Abstract
Viral infections affecting the lower respiratory tract place enormous burdens on hospitals. As neither vaccines nor antiviral agents exist for many viruses, understanding risk factors and outcomes in each patient using minimally invasive analysis, such as blood, can lead to improved health care delivery. A cohort of PAXgene RNA sequencing of infants admitted with moderate or severe acute bronchiolitis and respiratory syncytial virus were compared with case-control statistical analysis and cohort-based outlier mapping for precision transcriptomics. Patients with severe bronchiolitis had signatures connected to the immune system, interferon signaling, and cytokine signaling, with marked sex differences in XIST, RPS4Y1, KDM5D, and LINC00278 for severity. Several patients had unique secondary infections, cytokine activation, immune responses, biological pathways, and immune cell activation, highlighting the need for defining patient-level transcriptomic signatures. Balancing relative contributions of cohort-based biomarker discoveries with patient’s biological responses is needed to understand the totality of mechanisms of adverse outcomes in viral bronchiolitis.
Keywords: immune system, precision medicine, respiratory syncytial virus, transcriptomics, viral bronchiolitis
INTRODUCTION
Viral bronchiolitis (VB) is a syndrome of lower respiratory tract infection caused by multiple viruses including respiratory syncytial virus (RSV), human rhinovirus, parainfluenza virus, human metapneumovirus, coronavirus, adenovirus, influenza, and enterovirus. RSV is the most common cause of VB, with 50%–80% of VB cases attributable to RSV (1). In 2015, globally, there were 33 million estimated RSV cases with 3 million hospitalizations, of which 1 million were children under 6 mo, yielding around 30,000 deaths (2).
No treatments of RSV-associated bronchiolitis beyond supportive care have been supported by evidence, and no vaccine is presently available for prevention (3). These facts underscore the need for a more detailed understanding of pathophysiology together with determinants of outcomes (4). RSV can present in various ways, ranging from mild respiratory issues to life-threatening respiratory failure (5). Roughly 1%–3% of infected individuals require hospitalization, making it the main cause of lower respiratory tract illnesses in infants (6–8). A small proportion of these patients require the support of a pediatric intensive care unit (PICU). One PICU admission alone costs approximately $35,000–$89,000 (9).
It remains unclear why some patients recover without the need for medical attention, and yet others develop respiratory failure. Growing evidence supports a global immune activation phenotype to contribute significantly to respiratory failure (10, 11). A better understanding of how the virus disrupts patients’ immune system and metabolism could yield critical insights. The early identification of these risk factors could reduce clinical costs and improve the delivery of care. Epidemiological risk factors for infant RSV severity include premature birth, lung disorders, heart disease, immunodeficiencies, secondary infections, and nasopharyngeal microbiota (12–15). It is known that hospitalizations for RSV are themselves a risk factor for chronic conditions such as allergic asthma in adolescence (16). Although most pathology occurs in the lungs, easier accessible blood samples robustly show RSV response (17). Changes in the innate and adaptive immune responses are associated with RSV hospitalization (18). A cohort of 190 children revealed the power of blood RNA sequencing (RNAseq) in segregating patients with RSV who received inpatient versus outpatient care (11).
RNA expression profiles derived from peripheral blood samples can provide a crucial real-time snapshot of how the body responds to a wide range of disease processes. Previous work has defined gene expression signatures for RSV hospitalization, host interaction, and immune time course changes (17, 19–21). Recent findings found interferon signaling to correlate with increased risk for hospitalization in patients with RSV infection (11). Clinicians understand that not all pediatric patients with the same disease present the same way. There is a need to balance transcriptomic findings with patient-specific signals related to the clinical course.
Recently, we implemented a workflow that dissects host-pathogen interactions in a rare disease format to examine the transcriptome in severe infections with multiorgan dysfunction syndrome (MODS) over three time points (22). This work further quantified that each patient uniquely responds to infection and that host genomics and transcriptomics can elucidate precision insights into multiple mechanisms that could drive severity. The transcriptome provides an abundance of valuable information in understanding an individual’s infection timeline, concurrent diseases, organ dysfunction, and treatment targets. Herein, we define precision transcriptomics as the use of RNAseq data to define human and nonhuman RNA signatures in each patient. In this paper, we applied this precision transcriptomic approach to analyzing whole blood samples from a cohort early in the illness for severe (n = 15) or moderate (n = 5) RSV over two time points, addressing acute and recovery phases. This allowed us to address biomarkers that have been identified in larger cohorts while simultaneously focusing on patient-centric responses that are a hallmark of precision medicine.
METHODS
Samples
Parents of patients were consented, and samples were collected under institutional review board (IRB) Protocol No. 2107-049-SH/HDVCH by the Spectrum Health IRB committee. After written informed consent of parents/guardians, blood was collected from patients <6 mo of age (excluding premature births <34 wk of gestation) who were admitted to the PICU for bronchiolitis and who were positive for (at least) RSV by nasopharyngeal test (n = 20). Patient samples were collected at two time points, within 24 h of initiation of respiratory support and just before intravenous (IV) removal and discharge from the PICU/hospital, on average 3–4days later. Patients were divided into either severe or moderate groupings based on the need for invasive mechanical ventilation. A total of 10 healthy age-matched sedation-control patients with normal airways and lungs, presenting for routine IV sedation, had blood collected at one time. At least 1.25 mL of blood was collected in EDTA tubes followed by transfer to a PAXgene tube at 1:2.5 mixture with stabilizing agent. Data, deidentified and paired to RNA sample, was collected and managed using Research Electronic Data Capture (REDCap).
RNAseq and Bioinformatics
PAXgene tubes were collected at −80 C until all samples were obtained, performing RNA extraction, preparation, and sequencing in a single batch. RNA was isolated from PAXgene Blood RNA tubes using Qiagen’s QIAsymphony PAXgene Blood RNA Kit. Libraries were prepared using KAPA RNA HyperPrep Kit (v2.17) and QIASeq FastSelect human rRNA and globin removal. Sequencing of libraries was done using 50-bp, paired-end reads on Illumina NovaSeq in a single flow lane. Reads are available under NCBI BioProject PRJNA693881. Paired-end fastq reads were quasi-aligned to Homo sapiens Gencode v36 or our single gene to sequence transcriptome (22) using salmon_0.14.1 (23). Mapped transcripts per million (TPMs) for all samples were processed through NetworkAnalyst3.0 (24) using Limma (25) to identify gene- or transcript-level annotations that differ between groups. Our focus comparison was VB day 0 relative to controls, with an additional nested comparison of VB day 0 relative to day 3 to see if signatures were returning to normal. Pathways identified in Network Analyst to have variable gene expressions with an adjusted P value (< 0.01) and log2 fold change of 2 comparing VB day 0 with controls were extracted for Gene Ontology enrichment. Transcript-level annotations were compared for control relative to day 0 or day 3 VB cases. CIBERSORTx digital cytometry cell fraction imputation (26, 27) was performed using gene mapping on LM22 signature matrix without batch corrections and 500 permutations. Bacterial and viral read mapping was performed using kraken2-2.0.7-beta (28) with mapping normalized to every million mapped human reads. The immune repertoire CDR3 mapping was done with the MiXCR tools (29). PERSEVERE genes were obtained from previous reports (30), and cytokine annotations of genes were identified from UniProt. Cell-type-specific genes were identified from the human protein atlas (HPA) (31) single cell type atlas. From the gene-level annotation, z scores were calculated for each gene TPM value across all samples [(patient gene level − gene average)/standard deviation of gene values]. Genes for each patient greater than 4 TPM and 4 standard deviations were identified and assessed with STRING (32) for pathway enrichment.
RESULTS
Patient Demographics
We enrolled 20 patients with VB and 10 sedation controls. Controls were 0.5–6 mo of age (mean, 3.2; median, 2.0) with six males and four females. Patients with VB were 0–7 mo of age (mean, 2.0; median, 2.0) with seven males and 13 females. Demographics for patients with VB are presented in Table 1. We reviewed both comprehensive and daily Pediatric Logistic Organ Dysfunction (PELOD) scores, which revealed similar medians across time points (both day 0 and day 3) and over their entire PICU stay. When PELOD scores were reviewed based on severe versus moderate, by day 3, moderates had lower scores (2.75 vs. 7.93), whereby severe patient PELOD scores had remained relatively unchanged from day 0 (8.87). Fifteen patients were categorized as severe bronchiolitis, and five patients were categorized as moderate.
Table 1.
Patient demographics of RSV positive patients (N = 20)
| Range (n) | # | Mean | Median | SD | |
|---|---|---|---|---|---|
| Age | |||||
| Controls | 0.5–6 mo | 10 | 3.2 | 3.0 | 1.83 |
| RSV | 0–7 | 20 | 2.0 | 2.0 | 1.52 |
| Male | |||||
| Controls | 6 | ||||
| RSV | 7 | ||||
| Birth weight, kg | |||||
| Controls | 10 | 3.65 | 3.7 | 0.40 | |
| RSV | 20 | 3.10 | 3.2 | 0.84 | |
| Ethnicity | |||||
| Controls | 10 | ||||
| Caucasian/European | 9 | ||||
| Black or Hispanic | 1 | ||||
| RSV | |||||
| Caucasian/European | 17 | ||||
| Black or Hispanic | 3 | ||||
| Secondary bacterial infections | 7 | ||||
| Severity of illness scores | |||||
| PELOD comprehensive | 19 | 10.70 | 11.00 | 3.28 | |
| Day 0c | 19 | 8.63 | 11.00 | 4.21 | |
| Severe | 15 | 8.87 | 11.00 | 4.09 | |
| Moderate | 5 | 7.75 | 10.00 | 5.19 | |
| Day 3d | 19 | 6.78 | 11.00 | 5.45 | |
| Severe | 15 | 7.93 | 11.00 | 5.05 | |
| Moderate | 5 | 2.75 | 0.00 | 5.50 | |
| PRISMIII | 20 | 2.15 | 1.5 | 1.95 | |
| Severe | 15 | 2.13 | 1.0 | 2.03 | |
| Moderate | 5 | 2.2 | 2.0 | 1.92 | |
| PICU LOS, days | 20 | 10.60 | 10.04 | 5.56 | |
| Severe | 15 | 11.41 | 4.86 | ||
| Moderate | 5 | 8.56 | 5.53 | 7.58 | |
| Respiratory support, days | |||||
| Mechanical ventilation | 15 | 8.95 | 7.08 | 4.30 | |
| High flow oxygen | 5 | 0.96 | 0.2 | 1.56 |
a11/19 patients scored a 1 for PELOD pulmonary score; bpreterm newborn, gestational age 36 completed weeks. LOS, length of stay; PICU, pediatric intensive care unit; RSV, respiratory syncytial virus.
Transcriptional Changes in VB
Each transcriptome was assessed using traditional differential gene analysis and our precision transcriptomic workflow (Fig. 1). Following quasi alignment of fastq files to gene-level sequences, a clear separation of all VB day 0 samples from controls was observed, whereas VB day 3 samples begin clustering to controls (Fig. 2A). Most notably in principal component analysis is that gene signatures of severe day 3 samples more closely reflect day 0 severe samples, suggesting an extensive elevated blood profile, whereas most patients with moderate RSV return to control values by day 3. A nested comparison of control versus VB day 0 relative to VB day 0 versus VB day 3 revealed 266 genes significantly higher and 53 genes lower in patients with VB (Fig. 2B). The top two genes higher in VB day 0 are IFI27 and CD177 (Fig. 2C). Interferon-inducible protein IFI27 has previously been shown to segregate bacterial versus viral infections (33), and CD177 is a neutrophil gene involved in infection responses (34). FCRL6 and FGFB2 are the most significantly decreased genes in VB day 0 (Fig. 2C). The 266 genes higher in VB day 0 are significantly enriched for cytokine signaling, innate immune system, neutrophil degranulation, influenza A, and secretory granule biology (Fig. 2D). Two papers have previously seen many of the same genes describing blood cell components to interferon signatures (35) and monitoring viral infections (36). Genes lower in VB day 0 have less enriched pathways but show association to innate lymphoid cells and T-cell activation (37, 38).
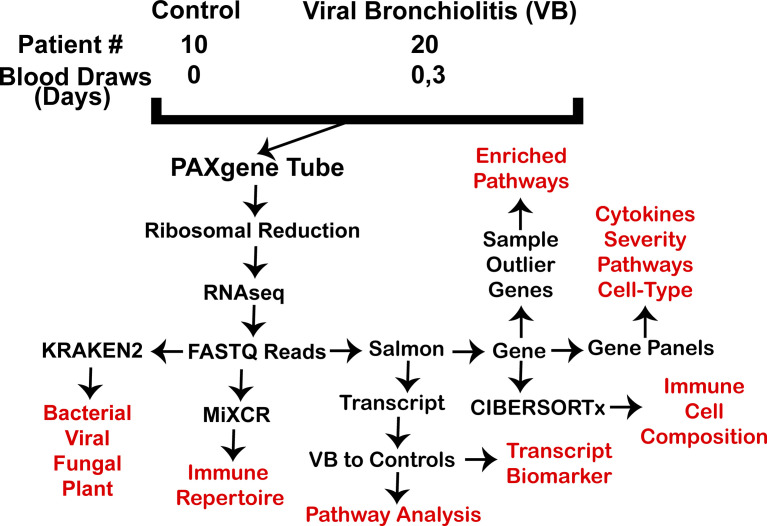
Figure. 1.
Sample and analysis workflow. In red are data returns assessed for patients.
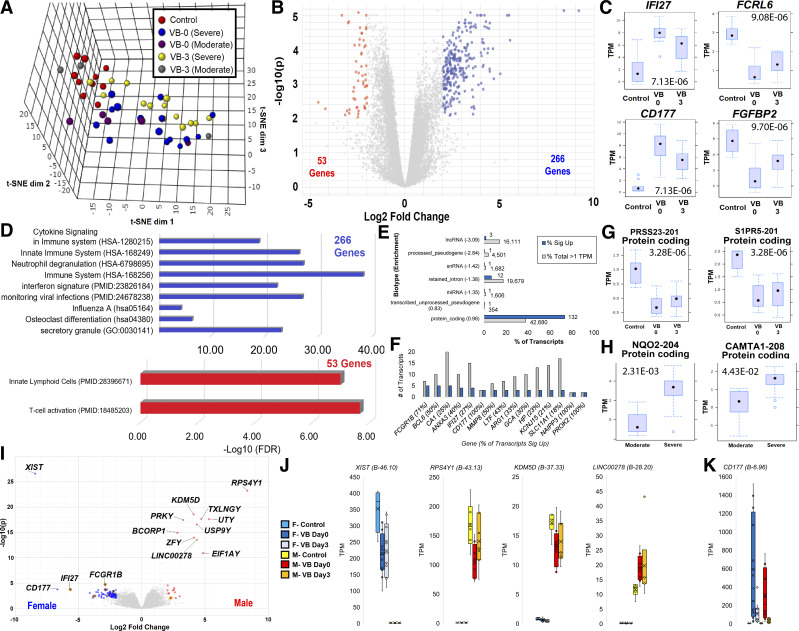
Figure. 2.
Transcriptome comparisons of viral bronchiolitis (VB) day 0 and 3 versus controls. A: sample segregation for all control (red), VB day 0 severe (blue), VB day 0 moderate (magenta), VB day 3 severe (yellow), and VB day 3 moderate (gray) transcriptomes for gene expression on three-dimensional reduction using t-SNE. B: volcano plot of genes with differential expression between nested control versus VB day 0 relative to VB day 0 versus VB day 3 for genes higher (blue) or lower (red). Cutoffs used were log2 fold change 2 and Adj-P < 0.01, with 319 identified genes. C: top four significant genes up or down shown as box and whisker plots with Adj-P value shown. D: STRING-based enriched pathways for genes higher (blue) or lower (red) in VB. Values are shown as the −log10 of P value and the term identification number is in parentheses. E: top segregating transcripts between controls and VB day 0 (blue) annotated at biotype level. The gray box shows the percent of mapped transcripts for each biotype and the blue box shows the percent of genes significantly different with log2 fold change 2 and Adj-P < 0.01. F: transcripts with multiple isoforms significant between controls and VB. The gray box shows the total number of mapped transcripts and the blue box shows how many were significantly changed. The percent of transcripts change for each gene is labeled in parentheses. G: each transcript is labeled with ENST, protein name in parenthesis, and biotype annotation with values shown as box and whisker plots. F: volcano plot of male control versus male day 0 relative to male day 0 versus female day 0. The top genes based on combined B metric are labeled. G and H: top two significant transcripts between controls and VB samples (G) or moderate versus severe VB (H) shown as a box and whisker plot. I: volcano plot for nested comparison of control versus VB relative to male versus female. J: top four genes for nested comparisons. Colors represent samples: cyan-F control, dark blue-F severe VB, light blue-F moderate VB, gray-M control, red-M severe VB, orange-M moderate VB. K: female versus male values for the highly significant CD177 using same colors as panel J. TPM, transcripts per million.
Gene-level annotation has 27,854 sequences, whereas transcript-level annotation has 231,288 unique sequences, including splicing and noncoding derivatives not present within gene annotations. A total of 117,396 of the transcripts are annotated with a TPM value above 1 within at least one sample. There are 180 significant elevated transcripts in VB day 0 enriched for protein-coding transcripts (Fig. 2E). Several genes have multiple altered isoforms, including FCGR1B, BCL6, CA1, ANXA1, and IFI27 (Fig. 2F). The top two significantly elevated transcripts were protein-coding transcripts in PRSS23 and S1PR5 (Fig. 2G). Several transcripts, including NQO2-204 and CAMTA1-208, are significant at day 0 in moderate versus severe patients (Fig. 2H). All gene and transcript mapping and significance lists can be found in Supplemental Table S1 (see https://doi.org/10.6084/m9.figshare.14265182.v1).
An in-depth analysis of sex differences within patients with VB relative to controls, using a nested comparison (control vs. VB day 0 males relative to VB day 0 males vs. VB day 0 females), revealed the X-chromosome gene XIST and multiple Y-chromosome genes to have significant changes in VB (Fig. 2I). Further analysis of XIST shows female-specific expression with a decrease in VB day 0/3 relative to the controls (Fig. 2J). From the Y-chromosome, both RPS4Y1 and KDM5D are lower in VB day 0 with a return to control values in VB day 3, and LINC00278 shows elevation in VB day 0/3 relative to the controls (Fig. 2G). This highlights that sex chromosomes contribute to VB response. None of the nonsex chromosome genes shows pronounced differences between males and females, as highlighted by CD177 analysis (Fig. 2K).
Precision Transcriptomics
Our initial analysis demonstrated widespread induction of specific components of inflammatory response among patients with VB relative to controls at the cohort level. However, we have previously demonstrated that inflammatory response to infection and outcome is patient specific (22). Therefore, we used a precision transcriptome strategy to assess individuals. Immune cell deconvolution, using CIBERSORTx, demonstrated considerable heterogeneity between samples in terms of the white blood cell-specific genes (Fig. 3A). The absolute score for immune cells was high in VB day 0 for severe and moderate patients (Fig. 3B). The 39 genes specific to neutrophils are elevated primarily in VB day 0 patients, with a few samples with extreme activation (Fig. 3C).
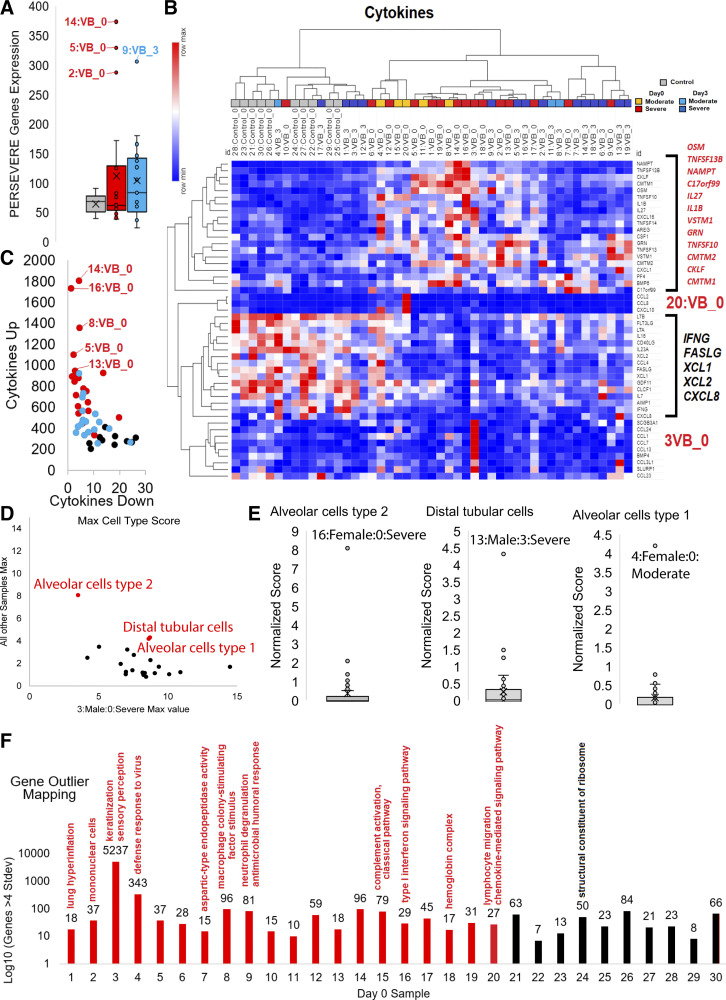
Figure. 3.
Gene deconvolution and bacteria/virus mapping from blood RNAseq reads. A: percent of the 22 annotated cells (far right color key) imputed for each of the RNAseq gene annotations using CIBERSORTx. Labeled below is sample ID:sex:day:group. B: box and whisker plot of absolute cell data from CIBERSORTx for controls (gray), day 0 moderate (orange), day 0 severe (red), day 3 moderate (cyan), day 3 severe (blue). C: gene analysis of neutrophil for each sample with colors corresponding to panel B. Top segregating genes from CIBERSORTx were pulled for each group and the z score of expression assessed. Z scores were added for genes (x-axis) or assessed for the % of genes at >1 standard deviation (stdev, y-axis). D: clustering of each group from panel B shown as box and whisker plots for 10 cell annotations. Outliers are labeled for VB day 0. E: read mapping from Kracken2 normalized to human, unmapped, bacteria, and virus with colors corresponding to panel B. Samples identified as outliers of the box and whisker plots are labeled. F: box and whisker plots for read mapping for the top species-level identifications for top outliers from panel E. Outliers from all samples are labeled. G: top segregating species annotations between controls and VB day 0 using mapped reads from Bacillus subtilis, Elizabethkingia meningoseptica, Cryobacterium arcticum, Labrenzia alexandrii, Spiroplasma corruscae. Plot shows the summed z score for the 5 species (x-axis) and the % of species above average across all samples (y-axis). H: statistics for CDR3 mapped reads. Panels show in order the % of reads mapped to CDR3, the number of clonotypes in each sample, and the number of reads per clonotype. Colors are the same as panel B. I: the number of chains (top) or clones (bottom) for IGH or IGK CDR3 mapping. J: the max % of one clonotype relative to all mapped clonotypes and the number of clonotypes with greater than 1% of the total clonotype mapping. K: meme analysis for the top two motifs. The top one is seen with highest E-value (red) and in the most samples. The second motif is only observed elevated in patient 16 samples.VB, viral bronchiolitis.
We further analyzed relative scores for each cell type based on CIBERSORTx to identify outliers (Fig. 3D). Neutrophils are elevated in VB day 0 severe and moderate patients relative to controls (P = 2E–4), with patients 14 (1-wk-old female, ventilated for 10 days or V = 10) and 16 (4-wk-old female, V= 18) as outliers. Patient 16 with the spike in neutrophil genes had the longest time on ventilator and longest hospital stay (40 days). Patient 16 developed feeding difficulties needing placement of a gastrostomy tube for feeding in the first year of life. We noted that the correlation of clinically measured neutrophils did not correspond to the cell annotations, suggesting that neutrophil elevation based on CIBERSORTx is weighted to activation states of neutrophil genes and less on the total blood neutrophil counts.
Other cell groups with elevation include monocytes in severe VB day 0/3 (P = 0.02), memory B cells in VB day 0 moderate (trend, P = 0.1), M0 macrophages in VB day 0/3 severe (P = 0.05), resting mast cells in all groups relative to controls (P = 8E-3), activated dendritic cells in VB day 0 (P = 8E–3), and regulatory T cells (Tregs) in VB day 0 (trend, P = 0.07). The memory B cells in moderate VB day 0 patients are increased, suggesting that these patients segregate from severe patients potentially based on the adaptive immune response. Several patients are outliers of these cell estimates, including patient 2 (12-wk-old male, V = 7; tracheal aspirates for Hemophilus influenza needing multiple fluid boluses and inotropic support for 48 h) having elevated genes for monocytes, M0 macrophages, and resting mast cells. Patient 14 had elevated genes for M0 macrophages and activated dendritic cells, and patient 9 (2-wk-old female, V = 5) had elevated genes for Tregs and eosinophils (clinical eosinophil value of 322). Patient 14 was a 7-day-old who also had clinically suspected Escherichia coli in the blood based on culturing and who needed fluid boluses and brief inotropic support. However, reads in the blood were unable to be mapped to E. coli in this patient.
Secondary infections are putative drivers for immune responses, and microbiome changes have been linked to altered airway physiology (39). Using >20,000 bacterial and viral transcriptomes, we identify sequences in all samples, including controls (Fig. 3E). Patient 1 (10-wk-old male, V = 6) is an outlier for bacteria on both day 0 and day 3. Patient 1 had a protracted clinical course requiring a bronchoscopy, which revealed Aspergillosis species and a tracheal aspirate positive for Streptococcus pneumonae. After multiple admissions for respiratory illness, the patient was labeled asthmatic in the first year post-VB admission. Patient 18 (5-wk-old male, V = 15) day 0 and patient 7 (6-wk-old female, V = 11) day 3 are outliers for viral read mapping. Individual species were annotated for outliers identifying Agromyces aureus and Caulobacter vibrioides in patient 1, Streptomyces in patient 9 (eosinophil/Treg, Fig. 3D), and a Proteus phage in several patients (Fig. 3F). Five species are mapped significantly in patients with VB relative to controls, including Bacillus subtilis, Elizabethkingia meningoseptica, Cryobacterium arcticum, Labrenzia alexandrii, and Spiroplasma corruscae. None of the control patients had mapping for the five species, whereas multiple patients with severe VB showed elevation of all five species (Fig. 3G). Bacillus subtilis has been known to cause pneumonia and sepsis as well as allergic reactions (40, 41). Elizabethkingia meningoseptica was isolated from the respiratory tracts of patients with cystic fibrosis and is considered to be of low pathogenicity to humans (42). The Cryobacterium genus is found in the normal respiratory tract (43). This would seem to suggest that normal flora of the respiratory tract is detectable in the blood of patients with severe VB, with patient 16 showing the highest levels. This may represent either opportunistic disseminated infection by normal flora in the face of inflammatory response skewed to viral response or simply a breakdown of the blood-alveolar barrier.
Utilizing RNA reads, the immune repertoire can be measured by addressing the extensions of CDR3 read alignments to determine the expansion of clonotypes in B and T cells. Patients show relatively even mapping of reads to CDR3, with VB day 0 samples having fewer clonotypes and more reads per clonotype (Fig. 3H). The percent of mapped chains and clones of IGK is elevated in VB day 0 (Fig. 3I). VB day 0 samples have multiple clonotypes elevated above 1% of the total mapped CDR3 sites suggesting clonal expansion due to RSV (Fig. 3J). Integrating across all samples with a clonotype above 3% shows a highly similar motif across samples, found elevated the most in severe VB day 0 (Fig. 3K, top). Interestingly, the only patient with an elevated motif outside the shared was patient 16, where the same motif was elevated at both day 0 and day 3 (Fig. 3K, bottom).
Several genes are known to be highly correlated to severity outcomes in pediatric patients, including septic shock-linked genes (PERSEVERE, CXCL8, CCL3, HSPA1B, GZMB, MMP8) (44) and cytokines. Three patients at day 0 with VB have a spike in PERSEVERE genes (patients 14, 5, and 2), whereas patient 9 (with Streptomyces and eosinophil/Treg outlier) had a spike at day 3 (Fig. 4A). Both patients 2 and 14 had spikes in M0 macrophages in addition to several other cell types. Patient 2, after admission, was found to have many comorbidities (dysmorphic feature, hemifacial abnormality, foramen ovale, and hearing loss) suggestive of a rare genetic syndrome.
Figure. 4.
Gene outlier mapping for samples. A: expression levels of the top five severity genes for PERSEVERE scores for controls (gray), VB day 0 (red) and VB day 3 (cyan) with outliers labeled. B: top cytokines expressed in samples shown as a heat map (red is highest value of each cytokine and blue the lowest). Clustering of samples was performed using one minus Pearson’s correlation. To the right are the top significant cytokines segregating controls versus VB day 0 samples that are higher (red) or lower (black) in VB day 0. C: additive cytokines from panel B for each sample that are down (x-axis) or up (y-axis) in VB day 0 samples. The highest expression of elevated factors are labeled. D: annotated of the normalized mapping value for cell-type-specific genes in patient 3 time point 0 relative to the max value of all other samples. The top three non patient 3 mapping values are labeled in red. E: outlier mapping for the top three in panel D, labeling the patient with highest mapping levels. F: genes significantly elevated (4 standard deviations above others) in each of the day 0 VB (red) or control (black) samples. Labeled above, each are GO enrichment terms identified from the sample elevated genes. VB, viral bronchiolitis.
A total of 12 cytokines are significantly elevated within patients with VB, whereas five cytokine genes are significantly higher in controls (Fig. 4B). An assessment of these significant cytokines’ additive expression provides further segregation of patients with VB, particularly day 0, relative to controls (Fig. 4C). Patient 16 had one of the highest VB cytokine profiles. Two patients showed a unique cytokine activation, patient 20 (11-wk-old male, V = 0) with CCL2, CCL8, and CXCL10 [all three tied into early responses in viruses like dengue (45)] and patient 3 (10-wk-old male, V = 6; SCGB3A1, CCL24, CCL1, CCL7, CCL13, BMP4, CCL3L1, SLURP1 enriched for neutrophil chemotaxis, FDR 8e-9). Patient 20 was later found to have a genetic disorder caused by PRRT2 associated with seizures, developmental delay, and later developed asthma. Some patients with variants in this gene have been associated with autoimmune-based pathologies (46). It should be noted that CXCL8 overlaps in the PERSEVERE and cytokine lists, where it is higher in control samples but with low mapped levels (the highest sample was patient 13 with 7 TPMs). CXCL8 activation in patients is associated with organ damage and fibrosis (47) and was not measured elevated in any patients’ blood in our cohort.
Using expression datasets of the HPA and single-cell RNAseq, genes unique to various cell types can be annotated for expression in the cohort for various cell types (Fig. 4D). Patient 3 day 0 shows an elevation of genes in nearly every cell type (Fig. 4D); however, three cell types (alveolar cell type 1/2 and distal tubular cells) are enriched in other patients (Fig. 4E). The elevation of alveolar cell type 2 genes in the blood of patient 16 day 0 should be noted.
The heterogeneity in terms of cytokine and cell-specific genes in these patients prompted us to look for transcriptome-wide differences in gene expression within each specific patient to shed additional light on mechanisms underlying individual responses to RSV. Each patient was assessed for genes activated 4 standard deviations higher than other patients, followed by Gene Ontology (GO) enrichment (Fig. 4F). Multiple patients had various immune-related terms. Patient 1 showed enrichment for lung hyperinflammation genes, the only patient with an identified lung fungal infection (Aspergillus). Patient 3 had the most unique genes with 5,237, although the patient does not have any distinguishing clinical measures. Patient 7, one of the sicker patients in our cohort, showed a spike of genes involved in aspartic-type endopeptidase activity and was later diagnosed with congenital adrenal hyperplasia. Patient 9 mentioned for Streptomyces, eosinophil/Treg outlier, and day 3 PERSEVERE genes has a spike in neutrophil degranulation and antimicrobial humoral response genes. Patient 16 mentioned multiple times (cytokines, neutrophils, longest hospital/ventilation times, unique immune repertoire, alveolar cell type 2 genes) has a spike in genes linked to type I interferon signaling and clinically appeared syndromic with failure to thrive. Patient 18 mapped with multiple genes linked to hemoglobin complex and has had a clinical course including failure to thrive, global developmental delay, and syndromic appearance. This work highlights that each patient with severe RSV has unique cofactors and responses that often overlap with clinical notes.
DISCUSSION
The respiratory tract is a portal of entry into the human body for pathogens. Suspended within the air and fluids within the lung are millions of microbes of terrestrial, animal, and human origin, including our microbiome that contains many potential pathogens. A few of these pathogens will cause relatively mild infections like the common cold, whereas others will become blood borne, leading to multiorgan failure. Almost every child growing up is exposed to RSV by the time they are 5 yr old. Yet only a few end up needing hospitalization and even fewer need ICU care. Studies have shown several risk factors related to severity in RSV infections (17, 19–21), yet the vast majority of hospitalized patients have few clinical risk factors (15, 48). This paper shows that a closer look at patients with VB without perceived risk factors may be warranted. These patients may have comorbidities related to unique active biological processes unrecognized by the current standard of care tests.
Humans have coevolved with RSV and other seasonal viruses to develop responses that are optimized to prevent severe systemic reactions that would result in lethality. Although we invest heavily in research that highlights shared responses through the development of cohort-based observational studies, the appreciation of individuals’ unique responses is much less explored. We continue to search for unique biomarkers that serve as an early warning for adverse outcomes, the discovery of which has been checkered at best, not so much in detecting biomarkers (49, 50), but rather in transitioning them into clinical care. The study not only confirms some of what other VB cohort-based studies have shown, such as IFI27 being one of the more expressed genes, but also showed sex-based differences in the way viruses cause dyshomeostasis. In comparing patients with VB with controls, we found differences in immune response genes, biological pathways, transcript changes, and immune cell dynamics. The overlap of so many cellular processes cooccurring highlights the human response’s complexity and why no single biomarker suffices (51).
Transcriptomics approaches allow us to simultaneously measure thousands of components of disease response and construct models of how a disease affects various systems in the body at the level of organs, tissues, and cells. The classification of all patients with genetic mutations into a single group using only case-control biomarker analysis does not have the power to define variants driving disease in each patient. Much like in rare diseases, it requires a detailed insight into each patient’s variants, identifying subtle changes that are rarely shared between two individuals.
Our current study is limited in sample size and the lower depth short-read sequencing. Although our cohort of 20 patients shows similar gene signatures as was described in the larger 190 patient multisite RNAseq analysis (11), our focus on precision transcriptomics could benefit from expanded collections. Also, our early transcriptomic analyses of this study utilized 50-bp reads. Ongoing work on blood samples from patients with COVID-19 has shown that utilizing longer reads and higher depth of sequencing is beneficial for variant calling using RNA, immune repertoire mapping, and multispecies KRAKEN analysis. An additional limitation of this precision transcriptomics is that it is challenging to sync patients’ time course in a critical care environment. Although we have used the admission date into the PICU as day 0, likely, some patients are further in their infection time course. It is nearly impossible to know when infections started within these patients, and the RSV titer in the lungs is also known to vary in severity outcomes (11). As the future cohort grows in size and if more time points are collected, it could become possible to build a progressive timeline of events in the blood to deconvolute the likely infection time course for each patient. When applying these precision medicine transcriptome approaches, the most important factor moving forward will be the decrease in time to transcriptome data. Currently, to reduce sequencing costs, samples are collected and analyzed together in a single batch in a retrospective approach. If samples could be sequenced within a shorter period, such as 24 h from collection, it will be possible to advance precision transcriptomics into a potential clinical assay.
Respiratory failure is often the result of overlapping pathologic responses based on unique dyshomeostasis. Our data analysis and transcriptomics were performed blinded to clinical notes and records, including medical treatments. We showed many overlapping biomarkers tied to clinical severity, such as mechanical ventilation and hospitalization duration. We also elucidated unique transcriptomic signals such as immune cell activations, secondary infections, cytokine activation, and syndromic connected transcripts that overlap with clinical notes. We note that many therapies patients received in clinical care can influence blood transcriptomics. In the future, we will modify our IRB protocols to enable the collection of this data and other clinical insights for larger cohorts to be used in precision medicine mapping of transcriptome changes.
Our outlier mapping specifically shows patient 16, whose mother had gestational diabetes, to have a high cytokine profile, a unique elevated clonotype of immune repertoire, activation of alveolar cell type 2, overactivation of neutrophils, and type I interferon signaling. The patient had the most extended duration of mechanical ventilation. This highlights an oddity in RSV response similar to the ∼8% of patients with COVID-19 with autoantibodies (52). The precision transcriptomics approach brings up the possibility that in some of the patients with syndrome-like expression, the respiratory failure results from the earliest interaction between the patient’s genetics and their environment. The infection serves as a tipping point leading to ICU admission. Patient 20 is an example of this type of manifestation. He was 11 wk old in the ICU and later was found to be with a PRRT2 genetic variant. These patients get diagnosed usually later in infancy due to seizures, developmental delay, and muscle weakness. This work suggests that in addition to cohort-based biomarker discoveries, we should also be attempting patient outlier mapping to address if individual biological responses, similar to genetic disorders, occur in adverse responses to viral or bacterial infections. This is particularly important as we continue to understand pandemic-based viruses like SARS-CoV-2 and endemic infant infections like RSV.
DATA AVAILABILITY
A total of 47 RNAseq samples are available, double de-identified, on NCBI BioProject PRJNA693881. The SRA codes are SRR13501042-SRR13501088. Gene/transcript TPM mapping data and statistics for significant genes are available at https://doi.org/10.6084/m9.figshare.14265182.v1.
GRANTS
This work was supported by the Helen DeVos Children’s Hospital, National Institutes of Health (K01ES025435 to J.W.P.), and the Michigan State University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S.C., D.J.S., J.W.P., S.R., M.L.L., M.A., A.S.B., and C.P.B. conceived and designed research; J.W.P., M.L.L. and M.A. performed experiments; R.G., S.S.C., J.W.P., M.L.L., and K.L.U. analyzed data; R.G., S.S.C., D.J.S., S.Y.L., J.W.P., S.R., M.L.L., K.L.U., C.P.B., N.L.H., E.J.K. and R.O. interpreted results of experiments; R.G., J.W.P., and M.L.L. prepared figures; R.G., J.W.P., S.R., and M.L.L. drafted manuscript; R.G., S.S.C., D.J.S., S.Y.L., J.W.P., S.R., M.L.L., M.A., A.S.B., K.L.U., C.P.B., N.L.H., E.J.K. and R.O. edited and revised manuscript; R.G., S.S.C., D.J.S., S.Y.L., J.W.P., S.R., M.L.L., M.A., A.S.B., K.L.U., C.P.B., N.L.H., E.J.K. and R.O. approved final version of manuscript.
REFERENCES
- 1.Meissner HC. Viral bronchiolitis in children. N Engl J Med 374: 62–72, 2016. doi: 10.1056/NEJMra1413456. [DOI] [PubMed] [Google Scholar]
- 2.Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD; RSV Global Epidemiology Network, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390: 946–958, 2017. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson LJ, Dormitzer PR, Nokes DJ, Rappuoli R, Roca A, Graham BS. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine 31 Suppl 2: B209–B215, 2013. doi: 10.1016/j.vaccine.2012.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simoes EA. Respiratory syncytial virus infection. Lancet 354: 847–852, 1999. doi: 10.1016/S0140-6736(99)80040-3. [DOI] [PubMed] [Google Scholar]
- 5.Eisenhut M. Extrapulmonary manifestations of severe respiratory syncytial virus infection–a systematic review. Crit Care 10: R107, 2006. doi: 10.1186/cc4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V , et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The lancet 380: 2095–2128, 2012. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, Poehling KA, Szilagyi PG, Griffin MR, Williams JV, Zhu Y, Grijalva CG, Prill MM, Iwane MK. Respiratory syncytial virus–associated hospitalizations among children less than 24 months of age. Pediatrics 132: e341–e348, 2013. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 8.Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng P-Y, Steiner C, Abedi GR, Anderson LJ, Brammer L, Shay DK. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis 54: 1427–1436, 2012. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaurin KK, Farr AM, Wade SW, Diakun DR, Stewart DL. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol 36: 990–996, 2016. doi: 10.1038/jp.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheeran P, Jafri H, Carubelli C, Saavedra J, Johnson C, Krisher K, Sánchez PJ, Ramilo O. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J 18: 115–122, 1999. doi: 10.1097/00006454-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Heinonen S, Velazquez VM, Ye F, Mertz S, Acero-Bedoya S, Smith B, Bunsow E, Garcia-Mauriño C, Oliva S, Cohen DM, Moore-Clingenpeel M, Peeples ME, Ramilo O, Mejias A. Immune profiles provide insights into respiratory syncytial virus disease severity in young children. Sci Transl Med 12: eaaw0268, 2020. doi: 10.1126/scitranslmed.aaw0268. [DOI] [PubMed] [Google Scholar]
- 12.Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr 143: S112–S117, 2003. doi: 10.1067/s0022-3476(03)00508-0. [DOI] [PubMed] [Google Scholar]
- 13.Thorburn K, Harigopal S, Reddy V, Taylor N, van Saene HKF. High incidence of pulmonary bacterial co-infection in children with severe respiratory syncytial virus (RSV) bronchiolitis. Thorax 61: 611–615, 2006. doi: 10.1136/thx.2005.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Steenhuijsen Piters WAA, Heinonen S, Hasrat R, Bunsow E, Smith B, Suarez-Arrabal M-C, Chaussabel D, Cohen DM, Sanders EAM, Ramilo O, Bogaert D, Mejias A. Nasopharyngeal microbiota, host transcriptome, and disease severity in children with respiratory syncytial virus infection. Am J Respir Crit Care Med 194: 1104–1115, 2016. doi: 10.1164/rccm.201602-0220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. The burden of respiratory syncytial virus infection in young children. N Engl J Med 360: 588–598, 2009. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, Kjellman B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 171: 137–141, 2005. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 17.Barral-Arca R, Gómez-Carballa A, Cebey-López M, Bello X, Martinón-Torres F, Salas A. A meta-analysis of multiple whole blood gene expression data unveils a diagnostic host-response transcript signature for respiratory syncytial virus. Int J Mol Sci 21: 1831, 2020. doi: 10.3390/ijms21051831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dapat C, Kumaki S, Sakurai H, Nishimura H, Labayo HKM, Okamoto M, Saito M, Oshitani H. Gene signature of children with severe respiratory syncytial virus infection. Pediatr Res, 2021. doi: 10.1038/s41390-020-01347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jong VL, Ahout IML, van den Ham H-J, Jans J, Zaaraoui-Boutahar F, Zomer A, Simonetti E, Bijl MA, Brand HK, van IJcken WFJ, de Jonge MI, Fraaij PL, de Groot R, Osterhaus ADME, Eijkemans MJ, Ferwerda G, Andeweg AC. Transcriptome assists prognosis of disease severity in respiratory syncytial virus infected infants. Sci Rep 6: 36603, 2016. doi: 10.1038/srep36603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dapat C, Oshitani H. Novel insights into human respiratory syncytial virus-host factor interactions through integrated proteomics and transcriptomics analysis. Expert Rev Anti Infect Ther 14: 285–297, 2016. doi: 10.1586/14787210.2016.1141676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ampuero S, Andaur R, Milano M, Moreno M, Lizama L, Larrañaga C, Urzúa U. Time-course of transcriptome response to respiratory syncytial virus infection in lung epithelium cells. Acta Virol 62: 310–325, 2018. doi: 10.4149/av_2018_225. [DOI] [PubMed] [Google Scholar]
- 22.Prokop JW, Shankar R, Gupta R, Leimanis ML, Nedveck D, Uhl K , et al. Virus-induced genetics revealed by multidimensional precision medicine transcriptional workflow applicable to COVID-19. Physiol Genomics 52: 255–268, 2020. doi: 10.1152/physiolgenomics.00045.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods 14: 417–419, 2017. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou G, Soufan O, Ewald J, Hancock REW, Basu N, Xia J. NetworkAnalyst 3.0: a visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acid Res 47: W234–W241, 2019. doi: 10.1093/nar/gkz240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43: e47, 2015. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 12: 453–457, 2015. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, Khodadoust MS, Esfahani MS, Luca BA, Steiner D, Diehn M, Alizadeh AA. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol 37: 773–782, 2019. doi: 10.1038/s41587-019-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 15: R46, 2014. doi: 10.1186/gb-2014-15-3-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolotin DA, Poslavsky S, Mitrophanov I, Shugay M, Mamedov IZ, Putintseva EV, Chudakov DM. MiXCR: software for comprehensive adaptive immunity profiling. Nat Methods 12: 380–381, 2015. doi: 10.1038/nmeth.3364. [DOI] [PubMed] [Google Scholar]
- 30.Wong HR, Salisbury S, Xiao Q, Cvijanovich NZ, Hall M, Allen GL, Thomas NJ, Freishtat RJ, Anas N, Meyer K, Checchia PA, Lin R, Shanley TP, Bigham MT, Sen A, Nowak J, Quasney M, Henricksen JW, Chopra A, Banschbach S, Beckman E, Harmon K, Lahni P, Lindsell CJ. The pediatric sepsis biomarker risk model. Crit Care 16: R174, 2012. doi: 10.1186/cc11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, Wernerus H, Björling L, Ponten F. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol 28: 1248–1250, 2010. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 32.Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C, Jensen LJ. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res 41: D808–D815, 2013. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang BM, Shojaei M, Parnell GP, Huang S, Nalos M, Teoh S, O’Connor K, Schibeci S, Phu AL, Kumar A, Ho J, Meyers AFA, Keynan Y, Ball T, Pisipati A, Kumar A, Moore E, Eisen D, Lai K, Gillett M, Geffers R, Luo H, Gul F, Schreiber J, Riedel S, Booth D, McLean A, Schughart KA. Novel immune biomarker IFI27 discriminates between influenza and bacteria in patients with suspected respiratory infection. Eur Respir J 49: 1602098, 2017. doi: 10.1183/13993003.02098-2016 [DOI] [PubMed] [Google Scholar]
- 34.Demaret J, Venet F, Plassais J, Cazalis M-A, Vallin H, Friggeri A, Lepape A, Rimmelé T, Textoris J, Monneret G. Identification of CD177 as the most dysregulated parameter in a microarray study of purified neutrophils from septic shock patients. Immunol Lett 178: 122–130, 2016. doi: 10.1016/j.imlet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Becker AM, Dao KH, Han BK, Kornu R, Lakhanpal S, Mobley AB, Li Q-Z, Lian Y, Wu T, Reimold AM, Olsen NJ, Karp DR, Chowdhury FZ, Farrar JD, Satterthwaite AB, Mohan C, Lipsky PE, Wakeland EK, Davis LS. SLE peripheral blood B cell, T cell and myeloid cell transcriptomes display unique profiles and each subset contributes to the interferon signature. PLoS One 8: e67003, 2013. doi: 10.1371/journal.pone.0067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carin L, Hero A, Lucas J, Dunson D, Chen M, Heñao R, Tibau-Puig A, Zaas A, Woods CW, Ginsburg GS. High-dimensional longitudinal genomic data: an analysis used for monitoring viral infections. IEEE Signal Process Mag 29: 108–123, 2012. doi: 10.1109/MSP.2011.943009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scoville SD, Freud AG, Caligiuri MA. Modeling human natural killer cell development in the era of innate lymphoid cells. Front Immunol 8: 360, 2017. doi: 10.3389/fimmu.2017.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang M, Windgassen D, Papoutsakis ET. Comparative analysis of transcriptional profiling of CD3+, CD4+ and CD8+ T cells identifies novel immune response players in T-cell activation. BMC Genomics 9: 225, 2008. doi: 10.1186/1471-2164-9-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Jackson D, Bacharier LB, Mauger D, Boushey H, Castro M, Durack J, Huang Y, Lemanske RF, Storch GA, Weinstock GM, Wylie K, Covar R, Fitzpatrick AM, Phipatanakul W, Robison RG, Beigelman A. The upper-airway microbiota and loss of asthma control among asthmatic children. Nat Commun 10: 5714, 2019. doi: 10.1038/s41467-019-13698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bais WJ. A case of pathogenicity of Bacillus subtilis. J Infect Dis 40: 313–315, 1927. doi: 10.1093/infdis/40.2.313. [DOI] [Google Scholar]
- 41.Flindt MLH. Pulmonary disease due to inhalation of derivatives of Bacillus subtilis containing proteolytic enzyme. The Lancet 293: 1177–1181, 1969. doi: 10.1016/S0140-6736(69)92165-5. [DOI] [PubMed] [Google Scholar]
- 42.Jean SS, Lee WS, Chen FL, Ou TY, Hsueh PR. Elizabethkingia meningoseptica: an important emerging pathogen causing healthcare-associated infections. J Hosp Infect 86: 244–249, 2014. doi: 10.1016/j.jhin.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Shima K, Coopmeiners J, Graspeuntner S, Dalhoff K, Rupp J. Impact of micro-environmental changes on respiratory tract infections with intracellular bacteria. FEBS Lett 590: 3887–3904, 2016. doi: 10.1002/1873-3468.12353. [DOI] [PubMed] [Google Scholar]
- 44.Wong HR, Cvijanovich NZ, Anas N, Allen GL, Thomas NJ, Bigham MT, Weiss SL, Fitzgerald JC, Checchia PA, Meyer K, Quasney M, Hall M, Gedeit R, Freishtat RJ, Nowak J, Raj SS, Gertz S, Grunwell JR, Lindsell CJ. Improved risk stratification in pediatric septic shock using both protein and mRNA biomarkers. PERSEVERE-XP. Am J Respir Crit Care Med 196: 494–501, 2017. doi: 10.1164/rccm.201701-0066OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tolfvenstam T, Lindblom A, Schreiber MJ, Ling L, Chow A, Ooi EE, Hibberd ML. Characterization of early host responses in adults with dengue disease. BMC Infect Dis 11: 209, 2011. doi: 10.1186/1471-2334-11-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blakeley J, Jankovic J. Secondary paroxysmal dyskinesias. Mov Disord 17: 726–734, 2002. doi: 10.1002/mds.10178. [DOI] [PubMed] [Google Scholar]
- 47.Russo RC, Garcia CC, Teixeira MM, Amaral FA. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol 10: 593–619, 2014. doi: 10.1586/1744666X.2014.894886. [DOI] [PubMed] [Google Scholar]
- 48.Stockman LJ, Curns AT, Anderson LJ, Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997-2006. Pediatr Infect Dis J 31: 5–9, 2012. doi: 10.1097/INF.0b013e31822e68e6. [DOI] [PubMed] [Google Scholar]
- 49.Brown PM, Schneeberger DL, Piedimonte G. Biomarkers of respiratory syncytial virus (RSV) infection: specific neutrophil and cytokine levels provide increased accuracy in predicting disease severity. Paediatr Respir Rev 16: 232–240, 2015. doi: 10.1016/j.prrv.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egron C, Roszyk L, Rochette E, Jabaudon M, Sapin V, Mulliez A, Labbé A, Coste K. Serum soluble receptor for advanced glycation end-products during acute bronchiolitis in infant: prospective study in 93 cases. Pediatr Pulmonol 53: 1429–1435, 2018. doi: 10.1002/ppul.24141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drucker E, Krapfenbauer K. Pitfalls and limitations in translation from biomarker discovery to clinical utility in predictive and personalised medicine. EPMA J 4: 7, 2013. doi: 10.1186/1878-5085-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann H-H, Zhang Y; HGID Lab, NIAID-USUHS Immune Response to COVID Group, COVID Clinicians, COVID-STORM Clinicians, Imagine COVID Group, French COVID Cohort Study Group, Milieu Intérieur Consortium, CoV-Contact Cohort, Amsterdam UMC Covid-19 Biobank, COVID Human Genetic Effort, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370: eabd4585, 2020. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A total of 47 RNAseq samples are available, double de-identified, on NCBI BioProject PRJNA693881. The SRA codes are SRR13501042-SRR13501088. Gene/transcript TPM mapping data and statistics for significant genes are available at https://doi.org/10.6084/m9.figshare.14265182.v1.