Abstract
Pulmonary arterial hypertension (PAH) affects more women than men, although affected females tend to survive longer than affected males. This sex disparity in PAH is postulated to stem from the diverse roles of sex hormones in disease etiology. In animal models, estrogens appear to be implicated not only in pathologic remodeling of pulmonary arteries, but also in protection against right ventricular (RV) hypertrophy. In contrast, the male sex hormone testosterone is associated with reduced survival in male animals, where it is associated with increased RV mass, volume, and fibrosis. However, it also has a vasodilatory effect on pulmonary arteries. Furthermore, patients of both sexes show varying degrees of response to current therapies for PAH. As such, there are many gaps and contradictions regarding PAH development, progression, and therapeutic interventions in male versus female patients. Many of these questions remain unanswered, which may be due in part to lack of effective experimental models that can consistently reproduce PAH pulmonary microenvironments in their sex-specific forms. This review article summarizes the roles of estrogens and related sex hormones, immunological and genetical differences, and the benefits and limitations of existing experimental tools to fill in gaps in our understanding of the sex-based variation in PAH development and progression. Finally, we highlight the potential of a new tissue chip-based model mimicking PAH-afflicted male and female pulmonary arteries to study the sex-based differences in PAH and to develop personalized therapies based on patient sex and responsiveness to existing and new drugs.
Keywords: PAH, pulmonary arterial hypertension, sex disparity, sex hormone, tissue chips
INTRODUCTION
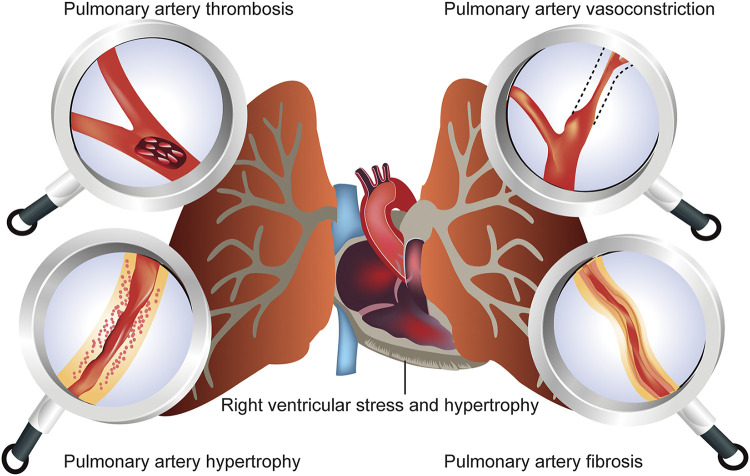
In patients with pulmonary arterial hypertension (PAH), a rare pulmonary vascular disease, pulmonary arterial pressure, becomes abnormally high, leading to increased pulmonary vascular resistance (PVR). A web of intricately intertwined biomolecular events is implicated in the elevated PVR, including vasoconstriction, narrowing of the pulmonary arterial lumen, aberrant and ectopic proliferation of primary cells of pulmonary arteries and arterioles, and development of in situ thromboses (Fig. 1) in the lungs. Chronic lung diseases and mitral valve defects may also contribute to the development of “pulmonary hypertension” (PH). Thus, it is necessary to distinguish between PAH and PH. The World Health Organization (WHO) categorizes patients with PH into five groups, wherein the term “PAH” applies strictly to group 1 patients, and “PH” refers to the other four groups. Furthermore, in 2018, based on the clinical presentations, PAH was further categorized into seven subgroups at the 6th World Symposium on Pulmonary Hypertension (1).
Figure 1.
Factors that increase pulmonary vascular resistance (PVR) and thus lead to the development of pulmonary arterial hypertension (PAH). Normal mean pulmonary arterial pressure (mPAP) at rest is between 12 and 16 mmHg. In PAH patients, mPAP rises to ≥20 mmHg at rest and PVR becomes >3 Wood units (normal range: ≤3). Neither elevated cardiac output alone nor high pulmonary venous pressure alone leads to pulmonary arterial hypertension (PAH), because the vascular bed undergoes relaxation as the blood flow increases. In most cases, elevated PVR is the major cause of PAH. Healthy blood vessels allow blood to flow without any resistance. Pathologies such as pulmonary arterial vasoconstriction, hypertrophy, fibrosis, or pulmonary thromboembolism can increase the PVR. Thus, a gradual increase in mPAP puts undue stress on the right ventricle, leading to the right heart failure.
Historically, PAH is known to affect more women than men. Depending on the disease subtype, epidemiological study, or PAH registry, women are 2–10 times more likely to have the disease than men, although the most commonly cited female to male ratio is 4:1 (2–5). However, defying the high incidence data, PAH-afflicted women, on average, appear to live 2 yr longer than PAH-afflicted men. This difference in the survival appears to hold true for patients older than 60 yr, but not for patients younger than 60 yr, suggesting that age is another confounding factor in PAH progression. However, despite the extended survival in females, women with PAH are hospitalized twice as often as men because women are the majority of patients with PAH (4, 6, 7). Furthermore, patients of both sexes appear to elicit a preferential response to one drug class over another (8). Thus, the sex disparity in PAH cannot be related to a single biological factor, but rather to multiple interconnected variables, including biological sex, age (pre- and postmenopause), sex hormones and their metabolites, genetic makeup, and drug therapy. Other factors that may play a role in the female bias in PAH include the use of contraceptive pills, hormone replacement therapy, and the higher propensity of females to suffer from autoimmune diseases (9–13).
The roles of sex hormones in PAH, especially the female sex hormones estrogens, remain puzzling. Furthermore, we know little regarding the roles of male sex hormones. Deeply implicated in the pathology of PAH is estrogen, because this hormone appears to have both harmful and healthful effects (14). Evidence supporting the hypothesis that estrogen is the cause of the female bias in PAH include: 1) estrogen and its cognate receptors are synthesized locally by the pulmonary arterial smooth muscle cells (PASMCs) of females; 2) estrogen causes localized proliferation of PASMC, and 3) inhibition of aromatase, an enzyme responsible for the synthesis of estrogen, improves pulmonary hemodynamics in animal models (15–20). In contrast, evidence supporting the hypothesis that estrogen is the reason for the improved survival observed in females are 1) estrogen offers protection against right ventricular hypertrophy (RVH) with improved right ventricular function (RV) in females, 2) estrogen pretreatment slows PAH progression in both male and female animals, 3) estrogen has antiproliferative effects on pulmonary arterial endothelial cells (PAECs), and 4) ovariectomized animals develop a more severe form of PAH (14, 18, 21–23). These contradictory findings for estrogen have been termed the “estrogen paradox,” which is the focal point of the debate surrounding the role of estrogen in PAH (14). Estrogen’s complex and compartmentalized roles need to be deciphered.
There is minimal information concerning why fewer men develop PAH and why those who do are more likely than women to die of the disease. Two observations suggest that the vasodilatory properties of testosterone and elevated levels of dehydroepiandrosterone (DHEA), a circulating steroid, may contribute to the reduced incidence of PAH in men (24). Testosterone is a much more powerful vasodilator of PASMC compared with estrogen (25–28). Furthermore, males have twice the levels of circulating DHEA and sulfated DHEA (DHEA-S) compared with females, which may have a vascular protective effect in males but not in females (29–31). In fact, DHEA inhibits PASMC proliferation and induces significant vasodilation in the pulmonary vasculature in both chronic hypoxia and the monocrotaline male rat models of PAH, but its specific role in PAH has not yet been elucidated (32–36). Testosterone increases the right ventricular mass and volumes via its profibrotic and prohypertrophic effects on cardiac myocytes (37–39). In the absence of cardioprotective effects (as from estrogen), increased afterload due to elevated pulmonary arterial pressure may explain the reduced survival in male patients with PAH.
In patients with PAH, elevated right ventricular afterload, resulting from narrowed and muscularized pulmonary arteries, causes RVH that progresses from adaptive to maladaptive hypertrophy. In adaptive cases, the size and function of the right ventricle (RV) change a little, but in maladaptive cases, the RV enlarges and undergoes major changes, including apoptosis of cardiac myocytes, reduction in the capillary density and oxidative capacity, and increased infiltration by various cytokines, peptides, and proteins (40). In animal models of PAH-induced RVH, the extent and the stages of hypertrophy (adaptive or maladaptive) vary depending on the animal species, and intervention (41–44). Although these models have provided valuable insights into the interactions between the pulmonary vasculature and the RV, the sex of the animals has not often been considered as a biological variable. Consequently, little is known regarding the communication that occurs among cardiomyocytes, PAH-afflicted cells (endothelial cells, smooth muscle cells, or adventitial cells), and extracellular matrix and the extent of apoptosis, fibrosis, and contractile dysfunction of the RV in patients with PAH.
Despite the known sex bias in PAH incidence and survival, PAH-afflicted patients receive the same treatments, which include endothelin receptors antagonists (ERA), phosphodiesterase-5-inhibitors (PD5-I), guanylate cyclase stimulators, and prostacyclin analogs (PCAs), regardless of their sex. However, an accumulating body of data suggests that, as with the sex differences in PAH etiology, patients with PAH respond to treatment in a sex-dependent fashion (8). Retrospective studies demonstrate that females have a better outcomes, assessed based on 6-min walk distance (6MWD) and hospitalization, when treated with ERA (45, 46) or PCA (47), but male patients seem to benefit more from PD5-I (48). True to the clinical data, animal models suggest that PAH-induced RVH shows a differential sex-based response to commonly used anti-PAH therapies (40, 49–51).
The aforementioned discussion suggests that the sex disparity in PAH is not related to a single variable but to various interconnected factors, including biological sex, age (pre- or postmenopausal), sex hormones and their metabolites, and genetics. In addition to innate biological variables, external factors such as therapy for PAH or concurrent drugs may also influence disease progression and survival in the two sexes. Here, we seek to enumerate and elucidate the influence of sex hormones in PAH pathogenesis, the role of immunity and genetics in the development of sex-specific PAH, and limitations of existing preclinical PAH models in deciphering the influence of sex and sex-specific therapies for patients with PAH.
PAH AFFECTS MORE WOMEN THAN MEN
Accurate data on the incidence and prevalence of PH, including subtypes of PAH, remain a contentious issue. The prevalence of PAH is unknown in the United States, whereas it is around 5–50 cases per million in Europe, confirming that PAH continues to be a rare disease (1, 3, 52). Comprehensive analysis of all the published registry data from 1981 to 2011 reveals that idiopathic PAH (IPAH) is the most prevalent form in developed countries (30%–50% of all PAH cases), whereas PAH associated with comorbidities (e.g., connective tissue disease) is more common in Asia (43% of all PAH cases). The NIH registry of 1981–1985 suggests that IPAH affects mostly young or middle-aged women. However, relatively new records such as US-REVEAL and the European-COMPERA registry confirm that relatively older women (avg. 52 ± 15 yr) with a preexisting illness such as diabetes and obesity are most at risk for IPAH. The exact reason for this age-dependent disparity is unknown, but it may stem from improved diagnosis and better therapeutic options, which were limited before the millennial period. Overall, data published over the past 30 years clearly demonstrate that among persons with PAH, women are disproportionately affected (62%–80%) (3, 53). The United States Pulmonary Hypertension Scientific Registry (USPHSR) is now conducting a study on 499 patients with PAH to identify genetic, environmental, and hormonal factors accounting for the female preponderance of IPAH (54).
ESTROGENS EXHIBIT DIVERSE PHYSIOLOGICAL EFFECTS THROUGH GENOMIC AND NONGENOMIC ACTIONS
Estrogens are steroidal sex hormones that have roles not only in the development of secondary sex characteristics, but also in memory, bone development, and cardioprotection (Fig. 2) (55). Estrogens have both beneficial and detrimental effects in males and females (Fig. 2). The female ovary produces three major estrogens: estrone (E1), estradiol (E2), and estriol (E3); estradiol is the primary circulating hormone in young females. Aromatase enzyme (CYP19A1) converts circulating androgen to estradiol (24). Estrone, which is less potent than estradiol, is the most prevalent estrogen in postmenopausal women (56). Estrogens are converted into hydroxy intermediates, including 4-hydroxyestradiol (4-OHE2) and 2-hydroxyestradiol (2-OHE2) by CYP1A1 and CYP1B1 phase I enzymes (57). A phase II enzyme, catechol-o-methyl transferase (COMT), further methylates these hydroxy metabolites. Adipose tissues also produce estrogens in both males and females (58).
Figure 2.
The sites of action of estrogens, clinical conditions, and the diseases associated with estrogen activity in females and males. In addition to estrogens’s dominant effect on the reproductive organs, estrogens also influence nonreproductive tissues such as the cardiovascular, skeletal, immune, gastrointestinal, and central nervous systems. BPH, benign prostatic hyperplasia; PCOS, polycystic ovary syndrome. [Modified from Hewitt and Korach (55) with permission from Walter Kluwers Health.]
Estrogens elicit their physiological action via nuclear estrogen receptors (ERα and ERβ) and via nongenomic activities (Fig. 3). Binding of estrogen or estrogen analogs to ER in the nucleus triggers a conformational change of the ligand-binding domain. ER agonists stimulate the ligand-binding domain to assume a conducive conformation, allowing interaction with coactivator molecules, whereas ER antagonists cause the same domain to adopt an unfavorable conformation, preventing the interaction with coactivators or recruiting corepressor molecules, ultimately changing the transcriptional rates of estrogen-responsive genes. ER can also be activated without estrogen. Both epidermal growth factor (EGF) and insulin-like growth factor-I (IGF-I) can activate ER target genes through membrane signaling. Estrogen also exerts nongenomic actions at various sites such as endothelial cells, cancer cells, and nervous tissue. This type of activity involves the binding of estrogen to the cell membrane and activation of mitogen-activated protein (MAP) kinase and extracellular signal-regulated kinase (ERK) pathways (55). Moreover, estrogen can also activate membrane-bound G protein-coupled receptor, GPR30.
Figure 3.
Production of estrogens and estrogen-mediated genomic and nongenomic actions. Aromatase converts dehydroepiandrosterone (DHEA) to estrogens, which diffuses across the cell membrane. The binding of estrogens to estrogen receptors (ER) in the cytoplasm triggers genomic actions. Estrogens also exhibit nongenomic effects by binding to cell membranes and thus rapidly activating mitogen-activated protein (MAP) kinase and extracellular signal-regulated kinase (ERK) pathways. These swift effects occur without any direct ER-gene interaction, but the resulting signals generated via these pathways indirectly regulate target genes. Various cytochrome P450 isoforms degrade estrogens and exert some nongenomic effects. DHEA is a prohormone because it must be converted to testosterone to elicit its genomic effects via cognate androgen receptors (AR). [Modified from Mair et al. (24) with permission from John Wiley and Sons.]
ESTROGEN HAS BOTH HELPFUL AND HARMFUL EFFECTS ON THE CARDIOVASCULAR SYSTEM
A series of studies suggest that estrogen can both help and hurt cardiovascular tissues (Fig. 4) (4). Mice deficient in ERα in their blood vessels exhibit carotid arterial injury, whereas ERβ-deficient mice show no morbidity. Thus, ERα mediates the protective action of estrogens. Lahm et al. (59) showed that estradiol (E2) administration improves right ventricular (RV) function both in Sugen/hypoxia (Su/Hx) and ovariectomized PAH female rats. The effect of estradiol (E2) on the RV function is less pronounced in Su/Hx male rats. Estrogen-based protective effects of RV are mediated via multiple pathways that include reduced RV fibrosis, elevation of the activity of endothelial nitric oxide synthase, promotion of apoptosis inhibitors such as Bcl-2, and reduction of RV collagen I/III ratio. ERα has been reported to offer protection against RV remodeling in female PAH rats (60). The expression of ERα in the RV of ovariectomized as well as Su/Hx PAH female rats was greater than that in the RV of male counterparts. E2 replacement therapy was more effective in alleviating RV malfunction in female rats than in male rats, as evidenced by reduced RV hypertrophy and systolic pressure. This finding suggests that the development of adaptive to maladaptive RV remodeling varies depending on the sex. Based on these observations, we believe that ERα may emerge as an important therapeutic target for the development drugs for PAH-induced RVH.
Figure 4.
Effects of sex hormones on pulmonary arterial smooth muscle cells (PASMCs) and pulmonary arterial endothelial cells (PAECs). Estrogen and one of its metabolites (16α-hydroxy estrogen) augment PASMC proliferation, which 2- and 4-methoxyestradiol and DHEA can reverse. Estrogens also adversely affect endothelium by releasing inflammatory mediators [C-reactive protein (CRP), IL-1 and IL-6]. Moreover, estrogen exerts beneficial effects on pulmonary endothelium by inducing nitric oxide (NO) mediated vasodilation and by reducing PAEC proliferation. DHEA and testosterone also cause vasodilation through multiple pathways including elevation of NO and reduction of cytosolic Ca2+ levels. Voltage-dependent calcium channel (VDCC); transient receptor potential channel (TRPC); calcium-sensing receptor (CaSR). [Figure redrawn from Martin and Pabelick (4) with permission from APS.]
Similarly, Cheng et al. (61) showed that ERα mutant female rats, but not male rats, exhibits maladaptive RV remodeling with elevated afterload. The authors hypothesized that functional ERα might offer a superior RV adaptation in female PAH animals compared with male PAH rats. A more detailed study by Philip et al. (62) showed that continuous E2 replacement therapy in female PAH rats and ovariectomized female rats reduce 1) the distal pulmonary arterial remodeling, 2) resistance to flow and distensibility, and 3) transpulmonary gradient. Overall, published studies suggest that E2 can potentially be used to treat PAH-induced RVH in females. Mice deficient in ER membrane signaling also failed to show any protective effects of estrogen on blood vessels (55). Genetic polymorphisms affecting the ERα gene were found to be associated with higher risks of myocardial infarction, stroke, and hypertension (63). In humans, genetic ERα deficiency in male patients causes endothelial malfunction and coronary arterial disease in individuals as young as 31 yr old, regardless of the average serum cholesterol concentrations (55). Oral estrogen treatment in young women or healthy older women (>60 yr) moderately improves lipid profiles. The same therapy also augments endothelial function in younger females but has no ameliorative effects in women older than 60 yr, who have coronary artery disease (64). Studies also showed that estrogen in females contributes to RV protection and better afterload (65).
On the other hand, estrogen also exerts proproliferative effects on the vasculature. The Heart and Estrogen/Progestin Replacement Study (HERS-I) trial confirmed that estrogen and progestin therapy in women does not elicit a statistically significant reduction in peripheral arterial disease. Oral estrogen elevates vascular inflammatory markers such as C-reactive protein (CRP) and serum triglycerides. The Women's Health Initiative (WHI) found that exogenous estrogen therapy increases venous thromboembolism by twofold (64). Therefore, clinicians now recommend menopausal hormone therapy (estrogen alone or in combination with progestin) only to young menopausal women (<60 yr) to alleviate vasomotor symptoms but not to reduce the risk of cardiovascular diseases or osteoporosis (66, 67).
LIMITATIONS OF CELL CULTURE MODELS IN STUDYING THE SEX DISPARITY IN PAH
Various two-dimensional (2-D), three-dimensional (3-D), and coculture cell systems have long been used as experimental tools to study the anatomical and physiological changes that occur in PAH-afflicted pulmonary arteries and arterioles, including hypertrophy, proliferation, inhibition of apoptosis, inflammatory changes, endothelial-mesenchymal transition, extracellular matrix accumulation, and metabolic alterations in different layers of the pulmonary arteries/arterioles: intimal (PAEC), medial (PASMC), and adventitial (ADC) (68, 69). The abnormal cellular activities expressed by these cells have also been used to study the effects of drugs and thus to screen and evaluate the efficacy of potential therapeutic agents. Of the cellular models, the 2-D cell culture systems are most widely used, which involves growing a monolayer of cells on a plastic dish. However, 2-D cellular models fail to mimic the true microenvironments of PAH, such as vascular hemodynamics, pulmonary artery remodeling, and cell-matrix cross talk. The 3-D cell culture system overcomes some of the limitations of 2-D systems, where cells are grown within extracellular matrix gels. The use of 3-D cell culture mimics some aspects of PAH microenvironments such as layer formation, arterial thickening, and cell-matrix communication. Adoption of a coculture model, such as growing immune cells along with PAH cells, helped delineating the interactions among various cellular and molecular components of the pulmonary arteries and arterioles. Although these models have been instrumental in illuminating the pathological basis of PAH, existing cellular models cannot fully recapitulate the tissue- or organ-level pathophysiology of human PAH. Cocultures or tissue-engineered 3-D models that use cells from rodents and humans cannot imitate the tissue-level function of PAH-afflicted pulmonary arteries and arterioles, nor can they reproduce the innate differences between the two sexes or replicate cell-cell interactions (68).
Moreover, not all previously published PAH cell culture studies state whether the cells were collected from male or female subjects. Therefore, it is imperative to develop cellular models to be able to investigate and confirm the sex-specific PAH findings. Over the past few years, tissue chips have generated much interest in the health sciences field (70). Tissue chips are microfluidic devices with micron-sized channels that allow cells to be grown in a more biologically relevant environment, including enabling cell-cell communication in a tissue-like fashion. Organ-on-a-chip or a human tissue-chip model may fill the gaps in our understanding of sex-based variation in PAH development, progression, and therapeutic response. The use of tissue-on-chip technologies may elucidate the effect of pulsatile release of estrogen, varying degrees of shear stress (vascular hemodynamics) on vascular tissue, and the interactions among the three layers of the vasculature (71). Using a PAH-on-chip model, the Ahsan laboratory studied the effect of E2 on pulmonary arterial remodeling. By designing and validating the male and female PAH chips mimicking PAH-afflicted pulmonary arterioles of male and female patients, the authors showed that male chips developed more severe form of pulmonary arterial remodeling compared with female chips. The number of smooth muscle cells in the intimal and luminal layers of the male chips was higher than those in female chips. However, E2 treatment worsened the arterial remodeling in female chips in a dose-dependent manner compared with male chips. The E2 treated female chips showed greater thickening of the intimal and medial layer and a higher expression of aromatase and CYP isozyme than the male chips (71).
ANIMAL MODELS FOR STUDYING THE SEX DISPARITY IN PAH YIELD CONFLICTING RESULTS
Various animal models of PAH are widely used for validating observations from cellular studies. Therefore, a combination of animal and cellular studies is a valid approach to study the large pathological spectrum of PAH. Unlike cellular models, several animal models have been used to explore the sex-specific differences in PAH, such as the monocrotaline model, the hypoxia model, and the Sugen plus hypoxia model (Su/Hx).
Chronic hypoxia induces potent, persistent vasoconstriction in rats, which mimics human pulmonary arterial remodeling such as muscularization, hypertrophy. The method is reproducible in rats, although it has its own limitations, such as the development of systemic hypertension and reversal of PAH in the presence of atmospheric oxygen, which does not occur in human IPAH. Furthermore, this model does not produce plexiform lesions, a hallmark of PAH. Mouse models do not accurately mimic human PAH. For example, the vascular remodeling that occurs in mouse models is less pronounced than that observed in human PAH (72).
The Su/Hx model is a robust animal model of PAH model that overcomes the limitations of the hypoxic model. In this model, rats are exposed to a VEGF receptor antagonist (Su5416/Sugen) in addition to chronic hypoxia (approximately 3 wk), after which they are reexposed to room air (73). This model emulates severe form of human PAH with pulmonary vascular remodeling, vasoocclusion, plexiform lesions, right ventricular failure, and death.
Monocrotaline (MCT) is a pneumotoxin which, upon subcutaneous administration, elicits PAH with arterial remodeling and ventricular hypertrophy in rats. Liver cytochrome P450 enzymes mediate MCT toxin formation. Therefore, the pathology of MCT-induced PAH in rats varies considerably depending on the species, possibly because of differential metabolism and pharmacokinetics of MCT in different species. Moreover, drugs such as the anorexigen, dexfenfluramine, can reverse the MCT-induced PAH, which is unlikely to happen in human PAH (72). Transgenic mouse models hold promise for studying PAH, but they also have some weaknesses. For example, mutation of the BMPR2 gene encoding bone morphogenetic protein receptor type II is implicated in heritable forms of PAH in humans. Homozygous knockout of BMPR2 in mice is a fatal condition, and heterozygous BMPR2+/− mice exert normal pulmonary arterial pressure at rest unless another triggering factor (e.g., IL-1β, serotonin) is added (72).
To study the sex disparity in PAH, investigators have used isolated pulmonary arteries from male and female mice and induced PAH by placing the arteries in hypoxia. Treatment of these arteries with estrogen elicited potent vasodilation in both male and female arteries (58). In intact swine and rats, hypoxia failed to generate PAH in females but did so in males (74). Circulating endogenous estrogen might diminish the hypoxia-induced vasoconstriction and PAH in intact female animals. This is supported by the observation that, in ovariectomized rats, hypoxia-induced a severe form of PAH, suggesting a protective role of estrogen in PAH. The MCT rat model showed similar results (58). RV dysfunction is one of the hallmarks of PAH. A series of studies revealed a complete reversal of preexisting PAH-induced RV remodeling upon estrogen administration (75). The RV dysfunction observed in female Su/Hx rats was significantly lower than in male rats, again indicating a protective role of estrogen in animals (76). However, data obtained from animal studies contradict the epidemiological data that PAH predominantly affects women.
Multiple overlapping pathways contribute to the protective effects of estrogen in females. These include a decrease in macrophage infiltration, enhanced apoptosis, increased levels of nitric oxide and prostacyclin, diminished phosphorylation of PI3K (phosphatidylinositol 3-kinase) and Akt (protein kinase B), and reduced levels of endothelin-1, a potent vasoconstrictor (77). Phase I and II enzymes such as CYP1A1, CYP1B1, and COMT convert estrogen to harmless metabolites and thus may confer some protection (78). For instance, methoxy- and hydroxyl-estradiol have proapoptotic and antiproliferative effects (22). Combination therapy with 2-methoxyestradiol or 1-ethoxyestradiol, bosentan, and sildenafil diminishes pulmonary arterial remodeling and lung inflammatory responses in MCT-induced PAH male rats (79).
Contradicting the data on the beneficial effects of estrogen in rat models of PAH, transgenic mouse models showed that estrogen exacerbates PAH development. In mice overexpressing serotonin transporter (SERT), under both normoxic and hypoxic conditions, estrogen promotes PAH development more so in females than in males (80). Furthermore, the whole lungs of female ovariectomized mice showed reduced expression of BMPR2 compared with their male counterparts (81). A study of lung tissue of patients with IPAH or secondary PAH reported higher levels of mRNA expression and signaling of the ERα compared with healthy controls (18). ERα may bind to the BMPR2 promoter and downregulate its expression (14). Thus, female dominance in PAH could be related to decreased BMPR2 expression, coupled with abnormal ERα signaling. We do not yet know the precise role ERβ plays in PAH. BMPR2 also modulates glucocorticoid receptor (GR) signaling, which suggests that ERα-mediated BMPR2 downregulation may promote altered GR signaling in PAH (82). In summary, all these preclinical and clinical data provide significant insights into the effects of sex hormones on PAH, but they do not illuminate the reasons for the higher prevalence of PAH in women and the higher risk of death from the disease in men.
THE ROLE OF OTHER SEX HORMONES IN PAH REMAINS UNCLEAR
Testosterone is the primary androgenic steroid synthesized in the testes, adrenal cortex, and ovaries. Testosterone and dihydrotestosterone are involved in the development of the male reproductive and cardiovascular systems (83). Testosterone binds to the androgen receptors (AR), which is profusely expressed in the pulmonary vasculature (83). Unlike estrogen, both testosterone and dihydrotestosterone promote RVH. (84).
Several studies suggest that rats treated with testosterone show a greater extent of RVH compared with rats that received no testosterone (85). Similar to estrogen, testosterone has vasodilatory effects in isolated rat pulmonary arteries. The degree of vasodilation is the same in both sexes (28). Testosterone, perhaps due to its vasoprotective effects, contributes to the reduced prevalence of PAH in men. The worse prognosis of PAH in males is probably due to dysregulation of RV function. Hence, by reversing RVH in male PAH patients, we could potentially extend survival in males.
DHEA, a weak but abundant androgen, is a precursor to both estrogen and testosterone. The adrenal gland generates DHEA and its sulfate ester, DHEA-S, both of which have a protective role against PAH. In the Su/Hx PAH model, DHEA ameliorated PAH endpoints and RV dysfunction in male rats (86). DHEA-S levels were twofold higher in human males as compared to females (31). Animal studies showed that DHEA enhances soluble guanylate cyclase levels and perhaps induces pulmonary vasodilation. Dumas de la Roque et al. (87)confirmed a complete reversal of hypoxia-induced pulmonary hypertension by the administration of DHEA.
In the clinical setting, DHEA is indicated only for women with primary adrenal insufficiency (cortisol and mineralocorticoid deficiency) as an add-on therapy to improve mood and psychological well being (88). However, DHEA therapy increases acne and hirsutism in women due to conversion to testosterone. A 12-wk clinical trial comprising men and women showed that DHEA therapy is ineffective in improving arterial rigidity and endothelial function or metabolic markers (CRP and lipids) in primary and secondary adrenal insufficiency (88). The Endocrine Society Guideline does not recommend using DHEA in postmenopausal women (low DHEA) because of its inadequate efficacy and lack of data on long-term safety (89).
CURRENT PROGRESS TOWARD OUR UNDERSTANDING SEX DISPARITY IN PAH
Mutation in human BMPR2 gene is the primary cause of heritable PAH (up to 80% of cases) and up to one-fourth of IPAH cases (90–92). Wild-type BMPR2 promotes vascular apoptosis, whereas mutated BMPR2 disrupts the balance between apoptosis and proliferation. Mutated BMPR2 elicits excessive proliferation of PAEC and thus leads to PAH. However, mutation alone cannot explain the progressive nature of PAH. Additional physiological factors are at play in disease progression or the risk of developing PAH. These factors include imbalances of vascular mediators (increased endothelin, reduced nitric oxide, and prostacyclin) and an abnormal estrogen response (1, 93). Mair et al. (19) showed that female mice overexpressing the SERT and Mts-1 genes (a calcium binding protein) developed PAH, and these mice had elevated levels of circulating endogenous estrogen (estradiol). Endogenous estrogen has a proliferation-inducing effect on PASMC, leading to remodeling of arteries, whereas exogenous estrogen has a vasodilatory effect. Higher expression of the aromatase enzyme (CYP19A1) in pulmonary arterial smooth cells promotes estrogen synthesis in both hypoxic mouse model and the Su/Hx rat models. Administration of an aromatase inhibitor (anastrozole) ameliorates PVR and RVH in female mice and rats but not in their male counterparts. Moreover, anastrozole therapy reduces circulating estrogen and restores BMPR2 expression in the female lung but does not affect the male lung. Overall, this study showed that a higher level of endogenous estrogen suppresses BMPR2 functions in females, and the reduction of estrogen synthesis or ER antagonism could be a potential therapeutic target for the treatment of female PAH. In support of these findings for endogenous estrogen, Chen et al. (94) showed that altered metabolism of endogenous estrogen leads to the development of PAH in BMPR2 mutant female mice. Contradicting this study, many preclinical and clinical studies suggest that endogenous estrogen has cardioprotective role (95).
Metabolism of estrogen to 16-estrogen (16αOHE1) is associated with severe PAH in BMPR2 mutant female mice, whereas estrogen, when converted to 2- or 4-estrogen, does not lead to the development of PAH in the same mutant mice. This work also showed that estrogen inhibition using a combination of anastrozole and fulvestrant, a pure estrogen receptor antagonist, reduces RV systolic pressure and uterine weight in BMPR2 mutant mice. Fulvestrant inhibits both ERα and ERβ, although the impact of ERβ inhibition was reported to be dominant. Currently, several phase II randomized clinical trials (PHANTOM, NCT02911844) are evaluating the potential role of estrogen inhibitors (anastrozole, fulvestrant, and tamoxifen) in female patients with PAH (96). Generally, tamoxifen is a competitive inhibitor of estrogen that binds to estrogen receptors (both α and β), but tamoxifen is reported to act as agonist or antagonist depending on the target tissue. For instance, tamoxifen triggers hot flashes due to its antagonist effect and endometrial hyperplasia because of its agonist effect. Because of its contradicting effect, tamoxifen is classified as a selective estrogen receptor modulator (SERM) instead of an estrogen antagonist. Currently, tamoxifen is used in ER positive breast cancer patients and thus its safety is already established. A clinical trial (NCT03528902), by the National Heart Lung and Blood Institute (NHLBI), is now studying the effect of tamoxifen (NCT03528902) for the treatment of PAH. Importantly, NHLBI is also investigating the efficacy of DHEA therapy on the right ventricle of patients with PAH (NCT03648385).
Nonetheless, these studies may help to untangle the estrogen paradox. More rigorous preclinical research and clinical trials may confirm the beneficial effects of estrogen inhibition in female PAH.
To address the unanswered questions concerning the prevalence and reduced survival of male PAH patients, Wu et al. (97) conducted a prospective study that enrolled 95 IPAH male patients and 95 healthy male controls. In that study, the authors measured the levels of various hormones (estradiol, testosterone, and progesterone) and correlated the levels against the risk of developing PAH and finally estimated the 5-yr mortality rate. The risk of developing PAH was higher with elevated estradiol, estradiol/testosterone ratio, reduced testosterone, and reduced progesterone. In addition, higher estradiol levels were alone associated with higher fatality in men with PAH. Ventetuolo et al. (98) found similar results in men, that is, an elevated level of estradiol is associated with a higher risk of developing PAH in males. However, the latter study did not find any correlation with lower testosterone and development of PAH in men. The dual but opposing effects of testosterone, adverse effect on the heart, and protective effects on the pulmonary vasculature may complicate efforts to correlate testosterone level with PAH outcome. To address the mystery of the estrogen paradox, Wu et al. postulated that too much or too little estradiol is a causative factor in the development of PAH. However, this speculation requires further investigation. Badlam et al. (99) conducted a case-control study in 634 patients with PAH (both men and women) and 132 healthy controls to investigate the impact of pharmacologic sex hormones (oral contraceptives, hormone replacement therapy) on PAH development. They found that neither oral contraceptives nor hormone replacement therapy appeared to raise the risk of developing PAH. A larger and multicenter study is needed to confirm this observation. Overall, these studies echo the data reported in multiple recent registries (3, 53).
IMMUNOPHYSIOLOGY AND GENETICS MAY HAVE A ROLE IN THE HIGHER PREVALENCE BUT REDUCED MORTALITY IN FEMALES
Estrogen exerts opposite effects on the innate and adaptive immune systems, conferring a balance between the two. It directly stimulates plasma cells for antibody production, enhances the survival of B cells, and reduces B-cell apoptosis. On the other hand, estrogen suppresses innate immunity through activation of T regulatory cells (Tregs) and impairs cytokine release by suppressing monocytes and neutrophils (100).
Generally, females have immune systems with higher sensitivity than males. The female X chromosomes host several genes that modulate the immune system (101, 102). The two alleles of these X chromosome genes are active in females (despite X chromosome inactivation), endowing women with a sensitive immunity than men because men have only one X chromosome. However, excessive activation of the immune system makes women more vulnerable to develop autoimmune diseases like lupus and systemic sclerosis (11). Melanie et al. (103) showed that overexpression of Toll-like receptor 7 (TLR7) on the X chromosome promotes the development of lupus more in females than in males. Thus, increased autoimmunity may be one of the risk factors in females for PAH development. Estrogen increases the production of inflammatory markers such as CRP and IL-6, which may augment PAH development in females.
Multiple reports suggest the extent of BMPR2 mutation is higher in females (42%) than in males (14%) (104). Umar et al. (105) showed that mice with an XY complement of chromosomes develop less severe form of PAH when compared with XX complement mice, suggesting that Y chromosome has a protective effect against the development of PAH. In a separate study, Yan et al. (106) showed that transcription factor SRY (sex-determining region Y) of the Y chromosome enhances BMPR2 expression in males. However, females are deficient in expression of SRY transcription factor and consequently females have reduced BMPR2 levels. These studies in part explain the biomolecular basis of the higher prevalence of PAH in females than in males. In summary, overactivation of immunity and the less protective action of X chromosome in BMPR2 mutation, may affect the development and progression of PAH in female patients.
CONCLUSIONS AND FUTURE DIRECTIONS
In summary, PAH is a highly progressive disease affecting more females than males, but having higher mortality among affected men. Therefore, it is clear that sex plays a significant role in PAH development and prognosis. Sex hormones and immune sensitivity have a broad impact on the pulmonary vasculature and the RV and may be correlated with PAH pathogenesis. Further studies using a robust preclinical model are needed to reveal all aspects of sex-specific differences in PAH and provide opportunities to develop better-targeted therapy.
Because of model-to-model variability and apparent differences between the pathophysiology of the disease in animals and humans, no animal models faithfully recapitulate human disease and capture the differences between men and women. In fact, the authors of influential editorials (95, 107) and reviews (14, 108) on the sex disparity in PAH recognize the limitations of existing animal models and reiterate the need for new models that better mimicking human PAH. New microengineering technology lend us an opportunity to design, fabricate and engineer models that can accommodate a single or multiple condition for a given experiment. Over the past decade, tissue chips have generated much interest in the health sciences field (70). Tissue chips are microfluidic devices with micron-sized channels that allow cells to be grown in a more biologically relevant environment, including enabling cell-cell communication in a tissue-like fashion (Fig. 5). To investigate the mechanism and sex-specific differences in PAH pathogenesis, the Ahsan laboratory has recently devised a tissue-chip model mimicking PAH-afflicted artery to 1) emulate the sex-based differences in PAH pathophysiology, 2) decipher the roles of sex and sex hormones in pulmonary arterial remodeling and RVH, and 3) develop sex-specific therapies for patients with PAH. We believe, if successful, the male and female PAH chips could transform the way experiments are conducted for studying the sex-based differences in PAH and for developing personalized therapies based on patient sex and responsiveness to a given drug.
Figure 5.
A: a bird’s eye view of a polydimethylsiloxane (PDMS)-based chip comprising five horizontal channels separated by pillars of different shapes. B: chips called male and female chips were prepared using three major cell types of the pulmonary arteries/arterioles: endothelial (PAEC), smooth muscle (PASMC), and adventitial cell (PADC). Seeding cells in different channels separated by pillars simulates the major features of pulmonary arteries. When cells collected from PAH-afflicted pulmonary arteries of male or female patients are seeded in their respective channels, the device can mimic the major pathological features of human PAH in a sex-dependent fashion. Growth media, drugs, and nonvascular cells can be instilled via the two outer channels (channels 1 and 5 in B). C: the pillars of various shapes (e.g., hexagons or trapezoids) placed every 200 µm keep the channels separated from each other, akin to elevated dividers in a multilane highway, but allow cell-cell communication to occur. Owing to the surface film created between two pillars by water in the cell medium, cells instilled into a given channel do not migrate to the neighboring channel in the absence of any cellular process or physical force.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute (NHLBI) Grants R01 HL114677 and R01 HL144590 and a Cardiovascular Medical Research and Education Fund grant (to F. Ahsan) and NHLBI Grants R01 HL114887 and P01 HL014985 (to K. R. Stenmark).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.H. and P.D. prepared figures; T.H. and P.D. drafted manuscript; W.L., T.L., E.N.-G., K.R.S., and F.A. edited and revised manuscript; F.A. approved final version of manuscript.
REFERENCES
- 1.Rubin LJ. The epidemiology and pathogenesis of pulmonary arterial hypertension (Group 1) www.uptodate.com. https://www.uptodate.com/contents/the-epidemiology-and-pathogenesis-of-pulmonary-arterial-hypertension-group-1?search=pulmonary%20arterial%20hypertension&source=search_result&selectedTitle=3∼150&usage_type=default&display_rank=3 [2020 Oct 1].
- 2.Chung L, Liu J, Parsons L, Hassoun PM, McGoon M, Badesch DB, Miller DP, Nicolls MR, Zamanian RT. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest 138: 1383–1394, 2010. doi: 10.1378/chest.10-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau EMT, Giannoulatou E, Celermajer DS, Humbert M. Epidemiology and treatment of pulmonary arterial hypertension. Nat Rev Cardiol 14: 603–614, 2017. doi: 10.1038/nrcardio.2017.84. [DOI] [PubMed] [Google Scholar]
- 4.Martin YN, Pabelick CM. Sex differences in the pulmonary circulation: implications for pulmonary hypertension. Am J Physiol Heart Circ Physiol 306: H1253–H1264, 2014. doi: 10.1152/ajpheart.00857.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White RJ. Estrogen: friend or foe in pulmonary hypertension? Am J Respir Crit Care Med 193: 1084–1086, 2016. doi: 10.1164/rccm.201512-2511ED. [DOI] [PubMed] [Google Scholar]
- 6.George MG, Schieb LJ, Ayala C, Talwalkar A, Levant S. Pulmonary hypertension surveillance: United States, 2001 to 2010. Chest 146: 476–495, 2014. doi: 10.1378/chest.14-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro S, Traiger GL, Turner M, McGoon MD, Wason P, Barst RJ. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest 141: 363–373, 2012. doi: 10.1378/chest.10-3114. [DOI] [PubMed] [Google Scholar]
- 8.Marra AM, Benjamin N, Eichstaedt C, Salzano A, Arcopinto M, Gargani L, D Alto M, Argiento P, Falsetti L, Di Giosia P, Isidori AM, Ferrara F, Bossone E, Cittadini A, Grünig E. Gender-related differences in pulmonary arterial hypertension targeted drugs administration. Pharmacol Res 114: 103–109, 2016. doi: 10.1016/j.phrs.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Masi AT. Editorial: pulmonary hypertension and oral contraceptive usage. Chest 69: 451–453, 1976. doi: 10.1378/chest.69.4.451b. [DOI] [PubMed] [Google Scholar]
- 10.Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. Genetic and hormonal factors in female-biased autoimmunity. Autoimmun Rev 9: 494–498, 2010. doi: 10.1016/j.autrev.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz-Irastorza G, Garmendia M, Villar I, Egurbide M-V, Aguirre C. Pulmonary hypertension in systemic lupus erythematosus: prevalence, predictors and diagnostic strategy. Autoimmun Rev 12: 410–415, 2013. doi: 10.1016/j.autrev.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney L, Voelkel NF. Estrogen exposure, obesity and thyroid disease in women with severe pulmonary hypertension. Eur J Med Res 14: 433–442, 2009. doi: 10.1186/2047-783x-14-10-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorne S, Nelson-Piercy C, MacGregor A, Gibbs S, Crowhurst J, Panay N, Rosenthal E, Walker F, Williams D, de Swiet M, Guillebaud J. Pregnancy and contraception in heart disease and pulmonary arterial hypertension. J Fam Plann Reprod Health Care 32: 75–81, 2006. doi: 10.1783/147118906776276486. [DOI] [PubMed] [Google Scholar]
- 14.Umar S, Rabinovitch M, Eghbali M. Estrogen paradox in pulmonary hypertension: current controversies and future perspectives. Am J Respir Crit Care Med 186: 125–131, 2012. doi: 10.1164/rccm.201201-0058PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamidi SA, Dickman KG, Berisha H, Said SI. 17β-estradiol protects the lung against acute injury: possible mediation by vasoactive intestinal polypeptide. Endocrinology 152: 4729–4737, 2011. doi: 10.1210/en.2011-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karas RH, Patterson BL, Mendelsohn ME. Human vascular smooth muscle cells contain functional estrogen receptor. Circulation 89: 1943–1950, 1994. doi: 10.1161/01.cir.89.5.1943. [DOI] [PubMed] [Google Scholar]
- 17.Kawut SM, Archer-Chicko CL, DeMichele A, Fritz JS, Klinger JR, Ky B, Palevsky HI, Palmisciano AJ, Patel M, Pinder D, Propert KJ, Smith KA, Stanczyk F, Tracy R, Vaidya A, Whittenhall ME, Ventetuolo CE. Anastrozole in pulmonary arterial hypertension. A randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med 195: 360–368, 2017. doi: 10.1164/rccm.201605-1024OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahm T, Albrecht M, Fisher AJ, Selej M, Patel NG, Brown JA, Justice MJ, Brown MB, Van Demark M, Trulock KM, Dieudonne D, Reddy JG, Presson RG, Petrache I. 17β-Estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am J Respir Crit Care Med 185: 965–980, 2012. doi: 10.1164/rccm.201107-1293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mair KM, Wright AF, Duggan N, Rowlands DJ, Hussey MJ, Roberts S, Fullerton J, Nilsen M, Loughlin L, Thomas M, MacLean MR. Sex-dependent influence of endogenous estrogen in pulmonary hypertension. Am J Respir Crit Care Med 190: 456–467, 2014. doi: 10.1164/rccm.201403-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White K, Johansen AK, Nilsen M, Ciuclan L, Wallace E, Paton L, Campbell A, Morecroft I, Loughlin L, McClure JD, Thomas M, Mair KM, MacLean MR. Activity of the estrogen-metabolizing enzyme cytochrome P450 1B1 influences the development of pulmonary arterial hypertension. Circulation 126: 1087–1098, 2012. doi: 10.1161/CIRCULATIONAHA.111.062927. [DOI] [PubMed] [Google Scholar]
- 21.Farhat MY, Chen MF, Bhatti T, Iqbal A, Cathapermal S, Ramwell PW. Protection by oestradiol against the development of cardiovascular changes associated with monocrotaline pulmonary hypertension in rats. Br J Pharmacol 110: 719–723, 1993. doi: 10.1111/j.1476-5381.1993.tb13871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tofovic SP, Zhang X, Jackson EK, Dacic S, Petrusevska G. 2-Methoxyestradiol mediates the protective effects of estradiol in monocrotaline-induced pulmonary hypertension. Vascul Pharmacol 45: 358–367, 2006. doi: 10.1016/j.vph.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Umar S, Iorga A, Matori H, Nadadur RD, Li J, Maltese F, van der Laarse A, Eghbali M. Estrogen rescues preexisting severe pulmonary hypertension in rats. Am J Respir Crit Care Med 184: 715–723, 2011. doi: 10.1164/rccm.201101-0078OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mair KM, Johansen AKZ, Wright AF, Wallace E, MacLean MR. Pulmonary arterial hypertension: basis of sex differences in incidence and treatment response. Br J Pharmacol 171: 567–579, 2014. doi: 10.1111/bph.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.English KM, Jones RD, Jones TH, Morice AH, Channer KS. Gender differences in the vasomotor effects of different steroid hormones in rat pulmonary and coronary arteries. Horm Metab Res 33: 645–652, 2001. doi: 10.1055/s-2001-18689. [DOI] [PubMed] [Google Scholar]
- 26.Jones RD, English KM, Pugh PJ, Morice AH, Jones TH, Channer KS. Pulmonary vasodilatory action of testosterone: evidence of a calcium antagonistic action. J Cardiovasc Pharmacol 39: 814–823, 2002. doi: 10.1097/00005344-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Rowell KO, Hall J, Pugh PJ, Jones TH, Channer KS, Jones RD. Testosterone acts as an efficacious vasodilator in isolated human pulmonary arteries and veins: evidence for a biphasic effect at physiological and supra-physiological concentrations. J Endocrinol Invest 32: 718–723, 2009. doi: 10.1007/bf03346526. [DOI] [PubMed] [Google Scholar]
- 28.Smith AM, Bennett RT, Jones TH, Cowen ME, Channer KS, Jones RD. Characterization of the vasodilatory action of testosterone in the human pulmonary circulation. Vasc Health Risk Manag 4: 1459–1466, 2008. doi: 10.2147/VHRM.S3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett-Connor E, Khaw KT, Yen SS. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med 315: 1519–1524, 1986. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- 30.Baulieu E-E. Androgens and aging men. Mol Cell Endocrinol 198: 41–49, 2002. doi: 10.1016/S0303-7207(02)00367-2. [DOI] [PubMed] [Google Scholar]
- 31.Parker CR, Jr. Dehydroepiandrosterone and dehydroepiandrosterone sulfate production in the human adrenal during development and aging. Steroids 64: 640–647, 1999. doi: 10.1016/S0039-128X(99)00046-X. [DOI] [PubMed] [Google Scholar]
- 32.de la Roque ED, Savineau J-P, Bonnet S. Dehydroepiandrosterone: a new treatment for vascular remodeling diseases including pulmonary arterial hypertension. Pharmacol Ther 126: 186–199, 2010. doi: 10.1016/j.pharmthera.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Farrukh IS, Peng W, Orlinska U, Hoidal JR. Effect of dehydroepiandrosterone on hypoxic pulmonary vasoconstriction: a Ca2+-activated K+-channel opener. Am J Physiol Lung Cell Mol Physiol 274: L186–L195, 1998. doi: 10.1152/ajplung.1998.274.2.l186. [DOI] [PubMed] [Google Scholar]
- 34.Hampl V, Bibova J, Povysilova V, Herget J. Dehydroepiandrosterone sulphate reduces chronic hypoxic pulmonary hypertension in rats. Eur Respir J 21: 862–865, 2003. doi: 10.1183/09031936.03.00084503. [DOI] [PubMed] [Google Scholar]
- 35.Oka M, Karoor V, Homma N, Nagaoka T, Sakao E, Golembeski SM, Limbird J, Imamura M, Gebb SA, Fagan KA, McMurtry IF. Dehydroepiandrosterone upregulates soluble guanylate cyclase and inhibits hypoxic pulmonary hypertension. Cardiovasc Res 74: 377–387, 2007. doi: 10.1016/j.cardiores.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paulin R, Meloche J, Jacob MH, Bisserier M, Courboulin A, Bonnet S. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 301: H1798–H1809, 2011. doi: 10.1152/ajpheart.00654.2011. [DOI] [PubMed] [Google Scholar]
- 37.Achar S, Rostamian A, Narayan SM. Cardiac and metabolic effects of anabolic-androgenic steroid abuse on lipids, blood pressure, left ventricular dimensions, and rhythm. Am J Cardiol 106: 893–901, 2010. doi: 10.1016/j.amjcard.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayward CS, Webb CM, Collins P. Effect of sex hormones on cardiac mass. Lancet 357: 1354–1356, 2001. doi: 10.1016/S0140-6736(00)04523-2. [DOI] [PubMed] [Google Scholar]
- 39.Hemnes AR, Maynard KB, Champion HC, Gleaves L, Penner N, West J, Newman JH. Testosterone negatively regulates right ventricular load stress responses in mice. Pulm Circ 2: 352–358, 2012. doi: 10.4103/2045-8932.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guihaire J, Bogaard HJ, Flecher E, Noly P-E, Mercier O, Haddad F, Fadel E. Experimental models of right heart failure: a window for translational research in pulmonary hypertension. Semin Respir Crit Care Med 34: 689–699, 2013. doi: 10.1055/s-0033-1355444. [DOI] [PubMed] [Google Scholar]
- 41.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121: 2747–2754, 2010. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 42.Gupta V, Gupta N, Shaik IH, Mehvar R, Nozik-Grayck E, McMurtry IF, Oka M, Komatsu M, Ahsan F. Inhaled PLGA particles of prostaglandin E1 ameliorate symptoms and progression of pulmonary hypertension at a reduced dosing frequency. Mol Pharm 10: 1655–1667, 2013. doi: 10.1021/mp300426u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kay JM, Suyama KL, Keane PM. Failure to show decrease in small pulmonary blood vessels in rats with experimental pulmonary hypertension. Thorax 37: 927–930, 1982. doi: 10.1136/thx.37.12.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rouleau JL, Kapuku G, Pelletier S, Gosselin H, Adam A, Gagnon C, Lambert C, Meloche S. Cardioprotective effects of ramipril and losartan in right ventricular pressure overload in the rabbit: importance of kinins and influence on angiotensin II type 1 receptor signaling pathway. Circulation 104: 939–944, 2001. doi: 10.1161/hc3401.093149. [DOI] [PubMed] [Google Scholar]
- 45.Gabler NB, French B, Strom BL, Liu Z, Palevsky HI, Taichman DB, Kawut SM, Halpern SD. Race and sex differences in response to endothelin receptor antagonists for pulmonary arterial hypertension. Chest 141: 20–26, 2012. doi: 10.1378/chest.11-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stauffer BL, Westby CM, Greiner JJ, Van Guilder GP, Desouza CA. Sex differences in endothelin-1-mediated vasoconstrictor tone in middle-aged and older adults. Am J Physiol Regul Integr Comp Physiol 298: R261–R265, 2010. doi: 10.1152/ajpregu.00626.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frantz RP, Schilz RJ, Chakinala MM, Badesch DB, Frost AE, McLaughlin VV, Barst RJ, Rosenberg DM, Miller DP, Hartline BK, Benton WW, Farber HW. Hospitalization and survival in patients using epoprostenol for injection in the PROSPECT observational study. Chest 147: 484–494, 2015. doi: 10.1378/chest.14-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathai SC, Hassoun PM, Puhan MA, Zhou Y, Wise RA. Sex differences in response to tadalafil in pulmonary arterial hypertension. Chest 147: 188–197, 2015. doi: 10.1378/chest.14-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borgdorff MAJ, Bartelds B, Dickinson MG, Boersma B, Weij M, Zandvoort A, Sillje HHW, Steendijk P, de Vroomen M, Berger RMF. Sildenafil enhances systolic adaptation, but does not prevent diastolic dysfunction, in the pressure-loaded right ventricle. Eur J Heart Fail 14: 1067–1074, 2012. doi: 10.1093/eurjhf/hfs094. [DOI] [PubMed] [Google Scholar]
- 50.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB, Light PE, Dyck JRB, Michelakis ED. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation 116: 238–248, 2007. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 51.van Albada ME, Berger RMF, Niggebrugge M, van Veghel R, Cromme-Dijkhuis AH, Schoemaker RG. Prostacyclin therapy increases right ventricular capillarisation in a model for flow-associated pulmonary hypertension. Eur J Pharmacol 549: 107–116, 2006. doi: 10.1016/j.ejphar.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 52.Prins KW, Thenappan T. World Health Organization group I pulmonary hypertension: epidemiology and pathophysiology. Cardiol Clin 34: 363–374, 2016. doi: 10.1016/j.ccl.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swinnen K, Quarck R, Godinas L, Belge C, Delcroix M. Learning from registries in pulmonary arterial hypertension: pitfalls and recommendations. Eur Respir Rev 28: 190050, 2019. doi: 10.1183/16000617.0050-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elliott CG, Austin ED, Badesch D, Badlam J, Benza RL, Chung WK, Farber HW, Feldkircher K, Frost AE, Poms AD, Lutz KA, Pauciulo MW, Yu C, Nichols WC. United States Pulmonary Hypertension Scientific Registry (USPHSR): rationale, design, and clinical implications. Pulm Circ 9: 2045894019851696, 2019. doi: 10.1177/2045894019851696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hewitt SC, Korach KS. Molecular biology and physiology of estrogen action www.uptodate.com. https://www.uptodate.com/contents/molecular-biology-and-physiology-of-estrogen-action?search=estrogen%20functions&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1 [2020 Oct 2].
- 56.Judd HL, Lucas WE, Yen SS. Serum 17β-estradiol and estrone levels in postmenopausal women with and without endometrial cancer. J Clin Endocrinol Metab 43: 272–278, 1976. doi: 10.1210/jcem-43-2-272. [DOI] [PubMed] [Google Scholar]
- 57.Roy D, Liehr JG. Temporary decrease in renal quinone reductase activity induced by chronic administration of estradiol to male Syrian hamsters. Increased superoxide formation by redox cycling of estrogen. J Biol Chem 263: 3646–3651, 1988. [PubMed] [Google Scholar]
- 58.Pugh ME, Hemnes AR. Development of pulmonary arterial hypertension in women: interplay of sex hormones and pulmonary vascular disease. Womens Health (Lond) 6: 285–296, 2010. doi: 10.2217/whe.09.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lahm T, Frump AL, Albrecht ME, Fisher AJ, Cook TG, Jones TJ, Yakubov B, Whitson J, Fuchs RK, Liu A, Chesler NC, Brown MB. 17β-Estradiol mediates superior adaptation of right ventricular function to acute strenuous exercise in female rats with severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 311: L375–L388, 2016. doi: 10.1152/ajplung.00132.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frump AL, Goss KN, Vayl A, Albrecht M, Fisher A, Tursunova R, Fierst J, Whitson J, Cucci AR, Brown MB, Lahm T. Estradiol improves right ventricular function in rats with severe angioproliferative pulmonary hypertension: effects of endogenous and exogenous sex hormones. Am J Physiol Lung Cell Mol Physiol 308: L873–L890, 2015. doi: 10.1152/ajplung.00006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng T-C, Philip JL, Tabima DM, Kumari S, Yakubov B, Frump AL, Hacker TA, Bellofiore A, Li R, Sun X, Goss KN, Lahm T, Chesler NC. Estrogen receptor-α prevents right ventricular diastolic dysfunction and fibrosis in female rats. Am J Physiol Heart Circ Physiol 319: H1459–H1473, 2020. doi: 10.1152/ajpheart.00247.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Philip JL, Tabima DM, Wolf GD, Frump AL, Cheng T-C, Schreier DA, Hacker TA, Lahm T, Chesler NC. Exogenous estrogen preserves distal pulmonary arterial mechanics and prevents pulmonary hypertension in rats. Am J Respir Crit Care Med 201: 371–374, 2020. doi: 10.1164/rccm.201906-1217LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vegeto E, Cuzzocrea S, Crisafulli C, Mazzon E, Sala A, Krust A, Maggi A. Estrogen receptor-α as a drug target candidate for preventing lung inflammation. Endocrinology 151: 174–184, 2010. doi: 10.1210/en.2009-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin KA, Rosenson RS. Menopausal hormone therapy and cardiovascular risk www.uptodate.com. https://www.uptodate.com/contents/menopausal-hormone-therapy-and-cardiovascular-risk?search=menopausal%20hormone%20therapy%20and%20cardiovascular%20risk&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1 [2020 Oct 2].
- 65.Ventetuolo CE, Ouyang P, Bluemke DA, Tandri H, Barr RG, Bagiella E, Cappola AR, Bristow MR, Johnson C, Kronmal RA, Kizer JR, Lima JA, Kawut SM. Sex hormones are associated with right ventricular structure and function: the MESA-right ventricle study. Am J Respir Crit Care Med 183: 659–667, 2011. doi: 10.1164/rccm.201007-1027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin KA, Barbieri RL. Menopausal hormone therapy: Benefits and risks. www.uptodate.com https://www.uptodate.com/contents/menopausal-hormone-therapy-benefits-and-risks?search=menopausal%20hormone%20therapy%20adn%20cardiovasculr%20risk&topicRef=7409&source=see_link [2020 Oct 2].
- 67.Martin KA, Barbieri RL. Treatment of menopausal symptoms with hormone therapy. www.uptodate.com. https://www.uptodate.com/contents/treatment-of-menopausal-symptoms-with-hormone-therapy?search=menopausal%20hormone%20therapy%20adn%20cardiovasculr%20risk&topicRef=7409&source=see_link [2020 Oct 2].
- 68.Bonnet S, Provencher S, Guignabert C, Perros F, Boucherat O, Schermuly RT, Hassoun PM, Rabinovitch M, Nicolls MR, Humbert M. Translating research into improved patient care in pulmonary arterial hypertension. Am J Respir Crit Care Med 195: 583–595, 2017. doi: 10.1164/rccm.201607-1515PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sakao S, Tatsumi K, Voelkel NF. Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir Res 10: 95, 2009. doi: 10.1186/1465-9921-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Low LA, Tagle DA. Tissue chips—innovative tools for drug development and disease modeling. Lab Chip 17: 3026–3036, 2017. doi: 10.1039/c7lc00462a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Hilal TA, Keshavarz A, Kadry H, Lahooti B, Al-Obaida A, Ding Z, Li W, Kamm R, McMurtry IF, Lahm T, Nozik-Grayck E, Stenmark KR, Ahsan F. Pulmonary-arterial-hypertension (PAH)-on-a-chip: fabrication, validation and application. Lab Chip 20: 3334–3345, 2020. doi: 10.1039/d0lc00605j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Firth AL, Mandel J, Yuan JX-J. Idiopathic pulmonary arterial hypertension. Dis Model Mech 3: 268–273, 2010. doi: 10.1242/dmm.003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15: 427–438, 2001. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 74.Rabinovitch M, Gamble WJ, Miettinen OS, Reid L. Age and sex influence on pulmonary hypertension of chronic hypoxia and on recovery. Am J Physiol Heart Circ Physiol 240: H62–H72, 1981. doi: 10.1152/ajpheart.1981.240.1.h62. [DOI] [PubMed] [Google Scholar]
- 75.Nadadur RD, Umar S, Wong G, Eghbali M, Iorga A, Matori H, Partow-Navid R, Eghbali M. Reverse right ventricular structural and extracellular matrix remodeling by estrogen in severe pulmonary hypertension. J Appl Physiol (1985) 113: 149–158, 2012. doi: 10.1152/japplphysiol.01349.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lahm T, Crisostomo PR, Markel TA, Wang M, Wang Y, Weil B, Meldrum DR. Exogenous estrogen rapidly attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction. Shock 30: 660–667, 2008. doi: 10.1097/SHK.0b013e31816f239f. [DOI] [PubMed] [Google Scholar]
- 77.Morecroft I, Dempsie Y, Bader M, Walther DJ, Kotnik K, Loughlin L, Nilsen M, MacLean MR. Effect of tryptophan hydroxylase 1 deficiency on the development of hypoxia-induced pulmonary hypertension. Hypertension 49: 232–236, 2007. doi: 10.1161/01.HYP.0000252210.58849.78. [DOI] [PubMed] [Google Scholar]
- 78.Zacharia LC, Gogos JA, Karayiorgou M, Jackson EK, Gillespie DG, Barchiesi F, Dubey RK. Methoxyestradiols mediate the antimitogenic effects of 17β-estradiol: direct evidence from catechol-O-methyltransferase-knockout mice. Circulation 108: 2974–2978, 2003. doi: 10.1161/01.cir.0000106900.66354.30. [DOI] [PubMed] [Google Scholar]
- 79.Tofovic SP, Jones TJ, Bilan VP, Jackson EK, Petrusevska G. Synergistic therapeutic effects of 2-methoxyestradiol with either sildenafil or bosentan on amelioration of monocrotaline-induced pulmonary hypertension and vascular remodeling. J Cardiovasc Pharmacol 56: 475–483, 2010. doi: 10.1097/FJC.0b013e3181f215e7. [DOI] [PubMed] [Google Scholar]
- 80.Dempsie Y, Nilsen M, White K, Mair KM, Loughlin L, Ambartsumian N, Rabinovitch M, Maclean MR. Development of pulmonary arterial hypertension in mice over-expressing S100A4/Mts1 is specific to females. Respir Res 12: 159, 2011. doi: 10.1186/1465-9921-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Austin ED, Hamid R, Hemnes AR, Loyd JE, Blackwell T, Yu C, Phillips Iii JA, Gaddipati R, Gladson S, Gu E, West J, Lane KB. BMPR2 expression is suppressed by signaling through the estrogen receptor. Biol Sex Differ 3: 6, 2012. doi: 10.1186/2042-6410-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fessel JP, Chen X, Frump A, Gladson S, Blackwell T, Kang C, Johnson J, Loyd JE, Hemnes A, Austin E, West J. Interaction between bone morphogenetic protein receptor type 2 and estrogenic compounds in pulmonary arterial hypertension. Pulm Circ 3: 564–577, 2013. doi: 10.1086/674312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res 53: 688–708, 2002. doi: 10.1016/s0008-6363(01)00527-2. [DOI] [PubMed] [Google Scholar]
- 84.Thum T, Borlak J. Testosterone, cytochrome P450, and cardiac hypertrophy. FASEB J 16: 1537–1549, 2002. doi: 10.1096/fj.02-0138com. [DOI] [PubMed] [Google Scholar]
- 85.Vander AJ, Moore LG, Brewer G, Menon KM, England BG. Effects of high altitude on plasma concentrations of testosterone and pituitary gonadotropins in man. Aviat Space Environ Med 49: 356–357, 1978. [PubMed] [Google Scholar]
- 86.Alzoubi A, Toba M, Abe K, O’Neill KD, Rocic P, Fagan KA, McMurtry IF, Oka M. Dehydroepiandrosterone restores right ventricular structure and function in rats with severe pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 304: H1708–H1718, 2013. doi: 10.1152/ajpheart.00746.2012. [DOI] [PubMed] [Google Scholar]
- 87.Dumas de la Roque ED, Quignard J-F, Ducret T, Dahan D, Courtois A, Begueret H, Marthan R, Savineau J-P. Beneficial effect of dehydroepiandrosterone on pulmonary hypertension in a rodent model of pulmonary hypertension in infants. Pediatr Res 74: 163–169, 2013. doi: 10.1038/pr.2013.73. [DOI] [PubMed] [Google Scholar]
- 88.Lynnette KN, Lacroix A, Martin KA. Treatment of adrenal insufficiency in adults. www.uptodate.com. https://www.uptodate.com/contents/treatment-of-adrenal-insufficiency-in-adults?search=DHEA&source=search_result&selectedTitle=4∼61&usage_type=default&display_rank=4 [2020 Oct 5].
- 89.Udoff LC, Barbieri RL, Crowley WF, Martin KA. Overview of androgen deficiency and therapy in women. www.uptodate.com. https://www.uptodate.com/contents/overview-of-androgen-deficiency-and-therapy-in-women?search=DHEA&source=search_result&selectedTitle=1∼61&usage_type=default&display_rank=1 [2020 Oct 5].
- 90.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE, Knowles JA. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 67: 737–744, 2000. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fessel JP, Loyd JE, Austin ED. The genetics of pulmonary arterial hypertension in the post-BMPR2 era. Pulm Circ 1: 305–319, 2011. doi: 10.4103/2045-8932.87293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Newman JH, Trembath RC, Morse JA, Grunig E, Loyd JE, Adnot S, Coccolo F, Ventura C, Phillips JA, 3rd, Knowles JA, Janssen B, Eickelberg O, Eddahibi S, Herve P, Nichols WC, Elliott G. Genetic basis of pulmonary arterial hypertension: current understanding and future directions. J Am Coll Cardiol 43: 33S–39S, 2004. doi: 10.1016/j.jacc.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 93.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol 8: 443–455, 2011. doi: 10.1038/nrcardio.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen X, Austin ED, Talati M, Fessel JP, Farber-Eger EH, Brittain EL, Hemnes AR, Loyd JE, West J. Oestrogen inhibition reverses pulmonary arterial hypertension and associated metabolic defects. Eur Respir J 50: 1602337, 2017. doi: 10.1183/13993003.02337-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lahm T, Kawut SM. Inhibiting oestrogen signalling in pulmonary arterial hypertension: sex, drugs and research. Eur Respir J 50: 1700983, 2017. doi: 10.1183/13993003.00983-2017. [DOI] [PubMed] [Google Scholar]
- 96.Badlam JB, Austin ED. Beyond oestrogens: towards a broader evaluation of the hormone profile in pulmonary arterial hypertension. Eur Respir J 51: 1801058, 2018. doi: 10.1183/13993003.01058-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu W-H, Yuan P, Zhang S-J, Jiang X, Wu C, Li Y, Liu S-F, Liu Q-Q, Li J-H, Pudasaini B, Hu Q-H, Dupuis J, Jing Z-C. Impact of pituitary-gonadal axis hormones on pulmonary arterial hypertension in men. Hypertension 72: 151–158, 2018. doi: 10.1161/HYPERTENSIONAHA.118.10963. [DOI] [PubMed] [Google Scholar]
- 98.Ventetuolo CE, Baird GL, Barr RG, Bluemke DA, Fritz JS, Hill NS, Klinger JR, Lima JA, Ouyang P, Palevsky HI, Palmisciano AJ, Krishnan I, Pinder D, Preston IR, Roberts KE, Kawut SM. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care Med 193: 1168–1175, 2016. doi: 10.1164/rccm.201509-1785OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Badlam JB, Badesch D, Brittain E, Cordell S, Ding T, Fox K, Hemnes A, Loyd J, Pugh M, Robbins I, Yu C, Austin ED. Sex hormone exposure and reproductive factors in pulmonary arterial hypertension: a case-control study. Pulm Circ 10: 2045894020908786, 2020. doi: 10.1177/2045894020908786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liao ZH, Huang T, Xiao JW, Gu RC, Ouyang J, Wu G, Liao H. Estrogen signaling effects on muscle-specific immune responses through controlling the recruitment and function of macrophages and T cells. Skelet Muscle 9: 20, 2019. doi: 10.1186/s13395-019-0205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fairweather D, Petri MA, Coronado MJ, Cooper LT. Autoimmune heart disease: role of sex hormones and autoantibodies in disease pathogenesis. Expert Rev Clin Immunol 8: 269–284, 2012. doi: 10.1586/eci.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 351: 1655–1665, 2004. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 103.Souyris M, Cenac C, Azar P, Daviaud D, Canivet A, Grunenwald S, Pienkowski C, Chaumeil J, Mejía JE, Guéry J-C. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol 3: eaap8855, 2018. doi: 10.1126/sciimmunol.aap8855. [DOI] [PubMed] [Google Scholar]
- 104.Larkin EK, Newman JH, Austin ED, Hemnes AR, Wheeler L, Robbins IM, West JD, Phillips JA, 3rd, Hamid R, Loyd JE. Longitudinal analysis casts doubt on the presence of genetic anticipation in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 892–896, 2012. doi: 10.1164/rccm.201205-0886OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Umar S, Cunningham CM, Itoh Y, Moazeni S, Vaillancourt M, Sarji S, Centala A, Arnold AP, Eghbali M. The Y chromosome plays a protective role in experimental hypoxic pulmonary hypertension. Am J Respir Crit Care Med 197: 952–955, 2018. doi: 10.1164/rccm.201707-1345LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yan L, Cogan JD, Hedges LK, Nunley B, Hamid R, Austin ED. The Y chromosome regulates BMPR2 expression via SRY: a possible reason “why” fewer males develop pulmonary arterial hypertension. Am J Respir Crit Care Med 198: 1581–1583, 2018. doi: 10.1164/rccm.201802-0308le. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lahm T. Sex differences in pulmonary hypertension: are we cleaning up the mess? Eur Respir J 47: 390–393, 2016. doi: 10.1183/13993003.01999-2015. [DOI] [PubMed] [Google Scholar]
- 108.Lahm T, Tuder RM, Petrache I. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 307: L7–L26, 2014. doi: 10.1152/ajplung.00337.2013. [DOI] [PubMed] [Google Scholar]