Abstract
The liver plays a central role that influences cardiovascular disease outcomes through regulation of glucose and lipid metabolism. It is recognized that the local liver molecular clock regulates some liver-derived metabolites. However, it is unknown whether the liver clock may impact cardiovascular function. Perivascular adipose tissue (PVAT) is a specialized type of adipose tissue surrounding blood vessels. Importantly, cross talk between the endothelium and PVAT via vasoactive factors is critical for vascular function. Therefore, we designed studies to test the hypothesis that cardiovascular function, including PVAT function, is impaired in mice with liver-specific circadian clock disruption. Bmal1 is a core circadian clock gene, thus studies were undertaken in male hepatocyte-specific Bmal1 knockout (HBK) mice and littermate controls (i.e., flox mice). HBK mice showed significantly elevated plasma levels of β-hydroxybutyrate, nonesterified fatty acids/free fatty acids, triglycerides, and insulin-like growth factor 1 compared with flox mice. Thoracic aorta PVAT in HBK mice had increased mRNA expression of several key regulatory and metabolic genes, Ppargc1a, Pparg, Adipoq, Lpl, and Ucp1, suggesting altered PVAT energy metabolism and thermogenesis. Sensitivity to acetylcholine-induced vasorelaxation was significantly decreased in the aortae of HBK mice with PVAT attached compared with aortae of HBK mice with PVAT removed, however, aortic vasorelaxation in flox mice showed no differences with or without attached PVAT. HBK mice had a significantly lower systolic blood pressure during the inactive period of the day. These new findings establish a novel role of the liver circadian clock in regulating PVAT metabolic gene expression and PVAT-mediated aortic vascular function.
Keywords: blood pressure, Bmal1, hepatocyte, PVAT, vascular function
INTRODUCTION
Healthy vascular function is maintained through systemic and local humoral and cellular function. The endothelium is a single cell layer on the luminal side of blood vessels and mediates vasodilation and antiatherogenic vascular homeostasis (1–4). Perivascular adipose tissue (PVAT), a specialized type of adipose tissue surrounding blood vessels, releases anticontractile and antiatherogenic vasoactive factors to regulate vascular function and is critical for vascular homeostasis (5–8). Recent data show that PVAT dysfunction precedes development of atherosclerosis (9–11). PVAT has characteristics of both brown adipose tissue (BAT) and white adipose tissue (WAT) depending on the anatomical location. PVAT and BAT share several similarities including expression of genes important in regulating energy metabolism and thermogenesis (12). The current literature indicates that PVAT surrounding the thoracic aorta shares more similarities with BAT, whereas abdominal aortic PVAT has characteristics of both BAT and WAT (6, 12, 13). Furthermore, there are regional differences in aortic PVAT function as abdominal aorta PVAT has reduced anticontractile function and lower levels of the vasoactive factor nitric oxide (NO) (14). Cross talk between the endothelium and PVAT via release of numerous factors is important for vascular function (15). Factors released from PVAT have direct effects on vascular smooth muscle mediated vasoconstriction and endothelial-dependent vasodilation. Multiple studies indicate a relationship between endothelial and PVAT dysfunction under pathophysiological conditions (16–19). Studies have also examined cross talk between the liver and WAT (20, 21), yet it is unknown whether there is cross talk between the liver and PVAT.
The transcription factor BMAL1 is essential for molecular circadian control through the timing of clock controlled gene expression (22). Importantly, the liver circadian clock system serves as an important regulator of metabolism. Mice with liver-specific Bmal1 deletion have reduced inactive period blood glucose, increased glucose clearance, and decreased insulin sensitivity (23, 24). Liver-specific deletion of Bmal1 also disrupts rhythmicity of hepatic glycogen and glucose metabolism gene expression and glycogen content in mice with alcohol-induced fatty liver disease (25). In addition to regulating hepatic glucose and glycogen metabolism, recent studies demonstrate a role of the liver clock in atherosclerosis development and lipid metabolism. Apolipoprotein E knockout mice with liver-specific Bmal1 deletion show increased atherosclerosis accompanied by hyperlipidemia and reduced cholesterol efflux (26). Furthermore, conditional liver-specific Bmal1 knockout mice show enhanced Western diet-induced hyperlipidemia (27). Liver-specific Bmal1 deletion also alters rhythms of circadian clock and lipid metabolism genes in the liver (28). Importantly, liver metabolism and function are now recognized as contributing to blood pressure regulation as studies show an association between hypertension and nonalcoholic fatty liver disease (29–31).
Although previous studies have established the importance of liver Bmal1 in metabolism (23–25, 27, 28) and atherosclerosis (26), it is unknown whether liver clock disruption impacts vascular function, including PVAT function and blood pressure control. Thus, we hypothesized that vascular function, including PVAT function and systemic cardiovascular function, is impaired in mice with liver-specific clock disruption. We designed studies to test this hypothesis utilizing young adult hepatocyte-specific Bmal1 knockout (HBK) mice with determinations of circulating metabolites, PVAT metabolic gene expression, vascular reactivity, including PVAT function, as well as rhythms in blood pressure and heart rate.
MATERIALS AND METHODS
Animals
Studies were conducted in 4- to 6-mo-old male hepatocyte-specific Bmal1 knockout (HBK) mice [Alb-Cre(+/−)/BMAL1(flox/flox)] and their control genotype littermates [AlbCre(−/−)/BMAL1(flox/flox)] on a C57BL/6J background (25, 28). Prior validation of the HBK mouse model shows an absence of Bmal1 and arrhythmic expression of several clock genes in the liver, as well as intact rhythmic expression of Bmal1 and the clock-controlled output gene Dbp in extra-hepatic tissues of HBK mice (25, 28). Mice were provided food and water ad libitum and kept on 12:12-h light-dark cycle (ZT0 = lights on and ZT12 = lights off) in a temperature and humidity controlled environment before and during studies. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham and were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th ed., National Academy of Sciences, 2011). Sample sizes of mice used in experiments are given in figure legends and table descriptions.
Plasma Collection and Assays
Mice were anesthetized with isoflurane and cardiac puncture was used for blood collection at ZT1 as described previously (32). Plasma was separated by centrifugation at 2,000 g for 15 min, aliquoted, snap frozen in liquid nitrogen, and stored at −80°C until analyses were conducted. Plasma ketone bodies and lipid levels were measured using enzymatic assay kits for β-hydroxybutyrate (β-HB) (Cayman Chemical, Ann Arbor, MI, Cat. No. 700190, 0–0.5 mM), nonesterified fatty acids/free fatty acids (NEFA/FFA) (ZenBio, Research Triangle Park, NC, Cat. No. SFA-1, 0–1,000 µM), triglycerides (TG) (Pointe Scientific, Canton, MI, Cat. No. T7532, 0–1,000 mg/dL), and cholesterol (Pointe Scientific, Canton, MI, Cat. No. C7510, 0–500 mg/dL). Plasma hormones were measured using ELISA kits for insulin-like growth factor-1 (IGF-1) (R&D Systems, Minneapolis, MN, Cat. No. MG100, 31.2–2,000 pg/mL), adiponectin (R&D Systems, Minneapolis, MN, Cat. No. MRP300, 0.2–10 ng/mL), and leptin (Crystal Chem, Elk Grove Village, IL, Cat. No. 90030, 0.2–12.8 ng/mL). eACh assay was conducted per the manufacturer’s instructions and values were within the linear portion of the standard curve. Plasma was also processed according to the manufacturer’s sample preparation protocol and the NO metabolites, nitrite, and nitrate, were measured by high-performance liquid chromatography (ENO-30, Eicom, Kyoto, Japan) (33–35). Nitrite and nitrate are both reported as micromolar.
RNA Isolation and Real-Time Polymerase Chain Reaction
PVAT was microdissected from thoracic aortas collected at ZT1, placed in RNAlater (Sigma Aldrich, St. Louis, MO), and stored at −20°C to prevent RNA degradation. Total RNA was isolated using TRI-Reagent (Sigma-Aldrich) and treated with DNA-free DNase Treatment and Removal Reagents (Thermo Fisher Scientific, Waltham, MA). DNase-treated RNA (260/280 ratio > 1.8) was converted to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA). Gene expression measurements were done by real-time polymerase chain reaction (RT-PCR) using gene-specific TaqMan primers from Thermo Fisher Scientific (Waltham, MA) and a QuantStudio6 Flex Real-time PCR system (Thermo Fisher Scientific). TaqMan gene assays are listed in Table 1. Gene expression was determined using the ΔΔCt method (36) with values normalized to the housekeeping gene Gapdh. Data are presented as a fold-change from the flox control genotype.
Table 1.
Primer sets for real-time PCR
| Gene Name | Thermo Fisher TaqMan Assay Number |
|---|---|
| Ppargc1a | Mm01208835_m1 |
| Pparg | Mm01184322_m1 |
| Adipoq | Mm00456425_m1 |
| Lpl | Mm00434764_m1 |
| Ucp1 | Mm01244861_m1 |
| Gapdh | Mm99999915_g1 |
Adipoq, adiponectin; Gapdh, glyceraldehyde 3-phosphate dehydrogenase; Lpl, lipoprotein lipase; Pparg, peroxisome proliferator-activated receptor g; Ppargc1a, peroxisome proliferator-activated receptor γ coactivator 1-α; Ucp1, uncoupling protein 1.
Vascular Function Studies
Thoracic aortas were isolated and prepared for vascular reactivity experiments at ZT1 as previously described (33, 37). Briefly, 2-mm thoracic aortic segments were dissected and placed in an ice-cold physiological salt solution before PVAT was either left attached or removed as described previously (38, 39). PVAT was carefully removed by cutting with microdissection scissors under a microscope to ensure integrity of the aortic ring, as described previously (38–40). Viability of aortic rings was confirmed by assessing the degree of maximal relaxation in response to acetylcholine (ACh) (38–40). Aortic rings with and without PVAT were prepared and vascular function was studied using the Multi Wire Myograph System - 620 M (DMT-USA, Inc., Ann Arbor, MI) with LabChart 8 software (ADInstruments, Colorado Springs, CO). Aortic rings were equilibrated and passive tension was set to 10 mN for aortic segments. To assess vasodilation, cumulative concentration-response curves to ACh (1 × 10−9 mol/L to 10−5 mol/L) and sodium nitroprusside (SNP; 1 × 10−10 mol/L to 10−5 mol/L) were performed after precontraction of arterial segments with phenylephrine (PE). To assess vasoconstriction, cumulative concentration‐responses to PE were generated and normalized to the maximum constriction elicited by 100 mmol/L KCl. GraphPad Prism 8 (GraphPad Software Inc., La Jolla, CA) was used for data analysis and data are presented as maximum relaxation (Emax) as a percentage of PE and sensitivity as logEC50. Area under the curve (AUC) was also calculated for PE and KCl dose-response curves.
Systemic Cardiovascular Function
Blood pressure (BP) and heart rate (HR) were monitored by telemetry (41). For telemetry transmitter implantation, mice were anesthetized with isoflurane and an arterial catheter was implanted into the right carotid artery connected to an implantable transmitter (PA-C10; Data Sciences International, St. Paul, MN) to continuously monitor arterial pressure and HR as previously described (32, 33, 35, 41, 42). Baseline recordings of systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and HR were collected every 10 min with the DataQuest System (Data Sciences International, St. Paul, MN). Telemetry files were then imported into Ponemah v6.3 (DSI, Minneapolis, MN) for analysis. Inactive and active period BP and HR for eACh mouse were calculated by averaging values during the inactive (ZT0-12) or active (ZT12-0) 12-h periods. Continuous 24-h data were calculated as the average of three consecutive days for each animal of combined telemetry data. Cosinor analysis and χ (43) periodogram (Actimetrics, ClockLab, Wilmette, IL) were used for circadian analysis of telemetry data (44–46). Mesor (midline estimating statistic of rhythms), amplitude, and period of BP and HR for eACh individual mouse were determined using a resolution of 10-min bins (45). HR and BP variability were assessed by frequency domain analysis using low-frequency (0.4–1.5 Hz) and high-frequency (1.5–4.0 Hz) ranges (32, 47).
Metabolic Cage Study and Urinary Sodium Excretion
Mice were maintained in metabolic cages with ad libitum access to food and water as previously described (35). Twelve hour food and water intake and urine output were measured each day for 4 days at ZT0 (7 AM) and ZT12 (7 PM) and reported as g/12 h or mL/12 h, respectively. Urinary sodium concentration was determined by atomic absorption 140 spectrometry (Analyst 200, Perkin Elmer, Waltham, MA) and reported as mEq/12 h (35, 41).
Statistical Analysis
GraphPad Prism 8 (GraphPad Software Inc., La Jolla, CA) was used for statistical analysis. Data are represented as means ± SE and statistical significance was set at P < 0.05. Unpaired Student’s t test was used for the following comparisons: baseline parameters of body and organ weights, plasma measurements, gene expression data, and telemetry data, individual values of each mouse for circadian analysis (mesor, amplitude, and period), and frequency domain analysis. Two-way repeated-measures ANOVA with Sidak’s multiple comparisons test was used to compare inactive and active period differences in BP, HR, food intake, water intake, urine production, and urinary sodium excretion. For vascular reactivity data, comparisons were made with two-way ANOVA with Tukey’s post hoc test.
RESULTS
Mouse Characteristics
Food intake, water intake, and urine production were similar between flox and HBK mice during both the inactive and active period (Table 2). Urinary sodium excretion during the inactive and active period was also similar in both groups of mice (Table 2). Furthermore, body weights were comparable between flox and HBK mice (Table 3). We did not detect a significant difference in kidney or liver weight between flox and HBK mice (Table 3). These results show that baseline physiological parameters are similar between flox and HBK mice.
Table 2.
Food intake, water intake, urine production, and urinary sodium excretion in flox and HBK mice
| Flox |
HBK |
|||
|---|---|---|---|---|
| Inactive Period | Active Period | Inactive Period | Active Period | |
| Food intake, g/12 h | 0.5 ± 0.0 | 3.9 ± 0.1‡ | 0.6 ± 0.1 | 3.7 ± 0.1‡ |
| Water intake, mL/12 h | 0.9 ± 0.1 | 3.7 ± 0.1‡ | 0.9 ± 0.1 | 3.5 ± 0.1‡ |
| Urine volume, mL/12 h | 0.1 ± 0.1 | 0.9 ± 0.2‡ | 0.1 ± 0.0 | 0.7 ± 0.1‡ |
| Sodium excretion, mEq/12 h | 0.02 ± 0.01 | 0.12 ± 0.02‡ | 0.01 ± 0.00 | 0.10 ± 0.01‡ |
Values are presented as means ± SE; n = 4. Two-way repeated-measures ANOVA was used to compare time of day and genotype effects with Sidak’s post hoc test for multiple comparisons. Time of day had a significant effect on food and water intake, urine volume, and urinary sodium excretion in both flox and HBK mice. Symbols indicate statistically significant differences (P < 0.05) between inactive and active periods.
‡P < 0.05 inactive vs. active.
HBK, hepatocyte-specific Bmal1 knockout.
Table 3.
Body weight, kidney weight, and liver weight in flox and HBK mice
| Parameter | Flox | HBK |
|---|---|---|
| Body weight, g | 29.6 ± 0.67 | 29.7 ± 0.44 |
| Kidney weight, g | 0.35 ± 0.02 | 0.34 ± 0.01 |
| Liver weight, g | 1.15 ± 0.11 | 1.16 ± 0.02 |
Values are presented as means ± SE; n = 7–12. Unpaired Student’s t test. HBK, hepatocyte-specific Bmal1 knockout.
Plasma Metabolites and Hormones are Altered in HBK Mice
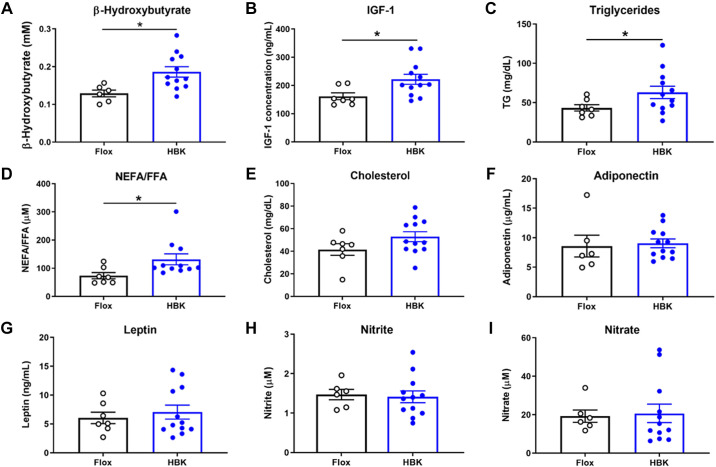
We determined if hepatocyte-specific circadian clock dysfunction led to systemic perturbations in plasma metabolites, hormones, and adipokines. The ketone body metabolite, β-HB, is mainly produced from fatty acids in the liver and contributes to metabolism in extrahepatic tissues (48). IGF-1 is a metabolic hormone primarily produced in liver with insulin-like effects (49), whereas TG, NEFA/FFA, and cholesterol are lipid metabolites. Adipokines have key metabolic roles as adiponectin is involved in glucose homeostasis at liver and muscle, and leptin functions as the “adipostat” by suppressing food intake (50). Nitrite and nitrate are NO metabolites that are important for vascular tone (51). We found that the liver-derived mediators, β-HB and IGF-1, and other lipid metabolites, TG and NEFA/FFA, were significantly elevated in plasma of HBK mice when compared with flox control mice (Fig. 1, A–D), whereas cholesterol was not different between flox and HBK mice (Fig. 1E). The adipokines, adiponectin, and leptin, were similar between genotypes (Fig. 1, F and G). Finally, circulating levels of the NO metabolites, nitrite, and nitrate, were also similar in flox and HBK mice (Fig. 1, H and I). These results indicate that circulating levels of liver-derived metabolites and lipids were found to be higher in HBK mice.
Figure 1.
Plasma metabolites and hormones are altered in HBK mice. β-Hydroxybutyrate (β-HB; A), IGF-1 (B), TG (C), NEFA/FFA (D), cholesterol (E), adiponectin (F), leptin (G), and as the NO metabolites nitrite (H) and nitrate (I) were measured in plasma from flox and HBK mice. Data are presented as means ± SE, n = 6 or 12 mice/genotype, unpaired Student’s t test, *P < 0.05. HBK, hepatocyte-specific Bmal1 knockout; NEFA/FFA, nonesterified fatty acids/free fatty acids; TG, triglycerides.
Disruption of the Liver Clock Increases Expression of Select Energy Metabolism Genes in PVAT
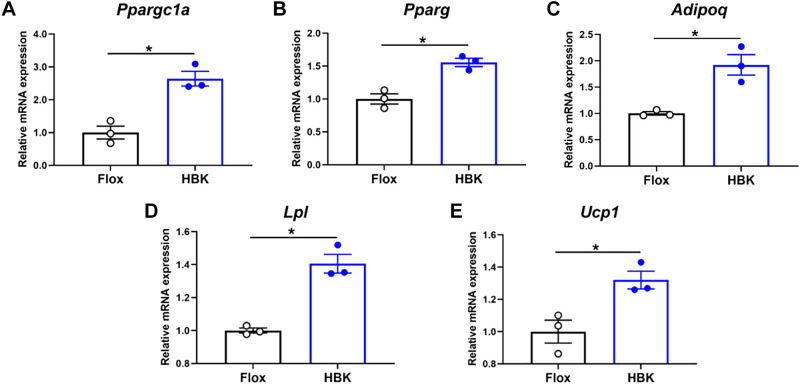
To examine whether liver circadian clock disruption might alter PVAT metabolism and function, we next determined mRNA expression of several key regulatory and energy metabolism genes in PVAT from flox and HBK mice. Interestingly, mRNA levels of peroxisome proliferator-activated receptor γ coactivator 1-α (Ppargc1a), peroxisome proliferator-activated receptor g (Pparg), adiponectin (Adipoq), lipoprotein lipase (Lpl), and uncoupling protein 1 (Ucp1), were significantly higher in PVAT of HBK mice compared with flox control mice (Fig. 2, A–E). These data suggest that the liver circadian clock distally affects PVAT gene expression.
Figure 2.
Disruption of the liver clock increases expression of select energy metabolism genes in PVAT. RT-PCR was used to assess the mRNA expression levels of peroxisome proliferator-activated receptor γ coactivator 1-α (Ppargc1a; A), peroxisome proliferator-activated receptor g (Pparg; B), adiponectin (Adipoq; C), lipoprotein lipase (Lpl; D), and uncoupling protein 1 (Ucp1; E) in PVAT of flox and HBK mice. (n = 3, unpaired Student’s t test, *P < 0.05). HBK, hepatocyte-specific Bmal1 knockout; PVAT, perivascular adipose tissue; RT-PCR, real-time polymerase chain reaction.
PVAT-Dependent Endothelial Function is Altered in HBK Mice
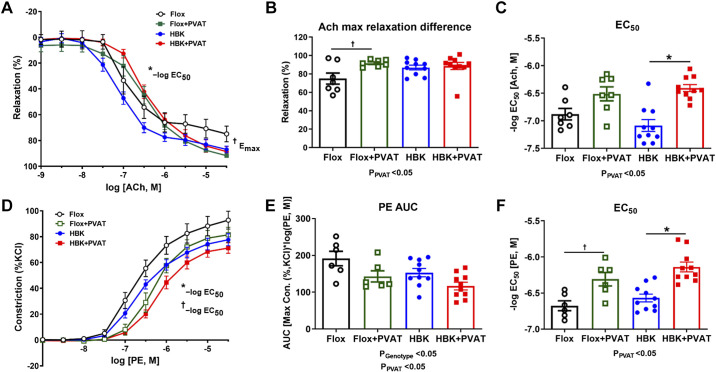
We performed studies to determine whether there are functional differences in thoracic aorta from HBK mice compared with flox mice. Specifically, we performed studies using aortic rings with the PVAT remaining intact or removed to determine the role of PVAT on endothelium-dependent and independent function. First, we assessed ACh-induced vasorelaxation in preconstricted aortic rings in the presence and absence of PVAT (Fig. 3A). There were no statistically significant differences in the maximum ACh-induced vasorelaxation (Emax) or sensitivity of ACh (−log EC50) observed in aortic rings without PVAT from HBK or flox mice (Fig. 3B). PVAT increased the Emax to ACh, but did not influence the sensitivity to ACh in aortic rings from flox mice (Fig. 3, B and C). In contrast, PVAT significantly decreased sensitivity to ACh in aortic rings from HBK mice, but did not statistically modify Emax to ACh (Fig. 3, B and C).
Figure 3.
PVAT-dependent endothelial function is altered in HBK mice. Vascular reactivity studies in the thoracic aorta of HBK and flox mice were assessed with acetylcholine (ACh)-induced vasorelaxation in the absence and presence of PVAT (A), Emax in the absence and presence of PVAT in flox and HBK mice (B), ACh EC50 in the absence and presence of PVAT in flox and HBK mice (C), phenylephrine (PE)-induced vasoconstriction curves in the absence and presence of PVAT (D), PE-induced responses assessed by AUC in flox and HBK mice in the presence and absence of PVAT (E), and PE EC50 in the absence and presence of PVAT in flox and HBK mice (F). Sensitivity (−log EC50) and maximal effect (Emax) were determined in response to agonists. Two-way ANOVA was used to compare effects of PVAT and genotype with Tukey’s post hoc test (n = 6 or 12, *P < 0.05). Refer to Table 4 for values of ACh, SNP, PE, and KCl curves and statistical analysis. Significant two-way ANOVA results are included in graphs. PVAT had a significant effect on ACh Emax (A and B), ACh EC50 (A and C), PE AUC (E), and PE EC50 (D and F). There was a significant effect of genotype on PE AUC (E). Symbols indicate statistically significant differences (P < 0.05) for post hoc tests showing differences within genotypes with (+) and without (−)PVAT. †P < 0.05 Flox + PVAT vs. Flox; *P < 0.05 HBK + PVAT vs. HBK. AUC, area under the curve; HBK, hepatocyte-specific Bmal1 knockout; PE, phenylephrine; PVAT, perivascular adipose tissue; SNP, sodium nitroprusside.
In contrast, PVAT did not statistically modify Emax to ACh, but did significantly decrease the sensitivity to ACh in aortic rings from HBK mice (Fig. 3, B and C). Vasorelaxation to the NO donor, sodium nitroprusside (SNP), which assesses endothelium-independent function, was similar in aortic rings with PVAT present or removed from both flox and HBK mice (Table 4). PVAT significantly increased sensitivity to SNP in a similar manner from aortic rings of flox and HBK mice (Table 4). These findings indicate that endothelium-dependent function (in the absence of PVAT) is unaltered in aorta from HBK mice. However, PVAT-dependent endothelial function is reduced in aorta from HBK mice compared with flox mice, suggesting liver clock disruption induces PVAT-derived factor(s) that mediate endothelial dysfunction.
Table 4.
Emax and −logEC50 in thoracic aorta with PVAT intact (+PVAT) or without PVAT
| Flox | Flox + PVAT | HBK | HBK + PVAT | |
|---|---|---|---|---|
| ACh-induced relaxation, % of PE | ||||
| Emax | 74.9 ± 6.0 | 91.8 ± 1.4† | 87.0 ± 2.5 | 88.6 ± 3.9 |
| −logEC50 | −6.88 ± 0.10 | −6.51 ± 0.13 | −7.09 ± 0.11 | −6.41 ± 0.06* |
| SNP-induced relaxation, % of PE | ||||
| Emax | 102.5 ± 1.1 | 101.8 ± 1.2 | 101.2 ± 0.6 | 102.4 ± 1.6 |
| −logEC50 | −7.52 ± 0.10 | −6.52 ± 0.18† | −7.49 ± 0.08 | −6.35 ± 0.08* |
| PE-induced constriction, % of KCl | ||||
| Emax | 92.8 ± 6.9 | 81.5 ± 5.7 | 77.6 ± 4.6 | 71.2 ± 4.1 |
| −logEC50 | −6.68 ± 0.07 | −6.31 ± 0.09† | −6.57 ± 0.05 | −6.14 ± 0.07* |
| AUC | 191.5 ± 19.6 | 143.0 ± 15.1 | 153.2 ± 11.2 | 116.8 ± 10.4 |
| KCl-induced constriction, % increase in force | ||||
| Emax | 122.5 ± 5.6 | 143.0 ± 6.6 | 121.0 ± 8.6 | 144.4 ± 3.5* |
| −logEC50 | −6.07 ± 0.09 | −6.60 ± 0.15† | −5.95 ± 0.07 | −6.50 ± 0.08* |
| AUC | 258.8 ± 18.2 | 364.8 ± 30.3† | 243.1 ± 22.1 | 351.6 ± 17.6* |
Values are presented as means ± SE; n = 6–10. Two-way ANOVA was used to compare effects of PVAT and genotype with Tukey’s post hoc test for cumulative concentration–response curves to assess vasorelaxation and vasoconstriction (Fig. 3). symbols indicate statistically significant differences (P < 0.05) for post hoc tests showing differences within genotypes with (+) and without (−)PVAT. AUC, area under the curve; HBK, hepatocyte-specific Bmal1 knockout; ACh, acetylcholine; PE, phenylephrine; PVAT, perivascular adipose tissue; SNP, sodium nitroprusside.
†P < 0.05 flox + PVAT vs. flox.
*P < 0.05 HBK + PVAT vs. HBK.
PVAT function is well recognized to be anticontractile. Therefore, we generated cumulative concentration response curves to the adrenergic agonist PE using aortic rings from HBK and flox mice with PVAT attached and PVAT removed (Fig. 3D) to ascertain the role of PVAT in contractile function of the vasculature. The response to PE-induced vasoconstriction in the absence of PVAT (AUC analysis) was significantly reduced in HBK mice compared with flox mice (Fig. 3E). PVAT significantly decreased the total response and significantly reduced the sensitivity of PE-induced vasoconstriction in both genotypes similarly (Fig. 3, E and F). Thus, the PVAT anticontractile function in aorta is similar in HBK and flox mice. Although KCl-induced vasoconstriction in the absence of PVAT was similar in flox and HBK mice, Emax was significantly increased in the aortae of HBK mice with intact PVAT (Table 4). Both genotypes showed a significantly increased AUC in the presence of PVAT and increased sensitivity to KCl-induced vasoconstriction (−log EC50) (Table 4). Thus, these data show that the liver clock disruption leads to a reduced response to PE-induced vasoconstriction compared with flox mice in the absence of PVAT, however the PVAT anticontractile function is not disrupted.
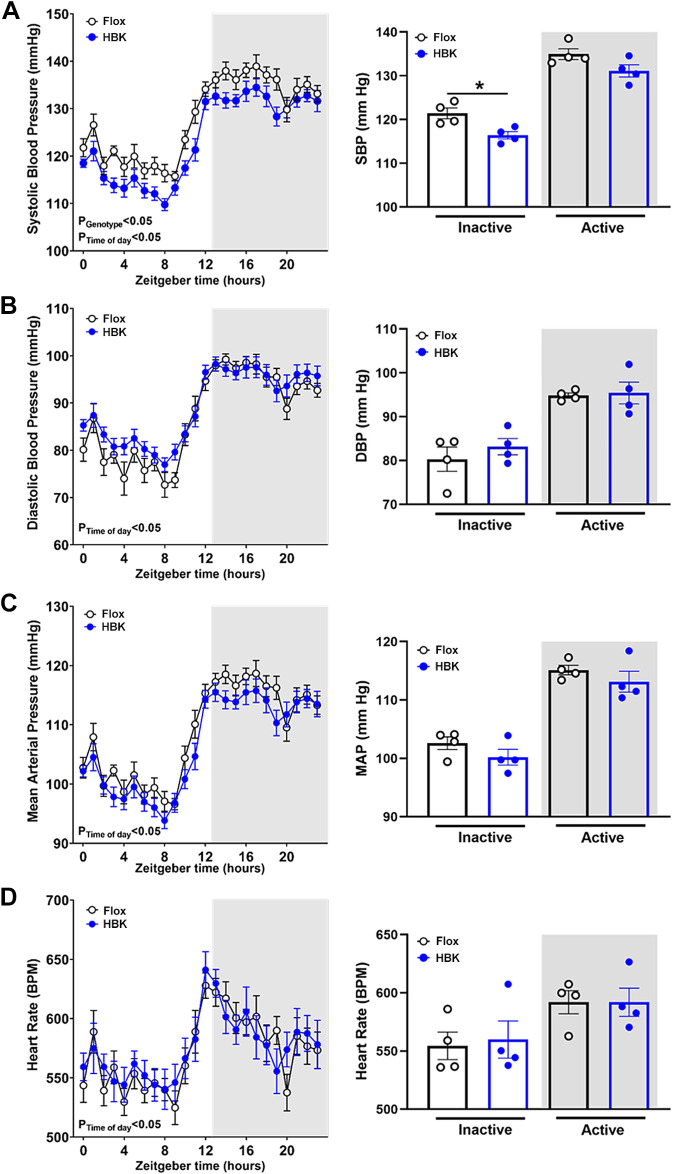
HBK Mice Display Lower Systolic Blood Pressure
To assess whether liver clock disruption changes cardiovascular rhythms, we performed telemetry studies to measure BP and HR in flox and HBK mice. The circadian periods of SBP, DBP, MAP, and HR were similar between flox and HBK mice (Table 5). SBP was significantly lower in HBK mice during the inactive period (Fig. 4A) with a reduced mesor (Table 5). DBP was not statistically different between flox and HBK mice (Fig. 4B). There were no significant differences between flox and HBK mice during the inactive and active period or in the amplitude and mesor of MAP (Fig. 4C) and HR (Fig. 4D). Time domain analysis of HR and frequency domain analysis of HR, SBP, and DBP revealed that autonomic activity was similar in both genotypes (Table 6). These data show that liver Bmal1 deletion does not affect circadian variation in cardiovascular rhythms, but reduces inactive period SBP.
Table 5.
Circadian analysis of telemetry data from flox and HBK mice
| Flox |
HBK |
|||||
|---|---|---|---|---|---|---|
| Parameter | Mesor | Amplitude | Period | Mesor | Amplitude | Period |
| Systolic blood pressure | 128 ± 1 | 22.3 ± 0.6 | 23.93 ± 0.03 | 125 ± 1* | 24.5 ± 1.6 | 23.95 ± 0.02 |
| Diastolic blood pressure | 88 ± 1 | 24.0 ± 3.5 | 23.92 ± 0.06 | 90 ± 2 | 20.7 ± 1.3 | 23.94 ± 0.03 |
| Mean arterial pressure | 109 ± 1 | 21.1 ± 1.2 | 23.93 ± 0.05 | 108 ± 1 | 21.9 ± 1.3 | 23.95 ± 0.02 |
| Heart rate | 594 ± 10 | 83.7 ± 10.1 | 23.80 ± 0.16 | 598 ± 10 | 78.9 ± 6.2 | 23.86 ± 0.06 |
Values are presented as means ± SE; n = 4. Unpaired Student’s t test. Symbols indicate statistically significant differences (P < 0.05) between genotypes. HBK, hepatocyte-specific Bmal1 knockout.
*P < 0.05 HBK vs. flox mice.
Figure 4.
HBK mice display lower systolic blood pressure. Telemetry data for flox and HBK mice were plotted as 1-h averages of 3 consecutive days. Inactive and active period BP and HR for eACh mouse were calculated by averaging values during the inactive (ZT0-12) or active (ZT12-0) 12-h periods and are indicated by white and gray shading, respectively. Systolic blood pressure (SBP; A), diastolic blood pressure (DBP; B), mean arterial pressure (MAP; C), and heart rate (HR; D) in flox controls and HBK mice are shown as traces (left) with 12-h inactive and active averages (right). Two-way repeated-measures ANOVA was used to compare time of day and genotype effects with Sidak’s post hoc test for multiple comparisons (n = 4, *P < 0.05). Significant two-way ANOVA results are included in graphs. Time of day had a significant effect on (A) SBP, (B) DBP, (C) MAP, and (D) HR in both flox and HBK mice. Genotype had a significant effect on SBP (A) with the post hoc test showing significance between genotypes during the inactive period. *P < 0.05 flox vs. HBK. HBK, hepatocyte-specific Bmal1 knockout.
Table 6.
Heart rate and blood pressure frequency and time domain parameters in flox and HBK mice
| Parameter | Flox | HBK |
|---|---|---|
| Time domain | ||
| SDNN, ms | 6.16 ± 0.27 | 6.27 ± 0.29 |
| RMSSD, ms | 4.85 ± 0.26 | 4.97 ± 0.44 |
| pNN6, % | 14.30 ± 1.6 | 13.31 ± 1.86 |
| HR frequency domain | ||
| LF/HF | 1.9 ± 0.1 | 1.9 ± 0.2 |
| LF, µs2 | 82.9 ± 4.0 | 87.7 ± 8.1 |
| HF, µs2 | 74.6 ± 2.0 | 80.5 ± 1.1 |
| TP, µs2 | 308.3 ± 4.5 | 324.9 ± 13.4 |
| SBP frequency domain | ||
| LF/HF | 2.35 ± 0.11 | 2.28 ± 0.14 |
| LF, mmHg2 | 0.075 ± 0.005 | 0.081 ± 0.008 |
| HF, mmHg2 | 0.052 ± 0.003 | 0.057 ± 0.004 |
| TP, mmHg2 | 0.291 ± 0.006 | 0.308 ± 0.013 |
| DBP frequency domain | ||
| LF/HF | 1.18 ± 0.04 | 1.37 ± 0.19 |
| LF, mmHg2 | 0.114 ± 0.005 | 0.113 ± 0.007 |
| HF, mmHg2 | 0.137 ± 0.007 | 0.112 ± 0.013 |
| TP, mmHg2 | 0.409 ± 0.010 | 0.396 ± 0.014 |
Values are presented as means ± SE; n = 4. Average values over 3 consecutive days of recording. DBP, diastolic blood pressure; HBK, hepatocyte-specific Bmal1 knockout; HF, high frequency (1.5–4.0 Hz); HR, heart rate; LF, low frequency (0.4–1.5 Hz); pNN6, proportion of NN intervals differing > 6 ms; RMSSD, root mean squared of successive differences; SBP, systolic blood pressure; SDNN, standard deviation of NN interval; TP, total power (0.01–5.0 Hz). Unpaired Student’s t test.
DISCUSSION
Prior studies have reported that mice with liver-specific Bmal1 deletion have alterations in lipid, glucose, and glycogen metabolism as well as insulin signaling (23–25, 28), which may negatively affect function in extrahepatic tissues. The major findings of this study demonstrate that liver clock disruption: 1) increases levels of circulating β-HB, IGF-1, TG, and NEFA/FFA, 2) increases expression of key metabolism genes in PVAT, 3) reduces aortic PVAT-mediated endothelium-dependent vasorelaxation, but not endothelial function in the absence of PVAT, 4) reduces aortic vasoconstriction in the absence of PVAT, even though PVAT-mediated anticontractile function remains intact, and 5) reduces inactive period SBP with no change in HR heart rate or autonomic function. These findings led us to propose that the liver clock regulates levels of circulating liver-derived metabolites and controls PVAT-derived metabolic gene expression, which may result in mediating PVAT-dependent signaling to the endothelium as well as reduced vasoconstriction and regulating SBP.
HBK Mice and Vascular Function
Previous studies have shown that mice with global (i.e., whole body) deletion of Bmal1 exhibit impaired vasorelaxation and endothelial dysfunction with reduced NO signaling and increased production of reactive oxygen species (52, 53). Smooth muscle-specific deletion of Bmal1 also impairs vascular contractile function, whereas endothelium-specific deletion of Bmal1 promotes thrombogenesis and vascular damage (54–56). Vasorelaxation and vasoconstriction are normal in aortic rings from mice with adipose-specific Bmal1 deletion (57). Although the aforementioned previous reports did not include determinations of PVAT-mediated vascular function, we hypothesized that PVAT function will be impaired in HBK mice. One of the main objectives of this study was to determine whether hepatocyte-specific deletion of Bmal1 alters vascular function. We also decided to test the hypothesis that PVAT function would be impaired in HBK mice. For these studies, we used young adult HBK mice maintained on an ad libitum feeding schedule and their flox littermate controls. Interestingly, we found reduced endothelium function in thoracic aortas with the PVAT still attached from HBK mice compared with controls, whereas there was no genotype difference in endothelial function when PVAT was removed from aortas before assays. In contrast, the total vascular response to adrenergic-induced vasoconstriction, in the absence of PVAT, was reduced. However, the PVAT anticontractile function remained intact in HBK mice. Taken together, these novel data suggest that the liver clock regulates adrenergic-mediated vasoconstriction, as well as PVAT-dependent cross talk to the endothelium.
We hypothesized that liver-derived metabolites, β-HB and IGF-1 (49, 58), may mediate liver clock-specific vascular function. Herein, we found increased plasma levels of β-HB and IGF-1 in HBK mice. Recent studies in endothelial and vascular smooth muscle cells demonstrate that β-HB has protective effects in the vasculature (59, 60). These protective effects prevent vascular senescence in endothelium and smooth muscle with reduced endothelial cell damage under low-glucose conditions (18, 61). The finding that HBK mice have elevated plasma IGF-1 is also consistent with previous studies reporting HBK mice have lower plasma glucose, disrupted rhythms of glucose and glycogen metabolism, and decreased expression of the GLUT-2 transporter in liver (23–25). IGF-1 has been reported to decrease vasoconstriction and vascular resistance by mechanisms including NO-mediated vasodilation and insulin-like effects (62). Furthermore, changes in glucose metabolism previously described (23–25) may also alter vascular function in HBK mice. Future studies are necessary to determine the mechanisms underlying the reduced vasoconstriction in the context of liver clock dysfunction.
To determine potential PVAT-specific mediator(s) regulating endothelium function, we assessed PVAT gene expression in HBK and flox mice. Interestingly, we found that liver-specific Bmal1 deletion regulates mRNA expression of several key metabolic genes Adipoq, Ppargc1a, Pparg, Ucp1, and Lpl in the PVAT of thoracic aorta. These genes were assessed because previous reports showed that PVAT surrounding the thoracic aorta has characteristics similar to BAT (6, 12, 13). Energy dissipation in BAT occurs through mitochondrial uncoupling (63), leading to thermogenesis (64), which is proposed to be protective against the development of cardiometabolic disorders (65). Adipoq encodes the adiponectin protein that is an adipokine with protective effects against cardiometabolic disorders including direct activation of vasorelaxing factors such as NO in the endothelium and thermogenesis (66). The energy metabolism regulators Ppargc1a and Pparg, regulators of adipogenesis and lipid storage, are also important in energy metabolism while Ucp1 mediates thermogenesis (67–69). Hydrolysis of TG by Lpl allows release of fatty acids for adipose tissue, including BAT, and other extrahepatic tissues (70, 71). Previous studies found that global Bmal1 deletion in mice disrupts systemic lipid homeostasis (72–74) with more lipid accumulation in depots of BAT (75) and that Bmal1 regulates adipogenesis (61). Our findings show that under ad libitum feeding, HBK mice have elevated plasma NEFA/FFA and TG, indicating perturbations in lipid metabolism. TG metabolism by hepatocytes is an important function of the liver (43), and elevated plasma TG levels increase vascular disease risk (76, 77). The increased levels of plasma lipids (e.g., NEFA) in HBK mice suggest possible changes in adipose tissue lipolysis as NEFAs are released from lipolysis of triglycerides in adipose tissue (78, 79).
It is well understood that clock disruption is associated with aging and cardiovascular diseases, thus elucidating the role of energy metabolism genes in the PVAT from HBK mice will reveal novel mechanisms for PVAT-mediated vascular function.
HBK Mice and Integrated Cardiovascular Function
An additional objective of this study was to examine whether young adult ad libitum fed HBK mice display a dysfunctional cardiovascular phenotype compared with flox mice. Surprisingly, we found that BP, HR, and autonomic function were maintained, or as observed with SBP during the inactive period, were improved. These findings suggest that there may be compensatory mechanisms having a positive influence on cardiovascular function in the young adult ad libitum fed HBK mice. HR variability and parasympathetic/sympathetic tone parameters were similar between genotypes, suggesting that the reduced SBP in HBK mice is not due to lower sympathetic tone. Plasma nitrite and nitrate were also unaffected in HBK mice, suggesting that NO signaling may not be playing a role in the reduced SBP. Interestingly, mice with adipose-specific Bmal1 deletion also display reduced resting phase SBP and DBP (57). In contrast, endothelium-specific Bmal1 deletion or deletion of Bmal1 in smooth muscle reduces BP during the active phase (55, 56). Previous studies show that higher levels of circulating mediators such as β-HB and IGF-1 may lower blood pressure (80–82). Hypertensive rats on a high-salt diet showed lower β-HB levels and supplementation of a β-HB precursor reduced the hypertension (80). IGF-1 deficient mice have higher blood pressure and low IGF-1 levels are associated with hypertension (81, 82). HBK mice had higher β-HB and IGF-1 levels, thus it is likely that these mediators are directly involved in regulating SBP and the observed changes in vascular function.
Perspectives and Significance
In conclusion, the findings of the current study provide evidence that liver circadian clock disruption alters PVAT gene expression and PVAT-mediated endothelial function, presumably through a change in circulating levels of liver-derived β-HB, IGF-1, and/or other lipid metabolites. These data suggest that a functional molecular circadian clock in the liver may be important for maintaining novel interorgan crosstalk pathways, involving the liver, PVAT, and endothelium and vascular function.
GRANTS
This study was supported by the University of Alabama at Birmingham AMC21 Multi-Investigator Grant to D. M. Pollock, S. M. Bailey, and J. S. Pollock; the National Institutes of Health R21 AA026906 Grant to S. M. Bailey; American Heart Association Grant postdoctoral fellowship 18POST34070051 to P. Pati; the National Institutes of Health postdoctoral fellowship 1F32HL146179-01 to P. Pati; National Institutes of Health predoctoral fellowship F31 DK111067 to R. Sedaka; American Heart Association Grant postdoctoral fellowship 19 POST34380109 to B. K. Becker; and National Institutes of Health 1P01HL136267 Grant to D. M. Pollock and J. S. Pollock.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.P., S.M.B., and J.S.P. conceived and designed research; P.P., J.A.V., D.Z., T.H.N., T.M-S., J.M.A., R.S., and C.J. performed experiments; P.P., J.A.V., D.Z., T.H.N., J.M.A., R.S., B.K.B., D.M.P., S.M.B., and J.S.P. analyzed data; P.P., D.Z., T.H.N., B.K.B., D.M.P., S.M.B., and J.S.P., interpreted results of experiments; P.P. and T.H.N. prepared figures; P.P., S.M.B., and J.S.P. drafted manuscript; P.P., J.A.V., D.Z., T.H.N., T.M-S., J.M.A., R.S., C.J., B.K.B., D.M.P., S.M.B., and J.S.P. edited and revised manuscript; P.P., J.A.V., D.Z., T.H.N., T.M-S., J.M.A., R.S., C.J., B.K.B., D.M.P., S.M.B., and J.S.P., approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Luke Dunaway for expert assistance with vascular function data analyses.
REFERENCES
- 1.Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch 459: 863–879, 2010. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 2.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 3.Widmer RJ, Lerman A. Endothelial dysfunction and cardiovascular disease. Glob Cardiol Sci Pract 2014: 291–308, 2014. doi: 10.5339/gcsp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaborska KE, Wareing M, Austin C. Comparisons between perivascular adipose tissue and the endothelium in their modulation of vascular tone. Br J Pharmacol 174: 3388–3397, 2017. doi: 10.1111/bph.13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baltieri N, Guizoni DM, Victorio JA, Davel AP. Protective role of perivascular adipose tissue in endothelial dysfunction and insulin-induced vasodilatation of hypercholesterolemic LDL receptor-deficient mice. Front Physiol 9: 229, 2018. doi: 10.3389/fphys.2018.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-γ deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation 126: 1067–1078, 2012. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Löhn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J 16: 1057–1063, 2002. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 8.Lynch FM, Withers SB, Yao Z, Werner ME, Edwards G, Weston AH, Heagerty AM. Perivascular adipose tissue-derived adiponectin activates BKCa channels to induce anticontractile responses. Am J Physiol Heart Circ Physiol 304: H786–H795, 2013. doi: 10.1152/ajpheart.00697.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohman MK, Luo W, Wang H, Guo C, Abdallah W, Russo HM, Eitzman DT. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis 219: 33–39, 2011. doi: 10.1016/j.atherosclerosis.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi X-Y, Qu S-L, Xiong W-H, Rom O, Chang L, Jiang Z-S. Perivascular adipose tissue (PVAT) in atherosclerosis: a double-edged sword. Cardiovasc Diabetol 17: 134–134, 2018. doi: 10.1186/s12933-018-0777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quesada I, Cejas J, Garcia R, Cannizzo B, Redondo A, Castro C. Vascular dysfunction elicited by a cross talk between periaortic adipose tissue and the vascular wall is reversed by pioglitazone. Cardiovasc Ther 36: e12322, 2018. doi: 10.1111/1755-5922.12322. [DOI] [PubMed] [Google Scholar]
- 12.Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol 34: 1621–1630, 2014. doi: 10.1161/ATVBAHA.114.303029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol 301: H1425–H1437, 2011. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Victorio JA, Fontes MT, Rossoni LV, Davel AP. Different anti-contractile function and nitric oxide production of thoracic and abdominal perivascular adipose tissues. Front Physiol 7: 295, 2016. doi: 10.3389/fphys.2016.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol 10: 191–196, 2010. doi: 10.1016/j.coph.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gil-Ortega M, Condezo-Hoyos L, García-Prieto CF, Arribas SM, González MC, Aranguez I, Ruiz-Gayo M, Somoza B, Fernández-Alfonso MS. Imbalance between pro and anti-oxidant mechanisms in perivascular adipose tissue aggravates long-term high-fat diet-derived endothelial dysfunction. PLoS One 9: e95312, 2014. doi: 10.1371/journal.pone.0095312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horimatsu T, Patel AS, Prasad R, Reid LE, Benson TW, Zarzour A, Ogbi M, Bruder do Nascimento T, Belin de Chantemele E, Stansfield BK, Lu XY, Kim HW, Weintraub NL. Remote effects of transplanted perivascular adipose tissue on endothelial function and atherosclerosis. Cardiovasc Drugs Ther 32: 503–510, 2018. doi: 10.1007/s10557-018-6821-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrammel A, MussbACher M, Wölkart G, Stessel H, Pail K, Winkler S, Schweiger M, Haemmerle G, Al Zoughbi W, Höfler G, Lametschwandtner A, Zechner R, Mayer B. Endothelial dysfunction in adipose triglyceride lipase deficiency. Biochim Biophys Acta 1841: 906–917, 2014. doi: 10.1016/j.bbalip.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Virdis A, Duranti E, Rossi C, Dell'Agnello U, Santini E, Anselmino M, Chiarugi M, Taddei S, Solini A. Tumour necrosis factor-alpha participates on the endothelin-1/nitric oxide imbalance in small arteries from obese patients: role of perivascular adipose tissue. Eur Heart J 36: 784–794, 2015. doi: 10.1093/eurheartj/ehu072. [DOI] [PubMed] [Google Scholar]
- 20.Nov O, Shapiro H, Ovadia H, Tarnovscki T, Dvir I, Shemesh E, Kovsan J, Shelef I, Carmi Y, Voronov E, Apte RN, Lewis E, Haim Y, Konrad D, Bashan N, Rudich A. Interleukin-1β regulates fat-liver crosstalk in obesity by auto-paracrine modulation of adipose tissue inflammation and expandability. PLoS One 8: e53626, 2013. doi: 10.1371/journal.pone.0053626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosso C, Kazankov K, Younes R, Esmaili S, Marietti M, Sacco M, Carli F, Gaggini M, Salomone F, Møller HJ, Abate ML, Vilstrup H, Gastaldelli A, George J, Grønbæk H, Bugianesi E. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J Hepatol 71: 1012–1021, 2019. doi: 10.1016/j.jhep.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 22.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72: 517–549, 2010. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 23.Lamia KA, Storch K-F, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA 105: 15172–15177, 2008. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou B, Zhang Y, Zhang F, Xia Y, Liu J, Huang R, Wang Y, Hu Y, Wu J, Dai C, Wang H, Tu Y, Peng X, Wang Y, Zhai Q. CLOCK/BMAL1 regulates circadian change of mouse hepatic insulin sensitivity by SIRT1. Hepatology 59: 2196–2206, 2014. doi: 10.1002/hep.26992. [DOI] [PubMed] [Google Scholar]
- 25.Udoh US, Valcin JA, Swain TM, Filiano AN, Gamble KL, Young ME, Bailey SM. Genetic deletion of the circadian clock transcription factor BMAL1 and chronic alcohol consumption differentially alter hepatic glycogen in mice. Am J Physiol Gastrointest Liver Physiol 314: G431–G447, 2018. doi: 10.1152/ajpgi.00281.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan X, Bradfield CA, Hussain MM. Global and hepatocyte-specific ablation of Bmal1 induces hyperlipidaemia and enhances atherosclerosis. Nat Commun 7: 13011, 2016. doi: 10.1038/ncomms13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma D, Liu T, Chang L, Rui C, Xiao Y, Li S, Hogenesch JB, Chen YE, Lin JD. The liver clock controls cholesterol homeostasis through Trib1 protein-mediated regulation of PCSK9/Low Density Lipoprotein Receptor (LDLR) axis. J Biol Chem 290: 31003–31012, 2015. doi: 10.1074/jbc.M115.685982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valcin JA, Udoh US, Swain TM, Andringa KK, Patel CR, Al DS, Baker PRS, Gamble KL, Bailey SM. Alcohol and liver clock disruption increase small droplet macrosteatosis, alter lipid metabolism and clock gene mRNA rhythms, and remodel the triglyceride lipidome in mouse liver. Front Physiol 11: 1048, 2020. doi: 10.3389/fphys.2020.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aneni EC, Oni ET, Martin SS, Blaha MJ, Agatston AS, Feldman T, Veledar E, Conceicao RD, Carvalho JA, Santos RD, Nasir K. Blood pressure is associated with the presence and severity of nonalcoholic fatty liver disease across the spectrum of cardiometabolic risk. J Hypertens 33: 1207–1214, 2015. doi: 10.1097/HJH.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 30.Donati G, Stagni B, Piscaglia F, Venturoli N, Morselli-Labate AM, Rasciti L, Bolondi L. Increased prevalence of fatty liver in arterial hypertensive patients with normal liver enzymes: role of insulin resistance. Gut 53: 1020–1023, 2004. doi: 10.1136/gut.2003.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Suarez A, Guerrero JM, Elvira-Gonzalez J, Beltran-Robles M, Canas-Hormigo F, Bascunana-Quirell A. Nonalcoholic fatty liver disease is associated with blood pressure in hypertensive and nonhypertensive individuals from the general population with normal levels of alanine aminotransferase. Eur J Gastroenterol Hepatol 23: 1011–1017, 2011. doi: 10.1097/meg.0b013e32834b8d52. [DOI] [PubMed] [Google Scholar]
- 32.Fox BM, Becker BK, Loria AS, Hyndman KA, Jin C, Clark H, Johns R, Yanagisawa M, Pollock DM, Pollock JS. Acute pressor response to psychosocial stress is dependent on endothelium-derived endothelin-1. J Am Heart Assoc 7: e007863, 2018. doi: 10.1161/JAHA.117.007863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho DH, Burch ML, Musall B, Musall JB, Hyndman KA, Pollock JS. Early life stress in male mice induces superoxide production and endothelial dysfunction in adulthood. Am J Physiol Heart Circ Physiol 310: H1267–H1274, 2016. doi: 10.1152/ajpheart.00016.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyndman KA, Dugas C, Arguello AM, Goodchild TT, Buckley KM, Burch M, Yanagisawa M, Pollock JS. High salt induces autocrine actions of ET-1 on inner medullary collecting duct NO production via upregulated ETB receptor expression. Am J Physiol Regul Integr Comp Physiol 311: R263–R271, 2016. doi: 10.1152/ajpregu.00016.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang D, Jin C, Obi IE, Rhoads MK, Soliman RH, Sedaka RS, Allan JM, Tao B, Speed JS, Pollock JS, Pollock DM. Loss of circadian gene Bmal1 in the collecting duct lowers blood pressure in male, but not female, mice. Am J Physiol Renal Physiol 318: F710–F719, 2020. doi: 10.1152/ajprenal.00364.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Kang KT, Sullivan JC, Sasser JM, Imig JD, Pollock JS. Novel nitric oxide synthase–dependent mechanism of vasorelaxation in small arteries from hypertensive rats. Hypertension 49: 893–901, 2007. doi: 10.1161/01.HYP.0000259669.40991.1e. [DOI] [PubMed] [Google Scholar]
- 38.Loria AS, Spradley FT, Obi IE, Becker BK, Miguel CD, Speed JS, Pollock DM, Pollock JS. Maternal separation enhances anticontractile perivascular adipose tissue function in male rats on a high-fat diet. Am J Physiol Regul Integr Comp Physiol 315: R1085–R1095, 2018. doi: 10.1152/ajpregu.00197.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spradley FT, Ho DH, Pollock JS. Dahl SS rats demonstrate enhanced aortic perivascular adipose tissue-mediated buffering of vasoconstriction through activation of NOS in the endothelium. Am J Physiol Regul Integr Comp Physiol 310: R286–R296, 2016. doi: 10.1152/ajpregu.00469.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loria AS, Kang KT, Pollock DM, Pollock JS. Early life stress enhances angiotensin II-mediated vasoconstriction by reduced endothelial nitric oxide buffering capacity. Hypertension 58: 619–626, 2011. doi: 10.1161/HYPERTENSIONAHA.110.168674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hyndman KA, Boesen EI, Elmarakby AA, Brands MW, Huang P, Kohan DE, Pollock DM, Pollock JS. Renal collecting duct NOS1 maintains fluid-electrolyte homeostasis and blood pressure. Hypertension 62: 91–98, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boesen EI, Sasser JM, Saleh MA, Potter WA, Woods M, Warner TD, Pollock JS, Pollock DM. Interleukin-1β, but not interleukin-6, enhances renal and systemic endothelin production in vivo. Am J Physiol Renal Physiol 295: F446–F453, 2008. doi: 10.1152/ajprenal.00095.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alves-Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol 8: 1–8, 2017. doi: 10.1002/cphy.c170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solocinski K, Holzworth M, Wen X, Cheng KY, Lynch IJ, Cain BD, Wingo CS, Gumz ML. Desoxycorticosterone pivalate-salt treatment leads to non-dipping hypertension in Per1 knockout mice. Acta Physiol (Oxf) 220: 72–82, 2017. doi: 10.1111/apha.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Speed JS, Hyndman KA, Kasztan M, Johnston JG, Roth KJ, Titze JM, Pollock DM. Diurnal pattern in skin Na+ and water content is associated with salt-sensitive hypertension in ETB receptor-deficient rats. Am J Physiology Regul Integr Comp Physiol 314: R544–R551, 2018. doi: 10.1152/ajpregu.00312.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vukolic A, Antic V, Van Vliet BN, Yang Z, Albrecht U, Montani JP. Role of mutation of the circadian clock gene Per2 in cardiovascular circadian rhythms. Am J Physiol Regul Integr Comp Physiol 298: R627–R634, 2010. doi: 10.1152/ajpregu.00404.2009. [DOI] [PubMed] [Google Scholar]
- 47.Thireau J, Zhang BL, Poisson D, Babuty D. Heart rate variability in mice: a theoretical and practical guide. Exp Physiol 93: 83–94, 2008. doi: 10.1113/expphysiol.2007.040733. [DOI] [PubMed] [Google Scholar]
- 48.Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab 25: 262–284, 2017. doi: 10.1016/j.cmet.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adamek A, Kasprzak A. Insulin-like growth factor (IGF) system in liver diseases. Int J Mol Sci 19: 1308, 2018. doi: 10.3390/ijms19051308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444: 847–853, 2006. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen K, Pittman RN, Popel AS. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal 10: 1185–1198, 2008. doi: 10.1089/ars.2007.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anea CB, Cheng B, Sharma S, Kumar S, Caldwell RW, Yao L, Ali MI, Merloiu AM, Stepp DW, Black SM, Fulton DJ, Rudic RD. Increased superoxide and endothelial NO synthase uncoupling in blood vessels of Bmal1-knockout mice. Circ Res 111: 1157–1165, 2012. doi: 10.1161/CIRCRESAHA.111.261750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD. Vascular disease in mice with a dysfunctional circadian clock. Circulation 119: 1510–1517, 2009. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhatwadekar AD, Beli E, Diao Y, Chen J, Luo Q, Alex A, Caballero S, Dominguez JM 2nd, Salazar TE, Busik JV, Segal MS, Grant MB. Conditional deletion of Bmal1 accentuates microvascular and macrovascular injury. Am J Pathol 187: 1426–1435, 2017. doi: 10.1016/j.ajpath.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westgate E, Cheng Y, Reilly D, Price T, Walisser J, Bradfield C, FitzGerald G. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation 117: 2087–2095, 2008. doi: 10.1161/CIRCULATIONAHA.107.739227. [DOI] [PubMed] [Google Scholar]
- 56.Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, Lefta M, Stauss HM, Guo Z, Gong MC. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Invest 125: 324–336, 2015. doi: 10.1172/JCI76881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang L, Xiong W, Zhao X, Fan Y, Guo Y, Garcia-Barrio M, Zhang J, Jiang Z, Lin JD, Chen YE. Bmal1 in perivascular adipose tissue regulates resting-phase blood pressure through transcriptional regulation of angiotensinogen. Circulation 138: 67–79, 2018. doi: 10.1161/CIRCULATIONAHA.117.029972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newman JC, Verdin E. β-Hydroxybutyrate: a signaling metabolite. Annu Rev Nutr 37: 51–76, 2017. doi: 10.1146/annurev-nutr-071816-064916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han YM, Bedarida T, Ding Y, Somba BK, Lu Q, Wang Q, Song P, Zou MH. β-Hydroxybutyrate prevents vascular senescence through hnRNP A1-mediated upregulation of Oct4. Mol Cell 71: 1064–1078.e5, 2018. doi: 10.1016/j.molcel.2018.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soejima E, Ohki T, Kurita Y, Yuan X, Tanaka K, Kakino S, Hara K, Nakayama H, Tajiri Y, Yamada K. Protective effect of 3-hydroxybutyrate against endoplasmic reticulum stress-associated vascular endothelial cell damage induced by low glucose exposure. PLoS One 13: e0191147, 2018. doi: 10.1371/journal.pone.0191147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, Wada T, Aoyagi T, Tezuka M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci USA 102: 12071–12076, 2005. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sowers JR. Insulin and insulin-like growth factor in normal and pathological cardiovascular physiology. Hypertension 29: 691–699, 1997. doi: 10.1161/01.hyp.29.3.691. [DOI] [PubMed] [Google Scholar]
- 63.Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev 64: 1–64, 1984. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- 64.Smith RE, Horwitz BA. Brown fat and thermogenesis. Physiol Rev 49: 330–425, 1969. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- 65.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature 281: 31–35, 1979. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- 66.Szmitko PE, Teoh H, Stewart DJ, Verma S. Adiponectin and cardiovascular disease: state of the art? Am J Physiol Heart Circ Physiol 292: H1655–H1663, 2007. doi: 10.1152/ajpheart.01072.2006. [DOI] [PubMed] [Google Scholar]
- 67.Busiello RA, Savarese S, Lombardi A. Mitochondrial uncoupling proteins and energy metabolism. Front Physiol 6: 36–36, 2015. doi: 10.3389/fphys.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol Metab 25: 293–302, 2014. doi: 10.1016/j.tem.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang H, Ward WF. PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ 30: 145–151, 2006. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 70.Hoeke G, Kooijman S, Boon Mariëtte R, Rensen PC, Berbée JF. Role of brown fat in lipoprotein metabolism and atherosclerosis. Circ Res 118: 173–182, 2016. doi: 10.1161/CIRCRESAHA.115.306647. [DOI] [PubMed] [Google Scholar]
- 71.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab 297: E271–E288, 2009. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 72.Kennaway DJ, Varcoe TJ, Voultsios A, Boden MJ. Global loss of bmal1 expression alters adipose tissue hormones, gene expression and glucose metabolism. PLoS One 8: e65255, 2013. doi: 10.1371/journal.pone.0065255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2: e377, 2004. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shimba S, Ogawa T, Hitosugi S, Ichihashi Y, Nakadaira Y, Kobayashi M, Tezuka M, Kosuge Y, Ishige K, Ito Y, Komiyama K, Okamatsu-Ogura Y, Kimura K, Saito M. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS One 6: e25231, 2011. doi: 10.1371/journal.pone.0025231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li S, Yu Q, Wang G-X, Lin JD. The biological clock is regulated by adrenergic signaling in brown fat but is dispensable for cold-induced thermogenesis. PLoS One 8: e70109, 2013. doi: 10.1371/journal.pone.0070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Toth PP, Granowitz C, Hull M, Liassou D, Anderson A, Philip S. High triglycerides are associated with increased cardiovascular events, medical costs, and resource use: a real‐world administrative claims analysis of statin‐treated patients with high residual cardiovascular risk. J Am Heart Assoc 7: e008740, 2018. doi: 10.1161/JAHA.118.008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van de Woestijne AP, Wassink AMJ, Monajemi H, Liem A-H, Nathoe HM, van der Graaf Y, Visseren FLJ; SMART study group. Plasma triglyceride levels increase the risk for recurrent vascular events independent of LDL-cholesterol or nonHDL-cholesterol. Int J Cardiol 167: 403–408, 2013. doi: 10.1016/j.ijcard.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 78.Frayn KN. Non-esterified fatty acid metabolism and postprandial lipaemia. Atherosclerosis 141, Suppl 1: S41–S46, 1998. doi: 10.1016/S0021-9150(98)00216-0. [DOI] [PubMed] [Google Scholar]
- 79.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 60: 2441–2449, 2011. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chakraborty S, Galla S, Cheng X, Yeo JY, Mell B, Singh V, Yeoh B, Saha P, Mathew AV, Vijay-Kumar M, Joe B. Salt-responsive metabolite, β-hydroxybutyrate, attenuates hypertension. Cell Rep 25: 677–689.e4, 2018. doi: 10.1016/j.celrep.2018.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Erlandsson MC, Lyngfelt L, Åberg ND, Wasén C, Espino RA, Silfverswärd ST, Nadali M, Jood K, Andersson KME, Pullerits R, Bokarewa MI. Low serum IGF1 is associated with hypertension and predicts early cardiovascular events in women with rheumatoid arthritis. BMC Med 17: 141, 2019. doi: 10.1186/s12916-019-1374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lembo G, Rockman HA, Hunter JJ, Steinmetz H, Koch WJ, Ma L, Prinz MP, Ross J Jr, Chien KR, Powell-Braxton L. Elevated blood pressure and enhanced myocardial contractility in mice with severe IGF-1 deficiency. J Clin Invest 98: 2648–2655, 1996. doi: 10.1172/JCI119086. [DOI] [PMC free article] [PubMed] [Google Scholar]