Abstract
Background
The prognosis of lung cancer with synchronous brain metastasis (LCBM) is very poor, and patients often die within a short time. However, little is known about the early mortality and related factors in patients with LCBM.
Methods
Patients diagnosed with LCBM between 2010 and 2016 were enrolled from the Surveillance, Epidemiology, and End Result (SEER) database. Univariate and multivariate logistic regression analysis were used to identify significant independent prognostic factors, which were used to construct nomograms of overall and cancer-specific early death. Then, the prediction ability of the model was verified by receiver operating characteristic (ROC) curve. At last, the clinical application value of the model was tested through decision curve analysis (DCA).
Results
A total of 29,902 patients with LCBM were enrolled in this study. Among them, 13,275 (44.4%) patients had early death, and 11,425 (38.2%) cases died of lung cancer. The significant independent risk factors for overall and cancer-specific early death included age, race, gender, Gleason grade, histological type, T stage, N stage, bone metastasis, liver metastasis and marital status, which were used to construct the nomogram. The ROC curve demonstrated good predictive ability and clinical application value. The areas under the curve (AUC) of the training group was 0.793 (95% CI: 0.788–0.799) and 0.794 (95% CI: 0.788–0.799), in the model of overall and cancer-specific early death respectively. And the AUC of the validation group were 0.803 (95% CI: 0.788–0.818) and 0.806 (95% CI: 0.791–0.821), respectively. The calibration plots of the model showed that the predicted early death is consistent with the actual value. The DCA analysis indicated a good clinical application value of this model.
Conclusions
We established a comprehensive nomogram to predict early death in lung cancer patients with synchronous brain metastases. Nomograms may help oncologists develop better treatment strategies, such as clinical trials and hospice care.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-021-08490-4.
Keywords: Lung cancer, Brain metastases, Nomogram, Early death
Introduction
Brain metastases (BM) are the most common malignant tumor in the central nervous system [1, 2]. It is reported that the incidence of brain metastases is 10 times higher than that of primary malignant brain tumors [3]. Most brain metastases progress rapidly, with an average survival time of 13 months [4]. Lung cancer is the leading cause of brain metastasis, accounting for more than 80% [5].
Currently, there is no reliable treatment for lung cancer with synchronous brain metastasis (LCBM). Surgical treatment is not recommended for patients with LCBM because it has no significant impact on the long-term prognosis, although the symptoms are temporarily relieved [6]. In comparison, intracranial tumor biopsy is the gold standard for the diagnosis of LCBM, which can not only determine the nature of intracranial lesions, but determine their source. The combination of radiotherapy and targeted therapy has gradually become the current treatment of primary lung cancer [7]. And regular MRI review was used to monitor the therapeutic effect. With the development of molecular biology, more and more abnormal signal transduction and tumor-driving genes have been found, and increasing targeted drugs have been designed to prolong the overall survival of patients [8–10]. However, it is difficult obtain effective drug concentration in cerebrospinal fluid, due to the existence of the blood-brain barrier (BBB) [11]. As a result, the treatment of lung cancer will be very poor once brain metastases occur.
Death within a short period of time after diagnosis is defined as early death. And many patients with LCBM die early death due to intracranial hypertension and tumor-related epilepsy [12]. An in-depth understanding of the relationship between tumor-related factors and early death may help us reveal the causes of early death in high-risk patients, and provide basis for further active treatment, clinical trial consideration and supportive treatment. However, few studies have focused on the early death of patients with LCBM. Little is known about the early mortality and related factors in patients with LCBM currently. Thus, it’s of great significance to identify the risk factors of early death for prognostic evaluation and clinical treatment guidance in patients with LCBM.
In this study, patients with LCBM in Surveillance, Epidemiology, and End Results (SEER) database were included as the research objects to evaluate the incidence of early death and explore the risk factors of early death (≤3 months). In addition, we developed a module containing prognostic factors to predict the early mortality of patients with LCBM.
Methods
Patients
The SEER project of the National Cancer Institute provides data on cancer incidence and survival rate of 28% of the population in the United States (http://seer.cancer.gov) [13]. In this study, data of malignant lung and bronchial cancer patients with synchronous brain metastasis was extracted from the SEER database (2010–2016) by the SEER*Stat software version 8.3.8 (reference number: 17293-November 2019). The study cohort included the following histological codes from the third edition of the International Classification of Diseases for Oncology (ICD-O-3): large cell carcinoma (LCLC): 8012, 8014; squamous cell carcinoma (SQLC):8070, 8071, 8072, 8073, 8074, 8083; adenoma (AD): 8140, 8200, 8230, 8250, 8255, 8260, 8290, 8310, 8323; small cell carcinoma (SCLC): 8041, 8043, 8044, 8045 and ICD-O-3 site code C34.0–34.8. The exclusion criteria are as follows: (1) Patients without histological examination; (2) Patients without complete follow-up; (3) Patients with missing or incomplete information about survival time, survival status, cause of death, or other important characteristics; (4) Patients not primary. In addition to topography and morphology, it also includes clinical information such as gender, race, age, TN stage, insurance status, and marital status. The patients diagnosed in 2010–2015 were used as a training cohort to develop a nomogram, and the patients diagnosed in 2016 were selected as the internal validation group.
28 patients with LCBM from Renmin Hospital of Wuhan University were included in the study. The definition of cancer-specific early death in the hospital cohort was: death within 3 months after initial diagnosis of primary lung cancer with brain metastases. This retrospective study of the hospital cohort was approved by the ethics committee of Renmin Hospital of Wuhan University in accordance with the ethical standards approved by the Helsinki declaration.
Statistical analysis
Categorized data were described by numbers and percentages (N, %). Early death, defined as death within 3 months after diagnosis, was the endpoint of interest for this study. Histogram and pie chart were drawn with SPSS25 (IBM Inc., Chicago, IL, USA). Univariate and multivariate logistic regression models were performed using SPSS25 to determine variables that were significantly related to early death of patients with LCBM. Two-tailed P values less than 0.05 were considered statistically significant. All statistical analysis below was performed using the R programming language and environment (http://www.r-project.org/). The “regplot” software package was used to construct a nomogram of independent factors predicting early death of patients with LCBM [14]. For calibration, the nomogram predicted probabilities were contrasted with the actual probabilities by bootstrapping with 1000 resamples. The receiver operating characteristic (ROC) curve was used to judge discrimination. The higher the area under the curve (AUC) was, the better the accuracy would be. AUC values vary from 0.5 to 1.0, where 0.5 represents random chance, and 1.0 represents full compliance. And AUC value greater than 0.7 means a reasonable estimate [15]. Decision curves analysis (DCA) was used to assess the clinical benefit and utility of the model. DCA is one way to evaluate the clinical benefit of alternative models, and is applied to nomograms by quantifying the net benefit under different threshold probabilities. The curves of the treatment plan (representing the highest clinical cost) and no treatment plan (representing no clinical benefit) for all patients are drawn as two references [16, 17].
Result
Demographic and clinical characteristics of lung cancer patients with synchronous brain metastasis
This study included 29,902 patients diagnosed with synchronous brain metastases of lung cancer from 2010 to 2016 in the SEER database (Fig. 1). Table 1 listed the demographic and clinicopathological characteristics of patients in the training cohort (n = 26,272) and validation cohort (n = 3630). In general, most of the patients were over 40 years old, and the male was slightly more than the female. 79.5% of the cases were white and 12.2% were black. Most of them were adenocarcinoma (51.6%), and SQCC, SCLC and LCLC accounted for 10.6, 17.0 and 2.3%, respectively. Gleason grade III lung cancer was significantly higher than other grades. Some cases were accompanied with liver metastases (20.7%) or bone metastases (33.5%). Very few patients received surgical treatment (3.2%), a small number of patients received radiotherapy (21.1%), and about half of the patients received chemotherapy (56.1%). There was no significant difference in composition between the training group and the validation group.
Fig. 1.
The flowchart of patient selection from SEER database
Table 1.
Demographic and tumor characteristics of lung cancer patients with brain metastases
| Variable | All subjects | [cases (%)] | Training cohort | [cases (%)] | Validation cohort | [cases (%)] |
|---|---|---|---|---|---|---|
| Total | 29,902 | 26,272 | 3630 | |||
| Gender | ||||||
| Male | 15,596 | 52.2 | 13,741 | 52.3 | 1855 | 51.1 |
| Female | 14,306 | 47.8 | 12,531 | 47.7 | 1775 | 48.9 |
| Age | ||||||
| < 40 | 252 | 0.8 | 212 | 0.8 | 40 | 1.1 |
| 40–49 | 1648 | 5.5 | 1479 | 5.6 | 169 | 4.7 |
| 50–59 | 7149 | 23.9 | 6308 | 24.0 | 841 | 23.2 |
| 60–69 | 10,447 | 34.9 | 9150 | 34.8 | 1297 | 35.7 |
| 70–79 | 7751 | 25.9 | 6775 | 25.8 | 976 | 26.9 |
| > =80 | 2655 | 8.9 | 2348 | 8.9 | 307 | 8.5 |
| Race | ||||||
| White | 23,759 | 79.5 | 20,947 | 79.7 | 2812 | 77.5 |
| Black | 3643 | 12.2 | 3190 | 12.1 | 453 | 12.5 |
| Other | 2500 | 8.4 | 2135 | 8.1 | 365 | 10.1 |
| Histology | ||||||
| AD | 15,427 | 51.6 | 13,467 | 51.3 | 1960 | 54.0 |
| SQCC | 3164 | 10.6 | 2770 | 10.5 | 394 | 10.9 |
| LCLC | 675 | 2.3 | 601 | 2.3 | 74 | 2.0 |
| SCLC | 5087 | 17.0 | 4480 | 17.1 | 607 | 16.7 |
| Other | 5547 | 18.6 | 4952 | 18.8 | 595 | 16.4 |
| Gleason grade | ||||||
| I | 463 | 1.5 | 409 | 1.6 | 54 | 1.5 |
| II | 2677 | 9.0 | 2398 | 9.1 | 279 | 7.7 |
| III | 7821 | 26.2 | 6927 | 26.4 | 894 | 24.6 |
| IV | 1046 | 3.5 | 954 | 3.6 | 92 | 2.5 |
| Unknown | 17,895 | 59.8 | 15,584 | 59.3 | 2311 | 63.7 |
| T Stage | ||||||
| T0 | 292 | 1.0 | 266 | 1.0 | 26 | 0.7 |
| T1 | 3244 | 10.8 | 2803 | 10.7 | 441 | 12.1 |
| T2 | 7386 | 24.7 | 6441 | 24.5 | 945 | 26.0 |
| T3 | 6534 | 21.9 | 5826 | 22.2 | 708 | 19.5 |
| T4 | 8802 | 29.4 | 7674 | 29.2 | 1128 | 31.1 |
| Tx | 3644 | 12.2 | 3262 | 12.4 | 382 | 10.5 |
| N Stage | ||||||
| N0 | 6446 | 21.6 | 5675 | 21.6 | 771 | 21.2 |
| N1 | 2531 | 8.5 | 2213 | 8.4 | 318 | 8.8 |
| N2 | 13,441 | 45.0 | 11,892 | 45.4 | 1549 | 42.7 |
| N3 | 5773 | 19.3 | 4994 | 19.0 | 779 | 21.5 |
| Nx | 1711 | 5.7 | 1498 | 5.7 | 213 | 5.9 |
| Bone Met | ||||||
| Yes | 10,024 | 33.5 | 8750 | 33.3 | 1274 | 35.1 |
| None | 19,306 | 64.6 | 17,006 | 64.7 | 2300 | 63.4 |
| Unknown | 572 | 1.9 | 516 | 2.0 | 56 | 1.5 |
| Liver Met | ||||||
| Yes | 6189 | 20.7 | 5390 | 20.5 | 799 | 22.0 |
| None | 22,971 | 76.8 | 20,226 | 77.0 | 2745 | 75.6 |
| Unknown | 742 | 2.5 | 656 | 2.5 | 86 | 2.4 |
| Surgery | ||||||
| Yes | 961 | 3.2 | 858 | 3.3 | 103 | 2.8 |
| None/ Unknown | 28,941 | 96.8 | 25,414 | 96.7 | 3527 | 97.2 |
| Chemotherapy | ||||||
| Yes | 16,767 | 56.1 | 14,762 | 56.2 | 2005 | 55.2 |
| None/ Unknown | 13,135 | 43.9 | 11,510 | 43.8 | 1625 | 44.8 |
| Radiotherapy | ||||||
| Yes | 6307 | 21.1 | 5497 | 20.9 | 810 | 22.3 |
| None/ Unknown | 23,595 | 78.9 | 20,775 | 79.1 | 2820 | 77.7 |
| Insurance | ||||||
| Yes | 28,191 | 94.3 | 24,723 | 94.1 | 3468 | 95.5 |
| None | 1221 | 4.1 | 1128 | 4.3 | 93 | 2.6 |
| Unknown | 490 | 1.6 | 421 | 1.6 | 69 | 1.9 |
| Marital | ||||||
| Single | 13,326 | 44.6 | 11,643 | 44.3 | 1683 | 46.4 |
| Married | 15,424 | 51.6 | 13,608 | 51.8 | 1816 | 50.0 |
| Unknown | 1152 | 3.9 | 1021 | 3.9 | 131 | 3.6 |
Mortality of early death
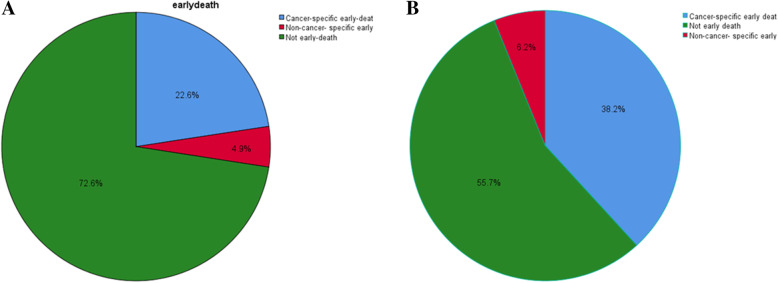
Among all lung cancer patients, 27.5% had early death, and 22.6% of them were caused by lung cancer (Fig. 2A). However, the early mortality of patients with LCBM was 44.4% (13275), and 38.2% (11425) of them were caused by lung cancer (Fig. 2B). From 2010 to 2016, the early mortality of patients with LCBM remained stable (Fig. 3A). The early mortality increased significantly with age, was slightly higher in white people than in other ethnic groups, and higher in male than in female (Fig. 3B, C, D).
Fig. 2.
Distribution of the incidence of overall and cancer-specific early death in all lung cancer patients (A) and lung cancer with synchronous brain metastasis patients (B)
Fig. 3.
Rate of overall and cancer-specific early death in lung cancer with synchronous brain metastasis patients stratified by year of diagnosis (A), age at diagnosis (B), race (C) and gender (D)
Identifying independent factors for early death
Univariate and multivariate logistic regression were used to analyze the risk factors of early death in patients with LCBM in the SEER training cohort. The results of univariate and multivariate analysis were shown in Table 2 and Table 3. In univariate analysis, most clinical and pathological characteristics such as gender, race, age at diagnosis, Gleason grade, histology, T stage, N stage, bone metastasis, liver metastasis and marital status were related to the probability of overall early death. All significant factors were included in the multivariate analysis. And multivariate analysis showed that gender, race, age at diagnosis, Gleason grade, histology, T stage, N stage, bone metastasis, liver metastasis and marital status were independent risk factors for predicting overall early death in patients with LCBM. The result of cancer-specific early death is consistent with that of overall early death.
Table 2.
Univariate logistic regression analysis of the training dataset
| Variable | Overall early-death | Cancer-specific early-death | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Gender | ||||||
| Male | Ref | Ref | ||||
| Female | 0.745 | 0.709–0.784 | < 0.001 | 0.773 | 0.734–0.815 | < 0.001 |
| Age | ||||||
| < 40 | Ref | Ref | ||||
| 40–49 | 1.57 | 1.046–2.356 | 0.03 | 1.441 | 0.942–2.205 | 0.092 |
| 50–59 | 2.43 | 1.645–3.589 | < 0.001 | 2.215 | 1.473–3.33 | < 0.001 |
| 60–69 | 3.205 | 2.173–4.727 | < 0.001 | 2.921 | 1.946–4.385 | < 0.001 |
| 70–79 | 4.687 | 3.176–6.917 | < 0.001 | 4.169 | 2.776 | < 0.001 |
| > =80 | 6.824 | 4.599–10.126 | < 0.001 | 5.571 | 3.69–8.411 | < 0.001 |
| Race | ||||||
| White | Ref | Ref | ||||
| Black | 0.907 | 0.839–0.981 | 0.014 | 0.891 | 0.821–0.966 | 0.005 |
| Other | 0.695 | 0.630–0.766 | < 0.001 | 0.705 | 0.637–0.781 | < 0.001 |
| Histology | ||||||
| AD | Ref | |||||
| SQCC | 1.784 | 1.642–1.939 | < 0.001 | 1.7 | 1.561–1.85 | < 0.001 |
| LCLC | 1.238 | 1.045–1.467 | 0.014 | 1.228 | 1.031–1.463 | 0.022 |
| SCLC | 1.192 | 1.111–1.28 | < 0.001 | 1.156 | 1.074–1.245 | < 0.001 |
| Other | 1.568 | 1.466–1.676 | < 0.001 | 1.433 | 1.337–1.535 | < 0.001 |
| Gleason grade | ||||||
| I | Ref | Ref | ||||
| II | 1.121 | 0.885–1.421 | 0.344 | 1.17 | 0.912–1.502 | 0.217 |
| III | 1.814 | 1.448–2.272 | < 0.001 | 1.813 | 1.43–2.298 | < 0.001 |
| IV | 1.682 | 1.302–2.172 | < 0.001 | 1.731 | 1.324–2.264 | < 0.001 |
| Unknown | 1.568 | 1.256–1.958 | < 0.001 | 1.519 | 1.202–1.921 | < 0.001 |
| T Stage | ||||||
| T0 | Ref | Ref | ||||
| T1 | 0.96 | 0.722–1.276 | 0.779 | 1.352 | 0.969–1.886 | 0.076 |
| T2 | 1.315 | 0.997–1.734 | 0.053 | 1.928 | 1.393–2.669 | < 0.001 |
| T3 | 1.674 | 1.269–2.207 | < 0.001 | 2.384 | 1.722–3.3 | < 0.001 |
| T4 | 1.826 | 1.387–2.406 | < 0.001 | 2.648 | 1.915–3.661 | < 0.001 |
| Tx | 1.925 | 1.454–2.548 | < 0.001 | 2.561 | 1.844–3.557 | < 0.001 |
| N Stage | ||||||
| N0 | Ref | Ref | ||||
| N1 | 0.93 | 0.838–1.034 | 0.18 | 0.961 | 0.86–1.072 | 0.474 |
| N2 | 1.203 | 1.126–1.286 | < 0.001 | 1.226 | 1.144–1.315 | < 0.001 |
| N3 | 1.154 | 1.065–1.25 | < 0.001 | 1.2 | 1.105–1.304 | < 0.001 |
| Nx | 1.553 | 1.383–1.745 | < 0.001 | 1.454 | 1.289–1.64 | < 0.001 |
| Bone Met | ||||||
| Yes | Ref | Ref | ||||
| None | 0.728 | 0.690–0.768 | < 0.001 | 0.728 | 0.689–0.769 | < 0.001 |
| Unknown | 0.998 | 0.833–1.196 | 0.984 | 0.903 | 0.748–1.09 | 0.286 |
| Liver Met | ||||||
| Yes | Ref | Ref | ||||
| None | 0.519 | 0.489–0.552 | < 0.001 | 0.543 | 0.51–0.577 | < 0.001 |
| Unknown | 0.771 | 0.654–0.909 | 0.002 | 0.796 | 0.673–0.942 | 0.008 |
| Insurance | ||||||
| Yes | Ref | Ref | ||||
| None | 0.894 | 0.791–1.01 | 0.073 | 0.947 | 0.834–1.076 | 0.407 |
| Unknown | 1.156 | 0.920–1.451 | 0.213 | 1.119 | 0.883–1.417 | 0.352 |
| Marital | ||||||
| Single | Ref | Ref | ||||
| Married | 0.805 | 0.764–0.847 | < 0.001 | 0.824 | 0.781–0.869 | < 0.001 |
| Unknown | 0.906 | 0.794–1.035 | 0.147 | 0.841 | 0.731–0.967 | 0.015 |
Table 3.
Multivariate logistic regression analysis of the training dataset
| Variable | Overall early-death | Cancer-specific early-death | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Gender | ||||||
| Male | Ref | Ref | ||||
| Female | 0.742 | 0.703–0.783 | < 0.001 | 0.773 | 0.731–0.817 | < 0.001 |
| Age | ||||||
| < 40 | Ref | Ref | ||||
| 40–49 | 1.715 | 1.133–2.596 | 0.011 | 1.558 | 1.011–2.4 | 0.044 |
| 50–59 | 2.634 | 1.769–3.923 | < 0.001 | 2.377 | 1.57–3.597 | < 0.001 |
| 60–69 | 3.494 | 2.35–5.197 | < 0.001 | 3.144 | 2.08–4.51 | < 0.001 |
| 70–79 | 5.296 | 3.559–7.881 | < 0.001 | 4.634 | 3.065–7.007 | < 0.001 |
| > =80 | 7.726 | 5.162–11.563 | < 0.001 | 6.176 | 4.062–9.389 | < 0.001 |
| Race | ||||||
| White | Ref | Ref | ||||
| Black | 0.941 | 0.867–1.022 | 0.151 | 0.923 | 0.847–1.005 | 0.064 |
| Other | 0.728 | 0.657–0.806 | < 0.001 | 0.733 | 0.659–0.816 | < 0.001 |
| Histology | ||||||
| AD | Ref | Ref | ||||
| SQCC | 1.555 | 1.425–1.697 | < 0.001 | 1.477 | 1.351–1.614 | < 0.001 |
| LCLC | 1.188 | 0.994–1.42 | 0.058 | 1.178 | 0.981–1.415 | 0.08 |
| SCLC | 0.975 | 0.901–1.056 | 0.541 | 0.96 | 0.885–1.043 | 0.336 |
| Other | 1.434 | 1.336–1.539 | < 0.001 | 1.315 | 1.223–1.414 | < 0.001 |
| Gleason grade | ||||||
| I | Ref | Ref | ||||
| II | 1.106 | 0.865–1.413 | 0.423 | 1.146 | 0.887–1.482 | 0.297 |
| III | 1.669 | 1.322–2.108 | < 0.001 | 1.673 | 1.31–2.136 | < 0.001 |
| IV | 1.555 | 1.187–2.038 | < 0.001 | 1.614 | 1.218–2.137 | 0.001 |
| Unknown | 1.446 | 1.148–1.800 | 0.002 | 1.412 | 1.109–1.799 | 0.005 |
| T Stage | ||||||
| T0 | Ref | Ref | ||||
| T1 | 0.951 | 0.71–1.273 | 0.734 | 1.334 | 0.951–1.872 | 0.095 |
| T2 | 1.221 | 0.919–1.624 | 0.169 | 1.787 | 1.283–2.489 | 0.001 |
| T3 | 1.512 | 1.137–2.01 | 0.004 | 2.138 | 1.535–2.978 | < 0.001 |
| T4 | 1.662 | 1.252–2.208 | < 0.001 | 2.389 | 1.717–3.323 | < 0.001 |
| Tx | 1.675 | 1.255–2.236 | < 0.001 | 2.247 | 1.608–3.141 | < 0.001 |
| N Stage | ||||||
| N0 | Ref | Ref | ||||
| N1 | 0.877 | 0.786–0.979 | 0.019 | 0.904 | 0.806–1.013 | 0.082 |
| N2 | 1.13 | 1.053–1.212 | 0.001 | 1.146 | 1.065–1.232 | < 0.001 |
| N3 | 1.108 | 1.018–1.207 | 0.018 | 1.145 | 1.048–1.25 | 0.003 |
| Nx | 1.257 | 1.107–1.426 | < 0.001 | 1.212 | 1.065–2.38 | 0.004 |
| Bone Met | ||||||
| Yes | Ref | Ref | ||||
| None | 0.791 | 0.746–0.839 | < 0.001 | 0.795 | 0.748–0.844 | < 0.001 |
| Unknown | 0.82 | 0.665–1.011 | 0.063 | 0.742 | 0.597–0.921 | 0.007 |
| Liver Met | ||||||
| Yes | Ref | Ref | ||||
| None | 0.566 | 0.529–0.605 | < 0.001 | 0.594 | 0.555–0.635 | < 0.001 |
| Unknown | 0.734 | 0.607–0.887 | 0.001 | 0.816 | 0.672–0.989 | 0.039 |
| Marital | ||||||
| Single | Ref | Ref | ||||
| Married | 0.757 | 0.717–0.8 | < 0.001 | 0.779 | 0.736–0.824 | < 0.001 |
| Unknown | 0.897 | 0.781–1.03 | 0.122 | 0.836 | 0.723–0.965 | 0.015 |
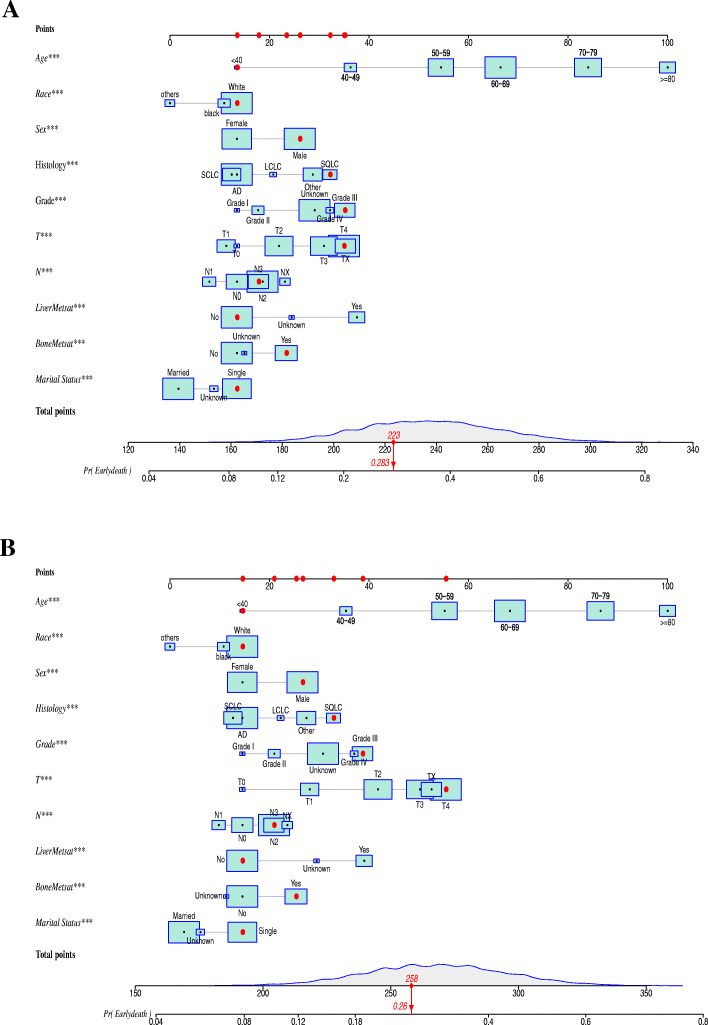
Nomogram construction
Depending on the multivariate logistic regression analysis model, the risk factor prediction nomogram of the SEER cohort was determined. An example of using nomogram to predict the survival probability of a given patient was shown in Fig. 4A. The total number of points can be attached to the overall probability of early death by calculating each variable point. And most patients had a total score of between 200 and 350 in this study. The predicting nomogram of the probability of cancer-specific early death was shown in Fig. 4B.
Fig. 4.
The predictive nomogram for the overall (A) and cancer-specific (B) early death of lung cancer with synchronous brain metastasis patients in the SEER database diagnosis between 2010 and 2015
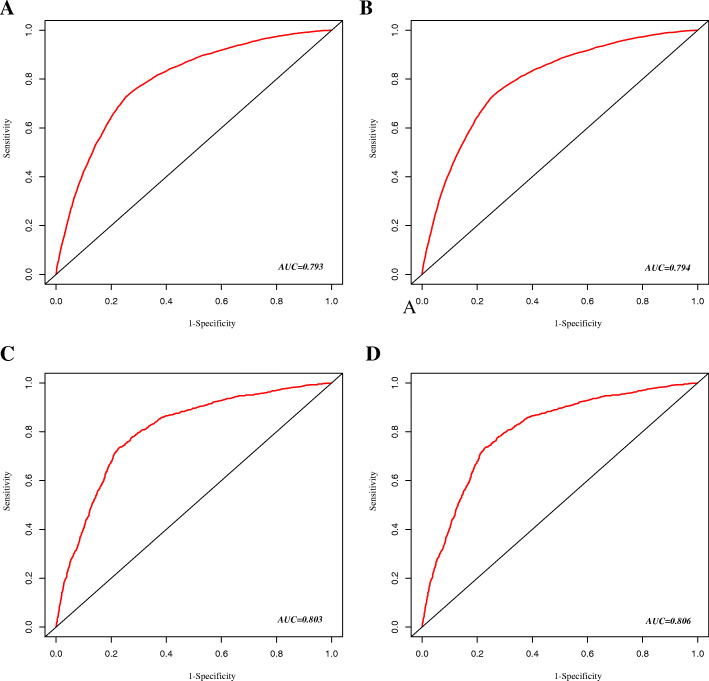
Nomogram validation
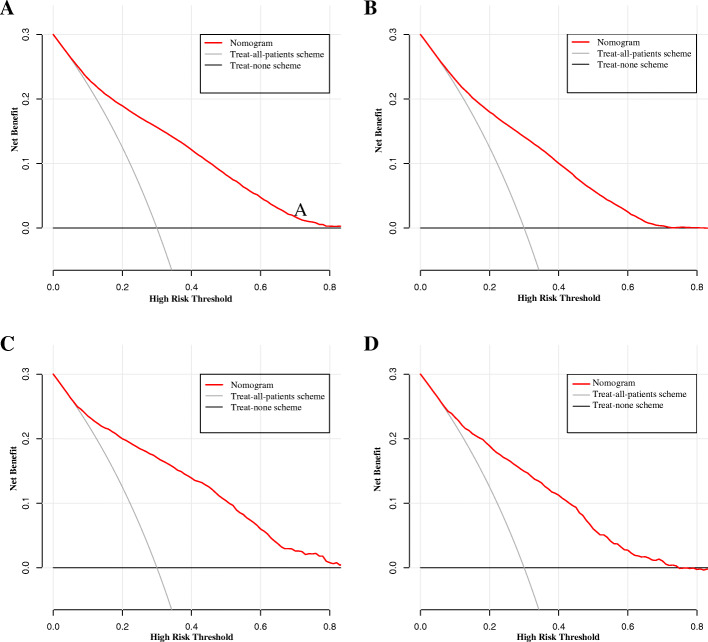
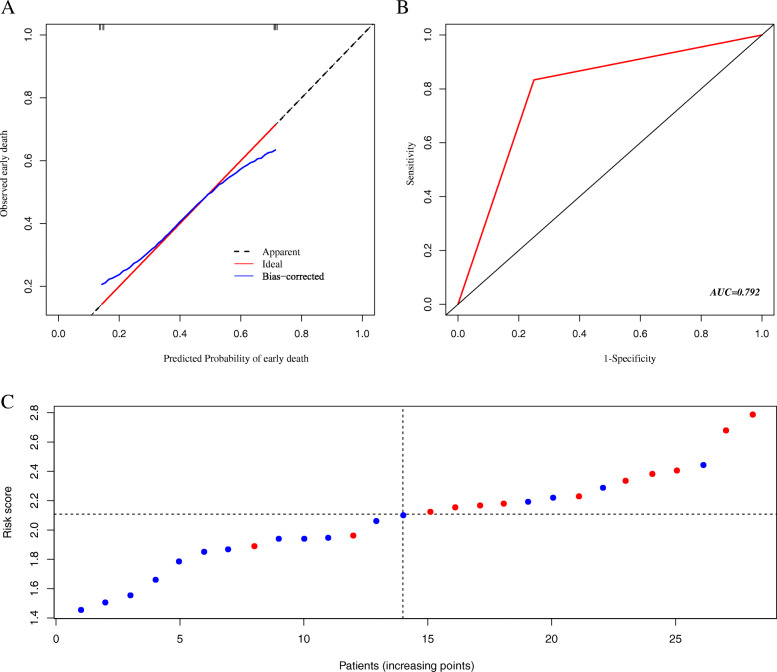
The nomogram showed good prediction efficiency on the probability of early death. The ROC curve used to assess the nomogram of overall and cancer-specific early death was shown in Fig. 5. The areas under the curve (AUC) of overall early death was 0.793 (Fig. 5A; 95% CI: 0.788–0.799), while the AUC of cancer-specific early death was 0.794 (Fig. 5B; 95% CI: 0.788–0.799) in the training group. And the AUC in the validation group were 0.803 for overall early death (Fig. 5C; 95% CI: 0.788–0.818) and 0.806 for cancer-specific early death (Fig. 5D; 95% CI: 0.791–0.821), respectively. The calibration plots of the model showed that the predicted early death was consistent with the actual value (Fig. 6). In addition, the DCA analysis indicated a good clinical application value of this model. (Fig. 7). Then, patients were scored by the nomogram in a hospital cohort. We only used the nomogram for cancer-specific early death, since the cause of early death in all patients was lung cancer and related factors. The total score of patients in the hospital cohort ranged from 145 to 279, which we divide them into high-score and low-score groups by setting the cutoff to 210 It showed that the early mortality in high score group was much higher than that in low score group, and the AUC was up to 0.792 (Fig. 8; 95% CI: 0.636–0.947).
Fig. 5.
ROC curves for the nomogram. (A) The ROC curve for the overall early death nomogram in the SEER database diagnosis between 2010 and 2015; (B) The ROC curve for the overall early death nomogram in the SEER database diagnosis at 2016; (C) The ROC curve for the cancer-specific early death nomogram in the SEER database diagnosis between 2010 and 2015; (D) The ROC curve for the cancer-specific early death nomogram in the SEER database diagnosis at 2016
Fig. 6.
Calibration plots for the nomogram of (A) overall early death in the SEER database diagnosis between 2010 and 2015; (B) overall early death in the SEER database diagnosis at 2016; (C) cancer-specific early death in the SEER database diagnosis between 2010 and 2015; (D) cancer-specific early death in the SEER database diagnosis at 2016
Fig. 7.
Decision curve analysis (DCA) for the nomogram of (A) overall early death in the SEER database diagnosis between 2010 and 2015; (B) overall early death in the SEER database diagnosis at 2016; (C) cancer-specific early death in the SEER database diagnosis between 2010 and 2015; (D) cancer-specific early death in the SEER database diagnosis at 2016
Fig. 8.
Validation in hospital cohort. (A) the calibration plots for the nomogram; (B) the ROC curve for the nomogram; (C) Total points distribution and early death status of patients in the hospital cohort
Web-based probability calculator
On the basis of the previous nomogram to predict the early death of patients with LCBM, the overall (Fig. 9A) and cancer specific (Fig. 9B) early death probability calculator based on Web was constructed (https://lcbmdynnom.shinyapps.io/lcbmofall/ and https://lcbmdynnom.shinyapps.io/lcbmcss/). The clinical characteristics of patients can be directly input to predict the early death probability of patients.
Fig. 9.
A web-based probability calculator. The graphical summary showed a rough range of overall (A) and cancer-specific (B) early death probability and its 95% confidence interval
Discussion
Brain metastasis is one of the common causes of death in cancer patients, and lung cancer is the main primary tumor of brain metastasis. Nowadays the survival time of patients has been significantly prolonged attributed to the early diagnosis and standardized treatment of lung cancer. However, once brain metastases occur, it will lead to neurological dysfunction, epilepsy and even delirium, which seriously endangers the survival of patients [18]. Thus, brain metastatic from lung cancer has become a major problem in neurosurgery.
The poor prognosis of brain metastatic lung cancer has always been a concern. However, most studies focus on the long-term survival of lung cancer patients with brain metastases, the early death of these patients has not been explored [19–22]. The definition of early death varies from studies and is usually defined as 30 days to 3 months after diagnosis. In this study, early death was defined as 3 months.
This study found that the overall early motality of lung cancer was 27.5%, and the rate increased to 44.4% once brain metastasis occurred, which indicated poor prognosis of lung cancer patients. Although the prognosis of lung cancer patients has improved in recent years, we found that the early mortality of patients with LCBM remained stable from 2010 to 2016, which indicates that we need to pay more attention to early death and related factors to reduce the risk of early death. Further studies found that age, race, gender, Gleason grade, histological type, T stage, N stage, bone metastasis, liver metastasis and marital status were independent risk factors for early death of patients with LCBM. Previous studies has shown that these risk factors have significant impact on the long-term survival of lung cancer patients (except bone metastases to SCLC), while it also shows that they have impact on the early death of patients with LCBM in this study [23].
The study on early death has been applied to advanced cancers in other systems and has shown important clinical significance. Song et al. established a nomogram chart to predict the early mortality of uterine sarcoma, which was significantly better than FIGO stage system [24]. Yang et al. established a model to predict the early mortality of stage IV gastric cancer, and the AUC was as high as 0.847 [25]. These studies demonstrate the feasibility and significance of nomograms in predicting early cancer motality. In our study, we established nomograms of overall and cancer-specific early death probability, according to the risk factors obtained from logistic regression analysis. The nomograms showed good predictive ability and clinical applicability. Internal validation of the nomogram showed good agreement between the predicted early deaths and the actual ones. DCA curves showed that our nomograms have good clinical value and practicability in predicting survival rate. It could provide a portable early death screening and clinical decision-making tool for clinicians, so as to customize the targeted therapy after diagnosis with LCBM.
This study also has several limitations. First of all, there is no information on molecular pathological indicators in SEER data set, and there are no positive prognostic variables. These variables may be an effective supplement to the existing system, which will be the main part of our future research. In addition, some indicators related to patients’ basic information, such as comorbidity rate, were not included in the study. In addition, although external validation is carried out, the amount of data is small, and the model still needs external validation of larger samples to estimate the accuracy.
Conclusion
In conclusion, we established a comprehensive nomogram to predict early death in lung cancer patients with synchronous brain metastases. Nomograms may help oncologists develop better treatment strategies, such as clinical trials and hospice care.
Supplementary Information
Acknowledgements
We gratefully acknowledge the SEER project organizers as well as all study participants for making data and results available.
Authors’ contributions
JQ and QC designed this study. HS and GD performed the data collection and wrote the paper. QC revised the manuscript. The author(s) read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The raw data of this study are derived from the SEER database, which is a publicly available database. The SEER detailed data of SEER database included in the study are available to all at https://seer.cancer.gov/. The hospital cohort database was available in the supplementary materials. More specific data used during the present study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study complied with the Declaration of Helsinki. The analysis dataset will be extracted without any identifiable information. Thus, informed consent has been waived. Ethical approval for use of clinical data of LCBM patients in this study was approved by the Renmin Hospital of Wuhan University’s Institutional Ethics Committee of the Faculty of Medicine (approval number: 2012LKSZ (010) H).
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Heng Shen and Gang Deng contributed equally to this work.
Contributor Information
Qianxue Chen, Email: chenqx666@whu.edu.cn.
Jin Qian, Email: Qianjin7601@163.com.
References
- 1.Sacks P, Rahman M. Epidemiology of brain metastases. Neurosurg Clin N Am. 2020;31(4):481–488. doi: 10.1016/j.nec.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Kotecha R, Gondi V, Ahluwalia MS, Brastianos PK, Mehta MP. Recent advances in managing brain metastasis. F1000Res. 2018 Nov 9;7: F1000 Faculty Rev-1772. doi: 10.12688/f1000research.15903.1. [DOI] [PMC free article] [PubMed]
- 3.Davis FG, Dolecek TA, McCarthy BJ, Villano JL. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro-Oncology. 2012;14(9):1171–1177. doi: 10.1093/neuonc/nos152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kromer C, Xu J, Ostrom QT, Gittleman H, Kruchko C, Sawaya R, Barnholtz-Sloan JS. Estimating the annual frequency of synchronous brain metastasis in the United States 2010-2013: a population-based study. J Neuro-Oncol. 2017;134(1):55–64. doi: 10.1007/s11060-017-2516-7. [DOI] [PubMed] [Google Scholar]
- 5.Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, Lee EQ, Wen PY, Dunn IF, Bi WL, Weiss SE, Haas-Kogan DA, Alexander BM, Aizer AA. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro-Oncology. 2017;19(11):1511–1521. doi: 10.1093/neuonc/nox077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Consonni D, Pierobon M, Gail MH, Rubagotti M, Rotunno M, Goldstein A, Goldin L, Lubin J, Wacholder S, Caporaso NE, Bertazzi PA, Tucker MA, Pesatori AC, Landi MT. Lung cancer prognosis before and after recurrence in a population-based setting. J Natl Cancer Inst. 2015;107(6):djv059. doi: 10.1093/jnci/djv059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naylor EC, Desani JK, Chung PK. Targeted therapy and immunotherapy for lung Cancer. Surg Oncol Clin N Am. 2016;25(3):601–609. doi: 10.1016/j.soc.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Janakiram M, Pareek V, Cheng H, Narasimhulu DM, Zang X. Immune checkpoint blockade in human cancer therapy: lung cancer and hematologic malignancies. Immunotherapy. 2016;8(7):809–819. doi: 10.2217/imt-2016-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yong CSM, Dardalhon V, Devaud C, Taylor N, Darcy PK, Kershaw MH. CAR T-cell therapy of solid tumors. Immunol Cell Biol. 2017;95(4):356–363. doi: 10.1038/icb.2016.128. [DOI] [PubMed] [Google Scholar]
- 10.Tan WL, Jain A, Takano A, Newell EW, Iyer NG, Lim WT, Tan EH, Zhai W, Hillmer AM, Tam WL, Tan DSW. Novel therapeutic targets on the horizon for lung cancer. Lancet Oncol. 2016;17(8):e347–e362. doi: 10.1016/S1470-2045(16)30123-1. [DOI] [PubMed] [Google Scholar]
- 11.Niranjan A, Lunsford LD, Ahluwalia MS. Targeted therapies for brain metastases. Prog Neurol Surg. 2019;34:125–137. doi: 10.1159/000493057. [DOI] [PubMed] [Google Scholar]
- 12.Yamanaka R, Koga H, Yamamoto Y, Yamada S, Sano T, Fukushige T. Characteristics of patients with brain metastases from lung cancer in a palliative care center. Support Care Cancer. 2011;19(4):467–473. doi: 10.1007/s00520-010-0838-5. [DOI] [PubMed] [Google Scholar]
- 13.Qiu MZ, Shi SM, Chen ZH, Yu HE, Sheng H, Jin Y, Wang DS, Wang FH, Li YH, Xie D, Zhou ZW, Yang DJ, Xu RH. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: a SEER-based study. Cancer Med. 2018;7(8):3662–3672. doi: 10.1002/cam4.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Xu G, Guo X, Ma W, Xu Y, Peltzer K, Chekhonin VP, Baklaushev VP, Hu N, Wang X, Liu Z, Zhang C. Early Death Incidence and Prediction in Stage IV Breast Cancer. Med Sci Monit. 2020;26:e924858. doi: 10.12659/MSM.924858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Zhang H, Li L, Hu M, Chen L, Xu B, Song Q. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: a population-based analysis. Cancer Commun (Lond) 2020;40(7):301–312. doi: 10.1002/cac2.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzgerald M, Saville BR, Lewis RJ. Decision curve analysis. JAMA. 2015;313(4):409–410. doi: 10.1001/jama.2015.37. [DOI] [PubMed] [Google Scholar]
- 17.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565-574. 27. Camp RL, Dolled-F DOI: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed]
- 18.Schmidt-Hansen M, Berendse S, Hamilton W. Symptomatic diagnosis of cancer of the brain and central nervous system in primary care: a systematic review. Fam Pract. 2015;32(6):618–623. doi: 10.1093/fampra/cmv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ascha MS, Ostrom QT, Wright J, Kumthekar P, Bordeaux JS, Sloan AE, Schumacher FR, Kruchko C, Barnholtz-Sloan JS. Lifetime occurrence of brain metastases arising from lung, breast, and skin cancers in the elderly: a SEER-Medicare study. Cancer Epidemiol Biomark Prev. 2019;28(5):917–925. doi: 10.1158/1055-9965.EPI-18-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Wu Q, Zhang J, Qin G, Yang T, Liu Y, Wang X, Zhang B, Wei Y. Prognostic impacts of extracranial metastasis on non-small cell lung cancer with brain metastasis: a retrospective study based on surveillance, epidemiology, and end results database. Cancer Med. 2020;10(2):471–482. doi: 10.1002/cam4.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu Y, Zhang J, Zhou Z, Liu D, Zhu H, Wen J, Xu X, Chen T, Fan M. Metastasis Patterns and Prognosis of Octogenarians with NSCLC: A Population-based Study. Aging Dis. 2020;11(1):82–92. doi: 10.14336/AD.2019.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Zhang Y, Sun X, Gusdon AM, Song N, Chen L, Jiang G, Huang Y. The prognostic value of multiorgan metastases in patients with non-small cell lung cancer and its variants: a SEER-based study. J Cancer Res Clin Oncol. 2018;144(9):1835–1842. doi: 10.1007/s00432-018-2702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goncalves PH, Peterson SL, Vigneau FD, Shore RD, Quarshie WO, Islam K, Schwartz AG, Wozniak AJ, Gadgeel SM. Risk of brain metastases in patients with nonmetastatic lung cancer: analysis of the metropolitan Detroit surveillance, epidemiology, and end results (SEER) data. Cancer. 2016;122(12):1921–1927. doi: 10.1002/cncr.30000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song Z, Wang Y, Zhang D, Zhou Y. A novel tool to predict early death in uterine sarcoma patients: a surveillance, epidemiology, and end results-based study. Front Oncol. 2020;10:608548. doi: 10.3389/fonc.2020.608548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, Chen ZJ, Yan S. The incidence, risk factors and predictive nomograms for early death among patients with stage IV gastric cancer: a population-based study. J Gastrointest Oncol. 2020;11(5):964–982. doi: 10.21037/jgo-20-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data of this study are derived from the SEER database, which is a publicly available database. The SEER detailed data of SEER database included in the study are available to all at https://seer.cancer.gov/. The hospital cohort database was available in the supplementary materials. More specific data used during the present study are available from the corresponding author upon reasonable request.