Abstract
Herein we report in a sample of healthy young men (n = 14) and women (n = 12) that hyperinsulinemia induces time-dependent decreases in total peripheral resistance and its contribution to the maintenance of blood pressure. In the same participants, we observe profound vasodilatory effects of insulin in the lower limb despite concomitant activation of the sympathetic nervous system. We hypothesized that this prominent peripheral vasodilation is possibly due to the ability of the leg vasculature to escape sympathetic vasoconstriction during systemic insulin stimulation. Consistent with this notion, we demonstrate in a subset of healthy men (n = 9) and women (n = 7) that systemic infusion of insulin blunts sympathetically mediated leg vasoconstriction evoked by a cold pressor test, a well-established sympathoexcitatory stimulus. Further substantiating this observation, we show in mouse aortic rings that insulin exposure suppresses epinephrine and norepinephrine-induced vasoconstriction. Notably, we found that such insulin-suppressing effects on catecholamine-induced constriction are diminished following β-adrenergic receptor blockade. In accordance, we also reveal that insulin augments β-adrenergic-mediated vasorelaxation in isolated arteries. Collectively, these findings support the idea that sympathetic vasoconstriction can be attenuated during systemic hyperinsulinemia in the leg vasculature of both men and women and that this phenomenon may be in part mediated by potentiation of β-adrenergic vasodilation neutralizing α-adrenergic vasoconstriction.
Keywords: autonomic nervous system, blood flow, insulin, muscle sympathetic nerve activity
INTRODUCTION
Insulin, in addition to its role on cellular glucose uptake, has vasodilatory effects that increase delivery of insulin and glucose to tissues such as skeletal muscle (1–14). In the context of insulin-mediated peripheral vasodilation, counter-regulatory mechanisms are required to maintain blood pressure and one such key mechanism is sympathetic activation. Indeed, insulin has centrally mediated effects that activate the sympathetic nervous system (15–21), thus limiting insulin-induced vasodilation via α-adrenergic vasoconstriction. In support of this, patients with regional sympathectomy display a more rapid vasodilation in response to insulin in the denervated limb than in the innervated limb (22). However, support for this notion is less ostensible during systemic measures in young healthy and lean individuals (8, 23). For example, we recently used neck suction as a maneuver to acutely reduce efferent sympathetic nerve activity (SNA) during systemic hyperinsulinemia and saw no effect on insulin-mediated vasodilation in the leg (8), thereby not providing evidence for ongoing sympathetic restraint in a large vascular territory.
Although it remains perplexing that skeletal muscle blood flow responses to insulin are not under a substantial SNA-mediated vasoconstrictor constraint, it is conceivable that, in healthy individuals, this is attributed to the sympatholytic effects of insulin (24–29). The concept that insulin signaling can “lyse” α-adrenergic vasoconstriction is well exemplified in experiments using the isolated human forearm model (25, 27) as well as in isolated arteries from rodents. In this regard, we reported in mouse aortas that vascular exposure to insulin suppresses the vasoconstrictor response to phenylephrine, a synthetic α1-adrenergic receptor agonist, and that this buffering effect of insulin is endothelium and nitric oxide dependent (8). In vivo, another plausible mechanism contributing to the insulin-induced blunting of sympathetic vasoconstriction could be related to the nonspecific binding of catecholamines to α- and β-adrenergic receptors. In this scenario, in addition to blunting α-adrenergic contractility, insulin may counteract constriction by potentiating the vasodilatory effects of β-adrenergic receptor activation. The existence of a cross talk between insulin signaling and β-adrenergic receptor activation is supported by the literature, particularly in the heart (30–35); however, more evidence is necessary to support this concept in vascular beds.
In this report, we first sought to document the magnitude and temporal changes in peripheral vasodilation and total peripheral resistance (TPR) in response to a systemic infusion of insulin in a robust cohort of young healthy men and women. Notably, prior work in this area has included men only (15, 24, 25), thereby preventing the examination of potential sex-related differences. Herein, we report a sex-independent fall in TPR and its contribution to the maintenance of blood pressure during hyperinsulinemia, in spite of an increase in SNA. This finding can be explained by the direct dilatory effects of insulin in the leg vasculature, but it is also mutually congruent with the possibility that insulin lessens sympathetic vasoconstriction, as abovementioned. To address the latter, we next examined in a subset of participants the extent to which hyperinsulinemia can blunt SNA-mediated peripheral vasoconstriction in the leg using a robust sympathoexcitatory stimulus. Specifically, we hypothesized that marked increases in muscle SNA in response to a cold pressor test would produce less vasoconstriction during hyperinsulinemia when compared with basal (nonhyperinsulinemic) conditions. Relevant to the present design is the examination of blood flow at the femoral artery during systemic insulin infusion, due to the importance of the lower limb circulation in the overall maintenance of blood pressure. Lastly, experiments were performed in isolated mouse arteries to gain a better understanding of the interactive effects of insulin and catecholamines in modulating vascular reactivity. We posited that insulin exposure would attenuate epinephrine and norepinephrine-induced vasoconstriction and any insulin-suppressing effects on catecholamine-induced constriction would be mitigated following β-adrenergic receptor blockade. Likewise, we hypothesized that insulin would potentiate β-adrenergic- (and particularly β2-adrenergic) mediated vasorelaxation.
METHODS
Human Participants
All participants were young adults (<40 yr of age), healthy, nonobese (BMI <30 kg/m2), normoglycemic (fasting glucose <100 mg/dL), and nonsmokers without chronic diseases and taking no medications known to affect endocrine, cardiovascular, or autonomic function. Participants (n = 26; 14 men, 12 women) identified as the following: Caucasian/non-Hispanic (n = 18, 69%), Caucasian/Hispanic (n = 4, 15%), Asian (n = 2, 8%), Black (n = 1, 4%), and other (n = 1, 4%). All women were premenopausal and had a confirmed negative pregnancy test on the morning of the study visit. Menstruating women were studied in the self-reported early follicular phase of the menstrual cycle (days 1–7, n = 5) or placebo phase of oral contraceptive use (n = 7). All participants were asked to refrain from alcohol, caffeine, and exercise for 24 h and fast for 12 h before the study visit following recently published guidelines (36). Written informed consent was obtained from all participants and all experiments and procedures were approved by the Institutional Review Board at the University of Missouri (protocols #2013126 and #2020286) and conformed to the Declaration of Helsinki. It should be noted that data from a subset of participants used in protocol 1 were also used in a previous publication (8).
Experimental Protocol 1 in Humans: Contribution of TPR to Mean Arterial Blood Pressure in the Setting of Systemic Hyperinsulinemia
The purpose of this protocol was to characterize the magnitude and temporal changes in leg vascular conductance and TPR in response to a systemic infusion of insulin in a cohort of young healthy men and women and, accordingly, calculate the percentage contribution of TPR to insulin-mediated changes in blood pressure. On the study day, participants were admitted to the Clinical Research Center at the University of Missouri between 0800 and 0830. Participants rested supine during instrumentation which included a 3-lead electrocardiogram (Bio Amp FE132, ADInstruments) and noninvasive beat-to-beat blood pressure via finger photoplethysmography (Finapres, Finapres Medical Systems) calibrated to upper arm blood pressure (automated sphygmomanometry). Muscle SNA was measured in a subset of participants (n = 4) using the technique of microneurography (37, 38). Briefly, a tungsten microelectrode was placed percutaneously into the peroneal nerve, posterior to the fibular head and the recorded signal was amplified, band-pass filtered (700–2,000 Hz), rectified and integrated (time constant 0.1 s). Data were analyzed using a semiautomated program (Ensemble-R, Elucimed LTD) and results are expressed as burst incidence (bursts/100 heartbeats) and burst frequency (bursts/min). Femoral artery diameter and blood velocity were measured using Doppler ultrasound as previously described (8). Briefly, an 11-MHz linear array transducer was placed over the superficial femoral artery. Simultaneous diameter and velocity signals were obtained in duplex mode at a pulsed frequency of 3.5–5 MHz and corrected with an insonation angle of 60°. Sample volume was adjusted to encompass the entire lumen of the vessel without extending beyond the walls and the cursor was set mid-vessel.
An intravenous catheter was placed in the antecubital vein of both arms, one for insulin and glucose infusion and the other for periodic blood sampling. Fasting measures of blood glucose and plasma insulin were used to calculate HOMA-IR (Table 1). After a baseline period, two priming infusion rates of insulin (Humulin R U-100) over 10 min were followed by a steady infusion rate of 40 mU/m2 body surface area/min for 60 min during which time cardiovascular measurements were obtained for an average of 4 min every 15 min and compared to baseline measures (See protocol timeline, Fig. 1A). The priming dose of insulin, introduced by DeFronzo (39), is designed to suppress hepatic glucose production. During insulin infusion, blood glucose was determined every ∼5 min at bedside (YSI 2300 STAT PLUS glucose analyzer) and maintained at baseline levels by infusing a dextrose solution at a variable rate. Plasma was also obtained and stored at −80°C for analysis of insulin, which was assessed using a commercially available kit (ALPCO Cat. No. 80-INSHU-E10.1, Salem, NH).
Table 1.
Participant demographics
| Protocol 1 | Protocol 2 | |
|---|---|---|
| Sex (M, W) | 14,12 | 9,7 |
| Age, yr | 27 ± 1 | 26 ± 1 |
| Height, cm | 173 ± 2 | 174 ± 3 |
| Weight, kg | 72 ± 2 | 72 ± 3 |
| Body mass index, kg/m2 | 24.0 ± 0.4 | 23.6 ± 0.5 |
| Body surface area, m2 | 1.9 ± 0.1 | 1.9 ± 0.1 |
| Fasting blood glucose, mg/dL | 75 ± 1 | 76 ± 2 |
| Fasting plasma insulin, µIU/mL | 5.2 ± 0.4 | 5.3 ± 0.5 |
| HOMA-IR | 1.0 ± 0.1 | 1.0 ± 0.1 |
| Systolic blood pressure, mmHg | 115 ± 2 | 114 ± 2 |
| Diastolic blood pressure, mmHg | 72 ± 2 | 71 ± 2 |
Data are presented as means ± SE. M, men; W, women.
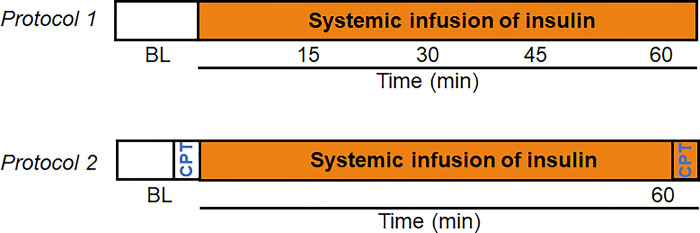
Figure 1.
Protocol timelines. In protocol 1, measures were collected at baseline (BL) and every 15 min for 60 min during systemic insulin infusion. In protocol 2, a change in femoral vascular conductance in response to a cold pressor test (CPT) was determined at BL and at 60 min of systemic insulin infusion.
Experimental Protocol 2 in Humans: Role of Hyperinsulinemia in Mitigating Sympathetic Vasoconstriction in the Leg
The purpose of this protocol was to examine the extent to which hyperinsulinemia can blunt SNA-mediated vasoconstriction in the leg using a robust sympathoexciatory stimulus (i.e., cold pressor test) in young healthy men and women. Participants were familiarized with the cold pressor test before initiation of the study and were instrumented as described in protocol 1. After a period of quiet rest and baseline cardiovascular measurements, a cold pressor test was initiated. During the cold pressor test, the participant’s hand was immersed (up to the wrist) in an ice-water bath maintained at 0°C–4°C. Following the baseline cold pressor test, insulin infusion began as described in protocol 1. Approximately 60 min after the start of the insulin infusion, the cold pressor test was repeated (Fig. 1B). Ratings of perceived cold and pain were obtained at the end of each cold pressor test using modified Borg scales (40). Importantly, the level of sympathetic nervous system activation in response to the cold pressor test is reliably repeatable within the study visit (41).
Analysis
Data were collected using PowerLab data acquisition system (analog to digital converter; ADInstruments, Inc.) with a sampling rate of 10,000 Hz for muscle SNA and 1,000 Hz for all other variables. Data are reported as an average across a minimum 4-min quiet resting period. Stroke volume was estimated from the arterial blood pressure waveform using the Modelflow method through LabChart (LabChart, ADInstruments), which incorporates age and sex. Cardiac output and TPR were calculated. In protocol 1, the percentage contributions of cardiac output and TPR to insulin-mediated changes in mean arterial pressure were also calculated. This was achieved by calculating the predicted change in mean arterial pressure during insulin infusion, if only the individual changes in cardiac output (MAPCO = COT15 × TPRT0; % Predicted = MAPCO ÷ MAPT15 × 100) or TPR (MAPTPR = COT0 × TPRT15; % Predicted = MAPTPR ÷ MAPT15 × 100) occurred and all other parameters remained at basal levels (42). TPRT0 is the TPR value before hyperinsulinemia and COT0 is the cardiac output value before hyperinsulinemia. In this example analysis (from min 15, T15), the predicted MAP (MAPCO) is the MAP response to 15 min of insulin infusion due to changes in cardiac output (CO T15) alone. Similarly, MAPTPR is the MAP response to 15 min of insulin infusion due to changes in TPR (TPRT15) alone (42).
Ultrasound recordings were analyzed offline using specialized edge-detection software (Cardiovascular Suite, Quipu srl, Pisa, Italy). Femoral artery blood flow was calculated from continuous diameter and mean blood velocity recordings using the following equation: 3.14 × [diameter (cm)/2]2 × mean blood velocity (cm/s) × 60 and reported as mL/min. Femoral vascular conductance (FVC) was calculated as femoral artery blood flow (mL/min)/mean arterial blood pressure (mmHg) × 100 and reported as mL/min/100 mmHg. In protocol 2, the peak reduction in FVC in response to the cold pressor test was determined using a 5-s moving average, and data are reported as a relative change from the period immediately before the cold pressor test (i.e., %FVC = ΔFVC/FVCPre CPT × 100). The increase in MSNA during the cold pressor test was analyzed as a 1-min average and data are reported as a relative change from the period immediately before the cold pressor test (i.e., %MSNA = ΔMSNA/MSNAPre CPT × 100).
Experimental Protocol 3 in Isolated Mouse Arteries: Insulin-Induced Suppression of Catecholamine-Induced Vasoconstriction and Role of β-Adrenergic Receptors
On the basis of findings from protocols 1 and 2 in humans, we designed a follow-up experiment in isolated arteries from mice to test the following hypotheses: 1) insulin can suppress norepinephrine and epinephrine-induced vasoconstriction; 2) the insulin suppressing effects are mitigated following β-adrenergic receptor blockade, and 3) activation of β-adrenergic receptors and consequent vasorelaxation is augmented by insulin. All animal study procedures received prior approval by the University of Missouri Institutional Animal Care and Use Committee. The University of Missouri is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. For this experiment, aortic rings were used from 93 C57BL/6NJ male mice (24.6 ± 0.7 g of body wt; 11–13 wk of age). Aortas were harvested and cleaned of perivascular adipose tissue in ice-cold physiological saline solution (pH 7.40) and cut into four 2-mm segments. The mouse aorta was selected as the model of choice because multiple adjacent arterial rings from the same artery can be obtained and exposed to different experimental conditions. Accordingly, comparisons are made within the artery, limiting the inherent between-artery and between-animal variability.
Aortic rings were mounted in wire myograph organ bath chambers (620 M, Danish Myo Technology, Hinnerup, Denmark) containing warmed physiological saline solution gassed with 95% O2–5% CO2 and maintained at 37°C as previously described (43, 44). Aortic rings were treated with 80 mM KCl to ensure viability. Next, aortas were incubated with or without insulin (100 nM) for 30 min before stimulation with increasing concentrations of norepinephrine (10−10–10−5 M) or epinephrine (10−10–10−5 M) while insulin (or lack thereof) remained in the bath. To test the interactive effects of insulin and β-adrenergic activation in modulating norepinephrine and epinephrine-induced vasoconstriction, experiments were performed in aortic rings treated with propranolol (600 nM), an FDA-approved β-adrenergic receptor inhibitor. The role of insulin in potentiating β-adrenergic vasorelaxation was examined in preconstricted aortic rings stimulated with increasing concentrations of isoproterenol (10−9–10−4 M, a nonspecific β-adrenergic receptor agonist) and salbutamol (10−8–10−4 M, a β2-adrenergic receptor agonist). Preconstriction was induced with phenylephrine (10−7 M). Under all these experimental conditions, the role of nitric oxide was examined by treating aortic rings with l-NAME (300 µM), a nitric oxide synthase inhibitor, whereas the role of the endothelium was examined by endothelial denudation. Aortic rings were mechanically denuded by gently rubbing the intima of the blood vessel against the myograph pins. This model of endothelial denudation was confirmed by the lack of relaxation response to acetylcholine (10−5 M). Vasoconstriction responses to norepinephrine and epinephrine were normalized to KCl-induced constriction. The magnitude of the vasodilator and vasoconstrictor responses were determined by calculating the area under the curve (AUC) for each concentration-response curve using the trapezoidal rule.
Statistical Analysis
Using previously published results (42, 45), we conducted an a priori power analyses in which we anticipated complete data from n = 21 and n = 10 would be sufficient to provide 80% power to detect differences in main outcome variables at α = 0.05 for protocols 1 and 2, respectively. In protocols 1 and 2, the effect of insulin (i.e., baseline vs. hyperinsulinemia) on main outcome variables was assessed using a one-way repeated measures analysis of variance (ANOVA), and pairwise multiple comparisons were made using the Holm–Sidak method. Normality was assessed with the Shapiro–Wilk test and equal variance by Brown–Forsythe. Data that were not normally distributed were assessed using the Friedman repeated measures ANOVA on Ranks and any pairwise multiple comparisons were made using the Tukey test. A two-way repeated-measures ANOVA was used to examine potential sex differences in the effect of insulin on main outcome variables. No sex differences were detected on primary outcome variables; therefore, data from men and women were pooled for analysis. A two-way repeated-measures ANOVA (Normality: Shapiro–Wilk; equal variance: Brown–Forsythe; multiple comparisons: Holm–Sidak) was also used in protocol 1 to examine the interaction between time and the relative contribution of cardiac output and/or TPR to blood pressure maintenance. In protocol 3, a paired two-tailed Student t test was used for AUC comparisons (without insulin vs. with insulin) under each different experimental condition. An α of P < 0.05 was considered statistically significant. Data are reported as means ± SE.
RESULTS
The Contribution of Total Peripheral Resistance to Systemic Blood Pressure Maintenance is Diminished during Hyperinsulinemia
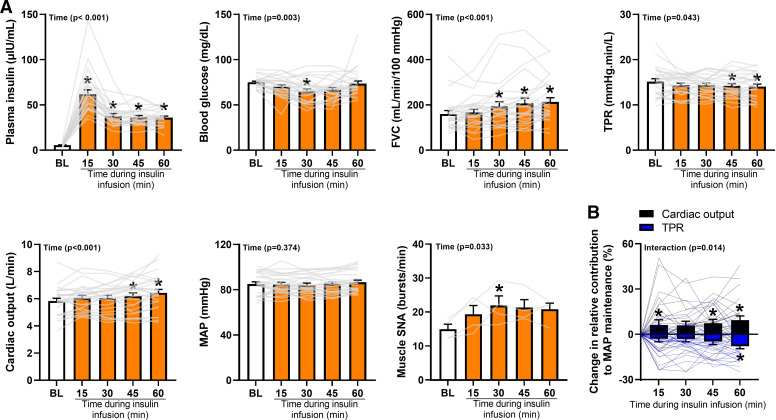
Twenty-six healthy men (n = 14) and women (n = 12) completed protocol 1. Participant characteristics are reported in Table 1. No sex differences were detected on the primary outcome variable (%TPR; main effect of sex, P = 0.44; interaction of time and sex, P = 0.26); therefore, data from men and women were pooled for analysis. Plasma insulin and blood glucose concentrations during systemic infusion of insulin are depicted in Fig. 2A and demonstrate successful achievement of hyperinsulinemia (P < 0.01). Resting heart rate increased in response to insulin infusion (P < 0.01, data not shown), while mean arterial blood pressure remained unchanged (P = 0.37, Fig. 2A). Data from ModelFlow showed an increase in cardiac output (P < 0.01) and reduction in TPR (P = 0.04) during insulin infusion (Fig. 2A). Muscle SNA (n = 4) increased from baseline during hyperinsulinemia when reported as burst incidence (baseline: 24 ± 3, min 15: 33 ± 4, min 30: 36 ± 5, min 45: 35 ± 4, min 60: 32 ± 4 bursts/100 heartbeats; P = 0.02) and burst frequency (bursts/min, P = 0.03; Fig. 2A). Femoral artery blood flow (baseline: 135 ± 13, min 15: 143 ± 12, min 30: 165 ± 21, min 45: 178 ± 21, min 60: 189 ± 18 mL/min) and FVC (Fig. 2A) increased from baseline in response to insulin infusion (P < 0.01). With the increasing duration of insulin infusion, the contribution of TPR to the maintenance of blood pressure decreased and the contribution of cardiac output increased (Interaction of time and variable, P = 0.01, Fig. 2B).
Figure 2.
The contribution of total peripheral resistance (TPR) to blood pressure maintenance is diminished during hyperinsulinemia. A: the time course of plasma insulin, blood glucose, muscle sympathetic nerve activity (SNA), and cardiovascular measures during insulin infusion. The higher plasma insulin concentration at min 15 can be attributed to the initial priming dose of insulin designed to suppress hepatic glucose production. B: the calculated decay in the contribution of TPR to the maintenance of mean arterial blood pressure (MAP). An attendant increase in the contribution of cardiac output to blood pressure maintenance is also noted. Data are reported as individual data points, as well as means ± SE (n = 26 participants; SNA, n = 4 participants). FVC, femoral vascular conductance. One-way repeated measures analysis of variance (ANOVA) (normality: Shapiro–Wilk, equal variance: Brown–Forsynthe) or ANOVA on Ranks (Insulin, Glucose, FVC) with pairwise multiple comparisons using the Holm–Sidak method or Tukey test, respectively (A). Two-way repeated-measures ANOVA (normality: Shapiro–Wilk, equal variance: Brown–Forsynthe, multiple comparisons: Holm–Sidak) (B). *Significance (P < 0.05) from BL.
Hyperinsulinemia Blunts Sympathetically Mediated Vasoconstriction in the Leg
Sixteen healthy young men (n = 9) and women (n = 7) from protocol 1 are included in protocol 2. Participant characteristics are reported in Table 1. No sex differences were detected in the primary outcome variable (%FVC; main effect of sex, P = 0.98; interaction of time and sex, P = 0.74); therefore, data from men and women were pooled for analysis. As in protocol 1, plasma insulin and blood glucose concentrations support the successful achievement of hyperinsulinemia (5.3 ± 0.5 to 36.2 ± 2.0 μIU/mL, P < 0.01) and maintenance of fasted blood glucose levels (76 ± 2 to 74 ± 4 mg/dL, P = 0.21). Consistent with protocol 1, insulin infusion increased resting heart rate (61 ± 3 to 64 ± 3 beats/min, P < 0.01), whereas mean arterial blood pressure was maintained (86 ± 2 to 88 ± 2 mmHg, P = 0.32). Also in alignment with protocol 1, insulin infusion increased cardiac output (6.2 ± 0.3 to 6.8 ± 0.4 L/min, P < 0.01) and tended to reduce TPR (14.2 ± 0.6 to 13.4 ± 0.7 mmHg/min/L, P = 0.11). Femoral artery blood flow and FVC increased from baseline in response to insulin infusion (136 ± 11 to 196 ± 25 mL/min and 160 ± 13 to 224 ± 27 mL/min/100 mmHg, respectively; P < 0.01).
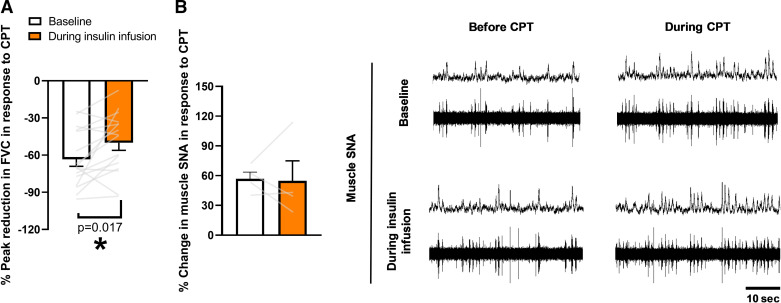
Consistent with our hypothesis, insulin infusion attenuated the peak reduction in FVC caused by the cold pressor test, relative to the reduction at baseline (P = 0.02, Fig. 3A). The effect of the cold pressor test on muscle SNA (n = 4) did not differ between baseline (burst incidence: 24 ± 3 to 34 ± 4 bursts/100 heartbeats; burst frequency: 15 ± 1 to 23 ± 2 bursts/min) and insulin conditions (burst incidence: 32 ± 4 to 43 ± 3 bursts/100 heartbeats; burst frequency: 21 ± 2 to 31 ± 1 bursts/min) (Interaction of insulin and CPT, P > 0.05) and this conclusion was maintained when assessed as a relative change (Burst incidence: 42 ± 9 to 40 ± 18%, P = 0.91; Burst frequency: P = 0.92, Fig. 3B). Although the rise in MSNA did not differ between conditions, greater perceived pain (10.6 ± 0.5 to 11.4 ± 0.5, P = 0.03) and cold (7.1 ± 0.4 to 7.4 ± 0.5, P = 0.08) scores were observed during hyperinsulinemia compared with baseline.
Figure 3.
Hyperinsulinemia blunts sympathetically mediated peripheral vasoconstriction. A: the peak reduction in FVC is attenuated during insulin infusion compared to baseline. B: representative traces of muscle sympathetic nerve activity (SNA) responses to CPT at baseline and during insulin infusion. Data are reported as individual data points, as well as means ± SE (n = 16 participants; SNA, n = 4 participants). CPT, cold pressor test. One-way repeated-measures ANOVA (normality: Shapiro–Wilk, equal variance: Brown–Forsynthe). *Significance (P < 0.05) from baseline.
Insulin Blunts Catecholamine-Induced Vasoconstriction, and This Effect is Diminished with β-Adrenergic Receptor Blockade
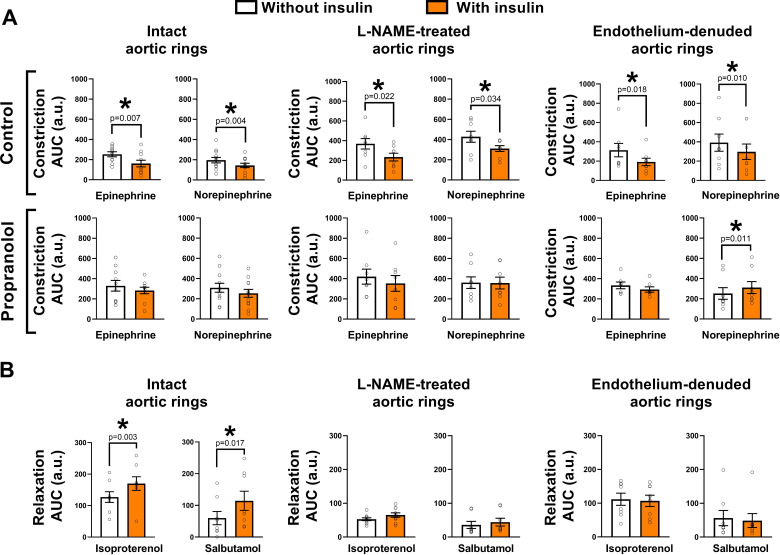
As summarized in Fig. 4A, insulin exposure blunted epinephrine and norepinephrine-induced vasoconstriction in isolated intact aortic rings (both P< 0.01). This insulin-suppressing effect on catecholamine-induced constriction persisted in aortic rings treated with nitric oxide synthase inhibitor L-NAME and in aortic rings devoid of the endothelium (all P < 0.05); however, it was largely diminished in rings treated with propranolol, a β-adrenergic receptor inhibitor [all P > 0.05, except for the norepinephrine constrictor responses in endothelium-denuded aortic rings which exhibited greater constriction in the presence of insulin (P< 0.05)].
Figure 4.
Insulin blunts catecholamine-induced vasoconstriction, in a β-adrenergic receptor-dependent manner, and augments β-adrenergic-mediated vasorelaxation. In protocol 3, mouse aortic rings were incubated with or without insulin for 30 min before stimulation with increasing concentrations of catecholamines (to assess vasoconstriction) or β-adrenergic receptor agonists (to assess vasorelaxation). A: insulin exposure blunts epinephrine- and norepinephrine-induced constriction in intact aortic rings. It also displays that this insulin-suppressing effect on catecholamine-induced constriction persists in aortic rings treated with nitric oxide synthase inhibitor l-NAME and in aortic rings devoid of the endothelium. Notably, such insulin-suppressing effect on catecholamine-induced constriction is diminished in rings treated with propranolol, a β-adrenergic receptor inhibitor. B: insulin exposure enhances isoproterenol (a nonspecific β-adrenergic receptor agonist)- and salbutamol (a β2-adrenergic receptor agonist)-induced vasorelaxation in intact aortic rings. This insulin-potentiating effect on β-adrenergic-mediated vasorelaxation is largely lost in aortic rings treated with nitric oxide synthase inhibitor l-NAME and in aortic rings devoid of the endothelium. Data are reported as means ± SE (n = 8–10/condition). a.u., arbitrary units; AUC, area under the curve. Paired two-tailed Student’s t test (without insulin vs. with insulin) under each different experimental condition. *Significance (P < 0.05).
Insulin Augments β-Adrenergic-Mediated Vasorelaxation
As summarized in Fig. 4B, insulin exposure enhanced isoproterenol (a nonspecific β-adrenergic receptor agonist)- and salbutamol (a β2-adrenergic receptor agonist)-induced vasorelaxation in isolated intact aortic rings (both P < 0.05). This insulin-potentiating effect on β-adrenergic-mediated vasorelaxation was largely lost in aortic rings treated with nitric oxide synthase inhibitor l-NAME for both isoproterenol and salbutamol relaxation responses and in aortic rings devoid of the endothelium (all P > 0.05).
DISCUSSION
In healthy individuals, systemic hyperinsulinemia causes robust peripheral vasodilation (1–14) and this occurs despite a concomitant activation of the sympathetic nervous system (15–21). It is conceivable that, in the healthy state, the potent vasodilatory effects of insulin in the face of sympathetic activation can be attained because insulin also exerts sympatholytic effects (24–29). Indeed, in support of this hypothesis, we provide evidence that the relative importance of TPR and sympathetic vasoconstriction in the leg circulation is attenuated during systemic hyperinsulinemia in young healthy individuals. Furthermore, we show in mouse aortic rings that insulin exposure suppresses epinephrine and norepinephrine-induced vasoconstriction and that such insulin-suppressing effects are diminished following β-adrenergic receptor blockade. Congruently, we also show that insulin augments β-adrenergic-mediated vasorelaxation in aortic rings. Thus, the present findings support the idea that sympathetic vasoconstriction can be tempered during hyperinsulinemia in the leg vasculature of healthy men and women and that this phenomenon may be in part mediated by potentiation of β-adrenergic vasodilation neutralizing α-adrenergic vasoconstriction.
Insulin has centrally mediated effects that activate the sympathetic nervous system (15–21). The prevailing premise is that hyperinsulinemia-associated increases in SNA limit insulin-induced peripheral vasodilation, thus contributing to the maintenance of blood pressure. This premise is largely founded on the classic observation by Sartori et al. (22) that patients with regional sympathectomy exhibit a more rapid vasodilation in response to insulin in the denervated limb than in the innervated limb. Results were further supported by Lembo and colleagues using an isolated forearm model (25, 27). However, the notion that during hyperinsulinemia there is sympathetic restraint of blood flow appears to be less supported in young healthy individuals when insulin is infused systemically (rather than locally) and a larger vascular territory is interrogated (e.g., leg vs. forearm circulations) (8, 23). Indeed, we recently used neck suction as a maneuver to acutely reduce SNA during systemic hyperinsulinemia and we did not detect an augmentation in leg vascular conductance (8), which would have been indicative of sympathetic restraint. Similarly, Randin et al. (23) reported that administration of prazosin, an α1-adrenergic receptor blocker, did not potentiate insulin-stimulated calf blood flow. Divergent findings across limbs may be due, at least in part, to differences in α-adrenergic receptor responsiveness and thus α-adrenergic mediated vasoconstriction in the leg versus forearm circulations (46).
Considering prominent activation of the sympathetic nervous system caused by hyperinsulinemia (15–21), it is surprising that the lower limb peripheral vasculature is not under a marked SNA-mediated vasoconstrictor constraint during systemic insulin infusion. By the same token, we presently show a fall in the contribution of TPR to maintain blood pressure during hyperinsulinemia, despite increases in SNA responses which were observed equally in both men and women. A plausible reconciliation would be that, in healthy individuals, systemic infusion of insulin attenuates sympathetic vasoconstriction in the leg vasculature; this is indeed what we report herein. Specifically, we found in young healthy men and women that systemic intravenous insulin infusion lessened leg vasoconstriction evoked during a cold pressor test, a well-established sympathoexcitatory stimulus (41), despite no difference in the muscle SNA response to the cold pressure test in the two conditions (baseline, hyperinsulinemia). Of note, muscle SNA was not a main outcome variable and data are only available from a subset of participants; therefore, future work in this area is necessary and results should be interpreted with caution.
In alignment with the idea that the vasculature can escape sympathetic vasoconstriction during insulin stimulation, we recently showed in isolated arteries from healthy mice that insulin exposure reduces α-adrenergic vasoconstriction and that this effect of insulin is endothelium-dependent as well as contingent upon an intact insulin signaling pathway (8). Of note, in vivo, insulin-induced blunting of sympathetic vasoconstriction could also be related to a potentiation of β-adrenergic vasodilation counteracting the vasoconstriction associated with α-adrenergic activation. In fact, there is strong precedence in the literature supporting a cross talk between insulin signaling and β-adrenergic signaling in various tissues, particularly in the heart (30–35). In accordance, we show in isolated mouse arteries that insulin exposure limits epinephrine and norepinephrine-induced vasoconstriction and that these insulin-suppressing effects are dampened with propranolol, a nonspecific β-adrenergic receptor inhibitor. Interestingly, this β-adrenergic-dependent insulin effect on suppressing catecholamine-induced constriction appears to be largely exerted on the smooth muscle, as neither endothelial denudation nor nitric oxide synthase inhibition abrogated the insulin effect. Correspondingly, we also show that vascular exposure to insulin enhances β-adrenergic-mediated vasorelaxation, an effect that is endothelium and nitric oxide dependent. These findings extend those of Gros et al. (47) who reported that insulin exposure augments isoproterenol-induced vasorelaxation in aortic rings from healthy rats but not in rings from spontaneously hypertensive rats. However, it should be acknowledged that the aorta is not a site of vascular resistance and thus experiments should be recapitulated in isolated resistance arteries.
The present findings support the notion that, in the healthy state, sympathetic vasoconstriction can be mitigated during hyperinsulinemia, as well as suggest that activation of β-adrenergic receptors may be implicated. In this regard, Creager et al. (48) demonstrated in the isolated human forearm model that insulin-induced vasodilation was inhibited with an intraarterial infusion of propranolol, further reinforcing the concept that insulin and β-adrenergic signaling interact in the modulation of vascular tone. Evidently, future studies using both reductionist and integrative approaches are warranted to better understand the mechanisms by which β-adrenergic activation contributes to insulin-induced suppression of sympathetic vasoconstriction in the peripheral circulation and the involvement of endothelial versus smooth muscle cells in this process. Furthermore, given that β-adrenergic receptor-mediated dilation in the peripheral circulation of humans is thought to be largely mediated by β2-receptors (49), it is credible that β2-receptors would be the primary β-adrenergic receptors implicated in any modulating effect of insulin on catecholamine-induced constriction. In support of this, herein we show for the first time that vasorelaxation in response to β2-receptor activation is heightened in the presence of insulin. The role of other β-adrenergic receptors in modulating the vasomotor effects of insulin remains to be determined. Along these lines, further research is also needed to determine how these mechanisms intersect with the coexisting mechanisms underlying insulin-induced lysing of α-adrenergic vasoconstriction and how they may differ in disease (e.g., insulin resistance, type 2 diabetes).
In aggregate, this investigation suggests that in the face of systemic hyperinsulinemia and resultant sympathetic activation, the profound peripheral vasodilation in healthy men and women may be enabled by the sympatho-attenuating effects of insulin. To that end, we provide evidence that vasoconstriction in the leg during a sympathoexcitatory stimulus is reduced during systemic hyperinsulinemia and this response does not differ between healthy men and women. These findings in vivo were corroborated in aortic rings where we demonstrate that insulin suppresses catecholamine-induced vasoconstriction. Notably, we show that this effect of insulin in dampening catecholamine-induced vasoconstriction requires unrestricted β-adrenergic signaling, implying that β-adrenergic vasodilation may offset α-adrenergic vasoconstriction. Accordingly, findings from this work suggest that targeting β-adrenergic receptors, most likely β2-receptors, may represent a strategy for enhancing the vasodilatory actions of insulin and, by extension, the delivery of insulin and glucose to end-organ tissues, particularly in the setting of vascular insulin resistance.
GRANTS
This work was supported in part by the Margaret W. Mangel Faculty Research Catalyst Fund (to J.K.L. and J.P.) and the National Heart, Lung, and Blood Institute Grants R00 HL130339 (to J.K.L.), R01 HL153523 (to J.K.L.), and R01 HL137769 (to J.P.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.K.L., R.N.S., and J.P. conceived and designed research; J.K.L., R.N.S., G.P., J.L.H., J.A.S., B.S., D.W.J., C.M.-A., and J.P. performed experiments; J.K.L., R.N.S., G.P., J.A.S., and D.W.J. analyzed data; J.K.L., R.N.S., C.M.-A., and J.P. interpreted results of experiments; R.N.S. prepared figures; J.K.L. and J.P. drafted manuscript; J.K.L., R.N.S., G.P., J.L.H., J.A.S., B.S., D.W.J., C.M.-A., and J.P. edited and revised manuscript; J.K.L., R.N.S., G.P., J.L.H., J.A.S., B.S., D.W.J., C.M.-A., and J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate the time and effort put in by all research participants. We also acknowledge the nursing team of the Clinical Research Center at the University of Missouri.
REFERENCES
- 1.Baron AD, Brechtel-Hook G, Johnson A, Cronin J, Leaming R, Steinberg HO. Effect of perfusion rate on the time course of insulin-mediated skeletal muscle glucose uptake. Am J Physiol Endocrinol Metab 271: E1067–E1072, 1996. doi: 10.1152/ajpendo.1996.271.6.E1067. [DOI] [PubMed] [Google Scholar]
- 2.Baron AD, Laakso M, Brechtel G, Edelman SV. Mechanism of insulin resistance in insulin-dependent diabetes mellitus: a major role for reduced skeletal muscle blood flow. J Clin Endocrinol Metab 73: 637–643, 1991. doi: 10.1210/jcem-73-3-637. [DOI] [PubMed] [Google Scholar]
- 3.Baron AD, Steinberg H, Chaker H, Leaming R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest 96: 786–792, 1995. doi: 10.1172/JCI118124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab 301: E252–E263, 2011. doi: 10.1152/ajpendo.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 52: 752–764, 2009. doi: 10.1007/s00125-009-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggleston EM, Jahn LA, Barrett EJ. Early microvascular recruitment modulates subsequent insulin-mediated skeletal muscle glucose metabolism during lipid infusion. Diabetes Care 36: 104–110, 2013. doi: 10.2337/dc11-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laakso M, Edelman S, Brechtel G, Baron A. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes 41: 1076–1083, 1992. doi: 10.2337/diab.41.9.1076. [DOI] [PubMed] [Google Scholar]
- 8.Limberg JK, Smith JA, Soares RN, Harper JL, Houghton KN, Jacob DW, Mozer MT, Grunewald ZI, Johnson BD, Curry TB, Baynard T, Manrique-Acevedo C, Padilla J. Sympathetically mediated increases in cardiac output, not restraint of peripheral vasodilation, contribute to blood pressure maintenance during hyperinsulinemia. Am J Physiol Heart Circ Physiol 319: H162–H170, 2020. doi: 10.1152/ajpheart.00250.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meijer RI, De BM, Groen MR, Eringa EC, Rattigan S, Barrett EJ, Smulders YM, Serne EH. Insulin-induced microvascular recruitment in skin and muscle are related and both are associated with whole-body glucose uptake. Microcirculation 19: 494–500, 2012. doi: 10.1111/j.1549-8719.2012.00174.x. [DOI] [PubMed] [Google Scholar]
- 10.Park LK, Parks EJ, Pettit-Mee RJ, Woodford ML, Ghiarone T, Smith JA, Sales ARK, Martinez-Lemus LA, Manrique-Acevedo C, Padilla J. Skeletal muscle microvascular insulin resistance in type 2 diabetes is not improved by eight weeks of regular walking. J Appl Physiol (1985) 129: 283–296, 2020. doi: 10.1152/japplphysiol.00174.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds L, Credeur DP, Manrique C, Padilla J, Fadel PJ, Thyfault JP. Obesity, type 2 diabetes, and impaired insulin stimulated blood flow: role of skeletal muscle NO synthase and endothelin-1. J Appl Physiol 122: 38–47, 2017. doi: 10.1152/japplphysiol.00286.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 94: 1172–1179, 1994. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. J Article 285: E123–E129, 2003. doi: 10.1152/ajpendo.00021.2003. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Vincent MA, Richards SM, Clerk LH, Rattigan S, Clark MG, Barrett EJ. Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes 53: 447–453, 2004. doi: 10.2337/diabetes.53.2.447. [DOI] [PubMed] [Google Scholar]
- 15.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest 87: 2246–2252, 1991. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berne C, Fagius J, Pollare T, Hjemdahl P. The sympathetic response to euglycaemic hyperinsulinaemia. Evidence from microelectrode nerve recordings in healthy subjects. Diabetologia 35: 873–879, 1992. doi: 10.1007/BF00399935. [DOI] [PubMed] [Google Scholar]
- 17.Lembo G, Napoli R, Capaldo B, Rendina V, Iaccarino G, Volpe M, Trimarco B, Sacca L. Abnormal sympathetic overactivity evoked by insulin in the skeletal muscle of patients with essential hypertension. J Clin Invest 90: 24–29, 1992. doi: 10.1172/JCI115842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherrer U, Sartori C. Insulin as a vascular and sympathoexcitatory hormone: implications for blood pressure regulation, insulin sensitivity, and cardiovascular morbidity. Circulation 96: 4104–4113, 1997. doi: 10.1161/01.cir.96.11.4104. [DOI] [PubMed] [Google Scholar]
- 19.Vollenweider L, Tappy L, Owlya R, Jequier E, Nicod P, Scherrer U. Insulin-induced sympathetic activation and vasodilation in skeletal muscle. Effects of insulin resistance in lean subjects. Diabetes 44: 641–645, 1995. doi: 10.2337/diabetes.44.6.641. [DOI] [PubMed] [Google Scholar]
- 20.Vollenweider P, Tappy L, Randin D, Schneiter P, Jequier E, Nicod P, Scherrer U. Differential effects of hyperinsulinemia and carbohydrate metabolism on sympathetic nerve activity and muscle blood flow in humans. J Clin Invest 92: 147–154, 1993. doi: 10.1172/JCI116542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol 588: 3593–3603, 2010. doi: 10.1113/jphysiol.2010.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sartori C, Trueb L, Nicod P, Scherrer U. Effects of sympathectomy and nitric oxide synthase inhibition on vascular actions of insulin in humans. Hypertension 34: 586–589, 1999. doi: 10.1161/01.HYP.34.4.586. [DOI] [PubMed] [Google Scholar]
- 23.Randin D, Vollenweider P, Tappy L, Jequier E, Nicod P, Scherrer U. Effects of adrenergic and cholinergic blockade on insulin-induced stimulation of calf blood flow in humans. Am J Physiol Regul Integr Comp Physiol 266: R809–R816, 1994. doi: 10.1152/ajpregu.1994.266.3.R809. [DOI] [PubMed] [Google Scholar]
- 24.Fujishima S, Imaizumi T, Abe I, Takeshita A, Fujishima M. Effects of intra-arterial infusion of insulin on forearm vasoreactivity in hypertensive humans. Hypertens Res 18: 227–233, 1995. doi: 10.1291/hypres.18.227. [DOI] [PubMed] [Google Scholar]
- 25.Lembo G, Iaccarino G, Rendina V, Volpe M, Trimarco B. Insulin blunts sympathetic vasoconstriction through the alpha 2-adrenergic pathway in humans. Hypertension 24: 429–438, 1994. doi: 10.1161/01.HYP.24.4.429. [DOI] [PubMed] [Google Scholar]
- 26.Lembo G, Iaccarino G, Vecchione C, Rendina V, Trimarco B. Insulin modulation of vascular reactivity is already impaired in prehypertensive spontaneously hypertensive rats. Hypertension 26: 290–293, 1995. doi: 10.1161/01.HYP.26.2.290. [DOI] [PubMed] [Google Scholar]
- 27.Lembo G, Rendina V, Iaccarino G, Lamenza F, Volpe M, Trimarco B. Insulin reduces reflex forearm sympathetic vasoconstriction in healthy humans. Hypertension 21: 1015–1019, 1993. doi: 10.1161/01.HYP.21.6.1015. [DOI] [PubMed] [Google Scholar]
- 28.Lembo G, Vecchione C, Iaccarino G, Trimarco B. The crosstalk between insulin and the sympathetic nervous system: possible implications in the pathogenesis of essential hypertension. Blood Press Suppl 1: 38–42, 1996. [PubMed] [Google Scholar]
- 29.Sakai K, Imaizumi T, Masaki H, Takeshita A. Intra-arterial infusion of insulin attenuates vasoreactivity in human forearm. Hypertension 22: 67–73, 1993. doi: 10.1161/01.HYP.22.1.67. [DOI] [PubMed] [Google Scholar]
- 30.Fu Q, Wang Q, Xiang YK. Insulin and β adrenergic receptor signaling: crosstalk in heart. Trends Endocrinol Metab 28: 416–427, 2017. doi: 10.1016/j.tem.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu Q, Xu B, Liu Y, Parikh D, Li J, Li Y, Zhang Y, Riehle C, Zhu Y, Rawlings T, Shi Q, Clark RB, Chen X, Abel ED, Xiang YK. Insulin inhibits cardiac contractility by inducing a Gi-biased β2-adrenergic signaling in hearts. Diabetes 63: 2676–2689, 2014. doi: 10.2337/db13-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jönsson C, Castor Batista AP, Kjølhede P, Strålfors P. Insulin and β-adrenergic receptors mediate lipolytic and anti-lipolytic signalling that is not altered by type 2 diabetes in human adipocytes. Biochem J 476: 2883–2908, 2019. doi: 10.1042/BCJ20190594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karoor V, Wang L, Wang HY, Malbon CC. Insulin stimulates sequestration of beta-adrenergic receptors and enhanced association of beta-adrenergic receptors with Grb2 via tyrosine 350. J Biol Chem 273: 33035–33041, 1998. doi: 10.1074/jbc.273.49.33035. [DOI] [PubMed] [Google Scholar]
- 34.Mandić M, Drinovec L, Glisic S, Veljkovic N, Nøhr J, Vrecl M. Demonstration of a direct interaction between β2-adrenergic receptor and insulin receptor by BRET and bioinformatics. PLoS One 9: e112664, 2014. doi: 10.1371/journal.pone.0112664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q, Liu Y, Fu Q, Xu B, Zhang Y, Kim S, Tan R, Barbagallo F, West T, Anderson E, Wei W, Abel ED, Xiang YK. Inhibiting insulin-mediated β2-adrenergic receptor activation prevents diabetes-associated cardiac dysfunction. Circulation 135: 73–88, 2017. doi: 10.1161/CIRCULATIONAHA.116.022281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Limberg JK, Casey DP, Trinity JD, Nicholson WT, Wray DW, Tschakovsky ME, Green DJ, Hellsten Y, Fadel PJ, Joyner MJ, Padilla J. Assessment of resistance vessel function in human skeletal muscle: guidelines for experimental design, Doppler ultrasound, and pharmacology. Am J Physiol Heart Circ Physiol 318: H301–H325, 2020. doi: 10.1152/ajpheart.00649.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curry TB, Charkoudian N. The use of real-time ultrasound in microneurography. Auton Neurosci 162: 89–93, 2011. doi: 10.1016/j.autneu.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hart EC, Head GA, Carter JR, Wallin BG, May CN, Hamza SM, Hall JE, Charkoudian N, Osborn JW. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am J Physiol Heart Circ Physiol 312: H1031–H1051, 2017. doi: 10.1152/ajpheart.00703.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Defronzo R, Tobin J, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 237: E214–E223, 1979. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 40.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. [PubMed] [Google Scholar]
- 41.Dillon GA, Lichter ZS, Alexander LM, Vianna LC, Wang J, Fadel PJ, Greaney JL. Reproducibility of the neurocardiovascular responses to common laboratory-based sympathoexcitatory stimuli in young adults. J Appl Physiology 129: 1203–1213, 2020. doi: 10.1152/japplphysiol.00210.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogoh S, Fadel PJ, Nissen P, Jans Ø, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003. doi: 10.1113/jphysiol.2003.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grunewald ZI, Jurrissen TJ, Woodford ML, Ramirez-Perez FI, Park LK, Pettit-Mee R, Ghiarone T, Brown SM, Morales-Quinones M, Ball JR, Staveley-O'Carroll KF, Aroor AR, Fadel PJ, Paradis P, Schiffrin EL, Bender SB, Martinez-Lemus LA, Padilla J. Chronic elevation of endothelin-1 alone may not be sufficient to impair endothelium-dependent relaxation. Hypertension 74: 1409–1419, 2019. doi: 10.1161/HYPERTENSIONAHA.119.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winn NC, Grunewald ZI, Gastecki ML, Woodford ML, Welly RJ, Clookey SL, Ball JR, Gaines TL, Karasseva NG, Kanaley JA, Sacks HS, Vieira-Potter VJ, Padilla J. Deletion of UCP1 enhances ex vivo aortic vasomotor function in female but not male mice despite similar susceptibility to metabolic dysfunction. Am J Physiol Endocrinol Metab 313: E402–E412, 2017. doi: 10.1152/ajpendo.00096.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Limberg JK, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. Alpha-adrenergic control of blood flow during exercise: effect of sex and menstrual phase. J Appl Physiol (1985) 109: 1360–1368, 2010. doi: 10.1152/japplphysiol.00518.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol 92: 2105–2113, 2002. doi: 10.1152/japplphysiol.00979.2001. [DOI] [PubMed] [Google Scholar]
- 47.Gros R, Borkowski KR, Feldman RD. Human insulin-mediated enhancement of vascular beta-adrenergic responsiveness. Hypertension 23: 551–555, 1994. doi: 10.1161/01.hyp.23.5.551. [DOI] [PubMed] [Google Scholar]
- 48.Creager MA, Liang CS, Coffman JD. Beta adrenergic-mediated vasodilator response to insulin in the human forearm. J Pharmacol Exp Ther 235: 709–714, 1985. [PubMed] [Google Scholar]
- 49.Harvey RE, Ranadive SM, Limberg JK, Baker SE, Nicholson WT, Curry TB, Barnes JN, Joyner MJ. Forearm vasodilatation to a β(2)-adrenergic receptor agonist in premenopausal and postmenopausal women. Exp Physiol 105: 886–892, 2020. doi: 10.1113/EP088452. [DOI] [PMC free article] [PubMed] [Google Scholar]