Abstract
Animal models have been highly informative for understanding the pathogenesis and progression of cystic fibrosis (CF) lung disease. In particular, the CF rat models recently developed have addressed mechanistic causes of the airway mucus defect characteristic of CF, and how these may change when cystic fibrosis transmembrane conductance regulator (CFTR) activity is restored using new modulator therapies. We hypothesized that inflammatory changes to the airway would develop spontaneously and progressively, and that these changes would be resolved with modulator therapy. To test this, we used a humanized-CFTR rat expressing the G551D variant that responds to the CFTR modulator ivacaftor. Markers typically found in the CF lung were assessed, including neutrophil influx, small airway histopathology, and inflammatory cytokine concentration. Young hG551D rats did not express inflammatory cytokines at baseline but did upregulate these in response to inflammatory trigger. As the hG551D rats aged, histopathology worsened, accompanied by neutrophil influx into the airway and increasing concentrations of TNF-α, IL-1α, and IL-6 in the airways. Ivacaftor administration reduced concentrations of these cytokines when administered to the rats at baseline but was less effective in the rats that had also received inflammatory stimulus. Therefore, we conclude that administration of ivacaftor resulted in an incomplete resolution of inflammation when rats received an external trigger, suggesting that CFTR activation may not be enough to resolve inflammation in the lungs of patients with CF.
Keywords: cystic fibrosis, G551D, inflammation, ivacaftor, rat
INTRODUCTION
Cystic fibrosis (CF) is a common autosomal recessive genetic disorder that results from disruptions to the cystic fibrosis transmembrane conductance regulator (Cftr) gene, resulting in reduced chloride and bicarbonate transport at the epithelial surface of exocrine organs (1). In the lung, this results in increased mucus accumulation, recurrent and chronic infections, and chronic airway inflammation (2). Recent evidence has shown that inflammatory markers are present before severe structural lung changes and in the absence of infection (3–5), suggesting that hyperactive inflammatory responses are a direct result of CFTR dysfunction.
Airway inflammation in lungs of people with CF (pwCF) is characterized by early and progressive appearance of neutrophilic influx (3), which releases reactive oxygen species and proteases that cause damage to the host. The epithelial cell layer secretes neutrophil chemokines and other cytokines, including TNF-α, interleukins, and IL-6 (6). Cytokines that are linked to macrophage activation, including INF-γ, are also altered in CF lung disease (7) although the role of macrophages has yet to be agreed upon (7–9). Until recently, the field has debated whether these changes to the inflammatory state in the CF lung were an inherent effect of absent CFTR or a secondary result of infection, a question that has been difficult to answer as pwCF become infected so early (10). New data from the CF AREST study found evidence of these markers before detectable infection (3). These results were corroborated by an experimental model that determined that CF ferrets raised on continuous antibiotics developed inflammatory infiltrates and mucus obstruction in the absence of detectable infection (11). Taken together, these studies indicate that inflammation is a primary pathology of CFTR dysfunction.
Therapeutic advances in CF treatments have escalated since the 1980s, adding intravenous and aerosolized antibiotics (12), mucolytics (13), and anti-inflammatory (14) treatment regimens. These supportive therapies (15) reduced morbidity and mortality in pwCF but did not address the underlying cause of the disease. In 2012, the FDA approved the first small molecule to change the function of the CFTR channel, ivacaftor (16). Known as a CFTR modulator, ivacaftor increases CFTR activity to roughly 50% of WT (17), resulting in increased lung function and decreased incidence of exacerbations in pwCF who have a number of “gating” mutations, most notably G551D. However, this patient population has had mixed results in the infection and inflammatory status of their airways (18). Why ivacaftor does not completely resolve airway inflammation is unclear but remains a question of high clinical importance.
To test this question, our laboratory created a humanized G551D-CFTR (hG551D) rat model (19), enabling highly controlled investigation into the efficacy of ivacaftor. The rat model has been shown to progressively develop abnormalities to airway mucus, including increased mucus viscosity and decreased mucus transport. These features of airway disease are present in larger animal models of CF but have not been found in murine models of the disease (20). The lack of mucus accumulation has been suggested to protect CF mice from the spontaneous infection and inflammation seen in human pathology. In the hG551D rat model, ivacaftor administration results in a restoration of mucus viscosity and transport, corresponding with increased CFTR activity.
We hypothesized that the hG551D rat would develop inflammation spontaneously, before overt bacterial infection. The objectives of this study were to identify the initial changes to inflammatory markers that would indicate development of disease pathology and test if ivacaftor would reduce inflammatory markers in this model.
MATERIALS AND METHODS
CF Rat Model
All animal experiments at University of Alabama at Birmingham (UAB) were conducted in accordance with UAB IACUC approved protocols. All experiments used humanized-G551D-CFTR rats (19), homozygous G551D (termed hG551D), or their littermate controls (termed WT). Heterozygote (CFTRhG551D/−) male and female were paired to generate WT and hG551D pups, and litters remained with lactating dams until 21 days of age. Animals were bred with housing in standard cages maintained on a 12 h light/dark cycle with ad libitum access to food and water, in temperatures ranging from 71°F–75°F. WT and hG551D were cohoused from weaning through to the end of the experiment and maintained on a standard rodent diet with supplemental DietGel 76 A (Clear H20, Westbrook, ME) and 50% Go-LYTLEY (Braintree Laboratories, Inc, Braintree, MA) added to the water from weaning, as a means to reduce mortality from gastrointestinal obstruction (21). hG551D pups have a 50% survival rate under these nutritional conditions (19). WT and hG551D rats were assayed at 1, 3, and 6 mo of age and groups were split evenly between males and females.
Ivacaftor Administration
Rats that received administration of ivacaftor, which was obtained from Selleckchem (Houston, TX), were dosed for 7 days with at 30 mg/kg/day or 3% methylcellulose vehicle by oral gavage. Rats in the stimulation study received LPS on day 7.
LPS Administration
Lipopolysaccharide (LPS) purified from Pseudomonas aeruginosa (Millipore Sigma, St. Louis, MO) was administered at a concentration of 5 μg/kg, via intratracheal administration. Rats were deeply anesthetized by isoflurane and suspended on an intubation stand (Braintree Scientific, Inc, Braintree, MA) by their incisors. The tongue was pulled out and aside using blunt forceps and a syringe attached to a blunt 18-gauge luer stub (Instech Laboratories, Plymouth Meeting, PA) inserted past the tongue and into the trachea to ensure lung delivery. In all, 100 μL of the solution was delivered via syringe, followed by 50 μL of air. Rats remained upright on the intubation stand for at least 5 s to complete inhalation and were allowed to recover. Rats were euthanized 24 h following administration for analysis.
Bronchoalveolar Lavage
Rats were euthanized via intraperitoneal injection of 500 μL pentobarbital sodium (390 mg/mL). Rats were exsanguinated and the thoracic cavity exposed for bronchoalveolar lavage. Before the thoracic cavity was punctured, rats were intubated via the trachea. The 5 mL of sterile PBS pushed into the lungs and recollected into a separate sterile syringe using a two-way stopcock. Bronchoalveolar lavage fluid (BALF) was centrifuged at 1,200 rpm for 7 min, and the supernatant transferred for cytokine and mucin analysis. The cell pellet was resuspended, counted, and adjusted to 1 × 104 cells/mL for cytospin. After centrifugation of the slide in the cytospin at 500 g for 5 min, slides were allowed to dry and stained with the Diff-Quick kit (Siemens Medical Solutions, Inc, Malvern, PA). Total cells, neutrophils, and macrophages were counted manually.
Histology
Tracheae and lungs were immersion fixed in 10% phosphate-buffered formalin and embedded in paraffin blocks for sectioning. Sections were stained with hematoxylin and eosin (H&E) or alcian blue-periodic acid Schiffs (ABPAS).
Cytokine Quantitation
Supernatant from BAL was used to analyze cytokine concentrations by ELISA. Protein concentration of each sample was determined with a bicinchoninic acid (BCA) kit (Millipore Sigma, St. Louis, MO). Undiluted samples were tested for initial hits using a multianalyte ELISA kit (Qiagen, Germantown, MD). Follow-up experiments tested for IL-1α, IFN-γ, TNF-α, and IL-6 using sandwich ELISA kits (Abcam, Cambridge, MA). Each cytokine was compared with a standard curve using known concentrations of recombinant rat cytokine. Signal was detected with HRP-secondary antibodies and the SuperSignal West Femto Maximum Sensitivity Substrate (Thermofisher Scientific, Grand Island, NY). Samples were normalized to protein concentration of the sample and age-matched WT controls.
Statistics
Statistical analysis was performed in GraphPad Prism (GraphPad, LaJolla, CA) version 7.0 or newer. Inferential statistics (mean, SD, and SEM) were computed using ANOVA. For multiple comparisons, post hoc testing was applied only if ANOVA was significant. P values of less than 0.05 were considered significant. Statistics are presented as mean ± SEM. Mean values per animal are reported.
Study Approvals
Procedures involving animals were approved by the IACUC at UAB (IACUC-09479, IACUC-21238, IACUC-21781, and IACUC-22072).
RESULTS
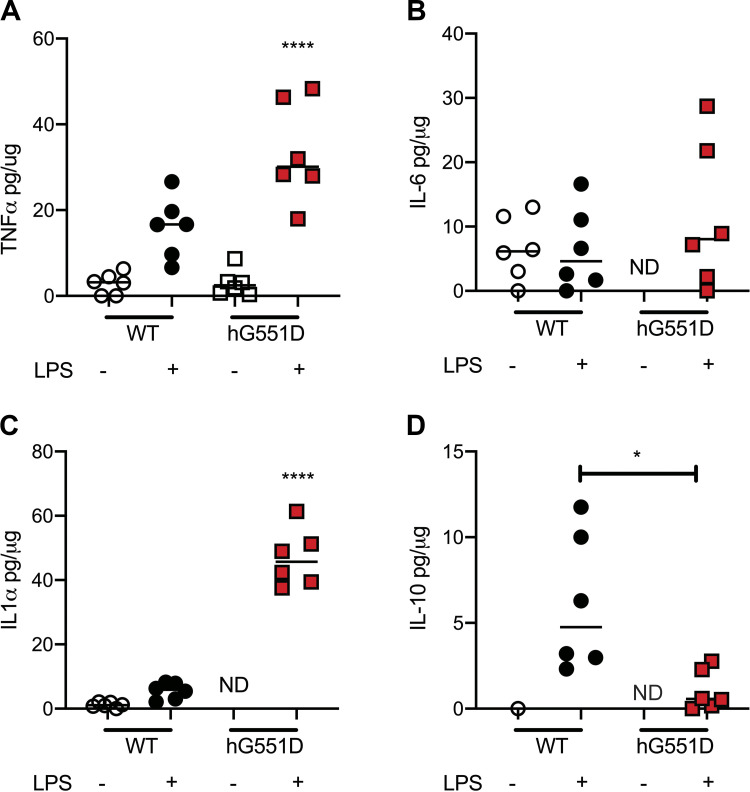
As has been previously documented in other experimental models of CF (22, 23), we expected that the hG551D rat would harbor some facets of inflammatory disease in their lungs from birth, with an exaggerated response to airway insults. Bronchoalveolar lavage fluid collected from hG551D rats and their WT littermates at 1 mo of age were analyzed for cytokine concentrations, in the presence or absence of lipopolysaccharide (LPS), a component of Gram-negative bacteria that induces inflammatory cascades in the airway via the TLR4 receptor (24). For our initial screen, we focused on general markers of inflammation, and found the most significant differences in TNF-α, IL-6, and IL1-α. In the rats that received PBS vehicle, there was no difference between the hG551D (open squares) and the WT (open circles) in TNF-α concentrations (Fig. 1A), whereas IL-6 and IL-1α were undetectable in the hG551D (Fig. 1, B and C). IL-10, an anti-inflammatory cytokine that is upregulated in response to TLR4 signaling (25), was undetectable in the hG551D in the absence of LPS. However, the hG551D rats that were exposed to LPS had a disproportionately higher inflammatory response compared with the WT exposed to LPS; hG551D rats responded with higher concentrations of TNF-α and IL-1α (Fig. 1, A and C). hG551D rats also responded with lower concentrations of IL-10 compared with the WT group that received LPS (Fig. 1D). IL-10 has been shown previously to be downregulated in pwCF (26–28). These data suggested to us that, although the young hG551D rat did not show signs of hyperinflammation at a young age, it may develop progressively, in a similar manner to the development of abnormal mucus (29). Therefore, we conducted a more extensive analysis of spontaneous appearance of inflammatory markers as the hG551D rat ages.
Figure 1.
Young hG551D rats have an exaggerated response to inflammatory stimulus. Rats were treated with PBS or lipopolysaccharide (LPS) by intratracheal instillation and euthanized for bronchoalveolar lavage fluid (BALF) collection 24 h later. Concentrations of cytokines were measured and presented as pg per μg of protein in the BALF. Cytokines detected in hG551D rats with LPS administration are presented. (A) TNF-α, (B) IL-6, (C) IL-1α, and (D) IL-10 were all present following stimulation. n = 6 animals/group. Data presented as mean ± SE. *P < 0.05, ****P < 0.0001. hG551D, homozygous G551D; ND, not determined; WT, wild type.
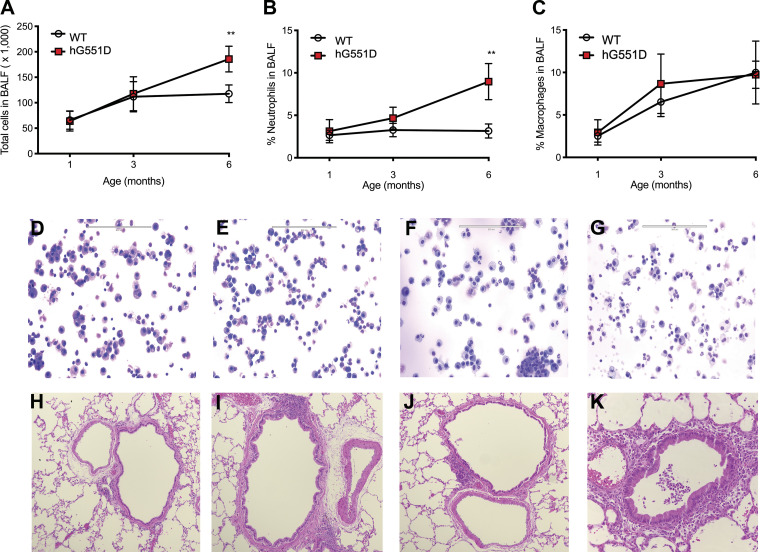
WT and hG551D rats were assayed at 1, 3, and 6 mo of age. Total cells present in the BALF were no different between the genotypes at 1 and 3 mo of age but became significantly higher in hG551D rats by 6 mo (Fig. 2A). The percentage of neutrophils was similarly unchanged in the hG551D rats at 1 and 3 mo of age but were significantly higher in 6-mo-old hG551D rats compared with age-matched WT (Fig. 2B). The percentage of macrophages in the BALF was no different between the two genotypes at any of the ages assayed (Fig. 2C). Representative cytospins of WT (Fig. 2D) and hG551D at 1 mo (Fig. 2E), 3 mo (Fig. 2F), and 6 mo (Fig. 2G) are shown. Corresponding histology images showed that, compared with the WT lung (Fig. 2H), 1-mo-old (Fig. 2I) and 3-mo-old (Fig. 3J) hG551D rats had comparative normal lung tissue. However, at 6 mo of age, the hG551D rat had evidence of increased neutrophilic infiltrates in the small airways (Fig. 2K).
Figure 2.
Inflammation develops as the hG551D rat ages. BALF and lungs from WT and hG551D rats at ages 1, 3, and 6 mo were collected to assess the influx of inflammatory cells. A: total cells in the BALF increased in hG551D lungs at 6 mo of age. B: the percentage of these cells that were neutrophils also increased in the hG551D rats by 6 mo of age. C: the percentage of macrophages in the BALF was not changed in the hG551D rat lungs at any age. Representative cytospins from (D) WT rats, (E) 1-mo-old hG551D rats, (F) 3-mo-old hG551D rats, and (G) 6-mo-old hG551D rats are shown. Representative histology of (H) WT rats, (I) 1-mo-old hG551D rats, (J) 3-mo-old hG551D rats, and (K) 6-mo-old hG551D rats indicated the presence of neutrophils in the small airways of the hG551D rats by 6 mo of age. n = 6 animals/group. Data presented as mean ± SE. **P < 0.01. BALF, bronchoalveolar lavage fluid; hG551D, homozygous G551D; WT, wild type.
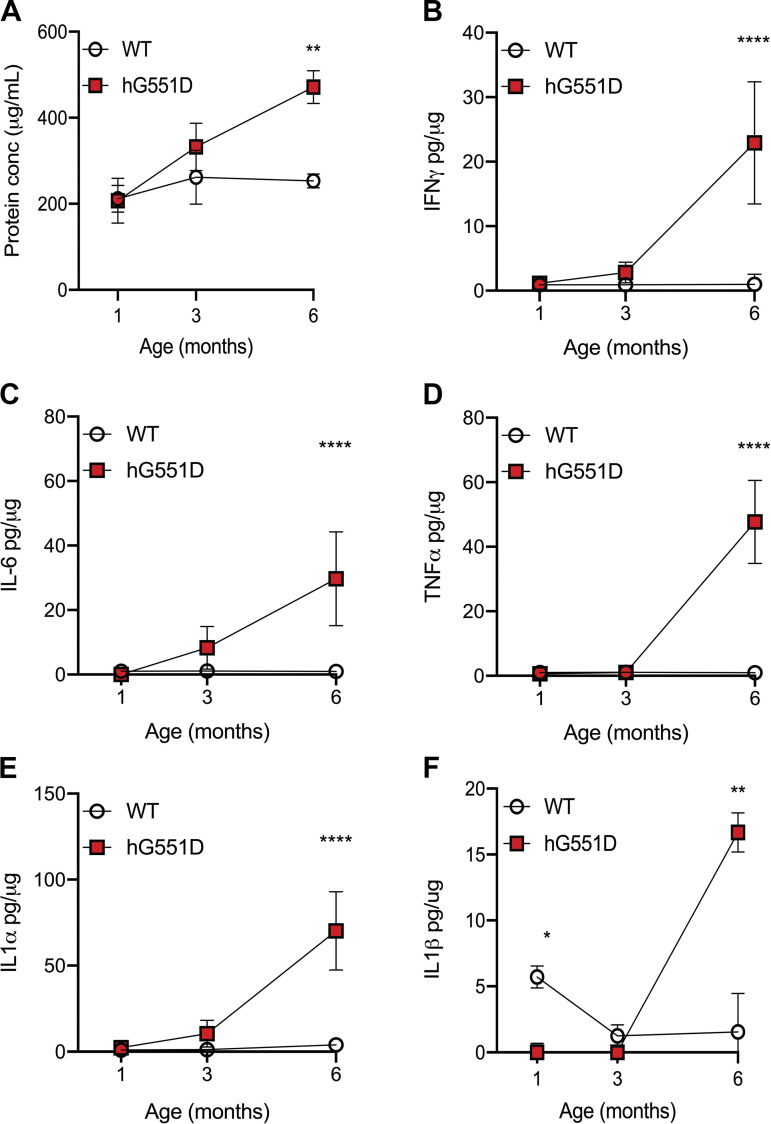
Figure 3.
Inflammatory cytokines are elevated in hG551D rat lungs by 6 mo of age. BALF collected from WT and hG551D rats at 1, 3, and 6 mo of age were analyzed for concentrations of inflammatory cytokines and presented as pg per μg of protein in the BALF. (A) Total protein concentrations in the BALF, (B) IFN-γ, (C) IL-6, (D) TNF-α, (E) IL-1α, and (F) IL-1β were all increased in BALF from 6-mo-old hG551D rats. n = 6 animals/group. Data presented as mean ± SE. **P < 0.01, ****P < 0.0001. BALF, bronchoalveolar lavage fluid; hG551D, homozygous G551D; WT, wild type.
To evaluate if the increased neutrophilic influx influenced secretion of inflammatory cytokines and chemokines, we measured total protein concentrations in the BALF (Fig. 3A) as well as concentrations of IFN-γ (Fig. 3B), IL-6 (Fig. 3C), TNF-α (Fig. 3D), IL-1α (Fig. 3E), and IL-1β (Fig. 3F) at 1, 3, and 6 mo of age, in both WT and hG551D rats. At 3 mo of age, the hG551D had slightly higher concentrations of IFN-γ, IL-1α, and IL-6, although the increase was not statistically significant. By 6 mo of age, hG551D rats had much higher concentrations of each cytokine present in the airway.
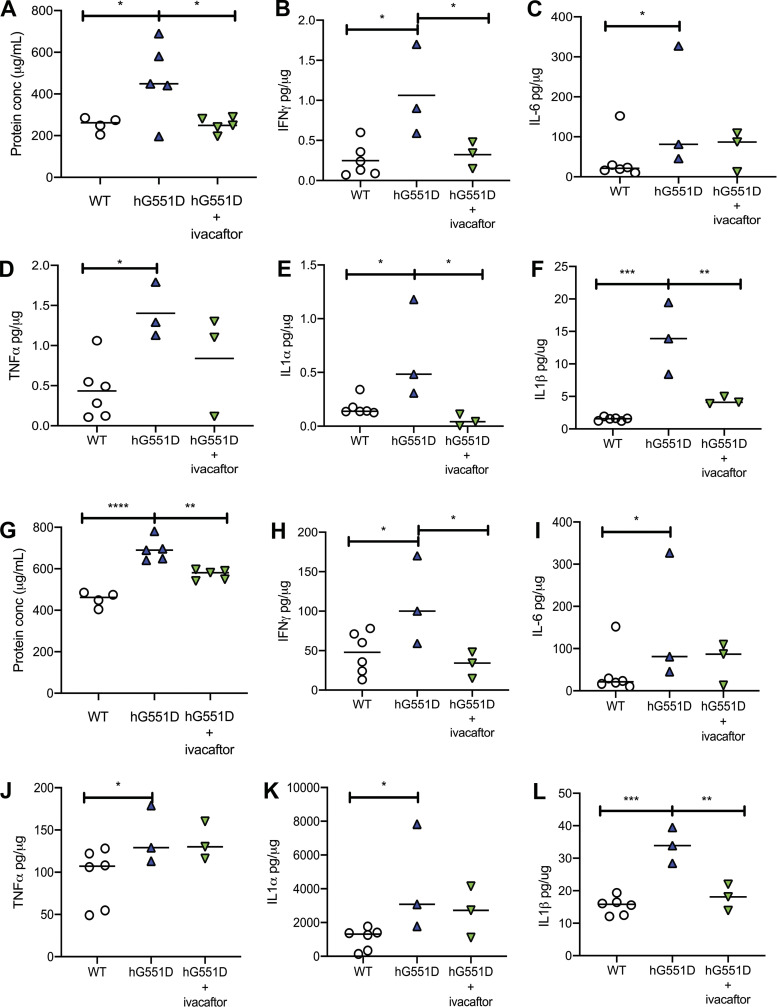
A major question facing the CF field is whether the administration of CFTR modulators will decrease airway inflammation through restoring CFTR function. To study that question in this model, we treated 6 month old hG551D rats with ivacaftor daily for seven days and then assessed cytokine concentrations compared to hG551D rats that were treated with methylcellulose vehicle. As before, we saw that the hG551D rats had higher concentrations of total protein (Fig. 4A), IFN-γ (Fig. 4B), IL-6 (Fig. 4C), TNF-α (Fig. 4D), IL-1α (Fig. 4E), and IL-1β (Fig. 4F) present in the BALF. However, the ivacaftor-treated group showed reductions in IFN-γ, IL-1α, and IL-1β. The decrease in IL-6 and TNF-α concentrations with ivacaftor was not statistically significant. To address if the administration of ivacaftor before insult would ameliorate the response, we treated hG551D rats for 7 days with ivacaftor, followed by an administration of LPS by intratracheal instillation. In this case, the hG551D rats that received ivacaftor had reduced concentrations of total protein (Fig. 4G), IFN-γ (Fig. 4H), and IL-1β (Fig. 4L) but no change to concentrations of IL-6 (Fig. 4I), TNF-α (Fig. 4J), or IL-1α (Fig. 4K). This suggests that inflammatory triggers or insults are able to overcome the effects of increased CFTR activity to some degree.
Figure 4.
Ivacaftor reduces inflammatory cytokines but does not prevent the response to LPS. hG551D rats were treated with methylcellulose vehicle or ivacaftor for 7 days, and BALF collected. Ivacaftor reduced (A) total protein concentration in the BALF, as well as concentrations of (B) IFN-γ, (C) IL-6, (D) TNF-α, (E) IL-1α, and (F) IL-1β. Rats pretreated with methylcellulose or ivacaftor and stimulated with LPS had (G) reduced protein concentration in the BALF, as well as concentrations of (H) IFN-γ and (L) IL-1β that were reduced, whereas concentrations of (I) IL-6, (J) TNF-α, and (K) IL-1α were unchanged. Data presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. BALF, bronchoalveolar lavage fluid; hG551D, homozygous G551D; LPS, lipopolysaccharide; WT, wild type.
DISCUSSION
The rat model presented here offers an opportunity to conduct studies of CF airway inflammation in a readily accessible rodent model that responds to ivacaftor. Although large animal models including the CF ferret and the CF pig have shown evidence of spontaneous inflammation (11, 23), the more commonly used murine models have been unable to replicate these events (30). The appearance of inflammatory markers in the CF rat also corresponds with the age at which these rats have developed abnormalities to airway mucus, including hyperviscosity and decreased transport (19, 29). The neutrophilic-driven changes seen here mimic the progression of human CF airway disease, making this an ideal model with which to study novel anti-inflammatory drugs, an important area of research and development (14).
The major question addressed by this work is whether ivacaftor will decrease inflammatory sequelae in the CF patient population. The results presented from hG551D rats who were treated with ivacaftor in the absence of external inflammatory stimulus are promising. These data indicate that airway inflammation may be purely secondary to the absence of CFTR activity and may be reduced with the restoration of channel activity. However, multiple studies of patients that have been treated with ivacaftor long term have had mixed results, suggesting that the expected reduction in airway inflammation with these new drugs may not be as dramatic as previously hoped (18, 31). Importantly, most patients have had infections in the past or have active infections at the time of therapy initiation (32, 33). This situation is replicated by the LPS-administration, ivacaftor-treated hG551D group. The outcomes showing only partial reduction of inflammatory cytokines stimulation with LPS may explain why patients with active infections do not see immediate or prolonged relief from this aspect of lung pathology. This patient population may benefit from supportive therapies to reduce or eliminate inflammation. Potential candidates include anti-inflammatory agents such as acebilistat or lenabasum (34), protease inhibitors to prevent neutrophilic damage (35), modification of TGF activity, or activation of additional chloride channels such as TMEM16A (36). The data presented in this report corroborate additional studies that show that CFTR restoration is not sufficient to return the CF lung to full health and indicate that these patients will continue to require supportive therapies, including anti-inflammatory agents, to maintain lung function.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute [1K08HL131867, S. E. Birket] and the Cystic Fibrosis Foundation [BIRKET18I0, BIRKET20G0, ROWE19R0].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.E.B. conceived and designed research; M.G., N.L., A.H., J.D.K., and A.M.O. performed experiments; M.G., N.L., A.H., J.D.K., and S.E.B. analyzed data; N.L., J.D.K., and S.E.B. interpreted results of experiments; M.G., N.L., and S.E.B. prepared figures; S.E.B. drafted manuscript; N.L. and S.E.B. edited and revised manuscript; M.G., N.L., A.H., J.D.K., A.M.O., and S.E.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the assistance of Dezhi Wang and the Histomorphometry and Molecular Analysis Core in the Center for Metabolic Bone Disease.
REFERENCES
- 1.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 352: 1992–2001, 2005. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 2.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med 372: 351–362, 2015. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esther CR, Jr., Muhlebach MS, Ehre C, Hill DB, Wolfgang MC, Kesimer M, Ramsey KA, Markovetz MR, Garbarine IC, Forest MG, Seim I, Zorn B, Morrison CB, Delion MF, Thelin WR, Villalon D, Sabater JR, Turkovic L, Ranganathan S, Stick SM, Boucher RC. Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci Transl Med 11: eaav3488, 2019. doi: 10.1126/scitranslmed.aav3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stick SM, Brennan S, Murray C, Douglas T, von Ungern-Sternberg BS, Garratt LW, Gangell CL, De Klerk N, Linnane B, Ranganathan S, Robinson P, Robertson C, Sly PD; Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF). Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr 155: 623–628.e1, 2009. doi: 10.1016/j.jpeds.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Rosenow T, Mok LC, TurkovIc L, Berry LJ, Sly PD, Ranganathan S, Tiddens HAWM, Stick SM. The cumulative effect of inflammation and infection on structural lung disease in early cystic fibrosis. Eur Respir J 54: 1801771, 2019. doi: 10.1183/13993003.01771-2018. [DOI] [PubMed] [Google Scholar]
- 6.Aldallal N, McNaughton EE, Manzel LJ, Richards AM, Zabner J, Ferkol TW, Look DC. Inflammatory response in airway epithelial cells isolated from patients with cystic fibrosis. Am J Respir Crit Care Med 166: 1248–1256, 2002. doi: 10.1164/rccm.200206-627OC. [DOI] [PubMed] [Google Scholar]
- 7.Bruscia EM, Bonfield TL. Cystic fibrosis lung immunity: the role of the macrophage. J Innate Immun 8: 550–563, 2016. doi: 10.1159/000446825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hisert KB, Schoenfelt KQ, Cooke G, Grogan B, Launspach JL, Gallagher CG, Donnelly SC, Welsh MJ, Singh PK, McKone EF, Becjer L. Ivacaftor-induced proteomic changes suggest monocyte defects may contribute to the pathogenesis of cystic fibrosis. Am J Respir Cell Mol Biol 54: 594–597, 2016. doi: 10.1165/rcmb.2015-0322LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Shrestha CL, Kopp BT. Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have differential effects on cystic fibrosis macrophage function. Sci Rep 8: 17066, 2018. doi: 10.1038/s41598-018-35151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chmiel JF, Davis PB. State of the art: why do the lungs of patients with cystic fibrosis become infected and why can't they clear the infection? Respir Res 4: 8, 2003. doi: 10.1186/1465-9921-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen BH, Evans TIA, Moll SR, Gray JS, Liang B, Sun X, Zhang Y, Jensen-Cody CW, Swatek AM, Zhou W, He N, Rotti PG, Tyler SR, Keiser NW, Anderson PJ, Brooks L, Li Y, Pope RM, Rajput M, Hoffman EA, Wang K, Harris JK, Parekh KR, Gibson-Corley KN, Engelhardt JF. Infection is not required for mucoinflammatory lung disease in CFTR-knockout ferrets. Am J Respir Crit Care Med 197: 1308–1318, 2018. doi: 10.1164/rccm.201708-1616OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flume PA, O'Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ, Jr., Willey-Courand DB, Bujan J, Finder J, Lester M, Quittell L, Rosenblatt R, Vender RL, Hazle L, Sabadosa K, Marshall B; Cystic Fibrosis Foundation; Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med 176: 957–969, 2007. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 13.Morrison CB, Markovetz MR, Ehre C. Mucus, mucins, and cystic fibrosis. Pediatr Pulmonol 54 Suppl 3: S84–S96, 2019. doi: 10.1002/ppul.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantin AM, Hartl D, Konstan MW, Chmiel JF. Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. J Cyst Fibros 14: 419–430, 2015. doi: 10.1016/j.jcf.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman LR, Ramsey BW. Cystic fibrosis therapeutics: the road ahead. Chest 143: 207–213, 2013. doi: 10.1378/chest.12-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermrt-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordonez C, Elborn JS; VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 365: 1663–1672, 2011. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, Sagel SD, Khan U, Mayer-Hamblett N, Van Dalfsen JM, Joseloff E, Ramsey BW; GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med 190: 175–184, 2014. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hisert KB, Heltshe SL, Pope C, Jorth P, Wu X, Edwards RM, Radey M, Accurso FJ, Wolter DJ, Cooke G, Adam RJ, Carter S, Grogan B, Launspach JL, Donnelly SC, Gallagher CG, Bruze JE, Stoltz DA, Welsh MJ, Hoffman LR, McKone EF, Singh PK. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit Care Med 195: 1617–1628, 2017. doi: 10.1164/rccm.201609-1954OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birket SE, Davis JM, Fernandez-Petty CM, Henderson AG, Oden AM, Tang L, Wen H, Hong J, Fu L, Chambers A, Fields A, Zhao G, Tearney GJ, Sorscher EJ, Rowe SM. Ivacaftor reverses airway mucus abnormalities in a rat model harboring a humanized G551D-CFTR. Am J Respir Crit Care Med 202: 1271–1282, 2020. doi: 10.1164/rccm.202002-0369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen BH, Chanson M, Gawenis LR, Liu J, Sofoluwe A, Zoso A, Engelhardt JF. Animal and model systems for studying cystic fibrosis. J Cyst Fibros 17: S28–S34, 2018. doi: 10.1016/j.jcf.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuggle KL, Birket SE, Cui X, Hong J, Warren J, Reid L, Chambers A, Ji D, Gamber K, Chu KK, Tearney G, Tang LP, Fortenberry JA, Du M, Cadillac JM, Bedwell DM, Rowe SM, Sorscher EJ, Fanucchi MV. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS One 9: e91253, 2014. doi: 10.1371/journal.pone.0091253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keiser NW, Birket SE, Evans IA, Tyler SR, Crooke AK, Sun X, Zhou W, Nellis JR, Stroebele EK, Chu KK, Tearney GJ, Stevens MJ, Hrris JK, Rowe SM, Engelhardt JF. Defective innate immunity and hyperinflammation in newborn cystic fibrosis transmembrane conductance regulator-knockout ferret lungs. Am J Respir Cell Mol Biol 52: 683–694, 2015. doi: 10.1165/rcmb.2014-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2: 29ra31, 2010. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 113: 153–162, 2004. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teixeira-Coelho M, Guedes J, Ferreirinha P, Howes A, Pedrosa J, Rodrigues F, Lai WS, Blackhear PJ, O’Garra A, Castro AG, Saraiva M. Differential post-transcriptional regulation of IL-10 by TLR2 and TLR4-activated macrophages. Eur J Immunol 44: 856–866, 2014. doi: 10.1002/eji.201343734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonfield TL, Konstan MW, Burfeind P, Panuska JR, Hilliard JB, Berger M. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol 13: 257–261, 1995. doi: 10.1165/ajrcmb.13.3.7544594. [DOI] [PubMed] [Google Scholar]
- 27.Bonfield TL, Konstan MW, Berger M. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J Allergy Clin Immunol 104: 72–78, 1999. doi: 10.1016/s0091-6749(99)70116-8. [DOI] [PubMed] [Google Scholar]
- 28.Dosanjh AK, Elashoff D, Robbins RC. The bronchoalveolar lavage fluid of cystic fibrosis lung transplant recipients demonstrates increased interleukin-8 and elastase and decreased IL-10. J Interferon Cytokine Res 18: 851–854, 1998. doi: 10.1089/jir.1998.18.851. [DOI] [PubMed] [Google Scholar]
- 29.Birket SE, Davis JM, Fernandez CM, Tuggle KL, Oden AM, Chu KK, Tearney GJ, Fanucchi MV, Sorscher EJ, Rowe SM. Development of an airway mucus defect in the cystic fibrosis rat. JCI Insight 3: e97199, 2018. doi: 10.1172/jci.insight.97199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guilbault C, Saeed Z, Downey GP, Radzioch D. Cystic fibrosis mouse models. Am J Respir Cell Mol Biol 36: 1–7, 2007. doi: 10.1165/rcmb.2006-0184TR. [DOI] [PubMed] [Google Scholar]
- 31.Harris JK, Wagner BD, Zemanick ET, Robertson CE, Stevens MJ, Heltshe SL, Rowe SM, Sagel SD. Changes in airway microbiome and inflammation with ivacaftor treatment in patients with cystic fibrosis and the G551D mutation. Ann Am Thorac Soc 17: 212–220, 2020. doi: 10.1513/AnnalsATS.201907-493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heltshe SL, Mayer-Hamblett N, Burns JL, Khan U, Baines A, Ramsey BW, Rowe SM; GOAL (the G551D Observation-AL) Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor. Clin Infect Dis 60: 703–712, 2015. doi: 10.1093/cid/ciu944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heltshe SL, Rowe SM, Skalland M, Baines A, Jain M; GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Ivacaftor-treated patients with cystic fibrosis derive long-term benefit despite no short-term clinical improvement. Am J Respir Crit Care Med 197: 1483–1486, 2018. doi: 10.1164/rccm.201710-2046LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roesch EA, Nichols DP, Chmiel JF. Inflammation in cystic fibrosis: an update. Pediatr Pulmonol 53: S30–S50, 2018. doi: 10.1002/ppul.24129. [DOI] [PubMed] [Google Scholar]
- 35.Griese M, Kappler M, Gaggar A, Hartl D. Inhibition of airway proteases in cystic fibrosis lung disease. Eur Respir J 32: 783–795, 2008. doi: 10.1183/09031936.00146807. [DOI] [PubMed] [Google Scholar]
- 36.Danahay HL, Lilley S, Fox R, Charlton H, Sabater J, Button B, McCarthy C, Collingwood SP, Gosling M. TMEM16A potentiation: a novel therapeutic approach for the treatment of cystic fibrosis. Am J Respir Crit Care Med 201: 946–954, 2020. doi: 10.1164/rccm.201908-1641OC. [DOI] [PMC free article] [PubMed] [Google Scholar]