Abstract
Resolution of the acute respiratory distress syndrome (ARDS) from pneumonia requires repair of the injured lung endothelium and alveolar epithelium, removal of neutrophils from the distal airspaces of the lung, and clearance of the pathogen. Previous studies have demonstrated the importance of specialized proresolving mediators (SPMs) in the regulation of host responses during inflammation. Although ARDS is commonly caused by Streptococcus pneumoniae, the role of lipoxin A4 (LXA4) and resolvin D1 (RvD1) in pneumococcal pneumonia is not well understood. In the present experimental study, we tested the hypothesis that endogenous SPMs play a role in the resolution of lung injury in a clinically relevant model of bacterial pneumonia. Blockade of formyl peptide receptor 2 (ALX/FPR2), the receptor for LXA4 and RvD1, with the peptide WRW4 resulted in more pulmonary edema, greater protein accumulation in the air spaces, and increased bacteria accumulation in the air spaces and the blood. Inhibition of this receptor was also associated with decreased levels of proinflammatory cytokines. Even in the presence of antibiotic treatment, WRW4 inhibited the resolution of lung injury. In summary, these experiments demonstrated two novel findings: LXA4 and RvD1 contribute to the resolution of lung injury due to pneumococcal pneumonia, and the mechanism of their benefit likely includes augmenting bacterial clearance and reducing pulmonary edema via the restoration of lung alveolar-capillary barrier permeability.
Keywords: acute lung injury, ARDS, infection, pneumonia, resolution
INTRODUCTION
Acute respiratory distress syndrome (ARDS) affects ∼200,000 critically ill patients annually in the United States (1). Despite therapeutic advances including low tidal volume ventilation and conservative fluid management (2, 3), ARDS has a mortality rate of ∼40% and is responsible for nearly 4 million hospital days per year (1). Injury to the alveolar epithelium and lung endothelium impairs normal ion and fluid transport, resulting in accumulation of protein-rich inflammatory edema in the alveolar space (4). Patient outcomes in ARDS secondary to pneumonia are related in part to the severity and duration of the host inflammatory response (5). Resolution of the inflammatory insult and removal of the pathogen enables restoration of tissue homeostasis and is essential for patient recovery and for prevention of long-term complications (6). Pharmacologic therapies that attenuate inflammation by inhibiting proinflammatory mediators, such as glucocorticoids, have not improved outcomes in patients with classical ARDS (7), though there is evidence that dexamethasone may improve survival in COVID-19 ARDS (8). As a result, it is important to test endogenous mechanisms that may dampen inflammation and support its resolution.
The resolution of ARDS requires removal of protein-rich edema fluid from the air spaces and restoration of the epithelial and endothelial barrier (9). This is an active, programmed response that requires molecular signaling and interactions among inflammatory cells (9, 10). This process is driven in part by specialized proresolving mediators (SPMs) including resolvins, protectins, lipoxins, and other mediators produced via enzymatic conversion of essential fatty acids (11). These mediators play an important role in regulating host responses and serve to inhibit the production of proinflammatory cytokines and chemokines, regulate neutrophil trafficking, and stimulate macrophage phagocytosis of apoptotic cells, bacteria, and cellular debris (12).
ARDS is commonly caused by bacterial pneumonia, for which the most frequent responsible pathogen is Streptococcus pneumoniae (13). Clinical outcomes in patients who are hospitalized with pneumonia are worse with longer time to antibiotic therapy (14, 15), though nearly all patients with severe ARDS receive effective antibiotics within hours of presentation. Thus the mechanisms involved in the evolution of bacterial pneumonia-induced ARDS may be considered in two phases: 1) before antibiotics and 2) after antibiotics. Given their potent effects on innate immune cell trafficking and phagocytosis, as well as clearance of debris and promotion of resolution of inflammation, SPMs may play important roles during both phases of bacterial pneumonia. In these studies, we used our well-characterized model of pneumococcal pneumonia in mice both with and without antibiotics to test the hypothesis that LXA4 and RvD1 play important roles in the innate immune response during both early pneumonia and the subsequent resolution of lung injury.
MATERIALS AND METHODS
Animals
Adult 8–10-wk-old female C57BL/6 mice were ordered from the National Cancer Institute (Frederick, MD) and housed in pathogen-free conditions. Excessive mouse injury was observed when male mice were housed together. Female mice were used due to the importance of testing for biological effects that are robust to hormonal differences. Additionally, female mice were used due to the National Institutes of Health (NIH)’s policy on sex as a biological variable and their guidance that addressing traditional overreliance on male animals can improve our understanding of key influences on health processes and outcomes. The mice were cared for in accordance with NIH guidelines by the Laboratory Animal Resource Center of the University of California, San Francisco (UCSF), and all experiments were conducted under protocols approved by the UCSF Institutional Animal Care and Use Committee (IACUC). Group size was determined to ensure adequate statistical power based on our prior experience with mouse models of ARDS (16–19). As specified in our IACUC protocol, group sizes of 8 mice were used in order to be able to detect a clinically relevant difference of 15% with power of 0.8 and alpha of 0.05, given that the standard deviation for in vivo mouse studies is 15%. Mice underwent overdose of ketamine and xylazine, bilateral thoracotomy, and exsanguination by right ventricular puncture.
Bacterial Infection, WRW4 Administration, and Antibiotic Treatment
S. pneumoniae serotype 19 F (American Type Culture Collection 49619, Manassas, VA) was grown in brain-heart broth (Becton Dickinson 237500, Sparks, MD) and harvested at the mid-log phase [optical density (OD) 0.50 at 600 nm]. This is a clinically relevant strain with high virulence. To improve reliability across experiments, all cultures were derived from aliquots of a single bacterial expansion frozen at −80°C in 30% glycerol. Once an OD of 0.5 was reached, the bacterial culture was spun at 4,000 revolutions/min (2,700 g) and resuspended in phosphate-buffered saline (PBS).
All mice were anesthetized deeply with isoflurane. In all studies, mice were inoculated intranasally with 1 × 108 colony-forming units (CFU) of Streptococcus pneumoniae in 50 µL of solution. Formyl peptide receptor 2 (ALX/FPR2), the receptor for LXA4 and RvD1, was blocked with the peptide antagonist WRW4 (16, 20). In the studies without antibiotics, the mice were either administered WRW4 (1 mg/kg) or PBS (1 mg/kg) intraperitoneally (i.p.) 4 and 20 h after inoculation with S. pneumo (21). In the later experiments that included antibiotics, mice were inoculated at time 0 using the same protocol as in the studies with no antibiotics. However, starting at 24 h after infection, the mice in the antibiotic model were then given five doses of ceftriaxone (150 mg/kg i.p.) and either WRW4 or PBS i.p. every 12 h. In our previous work, we found that it is important to balance delaying antibiotics long enough that the infection becomes established and lung injury occurs while delivering antibiotics early enough that the animal does not develop severe shock and die (18). We chose these time intervals because they afforded the best combination of lung injury and survival.
Measurement of Protein Biomarkers of Inflammation and Lung Injury
Cytokines were measured using a Magpix (Luminex) with a 20-plex kit (Mouse Magnetic 20-Plex, Thermo Fisher Scientific).
Oxygenation and Temperature Measurements during the Experiments
Pulse oximetry was measured using the MouseOx+ cervical collar system (Starr Life Sciences), as we have done in prior studies (17). The mean arterial oxygen saturation () during 5 min of recording was calculated for each time point. Temperature was measured with a rectal thermometer.
Lung Injury End Points and Microbiology
Based on our prior protocol (18), samples were collected from each mouse after 24 h for our studies of the early, preantibiotics phase of pneumonia and after 72 h for our studies of the late, postantibiotics phase of pneumonia. These samples were then used for assessment of the degree of lung injury and biochemical analysis. The lungs were removed and homogenized in 1 mL PBS, and samples of blood, lung homogenate, and homogenate supernatant were weighed before and after desiccation. The blood volume of the lung was calculated, permitting measurement of the excess extravascular lung water (i.e., pulmonary edema in the interstitial and air spaces above the level in normal mice of the same size). In other mice, after exsanguination, the trachea was cannulated and the lungs were lavaged twice with 250 µL of PBS. Bronchoalveolar lavage (BAL) protein was measured with the bicinchoninic acid protein assay (Thermo Fisher Scientific). Postmortem bacterial titers of BAL and blood were measured by serial dilution and plaque counting on sheep blood agar plates. Microbiologic studies were not performed in the mice treated with antibiotics, as our prior work has indicated that ceftriaxone sterilizes the blood and BAL fluid effectively (18, 22). Separate mice were utilized for the following two sets of measurements because of technical reasons: one set of experiments was conducted for 1) excess lung water, 2) oxygen saturation, and 3) bacterial titers in the blood and another set of simultaneous experiments involved measurements of 1) air space protein, 2) airspace bacteria, 3) air space cytokine levels, and 4) rectal temperature.
Statistical Analyses and Blinding
Comparisons between two groups were performed with unpaired t test or Wilcoxon rank sum test (for data not normally distributed), with P < 0.05 considered to be statistically significant. Log transformation was used for airspace bacteria and blood bacteria given the lack of normally distributed data within groups. Values below the level of detection were treated as zero post-log transformation rather than −1. Data are presented as mean ± SD for each group unless stated otherwise. Statistical analysis and graph production were done with Prism (GraphPad, La Jolla, CA). The measurements were undertaken by investigators who were blinded to treatment allocation. The analyses were not done in a blinded fashion.
RESULTS
FPR2 Inhibition Worsens Lung Injury during Early Pneumonia
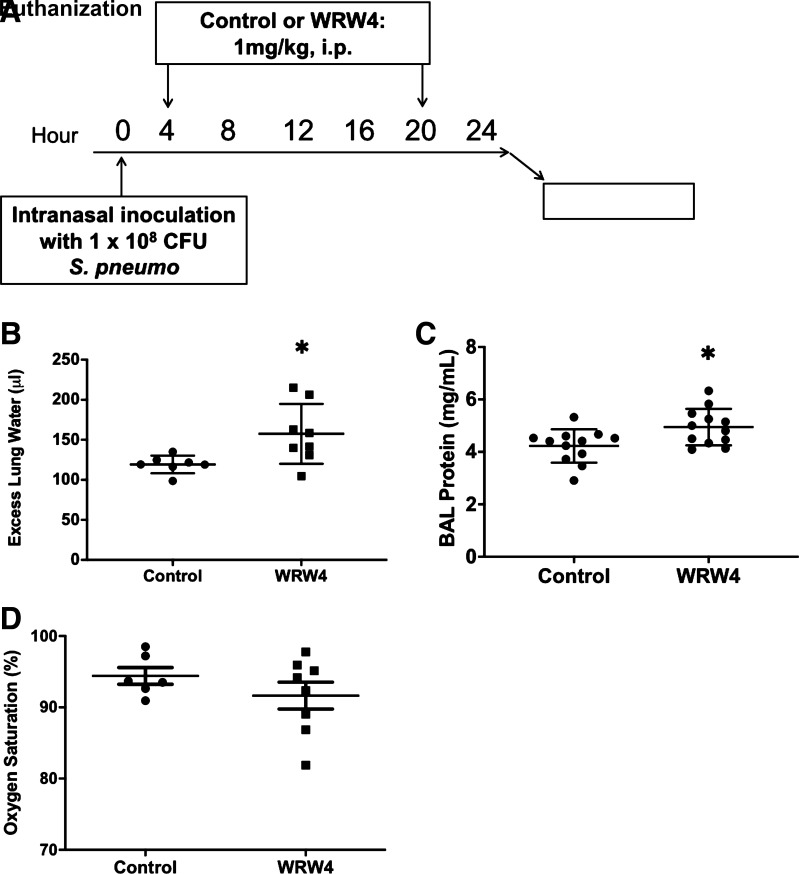
To test the potential role of SPMs in acute lung injury, we investigated the effects of FPR2 antagonism in our mouse model of pneumococcal pneumonia (18). The receptor for LXA4 and RvD1 was blocked with the short peptide WRW4, a potent antagonist (21). WRW4 was administered intraperitoneally (i.p.) at 4 and 20 h after intranasal inoculation with 1 × 108 CFU of Streptococcus pneumoniae. The mice were then euthanized after 24 h to study various physiologic variables in a model of early, preantibiotic pneumonia (Fig. 1A provides the timeline). The mice given WRW4 had a significant increase in excess lung water (Fig. 1B) and BAL protein (Fig. 1C). Mice treated with FPR2 blockade had a trend toward more hypoxemia that did not meet statistical significance (Fig. 1D).
Figure 1.
Pulmonary impact of formyl peptide receptor 2 (ALX/FPR2) inhibition. A: schematic depicting experimental procedures. Mice were inoculated with 1 × 108 CFU of Streptococcus pneumoniae intranasally. Four and 20 hours after induction of injury, mice were either given 1 mg/kg of WRW4 or control (PBS) intraperitoneally (i.p.). Mice were euthanized at 24 h postinfection for bronchoalveolar lavage (BAL). B: pulmonary edema, as measured by excess lung water (ELW) in the interstitial and alveolar spaces, was increased in the mice that received WRW4 (n = 8) compared with those that received control (n = 7). *P = 0.02. C: BAL protein was increased in the mice that received WRW4 (n = 12) compared with those that received control (n = 12). *P = 0.02. D: mean arterial oxygenation saturation in mice given either WRW4 (n = 8) or control (n = 6). P = 0.27. CFU, colony-forming units.
FPR2 Inhibition during Early Pneumonia Worsens Hypothermia and Increases the Burden of Airspace and Blood Bacteria in Association with Reduced Concentrations of Inflammatory Airspace Cytokines and Chemokines
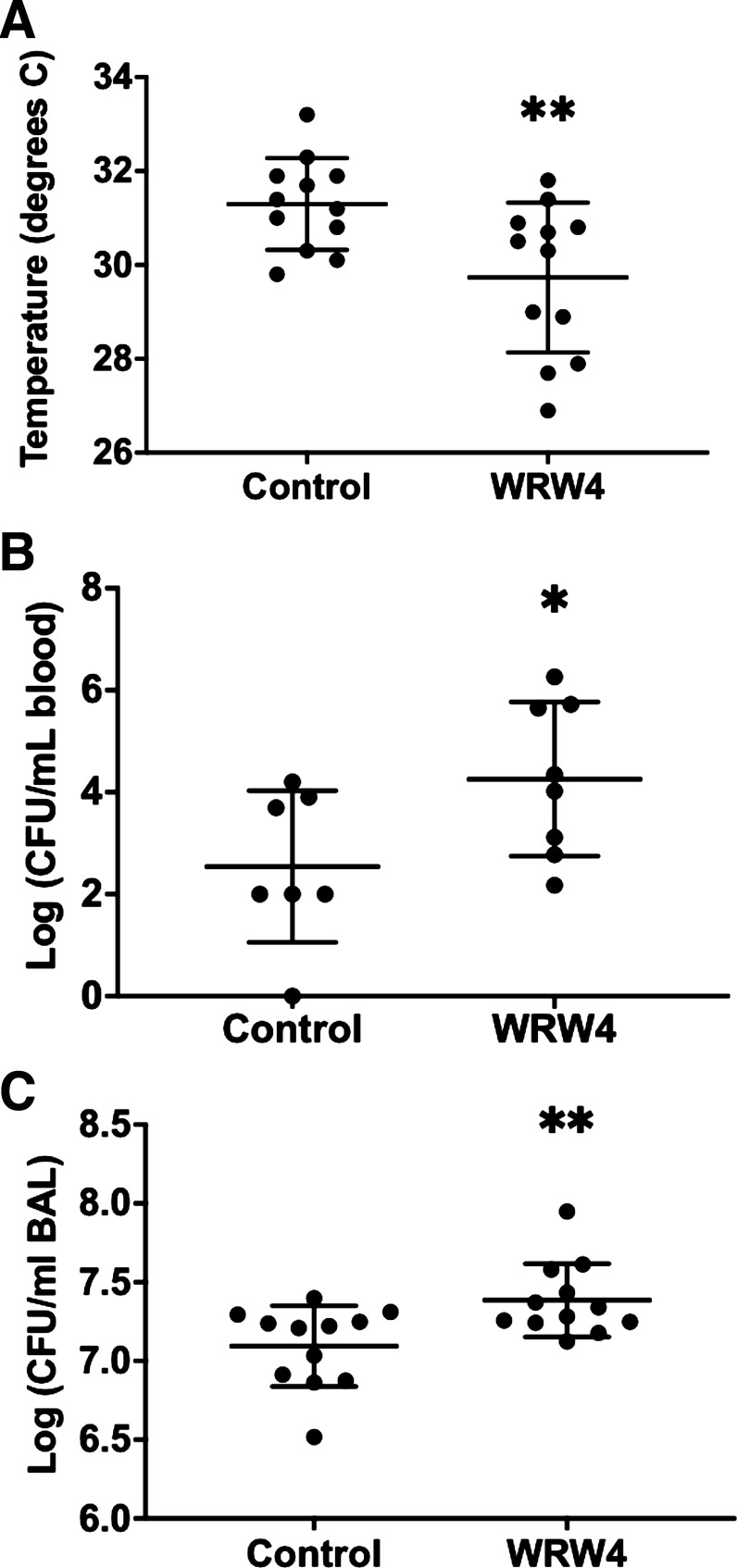
Prior work has shown that in mice, the severity of hypothermia predicts bacterial load and death (18, 22, 23). At 24 h (Fig. 1A), mice exposed to FPR2 antagonism after inoculation with pneumococcus experienced significantly more profound hypothermia than control mice (Fig. 2A). Bacteremia has been shown to be an independent risk factor for mortality during pneumonia caused by pneumococcus and other pathogens, and WRW4 administration was associated with higher levels of bacteremia (Fig. 2B) (24, 25). WRW4 was also associated with an increase in airspace bacteria (Fig. 2C). We next measured the concentration of selected chemokines and cytokines in BAL fluid. WRW4 exposure was associated with a significant decrease in the levels of several cytokines (IFN-γ, TNF-α, IL-1β, IL-2, CXCL9, and CXCL10; Table 1).
Figure 2.
Microbiologic effects of formyl peptide receptor 2 (ALX/FPR2) inhibition. A: core body temperature at 24 h following intranasal inoculation with Streptococcus pneumoniae in mice given either WRW4 (n = 13) or control (n = 12). Both developed hypothermia. The temperature was significantly lower in mice that received WRW4. **P = 0.008. B: bacterial counts [as measured by colony-forming units (CFU)] in the peripheral blood of mice treated with WRW4 (n = 8) or control (n = 7). Bacteremia was greater in mice that had been given WRW4. *P = 0.05. C: airspace pneumococcal density was increased in the mice that received WRW4 (n = 12) compared with those that received control (n = 12). **P = 0.008. BAL, bronchoalveolar lavage.
Table 1.
Airspace biomarkers of pneumococcal pneumonia-related lung injury in models 1) without and 2) with antibiotics
| Studies without Antibiotics | ||||
|---|---|---|---|---|
| Ligand | Control (n = 12) | WRW4 (n = 13) | *Ratio | Uncorrected P Value |
| IL-1β | 725 (581–947) | 566 (495–709) | 0.78 | 0.016 |
| TNF-α | 6,983 (4,907–8,480) | 5,517 (3,493–6,020) | 0.79 | 0.044 |
| CXCL9 | 2,749 (1,854–3,840) | 1,197 (875–1,929) | 0.44 | 0.011 |
| IFN-γ | 165 (123–214) | 95 (80–121) | 0.58 | 0.002 |
| IL-2 | 56 (53–62) | 54 (48–57) | 0.96 | 0.039 |
| CXCL10 | 11,182 (8,963–13,928) | 4,126 (896–5,166) | 0.37 | 0.0002 |
| MIP-1α | 61,246 (54,135–73,161) | 58,037 (24,421–73,015) | 0.95 | 0.201 |
| GM-CSF | 259 (139–309) | 201 (142–265) | 0.78 | 0.503 |
| IL-17 | 71 (49–135) | 70 (51–92) | 0.99 | 0.583 |
| IL-6 | 6,404 (4,032–7,839) | 4,922 (4,385–8,150) | 0.77 | 0.878 |
| IL-1α | 873 (766–1,194) | 673 (487–995) | 0.77 | 0.070 |
| MCP-1 | 1,089 (802–1,574) | 851 (349–1,335) | 0.78 | 0.096 |
| KC | 7,236 (6,269–8,079) | 7,378 (7,162–7,929) | 1.02 | 0.650 |
| Studies with Antibiotics | ||||
|---|---|---|---|---|
| Ligand | CTX (n = 8) | CTX + WRW4 (n = 10) | *Ratio | Uncorrected P Value |
| TNF-α | 149 (114–241) | 95 (78–167) | 0.64 | 0.046 |
| IL-1β | 80 (68–97) | 74 (56–110) | 0.93 | 0.760 |
| CXCL9 | 820 (444–1,011) | 967 (354–1,528) | 1.18 | 0.377 |
| IL-2 | 28 (27–31) | 29 (27–32) | 1.04 | 0.852 |
| CXCL10 | 554 (290–897) | 598 (330–1,026) | 1.08 | 0.566 |
| MIP-1α | 323 (200–498) | 314 (221–406) | 0.97 | 0.915 |
| IL-17 | 0.63 (0.35–0.93) | 0.44 (0.28–0.81) | 0.70 | 0.369 |
| IL-6 | 232 (130–326) | 240 (123–733) | 1.03 | 0.633 |
| IL-1α | 72 (49–94) | 54 (46–96) | 0.75 | 0.617 |
| MCP-1 | 976 (639–1551) | 1,011 (733–1,206) | 1.04 | 0.948 |
| KC | 213 (147–338) | 202 (123–309) | 0.95 | 0.662 |
| IFN-γ | Too low to measure | |||
| GM-CSF | Too low to measure | |||
Median (IQR) values at 24 h (above) or 72h (below) postinfection are expressed in pg/mL. *Ratio of the ligand in 1) WRW4 to control and 2) CTX + WRW4 to CTX only. CTX, ceftriaxone; CXCL, C-X-C ligand; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; IQR, interquartile range; KC, murine homolog of IL-8; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; TNF, tumor necrosis factor.
FPR2 Inhibition Worsens Lung Injury during Late, Antibiotic-Treated Pneumonia
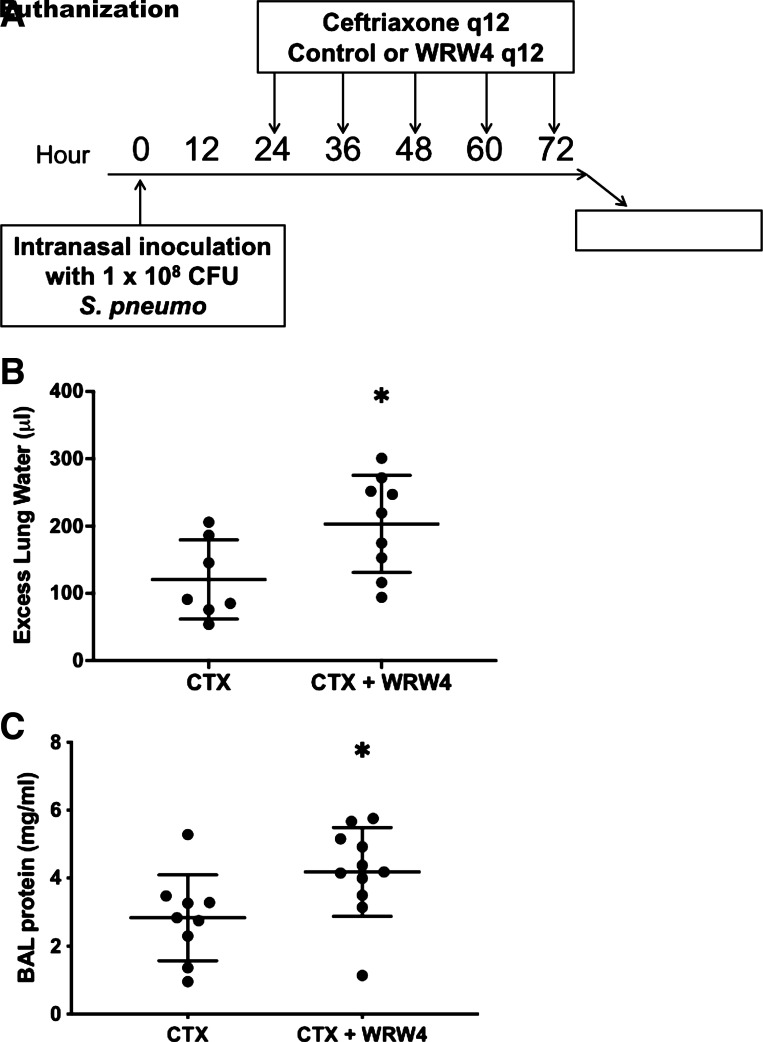
To study the late, postantibiotics phase of pneumonia and investigate potential mechanisms for LXA4 and RvD1 beyond the previously observed microbiologic effect, mice were treated with antibiotics after establishment of bacterial pneumonia. Mice were intranasally inoculated with 1 × 108 CFU of Streptococcus pneumoniae, and then starting at 24 h postinfection, all mice were treated with i.p. ceftriaxone at 150 mg/kg every 12 h for five doses, a regimen previously shown by our group to sterilize blood and airspaces (18). Mice were then administered either WRW4 or PBS control concurrently with each dose of ceftriaxone (Fig. 3A), followed by euthanization at 72 h. WRW4 administration resulted in significantly elevated excess lung water (Fig. 3B) and BAL protein (Fig. 3C). Neither temperature nor oxygen saturation were significantly different between the two groups at any of the 12-h intervals.
Figure 3.
Influence of formyl peptide receptor 2 (ALX/FPR2) inhibition on lung injury in an antibiotic-treated pneumococcal pneumonia model. A: schematic depicting experimental procedures. Mice were inoculated with 1 × 108 colony-forming units (CFU) of Streptococcus pneumoniae intranasally. The mice were then administered five doses of ceftriaxone (CTX) and five doses of either control (PBS) or WRW4 beginning 24 h after infection and then again every 12 h. The ceftriaxone, control, and WRW4 were all administered intraperitoneally. The mice were then euthanized for bronchoalveolar lavage (BAL) at 72 h postinfection. B: excess lung water was significantly higher in the mice that received both ceftriaxone and WRW4 (n = 9) as compared with the mice that only received ceftriaxone (n = 7). *P = 0.03. C: BAL protein was significantly higher in the mice that received both ceftriaxone and WRW4 (n = 11) as compared with the mice that only received ceftriaxone (n = 9). *P = 0.03.
Nearly all cytokine levels were lower in the model of late, antibiotic-treated pneumonia than in the model of early pneumonia (Table 1). Unlike in the model of preantibiotics pneumonia in which WRW4 decreased the levels of several cytokines, when mice were treated with ceftriaxone to model late pneumonia, WRW4 was associated with a significant reduction in TNF-α only (Table 1).
DISCUSSION
The major findings of this study can be summarized as follows. In an experimental model of pneumococcal pneumonia in mice, blockade of the receptor for LXA4 and RvD1 resulted in greater lung injury as evidenced by 1) more pulmonary edema, 2) increased air space protein, and 3) higher bacterial load in the blood and air spaces of the lung. Inhibition of this receptor was also associated with decreased levels of cytokines known to be important in host defense. This suggests that these SPMs have a role in the resolution of lung injury both in the early phase of pneumonia and in the late phase after antibiotics have been administered.
These findings fit well into the context of prior studies that have established a likely role for SPMs in the resolution of tissue injury following infection. In a mouse model of lipopolysaccharide (LPS)-induced acute lung injury, blocking the LXA4 receptor significantly reversed the protective effect of human bone marrow-derived mesenchymal stromal cells (MSCs) on both survival and the accumulation of pulmonary edema (16), suggesting that MSCs promote the resolution of lung injury in part through LXA4. In a rat model of oleic acid-induced lung injury, LXA4 stimulated alveolar fluid clearance which led to greater resolution of pulmonary edema, and this was reversed with an antagonist of ALX/FPR2. In mouse models of LPS-induced lung injury, RvD1 administration has been associated with reduced levels of leukocytes and protein in the BAL fluid, increased alveolar fluid clearance, and decreased mortality (26–28). Additionally, LXA4 administration has been associated with improved 48-h survival and less histological evidence of lung injury (16). In models of cecal ligation and puncture-initiated sepsis, RvD1 and LXA4 treatment have both been associated with increased 8-day survival and decreased blood bacterial load (29, 30). In murine bacterial pneumonia from Escherichia coli, the SPM 15-epi-LXA4 enhanced pathogen clearance and decreased lung inflammation (31). In a mouse model of E. coli peritonitis, resolvins stimulated phagocyte ingestion of the pathogen, reduced bacterial burden in the blood and peritoneal exudate, and reduced the degree of hypothermia (32). This consistency with the prior literature suggests that our findings may be relevant to a broad range of pathogens.
Streptococcus pneumoniae is one of the most common causes of ARDS and yet, little is understood about the role of SPMs in pneumococcal pneumonia. Although S. pneumoniae inoculation reduced overall plasma SPM levels after 48 and 168 h in a baboon model (33), that study did not address the functional impact of SPMs. Fpr2/3 knockout mice infected with S. pneumoniae had evidence of greater lung injury and worse mortality than wild-type mice (34). However, these mice were not treated with antibiotics to further our understanding of the mechanisms involved in the transformation of bacterial pneumonia-induced ARDS from the early, preantibiotics stage to the late, postantibiotics phase.
There are several reasons to investigate the role of FPR2 in a model of antibiotic-treated pneumococcal pneumonia. Given that we observed that WRW4 was associated with a higher bacterial load in a pneumococcal model without antibiotics, we tested whether there is a role for SPMs even after appropriate antibiotic therapy (18). Additionally, given that the Surviving Sepsis Campaign guidelines recommend administration of antibiotic therapy within an hour of recognizing severe sepsis or septic shock, antibiotics enable us to more accurately model human disease for patients under medical care (35). Antibiotics can also result in the release of products from the cell wall of Streptococcus pneumoniae, which can exacerbate inflammation and organ injury (36–38). Thus, treating a bacterial infection with antimicrobial therapy makes it possible to better understand the process of resolution.
Inhibition of the receptor for LXA4 and RvD1 resulted in an increase in bacterial counts in the blood and BAL fluid. This reduced clearance of bacteria was matched by more pulmonary edema and more protein exudate in the airspaces, consistent with pathogen-mediated lung injury. In the model of early pneumonia in which mice had not been treated with antibiotics, WRW4 exposure was associated with a significant decrease in the levels of several cytokines (IFN-γ, TNF-α, IL-1β, IL-2, CXCL9, and CXCL10). Cytokines are critical mediators of biologic pathways that can be harmful or beneficial depending on the clinical context. Although there are instances in which blocking a cytokine cascade can be beneficial, these data suggest that a potential mechanism for the microbiologic effect we observed may be inhibition of cytokine signaling, resulting in diminished host defense and innate immune response in the lung, including optimal phagocytosis of bacteria. In the field of lung injury research, both in animal models and with human patients, elevated pro-inflammatory cytokines are often thought to be injurious to the lung. However, proinflammatory cytokines play a critical role in host defense and are also a normal part of a healthy response to injury. The apparent paradox that blocking FPR2 results in lower cytokine levels is not incompatible with their critical role in host defense against bacterial pneumonia. SPMs are potent biologic mediators with complex activity and may be involved in multiple phases of inflammation.
In the model of late pneumonia that included antibiotics, there was approximately a 90% reduction in nearly all cytokine levels compared with the studies without antibiotics, and WRW4 was only associated with a small decrease in TNF-α. Administering antibiotics results in a different model, as evidenced by the much lower cytokine levels after only a few antibiotic doses. When antibiotics kill the pathogen, or during injury not caused by a live pathogen, the potentially beneficial effects of enhanced host defense may be mostly negated. This may partially explain why in murine Staphylococcus aureus sepsis, TNF-α inhibition alone increased bacterial load, whereas TNF-α inhibition in combination with antibiotics improved survival (39). This is also further evidence that there are two distinct phases of pneumococcal pneumonia (early, before antibiotic administration and late, postantibiotics) that both need to be modeled and studied carefully.
These studies utilized WRW4, a pharmacologic inhibitor of FPR2. This approach to inhibiting the activity of LXA4 and RvD1 has limitations, although WRW4 has been used extensively by other investigators and has proven effective (16, 20, 40, 41). WRW4 is a simple peptide with six amino acids that is reasonably specific for FPR2, although WRW4 also inhibits amyloid β42 peptide, which binds to the formyl peptide receptor-like 1 to generate superoxide and modulate cellular chemotactic migration in neutrophils (21). Although intra-alveolar accumulation of neutrophils can lead to pulmonary edema (9), the aforementioned off-target effects are not inconsistent with our findings because it is also possible to have recruitment of neutrophils into the airspaces without causing significant injury or change in the protein permeability of the epithelial barrier (42). WRW4 can also block the activation of ERK1/2 and p38 MAP kinase signaling (43). Although a knockout model would be another approach to SPM inhibition, knockout models themselves also have limitations, as they have the potential to cause alterations in endogenous signaling responses (44). Additionally, with this blockade we cannot discriminate between the relative impact of RvD1 and LXA4, the two SPMs for which ALX/FPR2 is the receptor. Finally, we did not measure arterial blood gases, but we did measure oxygen saturation.
In summary, these experiments indicate that the SPMs LXA4 and RvD1 contribute to the resolution of lung injury due to experimental pneumococcal pneumonia. The mechanism of SPM benefit includes a reduction in pulmonary edema, mediated by restoration of more normal lung alveolar-capillary barrier permeability. In addition, SPMs play a role in the augmentation of bacterial clearance, which may involve maintenance of host defense signaling from several cytokines, including IL-1β, IL-2, TNF-α, IFN-γ, CXCL9, and CXCL10. Given that Streptococcus pneumoniae is the most common cause of bacterial pneumonia and is frequently associated with ARDS, this research enhances understanding of the endogenous mechanisms of repair in ARDS and suggests that there may be therapeutic potential in systemic or lung-specific administration of LXA4 and RvD1 to improve host defense and reduce the severity of pneumonia.
GRANTS
This study was supported by NIH 5TL1TR001871-05 (E. R. Siegel), National Heart, Lung, and Blood Institute Grants HL134828 (M. A. Matthay and J. E. Gotts) and HL147127 (M. A. Matthay and J. E. Gotts), and National Institute of General Medical Sciences Training Grant T32-GM-008440 (R. H. Croze).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.H.C., M.A.M., and J.E.G. conceived and designed research; R.H.C., X.F., and J.E.G. performed experiments; E.R.S., R.H.C., and J.E.G. analyzed data; E.R.S., R.H.C., X.F., M.A.M., and J.E.G. interpreted results of experiments; E.R.S. and J.E.G. prepared figures; E.R.S. drafted manuscript; E.R.S., R.H.C., X.F., M.A.M., and J.E.G. edited and revised manuscript; E.R.S., R.H.C., X.F., M.A.M., and J.E.G. approved final version of manuscript.
REFERENCES
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Acute Respiratory Distress Syndrome Network; Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.National Heart Lung and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr., Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354: 2564–2575, 2006. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 4.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, Calfee CS. Acute respiratory distress syndrome. Nat Rev Dis Primers 5: 18, 2019. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Headley AS, Tolley E, Meduri GU. Infections and the inflammatory response in acute respiratory distress syndrome. Chest 111: 1306–1321, 1997. doi: 10.1378/chest.111.5.1306. [DOI] [PubMed] [Google Scholar]
- 6.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov 3: 401–416, 2004. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M; National Heart, Lung and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 354: 1671–1684, 2006. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 8.RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N Engl J Med 384: 693–704, 2021. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 122: 2731–2740, 2012. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol 2: 612–619, 2001. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 11.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510: 92–101, 2014. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serhan CN, Chiang N, Dalli J, Levy BD. Lipid mediators in the resolution of inflammation. Cold Spring Harb Perspect Biol 7: a016311, 2015. doi: 10.1101/cshperspect.a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musher DM, Roig IL, Cazares G, Stager CE, Logan N, Safar H. Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: results of a one-year study. J Infect 67: 11–18, 2013. doi: 10.1016/j.jinf.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel P, Rodrigo C, McKeever TM, Woodhead M, Welham S, Lim WS; British Thoracic Society. Time to first antibiotic and mortality in adults hospitalised with community-acquired pneumonia: a matched-propensity analysis. Thorax 71: 568–570, 2016. doi: 10.1136/thoraxjnl-2015-207513. [DOI] [PubMed] [Google Scholar]
- 15.Houck PM, Bratzler DW, Nsa W, Ma A, Bartlett JG. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med 164: 637–644, 2004. doi: 10.1001/archinte.164.6.637. [DOI] [PubMed] [Google Scholar]
- 16.Fang X, Abbott J, Cheng L, Colby JK, Lee JW, Levy BD, Matthay MA. Human mesenchymal stem (stromal) cells promote the resolution of acute lung injury in part through lipoxin A4. J Immunol 195: 875–881, 2015. doi: 10.4049/jimmunol.1500244. [DOI] [PubMed] [Google Scholar]
- 17.Gotts JE, Abbott J, Fang X, Yanagisawa H, Takasaka N, Nishimura SL, Calfee CS, Matthay MA. Cigarette smoke exposure worsens endotoxin-induced lung injury and pulmonary edema in mice. Nicotine Tob Res 19: 1033–1039, 2017. doi: 10.1093/ntr/ntx062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotts JE, Bernard O, Chun L, Croze RH, Ross JT, Nesseler N, Wu X, Abbott J, Fang X, Calfee CS, Matthay MA. Clinically relevant model of pneumococcal pneumonia, ARDS, and nonpulmonary organ dysfunction in mice. Am J Physiol Lung Cell Mol Physiol 317: L717–L736, 2019. doi: 10.1152/ajplung.00132.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JS, Su X, Rackley C, Matthay MA, Gupta N. Priming with endotoxin increases acute lung injury in mice by enhancing the severity of lung endothelial injury. Anat Rec (Hoboken) 294: 165–172, 2011. doi: 10.1002/ar.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sordi R, Menezes-de-Lima O, Jr., Horewicz V, Scheschowitsch K, Santos LF, Assreuy J. Dual role of lipoxin A4 in pneumosepsis pathogenesis. Int Immunopharmacol 17: 283–292, 2013. doi: 10.1016/j.intimp.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Bae YS, Lee HY, Jo EJ, Kim JI, Kang HK, Ye RD, Kwak JY, Ryu SH. Identification of peptides that antagonize formyl peptide receptor-like 1-mediated signaling. J Immunol 173: 607–614, 2004. doi: 10.4049/jimmunol.173.1.607. [DOI] [PubMed] [Google Scholar]
- 22.Gotts JE, Chun L, Abbott J, Fang X, Takasaka N, Nishimura SL, Springer ML, Schick SF, Calfee CS, Matthay MA. Cigarette smoke exposure worsens acute lung injury in antibiotic-treated bacterial pneumonia in mice. Am J Physiol Lung Cell Mol Physiol 315: L25–L40, 2018. doi: 10.1152/ajplung.00405.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bast DJ, Yue M, Chen X, Bell D, Dresser L, Saskin R, Mandell LA, Low DE, de Azavedo JC. Novel murine model of pneumococcal pneumonia: use of temperature as a measure of disease severity to compare the efficacies of moxifloxacin and levofloxacin. Antimicrob Agents Chemother 48: 3343–3348, 2004. doi: 10.1128/AAC.48.9.3343-3348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Vidal C, Fernandez-Sabe N, Carratala J, Diaz V, Verdaguer R, Dorca J, Manresa F, Gudiol F. Early mortality in patients with community-acquired pneumonia: causes and risk factors. Eur Respir J 32: 733–739, 2008. doi: 10.1183/09031936.00128107. [DOI] [PubMed] [Google Scholar]
- 25.Magret M, Lisboa T, Martin-Loeches I, Manez R, Nauwynck M, Wrigge H, Cardellino S, Diaz E, Koulenti D, Rello J; EU-VAP/CAP Study Group. Bacteremia is an independent risk factor for mortality in nosocomial pneumonia: a prospective and observational multicenter study. Crit Care 15: R62, 2011. doi: 10.1186/cc10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao Z, Dong J, Wu W, Yang T, Wang T, Guo L, Chen L, Xu D, Wen F. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARgamma/NF-kappaB pathway. Respir Res 13: 110, 2012. doi: 10.1186/1465-9921-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang B, Gong X, Wan JY, Zhang L, Zhang Z, Li HZ, Min S. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulm Pharmacol Ther 24: 434–441, 2011. doi: 10.1016/j.pupt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Zheng X, Cheng Y, Zhang YL, Wen HX, Tao Z, Li H, Hao Y, Gao Y, Yang LM, Smith FG, Huang CJ, Jin SW. Resolvin D1 stimulates alveolar fluid clearance through alveolar epithelial sodium channel, Na,K-ATPase via ALX/cAMP/PI3K pathway in lipopolysaccharide-induced acute lung injury. J Immunol 192: 3765–3777, 2014. doi: 10.4049/jimmunol.1302421. [DOI] [PubMed] [Google Scholar]
- 29.Chen F, Fan XH, Wu YP, Zhu JL, Wang F, Bo LL, Li JB, Bao R, Deng XM. Resolvin D1 improves survival in experimental sepsis through reducing bacterial load and preventing excessive activation of inflammatory response. Eur J Clin Microbiol Infect Dis 33: 457–464, 2014. doi: 10.1007/s10096-013-1978-6. [DOI] [PubMed] [Google Scholar]
- 30.Walker J, Dichter E, Lacorte G, Kerner D, Spur B, Rodriguez A, Yin K. Lipoxin a4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock 36: 410–416, 2011. doi: 10.1097/SHK.0b013e31822798c1. [DOI] [PubMed] [Google Scholar]
- 31.Sham HP, Walker KH, Abdulnour RE, Krishnamoorthy N, Douda DN, Norris PC, Barkas I, Benito-Figueroa S, Colby JK, Serhan CN, Levy BD. 15-epi-lipoxin A4, resolvin D2, and resolvin D3 induce NF-kappaB regulators in bacterial pneumonia. J Immunol 200: 2757–2766, 2018. doi: 10.4049/jimmunol.1602090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484: 524–528, 2012. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalli J, Kraft BD, Colas RA, Shinohara M, Fredenburgh LE, Hess DR, Chiang N, Welty-Wolf K, Choi AM, Piantadosi CA, Serhan CN. The regulation of proresolving lipid mediator profiles in baboon pneumonia by inhaled carbon monoxide. Am J Respir Cell Mol Biol 53: 314–325, 2015. doi: 10.1165/rcmb.2014-0299OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machado MG, Tavares LP, Souza GVS, Queiroz-Junior CM, Ascencao FR, Lopes ME, Garcia CC, Menezes GB, Perretti M, Russo RC, Teixeira MM, Sousa LP. The Annexin A1/FPR2 pathway controls the inflammatory response and bacterial dissemination in experimental pneumococcal pneumonia. FASEB J 34: 2749–2764, 2020. doi: 10.1096/fj.201902172R. [DOI] [PubMed] [Google Scholar]
- 35.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41: 580–637, 2013. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 36.Lutsar I, Friedland IR, Jafri HS, Wubbel L, Ahmed A, Trujillo M, McCoig CC, McCracken GH, Jr.. Factors influencing the anti-inflammatory effect of dexamethasone therapy in experimental pneumococcal meningitis. J Antimicrob Chemother 52: 651–655, 2003. doi: 10.1093/jac/dkg417. [DOI] [PubMed] [Google Scholar]
- 37.Tuomanen E, Hengstler B, Rich R, Bray MA, Zak O, Tomasz A. Nonsteroidal anti-inflammatory agents in the therapy for experimental pneumococcal meningitis. J Infect Dis 155: 985–990, 1987. doi: 10.1093/infdis/155.5.985. [DOI] [PubMed] [Google Scholar]
- 38.Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis 151: 859–868, 1985. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 39.Fei Y, Wang W, Kwieciński J, Josefsson E, Pullerits R, Jonsson I-M, Magnusson M, Jin T. The combination of a tumor necrosis factor inhibitor and antibiotic alleviates staphylococcal arthritis and sepsis in mice. J Infect Dis 204: 348–357, 2011. doi: 10.1093/infdis/jir266. [DOI] [PubMed] [Google Scholar]
- 40.Hughes EL, Becker F, Flower RJ, Buckingham JC, Gavins FNE. Mast cells mediate early neutrophil recruitment and exhibit anti-inflammatory properties via the formyl peptide receptor 2/lipoxin A4 receptor. Br J Pharmacol 174: 2393–2408, 2017. doi: 10.1111/bph.13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khaddaj-Mallat R, Sirois C, Sirois M, Rizcallah E, Marouan S, Morin C, Rousseau E. Pro-resolving effects of resolvin D2 in LTD4 and TNF-alpha pre-treated human bronchi. PLoS One 11: e0167058, 2016. doi: 10.1371/journal.pone.0167058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin TR, Pistorese BP, Chi EY, Goodman RB, Matthay MA. Effects of leukotriene B4 in the human lung. Recruitment of neutrophils into the alveolar spaces without a change in protein permeability. J Clin Invest 84: 1609–1619, 1989. doi: 10.1172/JCI114338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He HQ, Ye RD. The formyl peptide receptors: diversity of ligands and mechanism for recognition. Molecules 22: 455, 2017. doi: 10.3390/molecules22030455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lefrancais E, Mallavia B, Zhuo H, Calfee CS, Looney MR. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight 3: e98178, 2018. doi: 10.1172/jci.insight.98178. [DOI] [PMC free article] [PubMed] [Google Scholar]