Abstract
The transient receptor potential vanilloid 1 (TRPV1) channel is expressed in human bronchial epithelium (HBE), where it transduces Ca2+ in response to airborne irritants. TRPV1 activation results in bronchoconstriction, cough, and mucus production, and may therefore contribute to the pathophysiology of obstructive airway disease. Since children with asthma face the greatest risk of developing virus-induced airway obstruction, we hypothesized that changes in TRPV1 expression, localization, and function in the airway epithelium may play a role in bronchiolitis and asthma in childhood. We sought to measure TRPV1 protein expression, localization, and function in HBE cells from children with versus without asthma, both at baseline and after RSV infection. We determined changes in TRPV1 protein expression, subcellular localization, and function both at baseline and after RSV infection in primary HBE cells from normal children and children with asthma. Basal TRPV1 protein expression was higher in HBE from children with versus without asthma and primarily localized to plasma membranes (PMs). During RSV infection, TRPV1 protein increased more in the PM of asthmatic HBE as compared with nonasthmatic cells. TRPV1-mediated increase in intracellular Ca2+ was greater in RSV-infected asthmatic cells, but this increase was attenuated when extracellular Ca2+ was removed. Nerve growth factor (NGF) recapitulated the effect of RSV on TRPV1 activation in HBE cells. Our data suggest that children with asthma have intrinsically hyperreactive airways due in part to higher TRPV1-mediated Ca2+ influx across epithelial membranes, and this abnormality is further exacerbated by NGF overexpression during RSV infection driving additional Ca2+ from intracellular stores.
Keywords: airway hyperreactivity, bronchiolitis, calcium, endoplasmic reticulum, nerve growth factor
INTRODUCTION
The transient receptor potential vanilloid subtype 1 (TRPV1) channel belongs to a family of proteins originally characterized in neuronal cells as Ca2+-permeable, nonselective cation channels that sense physical and chemical stimuli such as noxious heat (>43°C), pH (<6.5 at 37°C), and vanilloid compounds like capsaicin (1). In dorsal root ganglia, TRPV1 activity is primarily modulated by the nerve growth factor (NGF), which controls its synthesis and translocation to the axonal plasma membrane (2–4).
Recent studies have demonstrated TRPV1 expression in nonneuronal cells (5–9). In particular, TRPV1 has been shown to be expressed and functional in adult human bronchial epithelial (HBE) cells (10), and to play a role in airway disease through increased mucus production by epithelial cells (11), stimulation of the cough reflex by neuronal cells (12), and smooth muscle contraction (13). Furthermore, increased TRPV1 expression has been shown by immunohistochemistry in HBE cells of bronchial biopsies from adults with severe asthma (14). Finally, a previous study from our group found that TRPV1 function in HBE cells from children, but not from adults, increases in response to respiratory syncytial virus (RSV) infection (15).
RSV is the most prevalent respiratory pathogen causing lower respiratory tract infections (LRTI) in infants and young children (16, 17). Moreover, solid epidemiological evidence suggests that acute RSV LRTI in early life predisposes to chronic airway dysfunction throughout childhood (18). RSV preferentially infects HBE cells resulting in increased expression of NGF and its cognate receptor tropomyosin-related kinase A (TrkA) (19, 20). Signaling through the NGF/TrkA axis facilitates RSV replication by interfering with host cell apoptosis (20–22) and promoting autophagy (23), and has also been shown to contribute to airway hyperreactivity and inflammation by amplifying neuroimmune interactions (19).
Although children with asthma face the greatest risk of developing airway obstruction as a consequence of viral LRTI, the mechanisms whereby viruses induce airway obstruction has not been fully elucidated. Also, the mechanisms by which viral infections induce changes in TRPV1 expression, localization, and function have never been studied, nor is it known if this channel is modulated by asthma predisposition. We hypothesized that, as a consequence of RSV infection, TRPV1 expression, localization, and function in bronchial cells is altered and augments downstream Ca2+ influx across epithelial membranes, and these effects are amplified in children with asthma.
To test this hypothesis, we studied TRPV1 expression, localization, and function in primary HBE cells from children with or without asthma. The goal of our study was to determine whether the observed changes in TRPV1 expression, localization, and function are regulated by RSV and/or the asthma status of donors. Our findings provide new insights into the mechanisms underlying virus-induced bronchiolitis and asthma in pediatric patients, and may help develop precise pharmacological strategies with enhanced specificity and effectiveness. Some of the results of these studies have been reported previously in abstract form (24, 25).
METHODS
Cells
Primary HBE cells derived from White, Black, or Hispanic children with or without asthma of both sexes aged 1 day to 8 yr were obtained from Lonza (Walkersville, MD) or from the International Institute for the Advancement of Medicine (IIAM, Edison, NJ; Table 1). We chose to study this age group because several epidemiological studies have shown increased risk of postRSV wheezing until the age of 11 –13 yr, with subsequent decrease and resolution before adulthood. Cells were cultured as described previously and were used between the 2nd and 5th passage (15).
Table 1.
Sources of primary HBE cells used in this study
| ID | Patient Code | Cause of Death (if provided) | Age | Sex | Race | Diagnosis | Notes | Source |
|---|---|---|---|---|---|---|---|---|
| 1 | 11121X01 | Anoxia | 2 yr | F | B | Nonasthmatic | IIAM | |
| 2 | 357048 | Not provided | 4 yr | M | C | Nonasthmatic | Lonza | |
| 3 | 489938 | Not provided | 2 yr | F | B | Nonasthmatic | Lonza | |
| 4 | 441099 | Not provided | 3 yr | F | B | Nonasthmatic | Lonza | |
| 5 | 11121002 | Head trauma | 3 mo | M | B | Nonasthmatic | IIAM | |
| 6 | ADIG208 | Anoxia/asphyxia | 1 mo | F | H | Nonasthmatic | IIAM | |
| 7 | ADC3443 | Motor vehicle accident | 6 yr | M | B | Nonasthmatic | IIAM | |
| 8 | 451411 | Not provided | 2 yr | M | H | Nonasthmatic | Lonza | |
| 9 | 366559 | Not provided | 6 yr | M | B | Nonasthmatic | Lonza | |
| 10 | ADHJ180 | Head trauma | 1 yr | M | C | Nonasthmatic | IIAM | |
| 11 | ACH1200 | Anencephaly | 1 day | M | C | Nonasthmatic | IIAM | |
| 12 | 427214 | Not provided | 8 yr | F | H | Asthmatic | Lonza | |
| 13 | 228044 | Not provided | 2 yr | M | B | Asthmatic | Lonza | |
| 14 | ADCQ492 | Head trauma | 4 yr | M | H | Asthmatic | IIAM | |
| 15 | 22121000 | Head trauma | 1 yr | M | B | Asthmatic | Inhaler used | IIAM |
HBE, human bronchial epithelium.
Cell Isolation from Donor Lungs
Lungs or bronchi from deceased donors were received within 24 h of surgical removal and shipped on wet ice in DMEM containing antibiotics. The bronchus was isolated from the whole lung if not already dissected by the surgeon before shipment, then the connective tissue was removed. The dissected bronchus was rinsed with DMEM containing antibiotics and antimycotics, opened longitudinally, and then placed in protease solution containing 0.1% dispase (Sigma-Aldrich, St. Louis, MO) in DMEM and 1% antibiotics/antimycotics overnight at 4°C. Dispase was inactivated by addition of 1% FBS, then cells were scraped from the surface, pelleted, washed, and cultured on dishes coated with plate-coating medium (1% Vitrogen, 1% fibronectin, and 0.01% BSA in LHC basal medium).
Ethics Statement
Our study was based on primary human bronchial epithelial cells derived from anonymous patient donors and provided by the International Institute for the Advancement of Medicine (IIAM, Edison, NJ) according to procedures approved by the Cleveland Clinic. As such, this study was deemed exempt from requiring IRB approval.
Measurement of Intracellular Calcium
The calcium response to TRPV1 channel stimulation with capsaicin was measured as described previously (15). The calcium response to TRPV1 channel stimulation with capsaicin was measured using the Fluorogenic Imaging Plate Reader (FLIPR) assay (Molecular Devices, Sunnyvale, CA) in 80%–90% confluent HBE grown in black-walled, clear-bottom Costar 96-well plates. Cells were loaded with Calcium-6 dye for 3 h and incubated at 37°C/5%CO2, according to manufacturer’s instructions. Plates were read in a Flexstation-3 reader, and fluorescence was measured over 3 min at 7-s intervals upon addition of ionomycin (0.5–1.0 µM in dimethyl sulfoxide; DMSO, Sigma-Aldrich, St. Louis, MO), capsaicin (150 µM in DMSO; Sigma-Aldrich), or vehicle. The ratio of maximum-minimum fluorescence/ionomycin fluorescence was used to calculate changes in intracellular Ca2+. For selected experiments measuring release of Ca2+ from intracellular stores, HBE were incubated overnight with medium from uninfected HEp-2 cells or with RSV, then loaded with Calcium-6 dye in Hanks’ balanced salt solution (HBSS) containing HEPES either with or without Ca2+ for 3 h.
All inhibitors were added during the last 30 min of incubation with Calcium-6 dye. An initial dose response of thapsigargin was performed where doses ranging from 0.01–5 µM was added then readings were taken at 10-s intervals over 15 min. At 15 min post thapsigargin application, intracellular calcium ([Ca2+]i) levels returned to baseline levels after peaking within the first 2 min. Where ER calcium depletion was performed, thapsigargin was applied 15 min before capsaicin application. All samples were measured in quadruplicate and experiments were repeated for a minimum of three times.
Quantitative Real-Time PCR
Total RNA was isolated from the cells using the TRI Reagent (Sigma-Aldrich) following manufacturer’s instructions, and 500 ng of RNA was used in a 20-µL SuperScript III RT (Invitrogen, Carlsbad, CA) reverse transcription reaction. One microliter of this reaction was used for qPCR, with 0.4 µM each of primer and SsoAdvancedSYBR Green Supermix (Bio-Rad, Hercules, CA). Transcript expression was normalized using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the housekeeping gene. The relative change in gene expression was calculated using the following formula: % change = 2−(ΔΔCq) = 2−[ΔCq (treated samples) − ΔCq (control samples)], where ΔCq = Cq (gene of interest) – Cq (GAPDH housekeeping gene) and Cq was the threshold number. Untreated was used to define 100% baseline for comparison purposes. Primer sequences are as follows: NGF forward 5′- TTCACCCCGTGTGCTGTTTA-3′ and reverse 5′- GAATTCGCCCCTGTGGAAGA-3′; TRPV1 forward 5′- GCTGTCTTCATCATCCTGCTG CT-3' and reverse 5′- GTTCTTGCTCTCCTGTGCGATCTTGT-3′; GAPDH forward 5'-CTA TAAATTGAGCCCGCAGCC-3′ and reverse 5′- GCGCCCAATACGACCAAATC-3′.
Gene Silencing
Exponentially growing HBE cells were transfected with SMARTpool ON-TARGET Plus (GE Dharmacon, Lafayette, CO) small interfering (si)RNA directed against NGF (50 nM) using Lipofectamine 2000 (Life Technologies, Carlsbad, CA), or with ON-TARGET Plus nontargeting pool siRNAs (50 nM) according to manufacturer’s instructions. After 6 h of transfection, the medium was replaced with fresh culture medium. Cells were harvested 48 h later and analyzed for knockdown of mRNA expression by qPCR analysis.
Western Blot
HBE cells, 70%–80% confluent, were infected with rrRSV (MOI of 1) at times indicated. Cells were lysed in RIPA buffer containing 25 mM Tris·HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS (Thermo Fisher Pierce) protease/phosphatase inhibitor cocktail (Thermo Fisher/Pierce), DNAse (Qiagen). Protein concentrations were determined using the BCA Protein assay reagent (Pierce). Equal amount of protein was separated by SDS-PAGE and transferred onto PVDF membrane (Thermo Scientific). Membranes were blocked in Tris-buffered saline (TBS)/T 5% bovine serum albumin or nonfat dry milk for 1 h at room temperature and probed with primary antibodies rabbit TRPV1 (Alomone Labs, Jerusalem, Israel) and anti-NGF (Santa Cruz). Anti-β actin (Sigma-Aldrich) was used to ensure equal loading. Luminol (Pierce) generated signal was used to detect bands.
Reagents
Capsaicin and ionomycin were purchased from Sigma-Aldrich. Thapsigargin (TG) was purchased from Cayman Chemical (Ann Arbor, MI) and rhNGF from Alomone Labs (Jerusalem, Israel).
Virus Propagation
To easily verify active viral replication in cells, recombinant RSV strain A2 expressing the red fluorescent protein (RFP) gene (rrRSV) was used, which was kindly provided by Dr. Mark Peeples (Nationwide Children’s Hospital, Columbus, OH) and Dr. Peter Collins (National Institutes of Health, Bethesda, MD). The rrRSV reporter gene provides direct evidence of viral replication because the transgene cannot be expressed unless the virus completes its replication cycle in the host cell cytosol (26). Thus, reporter gene expression manifested by the appearance of red fluorescence in the infected cells mirrors viral replication and can be used to monitor viral growth. The viral stock was propagated using HEp-2 cells grown at 37°C/5%CO2 in Eagle’s minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) and 1% each of Glutamax, penicillin/streptomycin, and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES). Cells at ∼50% confluence were inoculated with 3 mL/dish of virus stock diluted 1/10 with heat-inactivated FBS. After incubation for 2 h at 37°C, the inoculum was removed and replaced with 25 mL of fresh medium. The medium was replaced again 2 days later, and the virus was harvested after 1 to 2 additional days of incubation, at which point all cells appeared bright red when viewed under a fluorescent microscope. To harvest the virus, infected cells were scraped from the plate, separated with a pipette, mixed at medium speed, and pelleted by centrifugation at 1,200 g for 5 min. The supernatant was collected and cell debris removed by centrifugation at 9,500 g for 20 min in a centrifuge refrigerated at 4°C. One-milliliter aliquots of the supernatant were snap-frozen in liquid nitrogen and stored at −70°C until use. The final titer was determined with a modified plaque-forming unit assay. Control experiments were performed using culture medium from uninfected HEp-2 cells after cell debris were removed by centrifugation at 9,500 g for 20 min in a centrifuge refrigerated at 4°C. One-milliliter aliquots of the supernatant were snap-frozen in liquid nitrogen and stored at −70°C until use.
Immunocytochemistry
To visualize cytosol TRPV1 expression, HBE cells were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and blocked with 3% BSA. Cells were then incubated with primary anti-TRPV1 antibody followed by Alexa Fluor-conjugated secondary antibody (Molecular Probes, Carlsbad, CA). To visualize plasma membrane (PM)-associated TRPV1, HBE cells were incubated with anti-TRPV1 antibody (Abcam, Boston MA, Alomone Labs, Jerusalem Israel.) at 37°C/5%CO2 for 15 min with or without WGA Alexa Fluor 594 (Molecular Probes, Carlsbad CA), then fixed in 4% PFA followed by Alexa Fluor-conjugated secondary antibody (Molecular Probes, Carlsbad, CA). Coverslips were mounted and sealed before imaging using Vectashield mounting medium with 4–6 diamidino-2-phenylindole (DAPI; Vector Labs, Burlingame, CA). Photomicrographs were taken using an upright fluorescent microscope for cytosol TRPV1, or a confocal microscope for PM-associated TRPV1 (Leica Microsystems, Wetzlar, Germany) with a 405-diode laser to excite DAPI and HeNe laser to excite the Alexa Fluor 488-labeled secondary antibody. Cells were visualized using a ×40 or ×63/1.4 oil objective. Intensity plot profiles were acquired using the ImageJ software (NIH, Bethesda, MD) to measure the intensity of signal per pixel across the length of the cell. A minimum of 15 cells were measured. Total intensity was calculated by averaging signal intensity in each cell. ER Staining Kit − Red Fluorescence Cytopainter (Abcam) for staining of ER was used in colocalization experiments. Pearson’s Correlation coefficient analysis was performed using Volocity image analysis (Quorum Technologies, Inc. v 6.5.1). A library of files was generated by importing the original Leica Imaging LIF files into the software. Then using the 8 bit red (ER) and green (TRPV1) images, image sequences were generated to perform colocalization of the complete image (at ×40 magnification). Thresholding was done using area of image containing no cells set at background. A minimum of 5 images/condition/donor at ×40 magnification was analyzed.
Biotinylation and Immunoprecipitation of Plasma Membrane Proteins
HBE cells were washed twice with ice-cold PBS then incubated with sulfo-NHS-SS-biotin at 4°C with agitation. The reaction was stopped by adding the quenching solution provided with the kit (Abcam, Boston, MA). Cells were collected and washed in TBS before lysis in the buffer provided with the kit. Protein determination of the supernatant collected was performed using the BSA Protein Assay (Pierce Biotechnology, Rockford, IL), and an equal amount was incubated with streptavidin-coated beads for 60 min at room temperature with constant rotation. The beads were washed thrice in the buffer provided with the kit before elution of the labelled protein in 100 mM DTT in PBS. SDS/PAGE buffer was added to the samples and heated to 100°C for 5 min before separation on 7.5% polyacrylamide gel.
Statistical Analysis
All data are expressed as means ± SE. Comparisons between two groups were performed using the nonparametric Mann–Whitney U test. Comparisons among multiple groups were performed using the nonparametric, one-way Kruskal–Wallis test with Dunn’s multiple post hoc comparisons. Statistical analysis was performed with the software GraphPad Prism v 5.0 (La Jolla, CA). P values less than 0.05 were considered statistically significant. The box-plot graphs prepared with GraphPad Prism show whiskers down to the minimum and up to the maximum value.
RESULTS
Basal TRPV1 Expression in Primary HBE Cells from Children
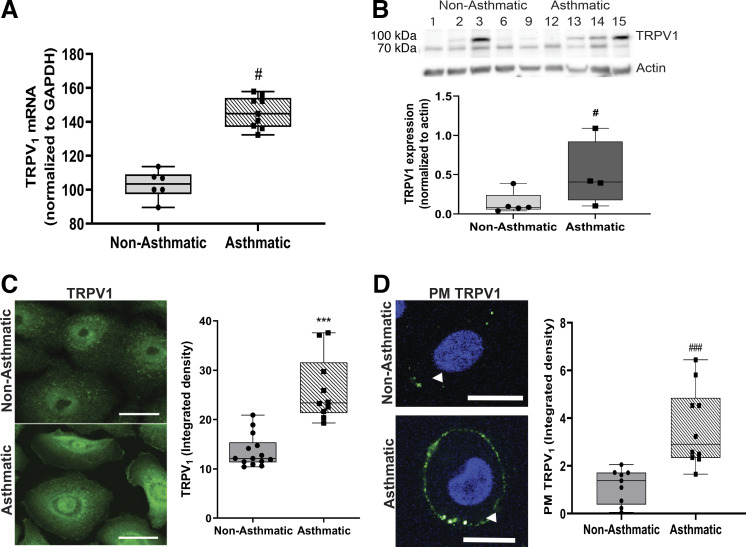
Our laboratory has previously shown that TRPV1-dependent calcium influx increases in response to RSV infection in primary cultures of HBE cells derived from children’s lungs (15). In addition, TRPV1 activation was greater in HBE cells from children with asthma in comparison with nonasthmatic controls. To understand the mechanisms responsible for these functional changes, we measured TRPV1 expression at the gene and protein level. TRPV1 mRNA (Fig. 1A) and protein (Fig. 1B) were significantly more abundant in HBE cells from children with asthma as compared with nonasthmatic controls (P < 0.05). Immunocytochemical analysis confirmed that TRPV1 protein expression was higher in HBE cells from children with asthma as compared with nonasthmatic controls (P < 0.001; Fig. 1C). Functionally, TRPV1 activation resulted in increased [Ca2+]i, as evidenced by the movement of calcium ions across the plasma membrane and into the cytosol. We therefore investigated the subcellular distribution of TRPV1 by immunocytochemistry. Again, under basal conditions the expression of TRPV1 in the PM compartment was greater in HBE from children with asthma as compared with nonasthmatic controls (P < 0.001; Fig. 1D).
Figure 1.
TRPV1 expression in HBE cells is greater in children with asthma versus without asthma. TRPV1 mRNA from children without asthma (1, 2, 9) and with asthma (12–15) (A) and TRPV1 protein expression in HBE cells from children without (1–3, 6, 9) and with asthma (12–15) (B) tested in two independent experiments. C: representative fluorescent micrographs of immunoreactive TRPV1 (green) localization at baseline in permeabilized HBE cells from children without (2, 6–8, 10) and with asthma (12–14) (left). Bars show the means ± SE of densitometry measurements on a minimum of 15 cells/donor (right). D: TRPV1 (green) abundance on plasma membranes (PMs) of nonpermeabilized HBE cells from children without (2–4, 8, 10) and with asthma (12, 13, 15). White arrowheads indicate the plasma membranes (left). Bars show the means ± SE of densitometry measurements on a minimum of 15 cells/donor (right). Data were analyzed using the nonparametric Mann–Whitney U test. #P < 0.05; ***P < 0.001; ###P < 0.001 compared with nonasthmatic controls. Scale bar = 20 µm. HBE, human bronchial epithelium; TRPV1, transient receptor potential vanilloid 1.
RSV-Mediated Changes in TRPV1 Expression and Localization
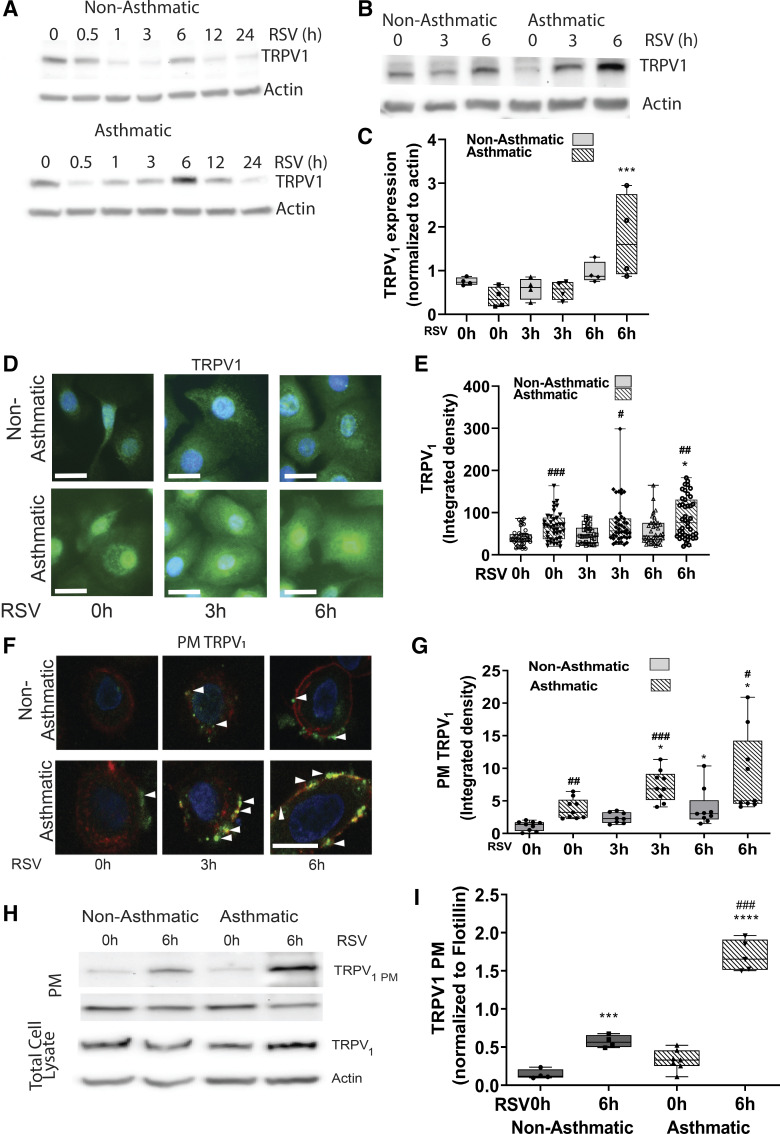
To determine the response of HBE cells to RSV infection in children with asthma and without asthma, the cells were incubated with control medium from uninfected HEp-2 cells or with rrRSV at a multiplicity of infection of 1 (MOI = 1). The cells were then collected at different time points, and the lysates were immunoblotted to measure TRPV1 expression. In preliminary experiments, TRPV1 expression showed a biphasic response with peak induction at 6 h postinfection followed by a progressive decline over time (Fig. 2A), and based on these data we used 6 h as the time point for the rest of our experiments. TRPV1 protein expression measured in response to RSV infection at 0, 3, and 6 h by Western blot (Fig. 2, B and C) and immunocytochemistry (Fig. 2, D and E) showed significantly higher expression in HBE cells from children with asthma as compared with nonasthmatic controls both with control medium (0-h time point) and in response to RSV infection (3 and 6 h time points). Next, we studied whether RSV infection modifies the relative distribution of TRPV1 between cellular compartments depending on the asthma status of the donor using both confocal microscopy and cell surface biotinylation assays. HBE cells were infected with RSV (MOI = 1) for 0, 3, or 6 h and then tested for plasma membrane-associated TRPV1 (TRPV1 PM). RSV infection resulted in increased TRPV1 PM in HBE cells from both children without and with asthma. However, TRPV1 PM in asthmatic HBE cells was higher under basal conditions (albeit not significantly), and increased earlier (within 3 h post infection) and farther compared with HBE cells from nonasthmatic donors (Fig. 2, F–I).
Figure 2.
TRPV1 expression in children’s HBE cells infected by RSV. HBE cells from children with and without asthma were infected with RSV (MOI = 1) and assessed for TRPV1 expression at 0–24 h post infection from children without (2, 9) and with asthma (14, 15) (A) or 0, 3, and 6 h post infection from children without (2, 6, 7, 10) and with asthma (12–15) (B). TRPV1 protein expression was measured by Western blot and normalized to actin. C: quantification of results from (A); n = 3 independent experiments. D: representative fluorescent micrographs of immunoreactive TRPV1 (green) expression in permeabilized HBE cells from children without (2, 8, 10) and with asthma (12, 13) at 0, 3, and 6 h after infection with RSV (MOI = 1). E: quantification of results from C, showing densitometry measurements on a minimum of 15 cells/donor. F: TRPV1 (green) expression in plasma membranes (PMs, red) of nonpermeabilized HBE of children without (2, 8, 10) and with asthma (12–14) at 0, 3, and 6 h post infection with RSV. White arrowheads indicate the plasma membranes. G: quantification of results from F showing TRPV1PM calculated as the average of densitometric measurements on a minimum of 15 cells/donor. Scale bar = 20 µm. H: TRPV1 expression in the biotinylated plasma membrane fraction of HBE cells from children without (2, 4, 7) and with asthma (12, 13, 15) incubated with sterile medium or RSV (MOI = 1) for 6 h. Cells were labeled with sulfo-NHS-biotin and purified with streptavidin. Input lysates are shown in the lower bands. I: quantification of the results from G normalized to flotillin. Data are expressed as means ± SE and were analyzed using the Kruskal–Wallis test with Dunn’s multiple comparisons. *P < 0.05, ***P < 0.001, ****P < 0.0001 compared with the 0-h time point; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with nonasthmatic controls. HBE, human bronchial epithelium; MOI, multiplicity of infection; RSV, respiratory syncytial virus; TRPV1, transient receptor potential vanilloid 1.
Altered TRPV1 Function in Response to RSV and Asthma Status
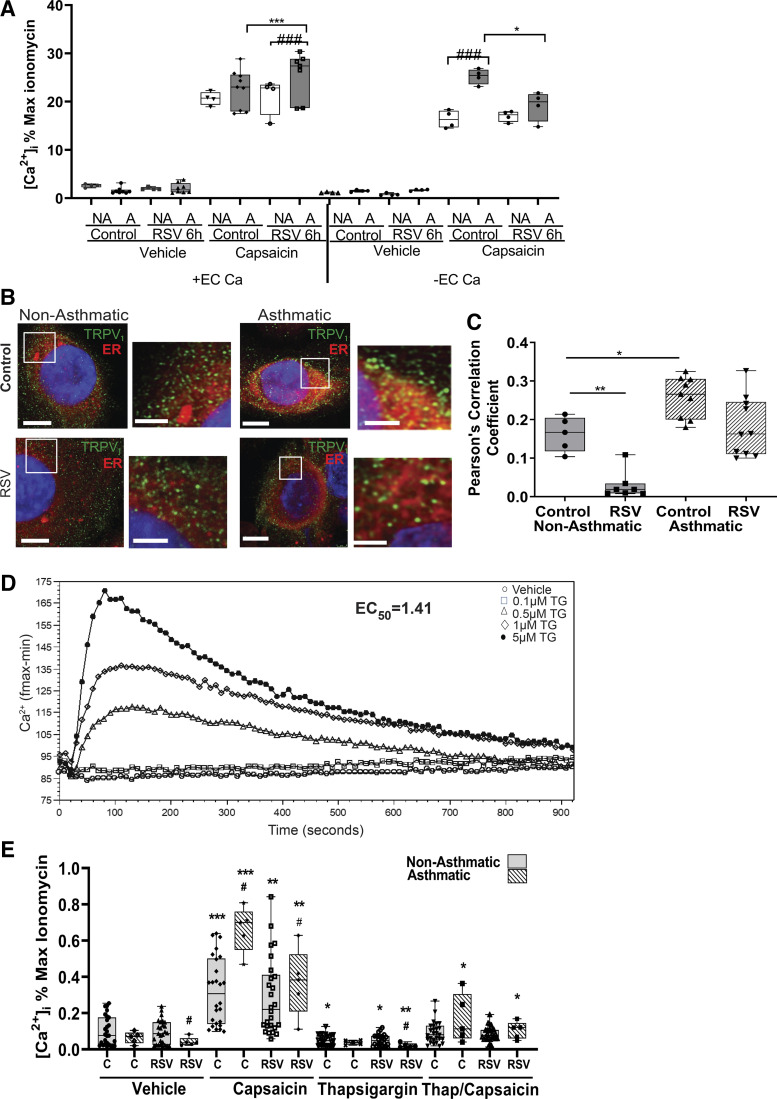
To determine the effects of TRPV1 cellular localization on its ion channel function, HBE cells were assessed for [Ca2+]i changes after stimulation with the selective TRPV1 agonist capsaicin, in the presence or absence of extracellular calcium ([Ca2+]ec). We have previously shown using pharmacological inhibition with capsazepine or siRNA-mediated gene knockdown, that RSV-mediated increase in TRPV1 activation is greater in HBE cells from children with asthma as compared with nonasthmatic controls when the assay is performed in the presence of [Ca2+]ec (19). Consistent with our previous findings, when TRPV1 activity was measured in the presence of [Ca2+]ec, HBE cells from children with asthma exhibited higher RSV-mediated increase in [Ca2+]i at 6 h after infection as compared with the control cells from children without asthma (Fig. 3A, left). In contrast, when TRPV1 activity was assessed in the absence of [Ca2+]ec, the [Ca2+]i increase noted in HBE cells from children with asthma upon RSV infection was reduced (Fig. 3A, right). These findings suggest that RSV-mediated increase in [Ca2+]i in HBE cells from children with asthma results primarily from TRPV1-dependent [Ca2+]ec influx, whereas basal elevated [Ca2+]i results primarily from internal stores. As TRPV1 localization and TRPV1-mediated release of Ca2+ from the endoplasmic reticulum (ER) has been demonstrated in sensory neurons (27, 28) and we saw an increase in [Ca2+]i in response to capsaicin in HBE cells upon depletion of [Ca2+]ec, this prompted an investigation into the source of increased [Ca2+]i in asthmatic cells upon RSV infection. HBE cells were immunostained for TRPV1 and costained with the red fluorescent ER marker CytoPainter (Abcam, Cambridge, MA) to determine if TRPV1 is localized to the ER (TRPV1 ER, Fig. 3B). HBE cells from both children with and without asthma confirmed TRPV1 localization within the ER, with a greater fraction of TRPV1 localized to the ER in HBE from children with asthma as compared with children without asthma (P < 0.05). After RSV infection (MOI = 1) for 6 h, both groups of HBE cells exhibited a reduction in TRPV1 ER localization (Fig. 3C). To determine if the TRPV1 ER was functional, HBE cells were treated with TG in order to deplete the ER of calcium. A dose-response curve of TG effect yielded an EC50 value of ∼1.5 µM for ER calcium depletion, and this concentration was used for all subsequent experiments (Fig. 3D). Pretreatment with TG for 30 min before the addition of capsaicin abrogated the TRPV1-mediated increase in [Ca2+]i when assayed in the absence of [Ca2+]ec regardless of RSV or asthma status (Fig. 3E), suggesting that ER-localized TRPV1 channels contribute to or interact with TRPV1 PM channels in response to capsaicin.
Figure 3.
Role of ER in TRPV1-mediated [Ca2+]i. HBE cells from either children without or with asthma were infected with sterile medium or RSV (MOI = 1) for 6 h before activation of TRPV1 with vehicle (DMSO) or capsaicin (150 µM) in the presence (left) or in the absence (right) of [Ca2+]ec (A). Calcium influx was measured using Calcium 6 dye after TRPV1 activation by vehicle or capsaicin of nonasthmatic (2–4, 7, 10) or asthmatic (12, 13, 15) intact HBE cells. B: representative fluorescent micrographs of colocalization of TRPV1 expression (green) with the ER (Cytopainter ER tracking Dye, red). Images shown are at ×100 magnification and insets are zoomed ×2. Scale bar = 15 µm in ×100 images; scale bar = 5 µm in the zoomed panels. C: Pearson’s correlation coefficient calculated for the colocalization of TRPV1 with ER using Volocity. HBE cells from children without (2–4, 7, 10) and with asthma (12–15) were used in the analysis. D: HBE cells were treated with increasing amounts of TG and calcium levels were measured over 15 min. Calcium influx was measured using Calcium-6 dye after vehicle or TG in intact HBE cells (2, 15). The Softmax-Pro software was used to calculate EC50 = 1.41 µM. Samples were run in triplicate. E: [Ca2+]i in HBE cells stimulated in the absence of [Ca2+]ec. HBE cells from children without (1–4, 7) and with asthma (12, 13, 15) were incubated with sterile medium or RSV (MOI = 1) for 6 h, then pretreated with vehicle or thapsigargin (TG, 1.5 µM) for 30 min before activation of TRPV1 with capsaicin (150 µM). Data are expressed as means ± SE and were analyzed using the Kruskal–Wallis test with Dunn’s multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001 compared with noninfected controls; #P < 0.05, ###P < 0.001 compared with nonasthmatic controls. All experiments were repeated ≥2 times in triplicate. [Ca2+]i, intracellular calcium; ER, endoplasmic reticulum; HBE, human bronchial epithelium; MOI, multiplicity of infection; RSV, respiratory syncytial virus; TRPV1, transient receptor potential vanilloid 1.
Role of NGF in TRPV1 Expression and Function
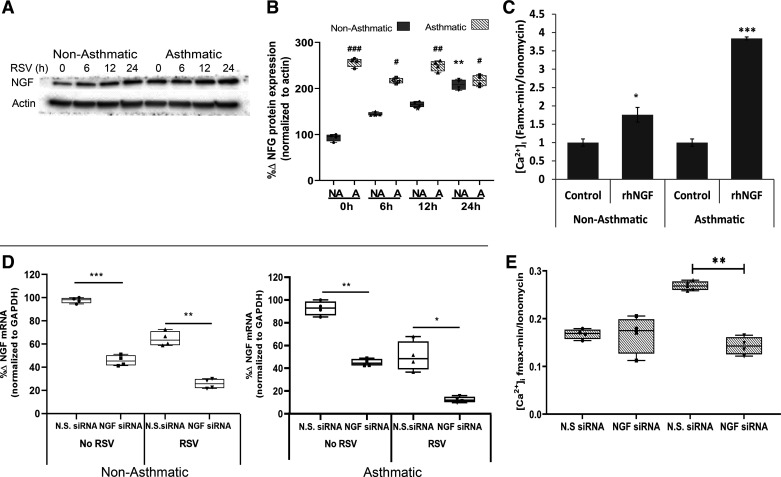
As NGF is known to be upregulated by RSV infection in HBE cells (29) and also controls TRPV1 translocation to the PM in neuronal cells by binding its cognate TrkA receptor (3), we investigated the role of NGF in regulating TRPV1 expression and function in HBE. To this end, the cells were infected with RSV (MOI = 1) and their lysates obtained at different time points were immunoblotted for NGF. Although RSV infection in HBE cells from nonasthmatic controls induced a time-dependent increase in NGF expression, NGF expression was significantly higher at baseline and remained higher upon RSV infection in HBE cells from children with asthma (Fig. 4, A and B). Next, we assessed NGF-mediated changes in capsaicin-induced Ca2+ influx. Although the administration of exogenous rhNGF (1 h before capsaicin) increased capsaicin-induced Ca2+ influx in both asthmatic HBE cells (P < 0.001, Fig. 4C) and nonasthmatic controls (P < 0.05), this effect was 3.5-fold larger in the asthmatic cells (P < 0.001). Silencing NGF expression with a specific siRNA (Fig. 4D) selectively abolished the effect of RSV infection on capsaicin-induced Ca2+ influx in asthmatic HBE cells, suggesting that NGF is both sufficient and necessary for the RSV-mediated increase of TRPV1 function in the bronchial epithelium of children with asthma (P < 0.001; Fig. 4E).
Figure 4.
NGF effect on TRPV1-mediated Ca2+ influx in children’s HBE. A: NGF protein expression measured by Western blot in primary HBE cells from children without (1, 2, 5, 11) and with asthma (12–15) infected with RSV (MOI = 1). B: quantification of results from A; n = 3 independent experiments. #P < 0.05, ##P < 0.01 non-asthmatic compared with asthmatic. C: [Ca2+]i in primary HBE cells from children without (1, 2, 5, 11) and with asthma (12–15) after treatment with rhNGF (50 ng/mL) for 1 h before activation of TRPV1 with capsaicin (150 µM); n = 3 independent experiments. D: primary HBE cells from children without asthma (1, 2) or with asthma (13, 15) were transiently transfected with nontargeting siRNA (NTsiRNA) or 50 nM of NGF-specific siRNA (NGFsiRNA) for 48 h, then infected with sterile medium or RSV (MOI = 1) for 12 h, and tested by qPCR for NGF mRNA expression normalized to GAPDH as the housekeeping gene. Data are expressed as means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001. E: inhibition of [Ca2+]i in primary HBE from children with asthma (12–15) transiently transfected with NGF-specific siRNA (NGFsiRNA, 50 nM) for 48 h, then incubated with RSV (MOI = 1) or sterile medium for 12 h before activation of TRPV1 with capsaicin. Controls were transfected with nontargeting siRNA (NTsiRNA). All experiments were repeated ≥2 times in quadruplicate. Data are expressed as means ± SE and were analyzed using the Kruskal–Wallis test with Dunn’s multiple comparisons. *P < 0.05, ***P < 0.001 compared with untreated controls; ###P < 0.001 compared with controls treated with NTsiRNA. [Ca2+]i, intracellular calcium; HBE, human bronchial epithelium; MOI, multiplicity of infection; NGF, nerve growth factor; qPCR, quantitative polymerase chain reaction; rhNGF, recombinant human nerve growth factor; RSV, respiratory syncytial virus; TRPV1, transient receptor potential vanilloid 1.
DISCUSSION
Our previous work revealed that the lower airway epithelium from patients with asthma displays increased capsaicin-mediated Ca2+ inflow compared with nonasthmatic controls, and that RSV infection leads to further significant increase in the effect of capsaicin in children but not in adults. Although capsaicin is a fairly selective ligand of the TRPV1 Ca2+ channel, that initial study was unable to provide conclusive evidence of a direct effect of asthma predisposition and RSV on TRPV1 expression and function because other calcium channels might have contributed to the increase in [Ca2+]i. For example, TRPA1 is activated upon rise in [Ca2+]i, and therefore activation of TRPV1 with consequent increase in [Ca2+]i may in turn activate TRPA1 leading to a greater effect. Our previous and present findings are important in that increased intracellular calcium in the airway epithelium has been shown to cause increased mucus production, disrupted barrier permeability, and increased bronchoconstriction—all of which are typical of the asthmatic phenotype—independently from the atopic diathesis that frequently underlies the pathophysiology of asthma in older children and adults.
Our current study took a deeper dive into the mechanisms underlying this different behavior of the asthmatic epithelium, demonstrating for the first time that the previously reported changes in Ca2+ permeability observed in HBE cells from children with asthma is due at least in part to intrinsically higher gene and protein expression of TRPV1. More importantly, TRPV1 expression also increases during infection with RSV—the most common lower respiratory tract pathogen in infants and young children—and the combined effect of underlying asthma predisposition and acute RSV infection synergistically increase Ca2+ entry into the bronchial epithelium. Another critical factor affecting TRPV1 function is its localization within the cell, and specifically whether it is expressed on the PM or within the cytosol. We show in this study that RSV infection of HBE cells results in increased TRPV1 PM, which is more pronounced in the cells derived from children with asthma compared with nonasthmatic controls and is critical for the RSV-mediated influx of [Ca2+]ec. As previous studies suggested that stimulation of PM-bound TRPV1 can induce epithelial cell apoptosis as well as the release of cytokines and tachykinins (10, 30), the observed TRPV1 PM overexpression in RSV-infected HBE cells could indirectly drive cytokine-induced goblet cell metaplasia, smooth muscle cell hyperreactivity, and increased vascular permeability (14).
Under basal conditions, TRPV1-mediated rise of [Ca2+]i in HBE cells from children with asthma is due in part to the release of Ca2+ from ER stores, as demonstrated by the loss of signal upon ER depletion with TG. In contrast, the increase in [Ca2+]i during RSV infection originates from the extracellular calcium pool, as demonstrated by loss of signal in the absence of [Ca2+]ec. These data suggest that the inflammatory effects of RSV involve TRPV1-mediated movement of Ca2+ from both extracellular and intracellular storage pools. Although previously shown in neuronal cells, our study is the first to suggest TRPV1 is capable of mediating the release of calcium from the ER of primary bronchial epithelial cells. However, caution must be taken in considering these conclusions, as we did not assess the potential role of other ion transporters, such as the NCX transporter or the ORAI family of channels that could be activated upon depletion of ER calcium by TG.
Finally, this study demonstrates that TRPV1 expression and translocation with consequent Ca2+ entry into the lower airway epithelium of children with asthma is modulated by signaling through the NGF/TrkA axis, and can be abolished by selective pharmacologic inhibition of the same pathway. NGF has been shown to contribute to airway inflammation and hyperreactivity in acute bronchiolitis and chronic asthma (29, 31, 32), and it was found to be elevated in the serum of patients with asthma and other inflammatory diseases of the lungs (33, 34). Importantly, NGF sensitizes nonadrenergic-noncholinergic neurons to capsaicin by increasing the expression and function of TRPV1 channels (35), and several lines of evidence in this study suggest it may exert a similar effect on the lower airway epithelium: 1) TRPV1 activity in children’s HBE is enhanced by the addition of rhNGF, and this effect is much stronger in asthmatic cells; 2) the effect of RSV on TRPV1 activity is abolished by silencing NGF gene expression; and 3) rhNGF mimics the effects of RSV on TRPV1 protein expression.
It is possible that other respiratory viruses might affect TRPV1 differently from RSV. In particular, human rhinovirus (HRV) becomes the more common respiratory pathogen after the first years of life and is predominant in adults. Therefore, a head to head comparison between RSV and different strains of HRV is needed and will require a separate study. We also recognize that the present studies were performed in vitro, which may not fully recapitulate what occurs in vivo. Finally, the HBE cells used in these studies were derived from deidentified donors, for whom we had no access to medical history. This was necessary because the availability of diseased samples is very limited, donors are deceased, and families must be consented.
Conclusions
As illustrated in our schematic model (Fig. 5), the results of this study show for the first time that the lower airway epithelium from children with asthma displays elevated basal and RSV-induced TRPV1 expression compared with nonasthmatic controls. This increase was measured both in total TRPV1 and TRPV1 localized to the plasma membrane. We have also shown that RSV-mediated increased TRPV1 expression and activation is a consequence of increased NGF-TrkA signaling. Finally, we have shown that TRPV1-mediated increase in [Ca2+]i can originate from either the extracellular pool or intracellular stores depending on asthma status and RSV infection. We speculate the virus-induced increase in [Ca2+]i mediated by TRPV1 activation might contribute to the clinical manifestations of asthma, including increased synthesis and release of TH2 cytokines, mucus overproduction, breakdown in barrier permeability, and increased bronchoconstriction in the context of endogenous TRPV1 activators known to be present in the inflamed airways of asthmatics. The present data are timely and important because of the increased morbidity for RSV bronchiolitis and asthma among pediatric patients (36). In addition, this study can shed light into the limited efficacy of the currently available therapies (37). More importantly, pharmacological inhibition of the NGF-TrkA-TRPV1 axis may become in the future a novel strategy to limit the impact of RSV infections in both children with and without asthma.
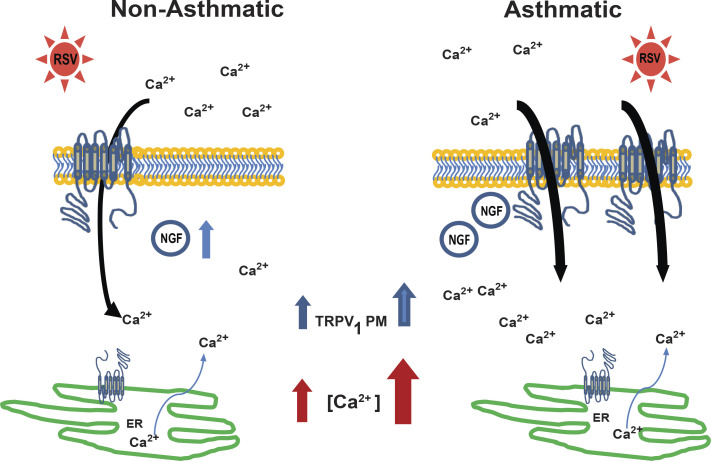
Figure 5.
Modulation of TRPV1-mediated Ca2+ influx in bronchial epithelium. RSV infection of nonasthmatic bronchial epithelial cells induces NGF synthesis. NGF binding to its high-affinity TrkA receptor starts a signaling cascade that leads to TRPV1 expression and its translocation to the plasma membrane, where it can be stimulated by multiple physical or chemical irritants and initiate the inward transfer of Ca2+ from the extracellular compartment to the cytosol. In contrast, basal expression of NGF is already increased at baseline in bronchial epithelial cells from children with asthma, which results in more TRPV1 channels expressed on the plasma membranes and lower threshold of activation. In addition, there is increased expression of intracellular TRPV1 localized to the ER, where it can release Ca2+ from the intracellular storage sites into the cytosol and lead to higher cytosolic Ca2+ concentration at rest. During RSV infection there is a shift of Ca2+ influx from both intracellular stores and extracellular sources to predominantly extracellular sources. Increased intracellular calcium stimulates mucus production, bronchoconstriction, airway barrier permeability, and release of proinflammatory cytokines resulting in the clinical manifestations of airway obstruction in bronchiolitis and asthma. ER, endoplasmic reticulum; NGF, nerve growth factor; RSV, respiratory syncytial virus; TrkA, tropomyosin-related kinase A; TRPV1, transient receptor potential vanilloid 1.
GRANTS
This work was supported in part by National Institutes of Health National Heart, Lung, and Blood Institute (NHLBI) grants: NHLBI R01 HL-061007 (to G.P.); NHLBI R01 HL148057 (to F.R.); NHLBI R01 HL133721 and NHLBI R01 HL11972 (to M.A.O.); and NHLBI K08 HL133380 (to R.S.). Funding was also provided by the Cystic Fibrosis Foundation Grant PIEDIM16G0 (to G.P.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.J.H., L.G., R.S., M.A.O., and G.P. conceived and designed research; T.J.H. performed experiments; T.J.H., M.A.O., and G.P. analyzed data; T.J.H., L.G., M.A.O., and G.P. interpreted results of experiments; T.J.H. prepared figures; T.J.H., M.A.O., and G.P. drafted manuscript; T.J.H., F.R., M.A.O., and G.P. edited and revised manuscript; T.J.H., L.G., F.R., R.S., M.A.O., and G.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We are indebted to Dr. Mark Peeples (Nationwide Children’s Hospital Research Institute, Columbus, OH) and Dr. Peter Collins (National Institutes of Health, Bethesda, MD) for providing the original batch of the RFP-expressing RSV virus. We also thank Dr. Paul Brown (Cleveland Clinic Center for Pediatric Research, Cleveland, OH) for preparing the virus stock used in some of the experiments.
REFERENCES
- 1.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 2.Eskander MA, Ruparel S, Green DP, Chen PB, Por ED, Jeske NA, Gao X, Flores ER, Hargreaves KM. Persistent nociception triggered by nerve growth factor (NGF) is mediated by TRPV1 and oxidative mechanisms. J Neurosci 35: 8593–8603, 2015. doi: 10.1523/jneurosci.3993-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol 128: 509–522, 2006. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu W, Oxford GS. Differential gene expression of neonatal and adult DRG neurons correlates with the differential sensitization of TRPV1 responses to nerve growth factor. Neurosci Lett 500: 192–196, 2011. doi: 10.1016/j.neulet.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue K, Koizumi S, Fuziwara S, Denda S, Inoue K, Denda M. Functional vanilloid receptors in cultured normal human epidermal keratinocytes. Biochem Biophys Res Commun 291: 124–129, 2002. doi: 10.1006/bbrc.2002.6393. [DOI] [PubMed] [Google Scholar]
- 6.Reilly CA, Taylor JL, Lanza DL, Carr BA, Crouch DJ, Yost GS. Capsaicinoids cause inflammation and epithelial cell death through activation of vanilloid receptors. Toxicol Sci 73: 170–181, 2003. doi: 10.1093/toxsci/kfg044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scotland RS, Chauhan S, Davis C, De Felipe C, Hunt S, Kabir J, Kotsonis P, Oh U, Ahluwalia A. Vanilloid receptor TRPV1, sensory C-fibers, and vascular autoregulation: a novel mechanism involved in myogenic constriction. Circ Res 95: 1027–1034, 2004. doi: 10.1161/01.res.0000148633.93110.24. [DOI] [PubMed] [Google Scholar]
- 8.Seki N, Shirasaki H, Kikuchi M, Sakamoto T, Watanabe N, Himi T. Expression and localization of TRPV1 in human nasal mucosa. Rhinology 44: 128–134, 2006. [PubMed] [Google Scholar]
- 9.Zhang F, Yang H, Wang Z, Mergler S, Liu H, Kawakita T, Tachado SD, Pan Z, Capo-Aponte JE, Pleyer U, Koziel H, Kao WW, Reinach PS. Transient receptor potential vanilloid 1 activation induces inflammatory cytokine release in corneal epithelium through MAPK signaling. J Cell Physiol 213: 730–739, 2007. doi: 10.1002/jcp.21141. [DOI] [PubMed] [Google Scholar]
- 10.Agopyan N, Bhatti T, Yu S, Simon SA. Vanilloid receptor activation by 2- and 10-micron particles induces responses leading to apoptosis in human airway epithelial cells. Toxicol Appl Pharmacol 192: 21–35, 2003. doi: 10.1016/s0041-008x(03)00259-x. [DOI] [PubMed] [Google Scholar]
- 11.Yu H, Li Q, Zhou X, Kolosov VP, Perelman JM. Transient receptor potential vanilloid 1 receptors mediate acid-induced mucin secretion via Ca2+ influx in human airway epithelial cells. J Biochem Mol Toxicol 26: 179–186, 2012. doi: 10.1002/jbt.20413. [DOI] [PubMed] [Google Scholar]
- 12.Groneberg DA, Niimi A, Dinh QT, Cosio B, Hew M, Fischer A, Chung KF. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med 170: 1276–1280, 2004. doi: 10.1164/rccm.200402-174oc. [DOI] [PubMed] [Google Scholar]
- 13.Lin RL, Hayes D, Jr, Lee LY. Bronchoconstriction induced by hyperventilation with humidified hot air: role of TRPV1-expressing airway afferents. J Appl Physiol 106: 1924–2009, 1917. doi: 10.1152/japplphysiol.00065.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGarvey LP, Butler CA, Stokesberry S, Polley L, McQuaid S, Abdullah H, Ashraf S, McGahon MK, Curtis TM, Arron J, Choy D, Warke TJ, Bradding P, Ennis M, Zholos A, Costello RW, Heaney LG. Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J Allergy Clin Immunol 133: 704–712, 2014. doi: 10.1016/j.jaci.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Harford TJ, Rezaee F, Scheraga RG, Olman MA, Piedimonte G. Asthma predisposition and respiratory syncytial virus infection modulate transient receptor potential vanilloid 1 function in children's airways. J Allergy Clin Immunol 141: 414–416.e4, 2018. doi: 10.1016/j.jaci.2017.07.015.[28982576] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J 21: 629–632, 2002. doi: 10.1097/00006454-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 282: 1440–1446, 1999. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 18.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 161: 1501–1507, 2000. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 19.Hu C, Wedde-Beer K, Auais A, Rodriguez MM, Piedimonte G. Nerve growth factor and nerve growth factor receptors in respiratory syncytial virus-infected lungs. Am J Physiol Lung Cell Mol Physiol 283: L494–L502, 2002. doi: 10.1152/ajplung.00414.2001. [DOI] [PubMed] [Google Scholar]
- 20.Othumpangat S, Gibson LF, Samsell L, Piedimonte G. NGF is an essential survival factor for bronchial epithelial cells during respiratory syncytial virus infection. PLoS One 4: e6444, 2009. doi: 10.1371/journal.pone.0006444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Othumpangat S, Walton C, Piedimonte G. MicroRNA-221 modulates RSV replication in human bronchial epithelium by targeting NGF expression. PLoS One 7: e30030, 2012. doi: 10.1371/journal.pone.0030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas KW, Monick MM, Staber JM, Yarovinsky T, Carter AB, Hunninghake GW. Respiratory syncytial virus inhibits apoptosis and induces NF-κB activity through a phosphatidylinositol 3-kinase-dependent pathway. J Biol Chem 277: 492–501, 2002. doi: 10.1074/jbc.m108107200. [DOI] [PubMed] [Google Scholar]
- 23.Chakraborty S, Castranova V, Perez MK, Piedimonte G. Nanoparticles increase human bronchial epithelial cell susceptibility to respiratory syncytial virus infection via nerve growth factor-induced autophagy. Physiol Rep 5: e13344, 2017. doi: 10.14814/phy2.13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harford TJ, Brown PM, Olman MA, Piedimonte G. Respiratory syncytial virus (RSV) induced changes in TRPV1 activation in pediatric bronchial epithelial cells (Abstract). Am J Respir Crit Care Med 201: A3384, 2015. https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2015.191.1_MeetingAbstracts.A3384. [Google Scholar]
- 25.Harford TJ, Brown PM, Olman MA, Piedimonte G. Respiratory syncytial virus (RSV) induced changes in TRPV1 expression and translocation. Am J Respir Crit Care Med 191: A4112, 2015. https://scholar-cnki-net-443.webvpn.zisu.edu.cn/en/Detail/index/GARJ2015/SJPD256062D637FBF8899FBEC030E99BDCCE. [Google Scholar]
- 26.Rameix-Welti MA, Ronan LGR, Herve PL, Sourimant J, Remot A, Riffault S, Yu Q, Galloux M, Gault E, Eleouet JF. Visualizing the replication of respiratory syncytial virus in cells and in living mice. Nat Commun 5: 5104, 2014. doi: 10.1038/ncomms6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallego-Sandin S, Rodriguez-Garcia A, Alonso MT, Garcia- SJ. The endoplasmic reticulum of dorsal root ganglion neurons contains functional TRPV1 channels. J Biol Chem 284: 32591–32601, 2009. doi: 10.1074/jbc.m109.019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karai LJ, Russell JT, Iadarola MJ, Olah Z. Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J Biol Chem 279: 16377–16387, 2004. doi: 10.1074/jbc.m310891200. [DOI] [PubMed] [Google Scholar]
- 29.Renz H, Kerzel S, Nockher WA. The role of neurotrophins in bronchial asthma: contribution of the pan-neurotrophin receptor p75. Prog Brain Res 146: 325–333, 2004. doi: 10.1016/s0079-6123(03)46020-2. [DOI] [PubMed] [Google Scholar]
- 30.Agopyan N, Li L, Yu S, Simon SA. Negatively charged 2- and 10-micron particles activate vanilloid receptors, increase cAMP, and induce cytokine release. Toxicol Appl Pharmacol 186: 63–76, 2003. doi: 10.1016/s0041-008x(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 31.Braun A, Lommatzsch M, Lewin GR, Virchow JC, Renz H. Neurotrophins: a link between airway inflammation and airway smooth muscle contractility in asthma? Int Arch Allergy Immunol 118: 163–165, 1999. doi: 10.1159/000024056. [DOI] [PubMed] [Google Scholar]
- 32.Micera A, Vigneti E, Pickholtz D, Reich R, Pappo O, Bonini S, Maquart FX, Aloe L, Levi-Schaffer F. Nerve growth factor displays stimulatory effects on human skin and lung fibroblasts, demonstrating a direct role for this factor in tissue repair. Proc Natl Acad Sci USA 98: 6162–6167, 2001. doi: 10.1073/pnas.101130898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonini S, Lambiase A, Bonini S, Angelucci F, Magrini L, Manni L, Aloe L. Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc Natl Acad Sci USA 93: 10955–10960, 1996. doi: 10.1073/pnas.93.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olgart C, Frossard N. Human lung fibroblasts secrete nerve growth factor: effect of inflammatory cytokines and glucocorticoids. Eur Respir J 18: 115–121, 2001. doi: 10.1183/09031936.01.00069901. [DOI] [PubMed] [Google Scholar]
- 35.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411: 957–962, 2001. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 36.Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, Poehling KA, Szilagyi PG, Griffin MR, Williams JV, Zhu Y, Grijalva CG, Prill MM, Iwane MK. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 132: e341–e348, 2013. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 37.Merckx J, Ducharme FM, Martineau C, Zemek R, Gravel J, Chalut D, Poonai N, Quach C, Pediatric Emergency Research Canada D; Pediatric Emergency Research Canada (PERC) DOORWAY team. Respiratory viruses and treatment failure in children with asthma exacerbation. Pediatrics 142: e20174105, 2018. doi: 10.1542/peds.2017-4105. [DOI] [PubMed] [Google Scholar]