Significance
Whether migratory populations are preadapted or constrained in responding to global climate change largely depends on which cues individuals use when deciding to start their migration. The identity of these cues is revealed by whether response thresholds are consistent within, but differ between, individuals (“repeatability”). By satellite tracking 48 individuals across multiple migrations, we show that 1) Asian houbara used the environmental cue of local temperature, which was correlated between wintering and breeding grounds, to time their spring migration departure; 2) departure responses to temperature varied between individuals but were individually repeatable; and 3) individuals’ use of temperature as a cue allowed for adaptive population-level change in migration timing, relative to annual variation in spring temperatures.
Keywords: Asian houbara, bustard, migratory cues, individual repeatability, climate connectivity
Abstract
A fundamental issue in migration biology is how birds decide when to start their journey, given that arriving too early or too late in a variable environment reduces individual fitness. Internal circannual regulation and predictable cues such as photoperiod prepare birds for migration, while variable external cues such as temperature and wind are thought to fine-tune departure times; however, this has not been demonstrated at the key point at which an individual animal decides to start migrating. In theory, environmental cues correlated between departure and arrival sites allow informed departure decisions. For 48 satellite-tracked Asian houbara Chlamydotis macqueenii, a medium-distance migrant with climatic connectivity between wintering and breeding areas, each tracked across multiple years, spring departure was under individually consistent temperature conditions, with greater individual repeatability than for photoperiod or wind. Individuals occupied a range of wintering sites latitudinally spanning 1,200 km but departed at lower temperatures from more northerly latitudes. These individual departure decisions produced earlier mean population-level departure and arrival dates in warmer springs. Phenological adjustments were fully compensatory, because individuals arrived on the breeding grounds under similar temperature conditions each year. Individuals’ autumn departure decisions were also repeatable for temperature but less distinct than for spring, likely because of relaxed time constraints on leaving breeding grounds and the use of wind as a supplementary departure cue. We show that individual-level departure decisions informed by local temperatures can preadapt a population to adjust its population-level phenology in response to annual variability in spring temperatures without requiring genetic change in reaction thresholds.
A fundamental mystery in avian migration biology has been the identity of the cues that individual birds use to start migrating in order to exploit seasonally favorable conditions in geographically different areas (1, 2). To maximize their fitness, individuals should time their journey so as to reach their destination when conditions are optimal (3, 4); mistiming can have costs (5). Understanding the cues that trigger migration is key to understanding the mechanisms underlying recent advances in the timing of migration schedules (6–11) and to predicting future migratory responses to climate and land-use change (7, 12–14). If the environment at a destination was stable between years, an individual’s timing of migration could simply depend on an internal “circannual rhythm” or a fixed external cue, such as photoperiod (12). However, environmental conditions at destinations, and therefore also the optimal time to depart for them, vary between years, so the trigger for an individual to migrate is likely to be an external environmental cue (2).
To yield useful information, environmental cues at a departure site must be temporally correlated with conditions at the target destination (15). Short- and intermediate-distance migrants are more likely to have strong correlations between conditions at departure sites and destinations, with an early spring at a wintering site indicating an early spring at the breeding site, whereas long-distance (e.g., transequatorial) migrants may use weather cues at the wintering site (16) or fixed departure times but then adjust their migration speed as environmental connectivity increases with proximity to destination (17). Wind, while not necessarily informative about conditions at destinations, can influence departure decisions by birds, with tailwinds favorable (18, 19) and headwinds not (20). Both theory (2) and modeling (15) suggest that environmental cues that are reliable indicators of conditions between sites will be involved in individual migration departure decisions, but this remains to be demonstrated quantitatively. To date, studies relating timing of migration to weather variables, particularly temperature, have been limited to correlations between spring passage/arrival dates and local breeding site conditions (9, 13, 17, 21, 22) and, more recently, between weather conditions at stopover/wintering areas and passage dates (16, 23), but not the local cues at the actual departure site that potentially influence the individual decisions underlying these patterns. While individuals can change their schedule between years (24), the functional link between external environment cues and individual migration departure dates, and how this translates into population-level responses, have not previously been demonstrated (21). Understanding individual behavior is needed to resolve the mechanism by which phenological change arises and thus predict how populations may respond to a changing climate; phenotypic plasticity and microevolution provide plausible but competing explanations (16).
Evidence to date suggests that birds’ migration departure decisions are under endogenous control in response to external cues (12). The identity of the particular cue should be apparent if its value during departure is similar (individually repeatable) across multiple migrations by the same individual. We tested the roles of three a priori environmental cues—photoperiod, temperature, and favorable/adverse (south–north) wind velocity—on individual migration departure decisions in spring and autumn by analyzing satellite tracking data from 48 adult Asian houbara Chlamydotis macqueenii from three different breeding populations in Uzbekistan across 8 y (averaging three migrations per individual for each season; Fig. 1). We explored how individual differences in responses to each cue are related to individual departure latitude, as this can influence departure dates (25) and as environmental conditions may vary along a geographic gradient of wintering sites. We also related population-level mean departure and arrival dates to annual mean spring and autumn temperatures at the breeding site, as a test of the population-level phenological response to annual environmental variability. Finally, we tested how the temperature at individual arrival in spring was related to annual mean spring breeding site temperatures, hypothesizing that there would be no relation if phenological changes were adaptive and fully compensatory, such that birds arrive at the breeding grounds under consistent conditions despite annual variation in weather.
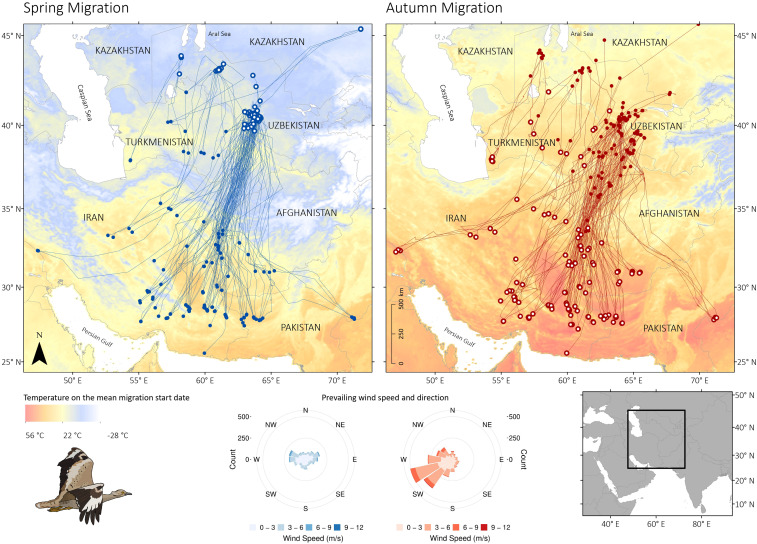
Fig. 1.
Spring and autumn migration of individual Asian houbara between breeding areas in Uzbekistan and wintering sites across southern Central Asia. Filled dots denote departure and hollow dots arrival locations. Background colors of the maps are the 8-d mean MODIS-derived land-surface daytime temperatures for the mean spring (February 27, 11.3 d SD) and autumn (October 17, 23.5 d SD) departure dates. The lower circular histograms show the prevailing wind directions and speed for the 25 d preceding the mean departure dates.
Results
Individual Repeatability of Departure Cues.
For spring migratory departure from the wintering site, individuals showed a repeatable response to temperature (R = 0.436 ± 0.09, P < 0.001 controlling for sex, Fig. 2 A and D) with a population-level mean departure temperature of 29.1 °C ± 0.8 SE (Fig. 2A, −6.59 °C ± 1.6 SE cooler for males, Δ Akaike Information Criterion [AICc] = 16.75). Repeatability with respect to temperature was significantly greater (z = 7.984, degrees of freedom [DF] = 1,998, and P < 0.001; Fig. 2D) than for photoperiod, which was also repeatable (R = 0.255 ± 0.10 and P = 0.004 controlling for sex; Fig. 2D) with a population-level mean of 11.5 h of daylight ± 0.04 SE at departure (Fig. 2B, −0.20 h shorter for males; ΔAICc = 3.00). In sex-specific models of repeatability, temperature was the only repeatable cue for males (R = 0.560 ± 0.142 and P < 0.01; Fig. 2D), with significantly greater repeatability with respect to this cue than females (z = 10.02, DF = 1,998, and P < 0.001), while females showed repeatable behavior with respect to both temperature (R = 0.335 ± 0.117 and P = 0.001; Fig. 2D) and, more weakly (z = 3.155, DF = 1,998, and P = 0.001; Fig. 2D), photoperiod (R = 0.268 ± 0.120 and P = 0.008; Fig. 2D). Individuals did not show repeatable behavior with respect to wind velocity at departure (R = 0.138 ± 0.09 and P = 0.08; Fig. 2D), but the population-level mean indicated exploitation of weak spring wind velocities toward the north (mean 0.93 metres per second [m/s] ± 0.26 SE), with no difference between the sexes (Fig. 2C; ΔAICc = −0.26). Null scenario simulations showed the observed R for temperature was significantly higher (SI Appendix, Fig. S1; z = −0.107, DF = 999, and P = 7.97 × 10−27) than could be expected (R = 0.265 ± 0.041 SD) solely from individual site fidelity and spatiotemporal correlation in temperatures within the geographic spread of spring departure sites (SI Appendix, Methods S1). In contrast, for daylength the observed repeatability (SI Appendix, Fig. S1, z = −0.615, DF = 999, and P = 0.539) did not differ from the null repeatability (R = 0.247 ± 0.009 SD).
Fig. 2.
Conditions of three potential migration departure cues, temperature (A), day length (B), and wind (C) at wintering sites during multiple spring migration departures (133) of individual Asian houbara (n = 45), showing annual values (dots) of individuals (linked by vertical line) sorted by sex and ranked by individual mean. (D) Individual repeatability (R) of the three migratory departure cues during consecutive spring departures showing the proportions of total population-level variance, explained by consistent, repeated individual behavior that is shown separately for all birds, sexes combined (black dots and bar), females (red), and males (black). Estimated repeatability does not differ significantly from zero (N.S.) where the 95% CI bar touches R = 0 (gray dotted horizontal line). Separately, for sexes combined, female, and male estimates, significant differences between cue-specific R estimates are shown with a horizontal bar and the P value but are not compared if one or more R estimate is N.S. (E) Mean individual temperatures at spring migration departure sites related to the mean latitude from which an individual Asian houbara departed. (F–H) The relation between annual mean spring temperature on the breeding grounds (8-d means that span the interannual mean of spring departure date, February 27, during springs from 2014 to 2020, with the year shown above the data) and individual spring migration departure (F) and arrival (G) dates and the temperature (H) at migration arrival sites on the arrival dates. Model means and relationships (separately for male and female) were estimated from GLMMs, including the fixed effect of sex and individual ID as a random factor, which are shown as colored lines with SEs.
For autumn migratory departure, individuals showed a repeatable response to both temperature (R = 0.678 ± 0.07 SE and P < 0.001 controlling for sex; Fig. 3 A and D) and photoperiod (R = 0.724 ± 0.06 SE and P < 0.001; Fig. 3 B and D). Repeatability with respect to these cues did not differ significantly (z = −2.35, DF = 1,998, and P = 0.018; nonsignificant after adjustment for the critical alpha value = 0.016 over multiple comparisons) potentially due to high collinearity between these cues (r = −0.905, DF = 150, and P < 0.001, SI Appendix, Fig. S2). Mean population-level departure conditions were 27.8 °C ± 1.4 SE (Fig. 3A; x −6.91 °C ± 2.7 SE cooler for males and ΔAICc = 3.487), and 11.1 h of daylight ± 0.12 SE (Fig. 3B; similar between the sexes, ΔAICc = 0.67). Sex-specific models of repeatability gave similar values to the combined estimates (Fig. 3D), and estimates of R did not differ between the sexes for either temperature (z = −0.702, DF = 1,998, and P = 0.483) or photoperiod (z = −0.764, DF = 1,998, and P = 0.445). In contrast to spring, wind velocity at autumn departure was individually repeatable (R = 0.432 ± 0.10 SE and P < 0.001; Fig. 3D), and at a population level, birds exploited fast winds toward the south (x = −2.47 m/s ± 0.228 SE; Fig. 3C) with no difference between the sexes (ΔAICc = 1.49). However, repeatability with respect to wind was weaker than for either temperature (Fig. 3D; z = 13.836, DF = 1,998, and P < 0.001) or photoperiod (Fig. 3D; z = −6.62, DF = 1,998, and P < 0.001). Sex-specific models showed males had a significantly higher repeatability for wind conditions at departure than females (z = 4.03, DF = 1,998, and P < 0.001; Fig. 3D).
Fig. 3.
Conditions of three potential migration departure cues, temperature (A), daylength (B), and wind (C) at postbreeding sites during multiple autumn migration departures (152) of individual Asian houbara (n = 48 individuals), showing annual values (dots) of individuals (linked by vertical line) sorted by sex and ranked by individual mean. (D) Individual repeatability (R) of the three migratory departure cues during consecutive autumn departures showing the proportions of total population-level variance explained by consistent repeated individual behavior. Estimated repeatability does not differ significantly from zero (N.S.), where the 95% CI bar touches R = 0 (shown as gray dotted horizontal line). Separately, for sexes combined, female, and male estimates, significant differences between cue-specific R estimates are shown with a horizontal bar and the P value but are not compared if one or more R estimate is N.S. (E) Mean individual temperatures at autumn migration departure sites related to the mean latitude from which an individual Asian houbara departed. (F and G) The relationship between annual mean spring temperature on the breeding grounds (8-d means that span the interannual mean autumn departure date, October 17, during autumns from 2013 to 2019 with the year shown above the data) and individual autumn migration departure (F) and arrival (G) dates. Model means and relationships (separately for male and female) were estimated from GLMMs, including the fixed effect of sex and individual ID as a random factor, which are shown as colored lines with SEs.
Latitude.
The mean temperature at which an individual left its wintering site during spring was colder for more northerly wintering latitudes (β = −1.155 ± 0.139 SE, controlling for sex for which ΔAICc = 45.48 on removal; Fig. 2E and SI Appendix, Table S1). The gradient of this relationship was similar (t70 = −0.022, P = 0.491, two-tailed Student's t test) to that of temperature to latitude across Central Asia in February (β = −1.223 ± 0.182 SE; SI Appendix, Figs. S3 and S4). In contrast, there was no support for a relation of spring departure date, photoperiod, or wind to latitude (SI Appendix, Tables S2–S4). For autumn departure decisions, the temperature response was again related to latitude (β = −0.892 ± 0.429 SE, controlling for sex for which ΔAICc = 2.07 on removal; Fig. 3E and SI Appendix, Table S5), with the gradient of this relationship again similar (t70 = −1.078, P = 0.143, two-tailed Student's t test) to that of temperature to latitude across Central Asia during October (β = −1.062 ± 0.118, SI Appendix, Figs. S3 and S4). There was no relation of autumn departure date, photoperiod, or wind to latitude (SI Appendix, Tables S6–S8).
Population-Level Phenology.
In spring, the overall mean population-level departure date was 27 February, SD = 11.3 d, but this varied between years (ΔAICc = 16.13 on removal of year [2014 to 2020] from a model controlling for sex but excluding 2012 and 2013 during which only three individuals were tracked; SI Appendix, Fig. S5). Both mean annual spring departure from the wintering site (Fig. 2F; ΔAICc = 14.62 on removal of year from a model controlling for sex) and arrival at the breeding grounds (Fig. 2G; ΔAICc = 34.67) were earlier in years with warmer mean spring temperatures. In contrast, the temperatures at which individuals arrived on the breeding grounds did not vary with mean annual spring temperature (ΔAICc = −1.97 on removal of term) with a slope (β = 0.059 ± 0.135 SE) that did not differ from zero (t = 0.435, P = 0.664 two-tailed Student's t test), indicating that individual adjustment of phenology was sufficient to enable birds to arrive on the breeding grounds at a similar temperature each year (Fig. 2H). In autumn, the overall mean population-level departure date was 17 October, SD = 23.5 d, but it varied between years (autumn ΔAICc = 4.92 on removal of year [2013 to 2019] from a model controlling for sex, excluding 2011 and 2012 when only three individuals were tracked), with fewer significant pairwise differences between years than for spring (SI Appendix, Fig. S5). In contrast to spring, mean population-level autumn migration departure and arrival dates were not related to mean autumn temperature on the breeding grounds (autumn departure, Fig. 3F, ΔAICc = −11.66; autumn arrival, Fig. 3G, ΔAICc = −9.72 on removal of temperature). Overall, within-year population-level departures dates in autumn were twice as variable as spring (variance test F132,151 = 0.191, P < 0.001).
Discussion
While there is a role played by photoperiod in migratory preparation (9, 12, 26, 27) and by wind in aiding migration (20, 28), individuals commencing migration from their wintering sites showed greater repeatability for temperature. In this flyway, temperatures on the wintering grounds were strongly correlated with those on the breeding grounds; this environmental connectivity thus provides a reliable cue to inform departure decisions (1, 29). Individually repeatable use of the temperature cue resulted in a measurable population-level phenological response to annual variation in mean spring temperatures at the breeding site, with earlier population departure and arrival in warmer springs. Phenotypic plasticity of individuals was fully compensatory across the range of variation in annual spring temperatures observed here, owing to the repeatable response to the temperature cue. Importantly, the interindividual variation in temperatures at spring departure was strongly and inversely related to departure latitude so that birds wintering in southerly latitudes departed at warmer local temperatures. This reflects the inverse relationship between climatic temperature and latitude (SI Appendix, Fig. S4). Despite birds starting from highly disparate locations in winter (latitudinally spanning 1,200 km), consistent individual decisions to depart according to latitudinally mediated temperature allowed a population-level adjustment to timing of departure according to variable annual temperature conditions.
Individuals separated by large geographic and climatic distances each displayed repeatable behavior in response to temperature, which indicates an endogenous control that likely includes a heritable genetic component to the use of cues for migration departure decisions (12). However, understanding how individual reaction thresholds are established, as well as the contribution of genetics, environment, and learning, requires further investigation. Genetics can contribute to migration distance (wintering latitude) in this species (30) and others (31), raising the possibility that wintering latitude and the individual’s temperature cue threshold may be genetically linked. However, early life experiences may also shape an individual’s phenotypic responses to temperature cues, given the geographic spread and potential uncertainties in the locations of wintering sites available to naïve juveniles on their first migration (32). Individual migration strategies (routes and wintering site) are often established during the first migration of naïve juveniles, which, on subsequent migrations, tend to be faithful to their first wintering site (32). Temperature responses may be shaped in a similar way. In support of this, experimental releases of captive-bred Asian houbara into Central Asian populations wintered in colder areas further north than wild counterparts, but their arrival times in breeding areas were similar to those of wild birds (32). Lastly, in long-lived species such as Asian houbara, accumulating experience may fine-tune individual phenotypes (14, 33). The relative importance of learning and genotype to migratory cue responses could be explored by parent-offspring comparisons, but the lekking behavior of this species makes establishing paternity challenging. The phenotypic flexibility in timing in Asian houbara can arise without genetic change but only because of an individually consistent phenotypic response to temperature that, in this case, is adaptive. In other species, the ability to adapt timing in response to environmental change without the need for genetic change may be constrained by the cues to which the response has evolved and how those cues are coupled to environmental change (6, 7, 34).
For autumn departure, individuals again showed repeatable responses in respect to temperature, but this could not be reliably separated from a potential response to photoperiod, highlighting the persistent problem of collinearity and autocorrelation when investigating environmental migration cues (16, 23). In autumn, the departure sites were much closer geographically than in spring, when there was enough spatiotemporal variation between individuals to separate the cues. Evolutionary constraints also differ between autumn and spring, with much greater fitness costs to mistiming spring migration (5, 34) consistent with twofold greater within-year variation of departure dates in autumn than in spring. Wind, which was uncorrelated with the other candidate cues, influenced the decision to migrate in autumn (tailwinds can be expected at that time of year and, free of reproductive pressures, birds can afford to wait for them), particularly for males that are heavier than females and thus face greater energetic costs of flight. In contrast, birds did exploit favorable winds in spring on average but did not appear to use wind as a cue and sometimes even departed into headwinds. This fits with previous models proposing that if the window for migration begins to close, due to a constraint such as food availability, then birds depart regardless of wind conditions (28).
Many bird populations have adjusted their migration schedules in response to climate change, but the mechanisms allowing such adjustment have remained unclear (24, 35), with phenotypic plasticity or microevolution often being proposed (23). Here, the evidence supports the conclusion that adjustments in migration departure can be driven by an individually consistent response to environmental cues that are correlated between breeding and nonbreeding areas, which, in turn, generate individual plasticity in terms of timing. That individual departure decisions are informed by local temperature preadapts population-level climate responses to earlier or more variable springs without genetic change in reaction thresholds.
Materials and Methods
Study System.
Asian houbaras breeding in Uzbekistan are migratory and winter hundreds to thousands of kilometers to the south (32, 36). Their migration is confined to the Central Asian flyway within the northern hemisphere, with substantial climate connectivity between breeding and wintering sites. Breeding areas in Uzbekistan have a semiarid continental climate, with hot summers (August mean 31.4 °C), cold winters (January mean −1 °C), and 134.5 mm of annual precipitation mostly falling as winter snow and spring showers (37). Wintering Asian houbara avoid areas >1,235 m above sea level, dispersing widely into warm desert landscapes experiencing some winter rainfall (38). A goose-sized bustard (Otididae), the species migrates using flapping flight, so tailwinds are desirable to reduce energetic costs (18, 19). We tracked birds from three subpopulations spanning 640 km longitudinally and 300 km latitudinally (Fig. 1). Individuals show high interannual fidelity to both wintering and breeding sites (30), and their migration behavior includes a potentially large genetic component (30, 32).
Migratory Data.
From autumn 2011 to spring 2019, we captured 48 houbaras in order to study their migrations; 44 were snared as adults at their spring display or nesting sites, one juvenile was caught by hand, and two adults and one adult-sized juvenile were caught using a talon-baffled falcon during autumn. Adults would previously have completed at least one return migration (32), and the two juveniles were included in the adult group from their second migration onwards. The sample comprised 18 adult males from three areas in Uzbekistan, namely the Ustyurt plateau (three males), Aral Kum (six males), and Bukhara region (nine males) (Fig. 1), and 30 adult females from Bukhara only [owing to difficulties tracking and locating nests elsewhere (30)]. Each was fitted with a backpack harness–mounted satellite transmitter (Microwave Telemetry Argos/global positioning system [GPS] Platform Transmitter Terminal [PTT]-100, either 30 [both sexes] or 45 g [males only] models) that recorded 5 to 12 GPS fixes per day. Transmitters weighed <3% of body mass (females = 1.25 kg ± 0.15 SD; males = 1.82 kg ± 0.31 SD weights at capture) and do not affect breeding performance (39), so they are assumed to have minimal or no effect on migration.
These 48 individuals provided 152 autumn migration departures (mean 3.2 per individual, ranging from 2 to 7), and 44 individuals (17 males and 27 females) provided 132 spring departures (mean 3.0 per individual, ranging from 2 to 7). Step distance, duration (time), and speed (distance/time) between sequential GPS fixes were calculated using "adehabitatLT" (40) in R (41), allowing steps to be classified as foraging (<2 kph) or transit (>2 kph), following ref. 32. Migration onset was indicated by a step length >54 km (the maximum width of a recorded postbreeding home range) away from the last fix in the postbreeding area after August or on the wintering grounds after February, followed by a series of stopovers and further migratory steps on a similar trajectory. Arrival dates for the population-level analysis were identified as the last transition from transit to foraging when the breeding or wintering site was reached. Female autumn departure dates were unaffected by breeding outcome (SI Appendix, Fig. S6), but we could not gauge the age of the tracked birds and acknowledge that migratory performance has the potential to improve with age/experience (14, 32, 33).
Migration Departure Cues.
For each individual in each year, environmental conditions were extracted for the date and location of departure. Photoperiod (day length in hours) was calculated using the R package “geosphere” (42). Temperature and wind data were downloaded using the Movebank Environmental Data Automated Track Annotation System (43). This provides mean daytime land surface temperature (Celsius) of the 8 d preceding the date of departure and 8 d following arrival (extracted from Moderate Resolution Imaging Spectroradiometer [MODIS] Land Surface Day Temperature 0.05° [∼5.6 km] 8-d Terra [MOD11C2 V6]), interpolated (by inverse distance weighting) from the four closest points as recommended by Movebank (43). An 8-d mean product was chosen as it was less prone to short-term fluctuations than daily values, while monthly means had greater temporal overlap between individuals, reducing predictor variance. As temperatures were daytime only and measured from the earth’s surface, they are higher than reported daily mean temperatures from weather stations. Wind velocity (in meters per second) of the south–north (meridional) component at 10 m above the earth’s surface was extracted from the European Centre for Medium-Range Weather Forecasts (ECMWF) Global Atmospheric Reanalysis ECMWF Re-Analysis (ERA)-Interim dataset (44) at a 0.75° (∼83 km) spatial resolution and six-hourly temporal resolution but was unavailable for 2020, as the ERA-Interim dataset was discontinued, giving reduced sample sizes of 126 departures from 41 individuals in spring and 111 departures from 39 individuals in autumn. Negative values indicate winds from the north (favorable in autumn) and positive values winds from the south (favorable in spring). As Asian houbara mostly migrate at night (36), we consistently extracted wind data from 22:00 hours (sunset is at ∼18:00 hours in autumn and spring) on the departure day, irrespective of the timing of the last fix prior to departure.
To be identified as triggering a migratory response, the candidate environmental cues under investigation must be uncorrelated (decoupled), ideally through long-term monitoring of individuals over multiple years. Date is spatially invariable, while date-specific photoperiod varies with latitude; nevertheless, the date and photoperiod (extracted per individual departure date/location) were near-perfectly correlated in both seasons (Pearson’s product-moment correlation, r = −0.996, DF = 150, and P < 0.001 for autumn; r = 0.928, DF = 130, and P < 0.001 for spring). Consequently, we examined photoperiod, not date, as this external cue is more likely to trigger migratory departure than an endogenous clock (2). Temperature varies with both date/photoperiod and latitude and stochastically within seasons and between years. Photoperiod and temperature were strongly negatively correlated for autumn departure (r = −0.905, DF = 150, and P < 0.001; SI Appendix, Fig. S2A) when departure locations were more spatially aggregated (Fig. 1) but showed greater independence for spring departure (r = 0.433, DF = 130, and P < 0.001; SI Appendix, Fig. S2B) when departure locations were widely spread (spanning 1,200 km latitude and 2,300 km longitude; Fig. 1). A latitudinal temperature gradient across the region changes seasonally (SI Appendix, Methods S2), with the rate at which temperature warms as a bird moves south being greatest during the winter months (November to February, circa 1.5 °C change per degree latitude) but reversing in July to August when it is warmer to the north (SI Appendix, Fig. S4).
To profile wind conditions in spring and autumn, we explored wind velocity on each day up to 5 d prior to departure and at further 5-d intervals up to 25 d before departure. Overall, prevailing autumn winds were significantly southward vectors prior to departure (for all lag intervals), while in spring no pattern emerged, with weaker speeds and overall neutral mean wind directions (SI Appendix, Fig. S7). Therefore, while birds may be expected to wait for favorable winds to decide when to start migration, conditions were predominantly favorable in autumn, so wind conditions are not likely to limit the departure options, while in spring, birds would have fewer opportunities to start with a favorable wind, and there may be costs and benefits to waiting for winds to aid migration.
Studies have linked migration movements to food availability and habitat conditions at large geographic scales, often measured with remotely sensed normalized density vegetation indices (NDVI) as a proxy (12, 13). However, we did not include NDVI as a candidate cue because it does not provide a consistent measure of habitat quality (or food availability) across the various semiarid ecoregions of the Central Asian wintering range, owing to differences in vegetation structure and species composition (38).
Analysis.
Unless otherwise stated, responses to candidate predictor variables were tested by their removal from a global generalized linear mixed model (GLMM) containing all covariates, incorporating individual bird identity (ID) as a random effect, and were considered influential if the AICc (adjusted for small sample sizes) increased by ≥2 (45). To examine the within-year variation of departure dates between spring and autumn, departure dates for each period were standardized, the years pooled, and the differences in variance examined using F tests. All statistics were performed using the software “R” (41).
Estimation of Individual Repeatability in Migration Cues.
Repeatability (R), also known as the intraclass correlation coefficient, measures the degree to which individuals are each consistent in their behavior or responses. R ranges from 0 to 1, with 0 indicating that individuals show a similar magnitude of variation in their repeated behavior as observed across the population, and 1 indicating that multiple measurements of a trait expressed by an individual are identical, with population-wide variance entirely due to differences between individuals (46). Repeatability, and its uncertainty, were estimated with the package “rptR” (47) using GLMMs and parametric bootstrapping with 1,000 iterations. A response to a candidate cue was considered individually repeatable if R differed significantly from zero, tested by a likelihood ratio test of the model including individual ID grouping factor against a model constraining the group-level variance to zero (47).
Systematic differences between groups of individuals can inflate estimates of R if not accounted for within the repeatability model. As migration behaviors of birds can vary between sexes (32, 48) and breeding populations (49), prior to estimating R, we tested whether the means of each cue differed between these groups using GLMMs. We found no support for any influence of breeding population identity, which was therefore excluded when estimating R. Where raw cue values differed between males and females, sex was included as an additive effect within the repeatability model. Additionally, R for each cue was estimated separately for each sex.
The geographic spread of wintering sites, coupled with the high winter site fidelity of Asian houbara (32), has the potential to generate a significant individual repeatability estimate in daylength or temperature departure conditions through spatiotemporal constraints and not an individual’s repeatable response to these conditions. This could occur when the variance in these cues between wintering sites is greater than interannual variation within individual sites for a limited departure date window. To address this, we carried out additional model simulations of null departure scenarios that quantified what repeatability could arise in daylength and temperature solely as an artifact of the geographic and temporal constraints on individuals within the dataset. Each individual’s departure days were shuffled between years within their own time series (constrained not to replicate the observed date–temperature departure decision) and were resampled independently over 1,000 simulations, re-extracting the daylength and temperatures for the null dates. Repeatability was then estimated for each iteration to generate a null distribution of R values for both cues. The null distribution for each cue was then tested against the respective observed repeatability estimate which arose from individual choice of daylength or temperature. The procedure is fully described in SI Appendix, Methods S1.
For those cues for which R differed from zero, post hoc tests were used to compare R estimates between cues (pooling sexes, controlling for sex if appropriate, and separately within each sex) and also separately for each cue between adult males and females. The simulated R values from each of the bootstrap iterations with the grouping variable (cue or sex) as a covariate were modeled using a generalized linear model (GLM) with binomial errors and difference in cue means assessed using the z distribution with the critical P values adjusted for multiple comparisons according to Chen (50).
Latitudinal Variation in Individual Migration Cues.
As temperature, daylength, and migratory distance all vary with latitude, we were curious whether repeatable individual responses to these cues might also vary systematically with latitude, with birds that have greater distance to travel beginning migration earlier, or at a cooler temperature. We separately related individuals’ departure day, photoperiod, 8-d mean daytime surface temperature, and wind velocity to departure latitude using GLMMs that incorporated the random effect of bird ID to account for repeated measures from each individual.
Annual Population-Level Migration Timing and Arrival Temperatures Relative to Year-Specific Seasonal Temperature.
We examined the extent to which these individual behaviors linked population-wide migration phenology to annual variation in temperature on the breeding grounds. For each year, we extracted the mean MODIS surface temperatures for the breeding ground centroid in Bukhara (a consistent reference location) over a consistent 8-d period spanning the mean 2013 to 2020 departure dates in spring, February 26 to March 5, and autumn, October 15 to 23. Then, separately for spring and autumn migration, we related individual departure and arrival dates to annual weather in Bukhara in a GLMM, incorporating bird ID as a random effect. Finally, we related the annual mean spring temperature to the 8-d mean temperature individuals experienced following arrival at their breeding site. We used a GLMM incorporating bird ID as a random effect and sex as an additive effect. We hypothesized that if birds were adjusting their phenology to arrive to the breeding sites at consistent temperatures each year (fully compensating), then there would be no relation between individual arrival temperature and annual spring temperatures. In contrast, if birds were either not adjusting or were under- or over-compensating their arrival times relative to annual conditions, then individual arrival temperatures would be related to annual spring temperatures.
Supplementary Material
Acknowledgments
We thank the State Committee for Nature Conservation of the Republic of Uzbekistan for fieldwork permission provided through the Emirates Bird Breeding Center for Conservation (EBBCC). We also thank EBBCC for collaboration and logistical support; E. Khaitov for tracking and finding houbara nests; A. Brighten, V. Dobrev, J. Guilherme, M. Koshkin, P. Saunders, J. St. Clair, and J. Willans for catching and tagging wild houbara; and M. Acácio, C. Panter, and J. Guilherme for remotely monitoring tagged birds.
Footnotes
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2026378118/-/DCSupplemental.
Data Availability
All R code and the dataset necessary to replicate the results of this study can be accessed at Zenodo, http://doi.org/10.5281/zenodo.4917565 (51).
References
- 1.Bauer S., et al., “Cues and decision rules in animal migration” in Animal Migration: A Synthesis, Milner-Gulland E. J., Fryxell J., Sinclair A., Eds. (Oxford Univeersity Press, Oxford, UK, 2011), pp. 68–87. [Google Scholar]
- 2.Newton I., Migration Ecology of Birds (Elsevier, London, UK, 2007). [Google Scholar]
- 3.Pulido F., Berthold P., Mohr G., Querner U., Heritability of the timing of autumn migration in a natural bird population. Proc. Biol. Sci. 268, 953–959 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthold P., Pulido F., Heritability of migratory activity in a natural bird population. Proc. Biol. Sci. 257, 311–315 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lerche-Jørgensen M., Korner-Nievergelt F., Tøttrup A. P., Willemoes M., Thorup K., Early returning long-distance migrant males do pay a survival cost. Ecol. Evol. 8, 11434–11449 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Both C., Visser M. E., Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411, 296–298 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Charmantier A., Gienapp P., Climate change and timing of avian breeding and migration: Evolutionary versus plastic changes. Evol. Appl. 7, 15–28 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill J. A., Alves J. A., Gunnarsson T. G., Mechanisms driving phenological and range change in migratory species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20180047 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordo O., Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Clim. Res. 35, 37–58 (2007). [Google Scholar]
- 10.Knudsen E., et al., Challenging claims in the study of migratory birds and climate change. Biol. Rev. Camb. Philos. Soc. 86, 928–946 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Lehikoinen E., Sparks T., “Changes in migration” in Effects of Climate Change on Birds, A. P. Møller, W. Fiedler, P. Berthold, Eds. (Oxford University Press, Oxford, UK, 2010), pp. 89–112. [Google Scholar]
- 12.Åkesson S., Helm B., Endogenous programs and flexibility in bird migration. Front. Ecol. Evol. 8, 78 (2020). [Google Scholar]
- 13.Kelly J. F., et al., Novel measures of continental-scale avian migration phenology related to proximate environmental cues. Ecosphere 7, e01434 (2016). [Google Scholar]
- 14.Teitelbaum C. S., et al., Experience drives innovation of new migration patterns of whooping cranes in response to global change. Nat. Commun. 7, 12793 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer S., McNamara J. M., Barta Z., Environmental variability, reliability of information and the timing of migration. Proc. Biol. Sci. 287, 20200622 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haest B., Hüppop O., Bairlein F., Weather at the winter and stopover areas determines spring migration onset, progress, and advancements in Afro-Palearctic migrant birds. Proc. Natl. Acad. Sci. U.S.A. 117, 17056–17062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marra P. P., Francis C. M., Mulvihill R. S., Moore F. R., The influence of climate on the timing and rate of spring bird migration. Oecologia 142, 307–315 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Erni B., Liechti F., Bruderer B., The role of wind in passerine autumn migration between Europe and Africa. Behav. Ecol. 16, 732–740 (2005). [Google Scholar]
- 19.Senner N. R., et al., High-altitude shorebird migration in the absence of topographical barriers: Avoiding high air temperatures and searching for profitable winds. Proc. Biol. Sci. 285, 20180569 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drake A., Rock C. A., Quinlan S. P., Martin M., Green D. J., Wind speed during migration influences the survival, timing of breeding, and productivity of a neotropical migrant, Setophaga petechia. PLoS One 9, e97152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tøttrup A. P., et al., Local temperature fine-tunes the timing of spring migration in birds. Integr. Comp. Biol. 50, 293–304 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Bauer S., Gienapp P., Madsen J., The relevance of environmental conditions for departure decision changes en route in migrating geese. Ecology 89, 1953–1960 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Haest B., Hüppop O., Bairlein F., The influence of weather on avian spring migration phenology: What, where and when? Glob. Change Biol. 24, 5769–5788 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Fraser K. C., Shave A., de Greef E., Siegrist J., Garroway C. J., Individual variability in migration timing can explain long-term, population-level advances in a songbird. Front. Ecol. Evol. 7, 324 (2019). [Google Scholar]
- 25.Madon B., Le Nuz E., Ferlat C., Hingrat Y., Insights into the phenology of migration and survival of a long migrant land bird. bioRxiv [Preprint] (2015). https://www.biorxiv.org/content/10.1101/028597v1 (Accessed 1 November 2020).
- 26.Gwinner E., Photoperiod as a modifying and limiting factor in the expression of avian circannual rhythms. J. Biol. Rhythms 4, 237–250 (1989). [PubMed] [Google Scholar]
- 27.Robart A. R., McGuire M. M. K., Watts H. E., Increasing photoperiod stimulates the initiation of spring migratory behaviour and physiology in a facultative migrant, the pine siskin. R. Soc. Open Sci. 5, 180876 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Åkesson S., Hedenström A., Wind selectivity of migratory flight departures in birds. Behav. Ecol. Sociobiol. 47, 140–144 (2000). [Google Scholar]
- 29.McNamara J. M., Barta Z., Klaassen M., Bauer S., Cues and the optimal timing of activities under environmental changes. Ecol. Lett. 14, 1183–1190 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burnside R. J., Buchan C., Salliss D., Collar N. J., Dolman P. M., Releases of Asian houbara must respect genetic and geographic origin to preserve inherited migration behaviour: Evidence from a translocation experiment. R. Soc. Open Sci. 7, 200250 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu Z., et al., Climate-driven flyway changes and memory-based long-distance migration. Nature 591, 259–264 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Burnside R. J., Collar N. J., Dolman P. M., Comparative migration strategies of wild and captive-bred Asian houbara Chlamydotis macqueenii. Ibis 159, 374–389 (2017). [Google Scholar]
- 33.Sergio F., et al., Individual improvements and selective mortality shape lifelong migratory performance. Nature 515, 410–413 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Shipley J. R., et al., Birds advancing lay dates with warming springs face greater risk of chick mortality. Proc. Natl. Acad. Sci. U.S.A. 117, 25590–25594 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gill J. A., et al., Why is timing of bird migration advancing when individuals are not? Proc. Biol. Sci. 281, 20132161 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Combreau O., Al Baidhani M. S., A Natural History of the Asian Houbara Bustard (International Fund for Houbara Conservation, Dubai, UAE, 2018). [Google Scholar]
- 37.Kholmatjanov B., Jaloliddinov B., Trends and variability of air temperature and precipitation from 1961 to 2016 in the Kyzylkum desert. Eur. Sci. Rev. 9–10, 91–96 (2018). [Google Scholar]
- 38.Formica A. F., Burnside R. J., Dolman P. M., Rainfall validates MODIS-derived NDVI as an index of spatio-temporal variation in green biomass across non-montane semi-arid and arid Central Asia. J. Arid Environ. 142, 11–21 (2017). [Google Scholar]
- 39.Burnside R. J., Guilherme J. L., Collar N. J., Dolman P. M., Backpack harness solar powered PTTs do not affect reproductive traits in Asian houbara Chlamydotis macqueenii. Eur. J. Wildl. Res., 10.1007/s10344-019-1332-0 (2019). [Google Scholar]
- 40.Calenge C., The package adehabitat for the R software: A tool for the analysis of space and habitat use by animals. Ecol. Modell. 197, 516–519 (2006). [Google Scholar]
- 41.R Development Core Team , R: A Language and Environment for Statistical Computing (Version 3.6.1, R Foundation for Statistical Computing, Vienna, Austria, 2019). https://www.R-Project.org. Accessed 20 March 2020.
- 42.Hijmans R., Williams E., Veness C., Geosphere: Spherical Trigonometry (Version 1.5-10, R package, R Foundation for Statistical Computing, Vienna, 2016).
- 43.Dodge S., et al., The environmental-data automated track annotation (Env-DATA) system: Linking animal tracks with environmental data. Mov. Ecol. 1, 3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dee D. P., et al., The ERA-interim reanalysis: Configuration and performance of the data assimilation system. Q. J. R. Meteorol. Soc. 137, 553–597 (2011). [Google Scholar]
- 45.Burnham K., Anderson D., Model Selection and Multi-Model Inference (Springer, New York, ed. 2, 2002). [Google Scholar]
- 46.Lessells C. M., Boag P. T., Unrepeatable repeatabilities: A common mistake. Auk 104, 116–121 (1987). [Google Scholar]
- 47.Stoffel M. A., Nakagawa S., Schielzeth H., rptR: Repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639–1644 (2017). [Google Scholar]
- 48.Alonso J. C., Palacín C., Alonso J. A., Martín C. A., Post-breeding migration in male great bustards: Low tolerance of the heaviest Palaearctic bird to summer heat. Behav. Ecol. Sociobiol. 63, 1705–1715 (2009). [Google Scholar]
- 49.Phipps W. L., et al., Spatial and temporal variability in migration of a soaring raptor across three continents. Front. Ecol. Evol. 7, 323 (2019). [Google Scholar]
- 50.Chen S.-Y., Feng Z., Yi X., A general introduction to adjustment for multiple comparisons. J. Thorac. Dis. 9, 1725–1729 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burnside R. J., Salliss D., Collar N. J., Dolman P. M., Code and data for: "Birds use individually consistent temperature cues to time their migration departure.“ Zenodo. 10.5281/zenodo.4917565. Deposited 10 June 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All R code and the dataset necessary to replicate the results of this study can be accessed at Zenodo, http://doi.org/10.5281/zenodo.4917565 (51).