Significance
The idea of precipitous continent-wide population decline beginning ca. 1492 has long influenced ecological and social narratives of North America. We analyze the largest systematic dataset of mortality records (n = 33,715 individuals) yet compiled across North America coupling archaeological and historic data to evaluate the nature and timing of Indigenous depopulation in what is now called central California. Findings indicate that mortality risk consistent with a plague-like age-at-death structure began after sustained contact with European invaders ca. AD 1770, suggesting that major depopulation began relatively late in this and possibly other isolated regions of North America. Our analysis does not support a "rebound" in native flora and fauna as a consequence of hypothesized epidemics before the first colonial settlements.
Keywords: epidemics, paleodemography, Indigenous California
Abstract
Catastrophic decline of Indigenous populations in the Americas following European contact is one of the most severe demographic events in the history of humanity, but uncertainty persists about the timing and scale of the collapse, which has implications for not only Indigenous history but also the understanding of historical ecology. A long-standing hypothesis that a continent-wide pandemic broke out immediately upon the arrival of Spanish seafarers has been challenged in recent years by a model of regional epidemics erupting asynchronously, causing different rates of population decline in different areas. Some researchers have suggested that, in California, significant depopulation occurred during the first two centuries of the post-Columbus era, which led to a “rebound” in native flora and fauna by the time of sustained European contact after 1769. Here, we combine a comprehensive prehistoric osteological dataset (n = 10,256 individuals) with historic mission mortuary records (n = 23,459 individuals) that together span from 3050 cal BC to AD 1870 to systematically evaluate changes in mortality over time by constructing life tables and conducting survival analysis of age-at-death records. Results show that a dramatic shift in the shape of mortality risk consistent with a plague-like population structure began only after sustained contact with European invaders, when permanent Spanish settlements and missions were established ca. AD 1770. These declines reflect the syndemic effects of newly introduced diseases and the severe cultural disruption of Indigenous lifeways by the Spanish colonial system.
Catastrophic decline of Indigenous populations in the Americas following the arrival of Europeans is arguably one of the most severe demographic collapses in the history of humanity (1–8). While it is generally accepted that diseases from Eurasia and Africa played a significant role in depopulation, scholars have long debated many aspects of the Indigenous population decline, including its pace, timing, and the exact causes of mortality. A long-standing theory suggests that the high mortality rate was influenced most profoundly by a lack of immunity among Native Americans to newly introduced Afro-Eurasian diseases (the so-called “virgin soils theory”; 9–12), but it is increasingly recognized that germs alone do not provide a full explanation for the precipitous die-offs (13, 14). Perhaps more culpable was the cultural chaos that spread through the Americas following European contact that would have dramatically exacerbated the vulnerability of Indigenous populations (13, 14). Extreme social disruption (14), altered food regimes (15, 16), famine and food insecurity (17), escalating violence (18, 19), forced relocation, land expropriation, enslavement, and captive-taking (20) certainly amplified the deadly potential of new diseases while also increasing mortality independently.
Recognizing that introduced pathogens by themselves do not provide a full explanation for Indigenous depopulation, the timing, pace, and magnitude of the decline have long histories of debate. At the mid-twentieth century, scholars suggested that coast-to-coast disease dispersion began almost immediately following the arrival of Columbus and that the demographic collapse was the result of a pandemic or series of pandemics (2, 4, 7) that ultimately contributed to an underestimation of the true precontact Indigenous population of the Americas. Diseases were argued to have reached some regions, such as what is now known as California, before arrival of the Europeans themselves. The size of the precontact human population and timing of its decline also affect reconstructions of associated ecology used as conservation targets (21), as some suggest there was a rebound in endemic game animals coincident with the rapid decline of Indigenous populations after 1492 (22–24) as well as changes in fire regimes (25, 26), reforestation (27), and altered patterns of carbon sequestration (28, 29).
Recent research has questioned the evidentiary basis for early and extensive post-Columbian population decline because much of it consists of anecdotal and/or circumstantial historic accounts (30–32). A particularly influential bioarchaeological study (33) noted that Native populations were not living in a disease‐free environment prior to contact, that the arrival of Europeans did not initiate a sudden pandemic, and that epidemic diseases probably struck different populations at different times. Similar findings concerning health and resiliency were ascribed to Canadian Indigenous populations where people appeared to suffer severe epidemiological impacts only following sustained contact with Europeans (34). More recent regional studies in the east (e.g., refs. 35 and 36), southeast (37), and southwest (38) also report evidence for severe Native population decline mostly after the establishment of an enduring European presence. In northwestern North America, historic studies on the Columbia River (39) report disease-induced population decline only around the late eighteenth century after Spanish missions were established in California. Subsequently, a continent-wide spatial meta-analysis of archaeological and historic evidence for the timing of disease spread and population decline found that while most populations experienced significant losses from disease only after sustained contact with Europeans, there was evidence in some regions for impacts prior to sustained contact and that disease dispersal in North America is probably best characterized as a series of regional epidemics rather than a continent-wide pandemic (40). The meta-analysis had lighter coverage for western North America and did not include systematic records of mortality, which are ultimately required to fully understand the impact of the European invasion.
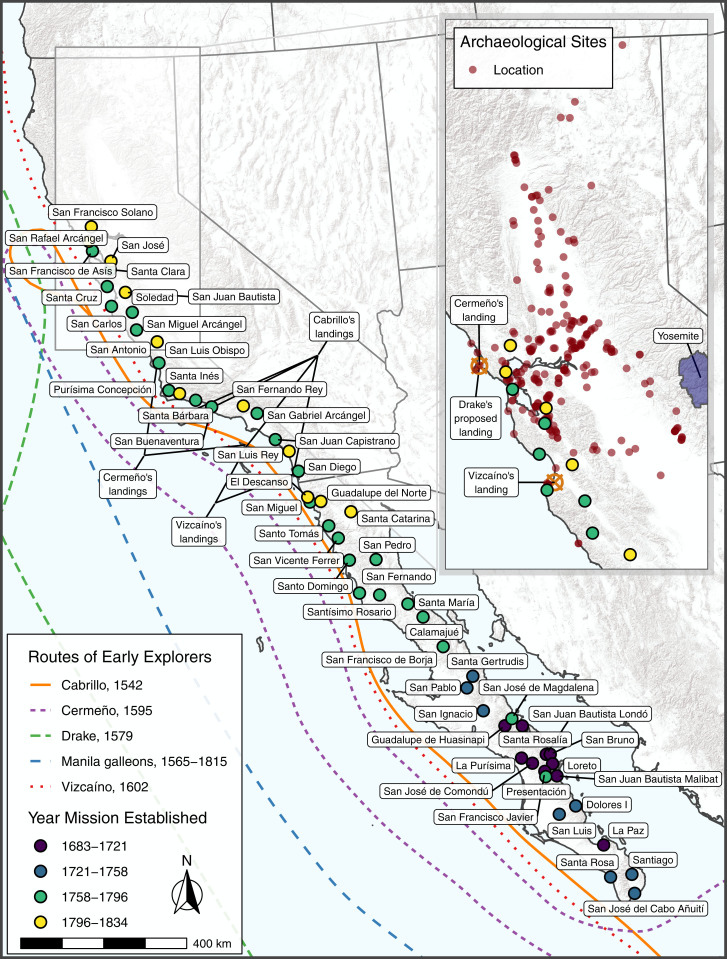
For California, a largely circumstantial case for severe disease-induced population decline beginning in the sixteenth century was advanced decades ago (41–44). Alternately, scholars have suggested that connections to Mexico via the Puebloan Southwest or contacts from European seafarers were sufficient to spread disease (42) and effect depopulation. Spanish explorer Juan Rodríguez Cabrillo made first contact with Native southern Californians by sea in 1542. After Cabrillo, there were four known European sea voyages before 1769 that included Native contacts (Fig. 1): de Unamuno in 1587, Cermeño in 1595, Vizcaíno in 1602 and 1603, and Francis Drake in 1579, although there may have been additional unrecorded, occasional contacts with Manila galleons that sailed along the California coast ca. 1566 to 1821. The Spanish also began establishing missions in southern Baja California in 1697 and were working their way northward, but the effort ended in 1767 with the establishment of Mission Santa Maria de Los Angeles, 340 km south of Alta California (Fig. 1). Sustained contact began in what is today California with the Portolá overland expedition in 1769 that made its way first to San Diego and eventually to San Francisco Bay. The expedition led to the establishment of Mission San Diego in 1769 and the first mission in central California (Mission San Carlos de Borromeo) in 1770.
Fig. 1.
Pre-1769 routes of colonial contact and California missions. (Inset) Northern California archaeological sites and Spanish missions in current study.
While it is possible that some infectious diseases were introduced into California as a result of pre-1769 contacts, the likelihood that they precipitated a radical reduction in Indigenous populations has proven difficult to test with bioarchaeological or other empirical evidence largely because the diseases thought most responsible for mortality do not leave an enduring skeletal signature (15, 45). A comprehensive archaeological study of the Yosemite Valley in central California (Fig. 1) that examined a variety of proxy data found evidence for disease-induced population decline prior to direct interaction between Indigenous and nonnative people but dating 1790 to 1800, decades after Spanish missions were established in the Coast Ranges 200 km to the west (46). Such a late date suggests that much of California may well have been relatively isolated from disease outbreaks during the sixteenth through eighteenth centuries.
Here, we attempt to systematically evaluate the impacts of pre-1769 diseases in order to determine if such a late date for initial depopulation applies to the whole of central California. We analyzed the single largest systematic dataset of mortality records yet compiled across North America that couples archaeological and historic data in order to contribute to an overarching portrait of regional epidemics effecting decline among Indigenous populations after the establishment of enduring European settlements. We systematically evaluated the impact of pre-1769 diseases by conducting survival analysis (refs. 47–49; SI Appendix) from age-at-death records of 33,715 Native people who lived on tribal lands within the area now referred to as central California between 5000 and 150 cal B.P. (AD 1870). These records come from 10,256 human burials from 252 archaeological sites (Datasets S1 and S2) and 23,459 historic records (Dataset S3) kept by Spanish missionaries at 10 central California missions (Fig. 1) dating between AD 1770 and 1825. We then compared the resulting survival curves, estimated mean age at death, and hazard ratios with historic plague populations (50) and simulated (51) records of stable and plague populations.
Results
Kaplan–Meier survival curves indicate that Indigenous populations exhibit a mortality profile resembling populations experiencing plague only after the permanent settlement of the Spanish and the establishment of their missions beginning AD 1770 (Fig. 2 and SI Appendix, Fig. S1 and Table S1). These results are consistent regardless of whether osteological cases are censored beyond age 50 to account for uncertainty in the age estimates of older individuals (SI Appendix, Figs. S2 and S3 and Tables S4 and S5).
Fig. 2.
Kaplan–Meier survival curves with confidence intervals (CIs) for all osteological (o) and mission (m) records relative to simulated (s) and archival (a) plague and stable populations. Osteological populations are censored at age 50. Uncensored model results reveal consistent results (SI Appendix, Fig. S2). Shaded regions illustrate the 99% CIs.
The calculated mean age at death for all pre-Mission populations resembles simulated stable attritional mortality, only overlapping with the 99% confidence intervals (CIs) of simulated and archival plague populations after AD 1770 (Fig. 3A and SI Appendix, Fig. S2A). The results of a Cox proportional hazards model confirm these findings, indicating that individuals only experienced hazards similar to a population exposed to the plague between AD 1770 and 1800 (P = 0.911), while all other periods differ significantly from a plague population (P < 0.0001, Fig. 3B and SI Appendix, Fig. S2B and Table S2).
Fig. 3.
Calculated mean age at death (A) and hazard ratio (B) with 99% CIs for each censored osteological (o) and mission (m) record relative to simulated (s) and archival (a) references. (A) Mean age at death derives from the Kaplan–Meier survival estimator with censored archaeological data (SI Appendix, Table S1). (B) Hazard ratio derives from a Cox proportional hazard model (SI Appendix, Table S2). Results hold when using uncensored osteological data (SI Appendix, Fig. S3 and Tables S4 and S5) and when examining median age at death (SI Appendix, Fig. S4).
To validate these results that evaluate mortality across different archives, we compared osteological data with mission records for a period of overlap (AD 1770 to 1870), which reveals the same median age-at-death estimates and overlapping hazards (SI Appendix, Figs. S4 and S5), indicating that the results are not driven by archive type.
Discussion
A comparison of pre- and postcontact age-at-death records does not support the long-standing circumstantial case for severe disease-induced Indigenous population decline in central California before AD 1770. The archaeological skeletal record shows an attritional age-at-death profile for 5,000 y of prehistory. Archaeological and historical mission death records show a shift toward an extreme plague-like profile after AD 1770. While there is anecdotal (15) evidence for unusual disease-related death in southern California within the memory of people within the mission system (slightly before AD 1770), as well as limited radiocarbon evidence for drop-off in population before AD 1770 (51, 52), our findings suggest that catastrophic decrease of Indigenous populations began in central California after the establishment of Spanish missions. Prior to that time, contacts with Europeans seem to have been too intermittent to initiate large-scale contagion (53), and diseases impacting eastern and southwestern North America at that time had not reached the west coast. If diseases did reach central California a decade or two before AD 1770, chances of surviving them would appear to have been much greater for Native people before they were subjected to conditions in the Spanish missions.
A variety of environmental, demographic, and social factors could have contributed to the delayed onset of disease. While central California had some of the highest documented Native population densities in North America (54, 55), it was bounded to the south and east by the deserts of Baja California and the Great Basin, where human populations were so low that they seem to have impeded the spread of disease. Dry climate may also have slowed the spread of disease northward from Mexico into the Puebloan area of the Southwest (40), and this effect would also have been in play in the deserts of southern California and Nevada. Furthermore, the Indigenous populations of California (prior to missionization) were probably also buffered from the spread of infectious disease by their autonomous political organization, which featured hundreds of independent, relatively small polities (32, 56) despite overall high population density.
Of paramount significance to the onset of major depopulation, however, were the social and living conditions imposed by the Spanish after AD 1770 that could not have been worse in their potential to encourage disease (16). Native people were settled into crowded communities adjacent to the missions, and as many died, the Spanish sought new recruits from further afield. Moreover, during the early years of the mission system, neophytes moved regularly between their traditional communities and the developing mission compounds, increasing, if not assuring, the likelihood that disease would be promulgated in the hinterlands away from the missions. The historic record also shows that a higher number of females (n = 13,166) than males (n = 10,293) died in the missions, suggesting that women especially suffered in these compounds. Because we could not determine sex for half of the age categories in the skeletal record, we did not evaluate that pattern further. Skeletal evidence using this same osteological database, however, indicates that violence increased during the mission period along with dietary stress (57). The net result was a staggering decline of Indigenous populations throughout the entire era of the Spanish occupation of central California that began with the establishment of the missions in AD 1770. An estimated population of 43,285 for the northern mission area at contact (58) was reduced to ca. 7,800 people in AD 1834, including mission residents and an estimated number of escapees (59).* For the entire northern and southern mission area combined, it is estimated that the Indigenous population was down to 15,000 in AD 1834 (60). Our findings support the idea of a patchwork disease-related depopulation for North America with many, but not all, regions seeing declines only at or shortly before a sustained European presence and the severe cultural, social, and economic disruption that went with it.
The late onset of diseases in California challenges historical ecological narratives that envision Native flora and fauna rebounding in response to reduced resource use by Indigenous populations (22–24, 42). Our results show that depopulation began coincident with permanent Spanish occupation, meaning there was no time for rebound prior to Spanish settlement. This is especially true when considering that the decline of Indigenous populations appears to coincide with the immediate ecological devastation wrought by the Spanish and other European settlers, followed by the subsequent invasion of gold seekers beginning in AD 1849. The period between AD 1770 and 1850 featured invasion of European livestock, agricultural plants, and weeds (61, 62) as well as climatic change associated with the tail end of the Little Ice Age. This is not to say that the decline in Indigenous populations had no perceptible ecological impact, especially in more isolated locations away from Spanish missions. For example, the frequency of controlled burning, a widespread Indigenous management practice noted repeatedly during the Portolá expedition in AD 1769 and by other early Spanish observers (63–66), would have declined precipitously, eventually leading to a buildup of dead fuel and greater potential for conflagration.
In line with this, evidence shows a doubling of the fire index in the Sierra Nevada for the period AD 1770 to 1865 over the previous 175 y (26). A similar pattern is documented for the American Southwest, where large wildfires in the Jemez Mountains followed declines in Indigenous populations after the expansion of Spanish missions post-AD 1600 (25). While evidence suggests that such changes in land use across North America may have also had significant global impact (29), the ecological effects of Indigenous depopulation were surely spatially and temporally variable. In central California, our findings suggest that rather than a period of rebound, the time between AD 1770 and 1850 might be perceived more as a time of ecological chaos with multiple large-scale forces at work, including Native depopulation and attempted cultural genocide associated with the colonial invasion.
Materials and Methods
Data Collection.
The skeletal record for this study was assembled by the second author (A.W.S.) from existing archaeological reports that include information on a total of 15,322 human burials excavated between 1900 and 2018 (Datasets S1 and S2 and Fig. 1). The burials are from 252 archaeological sites and date to six prehistoric periods between 5000 cal B.P. and AD 1770 and the historic period between AD 1770 and 1870; they include 1,376 burials that date to phase 2 of the Late Prehistoric (ca. AD 1510 to 1770), the period that some have suggested marked the onset of major depopulation (41, 42).
Temporal assignments for individual skeletons were based on artifact associations, obsidian–hydration values, radiocarbon dates, and stratigraphic position at archaeological sites with spatially and/or horizontally delineated temporal components. Radiocarbon dates were calibrated using CALIB Radiocarbon Calibration Version 7.1. (67). Dates from marine shells (including beads) were calibrated using the Marine 13 calibration curve with a local correction of 290 ± 35. Burials were assigned to one of seven time periods according to the most recent dating of the Central California Taxonomic System, which is based on directly dated, temporally diagnostic shell beads and ornaments (68) (SI Appendix, Table S3). Individual burials that could not be assigned to one of these periods were excluded from the study. This reduced the number of properly reported and temporally bracketed burials in the current study to 14,413 (Dataset S2).
Burial information was compiled from archaeological site reports, osteological appendixes, the Native American Graves Protection and Repatriation Act (NAGPRA) inventories, burial records, master’s theses, and doctoral dissertations (69). The ethical and legal circumstances surrounding the recovery of burials and burial-related information have changed considerably in California over the course of the twentieth and twenty-first centuries. Prior to the 1960s, burial recovery was undertaken almost exclusively by academic institutions with little, if any, consultation with living Native individuals. Burials were often recovered solely for academic purposes and were curated at academic institutions as well as state and federal repositories, which is counter to contemporary ethical standards of archaeological practice (70–72).
Beginning in the 1960s, the California Department of Parks and Recreation and other institutions began to limit burial recovery to cases where there was imminent threat of destruction and to include Native consultants in the on-site recovery process. Still, burials were usually not reinterred but were deposited in state and federal curation facilities. The process of repatriating those remains to culturally affiliated Native groups began following the passage of NAGPRA in 1990 and is ongoing. In 1976, California’s state government established the Native American Heritage Commission (NAHC) and in 1982 passed legislation giving the NAHC authority to identify a Most Likely Descendant (MLD) when Native American human remains are discovered at any place other than a dedicated cemetery. The MLDs were granted the legal authority to “make recommendations regarding the treatment and disposition of discovered remains” (73). Over the last 39 y, the vast majority of excavations in central California that included burial recovery were overseen by at least one Native American monitor who cedes authority to an NAHC-designated MLD over the disposition and treatment of remains.
Today, excavation teams almost always include a field bioarchaeologist. Remains are very rarely removed unless there is an unavoidable threat of destruction, typically from a construction project. While in the ground and after recovery, analysis and postexcavation treatment are strictly controlled by the MLD, who typically consults with other members of their tribe or community. The methods, degree of analysis, and documentation of remains are dictated by the MLD and vary from one excavation to another, but in most cases, burial posture is recorded, along with presence or absence of grave goods, age, sex, and observable skeletal pathological conditions or anomalies as noted by the field bioarchaeologist or (more rarely) in postfield laboratory analysis. The final disposition of remains is dictated by the MLD. In the vast majority of cases, remains are reinterred. Data on burials are compiled as part of excavation reports that are archived at regional archaeological information centers overseen by the California Office of Historic Preservation. These reports are accessible to professional archaeologists and Native people; these sources provide the vast majority of information in the current database. Details on the sources of information for specific sites and individual burials, including the name and qualifications of the principal bioarcheologist who evaluated the remains and disposition of the remains, are provided (Dataset S2). Additional information and a complete background history of the database are available (69).
Archaeological skeletal data were aggregated to include three categories for sex: male, female, and indeterminate (due to inadequate skeletal markers or immature remains). Following standards outlined in Buikstra and Ubelaker (74), burials were assigned to one of nine age classes: Fetal (<birth), Infant (0 to 3 y), Child (4 to 12 y), Adolescent (13 to 18 y), Young adult (19 to 34 y), Middle adult (35 to 49 y), Old adult (50+ years), or broader categories for individuals who lacked more precise age markers (i.e., Adult [18+ years] and skeletally/dentally mature). In some cases, with older collections, age and sex data in the original reporting that were inadequate for this purpose were upgraded in more recent NAGPRA inventories or thesis research. In other cases, those for which no age-at-death data were available, or the available data were incompatible with the age and sex categories employed here, the remains were not included in the current analysis. As discussed below, this dataset was further reduced by eliminating the youngest age classes, resulting in a total of 10,256 individuals (Dataset S1).
Sex estimations were based on skeletal morphological features as described in bioarcheological reporting and not archaeological data or mortuary context. Because sex estimations could not be made for the three youngest age categories and many individuals in the older age categories, sex was not considered in the current analysis; however, these data are provided in Dataset S1.
Data on mortuary patterns from the postcontact Spanish era come from records kept at 10 central California Spanish missions whose Native populations once lived in areas that overlap with the archaeological burial record (Dataset S3): Missions in San Antonio, San Carlos de Borromeo, Santa Clara, Santa Cruz, San Francisco de Assisi, San Francisco Solano, San Juan Bautista, San Jose, San Rafael, and Soledad (Fig. 1). These missions were established between AD 1770 (San Carlos de Borromeo) and 1823 (San Francisco Solano). Franciscan missionaries regularly recorded an individual’s estimated or actual age, sex, ranchería (village) of origin, and other data at the time of baptism. Burial registers documented dates of death, so age at death can be determined for most individuals who entered the mission system. Prior studies have utilized such records for studying death rates and changing demographic profiles among mission Indian populations (e.g., refs. 75 and 76). Our current study used a subset of comprehensive mission register data collected and compiled by the late Randall Milliken as part of his career-long research regarding ethnohistory, ethnogeography, and population history of Indigenous central California. This work is on file at the Bancroft Library, University of California, Berkeley, in CD-ROM format that was donated by Milliken to the institution prior to his death (Dataset S3).
Dataset S3 comprises individuals born in their Native communities prior to moving to the missions and their children born at the missions who survived beyond 3 y of age. The age at death was determined by adding the years between baptism and death to the estimated age listed in the baptismal register. In rare instances where no age was given in original records, Milliken estimated age at baptism in his database and provided justification in associated notes. When death records were missing (e.g., at Mission Soledad, which lacks a burial register), Milliken likewise was able to estimate dates of death by determining when a widowed spouse remarried or through other inferences. Such instances were always documented in his notes. Our analysis involved grouping ages at death into the categories comparable to those used for archaeological mortuary populations and dropping the youngest age categories. To respect the descendants of individuals listed in the Spanish mission records, these data were anonymized. We eliminated names and references to relatives from Dataset S3.
Paleodemography.
We evaluated these records using methods from paleodemography, the demographic study of past populations deriving from archaeological assemblages (77). In order to avoid potential pitfalls of this approach (e.g., ref. 78), we considered sample size and potential biasing of skeletal samples in relation to “demographic nonstationarity, selective mortality, and hidden heterogeneity in risks” (79). All these factors have the potential to affect which individuals enter the skeletal assemblage at a certain age (80).
Here, we compared mortality patterns over time based on age-at-death estimates of skeletal remains and mission records relative to both simulated and archival-derived records of populations experiencing plague events. The comparison to plague populations offered an extreme case of high extrinsic mortality across all age classes (51); however, this is certainly not the only possible outcome of exposure to an infectious disease. Studies analyzing the demography of cemeteries dating to the Black Death in the United Kingdom have provided insights into the different ways in which exposure can impact mortality across age classes. Both Gowland and Chamberlain (81) and Margerison and Knüsel (82) found that the Black Death cemetery in London was more reflective of the living population, indicating a catastrophic death event that indiscriminately affected the population. Conversely, Waldron (83) and DeWitte (84) did not find large differences between the plague cemeteries and other attritional cemeteries, although DeWitte (84) was careful to point out the differences in approach of each study and the benefits of including additional data, such as more precise age estimates, indicators of stress, and the use of more sophisticated statistical analyses. Other factors, such as sex differences or variation in age-specific growth, also play an important role (85).
The population growth rate (r) exerts a strong effect on the mean age at death (86–88). In a stable population, changes in r actually affect the mean age at death more than changes in life expectancy. Increased r depresses the mean age at death by increasing the relative proportion of young individuals in the living population. As such, evidence of high infant mortality in a skeletal sample correlates to high fertility and population growth. Conversely, decreased r, as would be expected in a time of disease stress, increases the mean age at death by lowering the relative proportion of young individuals in the living population. This would counter some of the effects of epidemic events on the mean age at death, although Paine (50) showed that shifts in the age distribution of death during a virgin soil epidemic would overwhelm stable death patterns and significantly decrease the mean age at death.
Data Analysis.
All analyses were run in the R environment for statistical computing (89). The code necessary to reproduce the analysis from the supplementary data files Datasets S1 and S3 is available in Code S1 (i.e., the file named Dataset S4). Data include osteological/bioarcheological-derived age-at-death estimates, archival age-at-death estimates from mission records, simulated plague and stable populations (50), and archival data (49) on age-at-death records from individuals experiencing plague in AD 1630 Italy.
The mission records were aggregated to three time periods: AD 1770 to 1800 (early Mission period), AD 1800 to 1825 (late Mission period), and AD 1770 to 1870 to include the period of overlap with the final osteological/archeological record for comparison and validation. To avoid potential bias introduced by differing precision of age-at-death estimates across the various records, we assigned all age-at-death records (osteological and mission) to the following coarse-grained osteological categories: Fetal (<birth), Infant (0 to 3 y), Child (4 to 12 y), Adolescent (13 to 18 y), Adult (18 to 50+ years), Young adult (19 to 34 y), Middle adult (35 to 49 y), and Old adult (50+ years).
We excluded the youngest two age classes (i.e., fetal and infant) from analysis to mitigate against the long-standing concern that the archaeological record tends to be incomplete for the youngest age categories (90). We then constructed life tables for each time period using the mortAAR package in R (47). This approach estimates the “living” population from the age-at-death records by assuming a uniform probability that each individual derived from any of the 5-y age classes that span the range of the age-at-death estimate (i.e., between the “from” and “to” columns in Datasets S1 and S3).
These data were then converted to case-form and fit with a Kaplan–Meier survival function and Cox proportional hazard model for each time period using the survival package in R (48). From the Kaplan–Meier model fits, we calculated the restricted mean and median age at death with 99% CIs per time period to compare with the reference stable and plague populations. We took two alternative approaches to account for the difficulty in assigning precise age estimates to older osteological individuals. First, we censored cases estimated as older than 50 y of age in the main analysis, which is common in survival analysis when the timing of the event (death in this case) is unknown past the last observation point (age 50 in this case). Second, we also ran the analysis without censoring by evenly distributing mortality across age classes over 50 in order to check if the results were consistent.
The censored analysis does limit our ability to estimate the median age at death for each time period because this is calculated as the age at which the model fit crosses the 0.5 survival threshold, which does not occur for many of the censored populations. While means do not accurately represent central tendency for skewed distributions, the results still offer a relative comparison between time periods. From the Cox proportional hazard model, we calculated hazards and 99% CIs for each time period relative to the reference archival plague population from ref. 49. For validation, we repeated these analyses comparing the osteological and mission records from the period of overlap from 1770 to 1870.
Supplementary Material
Acknowledgments
We acknowledge that the region covered by this study is the traditional and ancestral home of multiple Native California Tribes who continue to engage with their homelands through culture, ceremony, and stewardship. Their ancestors suffered devastating losses from the massive cultural disruption wrought by European colonization, as the present study demonstrates. We recognize the debt owed to our late colleague Randall Milliken for his lifetime of work collecting and making available information derived from California’s mission records. Our analysis would not have been possible without being able to use a subset of his meticulously compiled database. We also thank David Nicholson and Kenneth Blake Vernon for preparation of Fig. 1, Ken Smith and L. Brock James for analytical advice, and Jill Gardner for editorial assistance.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. S.D. is a guest editor invited by the Editorial Board.
*The geographic area associated with the 1834 northern mission Indigenous neophyte population estimate (59) is larger (it includes the southern San Joaquin Valley) than the area used for the AD 1770 estimate (58); as such, the actual population decline in the AD 1770 northern mission area was probably even greater.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2024802118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Mooney J., The aboriginal population of America north of Mexico. Smithson. Misc. Collect. 7, 1–40 (1928). [Google Scholar]
- 2.Dobyns H. F., Estimating aboriginal American population: An appraisal of techniques with a new hemispheric estimate. Curr. Anthropol. 7, 395–416 (1966). [Google Scholar]
- 3.Crosby A. W., The Columbian Exchange: Biological and Cultural Consequences of 1492 (Greenwood, Westport, CT, 1972). [Google Scholar]
- 4.Dobyns H. F., Their Number Became Thinned: Native American Population Dynamics in Eastern North America (University of Tennessee Press, Knoxville, TN, 1983). [Google Scholar]
- 5.Ramenofsky A. F., Vectors of Death: The Archaeology of European Contact (University of New Mexico Press, Albuquerque, NM, 1987). [DOI] [PubMed] [Google Scholar]
- 6.Thornton R., American Indian Holocaust and Survival (University of Oklahoma Press, Norman, OK, 1987). [Google Scholar]
- 7.Dobyns H. F., Disease transfer at contact. Annu. Rev. Anthropol. 22, 273–291 (1993). [Google Scholar]
- 8.Stannard D. E., American Holocaust (Oxford University Press, 1992). [Google Scholar]
- 9.Crosby A. W., Virgin soil epidemics as a factor in the aboriginal depopulation in America. William Mary Q. 33, 289–299 (1976). [PubMed] [Google Scholar]
- 10.Jones D. S., “Death, uncertainty, and rhetoric” in Beyond Germs: Native Depopulation in North America, Cameron C. M., Kelton P., Swedlund A. C., Eds. (University of Arizona Press, 2015), pp. 16–48. [Google Scholar]
- 11.Diamond J., Guns, Germs, and Steel: The Fates of Human Societies (W. W. Norton, New York, 1997). [Google Scholar]
- 12.Mann C., 1491: New Revelations about the Americas Before Columbus (Alfred A. Knopf, 2005). [Google Scholar]
- 13.Jones D. S., Virgin soils revisited. William Mary Q. 60, 703–742 (2003). [Google Scholar]
- 14.Cameron C. M., Kelton P., Swedlund A. C., Eds., Beyond Germs: Native Depopulation in North America (University of Arizona Press, Tucson, AZ, 2015). [Google Scholar]
- 15.Walker P. L., Lambert P., DeNiro M. J., “The effects of European contact on the health of Alta California Indians” in Columbian Consequences, Thomas D. H., Ed. (Smithsonian Institution, Washington, DC, 1989), pp. 349–364. [Google Scholar]
- 16.Cook S. F., The Conflict between the California Indian and White Civilization (University of California, Berkeley, CA, 1976). [Google Scholar]
- 17.Larsen C. S., “Colonialism and decline in the American Southeast: The remarkable record of La Florida” in Beyond Germs: Native Depopulation in North America, Cameron C. M., Kelton P., Swedlund A. C., Eds. (University of Arizona Press, Tucson, AZ, 2015), pp. 74–98. [Google Scholar]
- 18.Schwitalla A. W., Jones T. L., Pilloud M. A., Codding B. F., Wiberg R. S., Violence among foragers: The bioarchaeological record from central California. J. Anthropol. Archaeol. 33, 66–83 (2014). [Google Scholar]
- 19.Martin D. L., “Beyond epidemics a bioarchaeological perspective on Pueblo- Spanish encounters in the American Southwest” in Beyond Germs: Native Depopulation in North America, Cameron C. M., Kelton P., Swedlund A. C., Eds. (University of Arizona Press, Tucson, AZ, 2015), pp. 99–118. [Google Scholar]
- 20.Cameron C. M., “The effects of warfare and captive- taking on Indigenous mortality in postcontact North America” in Beyond Germs: Native Depopulation in North America, Cameron C. M., Kelton P., Swedlund A. C., Eds. (University of Arizona Press, Tucson, AZ, 2015), pp. 174–196. [Google Scholar]
- 21.Rick T. C., Lockwood R., Integrating paleobiology, archeology, and history to inform biological conservation. Conserv. Biol. 27, 45–54 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Broughton J. M., Declines in mammalian foraging efficiency during the late Holocene, San Francisco Bay. J. Anthropol. Archaeol. 13, 371–401 (1994). [Google Scholar]
- 23.Broughton J. M., “Pre-Columbian human impact on California vertebrates: Evidence from old bones and implications for wilderness policy” in Wilderness and Political Ecology: Aboriginal Influences and the Original State of Nature, Kay C., Simmons R. T., Eds. (University of Utah, Salt Lake City, UT, 2002), pp. 44–71. [Google Scholar]
- 24.Fisher J. L., Protohistoric artiodactyl rebound and resource deintensification in northern California. J. Archaeol. Sci. Rep. 19, 420–429 (2018). [Google Scholar]
- 25.Liebmann M. J., et al., Native American depopulation, reforestation, and fire regimes in the southwest United States, 1492-1900 CE. Proc. Natl. Acad. Sci. U.S.A. 113, E696–E704 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor A. H., Trouet V., Skinner C. N., Stephens S., Socioecological transitions trigger fire regime shifts and modulate fire-climate interactions in the Sierra Nevada, USA, 1600-2015 CE. Proc. Natl. Acad. Sci. U.S.A. 113, 13684–13689 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abrams M. D., Nowacki G. J., Native Americans as active and passive promoters of mast and fruit trees in the eastern USA. Holocene 18, 1123–1137 (2008). [Google Scholar]
- 28.Ferretti D. F., et al., Unexpected changes to the global methane budget over the past 2000 years. Science 309, 1714–1717 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Koch A., Brierley C., Maslin M. M., Lewis S. L., Earth system impacts of the European arrival and great dying in the Americas after 1492. Quat. Sci. Rev. 207, 13–36 (2019). [Google Scholar]
- 30.Henige D. P., Numbers from Nowhere (University of Oklahoma, 1998). [Google Scholar]
- 31.Johnson J. R., The earliest European contacts with the Chumash Islanders. Mains’l Haul: A J. Pacific Maritime Hist. 47, 38–45 (2011). [Google Scholar]
- 32.Lightfoot K. G., Simmons W. S., Culture contact in protohistoric California: Social contexts of native and European encounters. J. Calif. Gt. Basin Anthropol. 20, 138–170 (1998). [Google Scholar]
- 33.Larsen C. S., In the wake of Columbus: Native population biology in the postcontact Americas. Yearb. Phys. Anthropol. 37, 109–154 (1994). [Google Scholar]
- 34.Waldram J. B., Herring D. A., Young T. K., Eds., Aboriginal Health in Canada: Historical, Cultural, and Epidemiological Perspectives (University of Toronto, 1995). [Google Scholar]
- 35.Jones E. E., Population history of the Onondaga and Oneida Iroquois, AD 1500-1700. Am. Antiq. 75, 387–407 (2010). [Google Scholar]
- 36.Snow D. R., Lanphear K. M., European contact and Indian depopulation in the Northeast: The timing of the first epidemics. Ethnohistory 35, 15–33 (1988). [Google Scholar]
- 37.Larsen C., Ruff C., Schoeninger M., Hutchinson D., “Population decline and extinction in La Florida” in Disease and Demography in the Americas, Verano J. W., Ubelaker D. H., Eds. (Smithsonian, 1992), pp. 25–39. [Google Scholar]
- 38.Stodder A. L. W., Martin D. L., “Health and disease in the Southwest before and after Spanish contact” in Disease and Demography in the Americas, Verano J. W., Ubelaker D. H., Eds. (Smithsonian, Washington, DC, 1992), pp. 55–74. [Google Scholar]
- 39.Campbell S. K., Postcolumbian Culture History in the Northern Columbian Plateau, A.D. 1500-1900 (Garland, New York, 1990). [Google Scholar]
- 40.Jones E. E., Spatiotemporal analysis of Old World diseases in North America, A.D. 1519-1807. Am. Antiq. 79, 487–506 (2014). [Google Scholar]
- 41.Chartkoff J. L., Chartkoff K. K., The Archaeology of California (Stanford, Palo Alto, 1984). [Google Scholar]
- 42.Preston W., Serpent in Eden: Dispersal of foreign diseases into pre-Mission California. J. Calif. Gt. Basin Anthropol. 18, 2–37 (1996). [Google Scholar]
- 43.Erlandson J. M., Bartoy K., Cabrillo, the Chumash, and Old World diseases. J. Calif. Gt. Basin Anthropol. 17, 153–173 (1995). [Google Scholar]
- 44.Erlandson J. M., Bartoy K., Protohistoric California: Paradise or pandemic? Proc. Soc. California Archaeology 9, 304–309 (1996). [Google Scholar]
- 45.Ortner D. J., “Skeletal paleopathology: Probabilities, possibilities, and impossibilities” in Disease and Demography in the Americas, Verano J. W., Ubelaker D. H., Eds. (Smithsonian, Washington, DC, 1992), pp. 5–14. [Google Scholar]
- 46.Hull K. L., Pestilence and Persistence (University of California, Berkeley, CA, 2009). [Google Scholar]
- 47.Mueller-Scheessel N., et al., mortAAR: Analysis of Archaeological Mortality Data. https://cran.r-project.org/web/packages/mortAAR. Accessed 22 November 2020.
- 48.Therneau T. M., Grambsch P. M., Modeling Survival Data: Extending the Cox Model (Springer, New York, 2000). [Google Scholar]
- 49.Alfani G., Bonetti M., A survival analysis of the last great European plagues: The case of Nonantola (Northern Italy) in 1630. Popul. Stud. (Camb.) 73, 101–118 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Paine R. R., If a population crashes in prehistory, and there is no paleodemographer there to hear it, does it make a sound? Am. J. Phys. Anthropol. 112, 181–190 (2000). [DOI] [PubMed] [Google Scholar]
- 51.Erlandson J. M., Rick T. C., Kennett D. J., Walker P. L., Dates, demography, and disease: Cultural contacts and possible evidence for old world epidemics among the Protohistoric Island Chumash. Pac. Coast Archaeol. Soc. Q. 37, 11–26 (2001). [Google Scholar]
- 52.Scharlotta I., Determining temporal boundaries and land use patterns: Hunter-gatherer spatiotemporal patterning in San Diego County. Calif. Archaeol. 7, 205–244 (2015). [Google Scholar]
- 53.Hull K. L., “Quality of life: Native communities within and beyond the bounds of colonial Institutions in California” in Beyond Germs: Native Depopulation in North America, Cameron C. M., Kelton P., Swedlund A. C., Eds. (Univ. of Arizona, Tucson, AZ, 2015), pp. 222–248. [Google Scholar]
- 54.Baumhoff M. A., Ecological determinants of aboriginal California populations. Univ. Calif. Publ. Amer. Arch. and Ethno. 49, 155–336 (1963). [Google Scholar]
- 55.Ubelaker D. H., “Population size, contact to nadir” in Handbook of North American Indians 3, Ubelaker D. H., Ed. (Smithsonian Institution, Washington, DC, 2006), pp. 694–701. [Google Scholar]
- 56.Kroeber A. L., Nature of the land-holding group. Ethnohistory 2, 303–314 (1955). [Google Scholar]
- 57.Pilloud M. A., Schwitalla A. W., Broehl K. A., “Biological and cultural adaptations to climate change in prehistoric central California” in The Routledge Handbook of the Bioarchaeology of Climate and Environmental Change, Schug G. R., Ed. (Routledge, 2020), pp. 316–331. [Google Scholar]
- 58.Milliken R., The Central California Ethnographic Community Distribution Model, Version 2.0, with Special Attention to the San Francisco Bay Area (Office of Cultural Resources, California Department of Transportation, Oakland, CA, 2006). [Google Scholar]
- 59.Jackson R. H., Indian Population Decline: The Missions of Northwestern New Spain, 1687-1840 (University of New Mexico, Albuquerque, NM, 1994). [Google Scholar]
- 60.Hornbeck D., California Patterns: A Geographical and Historical Atlas (Mayfield, Palo Alto, CA, 1983). [Google Scholar]
- 61.Burcham L. T., California Range Land (California Division of Forestry, Sacramento, CA, 1957). [Google Scholar]
- 62.Hackel S. W., Children of Coyote, Missionaries of Saint Francis: Indian-Spanish Relations in Colonial California, 1769-1850 (UNC Press, Chapel Hill, NC, 2005). [Google Scholar]
- 63.Brown A. K., A Description of Distant Roads: Original Journals of the First Expedition into California 1769–1770 by Juan Crespi (San Diego State University, 2001). [Google Scholar]
- 64.Lightfoot K. G., Cuthrell R. Q., Anthropogenic burning and the Anthropocene in late-Holocene California. Holocene 25, 1581–1587 (2015). [Google Scholar]
- 65.Lightfoot K. G., et al., Anthropogenic burning on the central California coast in late Holocene and early historical times: Findings, implications, and future directions. Calif. Archaeol. 5, 368–388 (2013). [Google Scholar]
- 66.Timbrook J., Johnson J. R., Earle D. D., Vegetation burning by the Chumash. J. Calif. Gt. Basin Anthropol. 4, 163–186 (1982). [Google Scholar]
- 67.Stuiver M., Reimer P. J., Reimer R., CALIB Radiocarbon Calibration (Version 7.1, University Washington, Quaternary Isotope Laboratory, 2016). http://calib.org. Accessed 15 December 2019.
- 68.Groza R. G., Rosenthal J., Southon J., Milliken R., A refined shell bead chronology for late Holocene central California. J. Calif. Gt. Basin Anthropol. 31, 135–154 (2011). [Google Scholar]
- 69.Society for California Archaeology , Bylaws. https://scahome.org/aboutus/bylaws/. Accessed 7 February 2021.
- 70.Statement Concerning the Treatment of Human Remains. Society for American Archaeology, https://www.saa.org/career-practice/ethics-in-professionalarchaeology/. Accessed 7 February 2021.
- 71.Code of Conduct. Register of Professional Archaeologists, https://rpanet.org/code-and-standards. Accessed 7 February 2021.
- 72.About the Native American Heritage Commission . California Native American Heritage Commission, http://nahc.ca.gov/. Accessed 11 February 2021.
- 73.Schwitalla A. W., Global Warming in California: A Lesson from the Medieval Climatic Anomaly (A.D. 800–1350) (Center for Archaeological Research at Davis Publication No. 17, University of California, Davis, CA, 2013). [Google Scholar]
- 74.Buikstra J. E., Ubelaker D. H., Eds., Standards for Data Collection from Human Skeletal Remains (Arkansas Archeological Survey Research Series, Fayetteville, AR, 1994). [Google Scholar]
- 75.Cook S. F., Borah W. W., Essays in Population History: Mexico and California (University of California, Berkeley, CA, 1979), vol. III. [Google Scholar]
- 76.Walker P. L., Johnson J. R., “For everything there is a season: Chumash Indian births, marriages, and deaths at the Alta California missions” in Human Biologists in the Archives: Demography, Health, Nutrition, and Genetics in Historical Populations, Herring D. A., Swedlund A. C., Eds. (Cambridge University Press, 2003), pp. 53–77. [Google Scholar]
- 77.Hoppa R. D., “Paleodemography: Looking back and thinking ahead” in Paleodemography: Age Distributions from Skeletal Samples, Hoppa R. D., Vaupel J. W., Eds. (Cambridge University Press, 2002), pp. 9–28. [Google Scholar]
- 78.Bocquet-Appel J.-P., Masset C., Farewell to paleodemography. J. Hum. Evol. 11, 321–333 (1982). [Google Scholar]
- 79.Wood J. W., Milner G. R., Harpending H., The osteological paradox: Problems of inferring prehistoric health from skeletal samples [and comments and reply]. Curr. Anthropol. 33, 343–370 (1992). [Google Scholar]
- 80.Milner G., Wood J., Boldsen J., “Advances in paleodemography” in Biological Anthropology of the Human Skeleton, Katzenberg M. A., Saunders S. R., Eds. (John Wiley & Sons, Hoboken, NJ, 2008), pp. 561–600. [Google Scholar]
- 81.Gowland R. L., Chamberlain A. T., Detecting plague: Palaeodemographic characterisation of a catastrophic death assemblage. Antiquity 79, 146–157 (2005). [Google Scholar]
- 82.Margerison B. J., Knüsel C. J., Paleodemographic comparison of a catastrophic and an attritional death assemblage. Am. J. Phys. Anthropol. 119, 134–143 (2002). [DOI] [PubMed] [Google Scholar]
- 83.Waldron H. A., Are plague pits of particular use to palaeoepidemiologists? Int. J. Epidemiol. 30, 104–108 (2001). [DOI] [PubMed] [Google Scholar]
- 84.Dewitte S. N., Age patterns of mortality during the Black Death in London, A.D. 1349–1350. J. Archaeol. Sci. 37, 3394–3400 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Godde K., Pasillas V., Sanchez A., Survival analysis of the Black Death: Social inequality of women and the perils of life and death in medieval London. Am. J. Phys. Anthropol. 173, 168–178 (2020). [DOI] [PubMed] [Google Scholar]
- 86.Sattenspiel L., Harpending H., Stable populations and skeletal age. Am. Antiq. 48, 489–498 (1983). [Google Scholar]
- 87.Milner G. R., Humpf D. A., Harpending H. C., Pattern matching of age-at-death distributions in paleodemographic analysis. Am. J. Phys. Anthropol. 80, 49–58 (1989). [DOI] [PubMed] [Google Scholar]
- 88.Paine R. R., Model life table fitting by maximum likelihood estimation: A procedure to reconstruct paleodemographic characteristics from skeletal age distributions. Am. J. Phys. Anthropol. 79, 51–61 (1989). [DOI] [PubMed] [Google Scholar]
- 89.R Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2019). [Google Scholar]
- 90.Walker P. L., Johnson J. R., Lambert P. M., Age and sex biases in the preservation of human skeletal remains. Am. J. Phys. Anthropol. 76, 183–188 (1988). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.