Abstract
MK5 is a protein serine/threonine kinase activated by p38, ERK3, and ERK4 MAPKs. MK5 mRNA and immunoreactivity are detected in mouse cardiac fibroblasts, and MK5 haplodeficiency attenuates the increase in collagen 1-α1 mRNA evoked by pressure overload. The present study examined the effect of MK5 haplodeficiency on reparative fibrosis following myocardial infarction (MI). Twelve-week-old MK5+/− and wild-type littermate (MK5+/+) mice underwent ligation of the left anterior descending coronary artery (LADL). Surviving mice were euthanized 8 or 21 days post-MI. Survival rates did not differ significantly between MK5+/+ and MK5+/− mice, with rupture of the LV wall being the primary cause of death. Echocardiographic imaging revealed similar increases in LV end-diastolic diameter, myocardial performance index, and wall motion score index in LADL-MK5+/+ and LADL-MK5+/− mice. Area at risk did not differ between LADL-MK5+/+ and LADL-MK5+/− hearts. In contrast, infarct size, scar area, and scar collagen content were reduced in LADL-MK5+/− hearts. Immunohistochemical analysis of mice experiencing heart rupture revealed increased MMP-9 immunoreactivity in the infarct border zone of LADL-MK5+/− hearts compared with LADL-MK5+/+. Although inflammatory cell infiltration was similar in LADL-MK5+/+ and LADL-MK5+/− hearts, angiogenesis was more pronounced in the infarct border zone of LADL-MK5+/− mice. Characterization of ventricular fibroblasts revealed reduced motility and proliferation in fibroblasts isolated from MK5−/− mice compared with those from both wild-type and haplodeficient mice. siRNA-mediated knockdown of MK5 in fibroblasts from wild-type mice also impaired motility. Hence, reduced MK5 expression alters fibroblast function and scar morphology but not mortality post-MI.
NEW & NOTEWORTHY MK5/PRAK is a protein serine/threonine kinase activated by p38 MAPK and/or atypical MAPKs ERK3/4. MK5 haplodeficiency reduced infarct size, scar area, and scar collagen content post-myocardial infarction. Motility and proliferation were reduced in cultured MK5-null cardiac myofibroblasts.
Keywords: extracellular signal-regulated kinase 3/4, fibroblast, MAP kinase-activated protein kinase-5/p38-regulated/activated protein kinase, myocardial infarction, reparative fibrosis
INTRODUCTION
Following myocardial infarction (MI), the infarcted tissue undergoes wound healing and accelerated deposition of extracellular matrix proteins (10). Fibroblasts are the main source of extracellular matrix (ECM) (36). Reparative fibrosis, a critical component of cardiac wound healing, consists of 3 phases: 1) the inflammatory phase (≈3–4 days in mice), in which inflammatory and immune cells digest and clear the damaged cells and the extracellular matrix; 2) the reparative and proliferative phase, which includes resolution of inflammation, (myo)fibroblast migration and proliferation, scar formation, and angiogenesis; and 3) the maturation phase, which involves myofibroblast deactivation, cross-linking of the extracellular matrix, and stabilization of the scar (>14 days in mice). Over time, interstitial fibrosis also occurs within the noninfarcted myocardium and is associated with increased myocardial stiffness. The adverse cardiac remodeling that occurs post-MI contributes to the impaired function and ischemic heart failure that develops following the initial ischemic event (55). In addition, excessive wound repair, which is characterized by marked interstitial cardiac fibrosis and myofibroblast proliferation, can contribute to the development of cardiac hypertrophy and heart failure (31). Moreover, a fatal acute complication following an MI is cardiac rupture due to improper cardiac wound healing (64). Numerous studies have shown that matrix metalloproteinase (MMP)-2 and -9 play a pivotal role in cardiac rupture (18, 27, 28). Hence, appropriate wound healing following an MI involves the careful coordination of numerous processes.
The p38 MAPK pathway is activated during myocardial ischemia and reperfusion (6, 77). There are four known isoforms of p38 (α, β, δ, and γ); all are expressed in the murine heart, with p38α and p38γ being the most abundant (15, 17). MAP kinase-activated protein kinase (MK)-2, -3, and -5 are phosphorylated and activated by p38α and p38β (62). MK5, also known as p38-regulated/activated protein kinase (PRAK), is also phosphorylated by atypical MAPKs ERK3 and ERK4 (34, 66, 67). MK5 transcripts are detected in heart tissue (16, 25, 49) and present in high abundance in the left ventricle (25). In MK5-haplodeficient mice, LV hypertrophy and the increase in collagen type 1-α1 mRNA levels evoked by constriction of the transverse aorta are attenuated (46). Furthermore, MK5 immunoreactivity is detected in adult mouse cardiac fibroblasts, but not in myocytes (46). Hence, the present study to examine the effect of reduced MK5 expression on reparative fibrosis following myocardial infarction using MK5 haplodeficient (MK5+/−) mice (69) was undertaken. Mortality did not differ significantly when followed for up to 21 days post-MI. Although area at risk was unaffected by reduced MK5 expression, infarct size, scar size, and scar collagen content were significantly decreased in MK5+/− hearts. Motility and proliferation were reduced in cardiac ventricular fibroblasts isolated from MK5−/− mice in comparison with fibroblasts from MK5+/+ and MK5+/− mice. Hence, MK5 plays an important role in regulating cardiac fibroblast function and reparative fibrosis.
MATERIALS AND METHODS
Mice.
MK5-knockout mice employed in these studies have been described previously (69) and were produced by targeted disruption of exon 6 on a mixed 129/Ola × C57BL background. Twelve- to 13-wk-old male wild-type and heterozygous littermate male mice were used in this study. MK5−/− mice show embryonic lethality, with incomplete penetrance resulting in 50% of the expected number of MK5−/− embryos detectable after day E12. Hence, it was difficult to obtain MK5−/− mice in sufficient numbers for surgical procedures. As a result, MK5+/− mice were used in these studies. However, MK5−/− mice were available in sufficient numbers to allow a limited number of experiments using MK5-deficient cardiac ventricular fibroblasts. All animal experiments were approved by the local ethics committee and performed according to the guidelines of the Canadian Council on Animal Care.
Myocardial infarction and measurement of infarct size.
Mice (MK5+/+, n = 35; MK5+/−, n = 33) were anesthetized with 2% isoflurane (in 100% oxygen, 1.0 l/min), and myocardial infarction was achieved by permanent ligation of the left anterior descending coronary artery at a level ∼1 mm below the edge of the left atria using a 10-0 nylon suture. Sham operations were performed without the coronary artery being ligated. Buprenorphine (0.1 mg/kg) was administered subcutaneously before as well as 6 and 16 h after surgery. The surgeon was blinded to the genotype of the mice. Cardiac structure and function were assessed by transthoracic echocardiography (see below) 7 days post-MI on mice anesthetized using 2% isoflurane (in 100% oxygen, 1.0 l/min). Surviving mice were euthanized 8 or 21days post-surgery. To examine the possible effects of MK5 haplodeficiency on area at risk (AAR) and infarct size (IS), separate groups of MK5+/+ mice (n = 8) and MK5+/− mice (n = 7) underwent ligation of the left anterior descending coronary artery (LADL) and were perfused with 2% Evans blue dye 30 min after ligation. Hearts were placed at −80°C for 5 min, sliced into four to five transverse sections, photographed, incubated for 20 min in 1% 2,3,5-triphenyltetrazolium chloride (TTC) at 37°C, fixed using 10% formalin in PBS, and photographed again. The AAR (region unstained by Evans blue dye) and the myocardial infarct area (region within the AAR unstained by TTC) were determined using Image Pro Plus version 7.0 and 9.2 software (Media Cybernetics, Silver Spring, MD). The AAR was expressed as a percentage of the LV area [(AAR/LV) × 100], and infarct size (IS) was expressed as percentage of the AAR [(IS/AAR) × 100].
Diagnosis of cardiac rupture.
Necropsies were performed on all nonsurviving mice. Death due to cardiac rupture was diagnosed by the presence of a blood clot around the heart and in the chest cavity and perforation of the infarcted wall.
Cardiac magnetic resonance imaging.
Prior to surgery and 8 days post-MI, late gadolinium enhancement (LGE) contrast-enhanced MRI was performed, using a 30-cm 7T horizontal MR scanner (Agilent/Varian, Palo Alto, CA) on mice (n = 3–5) in a prone position, with a 12-cm inner diameter gradient coil, insert gradient strength 600 mT/m, and rise time of 130 µs. A four-channel receive-only surface coil positioned under the mouse was used in combination with a quadrature transmit/receive birdcage coil with an internal diameter of 69 mm (RAPID Biomedical). Anesthesia was maintained using 2.0–2.5% isoflurane (in 100% oxygen, 1.0 l/min), and body temperature was maintained at 37°C using a warm air fan (SA Instruments, Stony Brook, NY). ECGs were recorded via two metallic needles placed subcutaneously near the heart under the armpits. A pressure transducer for respiratory gating was placed under the abdomen near the sternum. The mice were positioned into the MRI after intraperitoneal injection of a 20-µl bolus of 1.0 mmol/kg Gd-DPTA (Gadovist; Bayer Schering Pharma). Shimming was performed using a combination of manual and automatic sequences to reach a line width of ≤150 Hz over the heart. Scout imaging was performed to obtain the proper orientation in the short-axis plane. LGE Cine-FLASH imaging, started 20–30 min after Gd injection, followed a technique validated by Protti and collaegues (56, 57) (also see Refs. 71 and 75). Imaging was performed with simultaneous ECG triggering and respiration gating (double gating) while steady state was maintained (9, 65). The imaging parameters were effective repetition time equal to the R-R interval, echo time = 1.1 ms, 16 cardiac time frames, field of view 30 × 30 mm, matrix size 128 × 128, slice thickness 1 mm, flip angle 40°, 3–5 averages, 147 kHz acquisition bandwidth, and eight to 10 short-axis slices covering the whole left ventricle from base to apex for a 20- to 30-min acquisition time. The operator was blinded as to the genotype and treatment group of the mice.
Transthoracic echocardiography and calculations.
Transthoracic echocardiography was performed using an i13L probe (10–14 MHz) and a Vivid 7 Dimension system (GE Healthcare Ultrasound, Horten, Norway) on mice sedated with 2% isoflurane in pure oxygen (1.0 l/min) for the duration of the procedure. Two-dimensional echocardiography was used to measure left ventricular outflow tract (LVOT) dimensions, visualize myocardial ischemia, and evaluate wall motion and score as being normal (1), hypokinetic (2), akinetic (3), dyskinetic (4), or aneurysmal (5). The wall motion score index (WMSI) was then calculated as (sum of all scores/no. of segments viewed) for six to 10 segments. The thickness of LV anterior (LVAWd) and posterior walls at end-diastole (LVPWd), LV dimension at end-diastole (LVDd) and end-systole (LVDs), and left atrial dimensions at end-systole (LADs) were measured by M-mode echocardiography. LV mass was calculated using the formula recommended by Liao et al. (42). LV fractional shortening (FS) and ejection fraction (EF) were obtained by formulae within the Vivid 7 system software. Trans-mitral flow peak velocity in early filling (E), E-wave deceleration time (58), deceleration rate (68), and the time interval from mitral valve closure to opening (MVCO) were determined by pulsed wave Doppler (PW), as were velocity of systolic (S) and diastolic (D) waves in both left lower and upper pulmonary venous flow. LVOT flow was obtained by pulsed-wave Doppler and traced to get stroke volume (SV) and cardiac output (CO). LV ejection time (LVET) was measured in LVOT flow. LV global myocardial performance index (MPIglobal) was calculated as (MVCO − LVET)/LVET × 100%. Enlarged pulsed-wave Doppler was used to measure the isovolumetric relaxation time (IVRT). Mitral lateral (SL) and septal annular velocity in systole (SS), early (e′) and atrial diastole (a′), and time interval from ending of a′ to beginning of e′ (b) and from beginning to end of SL/SS (a) were measured by tissue Doppler imaging. LV regional MPI was calculated by (b − a)/a × 100% for both lateral and septum. All measurements comprise the average of three consecutive cardiac cycles.
Histological analysis and immunostaining.
Formalin-fixed hearts were dehydrated by treatment with solutions of increasing alcohol content (70, 95, and 100%), followed by xylene, and then embedded in paraffin. The samples were then cut into 6-μm sections and mounted on charged slides. Each sample underwent histological and immunohistochemical analysis. Paraffin-embedded ventricle short-axis sections were stained with Masson’s trichrome. The thickness of the infarcted wall and the percentage of left ventricular wall that was scar tissue (stained blue) were measured (×20 magnification). Collagen content within the scar was determined by segmentation analysis wherein the intensity of blue (collagen) staining was quantified and expressed as a percentage of the intensity of red (non-collagen) staining within the scar (×20 magnification). Cardiomyocyte diameter was measured in four different regions of the left ventricular wall at ×100 magnification using Image Pro Plus version 7.0 software (Media Cybernetics, Silver Spring, MD).
Before immunohistochemistry, citrate antigen retrieval and endogenous peroxidase blocking (3% hydrogen peroxide) were performed. Sections were then blocked by incubating in phosphate-buffered saline (PBS) containing 10% normal serum (same species as secondary antibody) for 20 min. Tissues were incubated with a commercially available mouse monoclonal antibody against α-smooth muscle actin (α-SMA; Sigma), rabbit polyclonal antibodies against matrix metalloproteinase-9 (MMP-9; AB38898; Abcam), CD206 (anti-mannose receptor antibody, a macrophage marker, AB64693; Abcam), or CD31 (a marker for endothelial cells, SC1506, Santa Cruz Biotechnology) diluted in PBS containing 1% normal serum overnight at 4°C in a humidified chamber. Primary antibodies were omitted for negative controls. After washing, sections were incubated with a biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) for 30 min. Sections were then washed, incubated with streptavidin-HRP (Vector Laboratories) for 30 min, and visualized using a DAB-peroxidase substrate solution (Vector Laboratories). Finally, the sections were counterstained with hematoxylin and mounted with Permount. The number of α-SMA-positive vessels as well as CD31-positive capillary vessels located within noninfarcted myocardium and the infarct border zone was determined by manual counting. MMP-9 and CD206 immunoreactivity in the scar and scar border zone was evaluated by color segmentation. α-SMA-positive myofibroblasts in the infarct were quantified and expressed as numbers of cells per square millimeter of infarcted myocardium. All analyses were performed using Image Pro Plus version 7.0 and 9.2 software (Media Cybernetics).
Immunocytofluorescence.
MK5+/+ and MK5−/− fibroblasts were plated on glass coverslips and maintained in M199 media supplemented with 10% fetal bovine serum (FBS) until 80% confluence, rinsed with PBST, fixed with 2% paraformaldehyde in PBS for 20 min, and then permeabilized and blocked with PBST containing 0.2% Triton X-100 and 2% normal donkey serum. Cells were rinsed once with PBST and incubated overnight at 4°C with antisera against α-SMA (1:100) in blocking solution. Cells were then washed three times with PBST, incubated in the dark for 1 h with Alexa Fluor 555-conjugated secondary antibody (1:500) and DAPI (1:1,000) in PBST, rinsed three times with PBST, and mounted on glass slides using DABCO-glycerol. Images were acquired using a Zeiss LSM-510 confocal fluorescence microscope.
Isolation of myocardial fibroblasts.
Cardiac fibroblasts were isolated from 12- to 14-wk-old MK5+/+, MK5+/−, and MK5−/− mice, as described earlier (39). Briefly, mice were euthanized by cervical dislocation. The heart was excised and placed in sterile PBS (137 mM NaCl, 2.7 mM KCl, 4.2 mM Na2HPO4·H2O, 1.8 mM KH2PO4, pH 7.4) at 37°C. The atria were removed. Ventricular tissue was then macerated using scissors and subjected to a series of digestions in dissociation medium (116.4 mM NaCl, 23.4 mM HEPES, 0.94 mM NaH2PO4·H2O, 5.4 mM KCl, 5.5 mM dextrose, 0.4 mM MgSO4, 1 mM CaCl2, 1 mg/ml BSA, 0.5 mg/ml collagenase type IA, 1 mg/ml trypsin, and 20 μg/ml pancreatin, pH 7.4). Digestion was aided by gentle shaking on an orbital shaker maintained at 37°C. Digests were centrifuged at 1,500 rpm for 5 min, and the pellet was re-suspended in 4 ml of M199 media supplemented with 10% FBS and 2% penicillin-streptomycin. The cell suspension was plated into two 35-mm cell culture dishes and incubated in a humidified incubator at 37°C in a 5% CO2 atmosphere. The medium was changed after 150 min to remove unattached cells and debris. Cultures from passage 2 were used.
Proliferation assay.
Fibroblast proliferation was determined using the CyQUANT Cell Proliferation Assay kit (Invitrogen) according to the manufacturer’s protocol. Briefly, fibroblasts were plated in 96-well plates at a density of 1,000 cells/well. Cardiac fibroblasts were cultured for 24 h in M199 media containing 10% FBS in the presence or absence of 1 μM angiotensin II. After treatment, the medium was removed by gently inverting the microplate. The plates were then stored frozen at −80°C for ≤4 wk. At the time of processing, the plates were thawed at room temperature, 200 μl of CyQUANT dye/cell lysis buffer was added to each well, and plates were incubated for 2–5 min at room temperature in the dark. Fluorescence was measured using a BioTek Synergy 2 Microplate Reader (excitation λ = 480 nm, emission λ = 520 nm).
In addition to the CyQUANT Cell Proliferation Assay, proliferation was assessed as the increase in cell number over 24 h. Cardiac fibroblasts were maintained in culture at 37°C in an atmosphere of 5% CO2 until 80% confluence, washed with PBS, and maintained in serum-free M199 media for 24 h. In the case of fibroblasts from MK5+/+, MK5+/−, and MK5−/− mice, cells were again washed with PBS, followed by the addition of serum-free M199 media alone (control), serum-free M199 media containing 1 μM angiotensin II, M199 containing 10% FBS, or M199 containing 10% FBS and 1 μM angiotensin II. To examine the effect of MK5 knockdown, fibroblasts from MK5+/+ mice were isolated and treated as described below. Following knockdown, fibroblasts were incubated in serum-free M199 media for 12 h and washed with PBS, followed by the addition of serum-free M199 media alone (control), serum-free M199 media containing 1 μM angiotensin II, or M199 containing 10% FBS and incubated for for an additional 24 h. At times 0 and 24 h, images were captured using a Nikon inverted phase contrast microscope at ×4magnification. Cells were counted in a 1 × 1 mm square region of interest using ImageJ software version 1.51w, with the Cell Counter plugin and the cell density at 24 h normalized to the cell density on the same plate at time 0.
Scratch wound assay.
Cardiac fibroblasts were seeded at 3.3 × 104 cells/ml and maintained in culture at 37°C in an atmosphere of 5% CO2 until they were 80% confluent. Cells were then washed with PBS and maintained in serum-free M199 media for 24 h. On the day of the assay, scratches made using 100-μl pipette tips. Wells were then carefully washed with PBS to remove detached cells, followed by the addition of 1 ml of serum-free M199 media alone (control), 1 ml of serum-free M199 media containing 1 μM angiotensin II, 1 ml of M199 containing 10% FBS, or 1 ml of M199 containing 10% FBS and 1 μM angiotensin II. At times 0, 6, and 24 h, images were taken at ×4 magnification using a Nikon inverted phase-contrast microscope. “Wound” areas were quantified using the ImageJ software version 1.49p, and the reduction in wound area was calculated using the following formula: relative wound closure (%) = 100 – [(wound area on the indicated time point/original wound area) × 100].
MK5 knockdown.
Because MK5+/− and MK5−/− mice experience a chronic reduction in MK5 levels, compensatory effects may have occurred. Hence, we also characterized fibroblasts from MK5+/+ mice following a knockdown of MK5 using small inhibitory RNA (siRNA), referred to herein as MK5-kd. These experiments were performed using mice with the same genetic background. Cells (8 × 104/well) were seeded into 12-well plates. Twenty-four hours post-subculture, the media were replaced with Opti-MEM containing Ambion Silencer-Select MK5 siRNA (5 pmol; catalog no. 4390771; ID: s69588 and s69586) and Lipofectamine 2000 (2 µl; Invitrogen) and cells incubated for 19 h. Following a media change and an additional incubation in M199 media containing 10% FBS for 12 h, fibroblasts were incubated in serum-free M199 media for an additional 12 h. On the day of the assay, scratches were made in each well, and the cells were incubated with or without angiotensin II in serum-free M199 for 24 h. Images were captured at time 0 and 24 h later using an inverted phase-contrast microscope at ×4 magnification. Scratch wound areas were quantified using the ImageJ software 1.49p, and the reduction in open wound area relative to the original wound was calculated as described above.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism version 7.0d for the Mac OSX (GraphPad Software, La Jolla, CA). Student’s t-tests were employed for comparisons between two groups. When comparing between more than two groups, one-way ANOVA followed by Bonferroni’s multiple-comparisons test was performed. Two-way ANOVA followed by Bonferroni’s multiple-comparisons test was used between more than two groups with two grouping variables. Survival curves were compared using Mantel-Cox log-rank tests. The correlation between infarct size and collagen content was tested by linear regression analysis. Values were shown as means and SE. A value of P < 0.05 was considered statistically significant.
RESULTS
Survival rates of wild-type and MK5-haploinsufficient mice after LAD ligation.
We have shown previously that MK5 immunoreactivity is present in cardiac fibroblasts but not myocytes and that, compared with wild-type littermates, MK5 haplodeficiency is associated with attenuation of both cardiac hypertrophy and the increase in collagen type 1-α1 mRNA induced by constriction of the transverse aorta (46). In light of this apparent effect of reduced MK5 levels on collagen expression, the present study was undertaken to determine the effects of MK5 haplodeficiency (69) on reparative fibrosis (scar formation) secondary to a myocardial infarction. There are three overlapping phases to post-ischemic cardiac repair wherein dead myocytes are replaced by a stable scar: 1) inflammation, 2) proliferation/fibrosis, and 3) maturation (22). Hence, myocardial infarctions were induced by ligation of the left anterior descending coronary artery, and mice were examined 8 and 21 days later. The cardiac phenotype of MK5-haplodeficient mice has been described (46). Reduced MK5 levels did not predispose mice to early postoperative mortality; six of 35 wild-type mice died or were euthanized within 24 h of ligation, whereas two of 33 MK5-haplodeficient mice died. There were no deaths in the sham-operated groups. Within the first 7 days post-MI, corresponding to the inflammatory and scar formation phases of repair, survival rates for LADL-MK5+/+ and LADL-MK5+/− mice were 62 (20 of 32 survived, excluding 3 mice that were euthanized within 24 h of surgery, as they were in distress) and 71% (22 of 31 survived), respectively (Fig. 1A). Postmortem examination showed that 60% of the premature deaths were due to rupture of the left ventricular wall at the margin of the infarct in wild-type and MK5-haplodeficient mice, with the incidence peaking 4–7 days after surgery. In MK5+/− mice, rupture was associated with large scars (all 7 ruptured hearts were associated with a large scar), whereas MK5+/+ mice with both large- and medium-sized scars experienced rupture of the LV wall (5 out of 8 ruptured hearts were associated with a large scar, whereas 3 out of 8 ruptures were associated with medium-sized scars) (Fig. 1B). In the 21-day cohorts, seven LADL-MK5+/+ mice (out of 16 mice: 44% mortality in week 1) died within 1 wk post-MI, and none died thereafter. In contrast, six LADL-MK5+/− mice (out of 16 mice: 44%) died within 1 wk post-MI, and two additional mice died thereafter (Fig. 1C). Analysis of the survival curves (Fig. 1, A and C) indicated that the difference in mortality observed 7 or 21 days post-MI did not reach significance (Mantel-Cox tests, P = 0.4850, 7 days post-MI; P = 0.9160, 21 days post-MI).
Fig. 1.
MAP kinase-activated protein kinase-5 (MK5)+/+ and MK5+/− mice demonstrated similar mortality rates 1 and 3 wk after myocardial infarction. A: Kaplan-Meier survival curve for MK5+/− (dashed line; n = 31) and MK5+/+ littermate mice (solid line; n = 32) in the first 7 days post-myocardial infarction (MI). B: distribution of infarct sizes in MK5+/− and MK5+/+ mice undergoing cardiac rupture post-MI. C: Kaplan-Meier survival curve for MK5+/− (dashed line; n = 16) and MK5+/+ littermate mice (solid line; n = 16) in the first 21 days post-MI.
Echocardiographic characterization of cardiac structure and function following LAD ligation.
Echocardiographic examination of LADL-MK5+/+ and LADL-MK5+/− mice 7 days after surgery revealed that left ventricular systolic and diastolic diameters were similarly increased, indicating left ventricular dilation (Table 1). Thinning of the left ventricular anterior wall reached significance in MK5+/+ (sham: 0.786 ± 0.013 mm; LADL: 0.576 ± 0.041 mm; n = 20, P < 0.05) but not MK5+/− mice (sham: 0.784 ± 0.019 mm; LADL: 0.641 ± 0.049 mm; n = 17). Left ventricular ejection fraction was significantly reduced in both LADL-MK5+/+ (sham: 76.1 ± 1.1%; LADL: 37.1 ± 4.0%; n = 20, P < 0.0001) and LADL-MK5+/− mice (sham: 72.7 ± 2.0%; LADL: 44.5 ± 5.5%; n = 17, P < 0.01), indicating systolic dysfunction. In addition, the wall motion score index (WMSI), an assessment of regional systolic function, was increased from a value of 1 in the sham groups to 1.86 ± 0.12 and 1.90 ± 0.15 in the LADL-MK5+/+ and LADL-MK5+/− mice, respectively, indicating a similar extent of wall motion abnormality. E-wave deceleration times were reduced, and deceleration rates increased in both LADL-MK5+/+ and LADL-MK5+/− mice, indicating a restrictive filling pattern. Finally, myocardial performance index was increased to a similar extent in both LADL-MK5+/+ and LADL-MK5+/− mice. These observations suggest that reduced expression of MK5 had neither detrimental nor beneficial effects on cardiac function 1-wk post-MI.
Table 1.
Echocardiographic parameters of MK5+/+ and MK5+/− mice 1 wk post-MI
| Sham |
LAD |
|||
|---|---|---|---|---|
| MK5+/+ | MK5+/− | MK5+/+ (n = 20) | MK5+/− (n = 17) | |
| n | 14 | 8 | 20 | 17 |
| R-R interval, ms | 117 ± 4 | 117 ± 3 | 125 ± 6 | 121 ± 4 |
| Dimensions/mass | ||||
| LVAWd, mm | 0.786 ± 0.013 | 0.784 ± 0.019 | 0.576 ± 0.041‡ | 0.641 ± 0.049 |
| LVPWd, mm | 0.723 ± 0.011 | 0.725 ± 0.018 | 0.773 ± 0.029 | 0.783 ± 0.045 |
| LVDd, mm | 3.81 ± 0.07 | 4.00 ± 0.12 | 4.93 ± 0.12† | 4.61 ± 0.17§ |
| LVDs, mm | 2.31 ± 0.06 | 2.53 ± 0.07 | 4.17 ± 0.18† | 3.75 ± 0.26* |
| LV mass, mg | 100 ± 3 | 110 ± 6 | 135 ± 6‡ | 127 ± 6 |
| LV mass/LVDd, mg/mm | 26.4 ± 0.5 | 27.3 ± 0.8 | 27.3 ± 1.1 | 27.8 ± 1.1 |
| LVmass/BW, mg/g | 3.88 ± 0.08 | 4.03 ± 0.19 | 5.44 ± 0.33‡ | 5.11 ± 0.30§ |
| LVDd/BW, mm/g | 0.147 ± 0.002 | 0.148 ± 0.003 | 0.198 ± 0.009† | 0.186 ± 0.010§ |
| Systolic function | ||||
| LVFS, % | 39.4 ± 0.9 | 36.5 ± 1.6 | 16.1 ± 2.0† | 20.2 ± 3.0* |
| LVEF, % | 76.1 ± 1.1 | 72.7 ± 2.0 | 37.5 ± 4.0† | 44.5 ± 5.5* |
| Lateral Sm, cm/s | 2.73 ± 0.09 | 2.66 ± 0.11 | 1.84 ± 0.12† | 1.84 ± 0.13‡ |
| Septal Sm, cm/s | 2.91 ± 0.16 | 2.80 ± 0.12 | 2.18 ± 0.11‡ | 2.16 ± 0.15§ |
| WMSI | 1.000 ± 0.0 | 1.000 ± 0.0 | 1.86 ± 0.12 | 1.90 ± 0.15 |
| Diastolic function | ||||
| E velocity, cm/s | 84.9 ± 3.0 | 86.0 ± 3.6 | 83.2 ± 4.7 | 79.4 ± 3.8 |
| E DT, msec | 38.1 ± 1.9 | 39.9 ± 2.2 | 30.2 ± 1.5* | 28.1 ± 1.3† |
| E D rate, m/s2 | 22.4 ± 1.1 | 22.1 ± 1.4 | 28.2 ± 1.7§ | 28.6 ± 1.4* |
| S/D upper | 0.534 ± 0.044 | 0.509 ± 0.020 | 0.421 ± 0.053 | 0.353 ± 0.067 |
| S/D lower | 1.84 ± 0.09 | 2.03 ± 0.32 | 1.97 ± 0.20 | 2.06 ± 0.16 |
| LV isovolumetric relaxation time | ||||
| IVRT, ms | 7.81 ± 0.67 | 7.91 ± 0.70 | 8.53 ± 0.99 | 7.84 ± 0.81 |
| IVRTc | 0.719 ± 0.059 | 0.730 ± 0.059 | 0.747 ± 0.065 | 0.706 ± 0.06 |
| Myocardial performance index | ||||
| Global MPI, % | 42.9 ± 3.0 | 42.3 ± 3.3 | 63.3 ± 4.2* | 79.3 ± 8.0* |
Values are means ± SE. E, early diastolic transmitral filling velocity; EDT, early diastolic transmitral filling deceleration time; ED rate, early diastolic transmitral filling deceleration rate; FS, fractional shortening; IVRT, isovolumic relaxation time; IVRTc, rate-corrected isovolumic relaxation time; LVAWd, left ventricular anterior wall end-diastolic thickness; LVDd, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVDs, left ventricular end-systolic diameter; LVPWd, LV posterior wall end diastolic thickness; MI, myocardial infarction; MK5, MAP kinase-activated protein kinase-5; MPI, myocardial performance index; S/D, pulmonary venous systolic wave/pulmonary venous early diastolic wave; Sm, peak systolic tissue velocity; WMSI, wall motion score index.
P < 0.05 vs. sham;
P < 0.01 vs. sham;
P < 0.001 vs. sham;
P < 0.0001 vs. sham.
Tissue morphology.
Delayed-enhancement cardiac magnetic resonance imaging (CMRI) using a gadolinium contrast agent provides a noninvasive means to visualize and quantify the infarcted area. Estimates of scar size obtained by CRMI 8 days post-MI and segmentation of the infarct areas using short-axis images suggested reduced infarct size in MK5+/− mice (Fig. 2A). Nine mice in each group were euthanized 8 days post-MI, and their hearts were evaluated. After left ventricular myocardial infarction, the infarcted myocardium undergoes accelerated extracellular matrix deposition (10), and a major component of the scar is collagen, which is critical for the formation of a stable scar. Hence, the collagen content in the scar was examined in paraffin-embedded short-axis sections of the ventricular myocardium by Masson’s trichrome staining (Fig. 2B). Scar area was reduced in MK5-haplodeficient mice (Fig. 2C). In addition, LADL-MK5+/− hearts displayed significantly lower collagen content (stained blue) within the scar compared with LADL-MK5+/+ hearts (Fig. 2D). LV wall thickness across the scar did not differ significantly between LADL-MK5+/+ and LADL-MK5+/− mice (Fig. 2E); there was thinning of LV wall in both LADL-MK5+/+ and LADL-MK5+/− mice compared with their respective sham-operated mice. Finally, no changes in cardiomyocyte diameter in the surviving myocardium were observed 8 days post-MI (data not shown). Furthermore, the number of α-SMA-positive, spindle-shaped myofibroblasts in the infarcted area was assessed by immunohistochemical staining. Eight days post-MI, the number of myofibroblasts in the infarct area was significantly lower in MK5+/− compared with MK5+/+ mice (MK5+/+: 2,512 ±267/mm2; MK5+/−: 1,507 ± 231/mm2, P < 0.05; Fig. 3). No genotype-dependent differences in collagen content were observed in sham hearts (not shown).
Fig. 2.
Scar area and scar collagen content are reduced in MAP kinase-activated protein kinase-5 (MK5)-haplodeficient mice 1 wk post-myocardial infarction (MI). A: representative axial image series spanning the heart from base to apex of hearts from MK5+/+ and MK5+/− mice 8 days post-MI showing the normal myocardium (a) and scar region (b). B: Masson’s trichrome staining of short-axis sections showing collagen deposition (blue) and healthy tissue (red) in the interventricular septum (IVS) and left (LV) and right ventricular (RV) walls of sham and infarcted hearts in MK5+/+ and MK5+/− mice euthanized 8 days after myocardial infarction (MI). C: scar area (in mm2) determined from Masson’s trichrome-stained short-axis sections. D: quantitative analysis of the collagen content in the infarct area of mice euthanized 8 days after MI. Statistical analysis was by 2-way, unpaired t-test. E: LV wall thickness for sham and left anterior descending coronary artery (LADL) mice euthanized 8 days post-MI. Statistical analysis was by 2-way ANOVA, followed by Bonferroni’s multiple-comparisons test; n values are shown in parentheses. Results shown are means ± SE. *P < 0.05; ***P < 0.001.
Fig. 3.
The density of myofibroblasts within the scar was reduced in MAP kinase-activated protein kinase-5 (MK5)-haplodeficient mice 1 wk post-myocardial infarction (MI). Quantitative analysis of the number of α-smooth muscle actin (α-SMA)-positive myofibroblasts within the in MK5+/+ and MK5+/− mice. Top and middle: representative tissue sections stained for α-SMA immunoreactivity from MK5+/+ and MK5+/− mice. Bottom: histogram shows mean data taken from 8 MK5+/+ and 9 MK5+/− mice. Statistical analysis was by unpaired t-test. Bar, 250 μm. *P < 0.05.
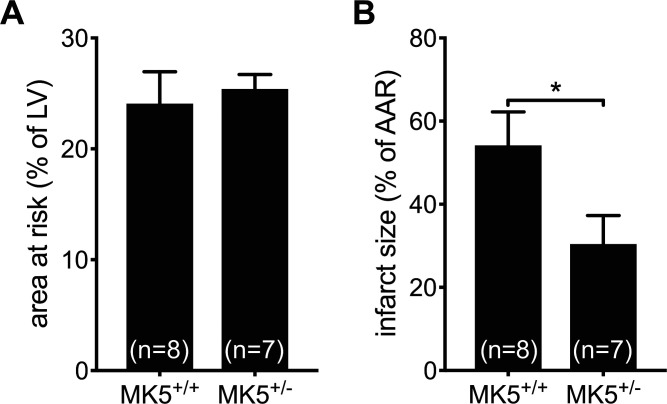
In light of the differences in scar area observed in MK5+/− mice 8 days post-MI (Fig. 4, B and C), the possible effects of MK5 haplodeficiency on the size of the area at risk (AAR) following LAD ligation were assessed. A cohort of MK5+/+ and MK5+/− mice were infused with Evans blue dye, which is excluded from the AAR, 30 min after ligation. MK5 haplodeficiency had no significant effect on the size of the AAR [expressed as %LV area (MK5+/+: 24.1 ± 2.9%, n = 8; MK5+/−: 25.4 ± 1.3%, n = 7, P > 0.05; Fig. 4A]. In contrast, 2,3,5-triphenyltetrazolium chloride (TTC), which stains viable myocardium, revealed that infarcts were significantly smaller in MK5+/− mice in comparison with MK5+/+ mice (expressed as %AAR: MK5+/+: 54.8 ± 8.0%, n = 8; MK5+/−: 30.5 ± 6.8%, n = 7, P < 0.05; Fig. 4B).
Fig. 4.
MAP kinase-activated protein kinase-5 (MK5)-haplodeficient mice demonstrated similar area at risk but reduced infarct size after myocardial infarction. A: area at risk (AAR) as assessed by infusion of Evans blue dye in MK5+/+ and MK5+/− mice (n = 7 or 8/group). The AAR, that area which excluded dye, is expressed as a percentage of the left ventricular (LV) wall area. B: infarct size assessed by triphenyltetrazolium chloride staining. Nonviable myocardium, which is not stained by this dye, is expressed as a percentage of the AAR. No. of animals in each group are indicated in parentheses. Statistical analysis was by 2-way, unpaired t-test. Results shown are means ± SE. *P < 0.05.
Inflammatory cell infiltration.
During post-MI healing, monocytes are recruited to the infarcted region, where they differentiate into macrophages. Macrophages contribute to the healing process by 1) phagocytosing dead cells and extracellular debris, 2) secreting growth factors and cytokines to regulate fibroblast function and angiogenesis, and 3) producing both matrix metalloproteinases (MMPs) and inhibitors of MMP activity (20). The presence of inflammatory cells in the scar was assessed by immunohistochemical staining for CD206 in both mice that died from scar rupture within the first 7 days post-MI and mice euthanized eight days post-MI. CD206 immunoreactivity was similar in the scar border regions of LADL-MK5+/+ and the LADL-MK5+/− mice that experienced scar rupture. However, there was a small but not significant reduction in CD206 immunoreactivity in the scar border regions in LADL-MK5+/− mice euthanized on day 8 compared with LADL-MK5+/+ mice (Fig. 5A).
Fig. 5.
MAP kinase-activated protein kinase-5 (MK5) haplodeficiency does not alter inflammatory cell or matrix metalloproteinase 9 (MMP-9) immunoreactivity in the scar region. A: quantitative analysis of CD206 (an inflammatory cell marker) immunoreactivity-positive cells in the infarct border region of MK5+/+ and MK5+/− mice that either died of heart rupture 5 days post-myocardial infarction (MI) or were euthanized 8 days post-MI. Immunoreactivity was evaluated by color segmentation and expressed as a percentage of the total field area. Statistical analysis was by 2-way ANOVA, followed by Bonferroni’s multiple-comparisons test (n = 3–6/group). B: quantitative analysis of MMP-9 immunoreactivity in the infarct border region of MK5+/+ and MK5+/− mice that either died of heart rupture 5 days post-MI or were euthanized 8 days post-MI. Immunoreactivity was evaluated by color segmentation and expressed as a percentage of the total field area. Statistical analysis was by 2-way ANOVA, followed by Bonferroni’s multiple-comparisons test (n = 3–6/group). Results shown are means ± SE. **P < 0.01; ****P < 0.0001. NS, not significant.
MMP-9 immunoreactivity.
Matrix metalloproteinases (MMPs), including MMP-9, play an important role in extracellular matrix remodeling and wound healing post-MI (18, 28, 60). We next assessed the presence of MMP-9 immunoreactivity in the scar border zone in both mice that experienced scar rupture within the first 7 days post-MI and mice euthanized 8 days post-MI. MMP-9 immunoreactivity was significantly increased in the scar border zone of LADL-MK5+/− hearts having undergone scar rupture (Fig. 5B); however, no differences were observed between LADL-MK5+/+ and LADL-MK5+/− hearts euthanized 8 days after surgery. Moreover, there were significantly lower levels of MMP-9 immunoreactivity in both LADL-MK5+/+ and LADL-MK5+/− hearts 8 days after surgery compared with those that experience scar rupture (Fig. 5B).
Vascularization.
Angiogenesis is an important step for the restoration of blood supply post-MI. Fibroblasts secrete soluble angiogenic growth factors such as vascular endothelial growth factor (VEGF) (23, 35), transforming growth factor-β (53), and platelet-derived growth factor (PDGF) (3). Moreover, fibroblasts promote endothelial cell sprouting and lumen formation (48). Thus, fibroblasts play a key role in angiogenesis. Hence, we next examined whether reduced MK5 expression altered angiogenesis using an immunohistochemical approach with antibodies directed against α-SMA and CD31. No significant differences in the number of α-SMA-positive vessels were observed in LV sections from sham-operated wild-type and MK5-haplodeficient mice (Fig. 6A) or in noninfarcted areas in LADL-MK5+/+ and LADL-MK5+/− hearts (Fig. 6B). In contrast, α-SMA-positive vessels (Fig. 6, C and D) were more abundant in the scar border zone in LADL-MK5+/− compared with LADL-MK5+/+ hearts. Similarly, capillary density was assessed in both healthy myocardium and the infarct border region by staining for CD31 immunoreactivity. The density of CD31-positive capillaries was similar in in LV sections from sham-operated wild-type and MK5-haplodeficient mice (Fig. 6E) but more abundant in the scar border zone in LADL-MK5+/− compared with LADL-MK5+/+ hearts (Fig. 6, F and G).
Fig. 6.
Vascularization is increased in the infarct border region of MAP kinase-activated protein kinase-5 (MK5)-haplodeficient mice 1 wk after myocardial infarction. A–C: quantitative analysis of α-smooth muscle actin (α-SMA)-positive vessels in the left ventricular myocardium of sham MK5+/+ and MK5+/− mice (A), healthy myocardium of the left anterior descending coronary artery (LADL)-MK5+/+ and LADL-MK5+/− hearts (B), and the infarct border region in both LADL-MK5+/+ and LADL-MK5+/− hearts of mice euthanized 8 days post MI (C). D: representative images of α-SMA-positive vessels as indicated by positive staining in the region of the infarct border. E and F: quantitative analysis of numbers of CD31-capillary vessels in sham MK5+/+ and MK5+/− mice (E) and in the infarct border region of both LADL-MK5+/+ and LADL-MK5+/− hearts (F). G: representative images of capillary vessels as indicated by positive staining for CD31 in the infarct border region. Results shown are means ± SE. Bar, 250 μm. *P < 0.05, as determined by unpaired t-test. NS, not significant.
Proliferation is decreased in MK5-deficient fibroblasts.
An essential component of the fibrotic response is an increase in fibroblast proliferation. To determine whether deleting MK5 altered proliferation, ventricular fibroblasts isolated from MK5+/+, MK5+/−, and MK5−/− mice were incubated for 24 h in media containing 10% serum, and then cell number was quantified using a fluorescent, double-stranded DNA-binding dye, CyQUANT. MK5−/− fibroblasts showed a significantly lower rate of proliferation than did the wild-type fibroblasts (Fig. 7). Haplodeficient fibroblasts showed a modest but not significant reduction. These results were then confirmed by direct cell counting in both fibroblasts isolated from MK5+/+, MK5+/−, and MK5−/− mice and following siRNA-mediated MK5 knockdown (Table 2). Cell numbers were reduced in both MK5-deficient fibroblasts and following siRNA knockdown when stimulated with serum. Interestingly, MK5 haplodeficiency did not appear to reduce cell density, and when incubated in the presence of serum plus angiotensin II, cell density was significantly greater for MK5-haplodeficient cells relative to MK5+/+ fibroblasts.
Fig. 7.
Proliferative activity is impaired in MAP kinase-activated protein kinase-5 (MK5)-deficient fibroblasts. Proliferative activity was assessed in cardiac fibroblasts isolated from the ventricular myocardium of MK5+/+ MK5+/− and MK5−/− mice. Fibroblasts were incubated in media supplemented with 10% fetal bovine serum for 24 h. Cell number was then assessed using CyQUANT cell proliferation assays. Statistical analysis was by 1-way ANOVA, followed by Bonferroni’s multiple-comparisons test. Results shown for each genotype are means ± SE of assessments performed in fibroblasts isolated from 3 or 6 different mice, as indicated by the numbers in parentheses. *P < 0.05.
Table 2.
Effect of reduced MK5 expression on cell density
| MK5+/+ | MK5+/− | MK5−/− | scRNA | siRNA | |
|---|---|---|---|---|---|
| n | 3 | 3 | 3 | 3 | 3 |
| Control | 1.13 ± 0.05 | 1.13 ± 0.04 | 1.01 ± 0.02 | 1.09 ± 0.01 | 1.07 ± 0.02 |
| ANG II | 1.25 ± 0.03 | 1.43 ± 0.08 | 1.13 ± 0.06c | 1.29 ± 0.04 | 1.14 ± 0.02e |
| Serum | 1.39 ± 0.05 | 1.41 ± 0.06 | 1.19 ± 0.01a,b | 1.57 ± 0.07 | 1.33 ± 0.03f |
| Serum + ANG II | 1.34 ± 0.06 | 1.54 ± 0.08a | 1.13 ± 0.05a,d |
Values are means ± SE; n = no. of independent fibroblast preparations. ANG II, angiotensin II; MK5, MAP kinase-activated protein kinase-5; scRNA, scrambled RNA; siRNA, small interfering RNA.
P < 0.05 vs. MK5+/+;
P < 0.05 vs. MK5+/−;
P < 0.01 vs. MK5+/−;
P < 0.0001 vs. MK5+/−;
P < 0.05 vs.scRNA;
P < 0.01 vs. scRNA.
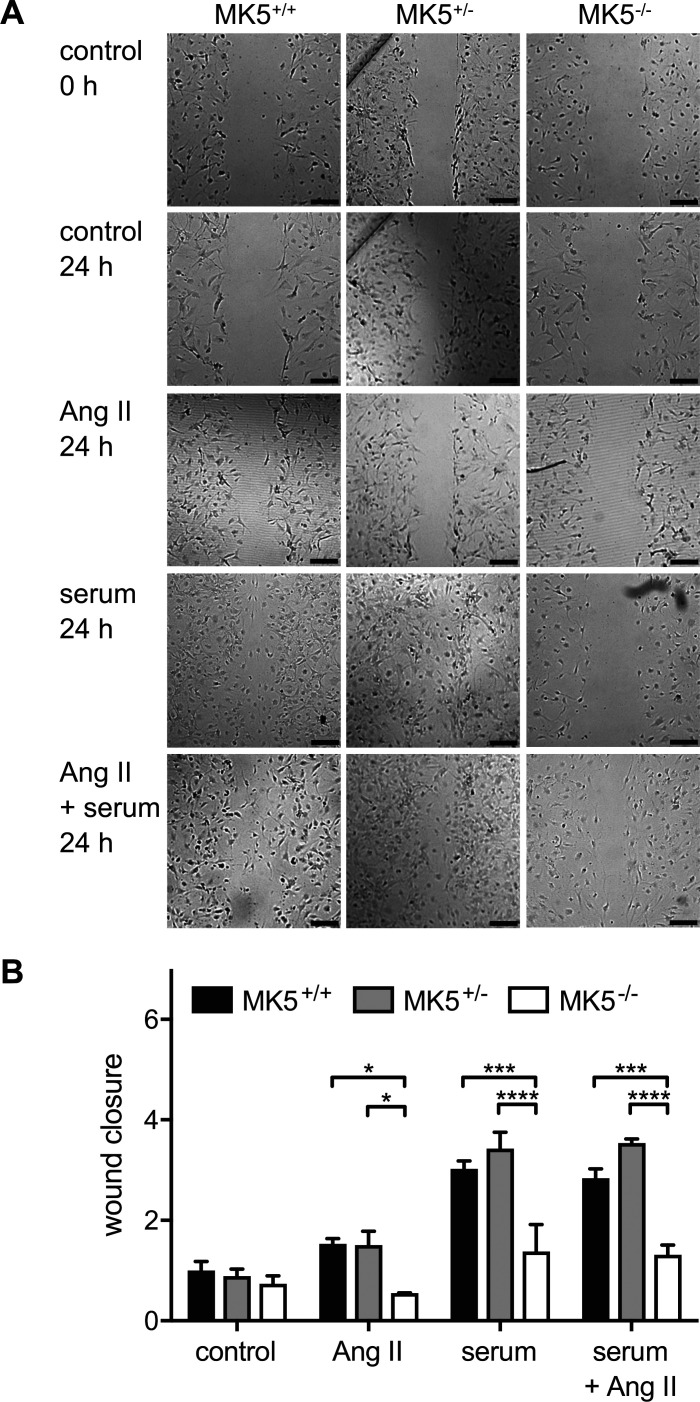
Motility is decreased in MK5-deficient fibroblasts.
Because the scar area and collagen content (Fig. 2, B and C) were reduced in LADL-MK5+/− mice and fibroblasts are the main source of collagen, we next examined the effect of reduced MK5 expression on fibroblast function in vitro. Migration was assessed by scratch wound assay in ventricular fibroblasts isolated from the hearts of MK5+/+, MK5+/−, and MK5−/− mice. Fibroblasts were grown to 80% confluence, “wounds” were created, and the cells were incubated for an additional 24 h with or without the addition of serum and/or angiotensin II to the media. Wound areas were measured morphometrically at time 0 and after 6 and 24 h. After 6 h in culture, no significant differences in wound closure were observed between serum- and/or angiotensin II-stimulated cardiac fibroblasts compared with unstimulated cells. The percentage of wound closure was also comparable between genotypes after 6 h (data not shown), with one exception; in the presence of serum plus angiotensin II, MK5+/− fibroblasts showed significantly greater wound closure than MK5−/− fibroblasts (MK5+/+: 8.0 ± 2.2%; MK5+/−: 20.1 ± 9.0%; MK5−/−: 26.9 ± 0.9%; MK5+/− vs. MK5−/−: P < 0.05; n = 3). Furthermore, after 24 h of culture in serum-free medium, wound closure for cardiac fibroblasts from all three genotypes still did not differ significantly (MK5+/+: 26.2 ± 4.7%; MK5+/−: 23.3 ± 3.7%; MK5−/−: 19.4 ± 4.0%; n = 3; Fig. 8, A and B). However, after 24 h, wound closure in MK5+/+ and MK5+/− fibroblasts treated with angiotensin II was increased significantly greater in comparison with MK5−/− fibroblasts. After 24 h of serum stimulation, wound closure MK5+/+ and MK5+/− fibroblasts was increased 3.03 ± 0.16-fold and 3.42 ± 0.33-fold, respectively, relative to untreated MK5+/+ fibroblasts, whereas that of MK5−/− fibroblasts increased only 1.38 ± 0.53-fold (Fig. 8, A and B). Although the effect of reduced MK5 expression on proliferation may have contributed to the decrease in wound closure, it was not the primary cause. In the presence of serum, the cell density of MK5+/+, MK5+/−, and MK5−/− fibroblasts showed a fold increase of 1.39 ± 0.05, 1.41 ± 0.06, and 1.19 ± 0.01 (n = 3; Table 2), respectively, over 24 h. Treatment with serum and angiotensin II for 24 h produced results comparable with those observed with serum alone.
Fig. 8.
Migration is impaired in MAP kinase-activated protein kinase-5 (MK5)-deficient ventricular fibroblasts. A: migration of cardiac fibroblasts isolated from the ventricular myocardium of MK5+/+, MK5+/−, and MK5−/− mice was assessed by scratch wound migration assay. After the scratch was imposed, fibroblasts were cultured in media alone (control) or media supplemented with angiotensin II (ANG II; 1 μM), serum, or angiotensin II plus serum. Open wound areas were measured at times 0 and 24 h, and wound closure was calculated. B: wound closure after 24 h normalized to the mean closure of the control MK5+/+ group (26.2 ± 4.7%). Statistical analysis was by 2-way ANOVA, followed by Bonferroni’s multiple-comparisons test. Data shown are the mean ± SE of assessments performed in fibroblasts isolated from 3 different mice. Bar, 250 μm. *P < 0.05; ***P < 0.001; ****P < 0.0001.
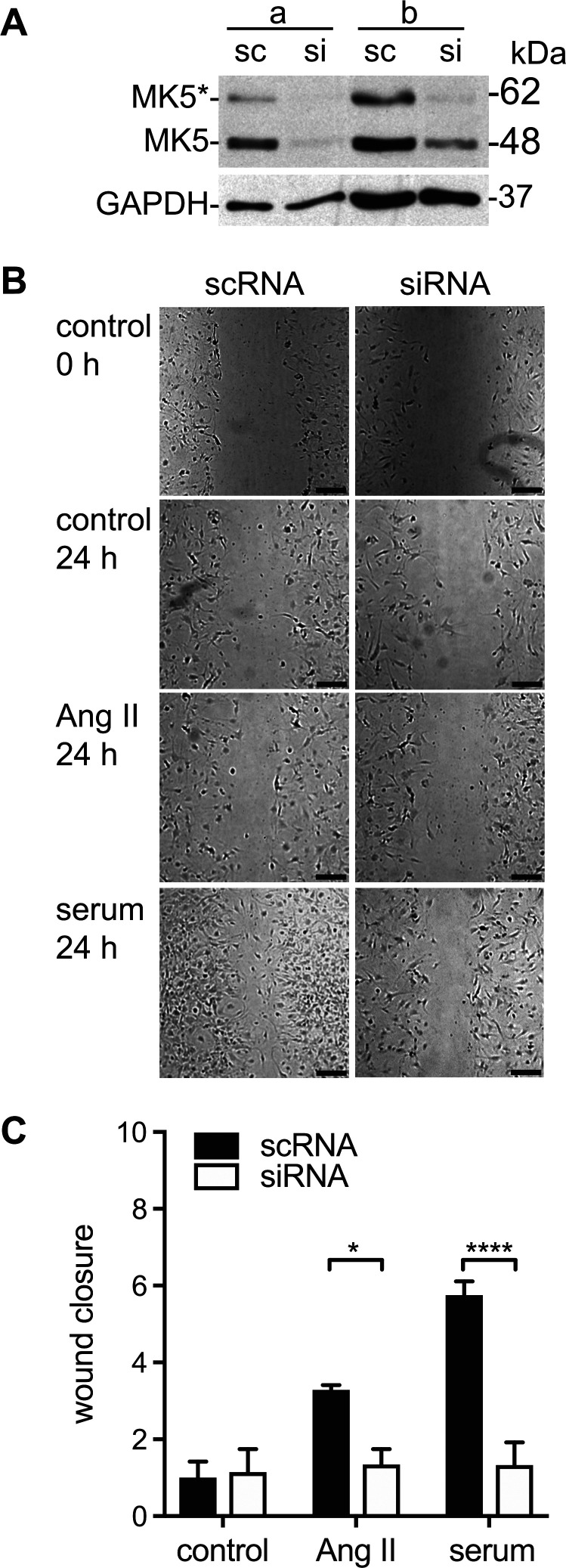
Because a life-long reduction in MK-5 expression may have resulted in compensatory changes, we next examined the effects of an acute reduction in MK5 expression on fibroblast motility. Small interfering RNA was used to silence MK5 expression (MK5-kd). Immunoblotting indicated that the siRNA-mediated knockdown using a single siRNA reduced the abundance of MK5 by 80–85% (Fig. 9A). Knocking down MK5 in cardiac fibroblasts using siRNA decreased wound closure when treated with either serum or angiotensin II for 24 h in comparison with fibroblasts transfected with a scrambled siRNA control (Fig. 9B). As with MK5-deficient fibroblasts, changes in proliferation likely contributed to the decrease in wound closure induced by siRNA-mediated MK5 knockdown, but it was not the primary cause. In the presence of serum, the cell density of fibroblasts treated with scRNA and siRNA showed a fold increase of 1.59 ± 0.07 and 1.33 ± 0.03 (n = 3; Table 2), respectively, over 24 h, whereas wound closure was 4.3-fold greater in fibroblasts treated with scRNA (Fig. 9C). Hence, either an acute or chronic depletion of MK5 markedly impaired cardiac fibroblast motility.
Fig. 9.
Small interfering (si)RNA-mediated knockdown of MAP kinase-activated protein kinase-5 (MK5) impairs fibroblast motility. A: immunoblot showing MK5 immunoreactive bands of 48 (MK5) and 62 kDa (MK5*) in cardiac fibroblast lysates from 2 separate cell preparations (a and b) from MK5+/+ mice transfected with either siRNA for MK5 (si) or a scrambled RNA sequence (sc). The intensity of both 48- and 62-kDa bands was reduced upon knockdown using a single siRNA. Numbers at right indicate the position of molecular mass markers (in kDa). MK5* represents a novel splice variant of MK5 (46). After probing for MK5 immunoreactivity, the membrane was stripped and reprobed for GAPDH immunoreactivity, which was used as a loading control. B: cardiac fibroblasts were isolated from the ventricular myocardium of MK5+/+ mice, split into two, and transfected with either siRNA for MK5 (si) or a scrambled RNA sequence (sc), and motility was assessed by scratch wound assay. After the scratch was imposed, fibroblasts were cultured in media alone (control) or media supplemented with angiotensin II (ANG II; 1 μM) or serum. Open wound areas were measured at times 0 and 24 h, and wound closure was calculated. C: wound closure after 24 h normalized to the mean closure of the control scRNA group (14.5 ± 6.1%). Statistical analysis was by 2-way ANOVA, followed by Bonferroni’s multiple-comparisons test. Data shown are the mean ± SE of assessments performed in fibroblasts isolated from 3 different mice. Bar, 250 μm. *P < 0.05; ****P < 0.0001.
In light of the reduced motility observed in MK5-deficient fibroblasts and the role played by the putative MK5 substrate hsp27 in actin cytoskeletal dynamics (see Ref. 63), we next examined the subcellular localization of α-SMA immunoreactivity in cardiac fibroblasts isolated from MK5+/+ and MK5−/− mice. Confocal immunofluorescence microscopy revealed the presence of stress fibers in both MK5+/+ and MK5−/− fibroblasts (Fig. 10). In contrast, whereas MK5+/+ fibroblasts contained prominent, intersecting bundles of α-SMA in the leading edge of the cells, MK5−/− fibroblasts had thin, parallel bundles of α-SMA in this region (Fig. 10).
Fig. 10.
The actin cytoskeleton is altered in MAP kinase-activated protein kinase-5 (MK5)-deficient fibroblasts. A: confocal immunofluorescent images of the distribution of the α-smooth muscle actin (α-SMA) immunoreactivity in cardiac fibroblasts isolated from MK5+/+ and MK5−/− mice. Cardiac fibroblasts were fixed, labeled with α-SMA antisera and DAPI, which stains DNA, and visualized with a Zeiss LSM 510 confocal fluorescent microscope. B: 3-fold enlargement of the cells in A plus differential interference contrast (DIC) images.
DISCUSSION
The findings in the present study demonstrate that 8 and 21 days post-MI the survival rate for MK5+/+ and MK5+/− mice did not differ significantly. Immediately after ligation, the area at risk in MK5-haplodeficient mice was similar in size to that of MK5+/+ mice, whereas the infarcts were smaller. Eight days post-MI, Masson’s trichrome staining indicated that scars were smaller in MK5-haplodeficient mice, and these scars contained less collagen. MMP-9 immunoreactivity, more abundant in the peri-infarct region of MK5+/− mice having experienced LV wall rupture, and inflammatory cell infiltration did not differ significantly 8 days post-MI, whereas α-SMA-positive vessels were more abundant in the scar border region of MK5+/− hearts. Both fibroblast proliferation and migration, which play important roles in wound healing, were decreased in fibroblasts isolated from MK5−/− mice compared with fibroblasts from MK5+/− or MK5+/+ mice. Because MK5 immunoreactivity is present in cardiac fibroblasts and not in cardiomyocytes (46), these results suggest a role for MK5 in regulating reparative fibrosis via alterations in fibroblast function.
There are three phases to post-MI tissue repair: inflammation, proliferation, and maturation. Myocardial infarction results in cell death and damage to the extracellular matrix, which in turn evoke a transient inflammatory response. Inflammation, in turn, plays a key role in the myocardial healing process (21). In response to cytokine (i.e., TNF, IL-1β, and IL-6) and chemokine release, mononuclear cells and neutrophils migrate into the infarcted myocardium, where cellular and ECM debris are removed by monocyte-derived macrophages. The inflammatory phase is also associated with a rapid activation of MMPs (50, 76). For tissue repair to proceed, the inflammatory response must end. An inflammatory response that is excessive or prolonged may result in left ventricular dilation and systolic dysfunction (21) or possibly cardiac rupture due to excessive MMP activity and ECM degradation (29, 33, 44, 54). Clinical studies have shown cardiac rupture to be associated with increased inflammatory cell infiltration and MMP-9 expression (32, 74). In the present study, the incidence of cardiac rupture as well as left ventricular dilation and reduced systolic function in MK5-haplodeficient mice 7 days post-MI did not differ significantly from wild-type mice, suggesting that the inflammatory phase of tissue repair was not adversely affected by reduced levels of MK5.
MMP-9 activity increases 3–5 days post-MI and then declines as the inflammatory phase of repair is resolved (4, 38). In the mice that died due to cardiac rupture, MK5 haplodeficiency was associated with increased MMP-9 immunoreactivity in the peri-infarct region, but no differences were observed in surviving mice 8 days post-MI. MMP-9 plays a significant role in both ECM remodeling and cardiac rupture following MI (12, 18, 30). Inflammatory cells, including neutrophils and macrophages, have been implicated as a source of MMP in cases of enhanced cardiac rupture (24, 54, 76), and M1 macrophages release MMP-9 (38, 45). However, cardiomyocytes also serve as a source of MMPs in the MI border zone (30). Because there was no difference in macrophage infiltration between MK5+/+ and MK5+/− mice and MK5 immunoreactivity is not detected in adult mouse cardiomyocytes (46), fibroblasts may be responsible for the enhanced MMP-9 production observed in the peri-infarct region of ruptured MK5+/− hearts.
In response to myocardial injury, fibroblasts become activated, differentiate into myofibroblast, and display increased proliferation, migration, and collagen production (8, 14). MAPK signaling plays a role in collagen production as well as migration and proliferation of cardiac fibroblasts (14, 43, 52). Although its mechanism of activation is controversial (69) and its substrates are largely unknown, MK5 has been implicated in several important biological processes, including transcription, cytoskeletal remodeling, migration, and tumorigenesis (reviewed in Ref. 63). Because debris is cleared from the infarcted myocardium, the inflammatory response subsides, and the proliferative phase of tissue repair begins. This process requires the proliferation and migration of fibroblasts as well as their activation to myofibroblasts. One-week post-MI, MK5-haplodeficient mice had smaller scars containing less collagen, with similar mortality rates through cardiac rupture, compared with wild-type littermates. In addition, the density of myofibroblasts within the scar area was lower. Reduced MK5 expression does not prevent murine ventricular fibroblast activation to α-SMA-expressing myofibroblasts in vitro (46). In contrast, we observed that MK5 deficiency reduced both the motility and proliferation of ventricular fibroblasts in culture relative to wild-type or haplodeficient fibroblasts. Previous studies have shown that the ectopic expression of a constitutively active MK5 (MK5 L335G) inhibits proliferation in an U2OS osteosarcoma cell line (37), whereas ectopic expression of wild-type MK5 inhibits Ras-induced proliferation in BJ human fibroblasts (72), murine skin fibroblasts (72), and NIH3T3 cells (11, 41). Furthermore, inhibition of proliferation in NIH3T3 requires nuclear localization of MK5, a characteristic of inactive MK5 (41). The apparent discrepancy on proliferation may be related to differences in cell type or the means whereby MK5 signaling was manipulated experimentally. For example, MK5-deficient mice generated by targeting exon 6 versus exon 8 display phenotypical differences in tumor suppression, although both models involved deletions within the kinase catalytic domain (61, 69, 72).
Cell migration involves cytoskeletal rearrangement, the formation of membrane protrusions, and cell spreading. In its unphosphorylated state, the small heat shock protein hsp27 sequesters globular actin and stabilizes filamentous actin (19). Phosphorylation of hsp27 at Ser15/78/82 causes hsp27 to dissociate from actin, permitting polymerization of cortical actin and microfilament rearrangement (5, 40). Because MK5 can phosphorylate hsp27 at these sites (47), it may be involved in regulating cytoskeletal remodeling. However, there is some debate about MK5 contributing to hsp27 phosphorylation in vivo, as phosphorylation of hsp25 (the rat and mouse homolog of hsp27) in response to cellular stress is unaffected by deletion of MK5 in murine embryonic fibroblasts (69). Alternatively, in other model systems, MK5 has been implicated in hsp27 phosphorylation and actin remodeling. We observed changes in the peripheral actin cytoskeleton in MK5-deficient fibroblasts. In addition, ectopic expression of MK5 in HeLa cells induces hsp27 phosphorylation and formation of actin stress fibers, whereas MK5 T182A, a form of MK5 that cannot be activated, has no effect (13, 73). Migration is reduced in MK5-deficient endothelial cells (78). HeLa cells transfected with MK5 show increased migration, whereas cells transfected with kinase-dead MK5 T182A do not (73). Transient transfection of HeLa cells with 14-3-3ε, which inhibits MK5, inhibits migration induced by either TNFα or ectopic expression of MK5 (73). Transfection of HeLa cells or human HaCaT keratinocytes with hTid-1S, a member of the hsp40 family that inhibits MK5, inhibits TNFα-induced migration, whereas downregulation of endogenous hTid-1S expression in HeLa cells increases both TNFα- and hypoxia-induced migration (13). In tumor-derived cell lines, knocking down IGF2BP1, which increases the abundance of MK5 activator ERK4, results in a decrease in cell migration that is rescued by knocking down MK5 (70). Similarly, VEGF induced migration in wild-type cells but not MK5-deficient mouse vascular endothelial cells or HUVECs (78). In transfected MDA-MB-231 and MCF-7 breast cancer cells, increasing ERK3 expression, an activator of MK5, increases cell motility (2). Hence, a role for MK5 in cytoskeletal remodeling and migration has been demonstrated in several different cell systems; however, the actual role played by MK5 appears to vary, depending upon the cellular model and possibly the cellular environment. In the present study, migration and proliferation were reduced in MK5-deficient fibroblast and following siRNA-mediated knockdown but not haplodeficient cells, whereas differences in reparative fibrosis were observed in MK5-haplodeficient mice. These apparent disparate results may be related to the contextual differences between fibroblasts in vivo and those maintained in 2D culture on a rigid matrix.
Revascularization of the infarct zone is essential for scar formation and the successful healing of the myocardium. Furthermore, mice normally lack collateral circulation in the heart (79). Within few hours of infarction, a rapid angiogenic response that leads to the formation of new blood vessels in the infarct zone to provide oxygen and nutrients to mesenchymal cells in the healing infarct is activated (22). Initially, the newly formed vessels have neither pericyte nor smooth muscle cell mural coat, resulting in hyperpermeable and proinflammatory vessels (22, 59). During the scar maturation phase, the infarct neovessels become coated with these mural cells in response to platelet-derived growth factor (55, 80). Defective vascular coating is associated with prolonged inflammatory extravasation and decreased collagen deposition in the healing infarct. Therefore, proper vessel maturation is required for the inhibition of granulation tissue formation, resolution of the inflammation post-MI, and the formation of a stable scar (55). Eight days post-MI, there was no significant difference in the number of α-SMA or CD31-positive vessels in the healthy myocardium of MK5+/+ and MK5+/− hearts. In contrast, α-SMA and CD31-positive vessels were more abundant in the peri-infarct zone in MK5+/− compared with MK5+/+ hearts. Further studies will be required to determine whether there exists a relationship between the smaller infarct size observed in the MK5-haplodeficient mice and the increased number of mature vessels.
Potential limitations.
The mice employed in the present study express lower amounts of MK5 at the protein level, not just the amount of activatable MK5. Because MK5 has been shown to form complexes with several signaling proteins, including p38 MAP kinase (47, 67), ERK3 (16, 66), ERK4 (1, 34), Cdc14A (26), kalirin-7 (7), and septins 7 and 8 (7), it is not presently clear whether the alterations in reparative fibrosis and fibroblast function we observed were a result of reduced MK5 activity per se or alterations in protein-protein interactions resulting from a reduction in MK5 as a molecular scaffold. In addition, the mice used in this study were a pan-knockout, and considering the important role played by other cell types in post-MI repair of the myocardium, further studies should be undertaken using a fibroblast-targeted deletion of MK5. Furthermore, MK5-haplodeficient mice showed alterations in wound repair, whereas MK5+/− fibroblasts behaved similarly to wild-type fibroblasts in vitro, and only MK5−/− fibroblasts showed marked alterations in motility or proliferation. This may be due to the absence of the many factors, such as stretch, a three-dimensional extracellular matrix, or contact with other cell types, that these cells normally experience in vivo. Finally, although the mouse myocardium lacks collateral circulation (79), which then develops 1–3 days post-MI (51, 79), the area at risk post-MI was unaffected by MK5 haplodeficiency, whereas infarct and scar areas were reduced; no differences in the density of α-SMA-positive vessels were observed between the MK5+/+ and MK5+/− sham mice, suggesting no differences in the microvasculature of the MK5-haplodeficient mouse heart.
Conclusions.
In conclusion, the present study shows that reduced MK5 was neither beneficial nor detrimental to viability, the extent of LV dilation, or systolic dysfunction 8 days post-MI. In contrast, MK5 haplodeficiency resulted in decreased infarct size, increased neovascularization in the peri-infarct region, and reduced scar size and scar collagen content. MK5 deficiency also resulted in impaired proliferative and migratory responses in cultured ventricular fibroblasts. Our study discloses a novel role of MK5 in reparative fibrosis and cardiac fibroblast function.
GRANTS
This work was supported by grants from the Heart and Stroke Foundation of Canada Grants G-14-0006060 and G-18-0022227) and Montreal Heart Institute Foundation (to B. G. Allen) and by the Faculty of Graduate and Postdoctoral Studies of the Université de Montréal and the Egyptian Supreme Council of Universities (to S. A. Nawaito). J.-C. Tardif holds the Canada Research Chair in translational and personalized medicine and the Université de Montréal Pfizer endowed research chair in atherosclerosis.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.N., P.S., M.G., and B.G.A. conceived and designed research; S.N., P.S., M.-É.C.-L., P.P., F.S., Y.S., M.-A.G., and M.G. performed experiments; S.N., P.S., M.-É.C.-L., P.P., F.S., Y.S., M.-A.G., F.L., M.G.S., A.C., J.-C.T., and B.G.A. analyzed data; S.N., P.S., M.-É.C.-L., P.P., Y.S., M.-A.G., F.L., M.G.S., A.C., J.-C.T., and B.G.A. interpreted results of experiments; S.N., P.S., and B.G.A. prepared figures; S.N. and B.G.A. drafted manuscript; S.N., P.S., M.-É.C.-L., P.P., F.S., Y.S., M.-A.G., F.L., M.G., M.G.S., A.C., J.-C.T., and B.G.A. approved final version of manuscript; P.S., M.-É.C.-L., P.P., F.L., M.G.S., A.C., J.-C.T., and B.G.A. edited and revised manuscript.
ACKNOWLEDGMENTS
Histological analysis was performed in the histology core facility in the laboratory of Dr. Martin Sirois at the Montreal Heart Institute. We thank Karine Bouthillier and Robert Parent for animal care and breeding and Dr. Dharmendra Dingar for help in analysis of fibroblast proliferation.
REFERENCES
- 1.Åberg E, Perander M, Johansen B, Julien C, Meloche S, Keyse SM, Seternes OM. Regulation of MAPK-activated protein kinase 5 activity and subcellular localization by the atypical MAPK ERK4/MAPK4. J Biol Chem 281: 35499–35510, 2006. doi: 10.1074/jbc.M606225200. [DOI] [PubMed] [Google Scholar]
- 2.Al-Mahdi R, Babteen N, Thillai K, Holt M, Johansen B, Wetting HL, Seternes OM, Wells CM. A novel role for atypical MAPK kinase ERK3 in regulating breast cancer cell morphology and migration. Cell Adhes Migr 9: 483–494, 2015. doi: 10.1080/19336918.2015.1112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoniades HN, Galanopoulos T, Neville-Golden J, Kiritsy CP, Lynch SE. Injury induces in vivo expression of platelet-derived growth factor (PDGF) and PDGF receptor mRNAs in skin epithelial cells and PDGF mRNA in connective tissue fibroblasts. Proc Natl Acad Sci USA 88: 565–569, 1991. doi: 10.1073/pnas.88.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anzai A, Anzai T, Nagai S, Maekawa Y, Naito K, Kaneko H, Sugano Y, Takahashi T, Abe H, Mochizuki S, Sano M, Yoshikawa T, Okada Y, Koyasu S, Ogawa S, Fukuda K. Regulatory role of dendritic cells in postinfarction healing and left ventricular remodeling. Circulation 125: 1234–1245, 2012. doi: 10.1161/CIRCULATIONAHA.111.052126. [DOI] [PubMed] [Google Scholar]
- 5.Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem 269: 20780–20784, 1994. [PubMed] [Google Scholar]
- 6.Bogoyevitch MA, Gillespie-Brown J, Ketterman AJ, Fuller SJ, Ben-Levy R, Ashworth A, Marshall CJ, Sugden PH. Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart. p38/RK mitogen-activated protein kinases and c-Jun N-terminal kinases are activated by ischemia/reperfusion. Circ Res 79: 162–173, 1996. doi: 10.1161/01.RES.79.2.162. [DOI] [PubMed] [Google Scholar]
- 7.Brand F, Schumacher S, Kant S, Menon MB, Simon R, Turgeon B, Britsch S, Meloche S, Gaestel M, Kotlyarov A. The extracellular signal-regulated kinase 3 (mitogen-activated protein kinase 6 [MAPK6])-MAPK-activated protein kinase 5 signaling complex regulates septin function and dendrite morphology. Mol Cell Biol 32: 2467–2478, 2012. doi: 10.1128/MCB.06633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burstein B, Libby E, Calderone A, Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation 117: 1630–1641, 2008. doi: 10.1161/CIRCULATIONAHA.107.748053. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy PJ, Schneider JE, Grieve SM, Lygate C, Neubauer S, Clarke K. Assessment of motion gating strategies for mouse magnetic resonance at high magnetic fields. J Magn Reson Imaging 19: 229–237, 2004. doi: 10.1002/jmri.10454. [DOI] [PubMed] [Google Scholar]
- 10.Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA 99: 12877–12882, 2002. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen G, Hitomi M, Han J, Stacey DW. The p38 pathway provides negative feedback for Ras proliferative signaling. J Biol Chem 275: 38973–38980, 2000. doi: 10.1074/jbc.M002856200. [DOI] [PubMed] [Google Scholar]
- 12.Chiao YA, Dai Q, Zhang J, Lin J, Lopez EF, Ahuja SS, Chou YM, Lindsey ML, Jin YF. Multi-analyte profiling reveals matrix metalloproteinase-9 and monocyte chemotactic protein-1 as plasma biomarkers of cardiac aging. Circ Cardiovasc Genet 4: 455–462, 2011. doi: 10.1161/CIRCGENETICS.111.959981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi JH, Choi DK, Sohn KC, Kwak SS, Suk J, Lim JS, Shin I, Kim SW, Lee JH, Joe CO. Absence of a human DnaJ protein hTid-1S correlates with aberrant actin cytoskeleton organization in lesional psoriatic skin. J Biol Chem 287: 25954–25963, 2012. doi: 10.1074/jbc.M111.313809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung CC, Kao YH, Yao CJ, Lin YK, Chen YJ. A Comparison of left and right atrial fibroblasts reveals different collagen production activity and stress-induced mitogen-activated protein kinase signaling in rats. Acta Physiol (Oxf) 220: 432–445, 2017. doi: 10.1111/apha.12835. [DOI] [PubMed] [Google Scholar]
- 15.Court NW, dos Remedios CG, Cordell J, Bogoyevitch MA. Cardiac expression and subcellular localization of the p38 mitogen-activated protein kinase member, stress-activated protein kinase-3 (SAPK3). J Mol Cell Cardiol 34: 413–426, 2002. doi: 10.1006/jmcc.2001.1523. [DOI] [PubMed] [Google Scholar]
- 16.Dingar D, Benoit MJ, Mamarbachi AM, Villeneuve LR, Gillis MA, Grandy S, Gaestel M, Fiset C, Allen BG. Characterization of the expression and regulation of MK5 in the murine ventricular myocardium. Cell Signal 22: 1063–1075, 2010. doi: 10.1016/j.cellsig.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dingar D, Merlen C, Grandy S, Gillis MA, Villeneuve LR, Mamarbachi AM, Fiset C, Allen BG. Effect of pressure overload-induced hypertrophy on the expression and localization of p38 MAP kinase isoforms in the mouse heart. Cell Signal 22: 1634–1644, 2010. doi: 10.1016/j.cellsig.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest 106: 55–62, 2000. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.During RL, Gibson BG, Li W, Bishai EA, Sidhu GS, Landry J, Southwick FS. Anthrax lethal toxin paralyzes actin-based motility by blocking Hsp27 phosphorylation. EMBO J 26: 2240–2250, 2007. doi: 10.1038/sj.emboj.7601687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res 58: 88–111, 2008. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 11: 255–265, 2014. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frangogiannis NG. Pathophysiology of Myocardial Infarction. Compr Physiol 5: 1841–1875, 2015. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 23.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B. Tumor induction of VEGF promoter activity in stromal cells. Cell 94: 715–725, 1998. doi: 10.1016/S0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 24.Gao XM, Ming Z, Su Y, Fang L, Kiriazis H, Xu Q, Dart AM, Du XJ. Infarct size and post-infarct inflammation determine the risk of cardiac rupture in mice. Int J Cardiol 143: 20–28, 2010. doi: 10.1016/j.ijcard.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Gerits N, Shiryaev A, Kostenko S, Klenow H, Shiryaeva O, Johannessen M, Moens U. The transcriptional regulation and cell-specific expression of the MAPK-activated protein kinase MK5. Cell Mol Biol Lett 14: 548–574, 2009. doi: 10.2478/s11658-009-0020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen CA, Bartek J, Jensen S. A functional link between the human cell cycle-regulatory phosphatase Cdc14A and the atypical mitogen-activated kinase Erk3. Cell Cycle 7: 325–334, 2008. doi: 10.4161/cc.7.3.5354. [DOI] [PubMed] [Google Scholar]
- 27.Hayashidani S, Tsutsui H, Ikeuchi M, Shiomi T, Matsusaka H, Kubota T, Imanaka-Yoshida K, Itoh T, Takeshita A. Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. Am J Physiol Heart Circ Physiol 285: H1229–H1235, 2003. doi: 10.1152/ajpheart.00207.2003. [DOI] [PubMed] [Google Scholar]
- 28.Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, Levi M, Nüβe O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med 5: 1135–1142, 1999. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- 29.Hilfiker-Kleiner D, Shukla P, Klein G, Schaefer A, Stapel B, Hoch M, Müller W, Scherr M, Theilmeier G, Ernst M, Hilfiker A, Drexler H. Continuous glycoprotein-130-mediated signal transducer and activator of transcription-3 activation promotes inflammation, left ventricular rupture, and adverse outcome in subacute myocardial infarction. Circulation 122: 145–155, 2010. doi: 10.1161/CIRCULATIONAHA.109.933127. [DOI] [PubMed] [Google Scholar]
- 30.Inoue T, Ikeda M, Ide T, Fujino T, Matsuo Y, Arai S, Saku K, Sunagawa K. Twinkle overexpression prevents cardiac rupture after myocardial infarction by alleviating impaired mitochondrial biogenesis. Am J Physiol Heart Circ Physiol 311: H509–H519, 2016. doi: 10.1152/ajpheart.00044.2016. [DOI] [PubMed] [Google Scholar]
- 31.Ju H, Zhao S, Jassal DS, Dixon IM. Effect of AT1 receptor blockade on cardiac collagen remodeling after myocardial infarction. Cardiovasc Res 35: 223–232, 1997. doi: 10.1016/S0008-6363(97)00130-2. [DOI] [PubMed] [Google Scholar]
- 32.Kameda K, Matsunaga T, Abe N, Fujiwara T, Hanada H, Fukui K, Fukuda I, Osanai T, Okumura K. Increased pericardial fluid level of matrix metalloproteinase-9 activity in patients with acute myocardial infarction: possible role in the development of cardiac rupture. Circ J 70: 673–678, 2006. doi: 10.1253/circj.70.673. [DOI] [PubMed] [Google Scholar]
- 33.Kandalam V, Basu R, Abraham T, Wang X, Awad A, Wang W, Lopaschuk GD, Maeda N, Oudit GY, Kassiri Z. Early activation of matrix metalloproteinases underlies the exacerbated systolic and diastolic dysfunction in mice lacking TIMP3 following myocardial infarction. Am J Physiol Heart Circ Physiol 299: H1012–H1023, 2010. doi: 10.1152/ajpheart.00246.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kant S, Schumacher S, Singh MK, Kispert A, Kotlyarov A, Gaestel M. Characterization of the atypical MAPK ERK4 and its activation of the MAPK-activated protein kinase MK5. J Biol Chem 281: 35511–35519, 2006. doi: 10.1074/jbc.M606693200. [DOI] [PubMed] [Google Scholar]
- 35.Kellouche S, Mourah S, Bonnefoy A, Schoëvaert D, Podgorniak MP, Calvo F, Hoylaerts MF, Legrand C, Dosquet C. Platelets, thrombospondin-1 and human dermal fibroblasts cooperate for stimulation of endothelial cell tubulogenesis through VEGF and PAI-1 regulation. Exp Cell Res 313: 486–499, 2007. doi: 10.1016/j.yexcr.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol 5: 123, 2014. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kress TR, Cannell IG, Brenkman AB, Samans B, Gaestel M, Roepman P, Burgering BM, Bushell M, Rosenwald A, Eilers M. The MK5/PRAK kinase and Myc form a negative feedback loop that is disrupted during colorectal tumorigenesis. Mol Cell 41: 445–457, 2011. doi: 10.1016/j.molcel.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 38.Kubota A, Hasegawa H, Tadokoro H, Hirose M, Kobara Y, Yamada-Inagawa T, Takemura G, Kobayashi Y, Takano H. Deletion of CD28 co-stimulatory signals exacerbates left ventricular remodeling and increases cardiac rupture after myocardial infarction. Circ J 80: 1971–1979, 2016. doi: 10.1253/circj.CJ-16-0327. [DOI] [PubMed] [Google Scholar]
- 39.Kumaran C, Shivakumar K. Calcium- and superoxide anion-mediated mitogenic action of substance P on cardiac fibroblasts. Am J Physiol Heart Circ Physiol 282: H1855–H1862, 2002. doi: 10.1152/ajpheart.00747.2001. [DOI] [PubMed] [Google Scholar]
- 40.Lavoie JN, Hickey E, Weber LA, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem 268: 24210–24214, 1993. [PubMed] [Google Scholar]
- 41.Li Q, Zhang N, Zhang D, Wang Y, Lin T, Wang Y, Zhou H, Ye Z, Zhang F, Lin SC, Han J. Determinants that control the distinct subcellular localization of p38α-PRAK and p38β-PRAK complexes. J Biol Chem 283: 11014–11023, 2008. doi: 10.1074/jbc.M709682200. [DOI] [PubMed] [Google Scholar]
- 42.Liao Y, Ishikura F, Beppu S, Asakura M, Takashima S, Asanuma H, Sanada S, Kim J, Ogita H, Kuzuya T, Node K, Kitakaze M, Hori M. Echocardiographic assessment of LV hypertrophy and function in aortic-banded mice: necropsy validation. Am J Physiol Heart Circ Physiol 282: H1703–H1708, 2002. doi: 10.1152/ajpheart.00238.2001. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell MD, Laird RE, Brown RD, Long CS. IL-1β stimulates rat cardiac fibroblast migration via MAP kinase pathways. Am J Physiol Heart Circ Physiol 292: H1139–H1147, 2007. doi: 10.1152/ajpheart.00881.2005. [DOI] [PubMed] [Google Scholar]
- 44.Monden Y, Kubota T, Tsutsumi T, Inoue T, Kawano S, Kawamura N, Ide T, Egashira K, Tsutsui H, Sunagawa K. Soluble TNF receptors prevent apoptosis in infiltrating cells and promote ventricular rupture and remodeling after myocardial infarction. Cardiovasc Res 73: 794–805, 2007. doi: 10.1016/j.cardiores.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 45.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41: 14–20, 2014. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nawaito SA, Dingar D, Sahadevan P, Hussein B, Sahmi F, Shi Y, Gillis MA, Gaestel M, Tardif JC, Allen BG. MK5 haplodeficiency attenuates hypertrophy and preserves diastolic function during remodeling induced by chronic pressure overload in the mouse heart. Am J Physiol Heart Circ Physiol 313: H46–H58, 2017. [Erratum in Am J Physiol Heart Circ Physiol 313: H458, 2017. 10.1152/ajpheart.00597.2017-corr.2017. 28801482.] doi: 10.1152/ajpheart.00597.2016. [DOI] [PubMed] [Google Scholar]
- 47.New L, Jiang Y, Zhao M, Liu K, Zhu W, Flood LJ, Kato Y, Parry GC, Han J. PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J 17: 3372–3384, 1998. doi: 10.1093/emboj/17.12.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CC. The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol Biol Cell 22: 3791–3800, 2011. doi: 10.1091/mbc.e11-05-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ni H, Wang XS, Diener K, Yao Z. MAPKAPK5, a novel mitogen-activated protein kinase (MAPK)-activated protein kinase, is a substrate of the extracellular-regulated kinase (ERK) and p38 kinase. Biochem Biophys Res Commun 243: 492–496, 1998. doi: 10.1006/bbrc.1998.8135. [DOI] [PubMed] [Google Scholar]
- 50.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 94: 1543–1553, 2004. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 51.Nishikido T, Oyama JI, Shiraki A, Tsukamoto I, Igarashi J, Node K. COA-Cl (2-Cl-C.OXT-A) can promote coronary collateral development following acute myocardial infarction in mice. Sci Rep 9: 2533, 2019. doi: 10.1038/s41598-019-39222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan Z, Zhao W, Zhang X, Wang B, Wang J, Sun X, Liu X, Feng S, Yang B, Lu Y. Scutellarin alleviates interstitial fibrosis and cardiac dysfunction of infarct rats by inhibiting TGFβ1 expression and activation of p38-MAPK and ERK1/2. Br J Pharmacol 162: 688–700, 2011. doi: 10.1111/j.1476-5381.2010.01070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paunescu V, Bojin FM, Tatu CA, Gavriliuc OI, Rosca A, Gruia AT, Tanasie G, Bunu C, Crisnic D, Gherghiceanu M, Tatu FR, Tatu CS, Vermesan S. Tumour-associated fibroblasts and mesenchymal stem cells: more similarities than differences. J Cell Mol Med 15: 635–646, 2011. doi: 10.1111/j.1582-4934.2010.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng H, Xu J, Yang XP, Dai X, Peterson EL, Carretero OA, Rhaleb NE. Thymosin-β4 prevents cardiac rupture and improves cardiac function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol 307: H741–H751, 2014. doi: 10.1152/ajpheart.00129.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 119: 91–112, 2016. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Protti A, Dong X, Andia ME, Yu B, Dokukina K, Chaubey S, Phinikaridou A, Vizcay-Barrena G, Taupitz M, Botnar RM, Shah AM. Assessment of inflammation with a very small iron-oxide particle in a murine model of reperfused myocardial infarction. J Magn Reson Imaging 39: 598–608, 2014. doi: 10.1002/jmri.24191. [DOI] [PubMed] [Google Scholar]
- 57.Protti A, Sirker A, Shah AM, Botnar R. Late gadolinium enhancement of acute myocardial infarction in mice at 7T: cine-FLASH versus inversion recovery. J Magn Reson Imaging 32: 878–886, 2010. doi: 10.1002/jmri.22325. [DOI] [PubMed] [Google Scholar]
- 58.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol 36: 598–606, 2004. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Ren G, Michael LH, Entman ML, Frangogiannis NG. Morphological characteristics of the microvasculature in healing myocardial infarcts. J Histochem Cytochem 50: 71–79, 2002. doi: 10.1177/002215540205000108. [DOI] [PubMed] [Google Scholar]
- 60.Rohde LE, Ducharme A, Arroyo LH, Aikawa M, Sukhova GH, Lopez-Anaya A, McClure KF, Mitchell PG, Libby P, Lee RT. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation 99: 3063–3070, 1999. doi: 10.1161/01.CIR.99.23.3063. [DOI] [PubMed] [Google Scholar]
- 61.Ronkina N, Johansen C, Bohlmann L, Lafera J, Menon MB, Tiedje C, Laaß K, Turk BE, Iversen L, Kotlyarov A, Gaestel M. Comparative analysis of two gene-targeting approaches challenges the tumor-suppressive role of the protein kinase MK5/PRAK. PLoS One 10: e0136138, 2015. doi: 10.1371/journal.pone.0136138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68: 320–344, 2004. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sahadevan P, Allen BG. MK5: A novel regulator of cardiac fibroblast function? IUBMB Life 69: 785–794, 2017. doi: 10.1002/iub.1677. [DOI] [PubMed] [Google Scholar]
- 64.Sane DC, Mozingo WS, Becker RC. Cardiac rupture after myocardial infarction: new insights from murine models. Cardiol Rev 17: 293–299, 2009. doi: 10.1097/CRD.0b013e3181bf4ab4. [DOI] [PubMed] [Google Scholar]
- 65.Schneider JE, Cassidy PJ, Lygate C, Tyler DJ, Wiesmann F, Grieve SM, Hulbert K, Clarke K, Neubauer S. Fast, high-resolution in vivo cine magnetic resonance imaging in normal and failing mouse hearts on a vertical 11.7 T system. J Magn Reson Imaging 18: 691–701, 2003. doi: 10.1002/jmri.10411. [DOI] [PubMed] [Google Scholar]
- 66.Schumacher S, Laaß K, Kant S, Shi Y, Visel A, Gruber AD, Kotlyarov A, Gaestel M. Scaffolding by ERK3 regulates MK5 in development. EMBO J 23: 4770–4779, 2004. doi: 10.1038/sj.emboj.7600467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seternes OM, Mikalsen T, Johansen B, Michaelsen E, Armstrong CG, Morrice NA, Turgeon B, Meloche S, Moens U, Keyse SM. Activation of MK5/PRAK by the atypical MAP kinase ERK3 defines a novel signal transduction pathway. EMBO J 23: 4780–4791, 2004. doi: 10.1038/sj.emboj.7600489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi Y, Denault AY, Couture P, Butnaru A, Carrier M, Tardif JC. Biventricular diastolic filling patterns after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg 131: 1080–1086.e3, 2006. doi: 10.1016/j.jtcvs.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 69.Shi Y, Kotlyarov A, Laaß K, Gruber AD, Butt E, Marcus K, Meyer HE, Friedrich A, Volk HD, Gaestel M. Elimination of protein kinase MK5/PRAK activity by targeted homologous recombination. Mol Cell Biol 23: 7732–7741, 2003. doi: 10.1128/MCB.23.21.7732-7741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stöhr N, Köhn M, Lederer M, Glass M, Reinke C, Singer RH, Hüttelmaier S. IGF2BP1 promotes cell migration by regulating MK5 and PTEN signaling. Genes Dev 26: 176–189, 2012. doi: 10.1101/gad.177642.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stuckey DJ, Carr CA, Camelliti P, Tyler DJ, Davies KE, Clarke K. In vivo MRI characterization of progressive cardiac dysfunction in the mdx mouse model of muscular dystrophy. PLoS One 7: e28569, 2012. doi: 10.1371/journal.pone.0028569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun P, Yoshizuka N, New L, Moser BA, Li Y, Liao R, Xie C, Chen J, Deng Q, Yamout M, Dong MQ, Frangou CG, Yates JR III, Wright PE, Han J. PRAK is essential for ras-induced senescence and tumor suppression. Cell 128: 295–308, 2007. doi: 10.1016/j.cell.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 73.Tak H, Jang E, Kim SB, Park J, Suk J, Yoon YS, Ahn JK, Lee JH, Joe CO. 14-3-3epsilon inhibits MK5-mediated cell migration by disrupting F-actin polymerization. Cell Signal 19: 2379–2387, 2007. doi: 10.1016/j.cellsig.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 74.van den Borne SW, Cleutjens JP, Hanemaaijer R, Creemers EE, Smits JF, Daemen MJ, Blankesteijn WM. Increased matrix metalloproteinase-8 and -9 activity in patients with infarct rupture after myocardial infarction. Cardiovasc Pathol 18: 37–43, 2009. doi: 10.1016/j.carpath.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 75.Vanhoutte L, Gerber BL, Gallez B, Po C, Magat J, Jean-Luc B, Feron O, Moniotte S. High field magnetic resonance imaging of rodents in cardiovascular research. Basic Res Cardiol 111: 46, 2016. [Erratum in Basic Res Cardiol 111: 55, 2016. 10.1007/s00395-016-0572-3. 27492500.] doi: 10.1007/s00395-016-0565-2. [DOI] [PubMed] [Google Scholar]
- 76.Yang Y, Ma Y, Han W, Li J, Xiang Y, Liu F, Ma X, Zhang J, Fu Z, Su YD, Du XJ, Gao XM. Age-related differences in postinfarct left ventricular rupture and remodeling. Am J Physiol Heart Circ Physiol 294: H1815–H1822, 2008. doi: 10.1152/ajpheart.00831.2007. [DOI] [PubMed] [Google Scholar]
- 77.Yin T, Sandhu G, Wolfgang CD, Burrier A, Webb RL, Rigel DF, Hai T, Whelan J. Tissue-specific pattern of stress kinase activation in ischemic/reperfused heart and kidney. J Biol Chem 272: 19943–19950, 1997. doi: 10.1074/jbc.272.32.19943. [DOI] [PubMed] [Google Scholar]
- 78.Yoshizuka N, Chen RM, Xu Z, Liao R, Hong L, Hu WY, Yu G, Han J, Chen L, Sun P. A novel function of p38-regulated/activated kinase in endothelial cell migration and tumor angiogenesis. Mol Cell Biol 32: 606–618, 2012. doi: 10.1128/MCB.06301-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang H, Faber JE. De-novo collateral formation following acute myocardial infarction: Dependence on CCR2+ bone marrow cells. J Mol Cell Cardiol 87: 4–16, 2015. doi: 10.1016/j.yjmcc.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zymek P, Bujak M, Chatila K, Cieslak A, Thakker G, Entman ML, Frangogiannis NG. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol 48: 2315–2323, 2006. doi: 10.1016/j.jacc.2006.07.060. [DOI] [PubMed] [Google Scholar]