Abstract
Hyperoxia can lead to respiratory failure and death. Our previous work demonstrates that oxidant and mitochondrial injury play a critical role in hyperoxia-induced acute lung injury (HALI). Recently, thyroid hormone has been demonstrated to promote mitochondrial survival in other models of lung injury, but its role in hyperoxia is unknown. Adult wild-type (WT) mice were pretreated with either nebulized triiodothyronine (T3, 40 μg/kg) for 1 or 3 days, or with propylthiouracil (PTU, 100 μg/kg), for 3 days. Following pretreatment, WT mice underwent 72 h of hyperoxia exposure. WT and PINK1−/− mice were pretreated with either nebulized T3 (40 μg/kg) for 3 days or no pretreatment before 72 h continuous hyperoxia exposure. Bronchoalveolar lavage (BAL), histological changes in cellular composition, and type I cytokine induction were assessed. Lung lysates for mitochondrial cellular bioenergetics markers were analyzed by Western blot. Hyperoxia caused a significant increase in BAL total cell counts and lung cellular infiltrates. Administration of PTU enhanced HALI, whereas T3 attenuated HALI, inflammation, and oxidants in WT mice. In addition, T3 pretreatment increased mitochondrial biogenesis/fusion/mitophagy and decreased ER stress and apoptosis. PINK1−/− mice were more susceptible to hyperoxia than WT mice. Notably, pretreatment with T3 did not attenuate HALI in PINK1−/− mice. In addition, T3 pretreatment increased mitochondrial anti-ROS potential, improved mitochondrial bioenergetics and mitophagy, and attenuated mitochondria-regulated apoptosis, all in a PINK1-dependent manner. Our results highlight a novel protective role for PINK1 in mediating the cytoprotective effects of thyroid hormone in HALI. Therefore, thyroid hormone may represent a potential therapy for ALI.
Keywords: HALI, hyperoxia, lung injury, PINK1, thyroid hormone
INTRODUCTION
Exposure of the lung to elevated levels of oxygen or hyperoxia, leads to a noninfectious form of lung injury called hyperoxia-induced lung injury (HALI) (1). HALI occurs clinically in patients with a variety of respiratory maladies who are placed on mechanical ventilation, treated with levels of inspired oxygen above ambient air. Although in one sense mechanical ventilation is lifesaving, ultimately the endothelial cells of the lung can be injured under such conditions, thus leading to poor patient outcomes. Reactive oxygen species (ROS) and other oxidant mediators lead to pulmonary epithelial cell injury and death in HALI (1). Efforts to prevent HALI may serve as agents to protect the lung from hyperoxia, ultimately benefiting patients who are critically ill.
Throughout the body and especially in the lung, a key pathway to maintain cellular homeostasis is the process of autophagy, where lysosomes recycle cellular building blocks (2–4). When autophagy involves mitochondria, it is termed mitophagy. Mitophagy is a key component of mitochondrial homeostasis or mitochondrial quality control (MQC), as mitochondria are under constant assault from ROS (2–4). A central pathway involved in mitophagy involves the interaction between PTEN-induced kinase I (PINK1) and the E3 ubiquitin ligase PARKIN. When PINK-1 and Parkin bind, a positive feedback loop leads to ubiquitination of mitochondrial proteins, autophagosome targeting, and removal of mitochondria (2–4). Prior work by Zhang et al. (5) has shown that PINK1 and PARKIN levels are elevated in HALI. In addition, PINK1 deficiency worsens HALI (5).
The thyroid hormone triiodothyronine (T3) is crucial to a variety of homeostatic processes (6, 7). Thyroid hormone has been shown to prevent lung fibrosis in a variety of models and T3 also promotes mitochondrial survival in other models of lung injury (8–16). Therefore, we assessed if T3 would protect lung mitochondria from injury under hyperoxia. The drug propylthiouracil (PTU) is an agent that counters T3 production, by inhibiting the action of the enzyme thyroid peroxidase. This is commonly used to manage conditions of T3 excess and was used in several of our experiments to preclude T3’s activities in alveolar tissues. Furthermore, given the critical role PINK1 has in MQC, we assessed the role of T3 in MCQ in both wild-type and PINK1 knockout mice.
MATERIALS AND METHODS
Mice
Six-week-old adult PINK1−/− mice and Parkin−/− mice were provided by Dr. Jack Elias, Brown University and Dr. Jie Shen, Harvard University. All mice were backcrossed for >10 generations onto a C57BL/6J background. C57BL/6J wild-type (WT) mice were purchased from Jackson Lab (Bar Harbor, ME). Half of the mice were male and half female. All protocols were reviewed and approved by the Yale University Institutional Animal Care and Use Committee (IACUC).
Hyperoxia Exposure
Mouse hyperoxia experiments have been described previously (5). Mice were placed a Plexiglas chamber with 5 L/min of 100% oxygen continuous flow. Mice were allowed food and water ad libitum. In injury experiments, after 72 h of hyperoxia exposure, mice were removed from the hyperoxia chamber and euthanized. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Yale University (IACUC).
Mouse Treatments
WT mice were nebulized with either triiodothyronine (T3, 40 µg/kg) for 1 or 3 days, or propylthiouracil (PTU, 100 µg/kg) for 3 days, before 72 h continuous hyperoxia exposure. WT mice were orally gavaged with GC-1 (5 mg/kg) followed by exposure to room air (RA) or hyperoxia for 72 h (H3), and had a period of observation of 1, 2, 3, or 6 days (R1–3, 1, 2, 3, and 6 days posthyperoxia).
Measurement of Lung Injury Markers
Bronchoalveolar lavage (BAL) and protein quantification have been previously described (5). In brief, BAL was performed twice with 0.8 mL phosphate-buffered saline (PBS) (pH 7.4) after mice were euthanized. Cells in BAL specimens were counted as previously described. The protein concentration was determined by the BCA Protein Assay Reagent (Thermo Fisher Scientific Inc., Waltham, MA).
Lactate Dehydrogenase Assay
Lactate dehydrogenase (LDH) was detected by Cytotoxicity Detection Kit (#11644793001, Roche, South San Francisco, CA) in mouse BAL, according to the manufacturer’s instructions.
Amplex Red Assay
Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (A22188, Invitrogen, Carlsbad, CA) was used to measure H2O2 released from mouse lung BAL samples.
ELISA
Mice BAL samples were assessed for IL1β, IL6, and TNFα by ELISA (BD Biosciences).
Western Blot Analysis
Lung protein analyses were performed as previously described (17). In brief, whole lung tissues were homogenized in M-PER mammalian protein extraction reagent (78501, Thermo Fisher Scientific Inc.). Protein concentrations were determined by BCA Protein Assay (Thermo Fisher Scientific Inc.). Samples were electrophoresed in 4%–20% ready-made Tris-HCl gels (Bio-Rad Laboratories) and electrophoretically transferred onto nitrocellulose membranes. After blocking for 1 h in 5% nonfat dry milk in 1X Tris-buffered saline, 0.1% Tween20 (M-TTBS), the membranes were incubated overnight with anti-light chain 3 isoform B (LC3B), -Caspase3, -Beclin-1 (Cell Signaling Technology), -PINK1 (Millipore, clone N4/15), -LAMP2A, -UCP2, -mitofusin-2 (MFN2), -dynamin-related protein 1 (DRP1; Abcam), -Proteasome 20S α6 subunit (Enzo Life Sciences), -STC1, -Parkin, -GADD153, -PGC-1α, and -β-actin (Santa Cruz Biotechnology) antibodies. After three washes in TTBS, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody in M-TTBS followed washing in TTBS and detection of signal with an ECL Western Blotting Detection Kit (Amersham).
Statistics
Statistical analyses of data were conducted with two-way analysis of variance (ANOVA). Bonferroni’s multiple comparisons test was used for pairwise comparisons. The Tukey honestly significant difference (HSD) post hoc test calculator was applied to the multiple comparisons. One factor comparison was analyzed by Mann–Whitney Test. Data are expressed as mean ± SD. Significant difference was accepted at P < 0.05.
RESULTS
T3 Pretreatment Attenuates Hyperoxia-Induced Lung Injury, Inflammation and Oxidants
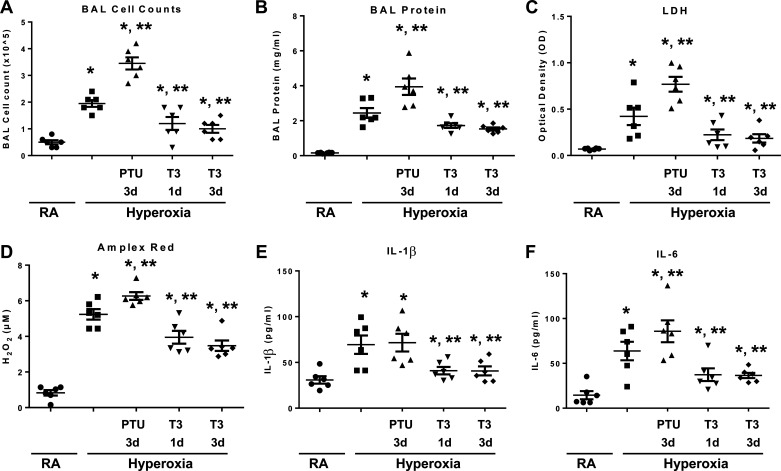
We treated WT mice with nebulized triiodothyronine (T3) or the inhibitor of thyroid hormone synthesis, propylthiouracil (PTU), before 72 h continuous hyperoxia exposure (Fig. 1). We previously reported 72 h of continuous hyperoxia as a time of maximal lung injury, which we used as a representative time point. T3 pretreated mice exhibited significantly decreased BAL cell counts, decreased BAL protein level, decreased lung injury, decreased reactive oxygen species generation (H2O2 release detected by Amplex red assay), and decreased IL1β and IL6 secretion, compared to WT mice (Fig. 2, A–F).
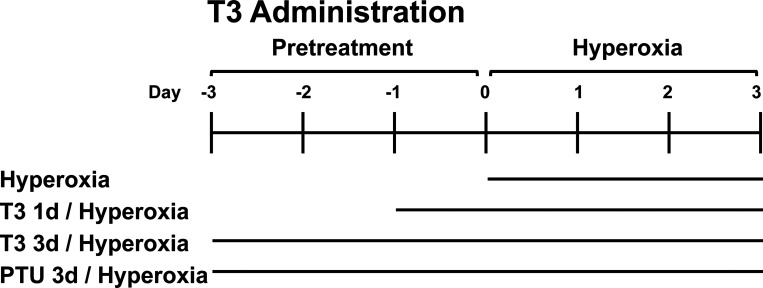
Figure 1.
Schema for triiodothyronine (T3) delivery. Wild-type (WT) mice were nebulized with triiodothyronine (T3, 40 µg/kg) for 1 day or 3 days, or propylthiouracil (PTU, 100 µg/kg) for 3 days before 72 h continuous hyperoxia exposure. Control mice were exposed to room air (RA).
Figure 2.
Triiodothyronine (T3) pretreatment attenuates hyperoxia-induced lung injury, inflammation, and oxidants in wild-type (WT) mice compared with mice treated with propylthiouracil (PTU). WT mice were nebulized with triiodothyronine (T3, 40 µg/kg) for 1 day or 3 days or propylthiouracil (PTU, 100 µg/kg) for 3 days before 72 h continuous hyperoxia exposure. Control mice were exposed to room air (RA). A: total cells recovered from bronchoalveolar lavage (BAL) were counted. B: lung permeability was assessed by BAL protein content. C) lactate dehydrogenase (LDH) activity assays were performed on BAL fluid. D: oxidant generation was detected by Amplex Red from BAL fluid. IL-1β (E) and IL-6 (F) were detected by ELISA in BAL fluid. The values are expressed as means ±SD and analyzed by Mann–Whitney test (n = 6 for each group). *P < 0.05, vs. RA; **P < 0.05, vs. hyperoxia without pretreatment.
T3 Pretreatment Increases Mitochondrial Biogenesis/Fusion/Mitophagy and Decreases ER Stress
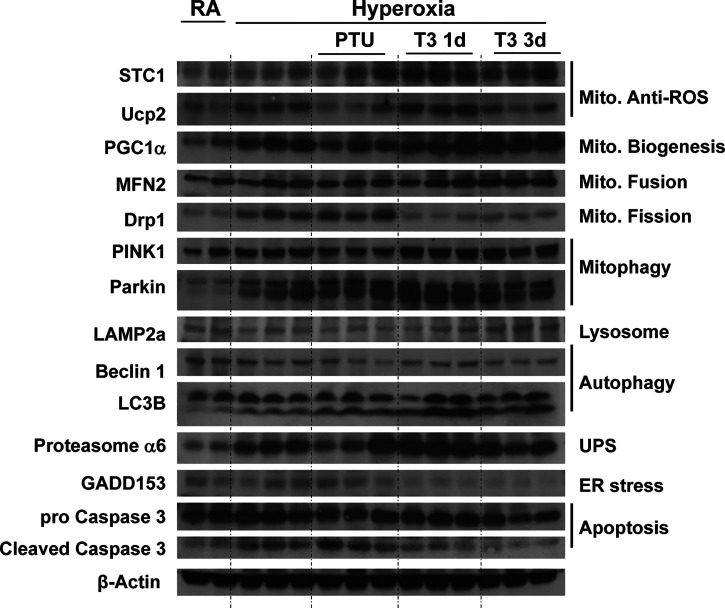
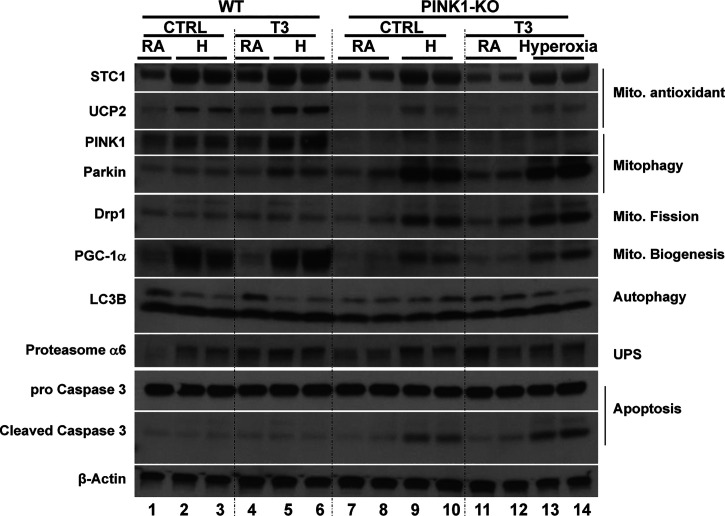
We found that lungs from mice with T3 pretreatment had higher mitochondrial anti-ROS protein STC1 and UCP2 expression (Fig. 3 and Supplemental Fig. S1; all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.14167196) (17, 18). T3 pretreatment also induced PGC-1α expression under hyperoxia, which is a key stimulator of mitochondrial biogenesis. In addition to biogenesis, T3 pretreatment modified the mitochondrial fusion/fission balance—exaggerated induction of mitochondrial fusion protein MFN2 and depressed mitochondrial fission protein Drp1—during hyperoxia compared to the control lungs, which might play an important role in determining the fate of depolarized mitochondria. We also found both mitophagy marker PINK1 and Parkin were induced with T3 pretreatment under hyperoxia.
Figure 3.
Triiodothyronine (T3) pretreatment increases mitochondrial biogenesis/fusion/mitophagy and decreases ER stress and apoptosis compared to mice treated with propylthiouracil (PTU). WT, PINK1−/−, and Parkin−/− mice were nebulized with triiodothyronine (T3, 40 µg/kg) for 3 days or no pretreatment before 72 h continuous hyperoxia exposure. Lysates were isolated and immunoblotted against antibodies as listed. β-Actin was used as protein loading control. Quantification based on densitometry of the Western blot is shown in Supplemental Fig. S1. The uncut Western blots listed in the Supplemental Fig. S3. PINK1, PTEN-induced kinase I; RA, room air; WT, wild-type.
Lysosome and proteasome degradation are major mechanisms whereby dysfunctional mitochondria and misfolded proteins are removed. We saw that LAMP2a (lysosome-associated membrane protein 2, subunit A), a receptor in the lysosomal membrane for chaperone-mediated autophagy, was induced with T3 pretreatment under hyperoxia. 20S Proteasome α6 was also induced. T3 pretreated lungs had higher LC3B ratios during hyperoxia than nonpretreated or PTU-treated mice, which indicated that T3 pretreatment activated autophagy. In addition, T3 pretreatment prevented hyperoxia induced ER stress (Fig. 3).
PINK1, Not Parkin, Mediates T3’s Protection against Hyperoxia-Induced Lung Injury
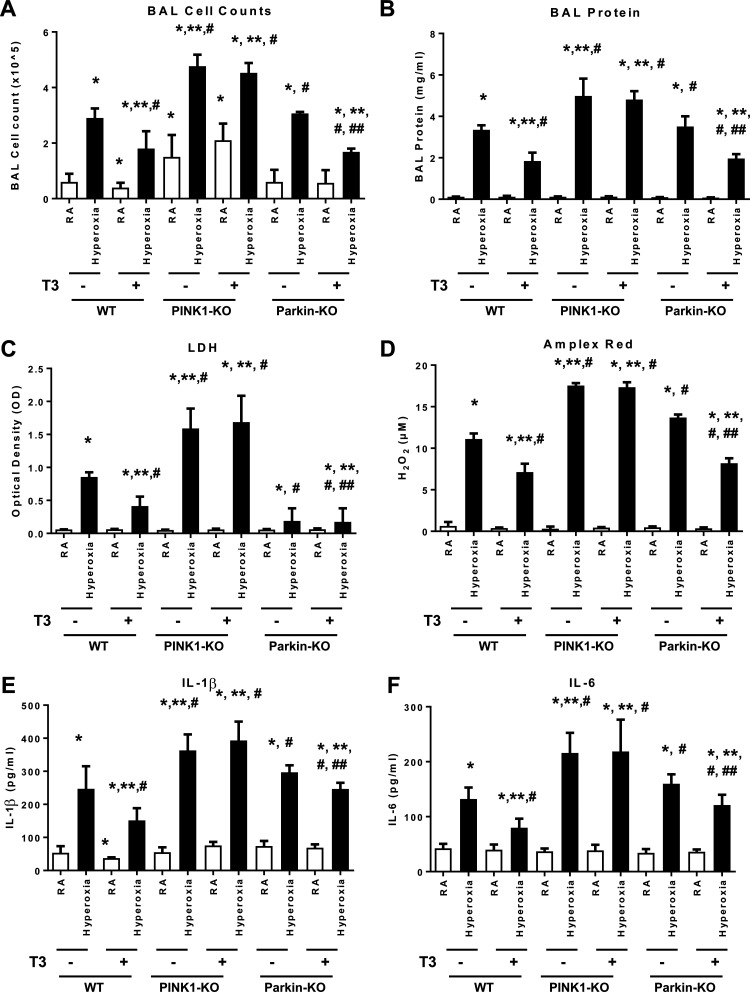
To determine whether PINK1 or Parkin mediated the protective effects of T3 under hyperoxia, we treated PINK1−/− mice, Parkin−/− mice and WT mice with T3 before 72 h continuous hyperoxia exposure (Fig. 4). PINK1−/− mice had the worst lung injury, and T3 pretreatment was not protective under hyperoxia in the background of PINK1 deficiency. Taken together, these results indicate that PINK1, but not Parkin, mediated the critical protective effects of T3 during hyperoxia.
Figure 4.
PINK1, not Parkin, mediates triiodothyronine (T3) effects against hyperoxia-induced lung injury. WT, PINK1−/−, and Parkin−/− mice were nebulized with triiodothyronine (T3, 40 µg/kg) for 3 days or no pretreatment before 72 h continuous hyperoxia exposure. A: total cells recovered from bronchoalveolar lavage (BAL) were counted. B: lung permeability was assessed by BAL protein content. C: lactate dehydrogenase (LDH) activity assays were performed on BAL fluid. D: oxidant generation was detected by Amplex Red from BAL fluid. IL-1β (E) and IL-6 (F) were detected by ELISA in BALF. The values are expressed as means ± SD and analyzed by Mann–Whitney test (n = 6 for each group). *P < 0.05, vs. WT no T3 RA; **P < 0.05, vs. WT no T3 hyperoxia; #P < 0.05, vs. corresponding RA; ##P < 0.05, vs. corresponding no T3 hyperoxia. PINK1, PTEN-induced kinase I; RA, room air; WT, wild-type.
T3 Pretreatment Increased Mitochondrial anti-ROS Potential, Biogenesis and Mitophagy under Hyperoxia and Is Dependent upon PINK1
To investigate the potential role of PINK1-mediated T3 protection under hyperoxia, we compared levels of marker proteins for mitochondrial potential, biogenesis, and mitophagy. We found that T3 pretreatment attenuated the induction of anti-ROS proteins STC1 and UCP2 in the PINK1 deficient lungs at basal level and under hyperoxia (Fig. 5 and Supplemental Fig. S2). By contrast, Parkin was dramatically induced under hyperoxia, even with T3 pretreatment. Induction of PCG-1α under hyperoxia was attenuated in the PINK1−/−. Although T3 pretreatment induced PGC-1α expression under hyperoxia, this response was lost in the PINK1 knockout. In addition to interruption of mitochondrial biogenesis, the mitochondrial fission protein Drp1 was induced in the PINK1 knockout, especially during hyperoxia, and T3 pretreatment prevented this induction in a PINK1-dependent manner. Importantly, PINK1 deficiency led to higher apoptosis during hyperoxia, and T3 pretreatment relieved the inhibition of apoptosis. (Fig. 5 and Supplemental Fig. S2).
Figure 5.
Triiodothyronine (T3) pretreatment increases in mitochondrial anti-ROS potential, biogenesis and mitophagy under hyperoxia are dependent upon PINK1. WT and PINK1−/− mice were nebulized with triiodothyronine (T3, 40 µg/kg) for 3 days before 72 h continuous hyperoxia exposure. Lysates were isolated and immunoblotted against antibodies as listed. β-Actin was used as protein loading control. Quantification based on densitometry of the western blots shown in Supplemental Fig. S2. The uncut Western blots are shown in Supplemental Fig. S4. PINK1, PTEN-induced kinase I; ROS, reactive oxygen species; WT, wild-type.
T3 Agonist GC-1 Pretreatment Prevents Hyperoxia Induced Lung Injury and Promotes Recovery
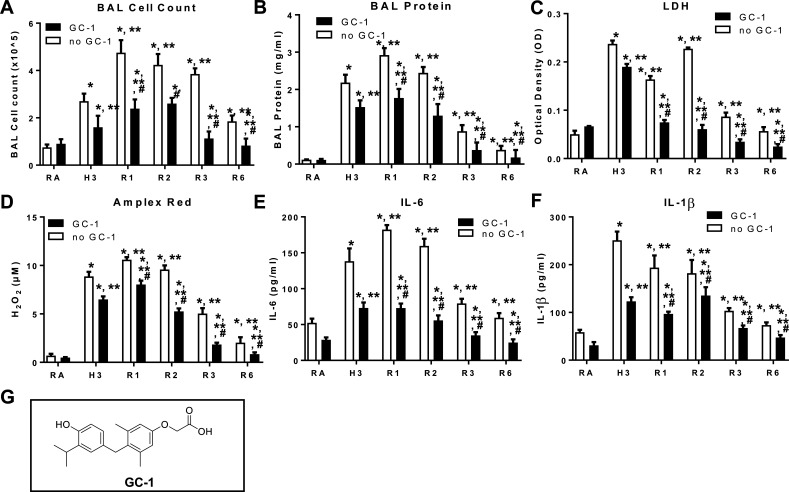
GC-1 is a synthetic analogue of T3 that is selective for both binding and activation of TRβ1 over TRα1. GC-1 binds TRβ1 with the same affinity as T3 but binds TRα1 with an affinity that is 10 times lower compared with that of T3. WT mice were orally gavaged with GC-1 (5 mg/kg) and exposed to RA or hyperoxia for 72 h (H3), followed by a recovery phase of 1-, 2-, 3-, and 6-days (R1–6). GC-1 pretreated mice exhibited significantly decreased BAL cell counts, decreased BAL protein level, decreased lung injury, decreased reactive oxygen species generation, and decreased IL1β and IL6 secretion compared with untreated mice (Fig. 6).
Figure 6.
Triiodothyronine (T3) agonist GC-1 pretreatment prevents hyperoxia induced lung injury and aids recovery. WT mice were orally gavaged with GC-1 (5 mg/kg) and then exposed to RA or to hyperoxia for 72 h (H3), followed by a recovery phase (R1–3, 1, 2, 3, and R6, 6 days posthyperoxia). Cells recovered from bronchoalveolar lavage (BAL) were counted as BAL total cell counts (A). B: lung permeability was assessed by BAL protein content. C: lactate dehydrogenase (LDH) activity assays were performed on BAL fluid. D: oxidant generation was detected by Amplex Red from BAL fluid. IL-6 (E) and IL-1β (F) was detected by ELISA in BALF. G: structural formula of GC-1. The values are expressed as means ± SD and analyzed by Mann–Whitney test (n = 4 for each group). *P < 0.05 vs. no GC-1 RA; **P < 0.05 vs. no GC-1 H3; #P < 0.05 vs. corresponding no GC-1. RA, room air.
During the early lung repair phase (recovery days 1∼3), lung inflammation and vascular leak quickly decreased to baseline levels in the GC-1 pretreated mice. Non-GC-1 mice still had high levels of cellular infiltrate and protein throughout their recovery period. Although the non-GC-1 mice improved their cellular infiltrate and protein leak, the levels of these markers were still much higher than the GC-1 pretreated mice. GC-1 pretreatment prevents hyperoxia induced lung injury and assists recovery (Fig. 7). GC-1 pretreatment induced mitochondrial anti-ROS, mitochondrial biogenesis, fusion, and decreased mitochondrial fission. GC-1 also induced mitophagy, autophagy, and decreased ER stress. More importantly, GC-1 decreased hyperoxia-induced apoptosis.
Figure 7.
GC-1 pretreatment prevents hyperoxia induced lung injury and aids recovery. Wild-type (WT) mice were orally gavaged with GC-1 (5 mg/kg) and then exposed to RA or to hyperoxia for 72 h (H3), followed by a recovery phase (R1-3, 1, 2, 3, and R6, 6 days posthyperoxia). Lysates from mouse lungs were immunoblotted against the listed Abs. The uncut Western blots are shown in Supplemental Fig. S5. All of the fold changes are compared with the room air control set as 1. For LC3B, comparison was made between the two bands of LC3B, but not to the loading control; the ratio of the two bands indicates the change in autophagy.
DISCUSSION
Hyperoxia caused a significant increase lung cellular infiltrates in mice. Administration of PTU enhanced HALI, whereas T3 attenuated HALI, inflammation, and oxidants in WT mice as measured by total protein content, LDH, and H2O2 production in BAL. In addition, T3 pretreatment increased mitochondrial biogenesis/fusion/mitophagy and decreased ER stress and apoptosis. Additionally, T3 pre-treatment also led to increased levels of PINK1. As expected, PINK1−/− mice were more susceptible to hyperoxia than WT mice. Notably, pretreatment with T3 did not attenuate HALI in PINK1−/− mice, suggesting that thyroid hormone requires PINK1 to protect against lung injury. In addition, T3 pretreatment increased mitochondrial anti-ROS potential, improved mitochondrial bioenergetics and mitophagy, and attenuated mitochondria-regulated apoptosis, all in a PINK1-dependent manner.
Looking at our results in a stepwise manner, we see that T3 treatment in WT mice leads to decreased BAL cell counts, BAL protein, LDH, IL-1B, and IL-6 levels, and an increase in ROS as measured by the Amplex Red assay. Drilling down on MCQ, we note several key findings in this work. Foremost, T3 treatment led to increased levels of STC1 and Ucp2, both proteins associated with defense against ROS in mitochondria. Increased levels of MFN2 and decreased levels of Drp1 in T3-treated mice show an increase in the mitochondrial fusion/fission ratio. Increased levels of both PINK1 and Parkin were noted in T3-treated mice, suggesting an uptick in mitophagy under hyperoxia. Elevated levels of LAMP2a suggest increased lysosomal activity under hyperoxia as well. Autophagy was also increased by T3 pretreatment, as signified by increased levels of Beclin 1 and LC3B. In addition, T3 pretreatment prevented hyperoxia induced ER stress (Fig. 3 and Supplemental Fig. S1).
The second series of experiments we performed involved T3 pretreatment of WT, PINK1−/−and Parkin−/− mice, again exposed to either room air of hyperoxia. PINK1−/− mice had substantially higher levels of BAL cell counts and BAL protein both under room air and hyperoxia, compared with WT and Parkin−/− mice. In addition, PINK1−/− mice had much higher levels of LDH and ROS, compared with WT and Parkin−/− mice. Finally, PINK1−/− mice had much higher levels of IL-1B and IL-6 than both WT and Parkin-/- mice. This shows PINK1 is critical to the anti-ROS and cytoprotective properties of T3 under both room air and hyperoxia. This suggests PINK1 has a crucial role in protecting the lung from HALI when T3 is used as a lung-protective agent (Figs. 4 and 5 and Supplemental Fig. S2).
Prior work by Bhargava (13) has shown a critical role for T3 in the resorption of fluid in the alveolus. In both in vivo and ex vivo lung models, T3 led to substantially greater alveolar fluid clearance than controls. Furthermore, the effect appears to be related to increased Na/K-ATPase activity, whereby fluid is cleared, and the effect further appears to be due to action directly on the alveolar cells. This was demonstrated both with intraperitoneal injection, and, interestingly, when T3 was instilled directly into the airspaces as well. This was further verified by the authors using a HALI model, which raises several interesting avenues of drug delivery, if inhaled T3 may be protective against HALI.
Regarding the protein expression profile of PINK1−/− mice compared with WT controls, pretreated with T3 under either room air or hyperoxia, there is a clear detrimental effect of knocking out PINK1 with regards to mitochondrial homeostasis. Higher levels of oxidant damage are noted in PINK1−/− mice, higher levels of mitophagy, the fission/fusion ratio is increased, autophagy is increased, biogenesis is decreased, and both lysosomal activity and apoptosis are increased in PINK1−/− mice. This clearly shows that PINK1 is critical to appropriate mitochondrial function and under hyperoxia, PINK1 deficiency clearly leads to increased mitochondrial stress and turnover.
Another experiment also showed a protective effect of pretreatment on inflammatory markers and mitochondrial homeostasis with the T3 agonist GC-1 (Figs. 6 and 7). Mice treated with GC-1 showed lower levels of inflammatory markers, cell counts, and ultimately lower levels of apoptosis. This shows a generally protective effect of GC-1 in the response to different models of acute lung injury.
One limitation of this work relates to the varying delivery methods of T3, PTU, and GC-1. We used nebulized T3 and PTU, as they both directly reach and act in the lung (11). GC-1, being an oral agent, does not directly enter the lungs and certainly some of the results may be due to variations in delivery method. A future experiment could utilize oral delivery of all agents to eliminate this possible confounder in some of the results. Practically speaking, a nebulized agent would be preferable to an oral agent in prevention of HALI as such patients may have great difficulty taking medications orally and an inhaled version has benefits both in terms of ease of administration and localizing the agent to the site of activity, that is, the lung.
An additional limitation, and an avenue for future study, involves assessment of the levels of circulating thyroid hormones in the bloodstream of animals with HALI. There are some data to suggest that serum levels of T3 and whether animals are hypothyroid, euthyroid, or hyperthyroid, will impact the lung’s response to hyperoxia (16, 19). These works, though, induced varying levels of thyroid function with exogenous thyroid hormone or blocked T3’s action, as opposed to observing the native response to HALI. Nonetheless, there may be a correlation with thyroid function and systemic thyroid hormone levels in HALI that is worthy of further investigation. In addition, the work of Rodriguez et al. (20), nearly 30 years ago, shows that the thyroid hormone axis has a role in the development of lung surfactant, another link in the protection mechanisms of the lung to hyperoxia. Further exploration of serum levels of thyroid hormone in such mice is therefore worthy of future study.
Our results highlight a novel protective role for PINK1 in mediating the cytoprotective effects of thyroid hormone in HALI. We conclude that the therapeutic properties of thyroid hormone are associated with improvement of MCQ and restoration of mitochondrial function. Therefore, thyroid hormone may represent a potential therapy for ALI.
SUPPLEMENTAL DATA
Supplemental Figs. S1–S5: https://doi.org/10.6084/m9.figshare.14167196.
GRANTS
This work was supported in part by National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) Grant R01 HL090660 (to P.J. Lee), U.S. Department of Veterans Affairs Office of Research and Development Grant ORD 11858595 (to P.J. Lee), and NIH NHLBI Grants R01HL127349, R01HL141852, and U01HL145567 (to N. Kaminski).
DISCLOSURES
Y.Z., G.Y., N.K., and P.J.L. have IP on the use of thyroid hormone mimetics in IPF and ARDS licensed to biotechnology. There are no conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
N.K. and P.J.L. conceived and designed research; Y.Z. and G.Y. performed experiments; Y.Z., G.Y., and P.J.L. analyzed data; Y.Z., G.Y., N.K., and P.J.L. interpreted results of experiments; Y.Z. prepared figures; Y.Z. drafted manuscript; Y.Z., G.Y., N.K., and P.J.L. edited and revised manuscript; Y.Z., N.K., and P.J.L. approved final version of manuscript.
REFERENCES
- 1.Dias-Freitas F, Metelo-Coimbra C, Roncon-Albuquerque R. Molecular mechanisms underlying hyperoxia acute lung injury. Respir Med 119: 23–28, 2016. doi: 10.1016/j.rmed.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Ornatowski W, Lu Q, Yegambaram M, Garcia AE, Zemskov EA, Maltepe E, Fineman JR, Wang T, Black SM. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol 36: 101679, 2020. doi: 10.1016/j.redox.2020.101679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha RA, Singh BK, Zhou J, Wu Y, Farah BL, Ohba K, Lesmana R, Gooding J, Bay B-H, Yen PM. Thyroid hormone induction of mitochondrial activity is coupled to mitophagy via ROS-AMPK-ULK1 signaling. Autophagy 11: 1341–1357, 2015. doi: 10.1080/15548627.2015.1061849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yau WW, Singh BK, Lesmana R, Zhou J, Sinha RA, Wong KA, Wu Y, Bay B-H, Sugii S, Sun L, Yen PM. Thyroid hormone (T3) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy 15: 131–150, 2019. doi: 10.1080/15548627.2018.1511263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Sauler M, Shinn AS, Gong H, Haslip M, Shan P, Mannam P, Lee PJ. Endothelial PINK1 mediates the protective effects of NLRP3 deficiency during lethal oxidant injury. J Immunol 192: 5296–5304, 2014. doi: 10.4049/jimmunol.1400653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng S-Y, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev 31: 139–170, 2010. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis PJ, Leonard JL, Lin H-Y, Leinung M, Mousa SA. Molecular basis of nongenomic actions of thyroid hormone. Vitam Horm 106: 67–96, 2018. doi: 10.1016/bs.vh.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Lei J, Nowbar S, Mariash CN, Ingbar DH. Thyroid hormone stimulates Na-K-ATPase activity and its plasma membrane insertion in rat alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 285: L762–L772, 2003. doi: 10.1152/ajplung.00376.2002. [DOI] [PubMed] [Google Scholar]
- 9.Ma S-F, Xie L, Pino-Yanes M, Sammani S, Wade MS, Letsiou E, Siegler J, Wang T, Infusino G, Kittles RA, Flores C, Zhou T, Prabhakar BS, Moreno-Vinasco L, Villar J, Jacobson JR, Dudek SM, Garcia JGN. Type 2 deiodinase and host responses of sepsis and acute lung injury. Am J Respir Cell Mol Biol 45: 1203–1211, 2011. doi: 10.1165/rcmb.2011-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mancini A, Raimondo S, Di Segni C, Persano M, Gadotti G, Silvestrini A, Festa R, Tiano L, Pontecorvi A, Meucci E. Thyroid hormones and antioxidant systems: focus on oxidative stress in cardiovascular and pulmonary diseases. Int J Mol Sci 14: 23893–23909, 2013. doi: 10.3390/ijms141223893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu G, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, Srivastava A, de Castro JP, Deluliis G, Ahangari F, Woolard T, Aurelien N, Drigo RAE, Gan Y, Graham M, Liu X, Homer RJ, Scanlan TS, Mannam P, Lee PJ, Herzog EL, Bianco AC, Kaminski N. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med 24: 39–49, 2018. doi: 10.1038/nm.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barca-Mayo O, Liao X-H, DiCosmo C, Dumitrescu A, Moreno-Vinasco L, Wade MS, Sammani S, Mirzapoiazova T, Garcia JGN, Refetoff S, Weiss RE. Role of type 2 deiodinase in response to acute lung injury (ALI) in mice. Proc Natl Acad Sci USA 108: E1321–E1329, 2011. doi: 10.1073/pnas.1109926108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhargava M, Runyon MR, Smirnov D, Lei J, Groppoli TJ, Mariash CN, Wangensteen OD, Ingbar DH. Triiodo-L-thyronine rapidly stimulates alveolar fluid clearance in normal and hyperoxia-injured lungs. Am J Respir Crit Care Med 178: 506–512, 2008. doi: 10.1164/rccm.200709-1429OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Vries EM, Fliers E, Boelen A. The molecular basis of the non-thyroidal illness syndrome. J Endocrinol 225: R67–R81, 2015. doi: 10.1530/JOE-15-0133. [DOI] [PubMed] [Google Scholar]
- 15.Fliers E, Bianco AC, Langouche L, Boelen A. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol 3: 816–825, 2015. doi: 10.1016/S2213-8587(15)00225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owji MS, Varedi M, Naghibalhossaini F, Pajouhi N. Thyroid function modulates lung fluid and alveolar viscoelasticity in mechanically ventilated rat. J Surg Res 253: 272–279, 2020. doi: 10.1016/j.jss.2020.03.060. [DOI] [PubMed] [Google Scholar]
- 17.Haslip M, Dostanic I, Huang Y, Zhang Y, Russell KS, Jurczak MJ, Mannam P, Giordano F, Erzurum SC, Lee PJ. Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arterioscler Thromb Vasc Biol 35: 1166–1178, 2015. doi: 10.1161/ATVBAHA.114.304865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Shan P, Srivastava A, Li Z, Lee PJ. Endothelial stanniocalcin 1 maintains mitochondrial bioenergetics and prevents oxidant-induced lung injury via toll-like receptor 4. Antioxid Redox Signal 30: 1775–1796, 2019. doi: 10.1089/ars.2018.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varedi M, Pajouhi N, Owji M, Naghibalhossaini F, Omrani GHR. Differential modulation of claudin 4 expression and myosin light chain phosphorylation by thyroid function in lung injury. Clin Respir J 11: 797–804, 2017. doi: 10.1111/crj.12418. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez MP, Sosenko IR, Antigua MC, Frank L. Prenatal hormone treatment with thyrotropin releasing hormone and with thyrotropin releasing hormone plus dexamethasone delays antioxidant enzyme maturation but does not inhibit a protective antioxidant enzyme response to hyperoxia in newborn rat lung. Pediatr Res 30: 522–527, 1991. doi: 10.1203/00006450-199112000-00005. [DOI] [PubMed] [Google Scholar]