Abstract
Leucine-rich repeat-containing 8 (LRRC8) volume-regulated anion channels (VRACs) play important physiological roles in diverse cell types and may represent therapeutic targets for various diseases. To date, however, the pharmacological tools for evaluating the druggability of VRACs have been limited to inhibitors, as no activators of the channel have been reported. We therefore performed a fluorescence-based high-throughput screening (HTS) of 1,184 Food and Drug Administration-approved drugs for compounds that increase VRAC activity. The most potent VRAC potentiator identified was zinc pyrithione (ZPT), which is used commercially as an antifouling agent and for treating dandruff and other skin disorders. In intracellular Yellow Fluorescent Protein YFP(F46L/H148Q/I152L)-quenching assays, ZPT potentiates the rate and extent of swelling-induced iodide influx dose dependently with a half-maximal effective concentration (EC50) of 5.7 µM. Whole cell voltage-clamp experiments revealed that coapplication of hypotonic solution and 30 µM ZPT to human embryonic kidney 293 or human colorectal carcinoma 116 cells increases the rate of swelling-induced VRAC activation by approximately 10-fold. ZPT potentiates swelling-induced VRAC currents after currents have reached a steady state and activates currents in the absence of cell swelling. Neither ZnCl2 nor free pyrithione activated VRAC; however, treating cells with a mixture of ZnCl2 and pyrithione led to robust channel activation. Finally, the effects of ZPT on VRAC were inhibited by reactive oxygen species (ROS) scavenger N-acetylcysteine (NAC) and NAD(P)H oxidase inhibitor diphenyleneiodonium chloride, suggesting the mechanism of action involves ROS generation. The discovery of ZPT as a potentiator/activator of VRAC demonstrates the utility of HTS for identifying small-molecule modulators of VRAC and adds to a growing repertoire of pharmacological tool compounds for probing the molecular physiology and regulation of this important channel.
Keywords: apoptosis, high-throughput screening, LRRC8, reactive oxygen species, VSOR
INTRODUCTION
The preservation of cell volume following osmotic perturbations is essential for survival of cells. Uncontrolled cell swelling can lead to the dilution of intracellular contents, destructive morphological changes, and, eventually, cell rupture (1–3). Cell swelling activates a process known as regulatory volume decrease (RVD), whereby chloride and potassium ions, organic osmolytes (e.g., glutamate, taurine), and osmotically obliged water are transported out of the cell to return cell volume back to its normal state. The volume-regulated anion channel, or VRAC, plays a critical role in RVD and other physiological process related to cell volume, including cell division, migration, and apoptotic cell death (4–7).
Since its initial discovery in T-lymphocytes and human intestinal epithelial cells in 1988, the biophysical, regulatory, and pharmacological properties of native VRAC have been studied extensively (8–11). The channel is activated by hypotonic cell swelling, lowering of intracellular ionic strength, and stimulation of apoptotic cell death. VRAC is inhibited by hypertonic cell shrinkage, depletion of intracellular ATP, and various nonspecific anion channel inhibitors (e.g., 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB), DIDS, niflumic acid). The most potent, efficacious, and selective VRAC inhibitor identified to date is 4-(2-butyl-6,7-dichlor-2-cyclopentylindan-1-on-5-yl) oxybutyric acid or DCPIB. However, even DCPIB has significant off-target activity toward the H-K-ATPase, inward rectifier and two-pore domain potassium channels, connexin hemichannels, glutamate transporters, and mitochondrial respiration (12–17).
The molecular identification of the gene family that encodes VRAC eluded investigators until 2014 when two laboratories independently discovered the leucine-rich repeat-containing 8A (LRRC8A) gene as an essential component of VRAC using genome-wide siRNA screens (18, 19). The LRRC8 gene family comprises five members and gives rise to five paralogs LRRC8A-LRRC8E (20). VRAC can be functionally reconstituted by heterologous expression of the obligatory subunit LRRC8A together with at least one other LRRC8 subunit LRRC8C/D/E (19). The relative expression of LRRC8 subunits and hence the molecular composition of LRRC8 heteromeric channel complex can differ between cell types, giving rise to channels with unique functional and regulatory properties (19, 21).
The identification of the LRRC8 gene family has revitalized the VRAC field, by confirming previously suspected roles and discovering new roles in adipocyte physiology, insulin signaling, vascular physiology, sperm development, drug uptake, immune responses to virus infection, and neurotransmitter release in the brain (22–31). Many of these studies have highlighted the need for developing potent and specific pharmacological tools for probing the physiology and therapeutic potential of VRAC in human diseases. We recently reported the discovery in a high-throughput screening (HTS) of the cysteinyl leukotriene 1 receptor (CysLT1) antagonist pranlukast that inhibits VRAC independently of the CysLT1 receptor (32). This discovery not only adds to the growing toolkit of inhibitors available for studying VRAC but also demonstrates the utility of modern drug discovery approaches for developing VRAC pharmacology.
There have been no small molecule potentiators of VRAC reported to date, which prompted us to interrogate a library of 1,184 Food and Drug Administration (FDA)-approved drugs for compounds that enhance VRAC activity. The most potent potentiator identified in this screen was the antifungal, bacteriostatic compound found in some antidandruff shampoos, zinc pyrithione (ZPT). ZPT activates VRAC activity under isotonic conditions and dramatically enhances the rate of channel activation during hypotonic cell swelling in a reversible manner. Experiments with constituents of ZPT, zinc, or pyrithione alone failed to activate the VRAC. Whereas, reconstitution in a 1:1 molar ratio of zinc and pyrithione was able to recapitulate ZPT-like activation of the channel, suggesting VRAC activation is zinc-pyrithione complex dependent.
MATERIALS AND METHODS
Chemicals
Zinc pyrithione (1-hydroxypyridine-2-thione zinc salt), free zinc pyrithione [1-hydroxy-2(1H)-pyridinethione], and salts of the highest available grade were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Culture
Human embryonic kidney 293 (HEK293) cells were cultured in 75-cm2 flasks with Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. HEK293 cells stably transfected with the YFP(F46L/H148Q/I152L) fluorophore (i.e., HEK293-Ozzy cells) (32) were cultured in the same medium supplemented with 700 µg/mL G418 sulfate (Corning, NY). Human colorectal carcinoma 116 (HCT116) cells were cultured in 75-cm2 flasks with McCoy’s 5A medium supplemented with 10% fetal bovine serum (FBS) and 0.5% penicillin/streptomycin.
Patch-Clamp Electrophysiology
On the day of experiments, HEK293 or HCT116 cells were rinsed with divalent-free Hanks’ balanced salt solution (HBSS), dissociated using 0.25% trypsin/1 mM EDTA for ∼10-s, diluted with complete medium, plated on poly-l-lysine-coated round glass coverslips, and allowed to recover at 37°C in a 5% CO2 incubator for at least 1 h before experiments. Patch electrodes were pulled from 1.5-mm–outer diameter silanized borosilicate microhematocrit tubes; electrode resistance ranged from 1 to 3 MΩ when filled with the following solution (in mM): 126 CsCl, 2 MgSO4, 20 HEPES, 1 EGTA, 2 Na2ATP, 0.5 GTP (pH 7.2 adjusted with CsOH, 275 mOsm. The isotonic bath solution contained (in mM): 75 CsCl, 5 MgSO4, 1 calcium-d-gluconate, 12 HEPES, 8 Tris base, 5 glucose, 2 glutamine, and 95 sucrose (pH 7.4 adjusted with CsOH, 300 mOsm). Hypotonic bath (200–250 mOsm) was made by reducing sucrose in the isotonic bath solution. DMSO, ZPT, and diphenyleneiodonium chloride (DPI) were added directly to bath solutions for experiments. Bath solutions containing NAC were made separately to ensure correct pH and osmolality.
Whole cell currents were recorded under voltage-clamp conditions using an Axopatch 200A (Molecular Devices, Sunnyvale, CA) patch-clamp amplifier. Electrical connections to the patch-clamp amplifier were made by using Ag/AgCl wires and 3 M KCl/agar bridges. Series resistance was compensated by >85% to minimize voltage errors. Data acquisition and analysis were performed by using pClamp 10 software (Axon Instruments).
Cells were voltage clamped at a holding potential of −30 mV, and whole cell currents were elicited by a voltage ramp or step protocol. For voltage ramps, membrane potential was first stepped to −100 mV for 50 ms and then ramped over 1 s to +100 mV. This was followed by a step back to 0 mV for 4 s before this protocol was repeated. Step changes in membrane voltage were induced by stepping membrane voltage to − 120 mV to +120 mV in 2 s, with 20-mV increments. Percent inhibition of compounds was determined by normalizing data to currents inhibited by 10 µM DCPIB.
As previously described (33), the initial rate of swelling-induced VRAC activation was calculated by linear regression analysis of whole cell currents over the first 60 s following current activation. Current activation is defined as the point for which current continuously increases above the current baseline (i.e., current measured with little fluctuation since the start of the experiment).
Cell Volume Measurements
Cell volume measurements were determined as previously described (34). Patch-clamped cells were visualized and image-captured by video-enhanced differential interference contrast microscopy by using a Nikon TE300 microscope and Nikon Plan Fluor ×60/0.7-numerical aperture extra-long working distance lens and a Dage-MTI charged-coupled device camera. The diameter of cells was measured at a single focal plane that showed maximum cell diameter. Cell morphology was assumed to approximate a sphere, and cell volume was calculated as 4/3 × π × r3, where r is the cell radius. Cell volumes were normalized to cell volume recorded 1 mi before solution exchange (i.e., time = −1 min).
Fluorescence Reporter Assay of VRAC Function
HEK293-Ozzy cells were dissociated using 0.25% trypsin/1 mM EDTA and plated at a density of 20,000 cells/well in clear-bottomed, black-walled Corning PureCoat amine-coated 384-well plates (Corning, NY) and cultured overnight at 37°C in a 5% CO2 incubator. The following day, cells were washed with isotonic solution containing (in mM): 140 NaCl, 5 KCl, and HEPES (pH 7.4, 310 mOsm). Compounds from the FDA library were dissolved in hypotonic solution containing (in mM): 5 KCl, 20 HEPES, and 90 mannitol (pH 7.4, 130 mOsm; 165 mOsm solution was used for submaximal activation of VRAC) and added simultaneously to all 384 wells at a final screening concentration of 10 µM. After 5 min allowed for cell swelling and VRAC activation, 100 mM NaI was added to all 384 wells simultaneously, and Ozzy fluorescence was measured at 1 Hz using a Panoptic kinetic imaging plate reader (WaveFront Bioscience, Franklin, TN. Fluorescence values from each well were normalized to baseline readings (i.e., average of the first 5 readings before hypotonic solution is added). Peak fluorescences are local maximum or minimum background-subtracted fluorescence values following NaI addition. Plate background subtraction is performed by averaging all fluorescence traces from the compound plate and subtracting the averaged traced from each compound trace of the same plate. Since a majority of compounds will not be active, this provides a larger sample of background signal. The local maximum or minimum fluorescence following NaI addition is determined and reported as peak fluorescence values. Percent quenching was calculated by normalizing slope values following NaI addition to DMSO control. Data were plotted with GraphPad Prism version 7.03 (GraphPad Software, San Diego, CA) to generate representative experiment traces and concentration-response curves using a nonlinear regression analysis.
Statistics
All data are presented as means ± SE; n represents the number of cells in patch-clamp recordings or the number of wells in fluorescence assays. Statistical significance was determined by using two-way analysis of variance (ANOVA), and Sidak’s multiple-comparisons test was used, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
RESULTS
Discovery of ZPT as a Novel VRAC Potentiator
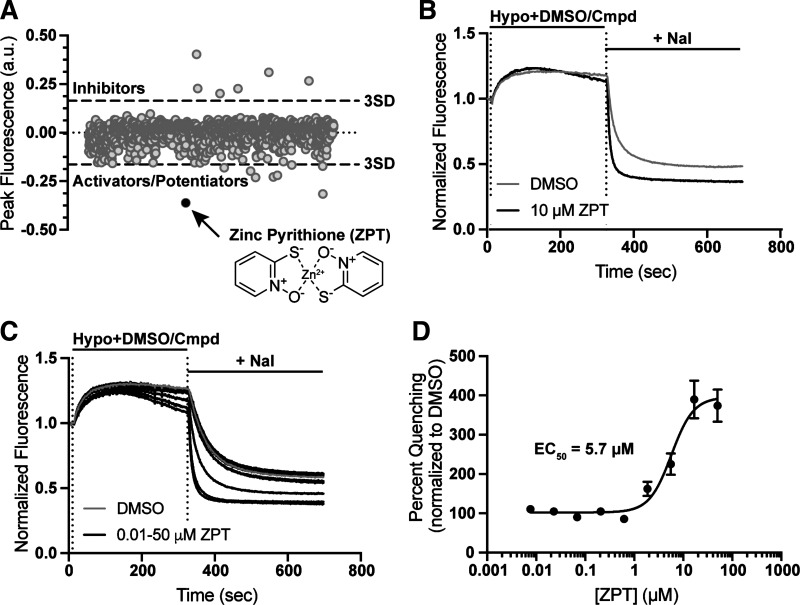
We recently reported the discovery of the CysLT1 receptor antagonist pranlukast as a novel VRAC inhibitor in a screen of the SelleckChem collection of 1,184 FDA-approved drugs (32). The HTS assay used in that screen reports the quenching of the YFP variant Ozzy by iodide as the anion enters osmotically swollen HEK293 cells through endogenously expressed VRACs (see materials and methods). We reasoned that compounds from the FDA library that potentiate VRAC activity should increase the rate and/or extent of iodide-induced fluorescence quenching. Figure 1A shows a scatter plot of the peak fluorescence difference from baseline for all 1,184 wells in the screen. Wells that exhibited a peak fluorescence difference 3 standard deviations away from the mean are shown lying above (inhibitors) or below (activators/potentiators) the dashed lines. Zinc pyrithione (ZPT), which led to the greatest apparent potentiation of VRAC of any compound in the screen, is indicated with a black circle.
Figure 1.
Discovery of zinc pyrithione (ZPT) in a screen of FDA-approved drugs. A: scatter plot of the 1,184 FDA drug compounds screened. A 3 standard deviation from the mean cutoff was implicated to identify compounds as “hits.” Compounds that slowed the rate of fluorescence quenching had positive values, and compounds that increased fluorescence quenching had negative values. ZPT is depicted as a black dot on the scatter plot and as a chemical structure (inset). B: representative traces of Ozzy-quenching assay with 10 µM ZPT. Traces were normalized to mean of five baseline fluorescent measurements taken at the start of the experiment. Hypotonic solution (130 mOsm) containing DMSO (vehicle) or drug compound was added 10 s after the start of the experiment. After 5-min incubation period, 100 mM NaI is added and fluorescence quenching is observed. Fluorescence measurements were taken throughout the entirety of the experiment. C: representative traces of ZPT dose-dependent fluorescence quenching using the Ozzy-quenching assay. D: ZPT concentration-response curve (CRC) generated from Ozzy-quenching assay using an EC50 osmolality solution (165 mOsm; means ± SE EC50 of 5.7 ± 1.1 µM; n = 13). FDA, Food and Drug Administration.
ZPT was ordered as a powder, freshly dissolved in DMSO and then assay buffer, and retested in fluorescence quenching assays. Figure 1B shows that compared with wells treated with the solvent control DMSO, addition of 10 µM ZPT during the 5-min hypotonic stimulus period led to an increase in the rate and extent of iodide-induced quenching. ZPT potentiated Ozzy quenching in a dose-dependent manner when evaluated at concentrations between 0.01 µM and 50 µM. Fitting these data with a four-parameter logistical function generated a 50% activation concentration (EC50) value of 5.7 ± 1.1 µM (Fig. 1, C and D; n = 13).
Characterization of ZPT Activity with Patch-Clamp Electrophysiology
To confirm that ZPT is a bona fide VRAC potentiator and does not enhance iodide-induced Ozzy quenching through some other mechanism unrelated to the channel, we evaluated its ability to potentiate VRAC activity in whole cell patch-clamp experiments. Cells were bathed and patch clamped with cesium chloride-based solutions designed to isolate anion currents from contaminating cation currents. Cell swelling was initiated by reducing bath osmolality from 300 mOsm (isotonic) to 200–250 mOsm (hypoosmotic) by reducing sucrose concentration.
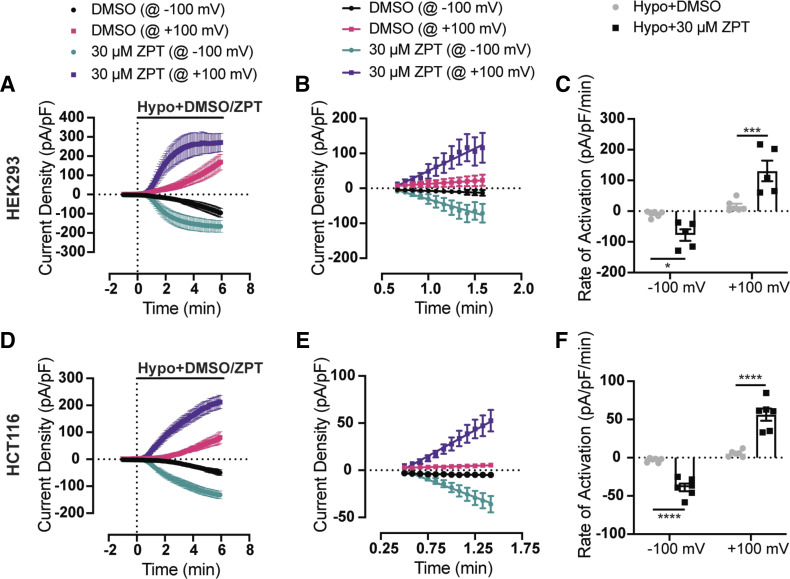
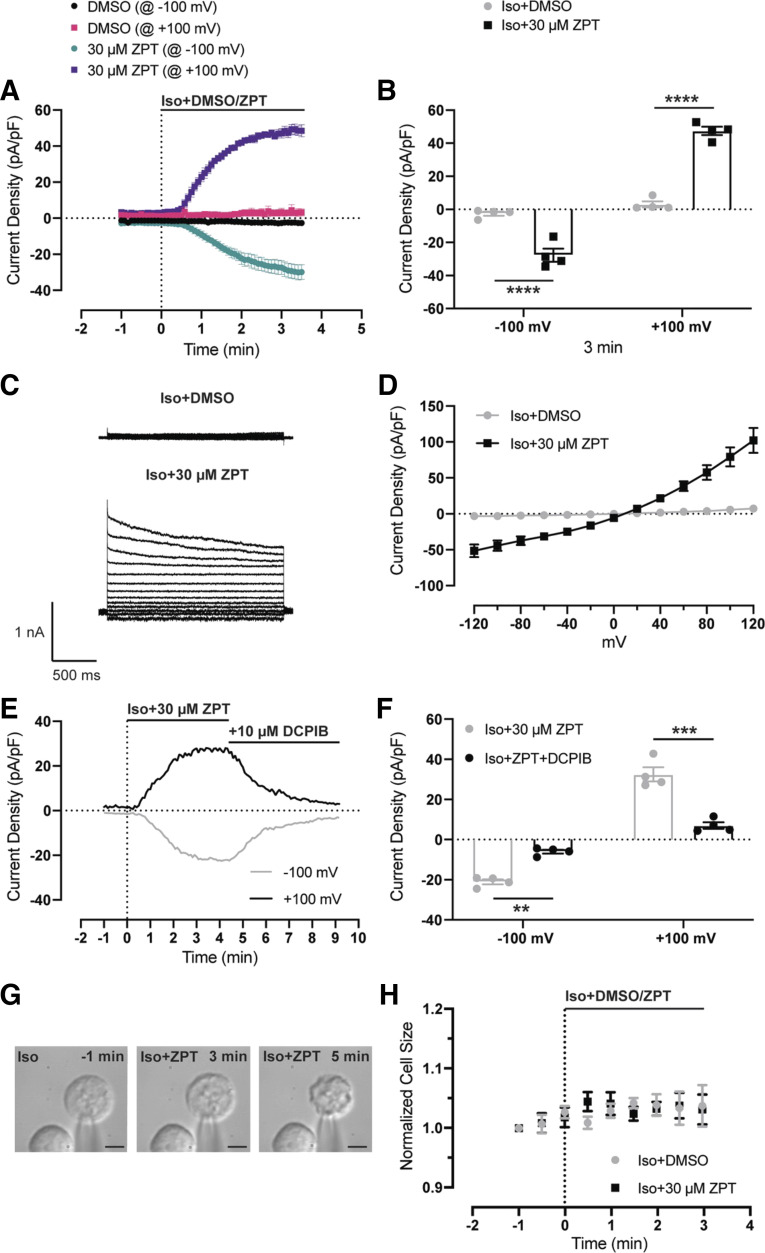
Figure 2A shows the time course of swelling-induced activation of VRAC currents recorded in voltage-ramp experiments at +100 mV or − 100 mV from HEK293 cells treated with a 200 mOsm bath solution containing either DMSO (n = 6) or 30 µM ZPT (n = 5). In contrast to DMSO-treated control cells, in which VRAC currents continue to activate over a 6-min period, VRAC currents in ZPT-treated cells reached a steady state within a few minutes after switching to a hypotonic bath. The initial rate of current activation in the two treatment groups was quantitated by a linear regression fit analysis of current density recorded over the first min of VRAC activation (Fig. 2B). Mean and individual activation rates ± SE are shown in Fig. 2C. VRAC in ZPT-treated cells activated approximately eightfold faster than that in DMSO-treated cells. These differences were significantly different between the two groups at −100 mV (P < 0.05) and +100 mV (P < 0.001) (Fig. 2C and Table 1). ZPT increased the rate of current activation significantly at both −100 mV (P < 0.05) and +100 mV (P < 0.05) by greater than 10-fold when cells were swollen with a milder hypotonic stimulus (i.e., 250 mOsm) (Table 1). Because subunit composition and hence regulatory properties of LRRC8-mediated VRAC channels can vary between cell types (19, 21), we tested whether ZPT could also potentiate VRAC in HCT116 colorectal carcinoma cells. As shown in Fig. 2, D–F, 30 µM led to a significant (P < 0.05) potentiation of swelling-induced VRAC activation rate compared with that seen in DMSO-treated cells. Therefore, the effect of ZPT on VRAC does not appear to be cell type specific.
Figure 2.
ZPT potentiates the rate of activation of swelling-activated VRAC currents. A: time course of swelling-activated whole cell Cl− currents from HEK293 cells. Currents were measured using a ramp protocol from − 100 mV to +100 mV. Cells are exposed to a hypotonic bath (200 mOsm) containing DMSO (vehicle) (n = 6) or 30 μM ZPT (n = 5) at time = 0 min. B: fitting initial current activation at − 100 mV and +100 mV of DMSO-treated (n = 6) and ZPT-treated (n = 5) cells with linear regression lines. C: summary of rate of activation at − 100 mV and +100 mV for individual DMSO-treated (@ −100 mV means ± SE −9.2 ± 4.1 pA/pF/min, @ +100 mV means ± SE 16.6 ± 7.6 pA/pF/min; n = 6) and ZPT-treated cells (@ −100 mV means ± SE −77.6 ± 18.9 pA/pF/min @ +100 mV means ± SE 130 ± 33.4 pA/pF/min; n = 5). D: time course of swelling-activated whole cell Cl− currents from HCT116 cells. Currents were measured using a ramp protocol from −100 mV to +100 mV. Cells are exposed to a hypotonic bath (250 mOsm) containing DMSO (vehicle) (n = 6) or 30 μM ZPT (n = 6) at time = 0 min. E: fitting initial current activation at −100 mV and +100 mV of DMSO-treated (n = 6) and ZPT-treated (n = 6) cells with linear regression lines. F: summary of rate of activation at −100 mV and +100 mV for individual DMSO-treated (@−100 mV means ± SE −3.2 ± 1.2 pA/pF/min, @ +100 mV means ± SE 5.4 ± 1.7 pA/pF/min; n = 6) and ZPT-treated cells (@ −100 mV means ± SE −38.9 ± 1.7 pA/pF/min @ +100 mV means ± SE 56.2 ± 7.9 pA/pF/min; n = 6). Data were analyzed by two-way ANOVA and Sidak’s multiple-comparisons test, *P < 0.05, ***P < 0.001, ****P < 0.0001. HCT, human colorectal carcinoma; HEK, human embryonic kidney; VRAC, volume-regulated anion channels; ZPT, zinc pyrithione.
Table 1.
The effect of 30 μM zinc pyrithione on rates of current activation for endogenously expressed VRACs in HEK293 and HCT116 cells
| Cell Type | Hypo Bath Solution; Osmolarity | Rate of Activation @ −100 mV, pA/pF/min (n) | Rate of Activation @ +100 mV, pA/pF/min (n) |
|---|---|---|---|
| HEK293 | DMSO; 250 mOsm | − 3.7 ± 1.6 (6) | 6.0 ± 2.5 (6) |
| HEK293 | 30 μM ZPT; 250 mOsm | − 44.3 ± 15.5 (6) | 68.1 ± 25.0 (6) |
| HEK293 | DMSO; 200 mOsm | − 9.2 ± 4.1 (6) | 16.6 ± 7.6 (6) |
| HEK293 | 30 μM ZPT; 200 mOsm | − 77.6 ± 18.9 (5) | 130 ± 33.4 (5) |
| HCT116 | DMSO; 250 mOsm | − 3.2 ± 1.2 (6) | 5.4 ± 1.7 (6) |
| HCT116 | 30 μM ZPT; 250 mOsm | − 38.9 ± 1.7 (6) | 56.2 ± 7.9 (6) |
Values are means ± SE (n). Swelling was induced 1 min after obtaining whole cell access by exchanging isotonic bath solution (300 mOsm) with specified hypotonic bath solution. HCT, human colorectal carcinoma; HEK, human embryonic kidney; VRAC, volume-regulated anion channels; ZPT, zinc pyrithione.
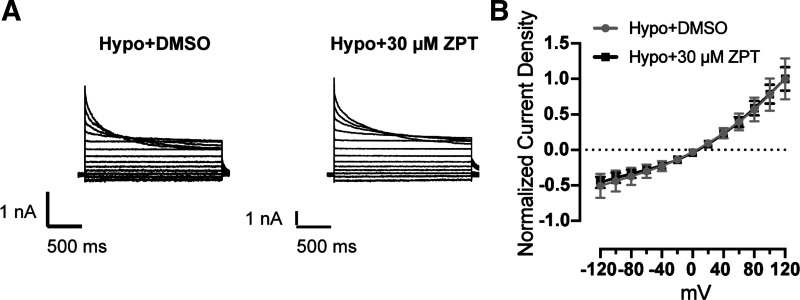
Voltage-step experiments in which cells are voltage clamped between −120 mV and +120 mV in 20-mV increments were performed to characterize the functional properties of the currents potentiated by ZPT. Cells were swollen with a hypotonic solution containing either DMSO (control) or 30 µM ZPT until whole cell currents reached a steady state. As shown in Fig. 3A, the voltage- and time-dependent inactivation properties of currents recorded from control- and ZPT-treated cells were virtually identical and displayed moderate outward rectification and inactivation at positive voltages—characteristic of VRAC. Furthermore, the normalized current-voltage relationships shown in Fig. 3B indicate that ZPT had no effect on the reversal potential and hence selectivity properties of the currents. These data strongly support the notion that ZPT is a potentiator of VRAC.
Figure 3.
Current-voltage (I–V) relationship of swelling-activated currents in the presence of DMSO or ZPT. A: whole cell currents from HCT116 cells were measured using a step protocol from −120 mV to +120 mV, with 20-mV steps. Cells were exposed to hypotonic bath (250 mOsm) containing DMSO (vehicle) or 30 μM ZPT. B: I–V plot from currents recorded in A (n = 4). Current density was normalized to currents at +120 mV. HCT, human colorectal carcinoma; ZPT, zinc pyrithione.
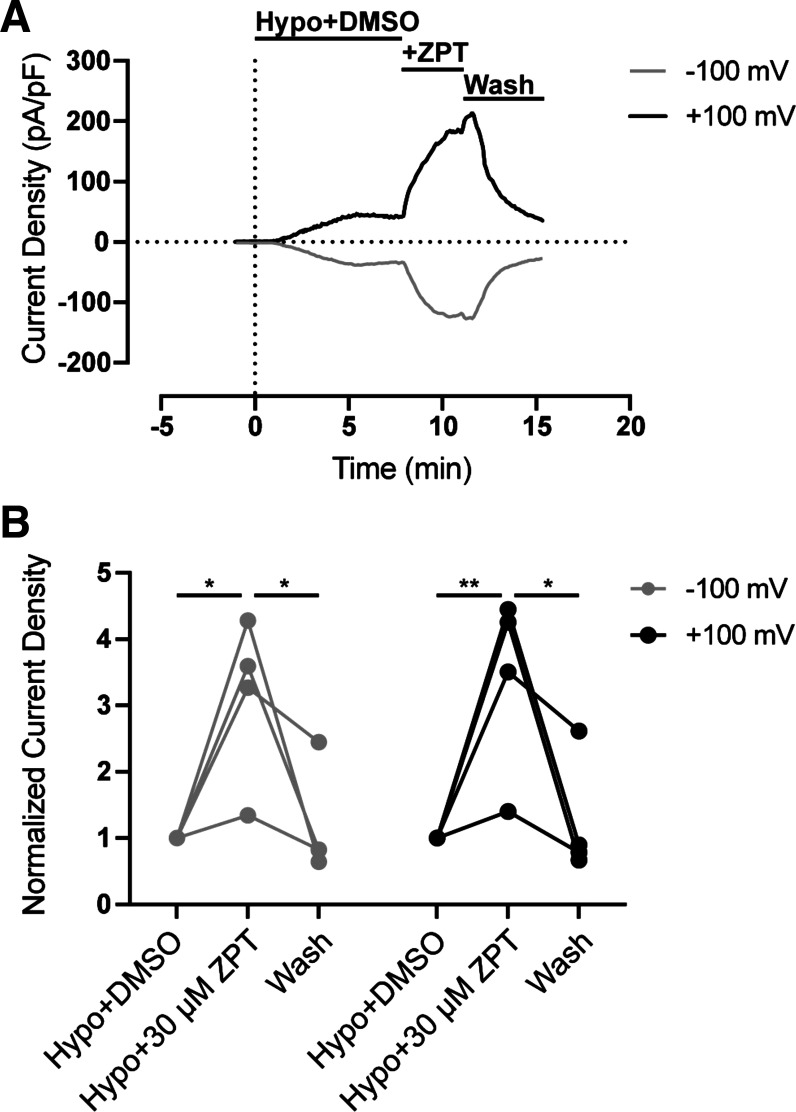
We questioned if ZPT potentiates the same regulatory process that activates VRAC during hypotonic cell swelling or if it acts through a distinct molecular mechanism. To address this question, we swelled HCT116 cells with hypotonic buffer (250 mOsm) containing DMSO (control) until VRAC currents reached a steady state and then applied 30 µM ZPT in the continuous presence of a hypotonic bath. As shown in Fig. 4, A and B, bath application of ZPT led to a striking and significant (P < 0.05) potentiation at −100 mV and +100 mV and was fully reversible (P < 0.05) following washout of ZPT.
Figure 4.
ZPT potentiates swelling-activated VRAC currents. A: representative time course of swelling-activated whole cell Cl− currents from HCT116 cells (n = 4). Currents were measured using a ramp protocol from −100 mV to +100 mV. Cells were exposed to a hypotonic bath (250 mOsm) containing DMSO at time = 0. Solution exchange with a hypotonic bath containing 30 μM ZPT occurred once steady state was reached (∼time = 7 min). Solution exchange with a hypotonic bath containing DMSO (wash) occurred once steady state with ZPT was reached (∼time = 12 min). B: summary of steady-state current density from A normalized to Hypo+DMSO. Data were analyzed by two-way ANOVA and Sidak’s multiple-comparisons test, *P < 0.05, **P < 0.01. HCT, human colorectal carcinoma; ZPT, zinc pyrithione.
ZPT Activates VRAC in the Absence of Hypotonic Cell Swelling
We next determined if ZPT can activate VRAC currents in the absence of hypotonic cell swelling. HCT116 cells were treated with either DMSO (control) or 30 µM ZPT in isotonic (300 mOsm) bath solution while voltage ramping the membrane potential between −100 mV and +100 mV. Apparent cell volumes were recorded by DIC video microscopy and calculated as described in materials and methods. As summarized in Fig. 5, A and B, ZPT significantly (P < 0.001) activated currents at −100 mV and +100 mV that reached a steady state within 3 min following ZPT addition. The currents activated by ZPT exhibited voltage- and time-dependent properties consistent with VRAC (Fig. 5, C and D) and were inhibited by the VRAC inhibitor DCPIB (Fig. 5, E and F). There was no significant difference in the volumes of DMSO- and ZPT-treated cells (Fig., 5, G and H). Thus, ZPT activates VRAC independently of a cell swelling-mediated mechanism.
Figure 5.
ZPT activates VRAC currents under isotonic conditions. A: time course of whole cell Cl− currents from HCT116 cells. Currents were measured using a ramp protocol from −100 mV to +100 mV. Cells are exposed to an isotonic bath (300 mOsm) containing DMSO (vehicle) (n = 4) or 30 μM ZPT (n = 4) at time = 0 min. B: summary of current density of DMSO-treated and ZPT-treated cells from −100 mV and +100 mV at time = 3 min. Data were analyzed by two-way ANOVA and Sidak’s multiple-comparisons test, ****P < 0.0001. C: whole cell currents from HCT116 cells were measured using a step protocol from −120 mV to +120 mV, with 20-mV steps. Cells were exposed to an isotonic bath (300 mOsm) containing DMSO (vehicle) or 30 μM ZPT. D: I–V plot from currents recorded in C [DMSO (n = 3); ZPT (n = 5)]. E: representative time course of ZPT-activated and DCPIB-inhibitable whole cell Cl− currents from HCT116 cells (n = 4). Currents were measured using a ramp protocol from −100 mV to +100 mV. Cells were exposed to a hypotonic bath (250 mOsm) containing 30 μM ZPT at time = 0. Solution exchange with a hypotonic bath containing 30 μM ZPT and 10 μM DCPIB occurred once steady state was reached (∼time = 4 min). F: summary of steady-state current density from E. Data were analyzed by two-way ANOVA and Sidak’s multiple-comparisons test, ***P < 0.001, ****P < 0.0001. G: images from a ZPT-treated cell at time = −1 min, +3 min, and +5 min. H: time course of cell volume measurements from HCT116 cells. Cells were exposed to an isotonic bath (300 mOsm) containing DMSO (n = 3) or 30 µM ZPT (n = 3) at time = 0 min. Data were analyzed by two-way ANOVA and Sidak’s multiple-comparisons test, not significant. DCPIB, 4-(2-butyl-6,7-dichlor-2-cyclopentylindan-1-on-5-yl) oxobutyric acid; HCT, human colorectal carcinoma; n.s., not significant; VRAC, volume-regulated anion channels; ZPT, zinc pyrithione.
VRAC Activation Requires Both Zn2+and Pyrithione
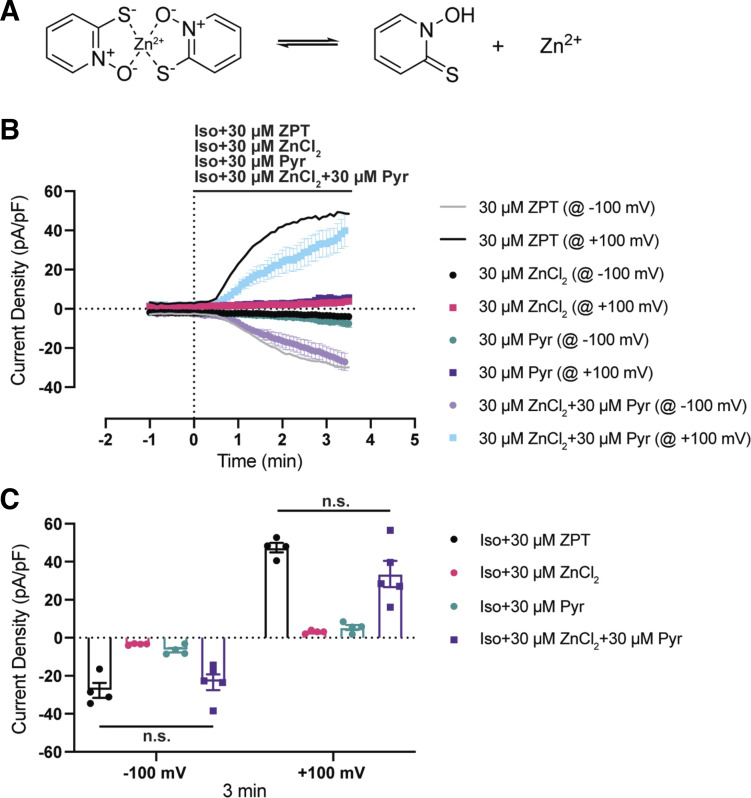
In solution, ZPT can dissociate into free pyrithione and Zn2+ (Fig. 6), raising the possibility that the effects of ZPT on VRAC are mediated through free pyrithione, free Zn2+, and/or the undissociated Zn2+-pyrithione complex. We set out to determine which form is responsible for activating VRAC by treating unswollen HCT116 cells bathed with 30 µM free pyrithione (n = 4), 30 µM ZnCl2 (n = 4), or pyrithione premixed with ZnCl2 (30 µM each; n = 5) in an isotonic bath. As shown in Fig. 6A, neither free pyrithione nor ZnCl2 led to activation of VRAC resembling that observed with ZPT (black line). However, adding a mixture of free pyrithione and ZnCl2 caused robust VRAC activation that was not significantly different from that induced by ZPT (Fig. 6B). Taken together, these data suggest that activation of VRAC requires either Zn2+ and pyrithione as separate entities or a pyrithione-Zn2+ complex.
Figure 6.
VRAC activation requires both zinc and pyrithione. A: a cartoon schematic showing ZPT existing in equilibrium as a complex and dissociated as free pyrithione and Zn2+. B: time course of whole cell Cl− currents from HCT116 cells. Currents were measured using a ramp protocol from −100 mV to +100 mV. Cells are exposed to an isotonic bath (300 mOsm) containing 30 μM ZPT (n = 4), 30 μM ZnCl2 (n = 4), 30 µM pyrithione (Pyr; n = 4), or 30 μM ZnCl2 +30 μM Pyr (n = 5) at time = 0 min. Iso+ZPT data are originally shown in Fig. 5. Average is depicted by solid lines. C: summary of current density of ZPT-treated, ZnCl2-treated, Pyr-treated, and ZnCl2+Pyr-treated cells from −100 mV and +100 mV at time = 3 min. Data were analyzed by two-way ANOVA and Sidak’s multiple-comparisons test. HCT, human colorectal carcinoma; n.s., not significant; VRAC, volume-regulated anion channels; ZPT, zinc pyrithione.
Activation of VRAC with ZPT Is Inhibited by Antioxidants
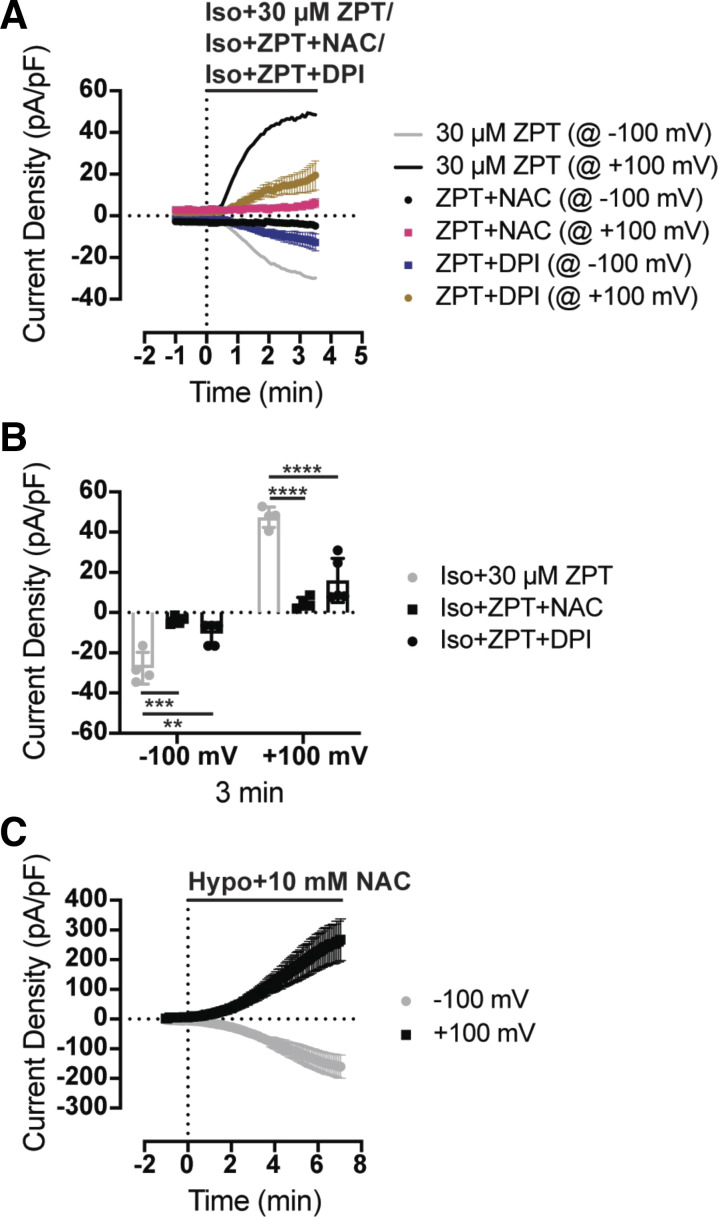
ZPT is reported to induce apoptosis through reactive oxygen species (ROS)-dependent mechanisms in several different cell types (35, 36). Induction of apoptosis triggers regulatory volume decrease (RVD) mediated in part through activation of VRAC currents. Okada and colleagues showed that induction of VRAC activation, RVD, and apoptosis in HeLa cells are inhibited by the ROS scavenger N-acetylcysteine (NAC) and NAD(P)H oxidase inhibitor diphenylene-iodonium (DPI). We therefore tested whether NAC or DPI could inhibit the effects of ZPT on VRAC. HCT116 cells were patch clamped in an isotonic bath solution and then treated with 30 µM ZPT in the presence of 10 mM NAC or 20 µM DPI in an isotonic bath. As shown in Fig. 7, A and B, both NAC and DPI significantly reduced the effects of ZPT on VRAC activation. Because NAC is used at high concentrations, we confirmed that it did not have a general effect on VRAC by demonstrating robust swelling-induced activation of VRAC in the presence of 10 mM NAC. These data support a model in which ZPT activates VRAC through an ROS-dependent mechanism.
Figure 7.
ZPT activates VRAC in an antioxidant-sensitive manner. A: time course of whole cell Cl− currents from HCT116 cells. Currents were measured using a ramp protocol from −100 mV to +100 mV. Cells are exposed to an isotonic bath (300 mOsm) containing 30 μM ZPT (n = 4), 30 μM ZPT + 10 mM NAC (n = 4), or 30 μM ZPT + 20 μM DPI (n = 5) at time = 0 min. Iso+ZPT data are originally shown in Fig. 5. Average is depicted by solid lines. B: summary of current density of ZPT-treated, ZPT+NAC-treated, and ZPT+DPI-treated cells from −100 mV and +100 mV at time = 3 min. C: time course of whole cell Cl− currents from HCT116 cells. Currents were measured using a ramp protocol from −100 mV to +100 mV. Cells are exposed to a hypotonic bath (250 mOsm) containing 10 mM NAC (n = 4). Data were analyzed by two-way ANOVA and Sidak’s multiple-comparisons test, **P < 0.01, ***P < 0.001, ****P < 0.0001. DPI, diphenyleneiodonium chloride; HCT, human colorectal carcinoma; VRAC, volume-regulated anion channels; ZPT, zinc pyrithione.
DISCUSSION
The major finding of this study is the discovery that ZPT, an over-the-counter medication used to treat common skin disorders such as dandruff, eczema, psoriasis, and acne, activates endogenous VRAC currents expressed in human cells. Whether VRAC activation mediates some of the medicinal benefits of ZPT is not yet unknown. The actions of ZPT on VRAC reach steady state within a few minutes and occur at low micromolar concentrations. When cells are treated simultaneously with hypotonic buffer and ZPT, the rate of swelling-induced VRAC activation increases by ∼10-fold compared with that of DMSO-treated cells. Adding ZPT to swollen cells in which VRAC currents have already reached a steady activates VRAC further, suggesting that ZPT does not simply enhance the same regulatory process that underlies swelling-induced activation. This notion is supported by observations that ZPT activates VRAC in the absence of cell swelling. Taken together, these data support the conclusion that hypotonic cell swelling and ZPT activate VRAC through distinct molecular mechanisms.
There is growing interest in the use of ZPT as a potential anticancer agent because it induces apoptosis in acute myeloid leukemia, oral squamous cell carcinoma, and prostate cancer cell lines (35–38). Histological analysis of noncancerous psoriatic plaques treated with topical ZPT revealed a dramatic increase in epidermal apoptotic bodies over a 48-h period (39). In HepG2 hepatoma cells, ZPT reduces cell viability after 12 h at concentrations between 0.5 and 5 µM, coincident with induction of classical markers of mitochondria-mediated apoptosis. One of the earliest cellular responses to ZPT reported was an increase in ROS production at 3 h at 0.5 µM and 1.0 µM ZPT, suggesting that induction of apoptosis is a result of oxidative damage. Consistent with this hypothesis, the ROS scavenger NAC and other exogenously applied antioxidants were shown to rescue cell viability and prevent induction of mitochondrial apoptotic markers caused by ZPT (36).
Our findings that NAC and the NAD(P)H oxidase inhibitor DPI inhibit VRAC activation suggest that ROS may play a role in ZPT’s mechanism of action. Indeed, both exogenous and endogenous ROS are widely known to modulate VRAC function. For example, oxidative stress induced by exogenous application of H2O2 activates VRAC in diverse cells types (40–42). In HeLA cells, stimulation of mitochondrial-mediated apoptosis with staurosporine leads to rapid (∼5 min) activation of VRAC (42) and generation of intracellular ROS over a similar time course. Both NAC and DPI almost completely block staurosporine-induced VRAC activation (42). Mechanical stimulation of ventricular myocytes activates VRAC currents following release of angiotensin II, stimulation of type I receptors, and activation of sarcolemmal NAD(P)H oxidase, the major source of endogenous H2O2 in these cells. Activation of VRAC with mechanical stimulation is completely blocked by DPI, suggesting a key role of NAD(P)H-mediated H2O2 in the mechanism of action (40). Recent evidence in support of this model is the discovery that the essential VRAC subunit LRRC8A colocalizes and coimmunoprecipitates with the NAD(P)H oxidase membrane subunits Nox2, Nox4, and p22phox (43). There appears to be a reciprocal regulation between VRAC and NAD(P)H oxidase systems, as both angiotensin II-induced cardiac hypertrophy and TNF-α-induced vascular inflammation are dependent on LRRC8A expression (24, 43). The impact of oxidative stress on VRAC function might vary with cell type due to cell type-specific expression of LRRC8 heteromeric subtypes. Pusch and colleagues (44) demonstrated that ROS induced with millimolar concentrations of chloramine-T led to activation of heterologously expressed LRRC8A/E channels but inhibited channels comprising LRRC8A/C and LRRC8A/D subunits. Activation of LRRC8A/E channels appears to be mediated by oxidation of intracellular cysteine residues, whereas inhibition of LRRC8A/C and LRRC8A/D is more complex. It is plausible that ZPT activates VRAC in HEK and HCT116 cells due to dominant expression of LRRC8A/8E channels; however, LRRC8C and LRRC8D subunits are also expressed in HEK and HCT116 cells. This raises the possibility that these subunits may differentially contribute to channel activation by ZPT.
Adding free pyrithione or Zn2+ individually to unswollen HCT116 cells had no effect on VRAC, whereas adding a mixture of the two compounds led to robust channel activation. The simplest interpretation of these data is that the Zn2+-pyrithione complex is required for ZPT’s mechanism of action. The cytotoxic effects of ZPT on various microorganisms and mammalian cells is widely believed to be initiated by an increase in intracellular Zn2+ concentration and disruption of cellular Zn2+ homeostasis (36, 45–48). The pyrithione ligand appears to function as a membrane ionophore that permits the movement of Zn2+ into the cell. For example, exposure of malignant human keratinocytes lines to 10 µM ZPT leads to an ∼50-fold increase in free Zn2+ concentrations between 10 and 30 min after treatment, with the first significant increases in intracellular Zn2+ being detected as early as 30 s after exposure. The ensuing Zn2+ loading is associated with oxidative stress, mitochondrial dysfunction, and cell death (49). Similarly, ZPT-induced apoptosis in HepG2 cells requires elevation of intracellular Zn2+, as treatment of cells with Na+-pyrithione or pyrithione analogs that lack Zn2+ binding capacity fails to induce apoptosis, and chelation of intracellular Zn2+ after ZPT treatment prevents induction of apoptosis (36). Taken together, these data support a model in which ZPT activates VRAC via increased intracellular Zn2+ concentration and induction of apoptosis. The role of these two processes in activation of VRAC will be systematically explored in future studies.
It is also conceivable that ZPT activates VRAC at least in part through direct interactions with the LRRC8 channel complex. ZPT was identified as a potentiator of heterologously expressed KCNQ2 (Kv7.2) potassium channels in an HTS of ∼20,000 compounds. ZPT exhibits an EC50 of ∼2 µM and is mediated through a ∼25-mV hyperpolarizing shift in the activation voltage and increase in open-state probability of the channel. Mutagenesis analysis identified three amino acids on transmembrane domains S5 and S6 that appear to participate in ZPT’s mechanism of action on homotetrameric KCNQ2. Importantly, ZPT can potentiate the function of KCNQ2 channels carrying loss-of-function mutations that underlie benign familial neonatal seizures (50). Using site-directed mutagenesis approaches to identify potential binding sites of ZPT in LRRC8 channels is complicated by the heteromeric nature of the channel as well as the unknown stoichiometry and order of LRRC8 subunit arrangement in the channel complex. Recently, Strange and colleagues developed a series of LRRC8A, LRRC8C, LRRC8D, and LRRC8E chimeras that can be functionally expressed as homomeric channels that exhibit regulatory and pharmacological properties resembling native VRAC. These chimeras may be useful for determining whether ZPT acts directly on the channel complex or indirectly through mechanisms involving oxidative stress.
In conclusion, we have used an HTS assay to identify a novel compound, ZPT, which potentiates VRAC activity in cultured mammalian cells. Given the newly emerging roles of VRAC in insulin secretion, adipocyte biology, sperm development, vascular biology, and innate immunity, as well as the availability of molecular tools for exploring VRAC structure-function-pharmacology relationships, the development of a diverse pharmacological toolkit of inhibitors and activators will facilitate studies aimed at probing the druggability and molecular pharmacology of this important channel family.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 1F31DK120225-01 (to E. Figueroa) and unrestricted funds (to J. Denton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.E.F. and J.S.D. conceived and designed research; E.E.F. performed experiments; E.E.F. analyzed data; E.E.F. and J.S.D. interpreted results of experiments; E.E.F. and J.S.D. prepared figures; E.E.F. and J.S.D. drafted manuscript; E.E.F. and J.S.D. edited and revised manuscript; E.E.F. and J.S.D. approved final version of manuscript.
REFERENCES
- 1.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev 89: 193–277, 2009. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 2.Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol Cell Physiol 270: C711–C730, 1996. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- 3.Wehner F, Olsen H, Tinel H, Kinne-Saffran E, Kinne RK. Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev Physiol Biochem Pharmacol 148: 1–80, 2003. doi: 10.1007/s10254-003-0009-x. [DOI] [PubMed] [Google Scholar]
- 4.Jentsch TJ. VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat Rev Mol Cell Biol 17: 293–307, 2016. doi: 10.1038/nrm.2016.29. [DOI] [PubMed] [Google Scholar]
- 5.Okada Y, Numata T, Sato-Numata K, Sabirov RZ, Liu H, Mori SI, Morishima S. Roles of volume-regulatory anion channels, VSOR and Maxi-Cl, in apoptosis, cisplatin resistance, necrosis, ischemic cell death, stroke and myocardial infarction. Curr Top Membr 83: 205–283, 2019. doi: 10.1016/bs.ctm.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Osei-Owusu J, Yang J, Vitery MDC, Qiu Z. Molecular biology and physiology of volume-regulated anion channel (VRAC). Curr Top Membr 81: 177–203, 2018. doi: 10.1016/bs.ctm.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strange K, Yamada T, Denton JS. A 30-year journey from volume-regulated anion currents to molecular structure of the LRRC8 channel. J Gen Physiol 151: 100–117, 2019. doi: 10.1085/jgp.201812138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahalan MD, Lewis RS. Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc Gen Physiol Ser 43: 281–301, 1988. [PubMed] [Google Scholar]
- 9.Friard J, Tauc M, Cougnon M, Compan V, Duranton C, Rubera I. Comparative effects of chloride channel inhibitors on LRRC8/VRAC-mediated chloride conductance. Front Pharmacol 8: 328, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazama A, Okada Y. Ca 2+ sensitivity of volume-regulatory K + and Cl - channels in cultured human epithelial cells. J Physiol 402: 687–702, 1988. doi: 10.1113/jphysiol.1988.sp017229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato-Numata K, Numata T, Inoue R, Okada Y. Distinct pharmacological and molecular properties of the acid-sensitive outwardly rectifying (ASOR) anion channel from those of the volume-sensitive outwardly rectifying (VSOR) anion channel. Pflugers Arch 468: 795–803, 2016. doi: 10.1007/s00424-015-1786-1. [DOI] [PubMed] [Google Scholar]
- 12.Afzal A, Figueroa EE, Kharade SV, Bittman K, Matlock BK, Flaherty DK, Denton JS. The LRRC8 volume-regulated anion channel inhibitor, DCPIB, inhibits mitochondrial respiration independently of the channel. Physiol Rep 7: e14303, 2019. doi: 10.14814/phy2.14303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Best L, Yates AP, Decher N, Steinmeyer K, Nilius B. Inhibition of glucose-induced electrical activity in rat pancreatic beta-cells by DCPIB, a selective inhibitor of volume-sensitive anion currents. Eur J Pharmacol 489: 13–19, 2004. doi: 10.1016/j.ejphar.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 14.Bowens NH, Dohare P, Kuo YH, Mongin AA. DCPIB, the proposed selective blocker of volume-regulated anion channels, inhibits several glutamate transport pathways in glial cells. Mol Pharmacol 83: 22–32, 2013. doi: 10.1124/mol.112.080457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng W, Mahajan R, Baumgarten CM, Logothetis DE. The ICl, swell inhibitor DCPIB blocks Kir channels that possess weak affinity for PIP2. Pflugers Arch 468: 817–824, 2016. doi: 10.1007/s00424-016-1794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii T, Takahashi Y, Takeshima H, Saitoh C, Shimizu T, Takeguchi N, Sakai H. Inhibition of gastric H+, K+-ATPase by 4-(2-butyl-6,7-dichloro-2-cyclopentylindan-1-on-5-yl)oxybutyric acid (DCPIB), an inhibitor of volume-regulated anion channel. Eur J Pharmacol 765: 34–41, 2015. doi: 10.1016/j.ejphar.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Minieri L, Pivonkova H, Caprini M, Harantova L, Anderova M, Ferroni S. The inhibitor of volume-regulated anion channels DCPIB activates TREK potassium channels in cultured astrocytes. Br J Pharmacol 168: 1240–1254, 2013. doi: 10.1111/bph.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 157: 447–458, 2014. doi: 10.1016/j.cell.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 344: 634–638, 2014. doi: 10.1126/science.1252826. [DOI] [PubMed] [Google Scholar]
- 20.Abascal F, Zardoya R. LRRC8 proteins share a common ancestor with pannexins, and may form hexameric channels involved in cell-cell communication. Bioessays 34: 551–560, 2012. doi: 10.1002/bies.201100173. [DOI] [PubMed] [Google Scholar]
- 21.Jentsch TJ, Lutter D, Planells-Cases R, Ullrich F, Voss FK. VRAC: molecular identification as LRRC8 heteromers with differential functions. Pflugers Arch 468: 385–393, 2016. [DOI] [PubMed] [Google Scholar]
- 22.Alghanem AF, Ta C, Maurer JM, Gunasekar SK, Kumar A, Fatima U, Kang C, Xie L, Adeola O, Abello J, Riker M, Elliot-Hudson M, Minerath RA, Stratman A, Grueter CE, Mullins RF, Sah R. The SWELL1-LRRC8 complex regulates endothelial AKT-eNOS-mTOR signaling and vascular function. eLife 10: e61313, 2021. doi: 10.7554/eLife.61313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao J, Perez CJ, Kim J, Zhang H, Murphy CJ, Hamidi T, Jaubert J, Platt CD, Chou J, Deng M, Zhou MH, Huang Y, Gaitan-Penas H, Guenet JL, Lin K, Lu Y, Chen T, Bedford MT, Dent SY, Richburg JH, Estevez R, Pan HL, Geha RS, Shi Q, Benavides F. Deficient LRRC8A-dependent volume-regulated anion channel activity is associated with male infertility in mice. JCI Insight 3: e99767, 2018. doi: 10.1172/jci.insight.99767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Choi H, Ettinger N, Rohrbough J, Dikalova A, Nguyen HN, Lamb FS. LRRC8A channels support TNFalpha-induced superoxide production by Nox1 which is required for receptor endocytosis. Free Radic Biol Med 101: 413–423, 2016. doi: 10.1016/j.freeradbiomed.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang C, Xie L, Gunasekar SK, Mishra A, Zhang Y, Pai S, Gao Y, Kumar A, Norris AW, Stephens SB, Sah R. SWELL1 is a glucose sensor regulating beta-cell excitability and systemic glycaemia. Nat Commun 9: 367, 2018. doi: 10.1038/s41467-017-02664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luck JC, Puchkov D, Ullrich F, Jentsch TJ. LRRC8/VRAC anion channels are required for late stages of spermatid development in mice. J Biol Chem 293: 11796–11808, 2018. doi: 10.1074/jbc.RA118.003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Planells-Cases R, Lutter D, Guyader C, Gerhards NM, Ullrich F, Elger DA, Kucukosmanoglu A, Xu G, Voss FK, Reincke SM, Stauber T, Blomen VA, Vis DJ, Wessels LF, Brummelkamp TR, Borst P, Rottenberg S, Jentsch TJ. Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J 34: 2993–3008, 2015. doi: 10.15252/embj.201592409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuhlmann T, Planells-Cases R, Jentsch TJ. LRRC8/VRAC anion channels enhance beta-cell glucose sensing and insulin secretion. Nat Commun 9: 1974, 2018. doi: 10.1038/s41467-018-04353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Vitery MDC, Chen J, Osei-Owusu J, Chu J, Qiu Z. Glutamate-releasing SWELL1 channel in astrocytes modulates synaptic transmission and promotes brain damage in stroke. Neuron 102: 813–827, 2019. doi: 10.1016/j.neuron.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Xie L, Gunasekar SK, Tong D, Mishra A, Gibson WJ, Wang C, Fidler T, Marthaler B, Klingelhutz A, Abel ED, Samuel I, Smith JK, Cao L, Sah R. SWELL1 is a regulator of adipocyte size, insulin signaling and glucose homeostasis. Nat Cell Biol 19: 504–517, 2017. doi: 10.1038/ncb3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou C, Chen X, Planells-Cases R, Chu J, Wang L, Cao L, Li Z, Lopez-Cayuqueo KI, Xie Y, Ye S, Wang X, Ullrich F, Ma S, Fang Y, Zhang X, Qian Z, Liang X, Cai SQ, Jiang Z, Zhou D, Leng Q, Xiao TS, Lan K, Yang J, Li H, Peng C, Qiu Z, Jentsch TJ, Xiao H. Transfer of cGAMP into bystander Cells via LRRC8 volume-regulated anion channels augments STING-mediated interferon responses and anti-viral immunity. Immunity 52: 767–781, 2020. doi: 10.1016/j.immuni.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Figueroa EE, Kramer M, Strange K, Denton JS. CysLT1 receptor antagonists pranlukast and zafirlukast inhibit LRRC8-mediated volume regulated anion channels independently of the receptor. Am J Physiol Cell Physiol 317: C857–C866, 2019. doi: 10.1152/ajpcell.00281.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bond T, Basavappa S, Christensen M, Strange K. ATP dependence of the ICl, swell channel varies with rate of cell swelling: evidence for two modes of channel activation. J Gen Physiol 113: 441–456, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada T, Strange K. Intracellular and extracellular loops of LRRC8 are essential for volume-regulated anion channel function. J Gen Physiol 150: 1003–1015, 2018. doi: 10.1085/jgp.201812016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carraway RE, Dobner PR. Zinc pyrithione induces ERK- and PKC-dependent necrosis distinct from TPEN-induced apoptosis in prostate cancer cells. Biochim Biophys Acta 1823: 544–557, 2012. doi: 10.1016/j.bbamcr.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Mo J, Lin D, Wang J, Li P, Liu W. Apoptosis in HepG2 cells induced by zinc pyrithione via mitochondrial dysfunction pathway: involvement of zinc accumulation and oxidative stress. Ecotoxicol Environ Saf 161: 515–525, 2018. [DOI] [PubMed] [Google Scholar]
- 37.Srivastava G, Matta A, Fu G, Somasundaram RT, Datti A, Walfish PG, Ralhan R. Anticancer activity of pyrithione zinc in oral cancer cells identified in small molecule screens and xenograft model: Implications for oral cancer therapy. Mol Oncol 9: 1720–1735, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tailler M, Senovilla L, Lainey E, Thepot S, Metivier D, Sebert M, Baud V, Billot K, Fenaux P, Galluzzi L, Boehrer S, Kroemer G, Kepp O. Antineoplastic activity of ouabain and pyrithione zinc in acute myeloid leukemia. Oncogene 31: 3536–3546, 2012. doi: 10.1038/onc.2011.521. [DOI] [PubMed] [Google Scholar]
- 39.Rowlands CG, Danby FW. Histopathology of psoriasis treated with zinc pyrithione. Am J Dermatopathol 22: 272–276, 2000. doi: 10.1097/00000372-200006000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Browe DM, Baumgarten CM. Angiotensin II (AT1) receptors and NADPH oxidase regulate Cl- current elicited by beta1 integrin stretch in rabbit ventricular myocytes. J Gen Physiol 124: 273–287, 2004. doi: 10.1085/jgp.200409040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiao JD, Xu CQ, Yue P, Dong DL, Li Z, Du ZM, Yang BF. Volume-sensitive outwardly rectifying chloride channels are involved in oxidative stress-induced apoptosis of mesangial cells. Biochem Biophys Res Commun 340: 277–285, 2006. doi: 10.1016/j.bbrc.2005.11.175. [DOI] [PubMed] [Google Scholar]
- 42.Shimizu T, Numata T, Okada Y. A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl(-) channel. Proc Natl Acad Sci USA 101: 6770–6773, 2004. doi: 10.1073/pnas.0401604101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huo C, Liu Y, Li X, Xu R, Jia X, Hou L, Wang X. LRRC8A contributes to angiotensin II-induced cardiac hypertrophy by interacting with NADPH oxidases via the C-terminal leucine-rich repeat domain. Free Radic Biol Med 165: 191–202, 2021. doi: 10.1016/j.freeradbiomed.2021.01.022. [DOI] [PubMed] [Google Scholar]
- 44.Gradogna A, Gavazzo P, Boccaccio A, Pusch M. Subunit-dependent oxidative stress sensitivity of LRRC8 volume-regulated anion channels. J Physiol 595: 6719–6733, 2017. doi: 10.1113/JP274795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen M, Ding Y, Ke Y, Zeng Y, Liu N, Zhong Y, Hua X, Li Z, Xiong Y, Wu C, Yu H. Anti-tumour activity of zinc ionophore pyrithione in human ovarian cancer cells through inhibition of proliferation and migration and promotion of lysosome-mitochondrial apoptosis. Artif Cells Nanomed Biotechnol 48: 824–833, 2020. doi: 10.1080/21691401.2020.1770266. [DOI] [PubMed] [Google Scholar]
- 46.Park M, Cho YJ, Lee YW, Jung WH. Understanding the mechanism of action of the anti-dandruff agent zinc pyrithione against malassezia restricta. Sci Rep 8: 12086, 2018. doi: 10.1038/s41598-018-30588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rudolf E, Rudolf K. Acute increases in intracellular zinc lead to an increased lysosomal and mitochondrial autophagy and subsequent cell demise in malignant melanoma. Int J Mol Sci 22: 667, 2021. doi: 10.3390/ijms22020667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuncay E, Turan B. Intracellular Zn(2+) increase in cardiomyocytes induces both electrical and mechanical dysfunction in heart via endogenous generation of reactive nitrogen species. Biol Trace Elem Res 169: 294–302, 2016. doi: 10.1007/s12011-015-0423-3. [DOI] [PubMed] [Google Scholar]
- 49.Justiniano R, Perer J, Hua A, Fazel M, Krajisnik A, Cabello CM, Wondrak GT. A topical zinc ionophore blocks tumorigenic progression in UV-exposed SKH-1 high-risk mouse skin. Photochem Photobiol 93: 1472–1482, 2017. doi: 10.1111/php.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong Q, Sun H, Li M. Zinc pyrithione-mediated activation of voltage-gated KCNQ potassium channels rescues epileptogenic mutants. Nat Chem Biol 3: 287–296, 2007. doi: 10.1038/nchembio874. [DOI] [PubMed] [Google Scholar]