Abstract
Circadian clocks control immunity and virus replication, as well as pharmacokinetics and efficacy therapeutics. The aim of this study was to investigate the extent of these relationships by measuring circadian gene expression in primary human-derived dermal fibroblast cultures (HDF) after remdesivir exposure. In the current study, we analysed circadian gene expression in a cohort of participants without a neuropsychiatric diagnosis. After ex vivo exposure to remdesivir to human dermal fibroblast (HDF) cultures and dexamethasone synchronization, the rhythmicity of circadian gene expression (Clock, Bmal1, Per1-3, Cry1) was analysed via qRT-PCR. In this study, D-MEQ scores indicated that participants without a neuropsychiatric diagnosis had no evening preference. Remdesivir leads to a slight phase-shift in Clock, Per1 and Per2. Significant different expressions of Bmal1 and Per3 were detected after remdesivir exposure: Bmal1 at ZT8 (t(22) = 3.26, p = 0.004), ZT24 (t(22) = − 2.66, p = 0.015), ZT28 (t(20) = − 2.14, p = 0.045) and Per3 at ZT8 (t(22) = − 4.27, p < 0.001) and ZT12 (t(22) = − 2.61, p = 0.016). A significant difference between chronotype and circadian gene expression for Bmal1, Cry1 and Per3 was observed. The present study shows that remdesivir has an impact on circadian function. It is well known that the circadian rhythm effects sleep and, moreover, sleep quality. The results suggest that remdesivir medication may alter sleep quality in participants without a neuropsychiatric diagnosis and shifts chronotype to eveningness; similar as prevalent in ADHD.
Keywords: Remdesivir, Human dermal fibroblasts, Circadian rhythm
Introduction
Sleep plays a fundamental role in mental and physical health. Adequate sleep duration is essential for coping with major life events such as the COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Daily rhythms regulate the pharmacokinetics and pharmacodynamics of several therapeutic measures (Smolensky et al. 2017). Therefore, to obtain the best possible clinical outcomes circadian rhythms should be considered when designing and administering possible therapeutic interventions against COVID-19. Most of these are antivirals which are directed against intracellular or even nuclear targets.
The core circadian rhythm is regulated by the clock genes represented by circadian locomotor output cycles kaput (Clock), brain and muscle Arnt-like protein-1 (Bmal1), period (Per) and cryptochrome (Cry) genes. The master clock resides in the suprachiasmatic nucleus (SCN) of the hypothalamus and is entrained in peripheral cells (Schibler and Sassone-Corsi 2002), including fibroblasts (Balsalobre 1998). The molecular clock has also been characterised in immune cells, including T and B as well as dendritic cells (Bollinger et al.2011; Silver et al. 2012). Circadian rhythms influence sleep propensity, alertness and performance (Dijk and von Schantz 2005). Sleep is linked to immune functions (Besedovsky et al. 2012; Irwin 2019), in particular, to increased production of pro-inflammatory cytokines (Prather et al. 2009). Macrophages, important regulators of the immune response, exhibit robust circadian oscillation in gene expression, including genes responsible for pathogen recognition and cytokine secretion (Hayashi et al. 2007; Keller et al.2009). Circadian disruption, mimicking jet lag in mouse models, can greatly stimulate the release of interleukin-8 and other inflammatory cytokines induced by lipopolysaccharide, the most abundant components within the cell wall of Gram-negative bacteria (Castanon-Cervantes et al. 2010). An increase in immune response molecules is linked to clock-controlled enhanced sensitivity. Desynchronisation of the clock impairs immune function (Curtis et al. 2014). Peritoneal macrophages lacking Bmal1 produce higher amounts of proinflammatory cytokine interleukin-6 in response to lipopolysaccharide after synchronisation in comparison to wild-type peritoneal macrophages (Gibbs et al.2012). Bmal1 depletion leads to inflammatory reaction in myeloid cells (Nguyen et al. 2013), and herpes as well as influenza A virus infections are enhanced by Bmal1 depletion (Edgar et al. 2016).
Sleep deprivation is linked to the alteration of the immune system. Partial sleep deprivation of fifty-two healthy volunteers for one night transiently impaired mitogen proliferation, decreased the human leukocyte antigen, isotype DR (HLA-DR) and upregulated the cluster of differentiation 14 (CD14), increasing susceptibility to respiratory infections (Wilder-Smith et al. 2013). Shorter sleep duration, measured behaviourally using actigraphy prior to rhinovirus exposure, was associated with increased susceptibility to the common cold (Prather et al. 2015).
The COVID-19 pandemic has produced significant stress, anxiety, and increased incidence of sleep disturbances (Morin et al. 2020). Psychiatric services are at risk of being overwhelmed at this time of unprecedented crisis, with an increased need to protect and defend the fundamental rights of patients (Thome et al. 2020a, b). Sleep disturbances are common among psychiatric disorders (Anderson and Bradley 2013). Silva et al. 2020, suggested that short sleep and sleep loss, which are common consequences of shift work, are associated with altered immune function, increasing the risk of COVID-19 (Silva et al. 2020). Shift-workers also report higher incidence and severity of respiratory infections (Archer et al. 2018; Archer and Oster 2015). Increased exposure to light at night may inhibit the production of melatonin, resulting in alterations in immune activity (Galano et al. 2018). Shift-workers show abnormal immune cell and cytokine levels (Loef et al. 2019). Vitale et al. (2020) assessed the influence of severe symptoms of COVID-19 infection on sleep quality in four patients during the sub-acute recovery stage of the disease. The authors observed lower sleep efficiency and immobility time and higher fragmentation index in patients with severe respiratory COVID-19 symptoms who were admitted to an intensive care unit (ICU) compared to patients with mild respiratory symptoms not requiring an ICU stay (Vitale et al. 2020). In contrast, Leone et al. reported overall improvement of sleep conditions in healthy subjects during lockdown (Leone et al. 2020). When comparing sleep duration, quality and timing, and social jetlag between control and lockdown conditions of 1021 subjects, the authors observed that participants slept longer and later during lockdown weekdays, and exhibited lower levels of social jetlag. Chronotype was, however, significantly delayed during the lockdown.
Based on behavioural manifestations of an individual's internal clock, one can display a morning, neutral or evening preference. A recent study suggests that chronotype plays an important role in the negative effects of home confinement of ADHD children during the COVID-19 outbreak (Cetin et al. 2020). Impaired sleep may occur as an adverse reaction of psychopharmacotherapy (Gahr et al. 2018).
Remdesivir is considered one of the most promising drug candidates for the treatment of COVID-19. Remdesivir, GS-5734 is a single diastereomer monophosphoramidate prodrug of a monophosphate nucleoside analog GS-441524. Intracellularly remdesivir is metabolised to the pharmacologically active analogous of adenosine triphosphate, GS-443902, that selectively inhibits viral RNA polymerases but not host RNA or DNA polymerases (Gordon et al. 2020). Remdesivir has a very short plasma half-life in non-human primates and mice (Ray and Reddy 2020), and has, in humans, shown 80–90% plasma protein binding (Tempestilli et al.2020).
Remdesivir demonstrated effectiveness against members of several virus families, including filoviruses and coronaviruses, such as SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) (Sheahan 2020; Tchesnokov et al. 2019; Wang et al. 2020). In vitro testing has also shown that remdesivir can be used against COVID-19 (Augustin et al. 2020; Beigel et al. 2020; Grein et al. 2020). For individuals who are diagnosed early during illness with a high risk of hyperinflammation, 10- day remdesivir therapy shortens the time to recovery and reduces the risk of disease progression (Spinner et al. 2020). Furthermore, among patients with moderate COVID-19, 5-day remdesivir therapy is effective with the lowest risk of serious adverse events (Szarpak et al. 2020).
Several studies have evaluated the antiviral efficiency and effectiveness of remdesivir in cellular models. In human airway epithelial cells, remdesivir efficiently inhibited both MERS-CoV and SARS-CoV replication at 0.1, 1 and 10 µM concentrations. No cytotoxicity was observed at 10 μM remdesivir, the highest concentration tested (Sheahan et al. 2017). The Wuhan Institute of Virology analysed the antiviral efficiency of five FDA-approved drugs including ribavirin, penciclovir, nitazoxanide, nafamostat, and chloroquine, as well as antiviral drugs remdesivir and favipiravir, using Vero cells lines infected with SARS-CoV-2. Remdesivir potently blocked virus infection at low-micromolar concentrations, with EC50 value of 0.77 μM (Wang et al. 2020).
Glucocorticoid receptor agonist, dexamethasone may be useful in the short-term for severe, intubated, COVID-19 patients (Group et al. 2020). Glucocorticoids contribute to the synchronisation of the cell-autonomous clocks in peripheral cells and dexamethasone stimulates G1-S phase transition in human airway fibroblasts obtained from asthmatics (Dickmeis et al. 2007; Fouty et al. 2006).
Our research group successfully established a model based on patient-derived human dermal fibroblast (HDF) for the investigation of the circadian rhythm, particularly, circadian gene expression (Coogan et al. 2019). In a study, at the molecular level, we observed alterations in the expression of Per2 and Cry1 between HDF cultures obtained from ADHD individuals with no medication compared to HDF from healthy participants or medicated ADHD patients after 30 min dexamethasone synchronisation (Coogan et al. 2019). HDFs are an advantageous model to study the influence of drugs. The synchronization of the circadian system of fibroblasts can be achieved by several substances with different effects on circadian gene expression (Faltraco et al. 2020). HDF cultures are advantageous for in vitro functional models examining various cellular and molecular mechanisms (Kalman et al. 2016).
Depletion of cryptochrome genes in immortalised fibroblasts regulates the expression of proinflammatory cytokines (Van Linthout et al. 2014). Fibroblasts play a critical role in the switch of acute to chronic persistent inflammation (Narasimamurthy et al. 2012). Fibroblasts also produce inflammatory mediators such as toll-like receptors, antimicrobial peptides, proinflammatory cytokines, chemokines, and growth factors, as a response to microorganisms (Bautista-Hernandez et al. 2017). In a study, using normal human fibroblast lung cells as a model, a series of anti-HIV nucleosides and their fatty acyl derivatives were compared with remdesivir for antiviral activity against human coronavirus 229E (HCoV-229E). Only remdesivir was found to be potent, with an EC50 value of 0.07 µM and a therapeutic index of more than 28.6 µM (Parang et al. 2020).
The metabolism of drugs in some cases is extensive and missing knowledge of pharmacokinetics often results in misinterpretation of symptoms. Some drugs, e.g. psychostimulant medication, are known to influence sleep (Hvolby 2015). Considering that circadian clocks control immunity and virus replication, as well as the pharmacokinetics and efficacy of several therapeutics, an evaluable approach is to evaluate the effect of remdesivir on circadian genes. To address these issues, the circadian rhythms at the molecular level are investigated in participants without a neuropsychiatric diagnosis. Also, human dermal fibroblasts obtained from the study participants were exposed to remdesivir.
Based on the assumption of the effectiveness of remdesivir for the treatment of COVID-19 its influence on the expression of circadian genes is hypothesized. Therefore, in this study the influence of remdesivir on circadian rhythmicity is investigated using fibroblasts as in vitro model.
Material and methods
Participant selection criteria
Ethics approval for the conduct of the study, including obtaining human dermal biopsy samples, was given by the ethical review committee of Rostock University (Registration-number: A2013-159) and written consent was obtained from each study participant. The study was conducted according to the ethical guidelines of the Declaration of Helsinki.
In total, 12 volunteers without a neuropsychiatric diagnosis participating in the study were recruited via the Department of Psychiatry and Psychotherapy, University Medical Centre Rostock. Human dermal fibroblasts (HDF) were obtained from skin biopsies of the dorsal forearm area. Only adult individuals, able to give informed consent, were included. Shift-workers were excluded. The IQ of the participants were measured by using MWT (Multiple Choice Word Test). The chronotype of the participants were determined by the D-MEQ (Morning-Eveningness-Questionnaire, German Version). No special cognitive testing was implemented in the study.
The four manuscripts of this special issue dealing with circadian rhythmicity describe unique research questions (Faltraco et al. 2021a, b; Palm et al. 2021). Although some samples have been used for more than one research question, the overall sample composition differs from each other and thus is different for each study. Experiments differ substantially in their conditions, thus, they each investigate unique cellular biochemical pathways.
Actigraphy
To obtain objective measures of participants’ sleep and circadian rhythm function, the rest–activity pattern of participants was recorded using wrist-worn actigraphs (Actiwatch 2, Philips Respironics, USA). Actigraphs were worn on the non-dominant wrist for a period of at least seven consecutive days. The recording interval of the device was set at 60-s epochs. Data occurring before the first and after the final midnight of each record were excluded, ensuring at least six complete days for each participant, with a complete weekend included in each record. The following sleep parameters were measured: mid-sleep on weekend (time of mid-sleep on free/weekend days), mid-sleep on weekdays (time of mid-sleep on work/weekdays), social jetlag (difference between mid-sleep on workdays and free days), sleep efficiency (difference between sleeping time and the time spending in bed), WASO (time spent in wakening after sleep onset) and total number of wake bouts (number of awakenings).
Tissue isolation and fibroblast cell culture
Human dermal fibroblasts (HDF) were isolated and cultured as described previously (Takashima 1998). Fibroblasts were cultivated (37 °C, 5% CO2) in Dulbecco's Modified Eagle Medium DMEM (Gibco, Thermo Fisher, UK) /1 mg/ml Liberase TM (Roche, Germany) containing 100 units/ml penicillin, 100 µg/ml streptomycin (Gibco, Thermo Fisher, UK) and 10% fetal bovine serum FBS (Gibco, Thermo Fisher, UK).
Measurement of cell viability
To determinate the optimal remdesivir concentration for human dermal fibroblasts, upon confluency of the respective primary fibroblast cell culture from each participant, cells were incubated with 0.00, 0.05, 0.50 and 1.00 µM remdesivir (MedChemExpress, Germany) for 24 h. Cell viability was measured using the Trypan Blue Exclusion Test (Strober 2015).
Measurement of circadian gene expression
Upon confluency of the respective primary fibroblast cell culture from each participant, eight culture flask replicates were prepared and cells were incubated with 0.5 µM remdesivir (MedChemExpress, Germany). Cultures without remdesivir were used as a negative control. After 24-h of incubation, the cells were synchronised with 100 nm dexamethasone (Sigma-Aldrich, Germany) for 30 min. Samples were harvested every fourth hour after synchronisation for a period of 28-h in solution D (4.5 M guanidinium thiocyanate, 0.5% sodium-N-lauryl sarcosine, 25 mM tri-sodium citrate, 0.1 M betamercaptoethanol) and stored at − 80 °C. Total RNA was isolated and purified with RNeasy Plus Mini Kit (Qiagen, Germany), subjected to reverse transcription using the Superscript III First-Strand Synthesis System (Invitrogen, Germany) and gene expression of Clock, Bmal1, Per1, Per2, Per3 and Cry1 as well as and housekeeping genes (Rpl13A, Rpl19A, GAPDH) was measured by real-time quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) with CFX Connect™ Real-Time PCR Detection System (Biorad, Germany). All primers were purchased from Eurofins (Alameda, CA). The oligonucleotide sequences are presented in Table 1. The qRT-PCR was performed in 96-well 0.1-ml thin-wall PCR plates (Applied Biosystems) in the CFX Connect™ Real-Time PCR Detection System (Biorad, München, Germany) as previously described (Coogan et al. 2019).
Table1.
Oligonucleotides for qRT-PCR to measure circadian gene expression
| Gene | Forward primer (5ʹ–3ʹ) | Reverse primer (5ʹ–3ʹ) |
|---|---|---|
| Clock | CCAGCAGTTTCATGAGATGC | GAGGTCATTTCATAGCTGAGC |
| Bmal1 | AAGGATGGCTGTTCAGCACATGA | CAAAAATCCATCTGCTGCCCTG |
| Per1 | TGGGGACAACAGAACAGAGAA | AGGACACTCCTGCGACCA |
| Per2 | GTATCCATTCATGCTGGGCT | TCGTTTGAACTGCGGTGAC |
| Per3 | TCAGTGTTTGGTGGAAGGAA | TCTGGGTCAGCAGCTCTACA |
| Cry1 | CACGAATCACAAACAGACGG | TACATCCTGGACCCCTGGT |
| Rpl13a | GCCAGAAATGTTGATGCCTT | AGATGGCGGAGGTGCAG |
| Rpl19a | GTGGCAAGAAGAAGGTCTGG | GCCCATCTTTGATGAGCTTC |
| GAPDH | GAAGGTGAAGGTCGGAGT | GAAGATGGTGATGGGATTTC |
Statistical methods
Circadian gene expression data were tested for significant circadian rhythmicity, using CircWave v. 1.4 software (generated by Dr. Roelof Hut; www.euclock.org) to determine the best-fitting linear harmonic regression with an assumed period of 24-h and with α set at 0.05. The center-of-gravity of each best-fitting waveform in CircWave was used as the circadian acrophase, and the associated estimation error was used as the SD. Inferential statistics were carried out in SPSS (IBM Corporation). qRT-PCR clock gene data were analysed via Student’s’ t Test. For all inferential tests, p < 0.05 was used to indicate a statistically significant groupwise difference. Sample sizes were calculated via GPower 3.1 software; for correlations the assumptions used were significance level of α = 0.05 and the power of 0.8 for one group with two measures (0.0 µM, 0.5 µM remdesivir). Although the research in this field is generally scarce, we assumed that the influence of remdesivir on the circadian gene expression will have an effect size d’ = 0.5, returning a required total sample size of 21. Taking into consideration an expected drop-out rate, n = 12 participants were allocated per each measure. One-way ANOVA was used to assess differences of circadian gene expression levels among chronotype groups. Associations between clock gene expression and chronotype obtained from the volunteers were studied by Spearman’s rank-order correlation. Data were analyzed via time-series statistics adequately powered by 12 samples each, which in this statistical model is mathematically sufficient and thus representative (Menet et al. 2012; Thaben and Westermark 2016).
Results
Demographic data
Human dermal fibroblasts (HDF) were obtained via skin biopsy from volunteers (four men, eight women; 41.50 ± 14.04 years, mean ± SD; BMI: 25.87 ± 5.42 kg/m2, mean ± SD). All participants completed the Multiple-Choice Word Test (IQ score: 110.25 ± 9.32, mean ± SD) and Morningness-Eveningness-Questionnaire, German Version (D-MEQ Score: HC: 58.83 ± 8.97, mean ± SD, p < 0.01). D-MEQ scores indicated that participants without a neuropsychiatric diagnosis displayed either neutral or morning preferences. The demographic data are presented in Table 2.
Table 2.
Demographic data
| Demographic data | Volunteers without a neuropsychiatric diagnosis (n = 12) |
|---|---|
| Age | 41.50 ± 14.04 years |
| Female | 8 (66.7%) |
| BMI | 25.87 ± 5.42 |
| IQ-Score | 110.25 ± 9.32 |
| D-MEQ | 58.83 ± 8.97 |
| Chronotype |
7 (58.3%) neutral 3 (25.0%) moderate morning 2 (16.7%) definite morning |
Actigraphy
The following sleep parameters were measured by actigraphy from participants without a neuropsychiatric diagnosis: mid-sleep on weekend days (3.99 ± 1.57, mean ± SD), mid-sleep on weekdays (3.00 ± 1.59, mean ± SD), social jetlag (0.98 ± 0.85, mean ± SD), sleep efficiency (82.50% ± 9.27%, mean ± SD), WASO (58.90 min. ± 32.40 min., mean ± SD) and total number of wake bouts (25.00 ± 4.86, mean ± SD).
Cell viability
To establish the optimal remdesivir concentrations, human dermal fibroblasts were incubated with 0.05, 0.50 and 1.00 µM remdesivir for 24 h and compared to negative controls without remdesivir. The viability of cells treated with 0.05 µM remdesivir (84.45 ± 8.62, mean ± SD) and 1.00 µM remdesivir (86.32 ± 5.02, mean ± SD) was decreased compared to control cells without remdesivir (0 µM remdesivir: 89.03 ± 5.66, mean ± SD). The incubation with 0.50 µM remdesivir (89.16 ± 4.88, mean ± SD) did not affect the cell viability.
Circadian gene expression in human dermal fibroblasts
The expression profiles of circadian genes after incubation with 0.50 µM remdesivir were examined in primary fibroblasts cultured from skin biopsies collected from participants without a neuropsychiatric diagnosis and synchronised with dexamethasone. Cultures without remdesivir were used as negative control.
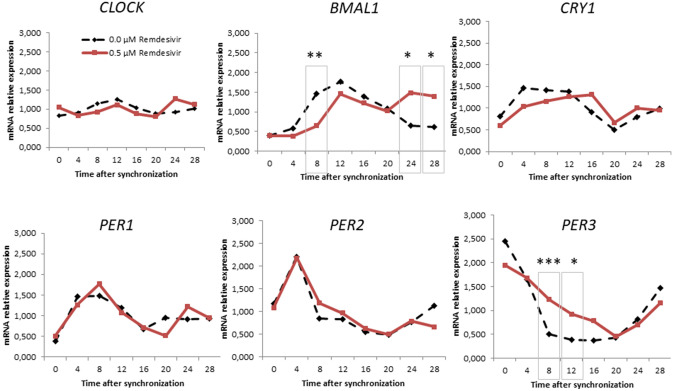
Cry1, Per1 and Per2 expression was rhythmic in both groups (CircWave, p < 0.001). No rhythmicity was detected for Clock in negative controls and cultures incubated with 0.5 µM remdesivir (CircWave, p > 0.05). Remdesivir eliminated the rhythmicity of Bmal1 and Per3 (CircWave, p > 0.05).
Data were normally distributed (Kolmogorov Smirnov). Differences in circadian gene expression levels among study groups were assessed using Student’s’ t Test (Fig. 1). Comparing the two groups, significant different expression levels of Bmal1 at ZT8 (t(22) = 3.26, p = 0.004), ZT24 (t(22) = − 2.66, p = 0.015), ZT28 (t(20) = − 2.14, p = 0.045) and Per3 at ZT8 (t(22) = − 4.27, p < 0.001) and ZT12 (t(22) = − 2.61, p = 0.016) were observed. Remdesivir increased the expression of Bmal1 at ZT24 and ZT28, as well as the expression of Per3 at ZT8 and ZT12 compared to the negative control cultures. The expression of Bmal1 at ZT8 was lower after remdesivir incubation whereas the expression levels at ZT24 and ZT28 was significantly higher compared to the negative control. Per3 expression level after remdesivir exposure was significantly higher at ZT8 and ZT12 compared to the negative control.
Fig. 1.
Relative mRNA gene expression of circadian genes in volunteers without a neuropsychiatric diagnosis with 0.0 and 0.5 μm Remdesivir. *p < 0.05, **p < 0.01,*p < 0.001
Chronotype and circadian gene expression
In the current study, 58.3% of participants without a neuropsychiatric diagnosis displayed circadian neutral preference, whereas 25.0% had moderate morning preference, and 16.7% definite morning preference.
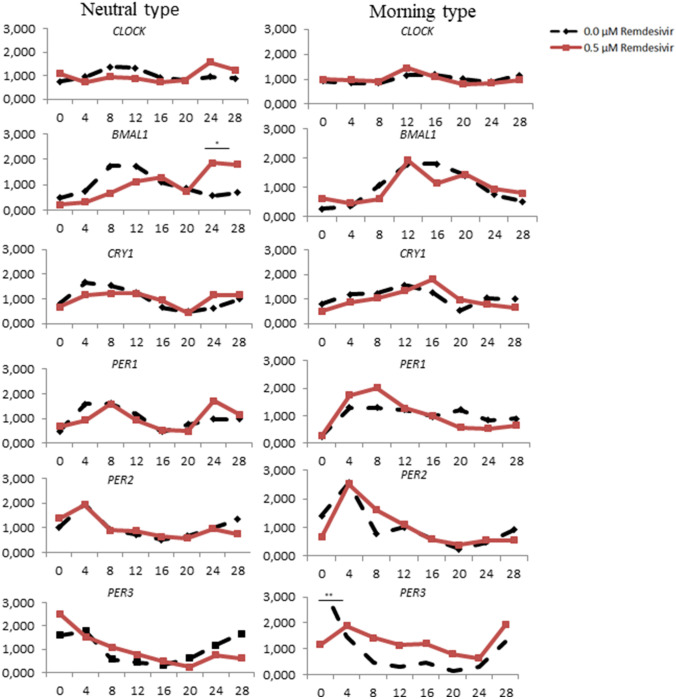
Differences of circadian gene expression levels among chronotypes were assessed using one-way ANOVA. Statistically significant differences were observed between chronotypes and circadian genes expression for Per3 (ZT 0, F = 7.518, p = 0.001; ZT8 F = 6.828, p = 0.002), Bmal1 (ZT8, F = 5.107, p = 0.009; ZT24, F = 4.151, p = 0.019) and Cry1 (ZT16, F = 4.216, p = 0.018; ZT20 F = 3.886, p = 0.024). A Bonferroni post hoc correction revealed a significantly decreased expression of Per3 (p = 0.007) at ZT0 in HDFs without remdesivir exposure exhibiting neutral chronotype compared to those with morning chronotype. Also differences in expression between negative controls and HDFs incubated with remdesivir were computed (Fig. 2). The incubation of HDFs with remdesivir significantly lowered the expression of Per3 (p = 0.002) at ZT0 compared to negative controls among participants with morning preferences. Compared to negative control cultures, remdesivir incubation decreased the expression of Bmal1 at ZT8 in cultures from participants with morning (p = 0.023) and neutral (p = 0.017) chronotype. In participants with neutral preferences, remdesivir incubation increased the expression of Bmal1 (p = 0.021) at ZT24. In HDFs from participants with morning chronotype, remdesivir also increased the expression of Per3 at ZT8 (p = 0.011) and Cry1 at ZT16 (p = 0.016) compared to negative control cultures from participants with neutral type.
Fig. 2.
Relative mRNA gene expression of circadian genes in volunteers without a neuropsychiatric diagnosis with 0.0 and 0.5 μm Remdesivir in neutral and morning type. *p < 0.05, **p < 0.01,*p < 0.001
Discussion
Remdesivir might shorten median recovery time and improve the clinical status of COVID-19 (Pimentel et al. 2020). In an uncontrolled, prospective, open observational study conducted at the Clinic I for Internal Medicine, Cologne University Hospital by Augustin et al., 10-day remdesivir therapy with a target follow-up time of 28 days was carried out (Augustin et al. 2020). The COVID-19 patients received 200 mg IV remdesivir on the first day, followed by 9 days of 100 mg IV remdesivir therapy. 36 of the 53 patients (68%) showed an improvement under remdesivir treatment. Adverse events were reported in 32 of the 53 patients (60%), the most common were elevated liver enzymes, as well as diarrhoea, rash, renal impairment and arterial hypotension. The mortality rate was 13%, with increased risk in patients older than 70 years as well as in patients with a higher serum creatinine level at the start of therapy (Augustin et al. 2020). In May 2020, Beigel et al. designed an adaptive platform to rapidly conduct a series of phase 3, randomized, double-blind, placebo-controlled trials. 1059 patients were randomly assigned to receive either 200 mg loading dose on day 1, followed by 100 mg daily remdesivir for up to nine additional days or placebo for up to 10 days. Beigel et al. observed that remdesivir treatment was superior to placebo in shortening the recovery time in adults hospitalized with COVID-19. Preliminary results indicated that those who received remdesivir had a median recovery time of 11 days as compared with 15 days in those who received placebo. Serious adverse events were reported for 21.1% patients of the remdesivir group and 27.0% patients of the placebo group. Mortality rate by 14 days was 7.1% with remdesivir treatment and 11.9% with placebo (Beigel et al. 2020). Recently, in a study including a cohort of patients hospitalised for severe COVID-19, treatment with compassionate-use remdesivir induced clinical improvement in 68% of patients. Of the 53 patients whose data were analysed, 22 were in the United States, 22 in Europe or Canada, and nine in Japan (Grein et al. 2020).
The risks and benefits of remdesivir in patients with COVID-19 are, however, uncertain. In a meta-analysis, the incidence of all adverse events was higher in the remdesivir group compared to the placebo group (Szarpak et al. 2020). The most common adverse reaction reported are hepatoxicity, cardiac adverse events, gastrointestinal symptoms and respiratory problems (Fan et al. 2020; Gupta et al. 2020; Pimentel et al. 2020).
To the best of our knowledge until now no studies have analysed the influence of remdesivir on circadian rhythm. The results of the present study illustrate that remdesivir impacts on circadian function, particularly, on the Bmal1 and Per3 gene expression. Previously, it has been shown that the expression of Bmal1 and Period genes is altered by chronic jet lag on the central and peripheral clocks of mice (Iwamoto et al. 2014). It has been observed, that sleep deprivation in jet lag may relate to a length polymorphism in the Per3 gene (Cheng et al. 2018; Leocadio-Miguel et al. 2018).
The study shows that Clock, Cry1, Per1 and Per2 expression is phase shifted in human dermal fibroblast cultures from participants without a neuropsychiatric diagnosis incubated with remdesivir. Significant different expression levels of Bmal1 were observed 8, 24 and 28 h after the dexamethasone synchronisation between negative controls and incubation with remdesivir. These results matched the expression of the Per3 gene, which was significant different at ZT8 and ZT12 in both groups. Remdesivir abolished the rhythmicity of the two genes. We observed a significant differences between chronotypes and circadian gene expression for Bmal1, Cry1 and Per3. In participants with neutral preference, remdesivir incubation increased the expression of Bmal1 at ZT24 and decreased Bmal1 expression at ZT8. The same effect for remdesivir was observed in participants with a morning preference. The incubation of HDFs with remdesivir decreased the expression of Per3 at ZT0 among participants with morning preferences.
It is to mention, that no special cognitive testing was implemented in this study. For further studies a connection between circadian disturbances, cognitive deficits and the effect of medication would be suitable.
In conclusion, human dermal fibroblasts present a suitable model to study circadian rhythms. Circadian rhythms have a profound impact on human health and regulate the pharmacokinetics and efficacy of many therapeutics. For example, dosing-time of drugs regulates the efficacy of viral vaccines (Ray and Reddy 2020). The results of the study suggest that remdesivir medication alters circadian rhythms and circadian gene expression. Remdesivir exposure may alter the sleep quality by inducing a jetlag effect. Circadian rhythmicity is shifted to eveningness similar to the most prevalent chronotype in ADHD.
Acknowledgements
We are grateful for the excellent assistance of Lena Borchert, Carolin Herzberg and Esther Kerstan for their support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of data and material
Data and material are available.
Code availability
Actiwatch 2, Philips Respironics, USA; CircWave v. 1.4 software (generated by Dr. Roelof Hut; www.euclock.org); SPSS (IBM Corporation).
Declarations
Conflict of interest
Johannes Thome has received financial support from pharmaceutical companies (Actelion, Astra Zeneca, Bristol-Myers Squibb, EVER Neuro Pharma GmbH, Janssen-Cilag, Lilly, Lundbeck, MEDICE, Merz, Novartis, Pfizer, Roche, Servier, Shire, Trommsdorff) some of which manufacture medication used in the treatment of ADHD patients. Frank Faltraco, Andrew Coogan, Oliver Tucha, Frederick Simon, Adriana Uzoni, Isabell Duwe and Denise Palm have no potential conflicts of interest to disclose.
Ethics approval
Ethical approval for the conduct of the study, including obtaining human dermal biopsy samples, was given by the ethical review committee of Rostock University (Registration-number: A2013-159).
Consent to participate
Written consent was obtained from each study participant.
Consent for publication
Written consent was obtained from each study participant.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anderson KN, Bradley AJ. Sleep disturbance in mental health problems and neurodegenerative disease. Nat Sci Sleep. 2013;5:61–75. doi: 10.2147/NSS.S34842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SN, Oster H. How sleep and wakefulness influence circadian rhythmicity: effects of insufficient and mistimed sleep on the animal and human transcriptome. J Sleep Res. 2015;24:476–493. doi: 10.1111/jsr.12307. [DOI] [PubMed] [Google Scholar]
- Archer AE, Von Schulze AT, Geiger PC. Exercise, heat shock proteins and insulin resistance. Philos Trans R Soc Lond B Biol Sci. 2018 doi: 10.1098/rstb.2016.0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin M, Hallek M, Nitschmann S. Remdesivir for patients with severe COVID-19. Internist (berl) 2020;61:644–645. doi: 10.1007/s00108-020-00800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/S0092-8674(00)81199-X. [DOI] [PubMed] [Google Scholar]
- Bautista-Hernandez LA, Gomez-Olivares JL, Buentello-Volante B, Bautista-de Lucio VM. Fibroblasts: the unknown sentinels eliciting immune responses against microorganisms. Eur J Microbiol Immunol (bp) 2017;7:151–157. doi: 10.1556/1886.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel JH, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger T, et al. Circadian clocks in mouse and human CD4+ T cells. PLoS ONE. 2011;6:e29801. doi: 10.1371/journal.pone.0029801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon-Cervantes O, et al. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin FH, Ucar HN, Turkoglu S, Kahraman EM, Kuz M, Gulec A. Chronotypes and trauma reactions in children with ADHD in home confinement of COVID-19: full mediation effect of sleep problems. Chronobiol Int. 2020 doi: 10.1080/07420528.2020.1785487. [DOI] [PubMed] [Google Scholar]
- Cheng P, Tallent G, Burgess HJ, Tran KM, Roth T, Drake CL. Daytime sleep disturbance in night shift work and the role of PERIOD3. J Clin Sleep Med. 2018;14:393–400. doi: 10.5664/jcsm.6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan AN, et al. Impact of adult attention deficit hyperactivity disorder and medication status on sleep/wake behavior and molecular circadian rhythms. Neuropsychopharmacology. 2019;44:1198–1206. doi: 10.1038/s41386-019-0327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AM, Bellet MM, Sassone-Corsi P, O'Neill LA. Circadian clock proteins and immunity. Immunity. 2014;40:178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Dickmeis T, et al. Glucocorticoids play a key role in circadian cell cycle rhythms. PLoS Biol. 2007;5:e78. doi: 10.1371/journal.pbio.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, von Schantz M. Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. J Biol Rhythms. 2005;20:279–290. doi: 10.1177/0748730405278292. [DOI] [PubMed] [Google Scholar]
- Edgar RS, Stangherlin A, Nagy AD, Nicoll MP, Efstathiou S, O'Neill JS, Reddy AB. Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proc Natl Acad Sci USA. 2016;113:10085–10090. doi: 10.1073/pnas.1601895113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faltraco F, Uzoni A, Shevchuk L, Thome J, Palm D. Synchronization of fibroblasts ex vivo in psychopharmacology. Pharmacopsychiatry. 2020 doi: 10.1055/a-1151-4947. [DOI] [PubMed] [Google Scholar]
- Faltraco F, Palm D, Uzoni A, Simon F, Tucha O, Thome J. Atomoxetine and circadian gene expression in human dermal fibroblasts from participants with a diagnosis of attention-deficit hyperactivity disorder. J Neural Transm. 2021 doi: 10.1007/s00702-021-02373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faltraco F, Palm D, Uzoni A, Borchert L, Simon F, Tucha O, Thome J. Dopamine adjusts the circadian gene expression of Per2 and Per3 in human dermal fibroblasts from ADHD patients. J Neural Transm. 2021 doi: 10.1007/s00702-021-02374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q, Zhang B, Ma J, Zhang S. Safety profile of the antiviral drug remdesivir: an update. Biomed Pharmacother. 2020;130:110532. doi: 10.1016/j.biopha.2020.110532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouty B, Moss T, Solodushko V, Kraft M. Dexamethasone can stimulate G1-S phase transition in human airway fibroblasts in asthma. Eur Respir J. 2006;27:1160–1167. doi: 10.1183/09031936.06.00078605. [DOI] [PubMed] [Google Scholar]
- Gahr M, Connemann BJ, Zeiss R, Frohlich A. Sleep disorders and impaired sleep as adverse drug reactions of psychotropic drugs: an evaluation of data of summaries of product characteristics. Fortschr Neurol Psychiatr. 2018;86:410–421. doi: 10.1055/s-0043-119800. [DOI] [PubMed] [Google Scholar]
- Galano A, Tan DX, Reiter RJ. Melatonin: a versatile protector against oxidative DNA damage. Molecules. 2018 doi: 10.3390/molecules23030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JE, et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CJ, Tchesnokov EP, Woolner E, Perry JK, Feng JY, Porter DP, Gotte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem. 2020;295:6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group RC et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- Gupta AK, Parker BM, Priyadarshi V, Parker J. Cardiac adverse events with remdesivir in COVID-19 infection. Cureus. 2020;12:e11132. doi: 10.7759/cureus.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Shimba S, Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol Pharm Bull. 2007;30:621–626. doi: 10.1248/bpb.30.621. [DOI] [PubMed] [Google Scholar]
- Hvolby A. Associations of sleep disturbance with ADHD: implications for treatment. Atten Defic Hyperact Disord. 2015;7:1–18. doi: 10.1007/s12402-014-0151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol. 2019;19:702–715. doi: 10.1038/s41577-019-0190-z. [DOI] [PubMed] [Google Scholar]
- Iwamoto A, Kawai M, Furuse M, Yasuo S. Effects of chronic jet lag on the central and peripheral circadian clocks in CBA/N mice. Chronobiol Int. 2014;31:189–198. doi: 10.3109/07420528.2013.837478. [DOI] [PubMed] [Google Scholar]
- Kalman S, Garbett KA, Janka Z, Mirnics K. Human dermal fibroblasts in psychiatry research. Neuroscience. 2016;320:105–121. doi: 10.1016/j.neuroscience.2016.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, et al. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci USA. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocadio-Miguel MA, Carneiro BT, Ximenes-da-Silva A, Caumo W, Grassi-Kassisse D, Pedrazzoli M. PER3 gene regulation of sleep-wake behavior as a function of latitude. Sleep Health. 2018;4:572–578. doi: 10.1016/j.sleh.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Leone MJ, Sigman M, Golombek DA. Effects of lockdown on human sleep and chronotype during the COVID-19 pandemic. Curr Biol. 2020;30:R930–R931. doi: 10.1016/j.cub.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loef B, et al. Immunological effects of shift work in healthcare workers. Sci Rep. 2019;9:18220. doi: 10.1038/s41598-019-54816-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menet JS, Rodriguez J, Abruzzi KC, Rosbash M. Nascent-Seq reveals novel features of mouse circadian transcriptional regulation. Elife. 2012;1:e00011. doi: 10.7554/eLife.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Carrier J, Bastien C, Godbout R, Canadian S, Circadian N. Sleep and circadian rhythm in response to the COVID-19 pandemic. Can J Public Health. 2020 doi: 10.17269/s41997-020-00382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci USA. 2012;109:12662–12667. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341:1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm D, Uzoni A, Simon F, Tucha F, Thome J, Faltraco F. Norepinephrine influences the circadian clock in human dermal fibroblasts from participants with a diagnosis of attention deficit hyperactivity disorder. J Neural Transm. 2021 doi: 10.1007/s00702-021-02376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parang K, El-Sayed NS, Kazeminy AJ, Tiwari RK. Comparative antiviral activity of remdesivir and anti-HIV nucleoside analogs against human coronavirus 229E (HCoV-229E) Molecules. 2020 doi: 10.3390/molecules25102343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel J, Laurie C, Cockcroft A, Andersson N. Clinical studies assessing the efficacy, effectiveness, and safety of remdesivir in management of COVID-19: a scoping review. Br J Clin Pharmacol. 2020 doi: 10.1111/bcp.14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Marsland AL, Hall M, Neumann SA, Muldoon MF, Manuck SB. Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biol Psychol. 2009;82:12–17. doi: 10.1016/j.biopsycho.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Janicki-Deverts D, Hall MH, Cohen S. Behaviorally assessed sleep and susceptibility to the common cold. Sleep. 2015;38:1353–1359. doi: 10.5665/sleep.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Reddy AB. COVID-19 management in light of the circadian clock. Nat Rev Mol Cell Biol. 2020;21:494–495. doi: 10.1038/s41580-020-0275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U, Sassone-Corsi P. A web of circadian pacemakers. Cell. 2002;111:919–922. doi: 10.1016/s0092-8674(02)01225-4. [DOI] [PubMed] [Google Scholar]
- Sheahan TP, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan TP, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva FRD, Guerreiro RC, Andrade HA, Stieler E, Silva A, de Mello MT. Does the compromised sleep and circadian disruption of night and shiftworkers make them highly vulnerable to 2019 coronavirus disease (COVID-19)? Chronobiol Int. 2020;37:607–617. doi: 10.1080/07420528.2020.1756841. [DOI] [PubMed] [Google Scholar]
- Silver AC, Arjona A, Hughes ME, Nitabach MN, Fikrig E. Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain Behav Immun. 2012;26:407–413. doi: 10.1016/j.bbi.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolensky MH, Reinberg AE, Sackett-Lundeen L. Perspectives on the relevance of the circadian time structure to workplace threshold limit values and employee biological monitoring. Chronobiol Int. 2017;34:1439–1464. doi: 10.1080/07420528.2017.1384740. [DOI] [PubMed] [Google Scholar]
- Spinner CD, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2015;111:A3 B 1 A3 B 3. doi: 10.1002/0471142735.ima03bs111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarpak L, Dzieciatkowski T, Jaguszewski MJ, Ladny JR, Filipiak KJ. Is remdesivir important in clinical practice as a treatment against COVID19? A study based on metaanalysis data. Pol Arch Intern Med. 2020 doi: 10.20452/pamw.15686. [DOI] [PubMed] [Google Scholar]
- Takashima A (1998) Establishment of fibroblast cultures. Curr Protoc Cell Biol. 10.1002/0471143030.cb0201s00 [DOI] [PubMed]
- Tchesnokov EP, Feng JY, Porter DP, Gotte M. Mechanism of inhibition of ebola virus RNA-dependent rna polymerase by remdesivir. Viruses. 2019 doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempestilli M, et al. Pharmacokinetics of remdesivir and GS-441524 in two critically ill patients who recovered from COVID-19. J Antimicrob Chemother. 2020 doi: 10.1093/jac/dkaa239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaben PF, Westermark PO. Differential rhythmicity: detecting altered rhythmicity in biological data. Bioinformatics. 2016;32:2800–2808. doi: 10.1093/bioinformatics/btw309. [DOI] [PubMed] [Google Scholar]
- Thome J, Coogan AN, Fischer M, Tucha O, Faltraco F. Challenges for mental health services during the 2020 COVID-19 outbreak in Germany. Psychiatry Clin Neurosci. 2020;74:407. doi: 10.1111/pcn.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome J, et al. The impact of the COVID-19 outbreak on the medico-legal and human rights of psychiatric patients. Eur Psychiatry. 2020;63:e50. doi: 10.1192/j.eurpsy.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Linthout S, Miteva K, Tschope C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res. 2014;102:258–269. doi: 10.1093/cvr/cvu062. [DOI] [PubMed] [Google Scholar]
- Vitale JA, Perazzo P, Silingardi M, Biffi M, Banfi G, Negrini F. Is disruption of sleep quality a consequence of severe Covid-19 infection? A case-series examination. Chronobiol Int. 2020 doi: 10.1080/07420528.2020.1775241. [DOI] [PubMed] [Google Scholar]
- Wang M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith A, Mustafa FB, Earnest A, Gen L, Macary PA. Impact of partial sleep deprivation on immune markers. Sleep Med. 2013;14:1031–1034. doi: 10.1016/j.sleep.2013.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and material are available.
Actiwatch 2, Philips Respironics, USA; CircWave v. 1.4 software (generated by Dr. Roelof Hut; www.euclock.org); SPSS (IBM Corporation).