Keywords: polycystic kidney disease, polycystin-2, primary cilium, PKD2, TRPP
Abstract
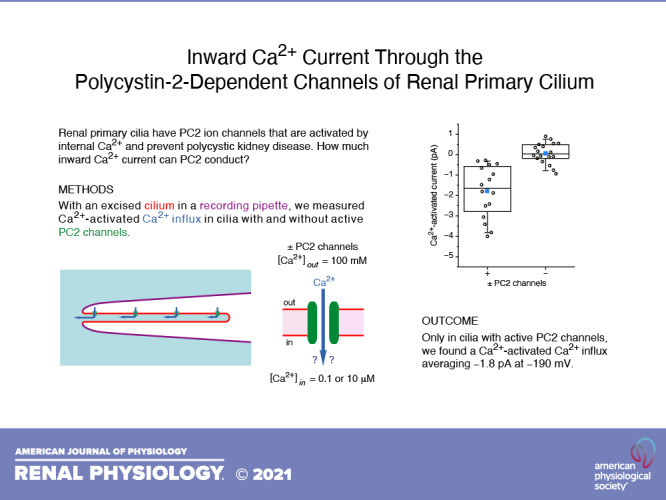
In 15% of cases, autosomal dominant polycystic kidney disease arises from defects in polycystin-2 (PC2). PC2 is a member of the polycystin transient receptor potential subfamily of cation-conducting channels and is expressed in the endoplasmic reticulum and primary cilium of renal epithelial cells. PC2 opposes a procystogenic influence of the cilium, and it has been proposed that this beneficial effect is mediated in part by a flow of Ca2+ through PC2 channels into the primary cilium. However, previous efforts to determine the permeability of PC2 channels to Ca2+ have yielded widely varying results. Here, we report the mean macroscopic Ca2+ influx through native PC2 channels in the primary cilia of mIMCD-3 cells, which are derived from the murine inner medullary collecting duct. Under conditions designed to isolate inward Ca2+ currents, a small inward Ca2+ current was detected in cilia with active PC2 channels but not in cilia lacking those channels. The current was activated by the addition of 10 µM internal Ca2+, which is known to activate ciliary PC2 channels. It was blocked by 10 µM isosakuranetin, which blocks the same channels. On average, the current amplitude was −1.8 pA at −190 mV; its conductance from −50 to −200 mV averaged 20 pS. Thus, native PC2 channels of renal primary cilia are able to conduct a small but detectable Ca2+ influx under the conditions tested. The possible consequences of this influx are discussed.
NEW & NOTEWORTHY In autosomal dominant polycystic kidney disease, it is proposed that Ca2+ entering the primary cilium through polycystin-2 (PC2) channels may limit the formation of cysts. Recent studies predict that any macroscopic Ca2+ influx through these channels should be small. We report that the native PC2 channels in primary cilia of cultured renal epithelial cells can allow a small macroscopic calcium influx. This may allow a significant accumulation of Ca2+ in the cilium in vivo.
INTRODUCTION
The autosomal dominant form of polycystic kidney disease (ADPKD) has a high incidence, between 1:400 and 1:1,000 (1), and is primarily caused by mutations in one of two membrane proteins: polycystin-1 (PC1; ∼78% of cases) or polycystin-2 (PC2; ∼15% of cases) (2). PC2 is a member of the polycystin transient receptor potential (TRPP) subfamily of transient receptor potential cation-conducting channels (3); PC2 protein has also been called PKD2, TRPP2, and TRPP1. It is expressed in both the endoplasmic reticulum (4) and the primary cilium (5–8). It has long been proposed that a ciliary defect underlies cystogenesis, a hallmark pathology of polycystic kidney disease (PKD). Evidence now strongly supports this hypothesis. In cells lacking PC1 or PC2, cystogenesis is greatly exacerbated by the presence of the cilium (9). In healthy cells, then, PC1 and PC2 likely protect against a procystogenic influence of the cilium. In PKD, renal epithelial cells have depressed intracellular Ca2+ (10–12), and this is proposed to be “the primary causative abnormality in PKD” (13). A common hypothesis links this observation to the ciliary dependence of the disease: it has been proposed that Ca2+ fluxes through ciliary ion channels, including PC2, help to maintain the resting cytoplasmic Ca2+ level in healthy cells (10–12, 14). Consistent with this idea, five missense mutations in PC2 that cause human ADPKD were recently shown to disrupt the protein’s channel function when expressed in the cilium (15).
The aforementioned hypothesis supposes that PC2 channels conduct an inward Ca2+ current, but just how well PC2 conducts Ca2+ has been difficult to establish. Early studies using both native channels and exogenously expressed PC2 have suggested a high permeability to Ca2+ (4, 16–21). Estimates of the permeability of Ca2+ relative to Na+ or K+ have ranged from 0.21 to 6, and single-channel conductances to Ca2+ have ranged from 35 to 95 pS. After recording the activity of native PC2 channels in the primary cilia of immortalized renal epithelial cells, we too have reported a substantial permeability to Ca2+ [permeability ratio of Ca2+ to K+ (PCa/PK) = 0.55] (7). Subsequently, though, Liu et al. (8) examined renal cells in primary cultures from the murine inner medullary collecting duct of mice. The resulting current reflected a much smaller Ca2+ permeability [permeability ratio of Ca2+ to Na+ (PCa/PNa) = 0.06], and the single-channel conductance for Ca2+ was just 4 pS. Such a low conductance to Ca2+, together with the low open probability of PC2 channels at negative membrane potentials (7, 8), suggests that the mean macroscopic Ca2+ influx at negative potentials might be very small.
We now report that PC2 channels in the renal primary cilium can support a measurable macroscopic Ca2+ influx. With high external Ca2+, a small Ca2+ influx is detectable. Three lines of evidence argue that this influx is attributable to PC2 channels. First, it is present in cilia with active PC2 channels but not in cilia lacking them. Second, the current is activated by 10 µM cytoplasmic Ca2+, as are the ciliary PC2 channels (7, 8). Finally, the ciliary Ca2+ influx is mostly blocked by isosakuranetin, which blocks ciliary PC2 channels (22). The result is discussed in the context of previous conflicting estimates, including our own.
MATERIALS AND METHODS
Electrical Recording
Electrical recordings were made from the primary cilia of mIMCD-3 cells as previously described (23). In short, mIMCD-3 cells [murine epithelial cells from the renal inner medullary collecting duct, CRL-2123, American Type Culture Collection (24)] were cultured on beads that were free to move in the recording chamber. Suction was applied to a recording pipette so that a single primary cilium entered the pipette. If the resistance between the membrane and the pipette was at least 1 GΩ, the cilium was excised from the cell. This left the cilium inside the recording pipette in the inside-out configuration. The pipette containing the cilium could then be transferred among the different solutions used to bathe the cytoplasmic face of the membrane. A schematic diagram appears elsewhere (7). All recordings were done under voltage clamp at room temperature (24°C) using equipment and software as previously described (7, 23). During acquisition, currents were low-pass filtered at 2 kHz and digitized at 5 kHz.
During recording, the beads coated with cells were stored in a standard external (extracellular) solution containing (in mM) 140 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 2 sodium pyruvate, 5 HEPES, and 9.4 d-glucose, adjusted to pH 7.4 with NaOH. The recording pipettes were filled with an external solution containing (in mM) 100 CaCl2, 5 HEPES, and 9.4 d-glucose, adjusted to pH 7.4 with Tris base. In all internal (cytoplasmic) solutions, Cl− was replaced with methanesulfonate− and gluconate− to minimize any possible inward current from Cl− efflux, and MgATP was included to block transient receptor potential melastatin 4 (TRPM4) channels present in these cilia (25). Two internal solutions were used, each with a different concentration of free Ca2+. The first contained (in mM) 145 potassium methanesulfonate, 0.8 calcium gluconate, 2 magnesium gluconate, 5 HEPES, 2 BAPTA, 2 MgATP, and 5 d-glucose, adjusted to pH 7.4 with KOH. The concentration of free Ca2+ in this solution was 0.1 µM. The second internal solution contained (in mM) 145 potassium methanesulfonate, 1.8 calcium gluconate, 2 magnesium gluconate, 5 HEPES, 2 dibromoBAPTA, 2 MgATP, and 5 d-glucose, adjusted to pH 7.4 with KOH. This solution contained 10 µM free Ca2+. Concentrations of free Ca2+ were estimated experimentally by the method of Bers (26) as previously described (27). Isosakuranetin (10 µM) was added to the second internal solution as noted.
Corrections were applied for each of two liquid junction potentials. The first, which was 8.8 mV, existed between each internal bath (which contained methanesulfonate−) and its salt bridge (which contained Cl−). The second potential, which was 6.9 mV, existed during the patch procedure because the pipette solution (which contained 100 mM CaCl2) was different from the solution bathing the cells (the standard external solution). All potentials are given as membrane potential (i.e., internal relative to external).
To establish a correlation between inward Ca2+ current and expression of PC2 channels, cilia with no detectable PC2 channels at positive potentials were used as controls. These were judged to be a closer control than mIMCD-3 cells with PC2 knocked out by CRISPR/Cas9 genome editing (7), since the latter had been subjected to genetic alteration.
Materials
BAPTA was purchased from Sigma-Aldrich, dibromoBAPTA from Santa Cruz Biotechnology, and isosakuranetin from Extrasynthese. Isosakuranetin was diluted from a 10 mM stock solution in DMSO.
Data Analyses
For membrane current-voltage relations, current was measured as voltage was continuously varied from −200 mV to +100 mV. Cilia used for these experiments had from 2 to ∼25 active PC2 channels. In all cases, the results of 10 voltage ramps were averaged to better represent the mean current from open channels. Current at −190 mV was figured as the mean of the values from −180 mV to −200 mV. Slope conductance of the inward current was determined by a linear least-squares fit of the relation from −50 mV to −200 mV. To determine the reversal potential (the voltage intercept of the current-voltage relation), the relation was first low-pass filtered at 10 Hz. Data in the immediate vicinity of the apparent reversal potential were fit to a straight line, and the voltage intercept of that line is reported. Current variance was determined from a 20-s recording at −100 mV that was not subjected to postacquisition filtering or averaging.
For the single-channel experiment, 20-s recordings were made at each voltage from cilia with one to three active channels. The external (pipette) solution contained 100 mM CaCl2 and the internal solution contained 10 µM free Ca2+ as described earlier. A single-channel current-voltage relation was fit to a Goldman–Hodgkin–Katz (GHK) current equation with terms representing the available conducting cations (external Ca2+ and internal K+) (28). For this purpose, ionic concentrations were converted to activities using published activity coefficients (see Appendix 8.10 of Ref. 29). The best fit of the experimental data was determined with the NonlinearModelFit function of Mathematica 11. This solved for PCa/PK and the reversal potential and reported the 95% confidence levels for both values.
For statistical analyses, all data sets were found to be normally distributed with equal variance by the Shapiro–Wilk test and to have equal variance by Levene’s test. Populations were compared with a parametric test (Student’s t test for independent measures, two-tailed). Results are expressed as means ± SE. We eliminated as outliers any values that differed from the mean by greater than four times the SD. Only one cilium gave outlying values, for both Ca2+-activated current and Ca2+-activated conductance.
RESULTS
Our goal was to determine the average amount of any inward Ca2+ current conducted through PC2 channels in the ciliary membrane of mIMCD-3 cells. Two steps were taken to maximize the likelihood that any inward currents measured were conducted by Ca2+. First, the external solution contained 100 mM Ca2+ as the only cation available to conduct an inward current. Second, to eliminate inward currents due to a possible efflux of Cl−, internal Cl− was replaced by methanesulfonate− and gluconate−. To increase the activity of PC2 channels, free Ca2+ in the internal solutions was elevated to 10 µM. We have observed just one other channel in these cilia that is activated by internal Ca2+ (TRPM4). This cannot account for any of the inward currents we measured for three reasons (25). First, it is not detectably activated at concentrations of internal Ca2+ as low as 10 µM. Second, it does not conduct Ca2+, which was the only ion available to conduct inward current. Third, we added 2 mM MgATP to the internal solutions, which blocks TRPM4.
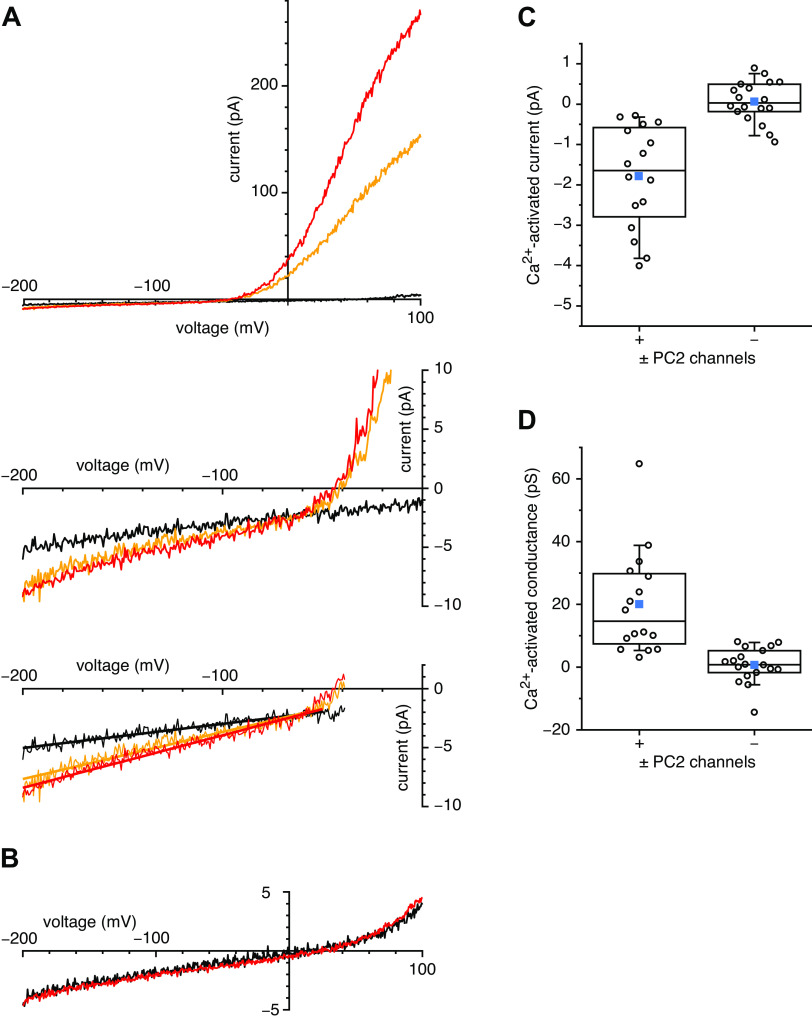
In our previous studies of mIMCD-3 primary cilia, 34% of cilia tested had detectable PC2 channels (7). The presence of these channels is easily detected by their large single-channel fluctuations at depolarizing potentials. Under the conditions used for the present study, fluctuations not characteristic of PC2 are very rare at all potentials tested. To look for inward Ca2+ currents through PC2 channels, we selected cilia that exhibited fluctuations at positive potentials due to PC2 channels. Such cilia were subjected to voltage ramps from −200 mV to +100 mV. To average out the fluctuations from individual channels, the results of 10 ramps were averaged for each cilium in each solution. In the presence of 10 µM internal Ca2+, a small inward current could be measured at negative potentials (Fig. 1A, red). A much larger outward current was visible at positive potentials; this outward rectification is characteristic of ciliary PC2 currents (7, 8). When internal free Ca2+ was lowered to 0.1 µM, the current was reduced at all potentials (Fig. 1A, black). The readdition of 10 µM Ca2+ again increased the current (Fig. 1A, orange). The current did not completely recover, particularly at positive potentials. This likely reflects the slow inactivation of ciliary PC2 channels in the presence of elevated internal Ca2+, as has been previously reported (7, 8). For each cilium, Ca2+ current and conductance were quantitated after subtracting the current-voltage relation measured with 0.1 µM free internal Ca2+ from that measured with 10 µM Ca2+. This difference represents the Ca2+-activated Ca2+ current and conductance and subtracts out any contributions from leaks. At −190 mV, the inward Ca2+ current activated by 10 µM internal Ca2+ averaged −1.8 ± 0.3 pA (Fig. 1C, column labeled “+,” n = 16 cilia). The conductance of the inward Ca2+ current (slope of the current-voltage relation), measured from −50 mV to −200 mV, averaged 20 ± 4 pS (Fig. 1D, column labeled “+,” n = 16 cilia). In cilia with sufficient Ca2+-activated conductance to measure a reversal potential, the average value was −50 ± 7 mV (n = 13 cilia). This establishes that internal Ca2+ activates a ciliary conductance that allows a Ca2+ influx at negative potentials.
Figure 1.
Ciliary Ca2+ influx activated by 10 µM internal free Ca2+. A: current-voltage relations as a cilium with ∼25 active polycystin-2 (PC2) channels was transferred from an internal solution with 10 µM free Ca2+ (red) to a solution with 0.1 µM Ca2+ (black) and then back to the original solution with 10 µM Ca2+ (orange). The external solution included 100 mM Ca2+ and no Na+ or K+. The middle image is an enlargement of part of the top image to better show the inward currents at negative potentials. The bottom image is a copy of the middle image with added lines showing the linear least-squares fits used to measure the conductances from −50 mV to −200 mV. Current amplitudes at −190 mV were −8.1 pA (10 µM Ca2+, red), −4.9 pA (0.1 µM Ca2+, black), and −7.7 pA (return to 10 µM Ca2+, orange). Slope conductances from −50 mV to −200 mV were 65 pS (10 µM Ca2+, red), 30 pS (0.1 µM Ca2+, black), and 56 pS (10 µM Ca2+, orange). The reversal potential of the Ca2+-activated current occurs where the three lines intersect (−60 mV). B: current-voltage relations as a cilium with no active PC2 channels was transferred from an internal solution with 10 µM free Ca2+ (red) to a solution with 0.1 µM Ca2+ (black). Current amplitudes at −190 mV were −3.9 pA (10 µM Ca2+, red) and −3.8 pA (0.1 µM Ca2+, black). Slope conductances from −50 mV to −200 mV were 28 pS (10 µM Ca2+, red) and 29 pS (0.1 µM Ca2+, black). C: Ca2+-activated inward current at −190 mV, i.e., the current with 10 µM internal Ca2+ minus the current with 0.1 µM internal Ca2+, measured in populations of cilia with (+) and without (−) active PC2 channels. n = 16 (+) and 18 (−) cilia. D: Ca2+-activated conductance from −50 mV to −200 mV, i.e., the conductance with 10 µM internal Ca2+ minus the conductance with 0.1 µM internal Ca2+. n = 16 (+) and 18 (−) cilia. These and subsequent box plots show comparisons between cilia with (+) and without (−) active PC2 channels. The individual data are shown as open circles. The top, middle, and bottom lines indicate the 75th, 50th, and 25th percentile values, respectively. Whiskers indicate the 90th and 10th percentile values. A blue square is shown at the level of the mean value.
To determine whether any of this Ca2+ influx depends on PC2 channels, we compared a separate population of cilia in which PC2 channels were not detectable at positive potentials. In wild-type mIMCD-3 cells, 66% of the cilia show no active PC2 channels, even at depolarized potentials that would make the activity obvious (7). In such cilia, no significant inward Ca2+ current or conductance was activated by 10 µM internal free Ca2+ (Fig. 1B). Inward current from the addition of internal Ca2+ averaged 0.06 ± 0.12 pA (Fig. 1C, column labeled “−,” n = 18 cilia), which is significantly different from the average in cilia with detectable PC2 channels (P < 0.001). In cilia lacking PC2 channels, the conductance activated by internal Ca2+ from −50 mV to −200 mV averaged 0.7 ± 1.3 pS (Fig. 1D, column labeled “−,” n = 18 cilia), which is also significantly different from the average in cilia with detectable PC2 channels (P < 0.001). Thus, the inward Ca2+ current and conductance activated by 10 µM internal free Ca2+ depend on the presence of active PC2 channels.
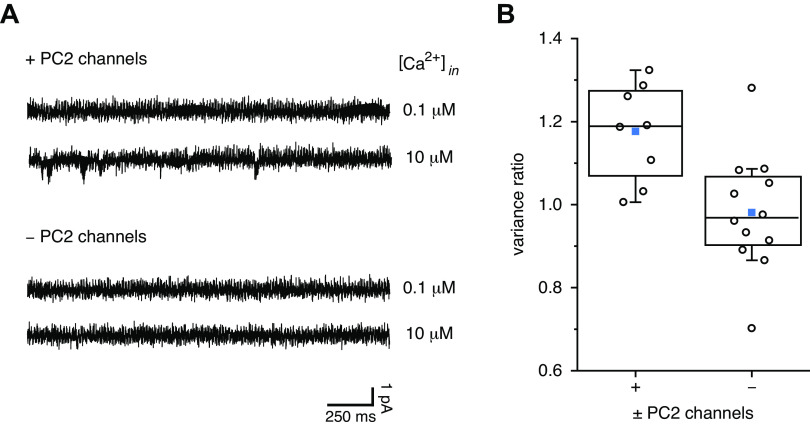
When the steady-state current was measured at −100 mV, current fluctuations were usually greatest in cilia with active PC2 channels in the presence of 10 µM internal Ca2+ (Fig. 2A, second recording). As an indirect measure of channel activity, the variance of the steady-state current was measured. Variance increases with the open probability of the underlying single channels for open probabilities up to 0.5 (30). In cilia with active PC2 channels, addition of 10 µM Ca2+ increased the current variance by 0.063 ± 0.016 pA2 (n = 8 cilia). In cilia with no active channels, the variance changed by −0.011 ± 0.016 pA2 (n = 12 cilia), which is significantly different from the value in cilia with PC2 channels (P = 0.006). The ratio of the variance measured in 10 µM Ca2+ to that measured in 0.1 µM Ca2+ averaged 1.17 ± 0.04 in cilia with PC2 channels and 0.98 ± 0.04 in those without (Fig. 2B). Again, the values in cilia with and without PC2 channels were significantly different (P = 0.005).
Figure 2.
Variance of the ciliary Ca2+ influx activated by 10 µM internal Ca2+. A: steady-state currents measured at −100 mV in each of two cilia, one with active polycystin-2 (PC2) channels and one without, each in both 0.1 and 10 µM internal Ca2+. In the cilium with active PC2 channels, the variance (averaged over a 20-s recording) was 0.24 pA2 in 0.1 µM Ca2+ and 0.29 pA2 in 10 µM Ca2+, and the ratio of the variances (10 µM Ca2+/0.1 µM Ca2+) was 1.20. In the cilium without active PC2 channels, the variance was 0.27 pA2 in both 0.1 and 10 µM Ca2. For presentation as a figure (but not for analysis), the two recordings were low-pass filtered at 500 Hz. B: ratio of current variances (10 µM internal Ca2+/0.1 µM internal Ca2+), both measured over 20 s at −100 mV in populations of cilia (+) with and without (−) active PC2 channels. n = 8 (+) and 12 (−). The individual data are shown as open circles. The top, middle, and bottom lines indicate the 75th, 50th, and 25th percentile values, respectively. Whiskers indicate the 90th and 10th percentile values. A blue square is shown at the level of the mean value.
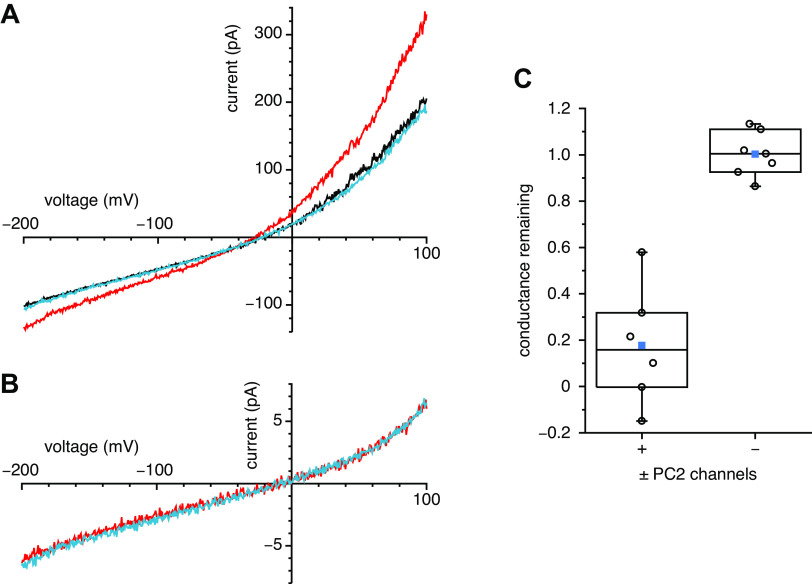
If the inward Ca2+ current is conducted through PC2 channels, it should be reduced by isosakuranetin, which irreversibly blocks ciliary PC2 channels (22). In cilia with PC2 channels, 10 µM internal Ca2+ activated a Ca2+ influx, as before (Fig. 3A, compare red and black). Further addition of 10 µM isosakuranetin reduced the current to the level measured with 0.1 µM internal Ca2+ (Fig. 3A, compare blue and black). To eliminate the possibility that isosakuranetin was blocking a component of the leak current, we measured its effect in cilia with no active PC2 channels. These cilia have no significant Ca2+-activated current (Fig. 1C), so the total current measured is attributable to the leak. In such cilia, isosakuranetin had no effect on the leak current (Fig. 3B). In a population of cilia with active PC2 channels, isosakuranetin eliminated most of the Ca2+-activated Ca2+ influx at −190 mV; the fraction remaining after treatment with isosakuranetin averaged 0.10 ± 0.22 (n = 7 cilia). In a previous study of single PC2 channels, the fraction of the current remaining in the presence of 10 µM isosakuranetin was 0.09 ± 0.04 (22). In cilia without active PC2 channels, no significant Ca2+-activated Ca2+ influx was present (Fig. 1C, column labeled “−”), and the leak current observed was not affected by isosakuranetin (fraction remaining 0.98 ± 0.04, n = 7 cilia). The effect of isosakuranetin on the Ca2+-activated Ca2+ influx was significantly different from the effect on the leak current (P = 0.002). The same was found on comparing the Ca2+-activated conductance from −50 mV to −200 mV. In cilia with PC2 channels, the fraction of the Ca2+-activated conductance remaining after treatment with isosakuranetin was 0.18 ± 0.10 (Fig. 3C, column labeled “+,” n = 6 cilia). In cilia without PC2 channels, isosakuranetin had no effect on the leak conductance (Fig. 3C, column labeled “−,” fraction remaining 1.00 ± 0.04, n = 7 cilia). The effect of isosakuranetin on the Ca2+-activated Ca2+ conductance was significantly different from the effect on the leak conductance (P < 0.001).
Figure 3.
Effects of isosakuranetin on the ciliary currents. A: effects of isosakuranetin on the current in a cilium with active polycystin-2 (PC2) channels. Current-voltage relations were measured as the cilium was transferred from an internal solution with 0.1 µM free Ca2+ (black) to a solution with 10 µM free Ca2+ (red) and finally to a solution with 10 µM Ca2+ and 10 µM isosakuranetin (blue). The external solution included 100 mM Ca2+ and no Na+ or K+. In this cilium, the fraction of the Ca2+-activated current at −190 mV remaining in the presence of isosakuranetin was 0.09; the fraction remaining of the Ca2+-activated conductance from −50 mV to −200 mV was 0.10. B: effects of isosakuranetin on the leak current in a cilium without active PC2 channels. Current-voltage relations were measured as the cilium was transferred from an internal solution with 10 µM free Ca2+ (red) to a solution with 10 µM Ca2+ and 10 µM isosakuranetin (blue). In this cilium, the fraction of the leak current at −190 mV remaining in the presence of isosakuranetin was 1.11; the fraction of the conductance from −50 mV to −200 mV was 1.02. C: effects of isosakuranetin in populations of cilia with and without active PC2 channels. The data on the left (+) show the fraction of the Ca2+-activated Ca2+ conductance from −50 mV to −200 mV remaining after treatment with 10 µM internal isosakuranetin in cilia with PC2 channels. The data on the right (−) represent the fraction of leak current remaining after treatment with 10 µM internal isosakuranetin in cilia without PC2 channels. n = 6 (+) and 7 (−). The individual data are shown as open circles. The top, middle, and bottom lines indicate the 75th, 50th, and 25th percentile values, respectively. Whiskers indicate the 90th and 10th percentile values. A blue square is shown at the level of the mean value.
In studies of single-channel properties of the native ciliary PC2 channels given Ca2+ as the only external cation, our group and another reported substantially different estimates of the channel permeability to Ca2+ (7, 8). For that reason, we conducted a more detailed reexamination in our system. As before, it was possible to measure single-channel fluctuations representing outward currents carried by K+ (Fig. 4). At potentials from −50 mV to −140 mV, current fluctuations greater than the background noise were rarely observed. At more negative potentials (−160 mV to −200 mV), the pipette-membrane seal showed occasional instability that precluded confident identification of single-channel events. The single-channel outward currents were fit to a GHK current equation representing the only cations present (external Ca2+ and internal K+; Fig. 4, black curve). The curve has a reversal potential of −50 mV, which matches the average reversal potential of the macroscopic current and corresponds to a permeability ratio PCa/PK of 0.09. Figure 4 also shows (as dashed gray lines) curves representing the 95% confidence levels of PCa/PK (0.04 and 0.14) from fitting the data to the GHK equation.
Figure 4.
Single-channel current of ciliary polycystin-2 (PC2) channels as a function of voltage. The external (pipette) solution contained 100 mM CaCl2, and the internal solution contained principally potassium methanesulfonate with 10 µM free Ca2+, both as described in materials and methods. Single-channel current amplitudes were taken from amplitude histograms, each from a 20-s recording. Each value shown is a mean from measurements in 5–11 cilia. The black curve is the best fit of the data to a Goldman–Hodgkin–Katz current equation, as described in materials and methods. This equation has a permeability ratio of Ca2+ to K+ (PCa/PK) of 0.09; slope conductances are 18 pS at −200 mV and 100 pS at +80 mV. The dashed gray curves show the equations with PCa/PK equal to 0.04 and 0.14, which are the 95% confidence limits from the best fit.
DISCUSSION
It is often proposed that conductance of Ca2+ through PC2 channels of the primary cilium of renal epithelial cells maintains renal health and counters cystogenesis (10–12, 14). However, the reported ability of PC2 channels to conduct Ca2+ has varied widely across preparations. We report that, in the renal primary cilium, cytoplasmic Ca2+ activates a very small macroscopic Ca2+ influx that is absent in cilia without active PC2 channels.
For 20 years, evidence has accumulated that PC2 channels conduct Ca2+, but estimates of the size of that conductance have varied greatly. Initial experiments suggested that PC2 channels conduct Ca2+ well. Those conclusions came from macroscopic currents arising from exogenous coexpression of PC2 and PC1. In Chinese hamster ovary cells, coexpression resulted in a conductance that was about six times more permeable to Ca2+ than to Na+ (16). Coexpression in rat sympathetic neurons gave a current with a PCa/PK value of 0.57 (20). More recently, Wang et al. (31) expressed PC1 together with a PC2 protein that contains a missense mutation conferring constitutive activity. Ca2+ permeability was detected but could not be quantified with confidence due to the presence of significant leak currents.
The Ca2+ conductance of PC2 has also been estimated from single-channel studies. When vesicles from human syncytiotrophoblast cells were incorporated into artificial bilayers, single channels with significant Ca2+ permeability (PCa/PK = 1.3) were seen (17). Exogenous expression of PC2 in oocytes produced channels with a smaller Ca2+ permeability (PCa/PK = 0.21) (18). In LLC-PK1 renal epithelial cells overexpressing PC2, PCa/PK was 0.28 in the channels observed (21). With bi-ionic conditions (external Ca2+ and internal K+), the single-channel conductances for inward Ca2+ flux in these studies ranged from 35 pS to 95 pS, and the current-voltage relation was linear or showed mild outward rectification (4, 17–19, 21). Shen et al. (32) constructed a chimeric channel by replacing the pore of a related channel (PKD2-L1) with the PC2 pore. The hybrid channel was expected to share the same ionic selectivity as native PC2; it showed a PCa/PNa value of 0.5 and a single-channel conductance for Ca2+ of 8 pS. Native channels in the studies cited above were identified as PC2 by comparison with channels seen by exogenous expression; controls with knockdown or knockout of PC2 were not available.
Three recent studies have used newer genetic methods to confirm that the channels characterized depend on the expression of PC2 (7, 8, 33). In primary cilia of renal cells from the murine inner medullary collecting duct in primary culture, Liu et al. (8) characterized a macroscopic PC2 current. Doxycycline-induced knockout of PC2 expression in those cells allowed a convincing demonstration that the channels studied require PC2. With external Ca2+ and internal Na+, the current-voltage relation showed strong outward rectification and a reversal potential of −49 mV. In a GHK analysis representing external Ca2+ and internal Na+, that reversal potential corresponds to a PCa/PNa value of just 0.06. At very negative potentials (−140 mV to −200 mV), rare single-channel events, likely representing Ca2+ influx, were seen. The single-channel slope conductance for Ca2+ influx over those potentials was 4 pS, and the reversal potential was −61 mV. In another report, native channels of primary cilia of mIMCD-3 cells had a single-channel conductance to Ca2+ of 47.5 pS at negative potentials when activated by an NH2-terminal fragment of PC1 and with 2 mM Ca2+ as the sole current carrier (33).
In a previous study, we characterized single channels that were eliminated when PC2 was knocked out by CRISPR/Cas9 genome editing in mIMCD-3 cells (7). With external Ca2+ and internal K+, single-channel outward currents, presumably reflecting K+ efflux, were recorded. The current-voltage relation for those currents was subjected to linear extrapolation, giving an inferred reversal potential of −13 mV. GHK analysis of that potential gave a PCa/PK value of 0.55, which we reported. It is clear now that our linear extrapolation of the current-voltage relation was not justified. By directly measuring rare channel events due to Ca2+ influx, Liu et al. (8) established that the true current-voltage relation is very nonlinear. We have been unable to see the rare channel openings that Liu et al. (8) observed from −140 mV to −200 mV, perhaps because of experimental limitations. At −100 mV, we found occasional current fluctuations (e.g., Fig. 2A, second recording), but they were not consistently well quantized. At more negative potentials, we observed current fluctuations that may have arisen from a reduced stability of the membrane-pipette seal. In the present report, the reversal potential of the macroscopic Ca2+ influx through ciliary PC2-dependent channels averages −50 mV, corresponding to a PCa/PK value of 0.09. This confirms the conclusion of Liu et al. (8) that, at least in the unstimulated renal primary cilium, PC2 channels have a small permeability to Ca2+. Errors likely remain in the estimated permeability ratios. They have been inferred from GHK analysis, but the measured current-voltage relation for single channels (8) shows much stronger outward rectification than predicted from GHK analysis (Fig. 4). GHK analysis does not account for some properties of PC2 channels. It is known, for example, that the addition of external Ca2+ reduces an influx primarily due to Na+ (8, 18, 34). This indicates that fluxes of Ca+ and Na+ through the channel are not independent, which violates an assumption of the GHK model.
In assessing the functional significance of ciliary PC2, one would like to know to what extent it allows a macroscopic Ca2+ influx. The amplitude of this influx will depend on the number of active channels; the single-channel conductance to Ca2+, which is known to be small (8); and the channel’s open probability, which is low at negative potentials (7, 8). One thus predicts that the mean Ca2+ influx should be very small. In this report, we provide the first estimate of the macroscopic Ca2+ conductance attributable to the population of native, wild-type PC2 channels in a renal primary cilium. Experimental conditions were designed to isolate the Ca2+ current from other inward currents. The Ca2+ conductance attributed to PC2 channels was small, averaging 20 pS from −50 mV to −200 mV. In cilia with no active PC2 channels, the Ca2+ influx was absent. In cilia with active PC2 channels, the Ca2+ influx was largely blocked by isosakuranetin, which blocks PC2 channels. This confirms that the small macroscopic Ca2+ influx is conducted through PC2 channels. At the same time, we have been unable to quantitatively establish the single-channel basis of that influx. Assuming a single-channel conductance to Ca2+ of 4 pS (8), the average conductance we measured between −50 mV and −200 mV (20 pS) is the conductance that would be expected from five channels open all the time. However, the open probability at such potentials is apparently very low (7, 8), so that it would require hundreds of channels to generate a mean macroscopic conductance of 20 pS. As judged at positive potentials, though, the cilia in this study had at most 25 active PC2 channels. The discrepancy is not understood. PC2 channels are known to have low-conductance substates (7, 17, 35–37), but no substate has been described that accounts for the Ca2+ influx reported here. In any case, it is clear that the cilium supports a Ca2+ influx attributable to PC2 channels.
It cannot yet be said whether such a small Ca2+ influx is consequential in vivo. At rest, the cilium is estimated to contain fewer than 200 Ca2+ ions (38). The mean inward current we measured, −1.8 pA, equals 9 amol of Ca2+/s (where amol = 10−18 mol), or ∼6 million Ca2+ ions/s. The internal volume of the cilium is ∼0.5 fL (38) (where fL = 10−15 L). Were Ca2+ to accumulate inside this volume at a rate of 9 amol/s, the concentration would increase by 18 mM/s. This would be greatly limited by diffusion into the cytoplasm (38) and perhaps also by buffering and extrusion. Furthermore, the Ca2+ influx should be smaller in the renal filtrate, given that urine has 0.7–4.9 mM Ca2+ [human (39)] instead of the 100 mM used in this study. Still, it is not unreasonable to propose that the small Ca2+ influx demonstrated here could elevate intraciliary Ca2+ to a level that is physiologically active. In mIMCD-3 cells, activation of ciliary PC2 channels with an NH2-terminal fragment of PC1 has been shown to increase intraciliary Ca2+, although the extent of the increase is not yet known (33). The consequences of that for the cell are also not known, but several possibilities have been discussed elsewhere (14, 40–43).
GRANTS
S.J.K. and N.K.K. received support from research grants from Dialysis Clinic, Inc., and from the UAB Hepato/Renal Fibrocystic Diseases Core Center (National Institute of Diabetes and Kidney Disease Grant P30DK074038).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.J.K. conceived and designed research; S.J.K. and N.K.K. performed experiments; S.J.K. analyzed data; S.J.K. interpreted results of experiments; S.J.K. prepared figures; S.J.K. drafted manuscript; S.J.K. and N.K.K. edited and revised manuscript; S.J.K. and N.K.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Gillian Bryant for excellent technical assistance.
REFERENCES
- 1.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 2.Cornec-Le Gall E, Torres VE, Harris PC. Genetic complexity of autosomal dominant polycystic kidney and liver diseases. J Am Soc Nephrol 29: 13–23, 2018. doi: 10.1681/ASN.2017050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev 62: 381–404, 2010. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4: 191–197, 2002. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 5.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12: R378–R380, 2002. doi: 10.1016/S0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 6.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516, 2002. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 7.Kleene SJ, Kleene NK. The native TRPP2-dependent channel of murine renal primary cilia. Am J Physiol Renal Physiol 312: F96–F108, 2017. doi: 10.1152/ajprenal.00272.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Vien T, Duan J, Sheu SH, DeCaen PG, Clapham DE. Polycystin-2 is an essential ion channel subunit in the primary cilium of the renal collecting duct epithelium. eLife 7: e33183, 2018. doi: 10.7554/eLife.33183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma M, Tian X, Igarashi P, Pazour GJ, Somlo S. Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet 45: 1004–1012, 2013. doi: 10.1038/ng.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi T, Hempson SJ, Reif GA, Hedge A-M, Wallace DP. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol 17: 178–187, 2006. doi: 10.1681/ASN.2005060645. [DOI] [PubMed] [Google Scholar]
- 11.Zaika O, Mamenko M, Berrout J, Boukelmoune N, O'Neil RG, Pochynyuk O. TRPV4 dysfunction promotes renal cystogenesis in autosomal recessive polycystic kidney disease. J Am Soc Nephrol 24: 604–616, 2013. doi: 10.1681/ASN.2012050442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin X, Muntean BS, Aal-Aaboda MS, Duan Q, Zhou J, Nauli SM. L-type calcium channel modulates cystic kidney phenotype. Biochim Biophys Acta 1842: 1518–1526, 2014. doi: 10.1016/j.bbadis.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvet JP. The role of calcium and cyclic AMP in PKD. In: Polycystic Kidney Disease, edited by Li X. Brisbane, Queensland, Australia: Codon Publications, 2015, Chapt. 8, p. 169–196. doi: 10.15586/codon.pkd.2015.ch8. [DOI] [PubMed] [Google Scholar]
- 14.Chebib FT, Sussman CR, Wang X, Harris PC, Torres VE. Vasopressin and disruption of calcium signalling in polycystic kidney disease. Nat Rev Nephrol 11: 451–464, 2015. doi: 10.1038/nrneph.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vien TN, Wang J, Ng LCT, Cao E, DeCaen PG. Molecular dysregulation of ciliary polycystin-2 channels caused by variants in the TOP domain. Proc Natl Acad Sci USA 117: 10329–10338, 2020. doi: 10.1073/pnas.1920777117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408: 990–994, 2000. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- 17.González-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci USA 98: 1182–1187, 2001. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vassilev PM, Guo L, Chen X-Z, Segal Y, Peng J-B, Basora N, Babakhanlou H, Cruger G, Kanazirska M, Ye C-P, Brown EM, Hediger MA, Zhou J. Polycystin-2 is a novel cation channel implicated in defective intracellular Ca2+ homeostasis in polycystic kidney disease. Biochem Biophys Res Commun 282: 341–350, 2001. doi: 10.1006/bbrc.2001.4554. [DOI] [PubMed] [Google Scholar]
- 19.Luo Y, Vassilev PM, Li X, Kawanabe Y, Zhou J. Native polycystin 2 functions as a plasma membrane Ca2+-permeable cation channel in renal epithelia. Mol Cell Biol 23: 2600–2607, 2003. doi: 10.1128/mcb.23.7.2600-2607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delmas P, Nauli SM, Li X, Coste B, Osorio N, Crest M, Brown DA, Zhou J. Gating of the polycystin ion channel signaling complex in neurons and kidney cells. FASEB J 18: 740–742, 2004. doi: 10.1096/fj.03-0319fje. [DOI] [PubMed] [Google Scholar]
- 21.Ma R, Li W-P, Rundle D, Kong J, Akbarali HI, Tsiokas L. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol 25: 8285–8298, 2005. doi: 10.1128/MCB.25.18.8285-8298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleene SJ, Siroky BJ, Landero-Figueroa JA, Dixon BP, Pachciarz NW, Lu L, Kleene NK. The TRPP2-dependent channel of renal primary cilia also requires TRPM3. PLoS One 14: e0214053, 2019. doi: 10.1371/journal.pone.0214053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleene NK, Kleene SJ. A method for measuring electrical signals in a primary cilium. Cilia 1: 17, 2012. doi: 10.1186/2046-2530-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rauchman MI, Nigam SK, Delpire E, Gullans SR. An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am J Physiol Renal Physiol 265: F416–F424, 1993. doi: 10.1152/ajprenal.1993.265.3.F416. [DOI] [PubMed] [Google Scholar]
- 25.Flannery RJ, Kleene NK, Kleene SJ. A TRPM4-dependent current in murine renal primary cilia. Am J Physiol Renal Physiol 309: F697–F707, 2015. doi: 10.1152/ajprenal.00294.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bers DM. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. Am J Physiol Cell Physiol 242: C404–C408, 1982. doi: 10.1152/ajpcell.1982.242.5.C404. [DOI] [PubMed] [Google Scholar]
- 27.Kleene SJ, Gesteland RC. Calcium-activated chloride conductance in frog olfactory cilia. J Neurosci 11: 3624–3629, 1991. doi: 10.1523/JNEUROSCI.11-11-03624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jan LY, Jan YN. L-Glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol 262: 215–236, 1976. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson RA, Stokes RH. Electrolyte Solutions, the Measurement and Interpretation of Conductance, Chemical Potential, and Diffusion in Solutions of Simple Electrolytes. London: Butterworths, 1970, p. 571. [Google Scholar]
- 30.Lecar H, Sachs F. Membrane noise analysis. In: Excitable Cells in Tissue Culture, edited by Nelson PG, Lieberman M.. New York: Plenum Press, 1981, p. 137–172. [Google Scholar]
- 31.Wang Z, Ng C, Liu X, Wang Y, Li B, Kashyap P, Chaudhry HA, Castro A, Kalontar EM, Ilyayev L, Walker R, Alexander RT, Qian F, Chen XZ, Yu Y. The ion channel function of polycystin-1 in the polycystin-1/polycystin-2 complex. EMBO Rep 20: e48336, 2019. doi: 10.15252/embr.201948336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen PS, Yang X, DeCaen PG, Liu X, Bulkley D, Clapham DE, Cao E. The structure of the polycystic kidney disease channel PKD2 in lipid nanodiscs. Cell 167: 763–773, 2016. doi: 10.1016/j.cell.2016.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ha K, Nobuhara M, Wang Q, Walker RV, Qian F, Schartner C, Cao E, Delling M. The heteromeric PC-1/PC-2 polycystin complex is activated by the PC-1 N-terminus. eLife 9: e60684, 2020. doi: 10.7554/eLife.60684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arif Pavel M, Lv C, Ng C, Yang L, Kashyap P, Lam C, Valentino V, Fung HY, Campbell T, Moller SG, Zenisek D, Holtzman NG, Yu Y. Function and regulation of TRPP2 ion channel revealed by a gain-of-function mutant. Proc Natl Acad Sci USA 113: E2363–E2372, 2016. doi: 10.1073/pnas.1517066113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González-Perrett S, Batelli M, Kim K, Essafi M, Timpanaro G, Moltabetti N, Reisin IL, Arnaout MA, Cantiello HF. Voltage dependence and pH regulation of human polycystin-2-mediated cation channel activity. J Biol Chem 277: 24959–24966, 2002. doi: 10.1074/jbc.M105084200. [DOI] [PubMed] [Google Scholar]
- 36.Zhang P, Luo Y, Chasan B, González-Perrett S, Montalbetti N, Timpanaro GA, Cantero MR, Ramos AJ, Goldmann WH, Zhou J, Cantiello HF. The multimeric structure of polycystin-2 (TRPP2): structural-functional correlates of homo- and hetero-multimers with TRPC1. Hum Mol Genet 18: 1238–1251, 2009. doi: 10.1093/hmg/ddp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cantero MR, Cantiello HF. Effect of lithium on the electrical properties of polycystin-2 (TRPP2). Eur Biophys J 40: 1029–1042, 2011. doi: 10.1007/s00249-011-0715-2. [DOI] [PubMed] [Google Scholar]
- 38.Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signalling organelles. Nature 504: 311–314, 2013. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thode J. The calcium ion activity and the standardized excretion rate of calcium in urine of healthy adults. Scand J Clin Lab Invest 45: 327–334, 1985. doi: 10.3109/00365518509161015. [DOI] [PubMed] [Google Scholar]
- 40.Abdul-Majeed S, Nauli SM. Calcium-mediated mechanisms of cystic expansion. Biochim Biophys Acta 1812: 1281–1290, 2011. doi: 10.1016/j.bbadis.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Praetorius HA. The primary cilium as sensor of fluid flow: new building blocks to the model. A review in the theme: cell signaling: proteins, pathways and mechanisms. Am J Physiol Cell Physiol 308: C198–C208, 2015. doi: 10.1152/ajpcell.00336.2014. [DOI] [PubMed] [Google Scholar]
- 42.Ong AC, Harris PC. A polycystin-centric view of cyst formation and disease: the polycystins revisited. Kidney Int 88: 699–710, 2015. doi: 10.1038/ki.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mangolini A, de Stephanis L, Aguiari G. Role of calcium in polycystic kidney disease: From signaling to pathology. World J Nephrol 5: 76–83, 2016. doi: 10.5527/wjn.v5.i1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]