and metabolism, including exercise (14, 32). Nevertheless, the importance of SIRT1 to contraction-stimulated glucose uptake has not been studied. To address this, we studied mice with muscle-specific knockout of SIRT1 deacetylase activity and measured glucose uptake in response to ex vivo electrical stimulation. Contrary to our hypothesis, our results demonstrate that SIRT1 deacetylase function is not necessary for contraction-stimulated glucose uptake, regardless of sex or muscle fiber type.
Contraction and/or exercise robustly increases glucose uptake into skeletal muscle (33–35). For many years, AMPK (via its kinase activity) was considered essential to this process, although robust recent work demonstrates that AMPK is not required for glucose uptake during exercise or contraction, but is required for the increase in glucose uptake seen 30–60 min after completing exercise (9–11). Considering this, the identity of the protein(s) and signaling steps controlling contraction-stimulated glucose uptake remains to be elucidated. To this point, a number of studies have noted a signaling interplay and interdependence between SIRT1 and AMPK. For example, some studies suggest that AMPK can regulate SIRT1 via effects on NAD+ and NAD+/NADH ratio (16, 17), while other studies propose the opposite, such that SIRT1 can regulate AMPK activity through its ability to regulate liver kinase B1 (the kinase upstream of AMPK) (19). Despite this purported interplay between AMPK and SIRT1, we find that loss of SIRT1 deacetylase activity does not impair either contraction-stimulated glucose uptake or AMPK phosphorylation/activation in mouse skeletal muscle. It is possible that normal contraction-stimulated glucose uptake in mKO mice is due to compensation and redundancy of signaling by other sirtuin family members. However, we found that pan-inhibition of sirtuins (with nicotinamide) also did not impact GLUT4 translocation in L6 myotubes when using the AMPK activator, MK8722. Together, this lack of a role for SIRT1 in the regulation of glucose uptake by skeletal muscle is in line with previous work from our laboratory demonstrating that SIRT1 overexpression does not increase basal glucose uptake (36–39), AICAR-stimulated glucose uptake is not impaired in muscle from mKO mice (24), AMPK phosphorylation and/or activity is not impacted by modulation of skeletal muscle SIRT1 activity (23, 24, 36, 38) and insulin-stimulated glucose uptake is not impaired in SIRT1 mKO mice (24) or by pan-sirtuin inhibition (40). Moreover, it assimilates with the aforementioned work demonstrating that AMPK is not required for contraction-stimulated glucose uptake (8–11). Taken together, these results demonstrate that contraction-stimulated glucose uptake does not require SIRT1 deacetylase function.
SIRT1 has long been considered as an important regulator of mitochondrial biogenesis in skeletal muscle, and by extension, the fatigability of skeletal muscle (41, 42). For instance, several studies have demonstrated that pharmacological activation of SIRT1, or activation of SIRT1 via elevation of cellular NAD+ concentration and/or NAD+/NADH ratio, increases skeletal muscle mitochondrial biogenesis (43–45). Furthermore, mice with whole body overexpression of SIRT1 (including in skeletal muscle) demonstrate increased mitochondrial abundance and function in skeletal muscle (46). Nevertheless, while mice with overexpression of SIRT1 in skeletal muscle have increases in the gene expression and/or protein abundance of some electron transport chain proteins and glycolytic and oxidative enzymes (39, 47), skeletal muscle-specific overexpression of SIRT1 did not induce functional changes in mitochondrial respiration (36), time to fatigue during treadmill running, or ex vivo fatigability in response to repeated electrical stimulation (37). In line with this, and our previous work in the EDL of the mKO mouse (23), we found that maximal tetanic tension and fatigability of both the soleus and EDL were not impacted in mKO mice, regardless of sex. Thus, when combined with other work from our laboratory (36, 37, 39, 48) and others (49), it is clear that SIRT1 is not a major regulator of skeletal muscle contractile function or fatigability.
To our knowledge, this is the first study to demonstrate a sex-based difference in contraction-stimulated glucose uptake. Specifically, we found that in the contracted muscle (but not rested), glucose uptake was ∼40% higher in females versus males in both the EDL and soleus. Considering the well-known difference in myosin heavy chain composition (i.e., fiber type) between the soleus and EDL of mice (50), and the fact that we see this difference with ex vivo muscle stimulation (i.e., independent of sex hormones or other humoral factors), this suggests that “intrinsic” factor(s) underlie this sex-based difference. Although they did not compare male versus female, providing support for this finding, Campbell and Febbraio (51) found that contraction-stimulated glucose uptake by skeletal muscle during treadmill running was significantly lower (∼50% reduction in red- and ∼30% reduction in white quadricep) in estrogen-deficient female rats as compared with the female controls. Notably, a study by Kim and colleagues (52) investigated sex differences in swimming-induced glucose uptake in soleus and EDL of mice at 20, 75, and 200 min after completing exercise. While basal glucose uptake was higher at all time points in the soleus and EDL of females versus males, in contrast to treadmill exercise (which was only performed in male mice), swimming did not increase (insulin-independent) glucose uptake 20 min after exercise; as a result, sex-based differences in contraction-stimulated glucose uptake were inconclusive from this study. Interestingly, the sex-based difference in contraction-stimulated glucose uptake that we found is in line with numerous studies in rodent and human skeletal muscle demonstrating that insulin-stimulated glucose uptake and insulin sensitivity in skeletal muscle is higher in females compared with the males (53–55). It is well-known that there are proximally distinct and distally common (i.e., convergence) points of control in the mechanics of GLUT4 translocation to the plasma membrane in response to insulin and contraction (35, 56); the data presented in this study considered together with insulin literature suggest that sex influences a signaling step or mechanism common to both contraction- or insulin-stimulated glucose uptake pathways.
Skeletal muscle glucose uptake has three primary points of control: delivery, transport, and metabolism of glucose in the cell (35, 57). Because we used an ex vivo contraction approach, delivery is controlled, and as such, in our model the steps at which glucose uptake might be differently regulated between female and male mice likely are at transport, which is regulated by GLUT4 (35, 58), and/or intracellular glucose metabolism, which is primarily (at least initially) regulated by glucose phosphorylation by HKII. While we did not assess GLUT4 abundance in this study, previous studies demonstrate that skeletal muscle GLUT4 protein abundance is not different in male versus female skeletal muscle (59, 60) or in ovariectomized rats (51); this suggests that differences in GLUT4 protein abundance do not underlie the effects we see on contraction-stimulated glucose uptake. Alternatively, Høeg et al. (61) demonstrated that HKII protein abundance is 56% higher in women compared with men. However, we found no sex difference in HKII abundance in this study, although it is possible that HKII activity or localization is differentially modulated. It should be noted that glucose uptake is closely related to the abundance of GLUT4 at the plasma membrane and not necessarily the total abundance of GLUT4. As such, it is possible that in female muscle the dynamics of GLUT4 retention/release, translocation, docking, and fusion are differentially regulated compared with males (independent of GLUT4 abundance), and it will be interesting in future work to dissect these potential points of control. For example, given their well-described contributions to glucose metabolism in skeletal muscle, candidate points of control for sex-based differences could include TBC1D1 (tre-2/USP6, BUB2, cdc16 domain family 1) (9, 62) or Rac1 (ras-related C3 botulinum toxin substrate 1), which plays an important role in regulating both contraction- and insulin-stimulated glucose uptake in skeletal muscle (63–65).
It should be noted that the contraction protocol that we used was only 10 min in duration and 40 total contractions. While this protocol clearly fatigued the soleus and EDL, it is possible that SIRT1 is important for contraction-stimulated glucose uptake during longer duration exercise (i.e., exercise that is hours in duration, rather than minutes). Moreover, we only measured contraction-stimulated glucose uptake using an ex vivo set-up. While this approach is commonly used in the field because it allows tight control of the surrounding environment and the contractile stimulus (i.e., number and strength of contractions), it is possible that SIRT1 is important to exercise-stimulated glucose uptake, in vivo; to this point, differences in 2DOGU in response to in vivo versus ex vivo contraction have been noted in other mouse models (66). Finally, SIRT1 is 1 of 18 known deacetylases in mammalian cells (67), so while we found that loss of SIRT1 or pan SIRT inhibition did not impact contraction-stimulated glucose uptake or AMPK-mediated GLUT4 translocation to the plasma membrane, respectively, it will be interesting to determine whether other specific deacetylases or the histone deacetylase class of deacetylases contributes to contraction-stimulated glucose uptake, as has been done with insulin-stimulated glucose uptake (40).
In conclusion, we investigated whether muscle-specific knockout of SIRT1 deacetylase activity reduces contraction-stimulated glucose uptake. Our results demonstrate SIRT1 deacetylase function is not required for contraction-mediated glucose uptake in adult mouse skeletal muscle. Interestingly, similar to findings related to insulin action, we did find sex differences in contraction-stimulated glucose uptake, such that glucose uptake was ∼40% higher in female versus male, regardless of muscle type; to our knowledge, this is the first study to describe sex-based differences in contraction-stimulated glucose by skeletal muscle. The goal of future work will be to identify the molecular mechanisms that underlie this sex-based difference in contraction-stimulated glucose uptake.
GRANTS
This work was supported, in part, by National Institutes of Health Grants R01-AG-043120 and R21-AR-069775.
DISCLOSURES
No conflicts of interest, financial or otherwise are reported by the authors.
AUTHOR CONTRIBUTIONS
J.K., S.N.B., and S.S. conceived and designed research; J.K., S.W.M., J.R.D., and S.S. performed experiments; J.K., J.E.P., J.D., S.W.M., and J.R.D. analyzed data; J.K., S.W.M., T.L.M., S.N.B., and S.S. interpreted results of experiments; J.K., J.E.P., J.D., and S.W.M. prepared figures; J.K. drafted manuscript; J.K., J.E.P., S.W.M., T.L.M., S.N.B., J.R.D. and S.S. edited and revised manuscript; J.K., J.E.P., J.D., S.W.M., T.L.M., S.N.B., J.R.D., and S.S. approved final version of manuscript.
REFERENCES
- 1.Ivy JL, Zderic TW, Fogt DL. Prevention and treatment of non-insulin-dependent diabetes mellitus. Exerc Sport Sci Rev 27: 1–35, 1999. doi: 10.1249/00003677-199900270-00002. [DOI] [PubMed] [Google Scholar]
- 2.Stanford KI, Goodyear LJ. Exercise and type 2 diabetes: Molecular mechanisms regulating glucose uptake in skeletal muscle. Adv Physiol Educ 38: 308–314, 2014. doi: 10.1152/advan.00080.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holloszy JO, Narahara HT. Studies of tissue permeability. J Biol Chem 240, 3493–3500, 1965. [PubMed] [Google Scholar]
- 4.Jørgensen SB, Richter EA, Wojtaszewski JF. Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. J Physiol 574: 17–31, 2006. doi: 10.1113/jphysiol.2006.109942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Neill HM. AMPK and exercise: glucose uptake and insulin sensitivity. Diabetes Metab J 37: 1–21, 2013. doi: 10.4093/dmj.2013.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birk JB, Wojtaszewski JF. Predominant α2/β2/γ3 AMPK activation during exercise in human skeletal muscle. J Physiol 577: 1021–1032, 2006. doi: 10.1113/jphysiol.2006.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richter EA, Nielsen JN, Jørgensen SB, Frøsig C, Birk JB, Wojtaszewski JF. Exercise signalling to glucose transport in skeletal muscle. Proc Nutr Soc 63: 211–216, 2004. doi: 10.1079/pns2004343.doi:. [DOI] [PubMed] [Google Scholar]
- 8.Fujii N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM, Goodyear LJ. AMP-activated protein kinase α2 activity is not essential for contraction-and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem 280: 39033–39041, 2005. doi: 10.1074/jbc.M504208200. [DOI] [PubMed] [Google Scholar]
- 9.Kjøbsted R, Roll JLW, Jørgensen NO, Birk JB, Foretz M, Viollet B, Chadt A, Al-Hasani H, Wojtaszewski JF. AMPK and TBC1D1 regulate muscle glucose uptake after, but not during, exercise and contraction. Diabetes 68: 1427–1440, 2019. doi: 10.2337/db19-0050. [DOI] [PubMed] [Google Scholar]
- 10.McConell GK. It’s well and truly time to stop stating that AMPK regulates glucose uptake and fat oxidation during exercise. Am J Physiol Endocrinol Metab 318: E564–E567, 2020. doi: 10.1152/ajpendo.00511.2019. [DOI] [PubMed] [Google Scholar]
- 11.Hingst JR, Kjøbsted R, Birk JB, Jørgensen NO, Larsen MR, Kido K, Larsen JK, Kjeldsen SAS, Fentz J, Frøsig C, Holm S, Fritzen AM, Dohlmann TL, Larsen S, Foretz M, Viollet B, Schjerling P, Overby P, Halling JF, Pilegaard H, Hellsten Y, Wojtaszewski JF. Inducible deletion of skeletal muscle AMPKα reveals that AMPK is required for nucleotide balance but dispensable for muscle glucose uptake and fat oxidation during exercise. Mol Metab 40: 101028, 2020. doi: 10.1016/j.molmet.2020.101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: the “magnificent seven,” function, metabolism and longevity. Ann Med 39: 335–345, 2007. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- 13.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol Mech Dis 5: 253–295, 2010. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White AT, Schenk S. NAD+/NADH and skeletal muscle mitochondrial adaptations to exercise. Am J Physiol Endocrinol Metab 303: 308–321, 2012. doi: 10.1152/ajpendo.00054.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantó C, Auwerx J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 20: 98–105, 2009. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantó C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab 11: 213–219, 2010. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458: 1056–1060, 2009. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1: possible role in AMP-activated protein kinase activation. J Biol Chem 283: 27628–27635, 2008. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suchankova G, Nelson LE, Gerhart-Hines Z, Kelly M, Gauthier MS, Saha AK, Ido Y, Puigserver P, Ruderman NB. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochem Biophys Res Commun 378: 836–841, 2009. doi: 10.1016/j.bbrc.2008.11.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suwa M, Nakano H, Radak Z, Kumagai S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor γ coactivator-1α protein expressions in rat skeletal muscle. Metabolism 57: 986–998, 2008. doi: 10.1016/j.metabol.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Dumke CL, Davis JM, Murphy EA, Nieman DC, Carmichael MD, Quindry JC, Triplett NT, Utter AC, Gross Gowin SJ, Henson DA, McAnulty SR, McAnulty LS. Successive bouts of cycling stimulates genes associated with mitochondrial biogenesis. Eur J Appl Physiol 107: 419–427, 2009. doi: 10.1007/s00421-009-1143-1. [DOI] [PubMed] [Google Scholar]
- 22.Guerra B, Guadalupe-Grau A, Fuentes T, Ponce-González JG, Morales-Alamo D, Olmedillas H, Guillén-Salgado J, Santana A, Calbet JA. SIRT1, AMP-activated protein kinase phosphorylation and downstream kinases in response to a single bout of sprint exercise: Influence of glucose ingestion. Eur J Appl Physiol 109: 731–743, 2010. doi: 10.1007/s00421-010-1413-y. [DOI] [PubMed] [Google Scholar]
- 23.Philp A, Chen A, Lan D, Meyer GA, Murphy AN, Knapp AE, Olfert IM, McCurdy CE, Marcotte GR, Hogan MC, Baar K, Schenk S. Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) deacetylation following endurance exercise. J Biol Chem 286: 30561–30570, 2011. doi: 10.1074/jbc.M111.26168.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schenk S, Mccurdy CE, Philp A, Chen MZ, Holliday MJ, Bandyopadhyay GK, Osborn O, Baar K, Olefsky JM. Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J Clin Invest 121: 4281–4288, 2011. doi: 10.1172/JCI58554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A 100: 10794–10799, 2003. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maganaris CN, Baltzopoulos V, Ball D, Sargeant AJ. In vivo specific tension of human skeletal muscle. J Appl Physiol (1985) 90: 865–872, 2001. doi: 10.1152/jappl.2001.90.3.865. [DOI] [PubMed] [Google Scholar]
- 27.Masson SWC, Sorrenson B, Shepherd PR, Merry TL. β-catenin regulates muscle glucose transport via actin remodelling and M-cadherin binding. Mol Metab 42: 101091, 2020. doi: 10.1016/j.molmet.2020.101091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Khayat Z, Kishi K, Ebina Y, Klip A. GLUT4 translocation by insulin in intact muscle cells: detection by a fast and quantitative assay. FEBS Lett 427: 193–197, 1998. doi: 10.1016/S0014-5793(98)00423-2. [DOI] [PubMed] [Google Scholar]
- 29.Romero-Calvo I, Ocón B, Martínez-Moya P, Suárez MD, Zarzuelo A, Martínez-Augustin O, de Medina FS. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem 401: 318–320, 2010. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 30.Thacker JS, Yeung DH, Staines WR, Mielke JG. Total protein or high-abundance protein: Which offers the best loading control for Western blotting? Anal Biochem 496: 76–78, 2016. doi: 10.1016/j.ab.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 31.Yu J, Auwerx J. Protein deacetylation by SIRT1: an emerging key post-translational modification in metabolic regulation. Pharmacol Res 62: 35–41, 2010. doi: 10.1016/j.phrs.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurd BJ. Deacetylation of PGC-1a by SIRT1: Importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl Physiol Nutr Metab 36: 589–597, 2011. doi: 10.1139/h11-070. [DOI] [PubMed] [Google Scholar]
- 33.Holloszy JO. A forty-year memoir of research on the regulation of glucose transport into muscle. Am J Physiol Endocrinol Metab 284: E453–E67, 2003. doi: 10.1152/ajpendo.00463.2002. [DOI] [PubMed] [Google Scholar]
- 34.Lund S, Holman GD, Schmitz O, Pedersen O. Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci U S A 92: 5817–5821, 1995. doi: 10.1073/pnas.92.13.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 93: 993–1017, 2013. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 36.Svensson K, LaBarge SA, Martins VF, Schenk S. Temporal overexpression of SIRT1 in skeletal muscle of adult mice does not improve insulin sensitivity or markers of mitochondrial biogenesis. Acta Physiol (Oxf) 221: 193–203, 2017. doi: 10.1111/apha.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svensson K, Tahvilian S, Martins VF, Dent JR, Lemanek A, Barooni N, Greyslak K, McCurdy CE, Schenk S. Combined overexpression of SIRT1 and knockout of GCN5 in adult skeletal muscle does not affect glucose homeostasis or exercise performance in mice. Am J Physiol Endocrinol Metab 318: E145–E151, 2020. doi: 10.1152/ajpendo.00370.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White AT, McCurdy CE, Philp A, Hamilton DL, Johnson CD, Schenk S. Skeletal muscle-specific overexpression of SIRT1 does not enhance whole-body energy expenditure or insulin sensitivity in young mice. Diabetologia 56: 1629–1637, 2013. doi: 10.1007/s00125-013-2912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White AT, Philp A, Fridolfsson HN, Schilling JM, Murphy AN, Hamilton DL, McCurdy CE, Patel HH, Schenk S. High-fat diet-induced impairment of skeletal muscle insulin sensitivity is not prevented by SIRT1 overexpression. Am J Physiol Endocrinol Metab 307: E764–E772, 2014. doi: 10.1152/ajpendo.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martins VF, Begur M, Lakkaraju S, Svensson K, Park J, Hetrick B, McCurdy CE, Schenk S. Acute inhibition of protein deacetylases does not impact skeletal muscle insulin action. Am J Physiol Cell Physiol 317: C964–C968, 2019. doi: 10.1152/ajpcell.00159.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pardo PS, Boriek AM. The physiological roles of Sirt1 in skeletal muscle. Aging (Albany NY) 3: 430–437, 2011. doi: 10.18632/aging.100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett 582: 46–53, 2008. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai P, Cantó C, Oudart H, Brunyánszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 13: 461–468, 2011. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 15: 838–847, 2012. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cerutti R, Pirinen E, Lamperti C, Marchet S, Sauve AA, Li W, Leoni V, Schon EA, Dantzer F, Auwerx J, Viscomi C, Zeviani M. NAD+-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab 19: 1042–1049, 2014. doi: 10.1016/j.cmet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banks AS, Kon N, Knight C, Matsumoto M, Gutiérrez-Juárez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab 8: 333–341, 2008. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price NL, Gomes AP, Ling AJY, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15: 675–690, 2012. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svensson K, LaBarge SA, Sathe A, Martins VF, Tahvilian S, Cunliffe JM, Sasik R, Mahata SK, Meyer GA, Philp A, David LL, Ward SR, McCurdy CE, Aslan JE, Schenk S. p300 and cAMP response element-binding protein-binding protein in skeletal muscle homeostasis, contractile function, and survival. J Cachexia Sarcopenia Muscle 11: 464–477, 2020. doi: 10.1002/jcsm.12522.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myers MJ, Shepherd DL, Durr AJ, Stanton DS, Mohamed JS, Hollander JM, Alway SE. The role of SIRT1 in skeletal muscle function and repair of older mice. J Cachexia Sarcopenia Muscle 10: 929–949, 2019. doi: 10.1002/jcsm.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol 221: 177–190, 1994. doi: 10.1002/jmor.1052210207. [DOI] [PubMed] [Google Scholar]
- 51.Campbell SE, Febbraio MA. Effect of the ovarian hormones on GLUT4 expression and contraction-stimulated glucose uptake. Am J Physiol Endocrinol Metab 282: E1139–E1146, 2002. doi: 10.1152/ajpendo.00184.2001. [DOI] [PubMed] [Google Scholar]
- 52.Kim J, Arias EB, Cartee GD. Effects of gender and prior swim exercise on glucose uptake in isolated skeletal muscles from mice. J Physiol Sci 56: 305–312, 2006. doi: 10.2170/physiolsci.RP009406. [DOI] [PubMed] [Google Scholar]
- 53.Hevener A, Reichart D, Janez A, Olefsky J. Female rats do not exhibit free fatty acid induced insulin resistance. Diabetes 51: 1907–1912, 2002. doi: 10.2337/diabetes.51.6.1907. [DOI] [PubMed] [Google Scholar]
- 54.Lundsgaard A-M, Kiens B. Gender differences in skeletal muscle substrate metabolism - molecular mechanisms and insulin sensitivity. Front Endocrinol (Lausanne) 5: 195, 2014. doi: 10.3389/fendo.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rattanavichit Y, Chukijrungroat N, Saengsirisuwan V. Sex differences in the metabolic dysfunction and insulin resistance of skeletal muscle glucose transport following high fructose ingestion. Am J Physiol Regul Integr Comp Physiol 311: R1200–R1212, 2016. doi: 10.1152/ajpregu.00230.2016. [DOI] [PubMed] [Google Scholar]
- 56.Lauritzen HPMM, Galbo H, Toyoda T, Goodyear LJ. Kinetics of contraction-induced GLUT4 translocation in skeletal muscle fibers from living mice. Diabetes 59: 2134–2144, 2010. doi: 10.2337/db10-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wasserman DH. Four grams of glucose. Am J Physiol Endocrinol Metab 296: E11–E21, 2009. doi: 10.1152/ajpendo.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klip A, McGraw TE, James DE. Thirty sweet years of GLUT4. J Biol Chem 294: 11369–11381, 2019. doi: 10.1074/jbc.REV119.008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Høeg L, Roepstorff C, Thiele M, Richter EA, Wojtaszewski JF, Kiens B. Higher intramuscular triacylglycerol in women does not impair insulin sensitivity and proximal insulin signaling. J Appl Physiol 107: 824–831, 2009. doi: 10.1152/japplphysiol.91382.2008. [DOI] [PubMed] [Google Scholar]
- 60.Houmard JA, Weidner MD, Dolan PL, Leggett-Frazier N, Gavigan KE, Hickey MS, Tyndall GL, Zheng D, Alshami A, Dohm GL. Skeletal muscle GLUT4 protein concentration and aging in humans. Diabetes 44: 555–560, 1995. doi: 10.2337/diabetes.44.5.555. [DOI] [PubMed] [Google Scholar]
- 61.Høeg LD, Sjøberg KA, Jeppesen J, Jensen TE, Frøsig C, Birk JB, Bisiani B, Hiscock N, Pilegaard H, Wojtaszewski JF, Richter EA, Kiens B. Lipid-induced insulin resistance affects women less than men and is not accompanied by inflammation or impaired proximal insulin signaling. Diabetes 60: 64–73, 2011. doi: 10.2337/db10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cartee GD. Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle. Diabetologia 58: 19–30, 2015. doi: 10.1007/s00125-014-3395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sylow L, Jensen TE, Kleinert M, Højlund K, Kiens B, Wojtaszewski J, Prats C, Schjerling P, Richter EA. Rac1 signaling is required for insulin-stimulated glucose uptake and is dysregulated in insulin-resistant murine and human skeletal muscle. Diabetes 62: 1865–1875, 2013. doi: 10.2337/db12-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sylow L, Jensen TE, Kleinert M, Mouatt JR, Maarbjerg SJ, Jeppesen J, Prats C, Chiu TT, Boguslavsky S, Klip A, Schjerling P, Richter EA. Rac1 is a novel regulator of contraction-stimulated glucose uptake in skeletal muscle. Diabetes 62: 1139–1151, 2013. doi: 10.2337/db12-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sylow L, Nielsen IL, Kleinert M, Møller LLV, Ploug T, Schjerling P, Bilan PJ, Klip A, Jensen TE, Richter EA. Rac1 governs exercise-stimulated glucose uptake in skeletal muscle through regulation of GLUT4 translocation in mice. J Physiol 594: 4997–5008, 2016. doi: 10.1113/JP272039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sylow L, Møller LLV, Kleinert M, D'Hulst G, De Groote E, Schjerling P, Steinberg GR, Jensen TE, Richter EA. Rac1 and AMPK account for the majority of muscle glucose uptake stimulated by ex vivo contraction but not in vivo exercise. Diabetes 66: 1548–1559, 2017. doi: 10.2337/db16-1138. [DOI] [PubMed] [Google Scholar]
- 67.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10: 32–42, 2009. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]