Keywords: acute kidney injury, animal model, ischemia, nephrotoxicity, pig, sepsis
Abstract
Pigs represent a potentially attractive model for medical research. Similar body size and physiological patterns of kidney injury that more closely mimic those described in humans make larger animals attractive for experimentation. Using larger animals, including pigs, to investigate the pathogenesis of acute kidney injury (AKI) also serves as an experimental bridge, narrowing the gap between clinical disease and preclinical discoveries. This article compares the advantages and disadvantages of large versus small AKI animal models and provides a comprehensive overview of the development and application of porcine models of AKI induced by clinically relevant insults, including ischemia-reperfusion, sepsis, and nephrotoxin exposure. The primary focus of this review is to evaluate the use of pigs for AKI studies by current investigators, including areas where more information is needed.
INTRODUCTION
Acute kidney injury (AKI) is a frequent complication of illness, characterized by a dramatic decrease in the glomerular filtration rate (GFR), a major indicator of renal function, with an incidence of 0.21%, occurring in nearly 5% of in-patients (1). The pathogenic factors of AKI are complex, including volume depletion, ischemia-reperfusion (IR) injury, sepsis, trauma, and nephrotoxins (2, 3). Preclinical studies in animal models and clinical trials have been carried out to improve short-term and long-term outcomes of patients with AKI, with limited progress. Treatment for AKI is still largely based on removing or reversing the proximal causes, supportive care for volume and electrolyte management while waiting for renal recovery, and renal replacement therapy if recovery is prolonged or fails to occur. Since there are still no approved specific treatments for AKI beyond treating the underlying cause, a considerable portion of patients with AKI progress to chronic kidney disease (CKD) (4). In-hospital mortality is also high.
A better understanding of the pathogenesis of AKI and its associated complications is essential to reduce the associated morbidity and mortality. Much of the current knowledge of AKI at the molecular level comes from experiments using small animals. However, those experiments often fail to simulate the corresponding clinical conditions, making it difficult to extrapolate information obtained from small animals to humans. Compared with the commonly used laboratory animals (mice, rats, or rabbits), larger animals like pigs possess physiological advantages in size equivalency to humans as well as pathophysiological responses, metabolic process, and immunology characteristics that closely resemble those of humans.
The use of pigs in biomedical study began in the early 1970s (5). In recent years, the application and interest of pigs in all fields of medical research have become increasingly widespread since genetic similarities between Sus scrofa domestica and Homo sapiens were identified (6, 7). Pigs share more similarities in size, anatomic structure, and physiology with human than small animals and act as a principal mammalian model in research into auto- and xenotransplantation, cardiovascular diseases, digestive disorders, respiratory-related diseases, diabetes, urinary system diseases, orthopedic and muscular disorders, dermatology, and neurological sequelae (8–13). Furthermore, pigs have been used for surgical training and practice (14) and preclinical evaluation of medical devices as well as pharmacokinetics, pharmacodynamics, and toxicology studies (15–17).
This article provides a comprehensive overview of up-to-date approaches to the development of porcine models for AKI research and discusses the characteristics of porcine models currently available. Furthermore, we compare the advantages and disadvantages of large and small animal models of AKI and discuss areas where more information is needed to define the role for pigs in research into causes and potential treatments for AKI and disease (18).
CHARACTERISTICS OF EXPERIMENTAL PIGS
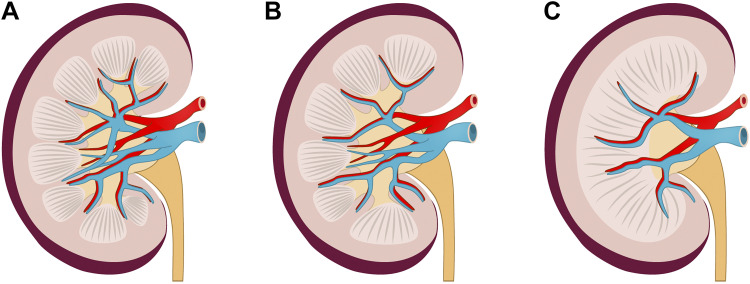
The urinary system of swine is anatomically and physiologically similar to that of humans, characterized by multilobular structures as opposed to the unilobular and unipapillary kidneys of rodents and dogs (Fig. 1) (8, 19–21). Porcine kidneys are multirenculate and multipapillate, with a calyceal system similar that in human kidneys. The total number of nephrons in swine kidneys is greater than in human kidneys (1.6−4.6 × 106 vs. 0.2−2.0 × 106), while the number of nephrons in rodents is approximately two orders of magnitude lower than in humans (9−18 × 103 in mice and 13−26 × 103 in rats) (12). The human and pig renal vasculature share many similarities. The porcine renal artery divides into cranial and caudal branches, whereas human renal arteries are divided into anterior and posterior branches. A horizontal branch in the ventral-mid kidney is described in both species. In the inferior pole of the kidney, the caudal branch fell into ventral and dorsal branches in 85% of swine and 62% of human kidneys (20). Porcine physiological function of the urological system and urodynamic parameters are also similar to humans (22). Taking advantage of similarities in the renal vasculature, researchers have designed pig models of renal vascular disease by inducing renal artery stenosis and hypertension that lead to progressive microvascular dysfunction and loss of the cortex and medulla and that correlated with loss of renal function (23). The model demonstrated reduction in renal blood flow, elevated serum creatinine, albuminuria, and increased urinary levels of kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL).
Figure 1.
The kidney architecture and vasculature in the human, miniature pig, and mouse. A: human kidneys are about 10−12 cm in length, with multiple calyxes. B: porcine kidneys are around 8 cm in length and have a similar structure to that of humans with multiple renal papillae and pyramids. C: mouse kidneys are about 1 cm in length, with only a single calyx. Renal arteries are originated from the abdominal aorta and divide into cranial and caudal branches at the hilar level in humans, miniature pigs, and mice. Furthermore, they split intro branches to supply the upper and lower poles and the middle segment of the kidney. Branching patterns of the renal vein in humans, miniature pigs, and mice are similar to those of the renal artery.
Domestic pigs, farm pigs, or large white pigs are frequently used in biomedical studies. However, fully grown adult pigs can weigh up to 250 kg, which limits their general experimental application (20). As a solution, several breeds of miniature pigs have been developed to make better laboratory models. Miniature pigs usually weigh 15−35 kg at 4−6 mo, with kidney size resembling that of the human kidney (20). They reach sexual maturity at 4−5 mo old or so. Different strains are often named after places where they were originally bred, such as the Yucatan, Hanford, Gottingen, Aachen, Sinclair, Bama, Diannan, and Tibet miniature pigs (24–30). Miniature pigs can be maintained at a lower cost than large pigs since they require less housing space, fodder, and drugs in pharmacological studies. The savings can be significant when the drug is under investigation only and manufactured at a small scale. In addition, piglets or porklings have been used in some studies, usually related to pediatrics, neonatology, or developmental biology, including research of renal diseases. In conclusion, there are many different types and strains of pigs that can be used as model animals, among which miniature pigs can be bred exclusively for scientific research since they have extra advantages compared with livestock pigs.
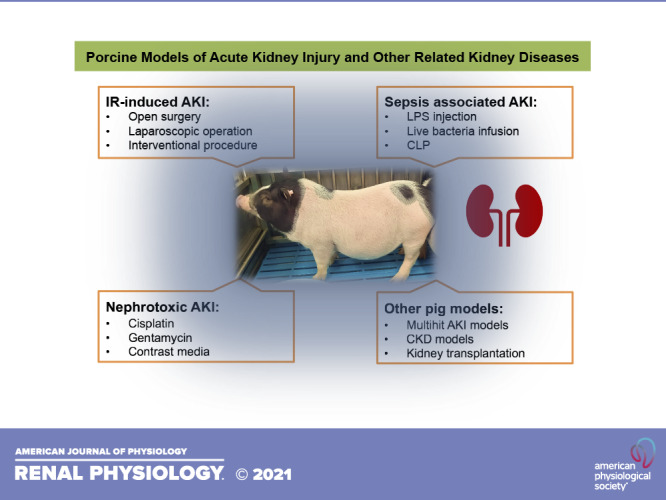
IR-INDUCED AKI MODEL
In humans, kidney damage from ischemic injury can commonly be seen as a result of reduced blood flow for a number of reasons, including structural or functional renal artery stenosis, hypoperfusion as a result of hemorrhagic, hypovolemic (including increased insensible losses from full-thickness burns), septic or cardiogenic shock, prolonged cold ischemic time in kidney transplantation, and during cardiopulmonary bypass in cardiac surgery. Effects of AKI due to hemorrhagic shock (31, 32), AKI produced by full-thickness burn injuries (33, 34), and cardiopulmonary bypass (31) have been studied in large animal models. In human and animal models, the principal feature of ischemia-induced AKI is renal blood flow decrease, resulting in a lower level of oxygen delivery to kidney tissue, ultimately leading to capillary endothelial cell damage, tubular cell detachment, apoptosis, and necrosis (35). IR kidney injury is characterized by inflammation and a sophisticated cascade of intracellular mechanisms leading to AKI in both animal models and humans (36).
Cells cultured under conditions of hypoxia or in medium deprived of glucose can be used as in vitro models of renal ischemic injury. However, few models of renal ischemia in vitro have been previously studied, and in vitro AKI studies cannot represent the global context expected to be observed because of cellular, focal, or systemic inflammatory, immune, and humoral factors involved in the process of ischemia and reperfusion in vivo. Characteristics including kidney development, anatomy, immunological patterns, urinary concentrating mechanism, and susceptibility to ischemic and IR injury indicate that the porcine kidney resembles the human kidney more than kidneys of small animals and can serve as a superior choice to study kidney IR consequences in humans (20).
Since IR is a leading cause of AKI, IR-induced AKI swine models are most frequently used in relevant studies (37–47). The methods used to establish such models are variable. Ischemia in porcine kidneys can be induced by laparoscopic or open surgery and closing the artery only, the artery and vein, or the whole renal pedicle, depending on the study. Bilateral or unilateral ligation, with or without contralateral nephrectomy, remains unstandardized (38, 48, 49). IR modeling can also be performed through interventional procedures, and the renal artery can be temporarily occluded by introducing and inflating a balloon. However, this method requires the use of a contrast agent, and the possible influence of the contrast agent on the kidney must be considered carefully (50). Moreover, the proper ischemic time is still under controversy. Generally, it was accepted by most researchers that the ischemic duration of <30 min would be not enough to cause kidney injury, and maintaining ischemia for over 120 min would probably result in irreversible damage to the kidney and even undesirable animal casualties (51). According to literature reports, renal function in pig models showed significant impairment around 1−3 days after reperfusion and began to recover gradually afterward (39, 51, 52).
Compared with small animals, pigs also have obvious advantages in the operation of IR modeling besides the anatomic and physiological resemblance to human kidneys. In laparoscopic surgery, the same instruments used in human adults or children can be used in small pigs. There is currently a lack of relevant equipment and microinstruments specifically designed for mice. In addition, because the pig has a more macroscopic anatomy structure, the renal artery, renal vein, and ureter can be easily separated during surgery, and subtle anatomic structures like arteriovenous branches can be clearly observed without a surgical microscope or other equipment.
Several examples are given below as well as in Table 1 to illustrate the specific application of the IR-induced AKI porcine model. Barrera-Chimal et al. (53) successfully established an IR model with white male pigs by clamping bilateral renal pedicles for 1 h. The renal function impairment induced from IR was evidenced through a rise in serum creatinine level, a substantial increase in the excretion of kidney injury-associated biomarkers (NGAL and KIM-1) in urine, and a loss of brush-border integrity and tubular dilation from biopsy and histopathological analysis on day 7 after IR (53). They then used this model to investigate the role of vascular mineralocorticoid receptor antagonism in IR-mediated AKI, and the beneficial results provided a rational basis for future clinical trials. Gozdzik et al. (68) used suprarenal aortic cross-clamping for 2 h to induce organ IR injury. This method not only involved the kidney but other distal organs. Similarly, multiorgan IR injury is reported during extracorporeal cardiopulmonary resuscitation (69–71). Although there were no generally accepted standardized procedures for modeling, IR-induced AKI in a porcine model has been widely used and has played an essential role in exploring the mechanisms and possible prevention of or therapeutic strategies for treating AKI.
Table 1.
Summary of porcine AKI-related literature concerning ischemia, infection, and nephrotoxicity
| First Author | Country | Publication Year | Journal | Animal | Age, mo | Body Weight, kg | Method | Sample Size | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Ischemia-reperfusion | ||||||||||
| Jayle | France | 2006 | Kidney International | Large white pigs | 3−4 | 30−35 | Clamping left renal pedicle for 45/60 min with a vascular clamp followed by a right nephrectomy | 60 | Creatinine clearance rate significantly decreased at day 1 and begin to recover afterward | (52) |
| Barrera-Chimal | France | 2017 | Journal of the American Society of Nephrology | White male pigs | NA | 42−51 | Clamping both renal pedicles for 60 min with a nontraumatic vascular clamp | NA | Plasma creatinine significantly increased at day 1 and begin to recover afterward | (53) |
| Silberstein | United States | 2013 | BJU International | Yorkshire farm pigs | 1.5−2 | 27−36 | Unilateral renal ischemia with left hilar clamping of timed duration (15, 30, and 60 min) | 12 | Median urine volume was lower (P = 0.04) at 6, 12, 24, and 48 h and median NGAL concentration was higher (P = 0.04) at 12 and 48 h compared with the control group | (37) |
| Baldwin | United States | 2004 | Urology | Farm pigs | NA | 46−69 | Unilateral laparoscopic right nephrectomy and, 2 wk later, left hilar clamp for 0, 30, 60, or 90 min | 16 | SCr level rose initially on days 2 and 4 in the 60- and 90-min ischemia groups and returned to baseline by day 7 | (38) |
| Bechara | Brazil | 2016 | Acta Cirurgica Brasileira | Pigs | NA | 20 | 30 min of warm ischemia by arteriovenous/arterial clamping of left kidney vessels | 24 | Renal warm ischemia of 30 min by arterial clamping did not caused significant glomerular damage, but arteriovenous clamping caused significant glomerular loss in a swine model | (49) |
| Brunswig-Spickenheier | Germany | 2010 | Stem Cells and Development | German hybrid pigs | 3 | 29−45 | Two balloon catheters were introduced into both renal arteries via the femoral arteries, and both renal arteries were occluded for 110 min by balloon inflation | 24 | Successful induction of AKI by balloon catheter occlusion of renal arteries and subsequent reperfusion were monitored angiographically as well as hemodynamically | (39) |
| Amdisen | Denmark | 2016 | PLoS One | Landrace pigs | NA | 42 | Unilateral renal ischemia-reperfusion injury was induced by clamping the left renal artery over a 2-h period | 20 | Unilateral kidney ischemia-reperfusion resulted in an immediate ∼50% GFR reduction, associated with a 4-fold increase in urinary NGAL-excretion | (54) |
| Gardner | United Kingdom | 2016 | American Journal of Physiology-Renal Physiology | Pigs | NA | 55−65 | Bilateral renal artery cross-clamping (40 min) with 48-h postsurgical recovery | 35 | 40 min of bilateral renal artery occlusion led to an increase (48%) in plasma creatinine that peaked at 24 h and an increase in the urinary albumin-to-creatinine ratio that peaked at 2 h | (40) |
| Malagrino | Brazil | 2017 | Journal of Proteomics | Pigs | 2−3 | 15−20 | A balloon catheter was introduced and inflated for 120 min (ischemia), followed by deflation (reperfusion) for 24 h | 5 | Only two animals showed levels of SCr above 150% of baseline | (42) |
| Matejkova | Germany | 2013 | Intensive Care Medicine | Cross-breed of Rapacz farm pig | 13−20 | 53−85 | 120 min of aortic occlusion by an aortic balloon catheter | 20 | Ischemia-reperfusion caused AKI with reduced creatinine clearance, increased fractional Na+ excretion and NGAL levels, and moderate to severe glomerular and tubular damage | (45) |
| Castellano | Italy | 2018 | Frontiers in Immunology | Large white pigs | 4 | 20 | Clamping the renal artery for 30 min followed by reperfusion | 10 | Oliguric kidney injury | (46) |
| Sepsis | ||||||||||
| Duburcq | France | 2018 | Shock | Large white pigs | 2 | 18−25 | Administration of 5 μg/kg/min Escherichia coli LPS intravenously | 10 | Endotoxic shock | (55) |
| Lima | The Netherlands/Italy | 2018 | Critical Care Medicine | Crossbred Landrace × Yorkshire pigs | 2 | 28 | Sepsis was induced through continuous intravenous infusion of LPS at 2 µg/kg/h | 15 | Increase in creatinine during shock and resuscitation | (56) |
| Castellano | Italy | 2014 | Critical Care | Domestic swine | 7 | 58 | 10 mL of saline solution containing 300 μg/kg LPS was infused | 28 | Acute tubulointerstitial fibrosis occurred within 9 h from LPS injection | (57) |
| Vassal | France | 2015 | Acta Anaesthesiology Scandinavica | Piglets | 2.5−3.5 | 20−25 | Intravenous infusion of Pseudomonas aeruginosa | 17 | A significant decrease in GFR was observed | (58) |
| Matejovic | Czech Republic | 2016 | Shock | Domestic Pigs | NA | 32− 0 | Continuous infusion of Pseudomonas aeruginosa | 17 | 43% reduction in glomerular filtration | (59) |
| Barth | Germany | 2008 | Critical Care Medicine | Domestic Pigs | NA | NA | 0.5 g/kg of autologous feces were suspended in 100 mL saline and cultivated at 38°C until the Matijevic induction of peritonitis | 20 | Septic shock | (60) |
| Merz | Germany | 2018 | Intensive Care Medicine Experimental | Pigs | 15−30 | 69 | 1 g/kg of autologous feces was collected during premedication, dissolved in 500 mL of 0.9 % saline, incubated at 38 °C for 12 h, and then infused into the abdominal cavity | 13 | Septic shock | (61) |
| Wang | China | 2015 | International Urology and Nephrology | Hybridized pigs | NA | 23.5−27.9 | Cecal ligation and puncture and clamping of the renal artery | 17 | Significantly higher renal function marker creatinine and blood urea nitrogen 24 h after surgery | (62) |
| Nephrotoxicity | ||||||||||
| Cui | China | 2019 | Shock | Chinese experimental mini-pigs | 3 | 8−10 | 80 mg/kg gentamicin sulfate intramuscularly daily for 10 days | 18 | AKI was well developed after 10 days of drug infusion | (63) |
| Robbins | United Kingdom/United States | 1992 | Cancer Chemotherapy Pharmacology | Large white pigs | 10 | NA | Single intravenous infusion of 1.5, 2, or 2.5 mg/kg (i.e., 90, 120, and 150 mg/m2) cisplatin | 12 | Significant cisplatin-induced reduction in GFR over the 24-wk period following drug treatment | (64) |
| Santiago | Spain | 2016 | PLoS One | Maryland pigs | 2−3 | 9 | Infused cisplatin to piglets through the peripheral vein in the Plow ear at doses of 2, 3, and 5 mg/kg | 41 | SCr significantly rose 48 h after cisplatin administration and largely fell back to the predose level in a week | (65) |
| Wu | China | 2019 | Biochemical and Biophysical Research Communications | Miniature pigs | 3 | 10.4 ± 0.23 | Injection of 25 mL/kg iohexol (350 mg iodine/mL) after dehydration | 16 | Increased blood levels of blood urea nitrogen, SCr, serum and urinary β-microglobulin, and injured kidney tissue, dilated tubules and foamy degeneration | (66) |
| Xu | China | 2020 | International Immunopharmacology | Miniature pig | 3 | NA | Iohexol (350 mg/mL) was injected via the external jugular vein at 25 mL/kg | 10 | Increased serum levels of blood urea nitrogen, SCr, and β-microglobulin and urinary levels of β-microglobulin and renal fibrosis and injury scores | (67) |
AKI, acute kidney injury; GFR, glomerular filtration rate; LPS, lipopolysaccharide; NA, not applicable; NGAL, neutrophil gelatinase-associated lipocalin; SCr, serum creatinine.
IR injury is an inevitable event and important cause of graft failure in kidney transplantation (72). Appropriate approaches to prevent and reduce IR damage in transplanted kidneys have not been fully elucidated. The ischemic phase duration, storage temperature, perfusion solution components, and preservation medium can all exert effects on ex vivo kidneys (73, 74). Large animal experiments or ex vivo experiments based on large animals will contribute to the progress in preclinical renal transplantation research and thereby possess potential to improve the prognosis of kidney recipients.
SEPSIS-ASSOCIATED AKI MODELS
As one of the most common causes of mortality in intensive care units, sepsis is a state of whole body inflammation caused by infection affecting around 20−50% of critical patients, with AKI a common complication (75). Gram-negative bacteria and the components of their cytoderm, in particular, lipopolysaccharide (LPS), play significant roles in the pathogenesis of sepsis-associated AKI (76). The pathophysiology characteristics of septic AKI include intrarenal hemodynamic alterations, capillary endothelial cell dysfunction, renal interstitial inflammatory cell infiltration, intraglomerular thrombosis, and tubular obstruction caused by exfoliated dead tubular cells or necrotic- and apoptotic-derived cellular debris (57). The large population of patients with sepsis and AKI has led to a strong need for further research, and studies concerning sepsis-associated AKI in porcine models have been carried in multiple medical subspecialties (55, 57, 59, 60, 77–81). Sepsis models developed using small animals showed mixed results when translated into clinical trials or practice (82). The response of mice to inflammatory stressors like endotoxins is quite different from human beings (83). In comparison, pigs reacted to infection in a manner that is similar to humans in terms of cytokine and immune cell profiles (84) and presented symptoms characteristic of human infections (85). As such, pigs appear to be an appropriate animal model for sepsis-induced AKI.
Several infectious options are available for the study of septic AKI in pigs. Commercialized Escherichia coli LPS can be administered intravenously (55–57, 79, 80, 86). In several studies, live E. coli or Pseudomonas aeruginosa was directly injected to pigs intravenously (59, 80, 87–89). These approaches are highly practicable, and it is easy to control the dose of endotoxin or bacteria, but there is a certain gap with the actual situation encountered in clinical practice. Another sepsis-related model of AKI in pigs is to induce peritonitis and subsequent multiorgan damage, including kidney injury. Autologous feces have introduced through drains into the pig abdomen after suspension in saline, with or without in vitro culturing first (58, 60, 61, 78, 79, 81, 90–92). Surgically, cecal ligation and puncture (CLP) results in abdominal infection, and then fecal peritonitis could induce sepsis and AKI (62, 93). The mortality rate during CLP modeling is relatively high. Many animals die of acute lung failure, and the degree of kidney damage is difficult to manage. Nevertheless, this model mimics changes in humans with digestive tract perforations and has the potential for clinical translational value.
For an E. coli peritoneal injection porcine model, without intervention, death usually occurred at 7.6 ± 0.5 h (89). AKI was demonstrated by a 30% rise in serum creatinine concentrations and subtle histological changes on renal biopsy 18−22 h after the administration of bacteria or feces in some reports (but not all studies) (81, 94). Castellano et al. (57) analyzed renal fibrosis with Masson trichrome staining and demonstrated extensive collagen deposition surrounding peritubular capillaries after LPS administration. Diffuse glomerular thrombi and tubular vacuolization were also observed via hematoxylin and eosin staining (57). Moreover, serum KIM-1 and cystatin C levels significantly increased 6 h after LPS infusion before serum creatinine began to rise (57). In addition, inflammatory factors are key indicators in the evaluation of infection-induced AKI models. IL-6 and TNF-α levels significantly elevated 12−18 h after LPS infusion (80).
Clinically, septic AKI affects ∼40−50% of all patients with sepsis (80). Therefore, inducement of sepsis does not mean the successful development of septic AKI. This is a concern for the establishment of septic AKI pig models. For example, in 2011, Benes et al. (78) used two procedures to develop septic AKI: either by peritonitis (n = 13) induced by injecting autologous feces into the abdominal cavity after in vitro culture or through intravenous administration of live P. aeruginosa (n = 15). The results showed that AKI developed only in 14 pigs (50%), 62% in peritonitis, and 40% in the bacteria infusion model.
Another concern regarding porcine septic AKI animal models is the complex and heterogeneous nature of sepsis (61, 95). Simon et al. (79) developed a septic shock pig model caused by peritonitis induced through intraperitoneal infusion of 1.0 g/kg autologous excrement after incubation with 100 mL of 0.9% saline for 12 h at 38°C. According to their report, liver, kidney, and heart dysfunction were all observed during resuscitation following septic shock, which can evolve out of systemic inflammatory response syndrome or multiple organ dysfunction syndrome. Thus septic AKI models were not perfectly applicable to studies aimed at exploring mechanisms or interventions to treat AKI alone.
Taken together, the above considerations pose challenges for applying specific models of septic AKI models to the pig. Still, the model holds considerable potential for studying septic AKI because of multiple choices of methods and simulation of the pathophysiological process of the human body.
NEPHROTOXIC AKI MODEL
AKI induced by known nephrotoxic drugs also occurs frequently in hospitalized patients. Nephrotoxin-induced AKI is a common complication of diagnostic and therapeutic procedures and is becoming an important cause of AKI both in China and worldwide. In studies of rodent models of AKI, renal injury has been induced by contrast media, vancomycin, aristolochic acid, and folic acid (96). In large animal research, the most commonly used modeling drugs are cisplatin, gentamicin, and contrast medium (63, 64, 66, 67, 97).
Cisplatin-Induced AKI
Cisplatin is a cytotoxic platinum derivative and has been widely used as potent chemotherapeutic agent for a variety of tumors, usually in patients with solid tumors, including nonsmall cell lung cancer, gastric tumor, and prostate cancer (98). A notable side effect of cisplatin is nephrotoxicity, which induces DNA damage, mitochondrial disorders, oxidative stress, and apoptosis in kidneys. Freely filtered at glomeruli and reabsorbed by tubular epithelial cells, cisplatin dominantly affects tubular areas of the kidney. Histopathological results show signs of a cisplatin dose-dependent increase in renal injury (65). A single administration of cisplatin at high dose could lead to AKI in animal models, while repeated low-dose injections of cisplatin might result in renal fibrosis and CKD (99).
In an effort to clarify the mechanism of cisplatin-induced renal injury through large animal model studies, Santiago et al. (65) infused cisplatin into piglets through a peripheral vein in the ear at doses of 2, 3, and 5 mg/kg. According to their results, 3 mg/kg cisplatin injection led to a significant rise in plasma creatinine and urea levels without an evident increase in plasma K+ concentration. Serum creatinine significantly rose 48 h after cisplatin administration and fell back to the predose level in a week (65). However, the data of an earlier study in 1992 presented with different results. Robbins et al. (64) developed a large white pig AKI model by a single intravenous administration of cisplatin at doses including 1.5, 2, and 2.5 mg/kg (i.e., 90, 120, and 150 mg/m2) and concluded that GFR significantly decreased during 8−12 wk after dosing. Histopathology lesions were observed in the corticomedullary junction region and extended into the medullary area, as far as calyces and the renal pelvis in some kidneys. Most histological changes were found in the inner one-half to one-third of the kidney cortex. Occasionally, corresponding renal tubules were moderately compressed. Neither severe glomerular abnormalities nor tubular epithelial degeneration was observed in this study. Considering a lack of documented reports and the differences in pig breeds and age, it is difficult to derive precise dose or time data on cisplatin-induced AKI. This also means that more research is needed to clarify the key information such as the dose required for cisplatin modeling, timing of the injury after administration of the drug, and how severe the renal damage will be.
The clinical significance of this model lies in formulating renoprotective strategies for cancer patients who receive chemotherapy. Cisplatin-induced AKI presents with relatively shorter immunosuppressive effect on animals, so this model is particularly suitable for studies that require long-term observation of animals, such as observation of long-term prognosis or potential delayed adverse events caused by drugs or therapies. Cisplatin-induced AKI can also be used for small animals and in cell cultures, allowing researchers to conduct multilevel studies. The porcine model of cisplatin-induced AKI is worthy of further exploration. Researchers have used a cisplatin-induced AKI model in miniature pigs to examine the effects of a probiotic cocktail on preventing renal injury, hypothesizing that the probiotic mix reduced inflammation and apoptosis and thereby slowed the progression of CKD (100).
Gentamicin-Induced AKI
Gentamicin belongs to aminoglycosides family, a category of antibiotics used clinically to treat serious infections caused by gram-negative bacteria. Nephrotoxicity mediated by aminoglycosides affects 10−20% of patients under associated treatment regimes. Gentamicin accumulates in renal tubular epithelial cells, leading to nephrotoxicity and AKI. The pathogenesis of gentamicin-mediated kidney injury is not totally clear. Studies have indicated that gentamicin may act on mitochondria and result in oxidative stress and apoptosis as cisplatin does. Ultimately, it can causes congestion of glomeruli, generation of free radicals in the kidney, impairment of renal antioxidant defense mechanisms, and acute tubular necrosis (5).
Cui et al. (63, 101) developed a gentamicin-associated AKI model using Chinese experimental miniature pigs. Pigs received intramuscular administration of gentamicin for up to 10 days at doses of 5, 6.85, 10, 15, 20, 40, 60, 80, 100, and 120 mg/kg. Marked dose-dependent effects were observed in survival rates, histopathology scores, renal function, and molecular biomarkers. The researchers established a protocol for inducing AKI by intramuscularly infusing 80 mg/kg gentamicin sulfate daily for 10 days. Histopathological findings indicated that gentamicin caused tubular epithelial cell degeneration, granular or protein cast tubular lumen obstruction, and inflammatory cell infiltration into the renal parenchyma.
Contrast-Induced AKI
With the development of imaging technology, many patients suffered from kidney damage induced by contrast medium (102). In a meta-analysis enrolling 29 studies, the incidence of contrast-induced AKI was reported to be 4.4−22.1% (103). Commonly used contrast agents included sodium iothalamate, iopamidol, and iodixanolare (102). These contrast agents can induce renal tubular cell apoptosis through the mechanisms involved in cell membrane damage, increased intracellular Ca2+, an activated proapoptotic unfolded protein response, and decreased ATP (102). Repeated administration of contrast medium also induced a transient increase in the renal resistance index in both pig models and clinical study (104, 105).
Porcine model of contrast-induced AKI model can be established by injecting a single dose of iohexol (350 mg/mL) at 25 mL/kg. This model highly mimics clinical contrast-induced AKI and is reproducible. After AKI, increased blood levels of blood urea nitrogen, serum creatinine, and β-microglobulin and urinary levels of β-microglobulin were observed; pathological changes included dilated tubules, foamy degeneration, and renal fibrosis (66, 67). Administration of retinoic acid and miR-30c can attenuate kidney injury in this model, respectively, providing new ideas for the prevention and treatment of contrast-induced AKI.
OTHER PIG MODELS
Beside IR, sepsis, and drug toxicity, other porcine models of AKI such as radiation-induced kidney damage exist (106). However, similar to septic AKI, most of those models produced not only kidney injury but damage to other organs, including acute liver injury, lung atelectasis, and intestinal damage, making it difficult to limit multiorgan systemic damage while trying to study renal injury alone. Furthermore, porcine kidneys were often used for ex vivo research (107), abiding by three “R” principles (the consensus to reduce the number of animals used, refine their life conditions, and replace animal models by in vitro or other procedure if possible) of animal welfare, and pigs have considerable application prospects in the field of transplantation (52, 108).
Clinically significant AKI is usually unpredictable and sometimes caused by multiple insults (ischemia, infection, nephrotoxicity, etc.) and their cross talk. For example, we can study the mechanism of renal IR injury in a diabetic model or explore a solution for cisplatin-mediated AKI in a tumor-bearing pig model. Compared with single insult-induced AKI models, “multihit” models share more similarities to actual clinical situations and show more translational medicine significance (94, 109).
CKD is an undesirable outcome of AKI, but the AKI-CKD transition and its underlying mechanism remain largely unclear. Rodent models for exploring post-AKI development of chronic renal lesions and the progression mechanism to CKD have been reviewed by Fu et al. (110). Recently, Lui et al. (111) established a porcine model of CKD by partial nephrectomy and radical contralateral nephrectomy (2/3, 3/4, or 5/6 nephrectomy) using laparoscopy, and Chade et al. (23) created a CKD porcine model by inducing bilateral renal artery stenosis. Similarly, Lerman and colleagues (112–114) developed a pig model with combination of unilateral renal artery stenosis and high-cholesterol/carbohydrate diet. The interplay between hyperlipidemia and CKD was discussed in detail in these studies; it seems that combined application of both hypercholesterolemia and renal artery stenosis could amplify, upregulate, and accelerate the cellular mechanisms leading to renal vascular, glomerular, and tubulointerstitial damage. In addition to metabolic similarities, the swine is an omnivorous animal with similar nutritional requirements to humans, so diet and regime can be designed exactly according to the needs of human (115–118). Thus porcine models are particularly suitable for research of nutrition- or metabolism-related disease, including CKD.
The pig is also the optimal animal for kidney transplant research and surgeon training, especially laparoscopic or robotic surgical training (50). For example, Kobayashi et al. (119) achieved orthotopic renal transplantation using pigs weighing 20–30 kg, and their methodology was reported in detail. The surgical method used in pigs can provide a direct reference for clinical settings. Swine can be used to explore the way to improve the survival rate of grafts and solve any problems that may arise during or after kidney transplantation (120). Pigs even possess the potential as organ donors for xenotransplantation, offering a new option for patients who need renal replacement. Studies are ongoing to overcome xenogeneic rejection through creation of genetically manipulated humanized swine (121). The advantages of porcine models in this aspect are beyond the scope of small animal models, indicating a great value of pigs.
Indeed, pig kidney xenotransplantation is not that far off, with some expecting clinical trials in the next couple of years (13). The authors of a recent review in the Journal of the American Society of Nephrology suggest that it would be ethical to offer a pig kidney transplant to a patient whose life expectancy was shorter than their projected waiting time on the list for a deceased donor (13). An important part of that process is to engineer pigs genetically to remove potential xenoantigens using technologies like CRISPR/Cas9 (13). Researchers have used flow cytometry to investigate whether patients on dialysis with high degrees of human leukocyte antigen sensitization had high cross-reactivity to pig antigens. They found no IgM or IgG binding to galactose-α-1,3 galactose or N-glycolylneuraminic acid in subjects who were highly sensitized [calculated panel reactive antibodies (cPRA) = 100%] versus those who were not (cPRA = 0%) but were significantly reduced compared with normal controls (122).
Miniature pigs have also been used in preclinical models of organ preservation (123) and prevention of rejection in marginal kidneys harvested in donation after cardiovascular death (123).
ADVANCES AND LIMITATIONS OF PIG MODELS IN AKI RESEARCH
The widespread use of laboratory animals has greatly contributed to the development of biology and medical science. In nephrology, a variety of mouse and rat AKI models are used to explore the mechanism of renal injury and potentially develop novel preventative, diagnostic, or therapeutic approaches for AKI. Currently, some models are only studied in small animals, such as warfarin-related AKI (124) and rhabdomyolysis-induced AKI (125). While the use of animals in experiments remains controversial and subject to many legal and ethical restrictions, large animal models are still frequently used when effective and safe preclinical protocols directly transferable to clinic are expected. Research using pigs has played a significant role in the assessment of new medical devices or efficacy of a pharmacological therapeutic regime before their entry into clinical trials, market, and application (22). Big physiological differences between small animals and humans limit the clinical translation significance of research based on rodents, particularly when a given model has been extensively carried out in small animals only. In that case, the United States Food and Drug Administration recommends that a therapeutic regime be tested in more than one animal model before an investigative new drug application is submitted. Thus at least one of the experimental models should be a large animal model (126). The ethical policy of porcine experimentation differs among countries and regions, and it is usually more complicated than rodents. At times, the ethics of pig models can reach a level approximate to that for human research, including the requirement that studies on pigs should be based on prior in vitro or even small animal experimentation (127).
To choose an optimal laboratory animal offering structural and functional similarities to humans, other important factors like size, manipulability, information transferability, economic efficiency, ecological consideration, ethical issues, and social impact should also be evaluated (128). Pigs possess many advantages over rodents (Table 2). A major advantage is the similarity in renal structural features between human and pig kidneys. While the structure of the pig kidney does not correspond completely to that of the human kidney, the analogy between porcine and human intrarenal vascular systems as well as relationships between intrarenal arteries and renal collecting systems is close, as previously described (20). The pig body size is also approximate that of humans, especially miniature pigs, whose body weight reaches 60−70 kg in adulthood. The importance of animal size in experimental or preclinical research is usually underestimated. Humans and rodents differ from each other in total number of cells, which matters in some experiments (127). Due to the similarity in body size, surgical techniques used in pig models can be directly transferred to clinical settings, including instruments and devices, operating methods, etc. (129, 130). AKI is a common complication of surgical patients, and surgical operations are required sometimes for AKI modeling, such as IR modeling. It is easier to control the amount of blood loss and to detect cardiac and hemodynamic parameters (i.e., electrocardiograph, blood pressure, etc.) in swine than in small animals. Despite the larger size, pigs are also docile animals and, when treated properly, are often gentle and tame (131).
Table 2.
Summary of advantages and limitations of the mouse and pig
| Advantages | Limitations |
|---|---|
| Mouse | |
| Small size: single-handed operation and relatively small housing space requirements | Small size: body weight is 3 orders of magnitude different from humans |
| Low food intake: low feeding cost | Anatomic characteristics, physiological mechanisms, genetic background, and immune response patterns are quite different from those of the human body |
| Complete data: gene sequence, proteomics, and transcriptomics information available | Research results cannot be directly translated into clinical applications |
| Applicable reagents, test kits, antibodies, and instruments available | Sometimes unable to obtain sufficient blood or tissue samples |
| Strong fertility and short reproductive cycle | Repeated blood sampling is usually not allowed |
| Different scales for blood biochemical parameters from humans | |
| Pig | |
| Large size: body weight and kidney size proximity to humans | Large size: complicated handling and large living space required |
| Anatomic characteristics, physiological features, genetic background, and immune response patterns are similar to those of the human body | High food intake: high feeding cost |
| Research results can be directly translated into clinical applications | Incomplete data: lack of full gene sequence, proteomics, and transcriptomics information |
| Sufficient amount of blood and tissue material in a lower total number of animals | Lack of applicable reagents, test kits, antibodies, and equipment |
| Repeated blood and tissues sampling | Relatively long breeding cycle |
| Comparable blood biochemical parameters and similar urodynamic parameters with humans | Ethical complexity |
Repeated blood sampling is another advantage for porcine models of AKI. Since pigs have large total blood volumes, this makes it possible for researchers to follow their renal function and other blood biochemical indexes in long-term studies. Moreover, renal biopsy can be performed on pig models to assess the longitudinal changes in histopathology and examine the expression of injury markers after AKI by repeated sampling of kidney tissue. It is also more convenient to collect 24-h urine specimens from pigs in metabolic cages or by catheters to monitor urinary parameters after renal injury (20). The ability to provide sufficient amounts of blood, urine, and kidney tissue or other biological material from relative fewer animals conforms to the three “R” principle. The human-resembling physiology patterns of swine assure a high relevance of the information obtained in studies using this species for clinical trials. The reference values, normal range, and threshold for many blood biochemical variables, such as serum creatinine and blood urea nitrogen levels, are almost identical in pigs and humans (132). Hannon et al. (132) analyzed over 100 porcine physiological parameters under baseline conditions and concluded that most variables were comparable to those collected under corresponding conditions in humans.
The inconsistency of innate and adaptive immunity between the human and mouse raises concerns about the relevance of rodents as preclinical AKI models (133) and calls for better models. Interestingly, the number of orthologous immune gene families between the pig and human is 10 times more than between the mouse and human (134). Pigs have a large proportion of immune cells in lymphoid tissues and similar distribution of their vascular-associated lymphoid tissues as do in humans (135). The Toll-like receptor expression profile is also close between swine and humans (136). Moreover, the literature on immune markers reported an evident analogy between the human and pig in antigen pattern and the immune response (137). Since the epitopes of T cells and B cells were comparable between human and pig, de León and colleagues (138) recently initiated a project to develop vaccine by using Landrace and large white cross-bred female pigs.
Genetics influence nearly every aspect of human biology and disease including AKI pathogenesis. The pig has 18 pairs of autosomes and 2 allosomes, totaling around 2.7 Gb, ∼7% smaller than the human genome. In contrast, mouse genomes are 14% smaller than humans. The overall similarity of the transcribed genome between the pig and human is nearly 85% (139). With the rapid development of gene editing technology, a great deal of genetically engineered pig models has been established by Cre-loxP, Tet-on/off, or CRISPR/Cas systems, as reviewed by Yum et al. (140). These transgene models not only mimic human diseases in symptoms but also achieve consistency in etiology.
Although the pig has many advantages for creating an AKI model, it has certain limitations. While pig anatomy and physiology show some correlation to those of humans, distinctions between the two species exist that may be associated with different responses to certain experimental protocols (12). How pigs are handled and how experiments are carried out can lead to certain variability. In addition, the immune response and disease susceptibility are slightly different among strains and even from one pig to another in a same breed. General attributes like breed, sex, age, and body weight are also reported to have an effect on results of certain tests (141). Experimental mice are mostly inbred strains, and pigs are usually closed colonies, so the individual variability of pigs is greater than that of mice. In clinical settings, the manifestation of patients varies from individual to individual and population to population. In this respect, pigs and humans are similar. As long as one controls the variables (strain, age, sex, etc.) well, one can still evaluate differences between experimental groups and obtain reliable data from swine.
One of the most important limitations of pig AKI models lies in a lack of elaborated data at the molecular level, including but not limited to accurate DNA sequencing, gene expression patterns, transcriptome features, and proteome composition. Applicability of protein-based techniques in pigs is limited since they have been developed at a relatively lower rate in contrast to humans or rodents. This limitation is a results of an imperfect porcine peptide mass database; using homologous sequences from databases of other species (such as mice) to carry out swine-based research is extremely inefficient since sufficiently identical or high-scored peptide matches are rare (8). The systematic creation and elaboration of porcine proteome database for different systems and organs are still underway (6, 8). With increased application of pig models, those issues are expected to be solved over time. In the past few decades, researchers have bred miniature pigs to settle the inconvenience of handling large livestock pigs. Aware of the drawbacks from lack of a swine database, researchers have initiated many genomic, transcriptomic, and proteome studies in pigs (142, 143). For example, the genome of Sus scrofa was sequenced for the first time in 2012, and data are continually being updated (144). Several microarray studies were also conducted in pigs (145, 146). Manufacturers nowadays can offer a variety of pig-specific products, including but not limited to cell lines, research agents, primary antibodies, and assay kits (115).
Another inconvenience may come from the high growth rate and subsequent large size of pigs. The gestation period for female pigs is around 114 days, with 2.4 litters a year during reproductive ages and 12 piglets per litter, which means large kennels are required (20). Procedures need to be performed at a time while pigs are still at a weight that allows them to fit into laboratories and associated equipment. Animals are therefore often piglets or young adults rather than mature adults. Moreover, the diet and experimental kits for pigs are more expensive than for rodents (20). It is generally believed that rodents are the most economical models since they present with short reproductive cycles, are easy to handle by research staff, and cost less to maintain. Large animals like pigs seem to be less cost effective and more complicated to manage but in the long run often prove to be cost efficient and worthwhile by providing more reliable, transferable, and solid data (8). Furthermore, total cost and number of euthanized pigs required can be reduced by using tissue materials from slaughtered big animals for ex vivo or in vitro studies. Samples from slaughtered large animals represent an abundant and inexpensive resource of viable cell material for applied renal study (147). This makes swine a convenient, reliable, and cost-efficient model system in AKI research.
SUMMARY
The application of pigs in AKI models is increasingly gaining traction because of anatomic, metabolic, and pathophysiological similarities between pigs and humans. Different types of porcine AKI models have been developed by researchers over the past decades. We believe that even if it is impossible for swine to completely replace rodents as a firstline choice, porcine AKI models can be used as complementary or alternative models to confirm the results obtained from small animal models. These modeling approaches of pigs provide a better understanding of the mechanisms of AKI and may eventually reveal new molecular targets for the prevention, early diagnosis, and effective treatment of AKI. Obstacles remain to be overcome in order to achieve the full potential of using pigs to study human disease and translate findings into clinical trials for potential therapies. Finally, as xenotransplantation moves to the clinical from preclinical arena, knowledge about AKI, acute kidney disease AKD, and CKD in the pig kidney will become crucial.
GRANTS
This work was supported by National Natural Science Foundation of China Grants 81830021 and 82070700 (to S.Z.), National Key R&D Program of China Grant 2018YFA0108802 (to S.Z.), and the National Institute of Diabetes and Digestive and Kidney Diseases Grant 1R01-DK-113256-01A1 (to S.Z).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.H. and G.B. drafted manuscript; S.Z. edited and revised manuscript; J.H., G.B. and S.Z. approved final version of manuscript.
REFERENCES
- 1.Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, Macleod A. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 18: 1292–1298, 2007. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 2.Ni J, Hou X, Wang X, Shi Y, Xu L, Zheng X, Liu N, Qiu A, Zhuang S. 3-deazaneplanocin A protects against cisplatin-induced renal tubular cell apoptosis and acute kidney injury by restoration of E-cadherin expression. Cell Death Dis 10: 355, 2019. [Erratum in Cell Death Dis 10: 543, 2019]. doi: 10.1038/s41419-019-1589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, Vanholder R. Acute kidney injury: an increasing global concern. Lancet 382: 170–179, 2013. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez K, Dicks N, Glanzner WG, Agellon LB, Bordignon V. Efficacy of the porcine species in biomedical research. Front Genet 6: 293, 2015. doi: 10.3389/fgene.2015.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schachtschneider KM, Madsen O, Park C, Rund LA, Groenen MA, Schook LB. Adult porcine genome-wide DNA methylation patterns support pigs as a biomedical model. BMC Genomics 16: 743, 2015. doi: 10.1186/s12864-015-1938-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H, Song KD, Kim HJ, Park W, Kim J, Lee T, Shin DH, Kwak W, Kwon YJ, Sung S, Moon S, Lee KT, Kim N, Hong JK, Eo KY, Seo KS, Kim G, Park S, Yun CH, Kim H, Choi K, Kim J, Lee WK, Kim DK, Oh JD, Kim ES, Cho S, Lee HK, Kim TH, Kim H. Exploring the genetic signature of body size in Yucatan miniature pig. PLoS One 10: e0121732, 2015. doi: 10.1371/journal.pone.0121732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma N, Rettenmeier AW, Schmitz-Spanke S. Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics 11: 776–793, 2011. doi: 10.1002/pmic.201000320. [DOI] [PubMed] [Google Scholar]
- 9.Yu HH, Zhao H, Qing YB, Pan WR, Jia BY, Zhao HY, Huang XX, Wei HJ. Porcine zygote injection with Cas9/sgRNA results in DMD-modified pig with muscle dystrophy. Int J Mol Sci 17: 1668, 2016. doi: 10.3390/ijms17101668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callesen MM, Arnadottir SS, Lyskjaer I, Orntoft MW, Hoyer S, Dagnaes-Hansen F, Liu Y, Li R, Callesen H, Rasmussen MH, Berthelsen MF, Thomsen MK, Schweiger PJ, Jensen KB, Laurberg S, Orntoft TF, Elverlov-Jakobsen JE, Andersen CL. A genetically inducible porcine model of intestinal cancer. Mol Oncol 11: 1616–1629, 2017. doi: 10.1002/1878-0261.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staunstrup NH, Stenderup K, Mortensen S, Primo MN, Rosada C, Steiniche T, Liu Y, Li R, Schmidt M, Purup S, Dagnaes-Hansen F, Schroder LD, Svensson L, Petersen TK, Callesen H, Bolund L, Mikkelsen JG. Psoriasiform skin disease in transgenic pigs with high-copy ectopic expression of human integrins alpha2 and beta1. Dis Model Mech 10: 869–880, 2017. doi: 10.1242/dmm.028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renner S, Blutke A, Clauss S, Deeg CA, Kemter E, Merkus D, Wanke R, Wolf E. Porcine models for studying complications and organ crosstalk in diabetes mellitus. Cell Tissue Res 380: 341–378, 2020. doi: 10.1007/s00441-019-03158-9. [DOI] [PubMed] [Google Scholar]
- 13.Cooper DK, Hara H, Iwase H, Yamamoto T, Jagdale A, Kumar V, Mannon RB, Hanaway MJ, Anderson DJ, Eckhoff DE. Clinical pig kidney xenotransplantation: how close are we? J Am Soc Nephrol 31: 12–21, 2020. doi: 10.1681/ASN.2019070651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman ME, Musk GC, He B. Establishment of laparoscopic live donor nephrectomy in a porcine model: techniques and outcomes in 44 pigs. J Surg Res 222: 132–138, 2018. doi: 10.1016/j.jss.2017.09.042. [DOI] [PubMed] [Google Scholar]
- 15.Tang H, Mayersohn M. Porcine prediction of pharmacokinetic parameters in people: a pig in a poke? Drug Metab Dispos 46: 1712–1724, 2018. doi: 10.1124/dmd.118.083311. [DOI] [PubMed] [Google Scholar]
- 16.Helke KL, Nelson KN, Sargeant AM, Jacob B, McKeag S, Haruna J, Vemireddi V, Greeley M, Brocksmith D, Navratil N, Stricker-Krongrad A, Hollinger C. Pigs in toxicology: breed differences in metabolism and background findings. Toxicol Pathol 44: 575–590, 2016. doi: 10.1177/0192623316639389. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz RS, Edelman E, Virmani R, Carter A, Granada JF, Kaluza GL, Chronos NA, Robinson KA, Waksman R, Weinberger J, Wilson GJ, Wilensky RL. Drug-eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv 1: 143–153, 2008. doi: 10.1161/CIRCINTERVENTIONS.108.789974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellum JA, Ronco C, Bellomo R. Conceptual advances and evolving terminology in acute kidney disease. Nat Rev Nephrol. In press. doi: 10.1038/s41581-021-00410-w. [DOI] [PubMed] [Google Scholar]
- 19.Bagetti Filho HJ, Pereira-Sampaio MA, Favorito LA, Sampaio FJ. Pig kidney: anatomical relationships between the renal venous arrangement and the kidney collecting system. J Urol 179: 1627–1630, 2008. doi: 10.1016/j.juro.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 20.Giraud S, Favreau F, Chatauret N, Thuillier R, Maiga S, Hauet T. Contribution of large pig for renal ischemia-reperfusion and transplantation studies: the preclinical model. J Biomed Biotechnol 2011: 532127, 2011. doi: 10.1155/2011/532127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sampaio FJ, Pereira-Sampaio MA, Favorito LA. The pig kidney as an endourologic model: anatomic contribution. J Endourol 12: 45–50, 1998. doi: 10.1089/end.1998.12.45. [DOI] [PubMed] [Google Scholar]
- 22.Roth JA, Tuggle CK. Livestock models in translational medicine. ILAR J 56: 1–6, 2015. doi: 10.1093/ilar/ilv011. [DOI] [PubMed] [Google Scholar]
- 23.Chade AR, Williams ML, Engel J, Guise E, Harvey TW. A translational model of chronic kidney disease in swine. Am J Physiol Renal Physiol 315: F364–F373, 2018. doi: 10.1152/ajprenal.00063.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McInnes EF, McKeag S. A brief review of infrequent spontaneous findings, peculiar anatomical microscopic features, and potential artifacts in gottingen minipigs. Toxicol Pathol 44: 338–345, 2016. doi: 10.1177/0192623315622423. [DOI] [PubMed] [Google Scholar]
- 25.Alstrup AK. Blood lactate concentrations in gottingen minipigs compared with domestic pigs. J Am Assoc Lab Anim Sci 55: 18–20, 2016. [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon DJ, Lee YS, Shin D, Won KH, Song KD. Genome analysis of Yucatan miniature pigs to assess their potential as biomedical model animals. Asian-Australas J Anim Sci 32: 290–296, 2019. doi: 10.5713/ajas.18.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi NR, Seo DW, Choi KM, Ko NY, Kim JH, Kim HI, Jung WY, Lee JH. Analysis of swine leukocyte antigen haplotypes in Yucatan miniature pigs used as biomedical model animal. Asian-Australas J Anim Sci 29: 321–326, 2016. doi: 10.5713/ajas.15.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang SB, Guo KN, Xie F, Liu Y, Shang HT, Wei H. Normal electrocardiogram of bama miniature pigs (Sus scrofa domestica). J Am Assoc Lab Anim Sci 55: 152–154, 2016. [PMC free article] [PubMed] [Google Scholar]
- 29.Shen Y, Xu K, Yuan Z, Guo J, Zhao H, Zhang X, Zhao L, Qing Y, Li H, Pan W, Jia B, Zhao HY, Wei HJ. Efficient generation of P53 biallelic knockout Diannan miniature pigs via TALENs and somatic cell nuclear transfer. J Transl Med 15: 224, 2017. doi: 10.1186/s12967-017-1327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W, Wu LH, Yue M, Nashun B, Tang H, Chen Y, Chen BZ, Yuan J, Xiao D, Gu WW. Generation of DKK1 transgenic Tibet minipigs by somatic cell nuclear transfer (SCNT). Oncotarget 8: 74331–74339, 2017. doi: 10.18632/oncotarget.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groger M, Scheuerle A, Wagner F, Simon F, Matallo J, McCook O, Seifritz A, Stahl B, Wachter U, Vogt JA, Asfar P, Matejovic M, Moller P, Lampl L, Bracht H, Calzia E, Georgieff M, Radermacher P, Stahl W. Effects of pretreatment hypothermia during resuscitated porcine hemorrhagic shock. Crit Care Med 41: e105–117, 2013. doi: 10.1097/CCM.0b013e31827c0b1f. [DOI] [PubMed] [Google Scholar]
- 32.Hoareau GL, Tibbits EM, Simon MA, Davidson AJ, DeSoucy ES, Faulconer ER, Grayson JK, Stewart IJ, Neff LP, Williams TK, Johnson MA. Renal effects of three endoaortic occlusion strategies in a swine model of hemorrhagic shock. Injury 50: 1908–1914, 2019. doi: 10.1016/j.injury.2019.08.037. [DOI] [PubMed] [Google Scholar]
- 33.Gomez BI, McIntyre MK, Gurney JM, Chung KK, Cancio LC, Dubick MA, Burmeister DM. Enteral resuscitation with oral rehydration solution to reduce acute kidney injury in burn victims: Evidence from a porcine model. PLoS One 13: e0195615, 2018. doi: 10.1371/journal.pone.0195615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burmeister DM, McIntyre MK, Baker BA, Rizzo JA, Brown A, Natesan S, Chung KK, Christy RJ. Impact of isolated burns on major organs: a large animal model characterized. Shock 46: 137–147, 2016. doi: 10.1097/SHK.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 35.Matejovic M, Ince C, Chawla LS, Blantz R, Molitoris BA, Rosner MH, Okusa MD, Kellum JA, Ronco C, ADQI XIII Work Group. Renal hemodynamics in AKI: in search of new treatment targets. J Am Soc Nephrol 27: 49–58, 2016. doi: 10.1681/ASN.2015030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298: 229–317, 2012. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silberstein JL, Sprenkle PC, Su D, Power NE, Tarin TV, Ezell P, Sjoberg DD, Feifer A, Fleisher M, Russo P, Touijer KA. Neutrophil gelatinase-associated lipocalin (NGAL) levels in response to unilateral renal ischaemia in a novel pilot two-kidney porcine model. BJU Int 112: 517–525, 2013. doi: 10.1111/bju.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baldwin DD, Maynes LJ, Berger KA, Desai PJ, Zuppan CW, Zimmerman GJ, Winkielman AM, Sterling TH, Tsai CK, Ruckle HC. Laparoscopic warm renal ischemia in the solitary porcine kidney model. Urology 64: 592–597, 2004. doi: 10.1016/j.urology.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Brunswig-Spickenheier B, Boche J, Westenfelder C, Peimann F, Gruber AD, Jaquet K, Krause K, Zustin J, Zander AR, Lange C. Limited immune-modulating activity of porcine mesenchymal stromal cells abolishes their protective efficacy in acute kidney injury. Stem Cells Dev 19: 719–729, 2010. doi: 10.1089/scd.2009.0494. [DOI] [PubMed] [Google Scholar]
- 40.Gardner DS, De Brot S, Dunford LJ, Grau-Roma L, Welham SJ, Fallman R, O'Sullivan SE, Oh W, Devonald MA. Remote effects of acute kidney injury in a porcine model. Am J Physiol Renal Physiol 310: F259–F271, 2016. doi: 10.1152/ajprenal.00389.2015. [DOI] [PubMed] [Google Scholar]
- 41.Gardner DS, Welham SJ, Dunford LJ, McCulloch TA, Hodi Z, Sleeman P, O'Sullivan S, Devonald MA. Remote conditioning or erythropoietin before surgery primes kidneys to clear ischemia-reperfusion-damaged cells: a renoprotective mechanism? Am J Physiol Renal Physiol 306: F873–F884, 2014. doi: 10.1152/ajprenal.00576.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malagrino PA, Venturini G, Yogi PS, Dariolli R, Padilha K, Kiers B, Gois TC, Cardozo KH, Carvalho VM, Salgueiro JS, Girardi AC, Titan SM, Krieger JE, Pereira AC. Proteome analysis of acute kidney injury–discovery of new predominantly renal candidates for biomarker of kidney disease. J Proteomics 151: 66–73, 2017. doi: 10.1016/j.jprot.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 43.Lyon MB, Orvieto MA, Zorn KC, Tolhurst SR, Rapp DE, Mikhail AA, Brendler CB, Shalhav AL. Effect of renal ischemia in laparoscopic acute versus chronic solitary kidney model. Urology 69: 402–406, 2007. doi: 10.1016/j.urology.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Malagrino PA, Venturini G, Yogi PS, Dariolli R, Padilha K, Kiers B, Gois TC, da Motta-Leal-Filho JM, Takimura CK, Girardi AC, Carnevale FC, Zeri AC, Malheiros DM, Krieger JE, Pereira AC. Catheter-based induction of renal ischemia/reperfusion in swine: description of an experimental model. Physiol Rep 2: e12150, 2014. doi: 10.14814/phy2.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matějková Š, Scheuerle A, Wagner F, McCook O, Matallo J, Gröger M, Seifritz A, Stahl B, Vcelar B, Calzia E, Georgieff M, Möller P, Schelzig H, Radermacher P, Simon F. Carbamylated erythropoietin-FC fusion protein and recombinant human erythropoietin during porcine kidney ischemia/reperfusion injury. Intensive Care Med 39: 497–510, 2013. doi: 10.1007/s00134-012-2766-y. [DOI] [PubMed] [Google Scholar]
- 46.Castellano G, Franzin R, Stasi A, Divella C, Sallustio F, Pontrelli P, Lucarelli G, Battaglia M, Staffieri F, Crovace A, Stallone G, Seelen M, Daha MR, Grandaliano G, Gesualdo L. Complement activation during ischemia/reperfusion injury induces pericyte-to-myofibroblast transdifferentiation regulating peritubular capillary lumen reduction through pERK signaling. Front Immunol 9: 1002, 2018. doi: 10.3389/fimmu.2018.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castellano G, Melchiorre R, Loverre A, Ditonno P, Montinaro V, Rossini M, Divella C, Battaglia M, Lucarelli G, Annunziata G, Palazzo S, Selvaggi FP, Staffieri F, Crovace A, Daha MR, Mannesse M, van Wetering S, Paolo Schena F, Grandaliano G. Therapeutic targeting of classical and lectin pathways of complement protects from ischemia-reperfusion-induced renal damage. Am J Pathol 176: 1648–1659, 2010. doi: 10.2353/ajpath.2010.090276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Damasceno-Ferreira JA, Abreu LA, Bechara GR, Costa WS, Pereira-Sampaio MA, Sampaio FJ, De Souza DB. Mannitol reduces nephron loss after warm renal ischemia in a porcine model. BMC Urol 18: 16, 2018. doi: 10.1186/s12894-018-0328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bechara GR, Damasceno-Ferreira JA, Abreu LA, Costa WS, Sampaio FJ, Pereira-Sampaio MA, Souza DB. Glomerular loss after arteriovenous and arterial clamping for renal warm ischemia in a swine model. Acta Cir Bras 31: 753–758, 2016. doi: 10.1590/s0102-865020160110000008. [DOI] [PubMed] [Google Scholar]
- 50.Doulamis IP, Guariento A, Saeed MY, Nomoto RS, Duignan T, Del Nido PJ, McCully JD. A large animal model for acute kidney injury by temporary bilateral renal artery occlusion. J Vis Exp, 168: e62230, 2021. doi: 10.3791/62230. [DOI] [PubMed] [Google Scholar]
- 51.Orvieto MA, Tolhurst SR, Chuang MS, Lyon MB, Ritch CR, Rapp DE, Shalhav AL. Defining maximal renal tolerance to warm ischemia in porcine laparoscopic and open surgery model. Urology 66: 1111–1115, 2005. doi: 10.1016/j.urology.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 52.Jayle C, Milinkevitch S, Favreau F, Doucet C, Richer JP, Deretz S, Mauco G, Rabb H, Hauet T. Protective role of selectin ligand inhibition in a large animal model of kidney ischemia-reperfusion injury. Kidney Int 69: 1749–1755, 2006. doi: 10.1038/sj.ki.5000335. [DOI] [PubMed] [Google Scholar]
- 53.Barrera-Chimal J, Andre-Gregoire G, Nguyen Dinh Cat A, Lechner SM, Cau J, Prince S, Kolkhof P, Loirand G, Sauzeau V, Hauet T, Jaisser F. Benefit of mineralocorticoid receptor antagonism in AKI: role of vascular smooth muscle Rac1. J Am Soc Nephrol 28: 1216–1226, 2017. doi: 10.1681/ASN.2016040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amdisen C, Keller AK, Hansen RS, Norregaard R, Krag SP, Moldrup U, Pedersen M, Jespersen B, Birn H. Testing danegaptide effects on kidney function after ischemia/reperfusion injury in a new porcine two week model. PLoS One 11: e0164109, 2016. doi: 10.1371/journal.pone.0164109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duburcq T, Durand A, Tournoys A, Gnemmi V, Bonner C, Gmyr V, Hubert T, Pattou F, Jourdain M. Single low dose of human recombinant antithrombin (Atryn(R)) has no impact on endotoxin-induced disseminated intravascular coagulation: an experimental randomized open label controlled study. Shock 52: e60–e67, 2019. doi: 10.1097/SHK.0000000000001274. [DOI] [PubMed] [Google Scholar]
- 56.Lima A, van Rooij T, Ergin B, Sorelli M, Ince Y, Specht PA, Mik EG, Bocchi L, Kooiman K, de Jong N, Ince C. Dynamic contrast-enhanced ultrasound identifies microcirculatory alterations in sepsis-induced acute kidney injury. Crit Care Med 46: 1284–1292, 2018. doi: 10.1097/CCM.0000000000003209. [DOI] [PubMed] [Google Scholar]
- 57.Castellano G, Stasi A, Intini A, Gigante M, Di Palma AM, Divella C, Netti GS, Prattichizzo C, Pontrelli P, Crovace A, Staffieri F, Fiaccadori E, Brienza N, Grandaliano G, Pertosa G, Gesualdo L. Endothelial dysfunction and renal fibrosis in endotoxemia-induced oliguric kidney injury: possible role of LPS-binding protein. Crit Care 18: 520, 2014. doi: 10.1186/s13054-014-0520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vassal O, Bonnet JM, Barthelemy A, Allaouchiche B, Goy-Thollot I, Louzier V, Paquet C, Ayoub JY, Dauwalder O, Jacquet-Lagreze M, Junot S. Renal haemodynamic response to amino acids infusion in an experimental porcine model of septic shock. Acta Anaesthesiol Scand 59: 598–608, 2015. doi: 10.1111/aas.12507. [DOI] [PubMed] [Google Scholar]
- 59.Matejovic M, Tuma Z, Moravec J, Valesova L, Sykora R, Chvojka J, Benes J, Mares J. Renal proteomic responses to severe sepsis and surgical trauma: dynamic analysis of porcine tissue biopsies. Shock 46: 453–464, 2016. doi: 10.1097/SHK.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 60.Barth E, Bassi G, Maybauer DM, Simon F, Groger M, Oter S, Speit G, Nguyen CD, Hasel C, Moller P, Wachter U, Vogt JA, Matejovic M, Radermacher P, Calzia E. Effects of ventilation with 100% oxygen during early hyperdynamic porcine fecal peritonitis. Crit Care Med 36: 495–503, 2008. doi: 10.1097/01.CCM.0B013E318161FC45. [DOI] [PubMed] [Google Scholar]
- 61.Merz T, Wepler M, Nußbaum B, Vogt J, Calzia E, Wang R, Szabo C, Radermacher P, McCook O. Cystathionine-gamma-lyase expression is associated with mitochondrial respiration during sepsis-induced acute kidney injury in swine with atherosclerosis. Intensive Care Med Exp 6: 43, 2018. doi: 10.1186/s40635-018-0208-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Zhang M, Mao H, Cheng Z, Zhang Q, Jiang C, Sun C, Sun L. Serum neutrophil gelatinase-associated lipocalin and proinflammatory cytokines in pigs with septic versus non-septic acute kidney injury. Int Urol Nephrol 47: 413–420, 2015. doi: 10.1007/s11255-014-0878-8. [DOI] [PubMed] [Google Scholar]
- 63.Cui J, Li T, Hong Q, Lin S, Sun X, Cai G, Bai XY, Chen X. N-acetylcysteine ameliorates gentamicin-induced nephrotoxicity by enhancing autophagy and reducing oxidative damage in miniature pigs. Shock 52: 622–630, 2019. doi: 10.1097/SHK.0000000000001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robbins ME, Bywaters TB, Jaenke RS, Hopewell JW, Matheson LM, Tothill P, Whitehouse E. Long-term studies of cisplatin-induced reductions in porcine renal functional reserve. Cancer Chemother Pharmacol 29: 309–315, 1992. doi: 10.1007/BF00685950. [DOI] [PubMed] [Google Scholar]
- 65.Santiago MJ, Fernandez SN, Lazaro A, Gonzalez R, Urbano J, Lopez J, Solana MJ, Toledo B, Del Castillo J, Tejedor A, Lopez-Herce J. Cisplatin-induced non-oliguric acute kidney injury in a pediatric experimental animal model in piglets. PLoS One 11: e0149013, 2016. doi: 10.1371/journal.pone.0149013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu J, Wan X, Zhang H, Li W, Ma M, Pan B, Liang X, Cao C. Retinoic acid attenuates contrast-induced acute kidney injury in a miniature pig model. Biochem Biophys Res Commun 512: 163–169, 2019. doi: 10.1016/j.bbrc.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 67.Xu J, Ma L, Fu P. MicroRNA-30c attenuates contrast-induced acute kidney injury by suppressing NLRP3 inflammasome. Int Immunopharmacol 87: 106457, 2020. doi: 10.1016/j.intimp.2020.106457. [DOI] [PubMed] [Google Scholar]
- 68.Gozdzik W, Zielinski S, Zielinska M, Ratajczak K, Skrzypczak P, Rodziewicz S, Kubler A, Lofstrom K, Dziegiel P, Olbromski M, Adamik B, Ryniak S, Harbut P, Albert J, Frostell C. Beneficial effects of inhaled nitric oxide with intravenous steroid in an ischemia-reperfusion model involving aortic clamping. Int J Immunopathol Pharmacol 32: 394632017751486, 2018. doi: 10.1177/0394632017751486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mehaffey JH, Money D, Charles EJ, Schubert S, Pineros AF, Wu D, Bontha SV, Hawkins R, Teman NR, Laubach VE, Mas VR, Tribble CG, Maluf DG, Sharma AK, Yang Z, Kron IL, Roeser ME. Adenosine 2A receptor activation attenuates ischemia reperfusion injury during extracorporeal cardiopulmonary resuscitation. Ann Surg 269: 1176–1183, 2019. doi: 10.1097/SLA.0000000000002685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van de Wouw J, Sorop O, van Drie RW, van Duin RW, Nguyen IT, Joles JA, Verhaar MC, Merkus D, Duncker DJ. Perturbations in myocardial perfusion and oxygen balance in swine with multiple risk factors: a novel model of ischemia and no obstructive coronary artery disease. Basic Res Cardiol 115: 21, 2020. doi: 10.1007/s00395-020-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sadeghi M, Dogan EM, Karlsson C, Jansson K, Seilitz J, Skoog P, Horer TM, Nilsson KF. Total resuscitative endovascular balloon occlusion of the aorta causes inflammatory activation and organ damage within 30 minutes of occlusion in normovolemic pigs. BMC Surg 20: 43, 2020. doi: 10.1186/s12893-020-00700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao Z, Yang C, Li L, Zhao T, Wang L, Rong R, Yang B, Xu M, Zhu T. Increased peripheral and local soluble FGL2 in the recovery of renal ischemia reperfusion injury in a porcine kidney auto-transplantation model. J Transl Med 12: 53, 2014. doi: 10.1186/1479-5876-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giraud S, Steichen C, Couturier P, Tillet S, Mallet V, Coudroy R, Goujon JM, Hannaert P, Hauet T. Influence of hypoxic preservation temperature on endothelial cells and kidney integrity. BioMed Res Int 2019: 8572138, 2019. doi: 10.1155/2019/8572138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaminski J, Delpech PO, Kaaki-Hosni S, Promeyrat X, Hauet T, Hannaert P. Oxygen Consumption by warm ischemia-injured porcine kidneys in hypothermic static and machine preservation. J Surg Res 242: 78–86, 2019. doi: 10.1016/j.jss.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 75.Jimenez-Fuertes M, Garcia-Olmo DC, Puy S, Beisani M, Planells F, Boldo A, Ruiz-Tovar J, Duran M, Garcia-Olmo D. Effects of negative-pressure therapy with and without ropivacaine instillation in the early evolution of severe peritonitis in pigs. Eur J Trauma Emerg Surg 47: 597–606, 2021. doi: 10.1007/s00068-019-01244-9. [DOI] [PubMed] [Google Scholar]
- 76.Zhang W, Guan Y, Bayliss G, Zhuang S. Class IIa HDAC inhibitor TMP195 alleviates lipopolysaccharide-induced acute kidney injury. Am J Physiol Renal Physiol 319: F1015–F1026, 2020. doi: 10.1152/ajprenal.00405.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Netti GS, Sangregorio F, Spadaccino F, Staffieri F, Crovace A, Infante B, Maiorano A, Godeas G, Castellano G, Di Palma AM, Prattichizzo C, Cotoia A, Mirabella L, Gesualdo L, Cinnella G, Stallone G, Ranieri E, Grandaliano G. LPS removal reduces CD80-mediated albuminuria in critically ill patients with gram-negative sepsis. Am J Physiol Renal Physiol 316: F723–F731, 2019. doi: 10.1152/ajprenal.00491.2018. [DOI] [PubMed] [Google Scholar]
- 78.Benes J, Chvojka J, Sykora R, Radej J, Krouzecky A, Novak I, Matejovic M. Searching for mechanisms that matter in early septic acute kidney injury: an experimental study. Crit Care 15: R256, 2011. doi: 10.1186/cc10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Simon F, Giudici R, Scheuerle A, Groger M, Asfar P, Vogt JA, Wachter U, Ploner F, Georgieff M, Moller P, Laporte R, Radermacher P, Calzia E, Hauser B. Comparison of cardiac, hepatic, and renal effects of arginine vasopressin and noradrenaline during porcine fecal peritonitis: a randomized controlled trial. Crit Care 13: R113, 2009. doi: 10.1186/cc7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matejovic M, Valesova L, Benes J, Sykora R, Hrstka R, Chvojka J. Molecular differences in susceptibility of the kidney to sepsis-induced kidney injury. BMC Nephrol 18: 183, 2017. doi: 10.1186/s12882-017-0602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chvojka J, Sykora R, Krouzecky A, Radej J, Varnerova V, Karvunidis T, Hes O, Novak I, Radermacher P, Matejovic M. Renal haemodynamic, microcirculatory, metabolic and histopathological responses to peritonitis-induced septic shock in pigs. Crit Care 12: R164, 2008. doi: 10.1186/cc7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dyson A, Singer M. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? Crit Care Med 37: S30–37, 2009. doi: 10.1097/CCM.0b013e3181922bd3. [DOI] [PubMed] [Google Scholar]
- 83.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 110: 3507–3512, 2013. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zakaryan H, Cholakyans V, Simonyan L, Misakyan A, Karalova E, Chavushyan A, Karalyan Z. A study of lymphoid organs and serum proinflammatory cytokines in pigs infected with African swine fever virus genotype II. Arch Virol 160: 1407–1414, 2015. doi: 10.1007/s00705-015-2401-7. [DOI] [PubMed] [Google Scholar]
- 85.Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol 20: 50–57, 2012. doi: 10.1016/j.tim.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duburcq T, Durand A, Tournoys A, Gnemmi V, Gmyr V, Pattou F, Jourdain M, Tamion F, Besnier E, Preau S, Parmentier-Decrucq E, Mathieu D, Poissy J, Favory R. Sodium lactate improves renal microvascular thrombosis compared to sodium bicarbonate and 0.9% NaCl in a porcine model of endotoxic shock: an experimental randomized open label controlled study. Ann Intensive Care 8: 24, 2018. doi: 10.1186/s13613-018-0367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Castellheim A, Thorgersen EB, Hellerud BC, Pharo A, Johansen HT, Brosstad F, Gaustad P, Brun H, Fosse E, Tonnessen TI, Nielsen EW, Mollnes TE. New biomarkers in an acute model of live Escherichia coli-induced sepsis in pigs. Scand J Immunol 68: 75–84, 2008. doi: 10.1111/j.1365-3083.2008.02122.x. [DOI] [PubMed] [Google Scholar]
- 88.Izquierdo-Garcia JL, Nin N, Cardinal-Fernandez P, Rojas Y, de Paula M, Granados R, Martínez-Caro L, Ruíz-Cabello J, Lorente JA. Identification of novel metabolomic biomarkers in an experimental model of septic acute kidney injury. Am J Physiol Renal Physiol 316: F54–F62, 2019. doi: 10.1152/ajprenal.00315.2018. [DOI] [PubMed] [Google Scholar]
- 89.Westover AJ, Buffington DA, Johnston KA, Smith PL, Pino CJ, Humes HD. A bio-artificial renal epithelial cell system conveys survival advantage in a porcine model of septic shock. J Tissue Eng Regen Med 11: 649–657, 2017. doi: 10.1002/term.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nuβbaum BL, McCook O, Hartmann C, Matallo J, Wepler M, Antonucci E, Kalbitz M, Huber-Lang M, Georgieff M, Calzia E, Radermacher P, Hafner S. Left ventricular function during porcine-resuscitated septic shock with pre-existing atherosclerosis. Intensive Care Med Exp 4: 14, 2016. [Erratum in Intensive Care Med Exp 4: 18, 2016]. doi: 10.1186/s40635-016-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wepler M, Hafner S, Scheuerle A, Reize M, Groger M, Wagner F, Simon F, Matallo J, Gottschalch F, Seifritz A, Stahl B, Matejovic M, Kapoor A, Moller P, Calzia E, Georgieff M, Wachter U, Vogt JA, Thiemermann C, Radermacher P, McCook O. Effects of the PPAR-beta/delta agonist GW0742 during resuscitated porcine septic shock. Intensive Care Med Exp 1: 28, 2013. doi: 10.1186/2197-425X-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Corrêa TD, Pereira AJ, Brandt S, Vuda M, Djafarzadeh S, Takala J, Jakob SM. Time course of blood lactate levels, inflammation, and mitochondrial function in experimental sepsis. Crit Care 21: 105, 2017. doi: 10.1186/s13054-017-1691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Soni H, Adebiyi A. Early septic insult in neonatal pigs increases serum and urinary soluble Fas ligand and decreases kidney function without inducing significant renal apoptosis. Ren Fail 39: 83–91, 2017. doi: 10.1080/0886022X.2016.1244082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simon TP, Schuerholz T, Hüter L, Sasse M, Heyder F, Pfister W, Marx G. Impairment of renal function using hyperoncotic colloids in a two hit model of shock: a prospective randomized study. Crit Care 16: R16, 2012. doi: 10.1186/cc11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Merz T, Stenzel T, Nußbaum B, Wepler M, Szabo C, Wang R, Radermacher P, McCook O. Cardiovascular disease and resuscitated septic shock lead to the downregulation of the H2S-producing enzyme cystathionine-gamma-lyase in the porcine coronary artery. Intensive Care Med Exp 5: 17, 2017. doi: 10.1186/s40635-017-0131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin-Sanchez D, Ruiz-Andres O, Poveda J, Carrasco S, Cannata-Ortiz P, Sanchez-Nino MD, Ruiz Ortega M, Egido J, Linkermann A, Ortiz A, Sanz AB. Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J Am Soc Nephrol 28: 218–229, 2017. doi: 10.1681/ASN.2015121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang H, Jin WW, Huang M, Ji H, Capen DE, Xia Y, Yuan J, Păunescu TG, Lu HA. Gentamicin-induced acute kidney injury in an animal model involves programmed necrosis of the collecting duct. J Am Soc Nephrol 31: 2097–2115, 2020. doi: 10.1681/ASN.2019020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Song J, Liu D, Feng L, Zhang Z, Jia X, Xiao W. Protective effect of standardized extract of ginkgo biloba against cisplatin-induced nephrotoxicity. Evid Based Complement Alternat Med 2013: 846126, 2013. doi: 10.1155/2013/846126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fu Y, Cai J, Li F, Liu Z, Shu S, Wang Y, Liu Y, Tang C, Dong Z. Chronic effects of repeated low-dose cisplatin treatment in mouse kidneys and renal tubular cells. Am J Physiol Renal Physiol 317: F1582–F1592, 2019. doi: 10.1152/ajprenal.00385.2019. [DOI] [PubMed] [Google Scholar]
- 100.Lee YJ, Li KY, Wang PJ, Huang HW, Chen MJ. Alleviating chronic kidney disease progression through modulating the critical genus of gut microbiota in a cisplatin-induced Lanyu pig model. J Food Drug Anal 28: 103–114, 2020. doi: 10.1016/j.jfda.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 101.Cui J, Bai XY, Sun X, Cai G, Hong Q, Ding R, Chen X. Rapamycin protects against gentamicin-induced acute kidney injury via autophagy in mini-pig models. Sci Rep 5: 11256, 2015. doi: 10.1038/srep11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fahling M, Seeliger E, Patzak A, Persson PB. Understanding and preventing contrast-induced acute kidney injury. Nat Rev Nephrol 13: 169–180, 2017. doi: 10.1038/nrneph.2016.196. [DOI] [PubMed] [Google Scholar]
- 103.Eng J, Wilson RF, Subramaniam RM, Zhang A, Suarez-Cuervo C, Turban S, Choi MJ, Sherrod C, Hutfless S, Iyoha EE, Bass EB. Comparative effect of contrast media type on the incidence of contrast-induced nephropathy: a systematic review and meta-analysis. Ann Intern Med 164: 417–424, 2016. doi: 10.7326/M15-1402. [DOI] [PubMed] [Google Scholar]
- 104.Lamby P, Jung F, Falter J, Mrowietz C, Graf S, Schellenberg L, Platz Batista da Silva N, Prantl L, Franke RP, Jung EM. Effect of radiographic contrast media on renal perfusion-first results. Clin Hemorheol Microcirc 64: 287–295, 2016. doi: 10.3233/CH-168110. [DOI] [PubMed] [Google Scholar]
- 105.Treitl M, Rupprecht H, Wirth S, Korner M, Reiser M, Rieger J. Assessment of renal vasoconstriction in vivo after intra-arterial administration of the isosmotic contrast medium iodixanol compared to the low-osmotic contrast medium iopamidol. Nephrol Dial Transplant 24: 1478–1485, 2009. doi: 10.1093/ndt/gfn638. [DOI] [PubMed] [Google Scholar]
- 106.Tian YG, Yue M, Nashun B, Wu SJ, Gu WW, Wang YJ. The diagnostic value of [(18)F]-FDG-PET/CT in assessment of radiation renal injury in Tibet minipigs model. J Transl Med 16: 257, 2018. doi: 10.1186/s12967-018-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hosgood SA, Moore T, Qurashi M, Adams T, Nicholson ML. Hydrogen gas does not ameliorate renal ischemia reperfusion injury in a preclinical model. Artif Organs 42: 723–727, 2018. doi: 10.1111/aor.13118. [DOI] [PubMed] [Google Scholar]
- 108.Hauet T, Goujon JM, Baumert H, Petit I, Carretier M, Eugene M, Vandewalle A. Polyethylene glycol reduces the inflammatory injury due to cold ischemia/reperfusion in autotransplanted pig kidneys. Kidney Int 62: 654–667, 2002. doi: 10.1046/j.1523-1755.2002.00473.x. [DOI] [PubMed] [Google Scholar]
- 109.Agarwal A, Dong Z, Harris R, Murray P, Parikh SM, Rosner MH, Kellum JA, Ronco C, Acute Dialysis Quality Initiative XIII Working Group. Cellular and molecular mechanisms of AKI. J Am Soc Nephrol 27: 1288–1299, 2016. doi: 10.1681/ASN.2015070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fu Y, Tang C, Cai J, Chen G, Zhang D, Dong Z. Rodent models of AKI-CKD transition. Am J Physiol Renal Physiol 315: F1098–F1106, 2018. doi: 10.1152/ajprenal.00199.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu HF, Li H, Bai G, Zhang QZ, Xiang G, Liu T, Wang HB. Establishment of renal failure models by laparoscopy in bama pigs which underwent partial nephrectomy and radical contralateral nephrectomy. J Vet Res 63: 447–455, 2019. doi: 10.2478/jvetres-2019-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol 23: 1295–1301, 2003. doi: 10.1161/01.ATV.0000077477.40824.52. [DOI] [PubMed] [Google Scholar]
- 113.Meng Y, Eirin A, Zhu XY, O'Brien DR, Lerman A, Van Wijnen AJ, Lerman LO. The metabolic syndrome modifies the mRNA expression profile of extracellular vesicles derived from porcine mesenchymal stem cells. Diabetol Metab Syndr 10: 58, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Song T, Eirin A, Zhu X, Zhao Y, Krier JD, Tang H, Jordan KL, Woollard JR, Taner T, Lerman A, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles induce regulatory t cells to ameliorate chronic kidney injury. Hypertension 75: 1223–1232, 2020. doi: 10.1161/HYPERTENSIONAHA.119.14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schomberg DT, Tellez A, Meudt JJ, Brady DA, Dillon KN, Arowolo FK, Wicks J, Rousselle SD, Shanmuganayagam D. Miniature swine for preclinical modeling of complexities of human disease for translational scientific discovery and accelerated development of therapies and medical devices. Toxicol Pathol 44: 299–314, 2016. doi: 10.1177/0192623315618292. [DOI] [PubMed] [Google Scholar]
- 116.Renner S, Blutke A, Dobenecker B, Dhom G, Müller TD, Finan B, Clemmensen C, Bernau M, Novak I, Rathkolb B, Senf S, Zöls S, Roth M, Götz A, Hofmann SM, Hrabĕ de Angelis M, Wanke R, Kienzle E, Scholz AM, DiMarchi R, Ritzmann M, Tschöp MH, Wolf E. Metabolic syndrome and extensive adipose tissue inflammation in morbidly obese Göttingen minipigs. Mol Metab 16: 180–190, 2018. doi: 10.1016/j.molmet.2018.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lykke M, Hother AL, Hansen CF, Friis H, Mølgaard C, Michaelsen KF, Briend A, Larsen T, Sangild PT, Thymann T. Malnutrition induces gut atrophy and increases hepatic fat infiltration: studies in a pig model of childhood malnutrition. Am Trans Res 5: 543–554, 2013. [PMC free article] [PubMed] [Google Scholar]
- 118.Curtasu MV, Knudsen KE, Callesen H, Purup S, Stagsted J, Hedemann MS. Obesity Development in a miniature Yucatan pig model: a multi-compartmental metabolomics study on cloned and normal pigs fed restricted or ad libitum high-energy diets. J Proteome Res 18: 30–47, 2019. doi: 10.1021/acs.jproteome.8b00264. [DOI] [PubMed] [Google Scholar]
- 119.Kinoshita Y, Iwami D, Fujimura T, Kume H, Yokoo T, Kobayashi E. Techniques of orthotopic renal transplantation in pigs. One donor to two recipients via inverted grafting. Acta Cir Bras 36: e360208, 2021. doi: 10.1590/ACB360208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yoshida J, Ozaki KS, Nalesnik MA, Ueki S, Castillo-Rama M, Faleo G, Ezzelarab M, Nakao A, Ekser B, Echeverri GJ, Ross MA, Stolz DB, Murase N. Ex vivo application of carbon monoxide in UW solution prevents transplant-induced renal ischemia/reperfusion injury in pigs. Am J Transplant 10: 763–772, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295: 1089–1092, 2002. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Z, Hara H, Long C, Iwase H, Qi H, Macedo C, Mangiola M, Zeevi A, Ezzelarab M, Ayares D, Cooper DK, Wijkstrom M. Immune responses of HLA highly sensitized and nonsensitized patients to genetically engineered pig cells. Transplantation 102: e195–e204, 2018. doi: 10.1097/TP.0000000000002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kobayashi E, Sano M. Organ preservation solution containing dissolved hydrogen gas from a hydrogen-absorbing alloy canister improves function of transplanted ischemic kidneys in miniature pigs. PLoS One 14: e0222863, 2019. doi: 10.1371/journal.pone.0222863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ware K, Qamri Z, Ozcan A, Satoskar AA, Nadasdy G, Rovin BH, Hebert LA, Nadasdy T, Brodsky SV. N-acetylcysteine ameliorates acute kidney injury but not glomerular hemorrhage in an animal model of warfarin-related nephropathy. Am J Physiol Renal Physiol 304: F1421–F1427, 2013. doi: 10.1152/ajprenal.00689.2012. [DOI] [PubMed] [Google Scholar]
- 125.Wang J, Wang D, Li Y, Zuo H, Wang S, Xu X, Guo X, Gao Y, Wang S, Peng R. Rhabdomyolysis-induced acute kidney injury under hypoxia and deprivation of food and water. Kidney Blood Press Res 37: 414–421, 2013. doi: 10.1159/000350154. [DOI] [PubMed] [Google Scholar]
- 126.Packialakshmi B, Stewart IJ, Burmeister DM, Chung KK, Zhou X. Large animal models for translational research in acute kidney injury. Ren Fail 42: 1042–1058, 2020. doi: 10.1080/0886022X.2020.1830108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dehoux JP, Gianello P. The importance of large animal models in transplantation. Front Biosci 12: 4864–4880, 2007. doi: 10.2741/2434. [DOI] [PubMed] [Google Scholar]
- 128.Rollin BE. Animal research: a moral science. Talking Point on the use of animals in scientific research. EMBO Rep 8: 521–525, 2007. doi: 10.1038/sj.embor.7400996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Snoeijs MG, Matthijsen RA, Seeldrayers S, Marcus MA, Daemen JW, Peutz-Kootstra CJ, Buurman WA, Schurink GW, Ernest van Heurn LW. Autologous transplantation of ischemically injured kidneys in pigs. J Surg Res 171: 844–850, 2011. doi: 10.1016/j.jss.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 130.He B, Musk GC, Mou L, Waneck GL, Delriviere L. Laparoscopic surgery for kidney orthotopic transplant in the pig model. JSLS 17: 126–131, 2013. doi: 10.4293/108680812x13517013318021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smith AC, Swindle MM. Preparation of swine for the laboratory. ILAR J 47: 358–363, 2006. doi: 10.1093/ilar.47.4.358. [DOI] [PubMed] [Google Scholar]
- 132.Hannon JP, Bossone CA, Wade CE. Normal physiological values for conscious pigs used in biomedical research. Lab Anim Sci 40: 293–298, 1990. [PubMed] [Google Scholar]
- 133.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 172: 2731–2738, 2004. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 134.Dawson HD, Loveland JE, Pascal G, Gilbert JG, Uenishi H, Mann KM, et al. Structural and functional annotation of the porcine immunome. BMC Genomics 14: 332, 2013. doi: 10.1186/1471-2164-14-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Langohr IM, HogenEsch H, Stevenson GW, Sturek M. Vascular-associated lymphoid tissue in swine (Sus scrofa). Comp Med 58: 168–173, 2008. [PMC free article] [PubMed] [Google Scholar]
- 136.Tohno M, Shimosato T, Moue M, Aso H, Watanabe K, Kawai Y, Yamaguchi T, Saito T, Kitazawa H. Toll-like receptor 2 and 9 are expressed and functional in gut-associated lymphoid tissues of presuckling newborn swine. Vet Res 37: 791–812, 2006. doi: 10.1051/vetres:2006036. [DOI] [PubMed] [Google Scholar]
- 137.Piriou-Guzylack L, Salmon H. Membrane markers of the immune cells in swine: an update. Vet Res 39: 54, 2008. doi: 10.1051/vetres:2008030. [DOI] [PubMed] [Google Scholar]
- 138.de León P, Cañas-Arranz R, Defaus S, Torres E, Forner M, Bustos MJ, Revilla C, Dominguez J, Andreu D, Blanco E, Sobrino F. Swine T-cells and specific antibodies evoked by peptide dendrimers displaying different FMDV T-cell epitopes. Front Immunol 11: 621537, 2020. doi: 10.3389/fimmu.2020.621537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fang X, Mou Y, Huang Z, Li Y, Han L, Zhang Y, et al. The sequence and analysis of a Chinese pig genome. GigaScience 1: 16, 2012. doi: 10.1186/2047-217X-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yum SY, Yoon KY, Lee CI, Lee BC, Jang G. Transgenesis for pig models. J Vet Sci 17: 261–268, 2016. doi: 10.4142/jvs.2016.17.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rothkotter HJ, Sowa E, Pabst R. The pig as a model of developmental immunology. Hum Exp Toxicol 21: 533–536, 2002. doi: 10.1191/0960327102ht293oa. [DOI] [PubMed] [Google Scholar]
- 142.Wernersson R, Schierup MH, Jørgensen FG, Gorodkin J, Panitz F, Staerfeldt HH, Christensen OF, Mailund T, Hornshøj H, Klein A, Wang J, Liu B, Hu S, Dong W, Li W, Wong GK, Yu J, Wang J, Bendixen C, Fredholm M, Brunak S, Yang H, Bolund L. Pigs in sequence space: a 0.66X coverage pig genome survey based on shotgun sequencing. BMC Genomics 6: 70, 2005. doi: 10.1186/1471-2164-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hesselager MO, Codrea MC, Sun Z, Deutsch EW, Bennike TB, Stensballe A, Bundgaard L, Moritz RL, Bendixen E. The Pig PeptideAtlas: A resource for systems biology in animal production and biomedicine. Proteomics 16: 634–644, 2016. doi: 10.1002/pmic.201500195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Groenen MA, Archibald AL, Uenishi H, Tuggle CK, Takeuchi Y, Rothschild MF, Rogel-Gaillard C, et al. Analyses of pig genomes provide insight into porcine demography and evolution. Nature 491: 393–398, 2012. doi: 10.1038/nature11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhao SH, Recknor J, Lunney JK, Nettleton D, Kuhar D, Orley S, Tuggle CK. Validation of a first-generation long-oligonucleotide microarray for transcriptional profiling in the pig. Genomics 86: 618–625, 2005. doi: 10.1016/j.ygeno.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 146.Tsai S, Cassady JP, Freking BA, Nonneman DJ, Rohrer GA, Piedrahita JA. Annotation of the Affymetrix porcine genome microarray. Anim Genet 37: 423–424, 2006. doi: 10.1111/j.1365-2052.2006.01460.x. [DOI] [PubMed] [Google Scholar]
- 147.Tuma Z, Kuncova J, Mares J, Matejovic M. Mitochondrial proteomes of porcine kidney cortex and medulla: foundation for translational proteomics. Clin Exp Nephrol 20: 39–49, 2016. doi: 10.1007/s10157-015-1135-x. [DOI] [PubMed] [Google Scholar]