Keywords: caudate putamen, dorsal striatum, electrophysiology, estradiol, estrogen receptor
Abstract
Exposure to steroid sex hormones such as 17β-estradiol (estradiol) during early life potentially permanently masculinize neuron electrophysiological phenotype. In rodents, one crucial component of this developmental process occurs in males, with estradiol aromatized in the brain from testes-sourced testosterone. However, it is unknown whether most neuron electrophysiological phenotypes are altered by this early masculinization process, including medium spiny neurons (MSNs) of the rat caudate-putamen. MSNs are the predominant and primary output neurons of the caudate-putamen and exhibit increased intrinsic excitability in females compared to males. Here, we hypothesize that since perinatal estradiol exposure occurs in males, then a comparable exposure in females to estradiol or its receptor agonists would be sufficient to induce masculinization. To test this hypothesis, we injected perinatal female rats with estradiol or its receptor agonists and then later assessed MSN electrophysiology. Female and male rats on postnatal day 0 and 1 were systemically injected with either vehicle, estradiol, the estrogen receptor (ER)α agonist PPT, the ERβ agonist DPN, or the G-protein-coupled receptor 1 (GPER-1) agonist G1. On postnatal days 19 ± 2, MSN electrophysiological properties were assessed using whole cell patch clamp recordings. Estradiol exposure abolished increased intrinsic excitability in female compared to male MSNs. Exposure to either an ERα or ERβ agonist masculinized female MSN evoked action potential firing rate properties, whereas exposure to an ERβ agonist masculinized female MSN inward rectification properties. Exposure to ER agonists minimally impacted male MSN electrophysiological properties. These findings indicate that perinatal estradiol exposure masculinizes MSN electrophysiological phenotype via activation of ERα and ERβ.
NEW & NOTEWORTHY This study is the first to demonstrate that estradiol and estrogen receptor α and β stimulation during early development sexually differentiates the electrophysiological properties of caudate-putamen medium spiny neurons, the primary output neuron of the striatal regions. Overall, this evidence provides new insight into the neuroendocrine mechanism by which caudate-putamen neuron electrophysiology is sexually differentiated and demonstrates the powerful action of early hormone exposure upon individual neuron electrophysiology.
INTRODUCTION
Biological sex and relevant sex steroid hormone exposure is increasingly appreciated as modulators of neuron electrophysiology across a wide range of brain regions (1–4). Rat striatal medium spiny neurons (MSNs), also called spiny projection neurons (5), exhibit sex differences and sensitivity to sex steroid hormones such as 17β-estradiol (estradiol). MSNs are present in all major striatal regions, including the nucleus accumbens core, shell, and the focus of this investigation, the caudate-putamen. MSNs are the chief output neuron of the striatal regions and constitute ∼95% of the caudate-putamen neuron population. Changes in caudate-putamen MSN electrical activity directly impacts animal behavior (6–8). In adulthood, female rat MSNs express membrane-associated estrogen receptors (ERs) α, β, and G-protein-coupled receptor 1 (GPER-1) (9–15). In early development, MSNs express nuclear ERs and GPER-1 (16). Dopamine receptors are also expressed by MSNs, and decades of ongoing research indicate that caudate-putamen dopaminergic and other modulatory systems exhibit sex differences and estradiol sensitivity as well (17–19). Overall, MSNs are a sexually differentiated neuron type which exhibit a complex pattern of sex differences in their electrophysiological properties that vary with developmental period, striatal region (including the caudate-putamen, nucleus accumbens core, and nucleus accumbens shell), species, estrous cycle phase, and acute estradiol and dopamine exposure (9, 11, 20–32).
Caudate-putamen MSNs recorded from females compared to males demonstrate increased intrinsic excitability, beginning just before puberty and persisting into adulthood, during which intrinsic excitability is modulated by the hormones associated with the estrous cycle (21, 32). This sex difference in intrinsic excitability manifests in several aspects of neuronal electrophysiology, but most prominently in increased evoked action potential firing rates in female compared to male MSNs. This increase in evoked action potential rate occurs before puberty, indicating that this sex difference is established early in life. In male rats, early development features a testicular-sourced testosterone surge which can be aromatized into estradiol. Thus, this sex difference could be potentially generated by the organizing actions of perinatal estradiol exposure, a process which would normally occur in males but not females. Recently, the presence of nuclear ERs and GPER-1 were established in the caudate-putamen of female and male rats during the perinatal developmental period (16), allowing for the possibility that estradiol acts on nuclear ER to masculinize MSNs during this developmental period. Whether these or other nuclear ER, or perinatal estradiol action at all, organizes sex differences in caudate-putamen MSNs is unknown.
Here, we elucidate the impact of perinatal estradiol and ER agonist exposure on caudate-putamen MSN electrophysiological properties. We reasoned that if perinatal exposure to masculinizing/defeminizing levels of estradiol decreases evoked firing rates in male MSNs, then a comparable estradiol exposure in females should also decrease MSN evoked firing rates. Based upon this reasoning, we articulated three hypotheses for testing. First, we test the hypothesis that perinatal estradiol exposure sexually differentiates caudate-putamen MSN electrophysiological properties. Second, we test the hypothesis that perinatal pharmacological stimulation of either ERα, ERβ, or GPER-1 would mimic the perinatal effects of estradiol in females. Third, we test the hypothesis that perinatal pharmacological stimulation of either ERα, ERβ, or GPER-1 would exert a less robust effect in males. To test these hypotheses, female and male rats on postnatal day 0 and 1 were systemically injected with either vehicle, estradiol, the ERα agonist PPT, the ERβ agonist DPN, or the GPER-1 agonist G1. On postnatal days 19 ± 2, a comprehensive battery of MSN electrophysiological properties were assessed via whole cell patch clamp technique. This age range was chosen, as it features mature and sexually differentiated MSN electrophysiological properties (21) but is before puberty and the influence of the estrous cycle (32).
MATERIALS AND METHODS
Animals
All animal protocols were approved by the Institutional Animal Care and Use Committee at North Carolina State University. Female (n = 55) and male (n = 39) Sprague Dawley CD IGS rats were born from 23 timed-pregnant females purchased from Charles River (Charles River). Rats were housed with their littermates and dams in a temperature- and light-controlled room (23°C, 40% humidity, 12-h light, 12-h dark cycle) at the Biological Resource Facility of North Carolina State University. Age at recording was postnatal day (P) 19 ± 2 during the prepubertal developmental period and was matched between sexes (mean ± SE: female, P 19.7 ± 2.3; male, P 19.3 ± 2.2). Animals were not weaned before experimental use. All cages were washed polysulfone bisphenol A free and were filled with bedding manufactured from virgin hardwood chips (Beta Chip; NEPCO, Warrensburg, NY) to avoid the endocrine disruptors present in corncob bedding (33–35). Soy protein-free rodent chow (2020X; Teklad, Madison, WI) and glass-bottle provided water were available ad libitum. All pups, on P0 and P1, received a subcutaneous injection of either 50 µL vehicle (10% dimethyl sulfoxide in 90% sesame oil), or vehicle containing estradiol (100 μg 17β-estradiol, Sigma Life Science), 10 μg G1 (a selective agonist of GPER-1, Cayman Chemical), 100 μg PPT (subtype-selective ERα agonist, Tocris Bioscience), or 100 μg DPN (highly potent ERβ agonist, Tocris Bioscience). Dose protocols were adapted from previously published techniques (22, 36, 37). Injections were performed across at least two litters and were balanced in calendar time.
Acute Brain Slice Preparation
Brain slices for electrophysiological recordings were prepared as previously described (38). Briefly, rats were deeply anesthetized with isoflurane gas and killed by decapitation. The brain was then dissected rapidly into ice-cold, oxygenated sucrose artificial cerebrospinal fluid (ACSF) containing 75 mM sucrose, 1.25 mM NaH2PO4, 3 mM MgCl2, 0.5 mM CaCl2, 2.4 mM Na pyruvate, and 1.3 mM ascorbic acid from Sigma-Aldrich, and 75 mM NaCl, 25 mM NaHCO3, 15 mM dextrose, 2 mM KCl from Fisher. The osmolarity of the sucrose ACSF was 295–305 mOsm, and the pH was between 7.2 and 7.4. Coronal brain slices (300 μm) were prepared using a vibratome and then incubated in regular ACSF containing 126 mM NaCl, 26 mM NaHCO3, 10 mM dextrose, 3 mM KCl, 1.25 mM NaH2PO4, 1 mM MgCl2, and 2 mM CaCl2 (295–305 mOsm; pH 7.2–7.4) for 30 min at 30 ± 1°C, and then at least 30 min at room temperature (21°C–23°C). Slices were stored submerged in room temperature, oxygenated ACSF for up to 5 h after sectioning in a large volume bath holder.
Electrophysiological Recording
Slices were allowed to rest at least 1 h after sectioning. They were then placed in a Zeiss Axioscope equipped with IRDIC optics, a Dage IR-1000 video camera, and ×10 and ×40 lenses with optical zoom, and superfused with oxygenated ACSF heated to ∼28°C (female, 28.1 ± 0.4; male, 28.3 ± 0.5). Whole cell patch-clamp recordings were used to record the electric properties of MSNs in the caudate-putamen (Fig. 1), with glass electrodes containing 115 mM K d-gluconate, 8 mM NaCl, 2 mM EGTA, 2 mM MgCl2, 2 mM MgATP, 0.3 mM NaGTP, 10 mM phosphocreatine from Sigma-Aldrich, and 10 mM HEPES from Fisher (285 mOsm; pH 7.2–7.4). Signals were amplified, filtered (2 kHz), and digitized (10 kHz) with a MultiClamp 700B amplifier attached to a Digidata 1550 system and a personal computer using pClamp 10 software. Membrane potentials were corrected for a calculated liquid junction potential of −13.5 mV. Using previously described procedures (21), recordings were first made in current clamp to assess neuronal electrophysiological properties. MSNs were identified by their medium-sized somas, the presence of a slow ramping subthreshold depolarization in response to low-magnitude positive current injections, an action potential amplitude ≥25 mV, a hyperpolarized resting potential more negative than −65 mV, inward rectification, and prominent spike after hyperpolarization (AHP) (39, 40). In a subset of recordings from males and females exposed to vehicle or estradiol, oxygenated ACSF containing the GABAA receptor antagonist picrotoxin (150 µM; Fisher) and the voltage-gated sodium channel blocker tetrodotoxin (TTX, 1 µm, Abcam Biochemicals) was applied to the bath to abolish action potentials and inhibitory postsynaptic current events, respectively. Once positive current injection no longer elicited an action potential, MSNs were voltage-clamped at −70 mV and miniature excitatory postsynaptic current events (mEPSCs) were recorded for at least 5 min. These settings specifically target AMPA receptor-mediated mEPSCs, as confirmed via AMPA receptor antagonist experiments (26). mEPSC analysis was chosen to better differentiate between pre- and postsynaptic effects. Input and series resistance was monitored for changes throughout the experiment.
Figure 1.
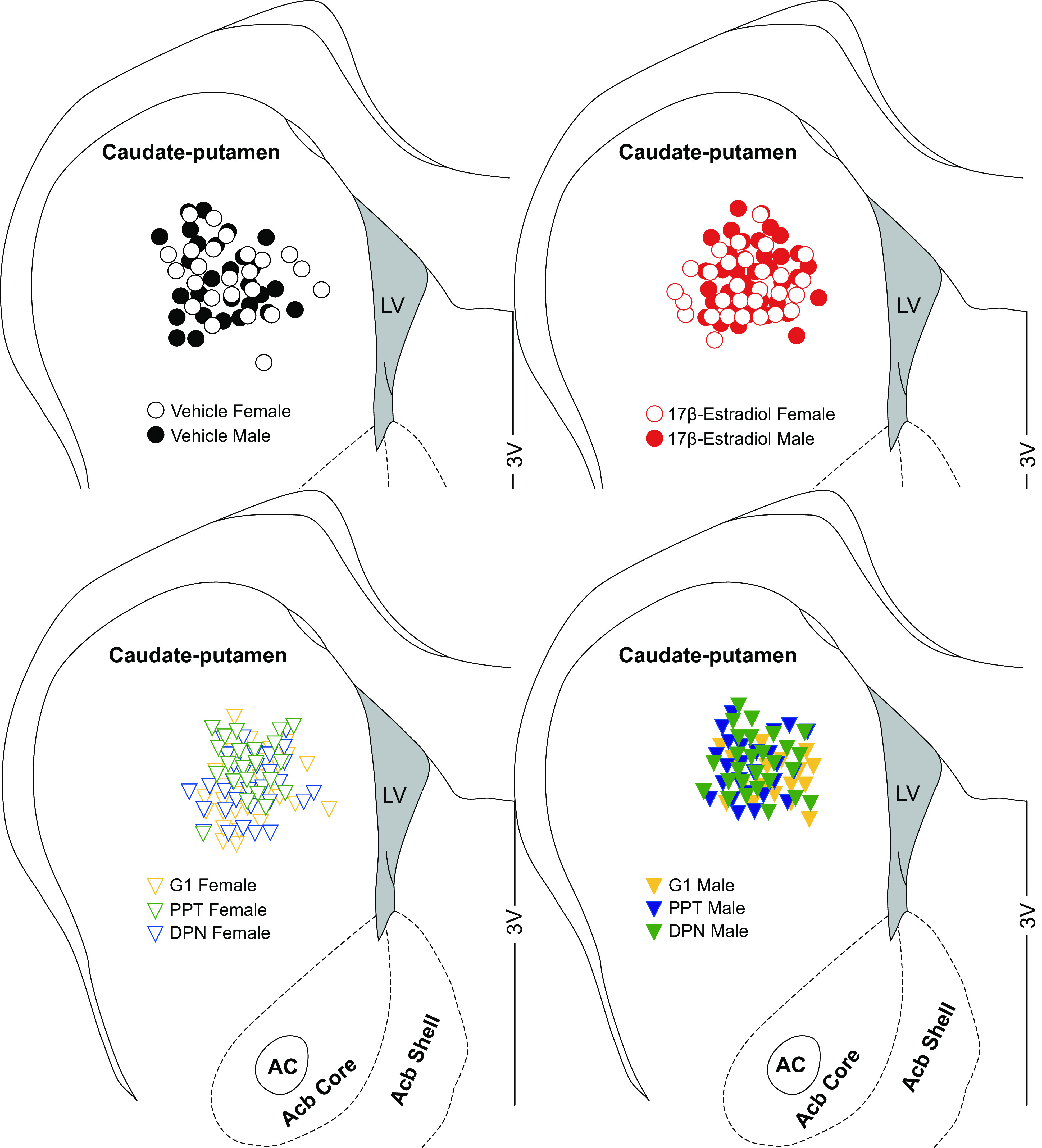
Location of whole cell patch clamped medium spiny neurons (MSNs) sorted by experimental group in the caudate-putamen of female and male rats. Depictions are deaggregated between four different schematics to enhance location clarity. Vehicle, 17β-estradiol (estradiol), G1, PPT, and DPN females and males represent MSNs recorded from animals receiving perinatal injections of vehicle, estradiol, or estrogen receptor agonists, respectively. AC, anterior commissure; Acb, nucleus accumbens; LV, lateral ventricle; 3V, third ventricle.
Data Recording and Analysis
Intrinsic electrophysiological properties and action potential characteristics were analyzed using pClamp 10. After break in, the resting membrane potential was first allowed to stabilize ∼1–2 min (41). Two to four series of depolarizing and hyperpolarizing current injections were applied to elicit basic neurophysiological properties (22). Most properties measured followed the definitions of Cao and colleagues (25), which were originally derived from those of Perkel and colleagues (42–45). For each neuron, measurements were made of two to four action potentials generated from minimal current injections. These measurements were then averaged to generate the reported action potential measurement for that neuron. For action potential measurements, only the first generated action potential was used. Action potential threshold was defined as the first point of sustained positive acceleration of voltage (δ2V/δt2) that was also more than three times the SD of membrane noise before the detected threshold (46). The slope of the linear range of the evoked firing rate to positive current curve (FI slope) was calculated from the first current stimulus which evoked an action potential to the first current stimulus that generated an evoked firing rate that reached the maximum, or persisted for at least three consecutive current stimuli. The evoked firing rate is defined as the number of action potentials evoked over the duration of the current injection. Initial firing rate is defined as the inverse of the first interspike interval and mean steady-state firing rate as the average firing rate over the last 300 ms of the current pulse (47). Input resistance in the linear, nonrectified range was calculated from the steady-state membrane potential in response to −0.02-nA hyperpolarizing pulses, the least hyperpolarizing current pulse injected into the neuron that produces a reliable measureable change in steady-state membrane potential. Rectified range input resistance, inward rectification, and percent inward rectification were calculated from the steady-state membrane potential in response to the most hyperpolarizing current pulse injected into the neuron (40). Inward rectification was defined as the difference between the measured input resistance from the linear and rectified ranges. A neuron with no rectification will have an inward rectification score of zero. Percent inward rectification was defined as the rectified range input resistance divided by the linear range input resistance multiplied by 100. A neuron with no rectification will have a percent inward rectification of 100%. A neuron exhibiting rectification will have a small percent inward rectification score. The membrane time constant was calculated by fitting a single exponential curve to the membrane potential change in response to −0.02 nA hyperpolarizing pulses. Membrane capacitance was calculated using the following equation: capacitance = time constant of the membrane/input resistance. mEPSCs frequency, amplitude, and decay were analyzed using Mini Analysis (Synaptosoft, http://www.synaptosoft.com/MiniAnalysis/), following Cao et al. (22). Recordings were filtered (1 kHz), and mEPSC threshold was set at a minimum value of 5 pA. Accurate event detection was validated by visual inspection.
Statistics
All experimental data was analyzed via Graphpad Prism version 6.07 (La Jolla, CA). Data analysis was a priori organized to address three hypothesis: the effects of perinatal estradiol exposure on female and male MSNs, the effects of perinatal ER agonist exposure on female MSNs, and the effects of perinatal ER agonist exposure on male MSNs. The effects of perinatal estradiol exposure on female and male MSNs were analyzed using two-way ANOVAs with Fisher’s least significant difference (LSD) post hoc tests to maximize statistical power. The effects of perinatal ER agonist exposure on female MSNs were analyzed using one-way ANOVAs with Fisher’s least significant difference (LSD) post hoc tests. The effects of perinatal ER agonist exposure on male MSNs were analyzed using one-way ANOVAs with Fisher’s least significant difference (LSD) post hoc tests. Within all three analysis groups differences in FI and IV curves were further decomposed via Pearson’s correlations accompanied by analyses of covariance (ANCOVAs) to detect differences in slope values. P values ≤ 0.05 were considered as significant and P values ≤ 0.10 were considered a trend. Data are presented as mean ± SE.
RESULTS
Perinatal Exposure to Estradiol Masculinizes Female Evoked Action Potential Firing Rates
To test the hypothesis that perinatal estradiol exposure sexually differentiates caudate-putamen MSN electrophysiological properties, we injected male and female rat pups with vehicle or estradiol solution on postnatal day 0 and 1. We made acute brain slices on postnatal day 19 ± 2 and assessed a comprehensive battery of caudate-putamen MSN electrophysiological properties via injections of a series of currents (Fig. 2A; Table 1). Complete statistical information as well as documentation of all assessed electrophysiological properties across all experimental groups are located in Tables 1–3. We reasoned that if perinatal exposure to masculinizing/defeminizing levels of estradiol naturally decreases evoked firing rates in males, then a comparable hormone exposure in females should also decrease evoked firing rates.
Figure 2.
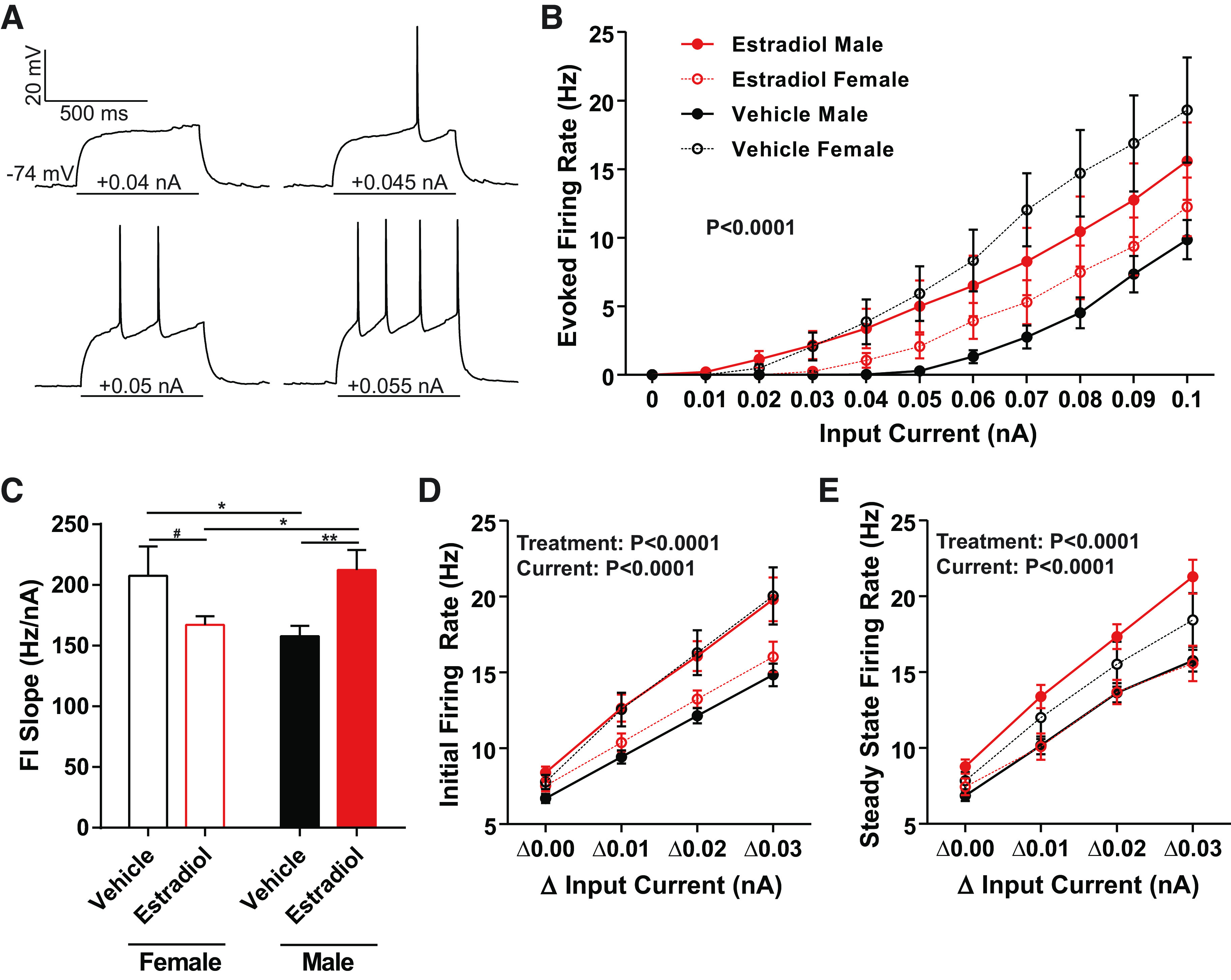
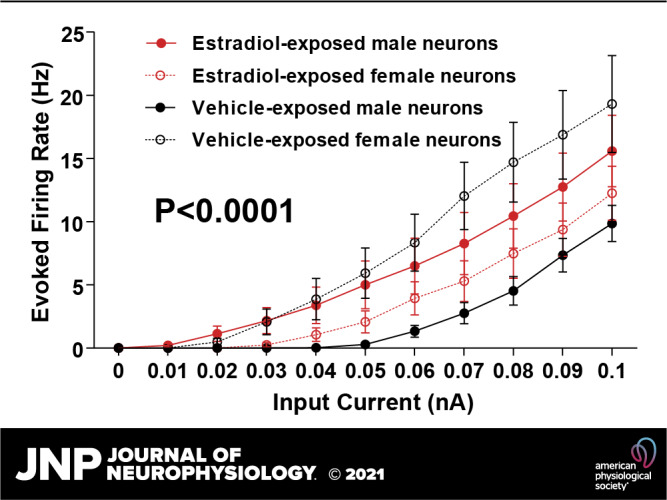
Medium spiny neuron (MSN) action potential (AP) firing rates evoked by depolarizing current injections vary by sex and perinatal exposure to estradiol. A: voltage response of an MSN to a series of depolarization current injections, recorded from a male with perinatal exposure to vehicle. “−74 mV” indicates resting membrane potential. B: AP firing rate evoked by depolarizing current injection. AP firing rate was decreased in MSNs recorded from males compared to females with perinatal exposure to vehicle. AP firing rate was masculinized in MSNs recorded from females with perinatal exposure to estradiol. MSNs recorded from males with perinatal exposure to estradiol exhibited increased AP firing rate compared to males with perinatal exposure to vehicle. C: the slope of the evoked AP firing rate to depolarizing current injection curve (FI Slope) is decreased in males with perinatal exposure to vehicle and females with perinatal exposure to estradiol compared to females with perinatal exposure to vehicle and males with perinatal exposure to estradiol. D: mean initial firing rate (the reciprocal of the 1st interspike interval) is decreased in MSNs recorded from males with perinatal exposure to vehicle and females with perinatal exposure to estradiol compared to females with perinatal exposure to vehicle and males with perinatal exposure to estradiol. E: mean steady-state firing rate (mean firing rate over the last 300 ms of the current injection) is decreased in MSNs recorded from males with perinatal exposure to vehicle and females with perinatal exposure to estradiol compared to females with perinatal exposure to vehicle and males with perinatal exposure to estradiol. FI slope: slope of the evoked action potential to depolarizing current injection curve. #P = 0.0802; *P < 0.05; **P < 0.01.
Table 1.
Caudate-putamen medium spiny neuron electrophysiological properties from females and males exposed to vehicle or estradiol during the perinatal period
| Property | Vehicle | Estradiol | Statistics (F, P) |
|---|---|---|---|
| Resting membrane potential, mV | F: −82.8 ± 1.7 (24) | −86.1 ± 1.6 (29) | 0.11, 0.74 |
| M: −84.2 ± 1.3 (28) | −86.6 ± 1.4 (37) | 0.38, 0.53 | |
| 3.48, 0.06 | |||
| Input resistance, MΩ | F: 260.2 ± 26.9 (24) | 213.9 ± 14.3 (29) | 0.90, 0.34 |
| M: 245.2 ± 15.3 (28) | 265.8 ± 17.2 (37) | 2.98, 0.09 | |
| 0.43, 0.51 | |||
| Rectified range input resistance, MΩ | F: 200.1 ± 13.7 (24) | 187.1 ± 11.1 (29) | 0.72, 0.39 |
| M: 209.6 ± 12.2 (28) | 218.0 ± 12.1 (37) | 2.61, 0.11 | |
| 0.03, 0.85 | |||
| Inward rectification, MΩ | F: 62.7 ± 14.8 (24) | 26.8 ± 4.6 (29) | 7.60, 0.0068 |
| M: 35.5 ± 4.7 (28) | 47.8 ± 8.5 (37) | 0.12, 0.73 | |
| 1.82, 0.18 | |||
| % Inward rectification, % | F: 82.2 ± 2.8 (24) | 89.0 ± 1.4 (29) | 6.70, 0.0109 |
| M: 86.5 ± 1.5 (28) | 83.6 ± 1.7 (37) | 0.09, 0.76 | |
| 1.08, 0.18 | |||
| Time constant of the membrane, ms | F: 16.6 ± 2.5 (24) | 12.0 ± 1.1 (29) | 6.70, 0.07 |
| M: 13.2 ± 1.0 (28) | 14.1 ± 1.2 (37) | 0.09, 0.68 | |
| 1.08, 0.21 | |||
| Capacitance, pF | F: 61.6 ± 4.8 (24) | 57.6 ± 4.6 (29) | 0.21, 0.65 |
| M: 54.9 ± 3.1 (28) | 54.4 ± 3.0 (37) | 1.63, 0.20 | |
| 0.33, 0.56 | |||
| Rheobase, pA | F: 101.9 ± 12.8 (24) | 161.5 ± 16.7 (29) | 5.5, 0.0204 |
| M: 111.7 ± 10.5 (28) | 112.2 ± 9.3 (37) | 2.5, 0.12 | |
| 5.7, 0.0182 | |||
| Delay to first AP, ms | F: 310.1 ± 27.0 (24) | 356.8 ± 22.8 (29) | 1.08, 0.30 |
| M: 344.1 ± 17.0 (28) | 348.9 ± 14.8 (37) | 0.42, 0.52 | |
| 1.64, 0.20 | |||
| AP threshold, mV | F: −48.4 ± 1.7 (24) | −44.4 ± 1.9 (29) | 3.64, 0.0589 |
| M: −45.8 ± 2.0 (28) | −48.7 ± 1.6 (37) | 0.24, 0.63 | |
| 0.09, 0.76 | |||
| AP width at half-peak amplitude, ms | F: 2.6 ± 0.2 (24) | 2.5 ± 0.1 (29) | 1.10, 0.30 |
| M: 3.0 ± 0.2 (28) | 2.5 ± 0.1 (37) | 3.41, 0.0672 | |
| 3.09, 0.0813 | |||
| AHP peak amplitude, mV | F: −7.6 ± 0.5 (24) | −8.6 ± 0.3 (29) | 6.5, 0.0120 |
| M: −8.4 ± 0.4 (28) | −7.5 ± 0.3 (37) | 0.2, 0.65 | |
| 0.1, 0.73 | |||
| AHP time to peak, ms | F: 33.5 ± 3.4 (24) | 33.7 ± 2.1 (29) | 1.1, 0.29 |
| M: 37.5 ± 2.3 (28) | 32.7 ± 1.7 (37) | 0.4, 0.52 | |
| 1.0, 0.31 | |||
| FI slope, Hz/nA | F: 207.1 ± 24.2 (24) | 167.2 ± 7.0 (29) | 9.4, 0.0027 |
| M: 157.9 ± 8.5 (28) | 212.3 ± 16.6 (37) | 0.0, 0.88 | |
| 0.2, 0.65 | |||
| mEPSC frequency, Hz | F: 2.3 ± 0.3 (21) | 3.3 ± 0.5 (24) | 0.32, 0.57 |
| M: 2.0 ± 0.3 (24) | 3.6 ± 0.8 (7) | 0.00, 0.99 | |
| 5.9, 0.0178 | |||
| mEPSC amplitude, pA | F: 10.0 ± 0.3 (21) | 10.8 ± 0.5 (24) | 0.31, 0.58 |
| M: 10.0 ± 0.7 (24) | 11.5 ± 1.2 (7) | 0.31, 0.58 | |
| 2.6, 0.11 | |||
| mEPSC decay, ms | F: 2.7 ± 0.2 (21) | 2.7 ± 0.2 (24) | 0.86, 0.36 |
| M:3.1 ± 0.2 (24) | 2.7 ± 0.4 (7) | 0.42, 0.52 | |
| 0.7, 0.40 |
Electrophysiological properties recorded from caudate-putamen MSNs from prepubertal female and male rats exposed to either vehicle or estradiol in the perinatal period. Values are means ± SE. Numbers in parentheses indicate the number of neurons in each group (experimental n). Statistics column lists F and P values for interaction, sex, and hormone from top to bottom for a two-way ANOVA analysis with sex and estradiol exposure as factors. Bold font indicates statistical significance. AHP, afterhyperpolarization; AP, action potential; F, female; FI, evoked firing rate-to-positive current curve; M, male; mEPSC, miniature excitatory postsynaptic current.
Table 3.
Caudate-putamen medium spiny neuron electrophysiological properties from males exposed to PPT, DPN, or G1 and females and males exposed to vehicle during the perinatal developmental period
| Property | Vehicle | PPT | DPN | G1 | Statistics (F, P) |
|---|---|---|---|---|---|
| Resting membrane potential, mV | F: −82.8 ± 1.7 (24) | −86.1 ± 1.8 (25) | −83.5 ± 2.0 (26) | −81.6 ± 3.0 (30) | 0.740 |
| M: −84.2 ± 1.3 (28) | 0.5665 | ||||
| Input resistance, MΩ | F: 260.2 ± 26.9 (24) | 279.3 ± 25.8 (25) | 270.9 ± 21.6 (26) | 247.5 ± 28.0 (17) | 0.405 |
| M: 245.2 ± 15.3 (28) | 0.8046 | ||||
| Rectified range input resistance, MΩ | F: 200.1 ± 13.7 (24) | 219.8 ± 12.5 (25) | 226.6 ± 17.5 (26) | 208.6 ± 19.4 (17) | 0.498 |
| M: 209.6 ± 12.2 (28) | 0.7371 | ||||
| Inward rectification, MΩ | F: 62.7 ± 14.8 (24) | 59.5 ± 16.4 (25) | 44.2 ± 7.0 (26) | 39.0 ± 11.9 (17) | 1.138 |
| M: 35.5 ± 4.7 (28) | 0.3423 | ||||
| % Inward rectification, % | F: 82.2 ± 2.8 (24) | 82.8 ± 2.5 (25) | 84.7 ± 2.1 (26) | 86.2 ± 2.0 (17) | 0.769 |
| M: 86.5 ± 1.5 (28) | 0.5474 | ||||
| Time constant of the membrane, ms | F: 16.6 ± 2.5 (24) | 14.4 ± 1.5 (25) | 15.0 ± 1.3 (26) | 14.0 ± 1.7 (17) | 0.591 |
| M: 13.2 ± 1.0 (28) | 0.6699 | ||||
| Capacitance, pF | F: 61.6 ± 4.8 (24) | 52.4 ± 2.7 (25) | 58.6 ± 5.3 (26) | 59.7 ± 5.4 (17) | 0.673 |
| M: 54.9 ± 3.1 (28) | 0.6117 | ||||
| Rheobase, pA | F: 101.9 ± 12.8 (24) | 107.1 ± 11.9 (25) | 116.7 ± 13.9 (26) | 130.2 ± 12.5 (17) | 0.623 |
| M: 111.7 ± 10.5 (28) | 0.6473 | ||||
| Delay to first AP, ms | F: 310.1 ± 27.0 (24) | 327.3 ± 21.0 (25) | 380.0 ± 21.0 (26) | 388.1 ± 28.9 (17) | 2.049 |
| M: 344.1 ± 17.0 (28) | 0.0922 | ||||
| AP threshold, mV | F: −48.4 ± 1.7 (24) | −45.8 ± 2.0 (25) | −47.3 ± 1.8 (26) | −47.0 ± 2.2 (17) | 0.294 |
| M: −45.8 ± 2.0 (28) | 0.8817 | ||||
| AP width at half-peak amplitude, ms | F: 2.6 ± 0.2 (24) | 2.6 ± 0.1 (25) | 2.7 ± 0.2 (26) | 2.6 ± 0.2 (17) | 0.962 |
| M: 3.0 ± 0.2 (28) | 0.4312 | ||||
| AHP peak amplitude, mV | F: −7.6 ± 0.5 (24) | −6.9 ± 0.3 (25) | −7.5 ± 0.5 (26) | −7.2 ± 0.3 (17) | 1.797 |
| M:−8.4 ± 0.4 (28) | 0.1341 | ||||
| AHP time to peak, ms | F: 33.5 ± 3.4 (24) | 32.1 ± 2.3 (25) | 33.0 ± 1.8 (26) | 33.4 ± 3.0 (17) | 0.749 |
| M: 37.5 ± 2.3 (28) | 0.5606 | ||||
| FI slope, Hz/nA | F: 207.1 ± 24.2 (24) | 229.2 ± 26.0 (25) | 185.7 ± 11.0 (26) | 186.4 ± 10.1 (17) | 2.409 |
| M: 157.9 ± 8.5 (28) | 0.0533 |
Electrophysiological properties recorded from caudate-putamen MSNs from prepubertal male rats exposed to vehicle, PPT, DPN, or G1 and females exposed to vehicle during the perinatal period. Values are means ± SE. Numbers in parentheses indicate the number of neurons in each group (experimental n). Statistics column lists F and P values for one-way ANOVA analysis. AHP, afterhyperpolarization; AP, action potential; F, female; FI, evoked firing rate-to-positive current curve; M, male; mEPSC, miniature excitatory postsynaptic current.
Female MSNs exposed to vehicle exhibited increased action potential firing rates compared to male MSNs exposed to vehicle, and this increase was masculinized in females by exposure to perinatal estradiol (Fig. 2B). A linear regression analysis revealed that the slopes of the action potential firing rates evoked by injected depolarizing current curves were significantly different between treatment groups [P < 0.0001; F(3,36) = 12.4255]. We further quantified these differences by comparing the slope of the evoked firing rate to positive current curve (FI slope) between groups (Fig. 2C). MSNs from females injected with vehicle and males injected with estradiol demonstrated steeper FI slope compared to MSNs recorded from male animals injected with vehicle and female animals injected with estradiol. This analysis indicates that perinatal estradiol exposure masculinized female neurons to generate fewer action potentials per injected current throughout the linear range, similar to vehicle-exposed males. Supporting this analysis, vehicle-treated females and estradiol-treated males showed increased initial firing rates compared to vehicle-treated males and estradiol-treated females at Δ0.01, Δ0.02, and Δ0.03 nA from the minimum current injection necessary for action potential generation [Fig. 2D; Treatment Group: F(3,440) = 13.85, P < 0.0001; Current: F(3,440) = 85.86, P < 0.0001; Interaction: F(9,440) = 0.8819, P = 0.5413]. A similar pattern was observed for steady state firing rate [Fig. 2E; Treatment Group: F(3,440) = 13.55, P < 0.0001; Current: F(3,440) = 89.78, P < 0.0001; Interaction: F(9,440) = 0.7604, P = 0.6532]. Males exposed to estradiol showed increased values compared to vehicle-treated males and estradiol-treated females, whereas vehicle-treated females demonstrated intermediate values at Δ0.01, Δ0.02, and Δ0.03 nA from the minimum current injection necessary for action potential generation.
Perinatal Exposure to Estradiol Masculinizes Female Afterhyperpolarization Peak and Rheobase
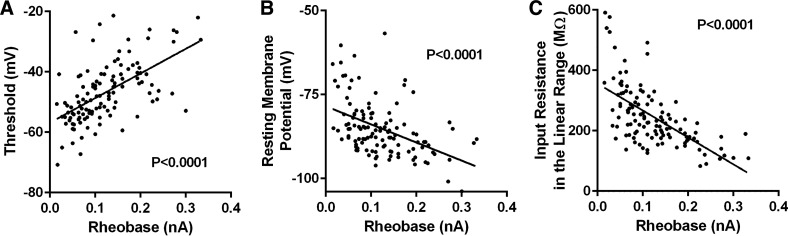
An estradiol-induced masculinization of evoked firing rate indicates that estradiol may likewise masculinize MSN action potential (AP) properties or the membrane properties. Regarding action potential properties, estradiol masculinized the amplitude of the afterhyperpolarization (AHP) peak in females (Fig. 3A). Females exposed to estradiol showed increased AHP peaks compared to females exposed to vehicle and males exposed to estradiol. Rheobase, the amount of positive injected current necessary to generate the first action potential, was also altered by estradiol in females (Fig. 3B). Females exposed to estradiol showed increased rheobase compared to females and males exposed to vehicle, and males exposed to estradiol. Typically, increased rheobase indicates decreased neuronal excitability. Differences in rheobase are typically accompanied by differences in electrophysiological properties such as AP threshold, resting membrane potential, or passive properties such as input resistance (Table 1). We calculated linear regressions between MSN rheobase and, respectively, AP threshold, resting membrane potential, and input resistance in the linear range. Increased rheobase strongly associated with depolarized AP threshold (Fig. 4A; Slope: 81.45, r2 = 0.3387, P < 0.0001). Rheobase and resting membrane potential values also correlated, with increased rheobase values associating with hyperpolarized resting membrane potential values (Fig. 4B; Slope: −53.95, r2 = 0.2105, P < 0.0001). Likewise, rheobase and input resistance in the linear range values also correlated, with increased rheobase values associating with decreased input resistance values (Fig. 4C; Slope: −890.7, r2 = 0.3866, P < 0.0001). This analysis is consistent with the conclusion that estradiol-induced masculinization in rheobase is dependent upon changes in AP threshold, neuron resting membrane potential, and input resistance in the linear range. Overall, these data indicate that estradiol acts to masculinize female MSN intrinsic excitability in a multifaceted manner, by robustly changing the amplitude of the AHP peak and the rheobase, while inducing smaller changes in other MSN properties.
Figure 3.
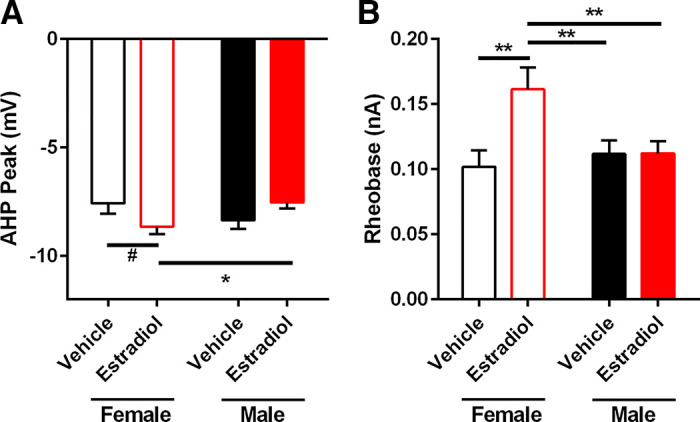
Action potential (AP) afterhyperpolarization (AHP) peak and AP rheobase varied by sex and perinatal exposure to estradiol. A: AP AHP peak amplitude was larger in MSNs recorded from females with perinatal exposure to estradiol compared to females with perinatal exposure to vehicle and males with perinatal exposure to estradiol. B: AP rheobase was larger in MSNs recorded from females with perinatal exposure to estradiol compared to females and males with perinatal exposure to vehicle and males with perinatal exposure to estradiol. MSN, medium spiny neuron. #P = 0.0522; *P < 0.05; **P < 0.01.
Figure 4.
Differences in AP threshold, MSN resting membrane potential, and input resistance in the linear range correlated with differences in AP rheobase. A: depolarized AP threshold values associated with increased AP rheobase values. B: hyperpolarized MSN resting membrane potential values associated with increased AP rheobase values. C: decreased MSN input resistance in the linear range values associated with increased AP rheobase values. AP, action potential; MSN, medium spiny neuron.
Perinatal Exposure to Estradiol Masculinizes Female Inward Rectification
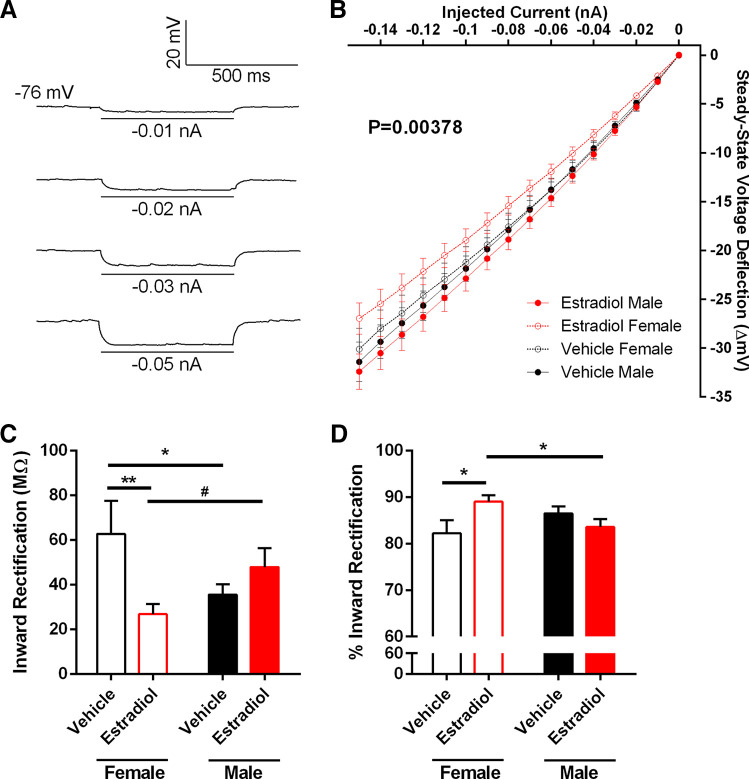
The strong association between increased rheobase and decreased input resistance in the linear range suggests that further analysis of input resistance properties is warranted. To investigate input resistance in the linear and rectified ranges, we injected a series of hyperpolarizing current injections in MSNs from estradiol- and vehicle-treated males and females (Fig. 5A) and first calculated the steady-state voltage deflection evoked by injected hyperpolarizing current curve (IV curve; Fig. 5B). A linear regression analysis revealed that estradiol masculinized the slopes of the steady-state voltage deflections evoked by injected hyperpolarizing current curves [P = 0.00378; F(3,1715) = 4.49544]. Interestingly, no significant differences were detected in the absolute values of input resistance in the linear and rectified ranges (Table 1). Linear range input resistance is calculated from the least hyperpolarizing current pulse injected into the neuron that produces a reliable measureable change in steady-state membrane potential. Rectified range input resistance is calculated from the steady-state membrane potential in response to the most hyperpolarizing current pulse injected into the neuron. A nonrectifying neuron would exhibit identical linear range and rectified range input resistances. This lack of a robust action of estradiol on absolute input resistance measurements suggests that estradiol may be acting to masculinize MSN inward rectification itself. To assess this question, we quantified both inward rectification and percent inward rectification. Inward rectification is calculated as the linear range input resistance minus the rectified range input resistance. Thus, an increased inward rectification value corresponds to a greater inwardly rectifying membrane potential. Estradiol masculinized inward rectification in females (Fig. 5C). Likewise, estradiol masculinized % inward rectification in females (Fig. 5D). Percent inward rectification is calculated as (linear range input resistance minus the rectified range input resistance)/100. Thus, decreased percent inward rectification values correspond with a greater inwardly rectifying membrane potential. Percent inward rectification is a useful additional analysis as it normalizes for differences in input resistance magnitude. This analysis is consistent with the conclusion that estradiol acts to masculinize the properties of female MSN inward rectification.
Figure 5.
Input resistance in the rectified range varied by sex and perinatal exposure to estradiol. A: voltage response of a MSN to a series of negative current injections, recorded from a male with perinatal exposure to vehicle. “−76 mV” indicates resting membrane potential. B: injected negative current to steady-stage voltage deflection curve (IV curve). C: inward rectification was increased in MSNs recorded from females with perinatal exposure to vehicle compared to females with perinatal exposure to estradiol and males with perinatal exposure to vehicle. Inward rectification was decreased MSNs recorded from females with perinatal exposure to estradiol compared to males with perinatal exposure to estradiol. D: percent inward rectification was decreased in MSNs recorded from females with perinatal exposure to vehicle compared to females with perinatal exposure to estradiol. Percent inward rectification was increased in MSNs recorded from females with perinatal exposure to estradiol compared to males with perinatal exposure to estradiol. Note that decreased percent inward rectification values typically correspond with increased inward rectification values. MSN, medium spiny neuron. #P = 0.0733; *P < 0.05; **P < 0.01.
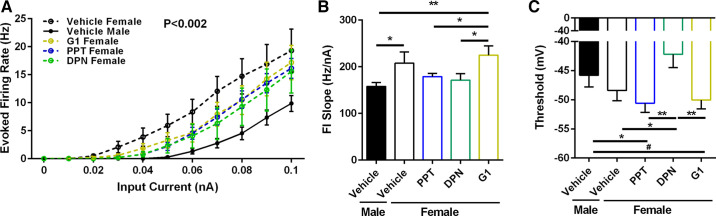
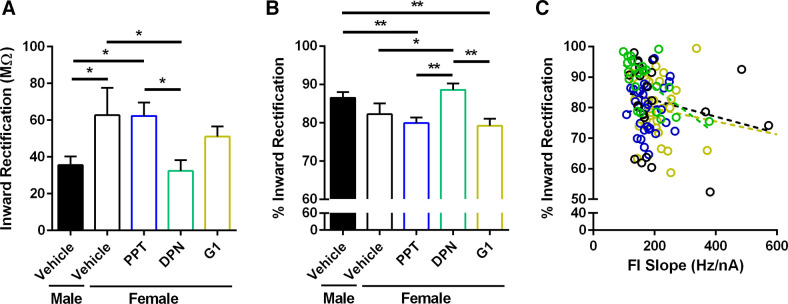
Perinatal Exposure to an ERα or ERβ Agonist Masculinizes Female MSN Evoked Action Potential Firing Rate
Given that perinatal estradiol exposure masculinized MSN intrinsic excitability in females, we reasoned that exposure to an ER agonist would mimic estradiol’s effects. Female rat pups were injected with either the ERα agonist PPT, the ERβ agonist DPN, or the GPER-1 agonist G1, and compared to female and male pups injected with vehicle solution (Fig. 6A). A linear regression analysis revealed that female MSNs exposed to either PPT or DPN significantly masculinized evoked action potential firing rate in females, unlike exposure to G1 [P = 0.001704; F(4,45) = 5.13574]. Evoked action potential firing rate differed between males and females exposed to vehicle. Regarding FI slope, female MSNs exposed to PPT or DPN exhibited decreased action potential firing rates compared to female MSNs exposed to vehicle, unlike female MSNs exposed to G1 (Fig. 6B). Interestingly, AP threshold values differed between groups, with MSNs from females injected with DPN exhibiting depolarized threshold values compared to females exposed to PPT and G1 (Fig. 6C). Rheobase and AP afterhyperpolarization values did not differ significantly (Table 2). Overall, these changes in evoked action potential firing rate induced by PPT or DPN exposure and subsequent ERα or ERβ activation largely mimic those changes induced by estradiol exposure.
Figure 6.
Perinatal exposure to an ERα or ERβ agonist masculinized female MSN evoked action potential firing rate. A: AP firing rate evoked by depolarizing current injection. AP firing rate was masculinized in MSNs recorded from females with perinatal exposure to the ERα agonist PPT and the ERβ agonist DPN. B: the slope of the evoked AP firing rate to depolarizing current injection curve (FI Slope) was masculinized in MSNs recorded from females with perinatal exposure to the ERα agonist PPT and the ERβ agonist DPN. C: AP threshold values were hyperpolarized in MSNs recorded from females with perinatal exposure to the ERα agonist PPT and the GPER-1 agonist G1. AP, action potential; ER, estrogen receptor; MSN, medium spiny neuron. #P = 0.0876; *P < 0.05; **P < 0.01.
Table 2.
Caudate-putamen medium spiny neuron electrophysiological properties from females exposed to PPT, DPN, or G1 and females and males exposed to vehicle during the perinatal period
| Property | Vehicle | PPT | DPN | G1 | Statistics (F, P) |
|---|---|---|---|---|---|
| Resting membrane potential, mV | F: −82.8 ± 1.7 (24) | −85.3 ± 1.3 (31) | −83.4 ± 1.2 (22) | −86.5.9 ± 1.5 (30) | 1.093 |
| M: −84.2 ± 1.3 (28) | 0.3628 | ||||
| Input resistance, MΩ | F: 260.2 ± 26.9 (24) | 287.1 ± 17.4 (31) | 261.9 ± 18.7 (22) | 232.9 ± 13.0 (30) | 1.395 |
| M: 245.2 ± 15.3 (28) | 0.2392 | ||||
| Rectified range input resistance, MΩ | F: 200.1 ± 13.7 (24) | 224.8 ± 11.5 (31) | 229.5 ± 15.3 (22) | 181.9 ± 9.9 (30) | 2.484 |
| M: 209.6 ± 12.2 (28) | 0.0468 | ||||
| Inward rectification, MΩ | F: 62.7 ± 14.8 (24) | 62.3 ± 7.3 (31) | 32.4 ± 5.9 (22) | 51.0 ± 5.6 (30) | 2.989 |
| M: 35.5 ± 4.7 (28) | 0.0212 | ||||
| % Inward rectification, % | F: 82.2 ± 2.8 (24) | 79.9 ± 1.5 (31) | 88.6 ± 1.7 (22) | 79.2 ± 1.8 (30) | 4.789 |
| M: 86.5 ± 1.5 (28) | 0.0012 | ||||
| Time constant of the membrane, ms | F: 16.6 ± 2.5 (24) | 18.0 ± 1.1 (31) | 12.8 ± 0.8 (22) | 15.7 ± 1.2 (30) | 2.425 |
| M: 13.2 ± 1.0 (28) | 0.0513 | ||||
| Capacitance, pF | F: 61.6 ± 4.8 (24) | 66.2 ± 4.2 (31) | 52.3 ± 4.9 (22) | 70.5 ± 5.3 (30) | 2.900 |
| M: 54.9 ± 3.1 (28) | 0.0245 | ||||
| Rheobase, pA | F: 101.9 ± 12.8 (24) | 95.5 ± 7.9 (31) | 135.2 ± 15.1 (22) | 99.2 ± 7.5 (30) | 2.077 |
| M: 111.7 ± 10.5 (28) | 0.0875 | ||||
| Delay to first AP, ms | F: 310.1 ± 27.0 (24) | 381.5 ± 21.4 (31) | 363.3 ± 20.5 (22) | 365.5 ± 18.3 (30) | 1.661 |
| M: 344.1 ± 17.0 (28) | 0.1629 | ||||
| AP threshold, mV | F: −48.4 ± 1.7 (24) | −50.6 ± 1.6 (31) | −42.2 ± 2.3 (22) | −50.1 ± 1.5 (30) | 3.424 |
| M: −45.8 ± 2.0 (28) | 0.0107 | ||||
| AP width at half-peak amplitude, ms | F: 2.6 ± 0.2 (24) | 3.1 ± 0.1 (31) | 2.9 ± 0.1 (22) | 2.4 ± 0.1 (30) | 4.643 |
| M: 3.0 ± 0.2 (28) | 0.0016 | ||||
| AHP peak amplitude, mV | F: −7.6 ± 0.5 (24) | −7.2 ± 0.4 (31) | −6.9 ± 0.5 (22) | −7.4 ± 0.4 (30) | 1.500 |
| M: −8.4 ± 0.4 (28) | 0.2059 | ||||
| AHP time to peak, ms | F: 33.5 ± 3.4 (24) | 36.0 ± 3.0 (31) | 29.8 ± 2.0 (22) | 29.5 ± 1.9 (30) | 2.003 |
| M: 37.5 ± 2.3 (28) | 0.0979 | ||||
| FI slope, Hz/nA | F: 207.1 ± 24.2 (24) | 178.9 ± 6.9 (31) | 171.0 ± 14.2 (22) | 224.8 ± 19.9 (30) | 3.181 |
| M: 157.9 ± 8.5 (28) | 0.0157 |
Electrophysiological properties recorded from caudate-putamen MSNs from prepubertal female rats exposed to vehicle, PPT, DPN, or G1 and males exposed to vehicle during the perinatal period. Values are means ± SE. Numbers in parentheses indicate the number of neurons in each group (experimental n). Statistics column lists F and P values for one-way ANOVA analysis. Bold font indicates statistical significance. AHP, afterhyperpolarization; AP, action potential; F, female; FI, evoked firing rate-to-positive current curve; M, male; mEPSC, miniature excitatory postsynaptic current.
Perinatal Exposure to an ERβ Agonist Masculinizes Female MSN Inward Rectification
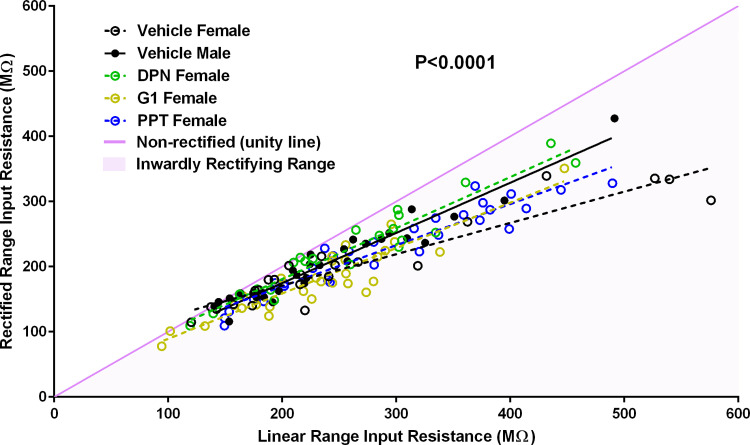
To assess whether ER agonist exposure induces differences in the input resistance between the linear and rectified ranges, we plotted linear range input resistance versus rectified range input resistance for females exposed to vehicle, PPT, DPN, and G1, and males exposed to vehicle (Fig. 7). A linear regression analysis revealed that the slopes between the calculated regression lines significantly differed [P < 0.0001; F(4,125) = 9.90]. Females with perinatal exposure to vehicle displayed the greatest difference between the linear range and rectified range input resistances (Slope: 0.48 ± 0.04, y-intercept: 75.13 ± 10.83, r2: 0.88, F: 165.8, P < 0.0001). Males with perinatal exposure to vehicle and females with perinatal exposure to the ERβ agonist DPN displayed the least differences between the linear and rectified range input resistances (Vehicle Males: Slope: 0.77 ± 0.04, y-intercept: 20.81 ± 10.28, r2: 0.93, F: 373.2, P < 0.0001; DPN Females: Slope: 0.78 ± 0.05, y-intercept: 24.34 ± 14.08, r2: 0.92, F: 234.9, P < 0.0001). Exposure to PPT or G1 did not masculinize the difference between the linear and rectified range input resistances (PPT Females: Slope: 0.63 ± 0.04, y-intercept: 43.16 ± 11.19, r2: 0.91, F: 292.2, P < 0.0001; G1 Females: Slope: 0.70 ± 0.06, y-intercept: 19.65 ± 14.20, r2: 0.84, F: 142.3, P < 0.0001). This finding indicates that perinatal exposure to an ERβ agonist masculinized female MSN inward rectification to male levels, mimicking the effects of perinatal estradiol exposure.
Figure 7.
Perinatal exposure to an ERβ agonist decreased female MSN inward rectification to male levels. Linear range input resistance versus rectified range input resistance plot. Females with perinatal exposure to vehicle displayed the greatest inward rectification. Males with perinatal exposure to vehicle and females with perinatal exposure to the ERβ agonist DPN displayed the least inward rectification. A linear regression analysis revealed that the slopes between the calculated regression lines significantly differ (P < 0.0001). Linear range input resistance was calculated from the least hyperpolarizing current pulse injected into the neuron that produces a reliable measureable change in steady-state membrane potential. Rectified range input resistance was calculated from the steady-state membrane potential in response to the most hyperpolarizing current pulse injected into the neuron. A nonrectifying neuron would exhibit identical linear range and rectified range input resistances and would fall on the nonrectified unity line (purple). A neuron exhibiting inward rectification in its membrane potential in response to increasing hyperpolarizing current pulses would fall in the bottom half of the plot, below the nonrectified unity line (mauve shading). ER, estrogen receptor; MSN, medium spiny neuron.
Consistent with this conclusion, DPN but not PPT exposure masculinized the calculated inward rectification in females (Fig. 8A). Inward rectification is calculated as the linear range input resistance minus the rectified range input resistance. Thus, an increased inward rectification value corresponds to a greater inwardly rectifying membrane potential. Consistent with an impact on inward rectification properties, DPN but not PPT exposure also masculinized percent inward rectification in females (Fig. 8B). Percent inward rectification is calculated as (linear range input resistance minus the rectified range input resistance)/100. Thus, decreased percent inward rectification values correspond with a greater inwardly rectifying membrane potential. A linear regression analysis revealed that DPN masculinization of % inward rectification in females correlated with FI Slope values, but not in females exposed to vehicle, PPT, and G1 [Fig. 8C; DPN: P = 0.0016; F(1,20) = 13.31; Vehicle: P = 0.2723; F(1,22) = 1.27; PPT P = 0.6202; F(1,29) = 0.25: G1: P = 0.2178; F(1,28) = 1.59]. This finding indicates that DPN strongly influences both firing rate and inward rectification in the same neurons, unlike other treatment groups. This result is highly interesting given that these neuronal attributes are typically controlled by different ion channel mechanisms. Overall, these analyses is consistent with the conclusion that changes in inward rectification induced by DPN exposure and subsequent respective activation of ERβ mimic changes induced by estradiol exposure.
Figure 8.
Perinatal exposure to an ERβ agonist masculinized female MSN inward rectification. A: inward rectification was decreased in MSNs recorded from females with perinatal exposure to the ERβ agonist DPN and males with perinatal exposure to vehicle compared to MSNs recorded from females with perinatal exposure to the ERα agonist PPT or vehicle. Inward rectification was calculated as the linear range input resistance minus the rectified range input resistance. Thus, an increased inward rectification value corresponds to a greater inwardly rectifying membrane potential. B: percent inward rectification was increased in MSNs recorded from females with perinatal exposure to the ERβ agonist DPN and males with perinatal exposure to vehicle compared to MSNs recorded from females with perinatal exposure to the ERα agonist PPT or vehicle. Percent inward rectification was calculated as (linear range input resistance minus the rectified range input resistance)/100. Thus, decreased percent inward rectification values correspond with a greater inwardly rectifying membrane potential. C: linear regression analysis revealed that masculinization of percent inward rectification in females exposed to DPN correlated with FI Slope values (P < 0.002), but not in females exposed to vehicle, PPT, and G1 (P > 0.05 for all). ER, estrogen receptor; FI Slope, slope of the evoked AP firing rate to depolarizing current injection curve; MSN, medium spiny neuron. *P < 0.05; **P < 0.01.
Perinatal Exposure to an ER Agonists Exert a Minimal Impact on Male MSN Electrophysiological Properties
We hypothesized that if perinatal exposure to ERβ or ERα is masculinizing female MSN electrophysiological properties, then a comparable exposure in males should exert a less robust impact, given that in male animals this process would normally already be occurring. To test this hypothesis, male rat pups were injected with either the ERα agonist PPT, the ERβ agonist DPN, or the GPER-1 agonist G1, and compared to female and male pups injected with vehicle solution. No differences were detected in the assessed battery of MSN electrophysiological properties (Table 3). A trend was noted regarding FI Slope (P = 0.0537), which is consistent with the differences detected between vehicle male and female MSNs (Table 3). Overall, exposure to PPT, DPN, or G1 exerted a minimal impact on male MSN electrophysiological properties.
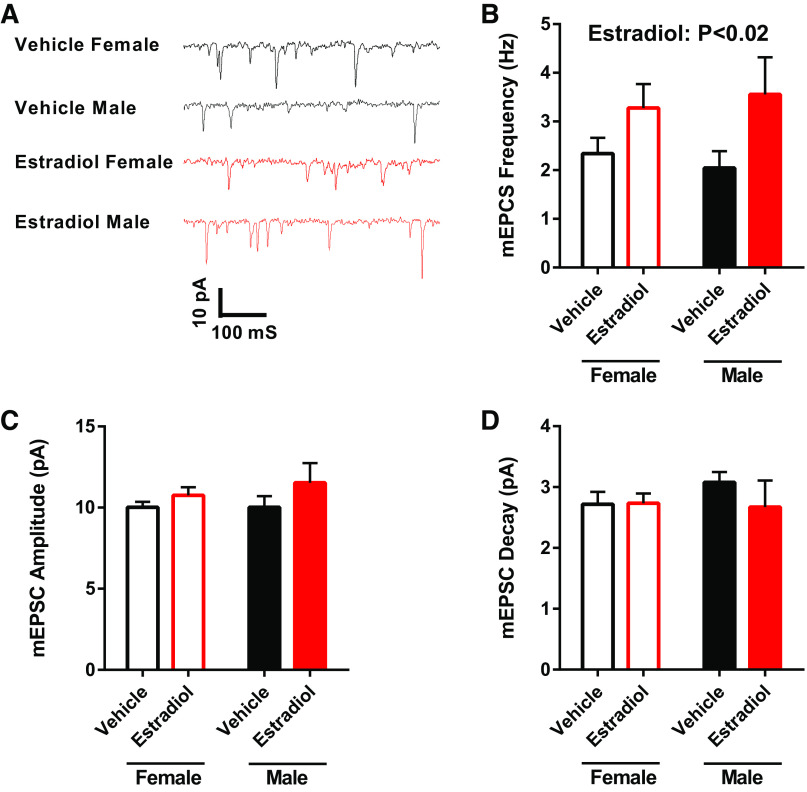
Perinatal Exposure to Estradiol Modulated mEPSC Frequency
To assess whether perinatal estradiol exposure modulated AMPA-mediated glutamatergic synapse properties, in a subset of recordings mEPSC were recorded at −70 mV in the presence of tetrodotoxin and picrotoxin (Fig. 9A). Perinatal estradiol exposure increased mEPSC frequency in both females and males (Fig. 9B); however, post hoc statistical tests did not detect significant differences between groups (Table 1). No differences were detected in mEPSC amplitude (Fig. 9C) or decay (Fig. 9D).
Figure 9.
MSN miniature excitatory postsynaptic current (mEPSC) properties from females and males with perinatal exposure to vehicle or estradiol. A: representative examples of mEPSCs recorded at −70 mV in the presence of tetrodotoxin and picrotoxin. B: an overall effect of perinatal estradiol exposure was detected for mEPSC frequency. Post hoc statistical tests did not detect statistical significance. C: no differences were found in mEPSC amplitude. D: no differences were detected in mEPSC decay. MSN, medium spiny neuron.
DISCUSSION
The experiments described in this manuscript make three key findings. First, that estradiol exposure during the perinatal sensitive period is sufficient to masculinize female caudate-putamen MSNs. Estradiol exposure decreased the intrinsic excitability of female MSNs to levels observed in male MSNs, as demonstrated by decreased evoked action potential firing rates and other electrophysiological metrics. Second, exposure to ERα and ERβ agonists during the perinatal period mimicked the action of estradiol upon female MSNs. Third, exposure to ERα and ERβ agonists during the perinatal period exerted a less robust impact upon male MSNs.
Overall, these findings help generate an intellectual framework wherein estradiol acts during the perinatal period upon ERα and ERβ to masculinize caudate-putamen MSN electrophysiological properties, a process which would normally occur in males. Specifically, these data indicate a working model in which testosterone secreted by the testes is first converted into estradiol by the enzyme aromatase, either in the striatum or elsewhere. Aromatase is present in all three striatal regions (16, 48–52), although there are differences across development and striatal regions and aromatase expression levels across adult hormone cycles have yet to be extensively explored. Perinatal estradiol sensitivity appears to be a characteristic of MSNs in both the caudate-putamen (this manuscript) and the nucleus accumbens as well as investigated select striatal-mediated behaviors (22, 24, 53–55). At least in the caudate-putamen, estradiol then is predicted to act on ERα and ERβ to masculinize specific MSN electrophysiological properties. ERα and ERβ both independently organize evoked action potential properties, whereas ERβ alone more predominantly organizes inward rectification and related properties. Regarding specific action potential properties, perinatal estradiol exposure in females appears to increase the AHP peak amplitude to levels commonly measured in males, consistent with prior findings that detected differences in AHP properties (21). The AHP peak amplitude attribute typically corresponds with a metric that other laboratory groups designate as the “fast afterhyperpolarization” (sAHP) (56). Changes in this portion of the AHP in MSNs suggest alteration of an A-type potassium current (56), whereas changes in inward rectification suggest alteration of an inwardly rectifying potassium current (56–60). Of course, other ion channels such as calcium-activated potassium channels could be at work, as demonstrated in both MSNs and striatal cholinergic interneurons (61, 62). We also note that the specific ionic mechanism generating the sex difference in evoked action potentials remains unexplored, as discussed in a previous study (21).
We currently favor a straightforward model, in which perinatal estradiol acts upon nuclear ERα and ERβ localized in MSNs. These nuclear ER then presumably dimerize and program gene expression by acting on estrogen response element (ERE) regions in the genome. After this action, nuclear ER cease to be expressed by the MSN. In adulthood (and perhaps before), estradiol would then play a classical activational role. This model is consistent with recent findings, which demonstrate that nuclear ERα and ERβ are present in striatal neurons in male and female rats during the perinatal period but disappear by puberty (16). This model also can incorporate recent findings that show differences in caudate-putamen and nucleus accumbens MSN electrophysiological properties across the estrous cycle and in response to acute estradiol exposure (26, 28, 30–32). Older work that shows differences in caudate-putamen MSN electrophysiological properties in response to either the estrous cycle or acute estradiol exposure (9, 63, 64) is also concordant with this model. Disambiguating whether sex differences in MSN electrophysiological properties are generated in this cell-autonomous process remains an important unanswered question and future direction. Consistent with this prediction of this working model, direct, cell-autonomous action of estradiol on female caudate-putamen MSN L-type calcium channel currents and CREB phosphorylation and male MSN caudate-putamen synaptic plasticity have been demonstrated in a rapid, acute timeframe (9, 11, 65). During the perinatal period, female and male rat caudate-putamen expresses at least nuclear ERα and GPER-1, as demonstrated by immunocytochemical studies (16). The absence of a validated antibody has stunted research into the presence of nuclear ERβ, especially during the perinatal period (66). Mitigating this unfortunate situation, ERβ and aromatase mRNA have been detected in female and male mouse caudate-putamen during the perinatal and fetal developmental periods (67, 68). Nuclear expression of ER declines after the perinatal period (16). ERα, β, and GPER-1 are detectable by electron microscopy studies, qPCR, and functional analysis in adult rat caudate-putamen, with evidence pointing toward membrane and cytoplasmic localization in MSNs and glia (9–15, 69). Immunocytochemical studies do not typically detect ERα in the adult rat caudate-putamen (12), potentially due to lower expression (70) or the blockade of the relevant epitope by plasma membrane. Many brain regions that project to the caudate-putamen express estrogen receptors (70), so a non-MSN cell autonomous mechanism remains an important potential, especially given previous evidence of such a mechanism from studies of songbirds (71–74). Cell autonomous and noncell autonomous neuroendocrine mechanisms are not mutually exclusive and may differ by developmental stage and neuron target.
GPER-1 is also present in both male and female caudate-putamen, but the current study did not generate evidence that GPER-1 organizes the assessed electrophysiological attributes. This lack of effect is perhaps consistent with GPER-1 not yet being expressed at the neuronal plasma membrane during the perinatal period (16). However, GPER-1 could potentially play a role in sexual differentiation in other metrics (75), and a modulatory role in adults. We also note that this current model does not preclude ER activation on other cell types either within or outside the striatum, which could then indirectly organize MSN properties. There is also evidence that direct chromosomal action may influence striatal neuron sexual differentiation, at least in terms of select dopamine receptor gene expression (76). Although particular details of this proposed model may ultimately turn out to be or to not be correct, overall, the data presented here demonstrate that caudate-putamen MSNs can be sexually differentiated by exposure to estradiol and its receptor agonists, which has profound implications for sex-sensitive caudate-putamen-mediated functions and disorders in adulthood. Importantly, the influence of steroid hormones such as estradiol in masculinizing the caudate-putamen reveals a potential vulnerability of caudate-putamen-relevant behaviors and disorders to the effects of endocrine disruption.
Pertinent limitations to this study exist. Caudate-putamen MSNs exhibit multiple subtypes, which were not addressed by the current study. MSN subtypes can differentially express D1 and D2-dopamine receptors and exhibit subtle differences in electrophysiological and dendritic properties (25, 29, 77, 78). Although at this point there is no evidence demonstrating differences in ER expression between MSN subtypes, it has not been ruled out that the MSN subtypes could be differentially sensitive to estradiol action during any developmental period. Other limitations also exist. Regarding the effects of ER agonists on males, it is possible that subtle effects of exposure to these compounds in males were not detected due to insufficient statistical power. Thus, we have conservatively interpreted the present study as ER agonists inducing “minimal” or “less robust” impact upon males compared to similar exposure in females, rather than these compounds having “no effects.” Another limitation is that the current study was intentionally designed to address whether estradiol and its receptors was sufficient rather than necessary for MSN masculinization, particularly in females. Future studies will be required to assess the necessity of estradiol and its receptors by blocking ER and perhaps aromatase action in perinatal and perhaps fetal males. This consideration is particularly pertinent, as perinatal estradiol exposure in males appears to increase MSN excitability, perhaps indicating paradoxical hyperfeminization (Fig. 2), a concept similar to that pioneered by studies in songbird model systems (79). This finding would seem to indicate that exogenous estradiol exposure, perhaps layered on top of the natural perinatal testosterone surge, may have an additional action in males. Testosterone can be metabolized into 17β-estradiol via the enzyme aromatase in the male rat brain. This metabolic action could be due to numerous mechanisms, given the complexity of brain sexual differentiation (80). One straightforward option may be that there is simply additional activation of ER, either the same ER normally activation by the natural hormone surge, or ER not normally activated by the natural hormone surge, which induces this effect. Normal sexual differentiation of the caudate-putamen may involve activation of both androgen and estrogen receptors, along with chromosomal action (76). We note that this study employed systemic exposures, so as discussed above it is possible that ER outside of MSNs may also be participating in organizing MSN electrical properties. This study was also not designed to differentiate between nuclear and membrane ER action, as ERα and ERβ can exist both nuclear and membrane form due to posttranscriptional modification (81, 82). At this point, it is well documented that nuclear ER are present in perinatal rat striatum, and that only membrane ER are present in adult rat striatum. It is not clear whether membrane ER are already present during the perinatal period, or if membrane ER emerge concurrent with or after the disappearance of nuclear ER. There is evidence from hippocampal neurons that estrogen action during the perinatal period can program membrane ER signaling from the plasma membrane (83). Future studies employing the use of biotinylated estradiol infusions or perhaps more sophisticated techniques could address whether estradiol is acting on nuclear or membrane ERα and ERβ to organize striatal neural substrate.
These data join previous work demonstrating that MSN excitability and excitatory synapse properties differs by sex in developmental-stage-specific, region-specific, sex-specific, and an estrous cycle-dependent manner (84). Most relevant to the current study, previously we and others have shown that perinatal estradiol exposure sexually differentiates MSNs in the nucleus accumbens core (22, 24). The experiments described in the current manuscript advance this body of research by demonstrating that perinatal estradiol exposure likewise sexually differentiates caudate-putamen MSNs, and that these effects are mimicked by exposure to ERα and ERβ agonists. Although estradiol during the perinatal period masculinizes MSNs in both the caudate-putamen and nucleus accumbens core, estradiol exerts differential effects upon specific MSN electrophysiological properties dependent upon striatal region. In the caudate putamen, female rat prepubertal caudate-putamen MSNs exhibit increased intrinsic excitability compared to male MSNs, as primarily demonstrated by increased evoked action potential firing rates in response to injected excitatory current (21).
Caudate-putamen MSNs specifically exhibit this sex difference, as it is not present in MSNs in nucleus accumbens shell and core (22, 23). In contrast, prepubertal rat nucleus accumbens core MSNs exhibit a sex difference in excitatory synapse properties, specifically a dramatic elevation in mEPSC frequency in female compared to males (22). Elevated mEPSC frequency in female nucleus accumbens core MSNs is masculinized by perinatal exposure to estradiol; however, intrinsic excitability properties such as evoked action potential frequency remains unchanged (22). It is unknown which ERs mediate nucleus accumbens core MSN masculinization, and this gap in knowledge is a critical omission given the importance of the nucleus accumbens in regulating motivated behaviors. The sex differences detected in rat caudate-putamen and nucleus accumbens core are either absent or less robust in inbred mice MSNs assessed during the same prepubertal developmental period, at least in the assessed strains (25, 29). The caudate-putamen and nucleus accumbens are both altered during the pubertal developmental period (85–89). After puberty, intrinsic excitability in adult rat female caudate-putamen MSNs are regulated by the estrous cycle (32). In the nucleus accumbens core, after puberty, mEPSC frequency, amplitude, and intrinsic excitability properties are regulated by the estrous cycle in female rats (26). In adult animals, select nucleus accumbens core MSN properties appear to be more tightly regulated by estradiol and some by progesterone (30, 31). Indeed, acute estradiol exposure rapidly modulates mEPSC frequency in adult female MSNs in the nucleus accumbens but not caudate-putamen (28).
One interesting question generated by the present study regards whether perinatal exposure to estradiol is changing synapse number onto caudate-putamen MSNs. Although the direct answer to this question remains unknown, the mEPSC frequency data presented in this manuscript are relevant (Fig. 8B). This mEPSC recording paradigm specifically targets AMPA mediated events (26). Perinatal exposure to estradiol increased mEPSC frequency in both female and males. There was no sex difference in either vehicle- or estradiol-treated animals, consistent with a previous study in this developmental age and striatal region (21). Differences in mEPSC frequency are typically associated with changes in the presynaptic terminal such as synapse number or vesicle release properties concomitant with calcium sensitivity. Thus, we conservatively conclude that the exogenous perinatal estradiol exposure employed here regulates presynaptic properties, but it does not mimic the natural neuroendocrine process given that no sex differences in mEPSC frequency has been detected in prepubertal caudate-putamen. At least two caveats exist for this conclusion. The first is that the imbalance in sample size between experimental groups in the mEPSC portion of the current study. The second caveat is that sex differences in spontaneous EPSCs have been differentially detected between different regions of the caudate-putamen in adult mice (90), indicating that there may be portions of the caudate-putamen that may exhibit increased sexual differentiation and potentially estradiol-sensitivity compared to other portions. In adult rat caudate-putamen, no differences specifically in mEPSC properties have been detected across the estrous cycle (32), unlike in the nucleus accumbens (26, 30, 31).
This divergent sensitivity to estradiol further indicates strong regional differences in the neuroendocrine profile of particular electrophysiological properties of MSNs. Consistent with this model are reports of aromatase-sensitive synaptic plasticity in male caudate-putamen MSNs (65), in calcium channel currents (9), CREB phosphorylation (11), DeltaFosB expression (91), as well as numerous reports of regional differences in sex- and hormone-specific modulation of MSN microanatomy such as dendritic spines (20, 92–98). Overall, the evidence provided by both the findings in this study and those of previous studies indicate that the striatal region’s principal and output neuron, the MSN, must be considered sexually differentiated. It is expected that the MSN is not unique in this regard. Sustained foundational research into other neuron types across varying regional, developmental, and neuroendocrine contexts will likely further demonstrate the malleability of fundamental cellular electrophysiological properties in response to the dynamic neuroendocrine environment of the nervous system.
GRANTS
This work was supported by the following funding sources: National Institutes of Health Grants R01 MH-109471 (J.M.) and P30 ES-025128 (Center for Human Health and the Environment), and North Carolina State University Faculty Research and Professional Development Award (J.M.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C. and J.M. conceived and designed research; J.C. performed experiments; J.C. and J.M. analyzed data; J.C. and J.M. interpreted results of experiments; J.C. and J.M. prepared figures; J.C. and J.M. drafted manuscript; J.C. and J.M. edited and revised manuscript; J.C. and J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank David Dorris for technical assistance and Dr. Amanda Krentzel for discussions regarding GPER-1 and other ER agonists.
REFERENCES
- 1.Blume SR, Freedberg M, Vantrease JE, Chan R, Padival M, Record MJ, DeJoseph MR, Urban JH, Rosenkranz JA. Sex- and estrus-dependent differences in rat basolateral amygdala. J Neurosci 37: 10567–10586, 2017. doi: 10.1523/JNEUROSCI.0758-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams C, DeFazio RA, Christian CA, Milescu LS, Schnell S, Moenter SM. Changes in both neuron intrinsic properties and neurotransmission are needed to drive the increase in GnRH Neuron firing rate during estradiol-positive feedback. J Neurosci 39: 2091–2101, 2019. doi: 10.1523/JNEUROSCI.2880-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain A, Huang GZ, Woolley CS. Latent sex differences in molecular signaling that underlies excitatory synaptic potentiation in the hippocampus. J Neurosci 39: 1552–1565, 2019. doi: 10.1523/JNEUROSCI.1897-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Popescu IR, Buraei Z, Haam J, Weng FJ, Tasker JG. Lactation induces increased IPSC bursting in oxytocinergic neurons. Physiol Rep 7: e14047, 2019. doi: 10.14814/phy2.14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meitzen J, Meisel RL, Mermelstein PG. Sex differences and the effects of estradiol on striatal function. Curr Opin Behav Sci 23: 42–48, 2018. doi: 10.1016/j.cobeha.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom MA. Mechanisms for selection of basic motor programs—roles for the striatum and pallidum. Trends Neurosci 28: 364–370, 2005. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci 14: 22–24, 2011. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan CL, Plotkin JL, Veno MT, von Schimmelmann M, Feinberg P, Mann S, Handler A, Kjems J, Surmeier DJ, O'Carroll D, Greengard P, Schaefer A. MicroRNA-128 governs neuronal excitability and motor behavior in mice. Science 342: 1254–1258, 2013. doi: 10.1126/science.1244193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci 16: 595–604, 1996. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB. Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci 29: 1897–1903, 2009. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience 170: 1045–1055, 2010. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almey A, Filardo EJ, Milner TA, Brake WG. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology 153: 5373–5383, 2012. doi: 10.1210/en.2012-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almey A, Milner TA, Brake WG. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav 74: 125–138, 2015. doi: 10.1016/j.yhbeh.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almey A, Milner TA, Brake WG. Estrogen receptor alpha and G-protein coupled estrogen receptor 1 are localized to GABAergic neurons in the dorsal striatum. Neurosci Lett 622: 118–123, 2016. doi: 10.1016/j.neulet.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorsch ZS, Loh YE, Purushothaman I, Walker DM, Parise EM, Salery M, Cahill ME, Hodes GE, Pfau ML, Kornman H, Hamilton PJ, Issler O, Labonte B, Symonds AE, Zucker M, Zhang TY, Meaney MJ, Russo SJ, Shen L, Bagot RC, Nestler EJ. Estrogen receptor alpha drives pro-resilient transcription in mouse models of depression. Nat Commun 9: 1116, 2018. doi: 10.1038/s41467-018-03567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krentzel AA, Willett JA, Johnson AG, Meitzen J. Estrogen receptor alpha, G-protein coupled estrogen receptor 1, and aromatase: Developmental, sex, and region-specific differences across the rat caudate-putamen, nucleus accumbens core and shell. J Comp Neurol 529: 786–801, 2021. doi: 10.1002/cne.24978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci 5: 27–41, 1994. doi: 10.1515/revneuro.1994.5.1.27. [DOI] [PubMed] [Google Scholar]
- 18.Song Z, Yang H, Peckham EM, Becker JB. Estradiol-induced potentiation of dopamine release in dorsal striatum following amphetamine administration requires estradiol receptors and mGlu5. eNeuro 6: ENEURO.0446-18.2019, 2019. doi: 10.1523/ENEURO.0446-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoest KE, Cummings JA, Becker JB. Oestradiol influences on dopamine release from the nucleus accumbens shell: sex differences and the role of selective oestradiol receptor subtypes. Br J Pharmacol 176: 4136–4148, 2019. doi: 10.1111/bph.14531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wissman AM, McCollum AF, Huang GZ, Nikrodhanond AA, Woolley CS. Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology 61: 217–227, 2011. doi: 10.1016/j.neuropharm.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorris DM, Cao J, Willett JA, Hauser CA, Meitzen J. Intrinsic excitability varies by sex in pre-pubertal striatal medium spiny neurons. J Neurophysiol 113: 720–729, 2015. doi: 10.1152/jn.00687.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao J, Dorris DM, Meitzen J. Neonatal masculinization blocks increased excitatory synaptic input in female rat nucleus accumbens core. Endocrinology 157: 3181–3196, 2016. doi: 10.1210/en.2016-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willett JA, Will T, Hauser CA, Dorris DM, Cao J, Meitzen J. No evidence for sex differences in the electrophysiological properties and excitatory synaptic input onto nucleus accumbens shell medium spiny neurons. eNeuro 3: ENEURO.0147-15.2016, 2016. doi: 10.1523/ENEURO.0147-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonansco C, Martínez-Pinto J, Silva RA, Velásquez VB, Martorell A, Selva MV, Espinosa P, Moya PR, Cruz G, Andrés ME, Sotomayor-Zárate R. Neonatal exposure to oestradiol increases dopaminergic transmission in nucleus accumbens and morphine-induced conditioned place preference in adult female rats. J Neuroendocrinol 30: e12574, 2018. doi: 10.1111/jne.12574. [DOI] [PubMed] [Google Scholar]
- 25.Cao J, Dorris DM, Meitzen J. Electrophysiological properties of medium spiny neurons in the nucleus accumbens core of prepubertal male and female Drd1a-tdTomato line 6 BAC transgenic mice. J Neurophysiol 120: 1712–1727, 2018. doi: 10.1152/jn.00257.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Proano SB, Morris HJ, Kunz LM, Dorris DM, Meitzen J. Estrous cycle-induced sex differences in medium spiny neuron excitatory synaptic transmission and intrinsic excitability in adult rat nucleus accumbens core. J Neurophysiol 120: 1356–1373, 2018. doi: 10.1152/jn.00263.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonso-Caraballo Y, Ferrario CR. Effects of the estrous cycle and ovarian hormones on cue-triggered motivation and intrinsic excitability of medium spiny neurons in the nucleus accumbens core of female rats. Horm Behav 116: 104583, 2019. doi: 10.1016/j.yhbeh.2019.104583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krentzel AA, Barrett LR, Meitzen J. Estradiol rapidly modulates excitatory synapse properties in a sex- and region-specific manner in rat nucleus accumbens core and caudate-putamen. J Neurophysiol 122: 1213–1225, 2019. doi: 10.1152/jn.00264.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willett JA, Cao J, Dorris DM, Johnson AG, Ginnari LA, Meitzen J. Electrophysiological properties of medium spiny neuron subtypes in the caudate-putamen of prepubertal male and female Drd1a-tdTomato Line 6 BAC transgenic mice. eNeuro 6: ENEURO.0016-19.2019, 2019. doi: 10.1523/ENEURO.0016-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proaño SB, Krentzel AA, Meitzen J. Differential and synergistic roles of 17β-estradiol and progesterone in modulating adult female rat nucleus accumbens core medium spiny neuron electrophysiology. J Neurophysiol 123: 2390–2405, 2020. doi: 10.1152/jn.00157.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proaño SB, Meitzen J. Estradiol decreases medium spiny neuron excitability in female rat nucleus accumbens core. J Neurophysiol 123: 2465–2475, 2020. doi: 10.1152/jn.00210.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willett JA, Cao J, Johnson A, Patel OH, Dorris DM, Meitzen J. The estrous cycle modulates rat caudate-putamen medium spiny neuron physiology. Eur J Neurosci 52: 2737–2755, 2020. doi: 10.1111/ejn.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Markaverich B, Mani S, Alejandro MA, Mitchell A, Markaverich D, Brown T, Velez-Trippe C, Murchison C, O'Malley B, Faith R. A novel endocrine-disrupting agent in corn with mitogenic activity in human breast and prostatic cancer cells. Environ Health Perspect 110: 169–177, 2002. doi: 10.1289/ehp.02110169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mani SK, Reyna AM, Alejandro MA, Crowley J, Markaverich BM. Disruption of male sexual behavior in rats by tetrahydrofurandiols (THF-diols). Steroids 70: 750–754, 2005. doi: 10.1016/j.steroids.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Villalon Landeros R, Morisseau C, Yoo HJ, Fu SH, Hammock BD, Trainor BC. Corncob bedding alters the effects of estrogens on aggressive behavior and reduces estrogen receptor-alpha expression in the brain. Endocrinology 153: 949–953, 2012. doi: 10.1210/en.2011-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bateman HL, Patisaul HB. Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology 29: 988–997, 2008. doi: 10.1016/j.neuro.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duarte-Guterman P, Lieblich SE, Chow C, Galea LA. Estradiol and GPER activation differentially affect cell proliferation but not GPER expression in the hippocampus of adult female rats. PLoS One 10: e0129880, 2015. doi: 10.1371/journal.pone.0129880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorris DM, Hauser CA, Minnehan CE, Meitzen J. An aerator for brain slice experiments in individual cell culture plate wells. J Neurosci Methods 238: 1–10, 2014. doi: 10.1016/j.jneumeth.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 39.O'Donnell P, Grace AA. Physiological and morphological properties of accumbens core and shell neurons recorded in vitro. Synapse 13: 135–160, 1993. doi: 10.1002/syn.890130206. [DOI] [PubMed] [Google Scholar]
- 40.Belleau ML, Warren RA. Postnatal development of electrophysiological properties of nucleus accumbens neurons. J Neurophysiol 84: 2204–2216, 2000. doi: 10.1152/jn.2000.84.5.2204. [DOI] [PubMed] [Google Scholar]
- 41.Mu P, Moyer JT, Ishikawa M, Zhang Y, Panksepp J, Sorg BA, Schluter OM, Dong Y. Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J Neurosci 30: 3689–3699, 2010. doi: 10.1523/JNEUROSCI.4063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farries MA, Perkel DJ. Electrophysiological properties of avian basal ganglia neurons recorded in vitro. J Neurophysiol 84: 2502–2513, 2000. doi: 10.1152/jn.2000.84.5.2502. [DOI] [PubMed] [Google Scholar]
- 43.Farries MA, Perkel DJ. A telencephalic nucleus essential for song learning contains neurons with physiological characteristics of both striatum and globus pallidus. J Neurosci 22: 3776–3787, 2002. doi: 10.1523/JNEUROSCI.22-09-03776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farries MA, Meitzen J, Perkel DJ. Electrophysiological properties of neurons in the basal ganglia of the domestic chick: conservation and divergence in the evolution of the avian basal ganglia. J Neurophysiol 94: 454–467, 2005. doi: 10.1152/jn.00539.2004. [DOI] [PubMed] [Google Scholar]
- 45.Meitzen J, Weaver AL, Brenowitz EA, Perkel DJ. Plastic and stable electrophysiological properties of adult avian forebrain song-control neurons across changing breeding conditions. J Neurosci 29: 6558–6567, 2009. doi: 10.1523/JNEUROSCI.5571-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baufreton J, Atherton JF, Surmeier DJ, Bevan MD. Enhancement of excitatory synaptic integration by GABAergic inhibition in the subthalamic nucleus. J Neurosci 25: 8505–8517, 2005. doi: 10.1523/JNEUROSCI.1163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gale SD, Perkel DJ. Physiological properties of zebra finch ventral tegmental area and substantia nigra pars compacta neurons. J Neurophysiol 96: 2295–2306, 2006. doi: 10.1152/jn.01040.2005. [DOI] [PubMed] [Google Scholar]
- 48.Jakab RL, Horvath TL, Leranth C, Harada N, Naftolin F. Aromatase immunoreactivity in the rat brain: gonadectomy-sensitive hypothalamic neurons and an unresponsive “limbic ring” of the lateral septum-bed nucleus-amygdala complex. J Steroid Biochem Mol Biol 44: 481–498, 1993. doi: 10.1016/0960-0760(93)90253-S. [DOI] [PubMed] [Google Scholar]
- 49.Wagner CK, Morrell JI. Distribution and steroid hormone regulation of aromatase mRNA expression in the forebrain of adult male and female rats: a cellular-level analysis using in situ hybridization. J Comp Neurol 370: 71–84, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 50.Horvath TL, Roa-Pena L, Jakab RL, Simpson ER, Naftolin F. Aromatase in axonal processes of early postnatal hypothalamic and limbic areas including the cingulate cortex. J Steroid Biochem Mol Biol 61: 349–357, 1997. [PubMed] [Google Scholar]
- 51.McArthur S, Murray HE, Dhankot A, Dexter DT, Gillies GE. Striatal susceptibility to a dopaminergic neurotoxin is independent of sex hormone effects on cell survival and DAT expression but is exacerbated by central aromatase inhibition. J Neurochem 100: 678–692, 2007. doi: 10.1111/j.1471-4159.2006.04226.x. [DOI] [PubMed] [Google Scholar]
- 52.Tobiansky DJ, Wallin-Miller KG, Floresco SB, Wood RI, Soma KK. Androgen regulation of the mesocorticolimbic system and executive function. Front Endocrinol (Lausanne) 9: 279, 2018. doi: 10.3389/fendo.2018.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meitzen J, Perry AN, Westenbroek C, Hedges VL, Becker JB, Mermelstein PG. Enhanced striatal β1-adrenergic receptor expression following hormone loss in adulthood is programmed by both early sexual differentiation and puberty: a study of humans and rats. Endocrinology 154: 1820–1831, 2013. doi: 10.1210/en.2012-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harp SJ, Martini M, Lynch WJ, Rissman EF. Sexual differentiation and substance use: a mini-review. Endocrinology 161: bqaa129, 2020. doi: 10.1210/endocr/bqaa129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elgueta-Reyes M, Martinez-Pinto J, Renard GM, Sotomayor-Zarate R. Neonatal programming with sex hormones: Effect on expression of dopamine D1 receptor and neurotransmitters release in nucleus accumbens in adult male and female rats. Eur J Pharmacol 902: 174118, 2021. doi: 10.1016/j.ejphar.2021.174118. [DOI] [PubMed] [Google Scholar]
- 56.Marty VN, Spigelman I. Long-lasting alterations in membrane properties, K+ currents, and glutamatergic synaptic currents of nucleus accumbens medium spiny neurons in a rat model of alcohol dependence. Front Neurosci 6: 86, 2012. doi: 10.3389/fnins.2012.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uchimura N, Cherubini E, North RA. Inward rectification in rat nucleus accumbens neurons. J Neurophysiol 62: 1280–1286, 1989. doi: 10.1152/jn.1989.62.6.1280. [DOI] [PubMed] [Google Scholar]
- 58.Uchimura N, Cherubini E, North RA. Cation current activated by hyperpolarization in a subset of rat nucleus accumbens neurons. J Neurophysiol 64: 1847–1850, 1990. doi: 10.1152/jn.1990.64.6.1847. [DOI] [PubMed] [Google Scholar]
- 59.Uchimura N, North RA. Muscarine reduces inwardly rectifying potassium conductance in rat nucleus accumbens neurones. J Physiol 422: 369–380, 1990. doi: 10.1113/jphysiol.1990.sp017989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nisenbaum ES, Wilson CJ. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J Neurosci 15: 4449–4463, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson CJ, Goldberg JA. Origin of the slow after hyperpolarization and slow rhythmic bursting in striatal cholinergic interneurons. J Neurophysiol 95: 196–204, 2006. doi: 10.1152/jn.00630.2005. [DOI] [PubMed] [Google Scholar]
- 62.Hopf FW, Bowers MS, Chang SJ, Chen BT, Martin M, Seif T, Cho SL, Tye K, Bonci A. Reduced nucleus accumbens SK channel activity enhances alcohol seeking during abstinence. Neuron 65: 682–694, 2010. doi: 10.1016/j.neuron.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arnauld E, Dufy B, Pestre M, Vincent JD. Effects of estrogens on the responses of caudate neurons to microiontophoretically applied dopamine. Neurosci Lett 21: 325–331, 1981. doi: 10.1016/0304-3940(81)90225-1. [DOI] [PubMed] [Google Scholar]
- 64.Tansey EM, Arbuthnott GW, Fink G, Whale D. Oestradiol-17 beta increases the firing rate of antidromically identified neurones of the rat neostriatum. Neuroendocrinology 37: 106–110, 1983. doi: 10.1159/000123527. [DOI] [PubMed] [Google Scholar]
- 65.Tozzi A, de Iure A, Tantucci M, Durante V, Quiroga-Varela A, Giampa C, Di Mauro M, Mazzocchetti P, Costa C, Di Filippo M, Grassi S, Pettorossi VE, Calabresi P. Endogenous 17beta-estradiol is required for activity-dependent long-term potentiation in the striatum: interaction with the dopaminergic system. Front Cell Neurosci 9: 192, 2015. doi: 10.3389/fncel.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Snyder MA, Smejkalova T, Forlano PM, Woolley CS. Multiple ERbeta antisera label in ERbeta knockout and null mouse tissues. J Neurosci Methods 188: 226–234, 2010. doi: 10.1016/j.jneumeth.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuppers E, Beyer C. Expression of aromatase in the embryonic and postnatal mouse striatum. Brain Res Mol Brain Res 63: 184–188, 1998. doi: 10.1016/s0169-328x(98)00279-4. [DOI] [PubMed] [Google Scholar]
- 68.Kuppers E, Beyer C. Expression of estrogen receptor-alpha and beta mRNA in the developing and adult mouse striatum. Neurosci Lett 276: 95–98, 1999. doi: 10.1016/s0304-3940(99)00815-0. [DOI] [PubMed] [Google Scholar]
- 69.Quigley JA, Becker JB. Activation of G-protein coupled estradiol receptor 1 in the dorsolateral striatum attenuates preference for cocaine and saccharin in male but not female rats. Horm Behav 130: 104949, 2021. doi: 10.1016/j.yhbeh.2021.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 388: 507–525, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 71.Brenowitz EA, Lent K. Afferent input is necessary for seasonal growth and maintenance of adult avian song control circuits. J Neurosci 21: 2320–2329, 2001. doi: 10.1523/JNEUROSCI.21-07-02320.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brenowitz EA, Lent K. Act locally and think globally: intracerebral testosterone implants induce seasonal-like growth of adult avian song control circuits. Proc Natl Acad Sci USA 99: 12421–12426, 2002. doi: 10.1073/pnas.192308799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brenowitz EA. Transsynaptic trophic effects of steroid hormones in an avian model of adult brain plasticity. Front Neuroendocrinol 37: 119–128, 2015. doi: 10.1016/j.yfrne.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alward BA, Madison FN, Parker SE, Balthazart J, Ball GF. Pleiotropic control by testosterone of a learned vocal behavior and its underlying neuroplasticity. eNeuro 3: ENEURO.0145-15.2016, 2016. doi: 10.1523/ENEURO.0145-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dovey JL, Vasudevan N. Does GPER1 play a role in sexual dimorphism? Front Endocrinol (Lausanne) 11: 595895, 2020. doi: 10.3389/fendo.2020.595895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen X, Grisham W, Arnold AP. X chromosome number causes sex differences in gene expression in adult mouse striatum. Eur J Neurosci 29: 768–776, 2009. doi: 10.1111/j.1460-9568.2009.06610.x. [DOI] [PubMed] [Google Scholar]
- 77.Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci 28: 10814–10824, 2008. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Planert H, Berger TK, Silberberg G. Membrane properties of striatal direct and indirect pathway neurons in mouse and rat slices and their modulation by dopamine. PLoS One 8: e57054, 2013. doi: 10.1371/journal.pone.0057054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mathews GA, Brenowitz EA, Arnold AP. Paradoxical hypermasculinization of the zebra finch song system by an antiestrogen. Horm Behav 22: 540–551, 1988. doi: 10.1016/0018-506x(88)90057-8. [DOI] [PubMed] [Google Scholar]
- 80.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci 14: 677–683, 2011. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levin ER. Minireview: extranuclear steroid receptors: roles in modulation of cell functions. Mol Endocrinol 25: 377–384, 2011. doi: 10.1210/me.2010-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gross KS, Mermelstein PG. Estrogen receptor signaling through metabotropic glutamate receptors. Vitam Horm 114: 211–232, 2020. doi: 10.1016/bs.vh.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 83.Meitzen J, Grove DD, Mermelstein PG. The organizational and aromatization hypotheses apply to rapid, nonclassical hormone action: neonatal masculinization eliminates rapid estradiol action in female hippocampal neurons. Endocrinology 153: 4616–4621, 2012. doi: 10.1210/en.2012-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cao J, Willett JA, Dorris DM, Meitzen J. Sex differences in medium spiny neuron excitability and glutamatergic synaptic input: heterogeneity across striatal regions and evidence for estradiol-dependent sexual differentiation. Front Endocrinol (Lausanne) 9: 173, 2018. doi: 10.3389/fendo.2018.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport 8: 1495–1498, 1997. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- 86.Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology 27: 683–691, 2002. doi: 10.1016/S0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- 87.Ghahramani NM, Ngun TC, Chen PY, Tian Y, Krishnan S, Muir S, Rubbi L, Arnold AP, de Vries GJ, Forger NG, Pellegrini M, Vilain E. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biol Sex Differ 5: 8, 2014. doi: 10.1186/2042-6410-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Staffend NA, Mohr MA, Doncarlos LL, Sisk CL. A decrease in the addition of new cells in the nucleus accumbens and prefrontal cortex between puberty and adulthood in male rats. Dev Neurobiol 74: 633–642, 2014. doi: 10.1002/dneu.22160. [DOI] [PubMed] [Google Scholar]
- 89.Kopec AM, Smith CJ, Ayre NR, Sweat SC, Bilbo SD. Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat Commun 9: 3769, 2018. doi: 10.1038/s41467-018-06118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rangel-Barajas C, Boehm SL , 2nd, Logrip ML. Altered excitatory transmission in striatal neurons after chronic ethanol consumption in selectively bred crossed high alcohol-preferring mice. Neuropharmacology 190: 108564, 2021. doi: 10.1016/j.neuropharm.2021.108564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sato SM, Wissman AM, McCollum AF, Woolley CS. Quantitative mapping of cocaine-induced DeltaFosB expression in the striatum of male and female rats. PLoS One 6: e21783, 2011. doi: 10.1371/journal.pone.0021783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Forlano PM, Woolley CS. Quantitative analysis of pre- and postsynaptic sex differences in the nucleus accumbens. J Comp Neurol 518: 1330–1348, 2010. doi: 10.1002/cne.22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Staffend NA, Loftus CM, Meisel RL. Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct Funct 215: 187–194, 2011. doi: 10.1007/s00429-010-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wissman AM, May RM, Woolley CS. Ultrastructural analysis of sex differences in nucleus accumbens synaptic connectivity. Brain Struct Funct 217: 181–190, 2012. doi: 10.1007/s00429-011-0353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peterson BM, Mermelstein PG, Meisel RL. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct 220: 2415–2422, 2015. doi: 10.1007/s00429-014-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wallin-Miller K, Li G, Kelishani D, Wood RI. Anabolic-androgenic steroids decrease dendritic spine density in the nucleus accumbens of male rats. Neuroscience 330: 72–78, 2016. doi: 10.1016/j.neuroscience.2016.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gross KS, Moore KM, Meisel RL, Mermelstein PG. mGluR5 Mediates dihydrotestosterone-induced nucleus accumbens structural plasticity, but not conditioned reward. Front Neurosci 12: 855, 2018. doi: 10.3389/fnins.2018.00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huijgens PT, Snoeren EMS, Meisel RL, Mermelstein PG. Effects of gonadectomy and dihydrotestosterone on neuronal plasticity in motivation and reward related brain regions in the male rat. J Neuroendocrinol 33: e12918, 2021. [DOI] [PubMed] [Google Scholar]