Significance
In complex biological systems, cells are constantly exposed to multiple physical and chemical stimuli. To decode such diverse inputs, cells use proteins embedded in the membrane to distinguish signal from noise and mount appropriate cellular responses. G protein-coupled receptors (GPCRs) represent the largest family of cell surface receptors in humans with more than 800 unique members. Here, we show that many GPCRs function as molecular coincidence detectors that couple proton (H+) binding to receptor agonism. Our findings provide insights into the fundamentals of GPCR signaling and avenues for exploring GPCR responses to coincident H+ signals generated by a variety of biological processes and pathological scenarios.
Keywords: GPCR, coincidence detection, proton gating, Boolean, acidosis
Abstract
The evolutionary expansion of G protein-coupled receptors (GPCRs) has produced a rich diversity of transmembrane sensors for many physical and chemical signals. In humans alone, over 800 GPCRs detect stimuli such as light, hormones, and metabolites to guide cellular decision-making primarily using intracellular G protein signaling networks. This diversity is further enriched by GPCRs that function as molecular sensors capable of discerning multiple inputs to transduce cues encoded in complex, context-dependent signals. Here, we show that many GPCRs are coincidence detectors that couple proton (H+) binding to GPCR signaling. Using a panel of 28 receptors covering 280 individual GPCR-Gα coupling combinations, we show that H+ gating both positively and negatively modulates GPCR signaling. Notably, these observations extend to all modes of GPCR pharmacology including ligand efficacy, potency, and cooperativity. Additionally, we show that GPCR antagonism and constitutive activity are regulated by H+ gating and report the discovery of an acid sensor, the adenosine A2a receptor, which can be activated solely by acidic pH. Together, these findings establish a paradigm for GPCR signaling, biology, and pharmacology applicable to acidified microenvironments such as endosomes, synapses, tumors, and ischemic vasculature.
Over hundreds of millions of years, eukaryotes have evolved vast sensor arrays of G protein-coupled receptors (GPCRs) (1). The size and functionality of this receptor superfamily stems primarily from the structural adaptability of its seven-transmembrane (7TM) core. The versatility of the 7TM architecture has facilitated an extensive diversification and expansion of species-specific GPCR repertoires that can detect thousands of physical and chemical signals. In humans, more than 800 GPCRs detect inputs such as light, neurotransmitters, metabolites, and protons (H+), the latter of which activate three receptors, GPR4, GPR65, and GPR68, in response to physiologic acidosis (2, 3). Outside of these three limited examples, the effect of pH on GPCR signaling and biology remains largely understudied.
Coincident signals are a well-recognized feature of neuronal and cellular communication (4, 5). By detecting simultaneous inputs, cells can filter signal from noise in complex chemical microenvironments to mount proper responses. As such, the combinatorial, spatial, and temporal nature of coincidence detection enables cells, tissues, and organs to integrate physiologic signals in a manner analogous to sensor array processing. At the molecular level, coincidence detection is mediated by proteins that sense distinct and simultaneous input signals, such as cofilin, which detects coincident phosphoinositide and proton signals to regulate actin filament dynamics (6, 7). Despite their abundance, similar roles for GPCRs as molecular coincidence detectors remain understudied and await exploration. Given that many GPCRs are regulated by multiple distinct inputs, it is likely this superfamily holds enormous potential for the discovery, understanding, engineering, and therapeutic utilization of molecular coincidence detection.
Several lines of evidence suggest that coincidence detection is hardwired into much of GPCR signaling biology. Recently, we have shown that acid-sensing GPCRs GPR4, GPR65, and GPR68 are molecular coincidence detectors of H+ and Na+ ions. In this case, H+ is an activator, and Na+ is a negative allosteric modulator of GPR4, GPR65, and GPR68 signaling (8). Additionally, GPR68 has recently been shown to integrate H+ and mechanical inputs in response to simultaneous extracellular acidification and membrane stretch (9). Other examples include the self-activation of the adhesion receptor GPR56 that is mediated by coincident collagen binding and shear force inputs (10) and input-specific synapse formation guided by the latrophilin-3 receptors and its coincident interactions with fibronectin leucine-rich repeat transmembrane proteins and teneurins (11). Here, we show that many other human GPCRs function as coincidence detectors that couple H+ binding to agonism, antagonism, and constitutive activity.
Results and Discussion
Yeast Is an Ideal Model System for Studying H+-Gated Coincidence Detection by GPCRs.
As transmembrane receptors, GPCRs can simultaneously experience different pH environments established across lipid bilayers. Localizing pH effects to the GPCR–ligand interface thus requires a model system with static intracellular pH (pHi) independent of dynamic extracellular pH (pHe). Such a model would avoid conflating pHe effects on GPCR signaling with changes in pHi or pH-induced stress responses that affect processes such as intracellular signal transduction and cellular homeostasis. For example, we have previously shown that changes in pHi affect signaling by Gα subunits, the principal transducers of GPCR signals, and small Ras-like GTPases (12, 13). To circumvent such complications and ensure the interpretability of our results, we sought to identify a suitable cell model for studying H+ gating of GPCR signaling.
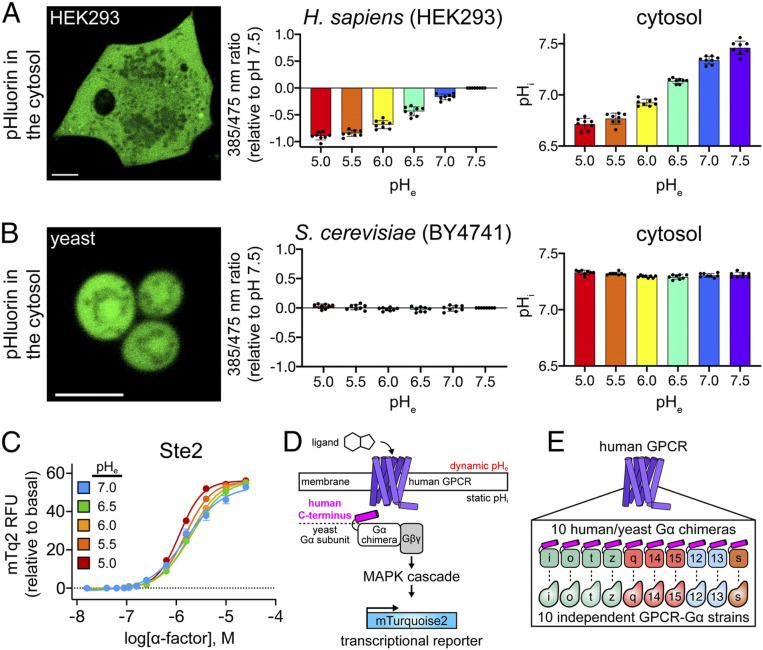
We began by testing the pH tolerance of human embryonic kidney cells (HEK293), the in vitro model most often used for cell-based GPCR assays. As shown in Fig. 1A, changes in pHe caused dramatic and unintended pHi alterations in HEK293 cells as reported by the pH biosensor pHluorin (14). This pH intolerance exhibited a sigmoidal shape centered at pH 6.25 and asymptotic pHi values that differed by almost 1 pH unit between high and low pHe. In stark contrast, we found that pHi in Saccharomyces cerevisiae, a yeast cell model used for studying human GPCRs (15–17), was independent of pHe (Fig. 1B). Based on this breakthrough, we concluded that the cross-species compatibility and pH tolerance of yeast was ideal for pH studies of human GPCRs. Having already developed a high-throughput platform for studying human GPCRs in yeast (17, 18), this insight provided us with an experimental framework for modeling biological scenarios in which only the GPCR–ligand interface is exposed to large pH changes, such as those that occur in acidified endosomes and tumor microenvironments.
Fig. 1.
Using yeast to study H+-gated coincidence detection by human GPCRs. Confocal images, wavelength ratios, and pHi values reported by the pH biosensor pHluorin in human embryonic kidney (HEK293) (A) and yeast cells (S. cerevisiae strain BY4741) (B) 10 min after pH treatment. Error bars represent SD of n = 8 biological replicates. (Scale bars, 5 μm.) pHluorin calibration experiments are provided in SI Appendix, Fig. S1. (C) The native yeast GPCR, Ste2, and its downstream pathway components are not affected by changes in pHe. Error bars represent SD of n = 4 experimental replicates. (D and E) Schematic summary of the DCyFIR platform. (D) In the DCyFIR platform, a human GPCR is coupled to a MAP kinase signaling cascade via a human/yeast C-terminal Gα chimera in which the last five residues of the native yeast Gα subunit, Gpa1, are replaced with the last five residues of a human Gα (pink helix). Upon activation, signaling by the genome-integrated human GPCR is quantified by the expression of a genome-integrated fluorescent transcriptional reporter mTurquoise2. (E) The DCyFIR platform covers all possible Gα coupling combinations for a given GPCR using 10 independent C-terminal Gα chimera strains (referred to as GPCR-Gα strains). Further details regarding the DCyFIR model can be found in Yeast DCyFIR Strains and Human Cell Lines.
Profiling H+-Gated GPCR Agonism.
The fundamentals of GPCR signaling are conserved from yeast to humans. In mammalian cells, many GPCRs transduce signals through complex networks of intracellular signaling pathways. In contrast, yeast have only one GPCR pathway, controlled by the native yeast receptor Ste2, that is largely insulated from signal crosstalk (19, 20). Unlike human cells, which transduce most GPCR signals through Gα subunits, Ste2 signals through the Gβγ heterodimer to activate a downstream MAP kinase cascade and transcriptional response. As shown in Fig. 1C, these processes are collectively insensitive to pHe. As such, pH responses observed for human GPCRs in the yeast model cannot be attributed to pH-induced changes in the underlying Ste2 pathway.
As shown in Fig. 1D, we previously engineered a high-throughput platform for studying human GPCRs that uses the isolated Ste2 pathway: Dynamic Cyan Induction by Functional Integrated Receptors, DCyFIR (8, 17, 18, 21). In the DCyFIR platform, a human GPCR is coupled to a MAP kinase signaling cascade via a human/yeast C-terminal Gα chimera in which the last five residues of the native yeast Gα subunit, Gpa1, are replaced with the last five residues of a human Gα (Fig. 1D). Upon activation, signaling by the genome-integrated human GPCR is quantified by the expression of a genome-integrated fluorescent transcriptional reporter mTurquoise2 (mTq2) (17). As shown in Fig. 1E, the DCyFIR platform covers all possible Gα coupling combinations for a given GPCR using 10 independent C-terminal Gα chimera strains, hereafter referred to as GPCR-Gα strains (17). Combining our DCyFIR technology with the unique pH tolerance of yeast, we performed the foremost assessment of H+-gated coincidence detection by GPCRs by profiling the pH responses of 280 individual GPCR-Gα combinations, covering 28 unique receptors.
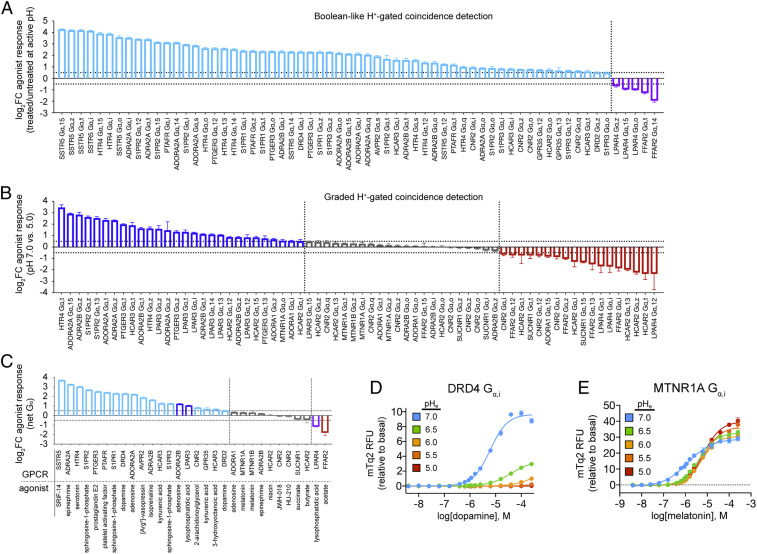
As shown in Fig. 2 A and B, we screened the set of 280 DCyFIR strains at pH 7 and 5, identifying 131 GPCR-Gα agonist responses. A total of 63 of these individual GPCR-Gα agonist responses exhibited signaling that was exclusive to pH 7 or 5. For example, the somatostatin receptor 5 (SSTR5) and all three sphingosine-1-phosphate receptors (S1PR1, S1PR2, and S1PR3) signaled only at pH 7. We characterized this all-or-nothing H+ gating as Boolean-like signaling behavior (Fig. 2A). In contrast, the remaining 68 individual GPCR-Gα agonist responses presented in Fig. 2B exhibited graded H+-gating behavior by signaling to some degree at both pH 7 and 5. For a small number of receptors, including free fatty acid receptor 2 (FFAR2) and lysophosphatidic acid receptor 4 (LPAR4), it was more challenging to quantify the effects of pH on agonism due to appreciable levels of constitutive activity. In these cases, constitutive activity had to be subtracted to observe and classify Boolean-like and graded agonist responses (SI Appendix, Fig. S2B). Considering both Boolean-like and graded sets, we found that 66% of agonist responses were greater at pH 7, 18% were greater at pH 5, and 16% were largely unaffected by pH (Fig. 2 A and B). Having selected agonists with pKa values outside of pH 7 and 5 (SI Appendix, Dataset S2), these effects were unlikely to be caused by changes in the agonist ionization state. Together, these findings show that signaling by many GPCR-Gα combinations is more likely to be greater at higher pH and dramatically diminished or turned off at lower pH values. However, in rare cases, we did find exceptions to this trend (e.g., FFAR2 and LPAR4 in Fig. 2 A and B).
Fig. 2.
H+-gated coincidence detection for 131 GPCR-Gα coupling combinations. Waterfall plots showing H+-gated agonist responses that exhibited Boolean-like (A) and graded (B) signaling behaviors between pH 7 and 5. For Boolean-like responses (A), differential agonism was quantified as the log2 fold change (log2FC) of the ratio of agonist-treated and untreated GPCR-Gα strains that signaled at high (cyan) or low (purple) pH. For graded responses (B), differential agonism was quantified as the log2FC of agonist responses between pH 7 and 5. GPCR-Gα strains that signaled more at pH 7, more at pH 5, or similarly at pH 7 and 5 are colored blue, red, and gray, respectively. Further details on the calculation of log2FC are available in Materials and Methods. (C) Waterfall plot showing the net Gα agonist responses for a given receptor using the ratio of summed activity for all agonist-active GPCR-Gα strains between pH 7 and 5. The color schemes used in A and B, and their associated classifications, are also used in C. (D) Boolean-like signaling behavior exhibited by dopamine receptor 4 (DRD4 Gα,i). (E) pH-insensitive GPCR signaling by melatonin receptor 1A (MTNR1A Gα,i). (A–C) Vertical dashed lines correspond to a log2FC of 0.5, and horizontal dashed lines separate examples that signaled more (log2FC ≥ 0.5) at pH 7 (blue/cyan bars) similarly (log2FC between ± 0.5) between both pH values (gray bars) or more (log2FC ≤ 0.5) at pH 5 (red/purple bars). Primary DCyFIR profiling data for A and B is provided in SI Appendix, Fig. S2. (A–E) Error bars represent SD of n = 4 experimental replicates. Further details regarding calculation of log2FC, experimental replicates, and experimental error are available in DCyFIR Profiling.
A major advantage of the yeast system is the ability to measure the pH responses of individual GPCR-Gα coupling combinations (Fig. 1 D and E). In most cases, we observed that the effects of pH on Gα coupling responses were similar for a given receptor and agonist. For example, all serotonin receptor 4 (HTR4) and LPAR4 Gα pairs were more active at pH 7 and 5, respectively. However, Gα coupling patterns were unexpectedly complex in several cases, such as niacin and butyrate agonism of hydroxycarboxylic acid receptor 2 (HCAR2) (Fig. 2 A and B and SI Appendix, Fig. S2). Whereas Gi/o coupling was generally stronger at low pH for niacin, Gq and G12/13 coupling favored high pH. In contrast, butyrate agonism through Gi/o and G13 generally favored low pH. These findings imply that H+ gating can be allosterically modulated by Gα coupling and that the net effects of H+ gating on GPCR agonism could depend on the Gα repertoire expressed in a given cell type.
Collectively, our DCyFIR profiling experiments demonstrate that H+-gated coincidence detection can lead to Boolean-like and graded GPCR signaling behaviors. As presented in Fig. 2 A–C, this observation is consistent for both individual GPCR-Gα pairs and net Gα agonist responses for a given GPCR. As shown in Fig. 2C, the net GPCR-Gα responses for 18 GPCR–agonist pairs exhibited Boolean-like behavior, with 17 signaling only at pH 7 and 1 only signaling at pH 5. A striking example of this type of H+-gated agonism is illustrated by pH titrations of dopamine receptor 4 (DRD4), for which signaling at pH 7 was switched off at pH 6 (Fig. 2D). Notably, of the 19 net GPCR-Gα responses more active at pH 7, only two exhibited graded behavior (ADORA2B and LPAR3) (Fig. 2C). In contrast, of the two net GPCR-Gα responses more active at pH 5 (LPAR4 and FFAR2), only LPAR4 exhibited Boolean-like signaling behavior. The remaining nine net GPCR-Gα responses in Fig. 2C were unaffected by pH as illustrated in Fig. 2E by pH titrations of the melatonin receptor 1A (MTNR1A). Together, these findings suggest that many GPCRs can respond to physiological pH changes with Boolean-like signaling behaviors that are switched on and off by high and low H+ concentrations.
Validating Boolean-Like versus Graded H+-Gated Coincidence Detection by GPCRs.
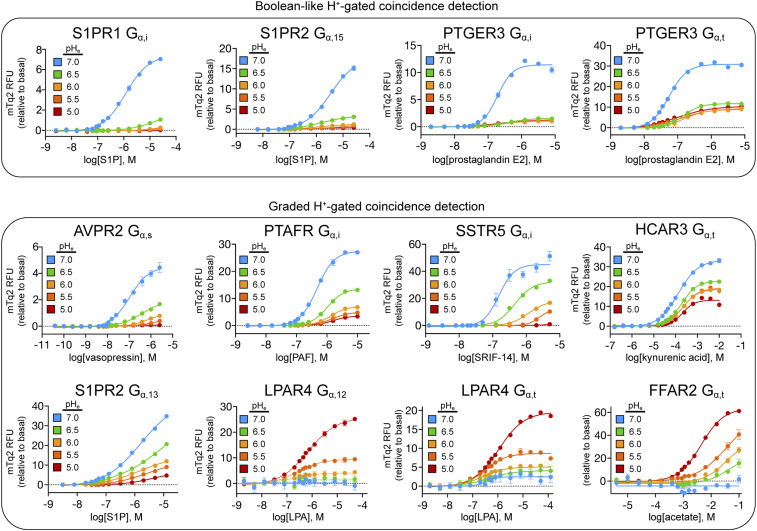
In addition to DRD4 and MTNR1A (Fig. 2 D and E), we next selected 21 additional GPCR-Gα strains for 125 follow-up pH titrations. As shown in Figs. 3 and 4 and SI Appendix, Fig. S3, we found these titrations to be in excellent agreement with the Boolean-like, graded, and pH-independent classifications in Fig. 2. For receptors that exhibited Boolean-like behaviors, such as sphingosine-1 phosphate receptor (S1PR1), arginine vasopressin receptor 2 (AVPR2), and prostaglandin E receptor 3 (PTGER3) (Fig. 3), full pH titrations confirmed that there is little to no signaling at pH 5 versus 7. However, at intermediate pH values, we found that S1PR1 signaled weakly (at pH 6.5) and AVPR2 and SSTR5 exhibited graded signaling responses. For receptors such as S1PR1 and AVPR2, which are known to signal from early endosomes (22–24), such findings imply that H+-gated coincidence detection will increasingly diminish or prevent GPCR signaling as endosomal pH decreases beyond ∼6.5. In our view, it is reasonable to speculate that other receptors capable of Boolean-like and graded signaling behaviors would respond similarly to other coincident H+ signals generated by a variety of biological processes and pathological conditions.
Fig. 3.
Representative pH titrations of H+-gated coincidence detection by GPCRs that exhibit Boolean-like and graded signaling behaviors. Select examples of Boolean-like and graded signaling behaviors by human GPCRs are shown. Titrations are the product of n = 4 independent experimental replicates with error bars representing the SD of each data point. Further details regarding the experimental setup, data processing, and experimental replicates are available in pH Titrations.
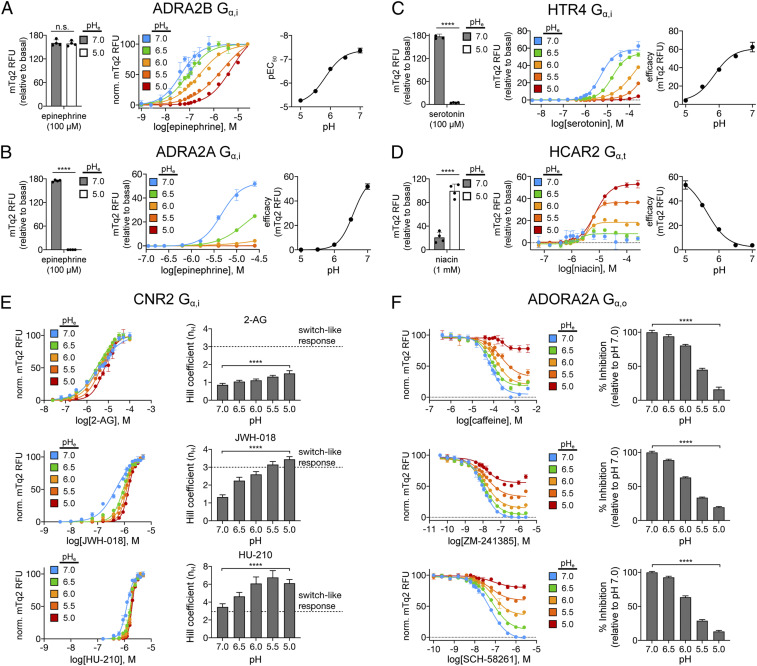
Fig. 4.
Pharmacological modalities of H+-gated coincidence detection by GPCRs. (A) Potentiation of agonist potency. Endpoint measurements of ADRA2B agonism (Left) at pH 7 (gray bars) and 5 (white bars). Epinephrine dose–response curves (Middle) and pEC50 values (Right) for ADRA2B. (B–D) Potentiation of agonist efficacy. Endpoint measurements (Left) of ADRA2A (B), HTR4 (C), and HCAR2 (D) agonism at pH 7 (gray bars) and 5 (white bars). Dose–response curves (Middle) and efficacy (Right) of each GPCR with its endogenous agonist. (E) Potentiation of sensitivity. 2-arachidonoylglycerol (2-AG) (Top), JWH-018 (Middle), and HU-210 (Bottom) dose–response curves (Left) and Hill coefficients (Right) for CNR2. (F) Potentiation of inhibition/antagonism. Caffeine (Top), ZM-241385 (Middle), and SCH-58261 (Bottom) dose–response curves (Left) and percent inhibition (Right) for ADORA2A in the presence of 10 μM adenosine. (A–F) Error bars represent SD of n = 4 experimental replicates. Statistical significance was calculated using a two-tailed Student’s t test (A–D) or Kruskal–Wallis followed by Dunnett’s multiple comparison test (E and F), ****P < 0.0001. Further details regarding the experimental setup, data processing, and experimental replicates are available in DCyFIR Profiling and pH Titrations.
The pH titration data in Figs. 2–4 and SI Appendix, Fig. S3 also serve to illustrate the underlying complexity of classifying Boolean-like versus graded GPCR signaling responses, as many receptors exhibit both behaviors depending on the pH range and agonist/inhibitor concentration considered. For example, PTGER3 exhibited both Boolean-like and weakly graded signaling behavior through Gi and Gt coupling, respectively (Fig. 3). However, between agonist concentrations of 10 and 100 nM, PTGER3 exhibited strictly Boolean-like signaling behavior through both Gα subunits. Similarly, the graded pH responses of AVPR2, SSTR5, and PTAFR were converted to Boolean-like responses at agonist concentrations between ∼10 to 100 nM, 50 to 100 nM, and 0.1 to 1 μM, respectively (Fig. 3). As such, in acidic scenarios such as maturing endosomes, tumor microenvironments, and inflammatory zones, we speculate that transitions from graded to Boolean-like GPCR signaling behaviors would depend on the magnitude change in pH and local concentrations of endogenous or synthetic agonists/inhibitors. Lastly, it is noteworthy that signaling by some GPCRs, such as MTNR1A and MTNR1B (Fig. 2E and SI Appendix, Fig. S3), appears unaffected by pH. As it stands, discriminating such receptors from their pH-sensitive counterparts a priori remains a formidable challenge, although we are beginning to make progress on this front (8, 12).
All Modes of GPCR Pharmacology Can Be Regulated by H+-Gated Coincidence Detection.
As shown in Fig. 4, we next investigated the effects of H+ gating on the major modes of GPCR pharmacology. Using a set of representative receptors and select Gα strains, we demonstrated that H+ inputs can modulate agonist potency and efficacy (Fig. 4 A–D) and receptor sensitivity and inhibition (Fig. 4 E and F, respectively). As shown in Fig. 4A, the α2B-adrenergic receptor (ADRA2B) appears to be insensitive to pH at high concentrations of the agonist epinephrine. However, pH has a profound effect on ADRA2B agonism at lower epinephrine concentrations, with increasingly lower pH values reducing epinephrine potency (pEC50 values) by >2 orders of magnitude (Fig. 4A). A consequence of these pH-dependent effects on epinephrine potency is that H+-gated agonism of ADRA2B exhibited Boolean-like behavior between pH 7 and 5 but only at concentrations between 10 and 1,000 nM. This finding further illustrates that Boolean-like H+ modulation of GPCR activity, or apparent lack thereof, can depend on agonist concentration.
In the case of ADRA2B, epinephrine elicits full agonist responses between pH 7 and 5 (Fig. 4A). As such, the set of ADRA2B dose–response curves all achieved full efficacy at high epinephrine concentration. In contrast, epinephrine agonism of the α2A-adrenergic receptor (ADRA2A) was weaker and only reached full efficacy at pH 7 (Fig. 4B). At lower pH, ADRA2A signaling did not achieve full efficacy (pH 6.5) or did not signal (pH ≤ 6). As a result, H+-gated ADRA2A agonism exhibited Boolean-like behavior between pH 7 and 6 at micromolar epinephrine concentrations. Similarly, agonism of serotonin receptor 4 (HTR4) achieved full efficacy at pH 6.5 and above (Fig. 4C), leading to Boolean-like H+ gating at micromolar serotonin concentrations. Lastly, as shown for hydroxycarboxylic acid receptor 2 (HCAR2), the effect of pH on agonism can be strictly limited to efficacy (Fig. 4D). In this case, niacin agonism of HCAR2 maintained the same pEC50 value at all pH values yet displayed a dramatic increase in HCAR2 efficacy with decreasing pH.
As illustrated in Fig. 4 E and F, H+-gated coincidence detection also extends to GPCR sensitization and inhibition. Fig. 4E shows how pH modulates cannabinoid receptor 2 (CNR2) responses to endogenous (2-arachidonoylglycerol, 2-AG) and synthetic (JWH-018 and HU-210) agonists. In the case of 2-AG, the sensitivity of CNR2 signaling was noncooperative and unaffected by pH as indicated by a near constant Hill coefficient (nH) between 0.9 to 1.5. In contrast, H+ gating of JWH-018 and HU-210 agonism elicited increasingly switch-like ultrasensitive responses (nH > 2) (25) as pH was decreased. As shown in Fig. 4F, we observed a similar trend in H+ gating of adenosine receptor A2A (ADORA2A) antagonism using the known competitive inhibitors caffeine, ZM-241385, and SCH-58261 in the presence of 10 μM adenosine. All three inhibitors were 50% effective at pH 6 and were almost completely ineffective at pH 5. Based on these findings, we concluded that the potential for H+ gating of GPCR pharmacology warrants far more attention in the development and evaluation of new and existing therapeutics. Our findings further suggest that as a principle of drug design, intentional H+ gating may find broad utility in the development of drugs that target GPCRs exclusively in acidic microenvironments.
Proton-Sensing GPCRs.
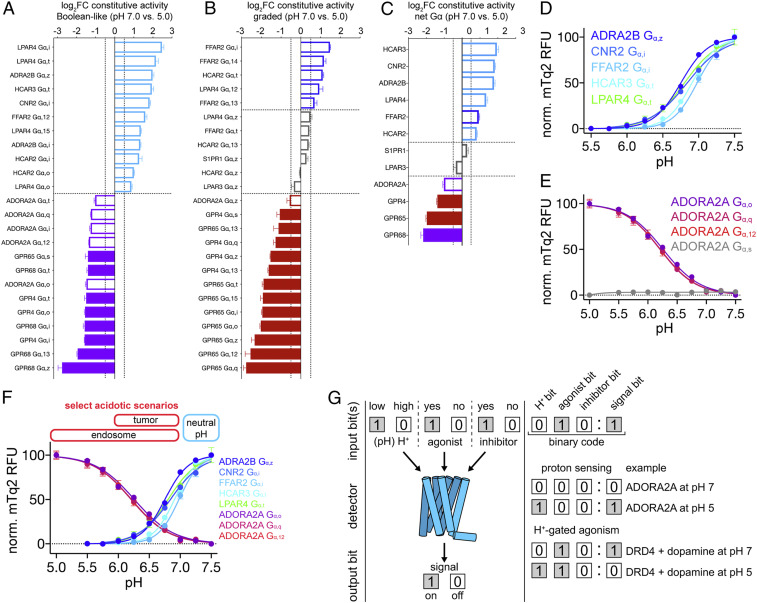
As shown by the waterfall plots in Fig. 5 A and B, 48 of the 280 individual GPCR-Gα strains screened at pH 7 and 5 were constitutively active, with 24 responses exhibiting Boolean-like behavior at pH 7 or 5 (Fig. 5A). For example, the known acid sensor GPR68 signaled exclusively below pH 7. The 24 remaining GPCR-Gα responses in Fig. 5B exhibited graded signaling behaviors between pH 7 and 5. Considering both Boolean-like and graded responses, we found that 33% of GPCR-Gα combinations signaled more at pH 7, 54% signaled more at pH 5, and 13% were largely unaffected by pH (Fig. 5 A and B). As shown in Fig. 5C, the net GPCR-Gα responses for the majority of constitutively active GPCRs (seven of 12) exhibited Boolean-like signaling behavior, with five being activated by high pH (HCAR3, CNR2, ADRA2B, LPAR4, and HCAR2) and two by low pH (GPR68 and ADORA2A). We next validated these Boolean-like signaling behaviors with detailed pH titrations.
Fig. 5.
New proton sensors and potential implications of Boolean-like H+-gated GPCR signaling. (A and B) Waterfall plots showing H+-gated GPCR-Gα constitutive activity that exhibited Boolean-like (A) and graded (B) signaling behaviors between pH 7 and 5. Differential signaling was quantified as the log2FC between pH 7 and 5. (C) Waterfall plot showing the net Gα responses for a given receptor using the ratio of summed mTq2 RFU for all constitutively active GPCR-Gα strains between pH 7 and 5, with Boolean-like responders colored cyan and purple, respectively. (D and E) New proton-sensing GPCRs inactivated (D) and activated (E) by low pH. (F) pH titrations of new proton-sensing GPCRs in the context of pH changes associated with select physiological processes and pathologies. (G) An illustration conceptualizing how H+-gated coincidence detection by Boolean-like responders (e.g., ADORA2A and DRD4) could lead to switch-like logic in GPCR signaling networks. (A–C) Vertical dashed lines correspond to a log2FC of 0.5, and horizontal dashed lines separate examples that signaled more (log2FC ≥ 0.5) at pH 7 (blue/cyan bars) similarly (log2FC between ± 0.5) between both pH values (gray bars) or more (log2FC ≤ 0.5) at pH 5 (red/purple bars). Filled bars correspond to strains of the known acid sensors (GPR4, GPR65, and GPR68). (A–F) Error bars represent SD of n = 4 experimental replicates. Further details regarding the calculation of log2FC, experimental replicates, and experimental error are available in DCyFIR Profiling and pH Titrations.
As shown in Fig. 5 D and E, we found excellent agreement between the Boolean-like classifications derived from our pH titrations and DCyFIR profiling experiments (Fig. 5 A–C). Furthermore, as reported in Fig. 5E, these pH titrations confirmed that the adenosine receptor A2a (ADORA2A) is activated by acidic pH in a manner strikingly similar to the known acid-activated proton sensor GPR68 (8). Furthermore, these pH profiles established that ADORA2A senses protons through the Gi/o, Gq, and G12/13 families of Gα subunits but did not indicate proton-sensing through the canonical ADORA2A Gα subunit Gα,s. However, we suspected that this was the result of the weak Gαs coupling we had previously reported in our yeast system (17). Informed by our earlier success of recovering and potentiating weak GPR68-Gα,q signaling by increasing receptor levels (18), we overexpressed ADORA2A from a high-copy yeast plasmid to confirm this hypothesis and demonstrate ADORA2A proton-sensing through Gα,s (SI Appendix, Fig. S4). Based on these findings, we concluded that ADORA2A is an acid-activated proton sensor capable of signaling through a variety of Gα subtypes.
H+-Gated GPCR Coincidence Detection Is an Avenue for Exploring GPCR Biology.
Our findings suggest that proton-sensing and H+-gated agonism are recurring features of GPCR signaling biology. Remarkably, in the subset of GPCRs we studied, the midpoint of proton-sensing and H+-gated responses (pH50 values) was tightly grouped within the physiologic pH range, having an average value of 6.25 ± 0.75 pH units (collectively for the pH titrations in Figs. 2–5). Using the acid sensor ADORA2A as an example, pH titrations through five ADORA2A-Gα combinations were in near-perfect agreement (Fig. 5E and SI Appendix, Fig. S4), all having pH50 values of 6.20 ± 0.03. This is similar to the pH50 of 6.35 ± 0.02 we previously reported for the known acid sensor GPR68 (8). Based on these findings, we can conclude that many but not all human GPCRs have evolved the ability to respond to physiologic pH changes between pH 6 and 7.
As shown in Fig. 5F, our findings further suggest that acid-sensing receptors such as ADORA2A and the known proton sensors (GPR4, GPR65, and GPR68) would be activated in acidic scenarios such as tumor microenvironments and maturing endosomes. Conversely, our data implies that acid-inactivated receptors (Fig. 5D), and most receptors that exhibit Boolean-like and/or graded H+-gated coincidence detection (Figs. 2–4), would be increasingly inhibited by decreasing pH. In the specific case of endosomal signaling, these insights suggest that luminal acidification may provide a coincident H+ signal that serves to gradually diminish (graded coincidence detection) or quench (Boolean-like coincidence detection) endosomal signaling by some internalized GPCRs, including S1PR1, AVPR2, and DRD4 (22–24, 26, 27).
In summary, we have shown that many human GPCRs are H+-gated coincidence detectors that are capable of Boolean-like and graded signaling responses. As illustrated for ADORA2A and DRD4 in Fig. 5G, our findings imply that to some degree, H+ gating of GPCRs might exhibit binary signaling behaviors within physiologically relevant pH ranges and agonist/inhibitor concentrations. Moving forward, we foresee that this concept of pH-regulated GPCR logic gating may be further developed, validated, and expanded to include a variety of additional inputs, such as allosteric modulators, and a variety of other GPCRs in organisms across the biological spectrum. As such, we believe that the technical and conceptual advances embodied in this work establish a paradigm for understanding and exploring the effects of pH on cell signaling that is likely to extend well beyond the GPCR superfamily.
Materials and Methods
Media.
All yeast strains were struck to yeast extract peptone dextrose (YPD) media plates (20 g/L peptone, 10 g/L yeast extract, 2% glucose, and 15 g/L agar) from 30% glycerol stock cultures stored at −80 °C. For all DCyFIR screen experiments and pH titrations, yeast were grown in low-fluorescence synthetic complete dropout media (SCD media; 50 mM potassium phosphate dibasic, 50 mM 2-(N-morpholino)ethanesulfonic acid [MES] hydrate, 5 g/L ammonium sulfate, 1.7 g/L yeast nitrogen base without amino acids, folic acid, and riboflavin [Formedium; CYN6505], 0.79 g/L complete amino acid mix [MP Biomedicals; 4500022], and 2% glucose) titrated to the desired pH using HCl or NaOH and filter sterilized. For yeast work using pHluorin to measure intracellular pH, yeast were selected for on plates lacking leucine and grown in synthetic defined media lacking leucine (5 g/L ammonium sulfate, 1.7 g/L yeast nitrogen base without amino acids [MP Biomedicals; 4510522], 1 NaOH pellet [VWR; BDH9292], 0.69 g/L CSM-LEU [amino acid mix lacking leucine], 2% glucose, and 15 g/L Bacto Agar for plates) that was filter sterilized. For pHluorin purification experiments, autoinduction media was prepared by combining 800 mL ZY media (10 g/L tryptone, 5 g/L yeast extract), 16 mL 50× 5052 (25% [wt/vol] glycerol, 2.5% [wt/vol] glucose, and 10% [wt/vol] α-lactose), 16 mL 50× M (1.25 M Na2HPO4, 1.25 M KH2PO4, 2.5 M NH4Cl, and 0.25 M Na2SO4), and 1.6 mL 1 M MgSO4. Phosphate-buffered saline (PBS) was made by first preparing solutions of mono- and dibasic potassium phosphate (100 mM potassium chloride and 25 mM potassium phosphate). Individual pH solutions of PBS (pH = 5.0 to 8.5, increments of 0.25) were prepared by mixing the mono- and dibasic solutions until the desired pH was reached, measured using an Accumet XL150 pH meter (Fisher Scientific). PBS-TCEP (tris(2-carboxyethyl)phosphine) was prepared as PBS (pH = 7.0) with 1 mM TCEP, 0.15 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM MgCl2, 1 mM ethylenediaminetetraacetic acid (EDTA), and 0.1% Triton, spiked with lysozyme and 10 μL deoxyribonuclease (DNase).
Plasmids.
Yeast plasmid pYEplac181-pHluorin was a gift from the Rajini Rao Laboratory at Johns Hopkins University. The pHluorin gene from pYEplac181-pHluorin was subcloned into a pLIC-His vector (pLIC-His-pHluorin) for recombinant overexpression and purification from Escherichia coli. Mammalian plasmid pcDNA3.1(+)-pHluorin was constructed by synthesizing and cloning a codon optimized sequence of pHluorin into the pcDNA3.1(+) backbone (GenScript). The yeast 2μ high-copy plasmid for overexpressing human ADORA2A (pYEplac181-ADORA2A) was created by subcloning ADORA2A into the pYEplac181 vector backbone using the NEBuilder HiFi DNA Assembly Kit (New England Biolabs, E2621S).
Yeast DCyFIR Strains and Human Cell Lines.
Yeast.
Yeast strains used in this work were described in our previous work (17). Briefly, the wild-type yeast strain BY4741 was modified using clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 editing to 1) delete the native yeast GPCR (ste2Δ) and negative regulators of the pheromone pathway (GTPase-activating protein sst2Δ and cell cycle arrest factor far1Δ); 2) install a CRISPR-addressable expression cassette into chromosome locus ×2 (×2 landing pad) that contained a constitutive promoter (PTEF), synthetic universal targeting sequence for CRISPR/Cas9 editing (i.e., knock-in of human GPCRs), and terminator (TCYC1B); and 3) install the mTurquoise2 transcriptional reporter in place of the pheromone-responsive Fig. 1 gene (fig1Δ::mTurquoise2). This base strain was then expanded to 10 GPCR-Gα strains using CRISPR/Cas9 editing to install human C termini (last five residues of each human Gα subunit) into the native yeast Gα subunit Gpa1. Lastly, human GPCR genes were integrated into the genomes of the 10 GPCR-Gα strains by installing them into the ×2 landing pad using high-throughput CRISPR/Cas9 editing. Refer to ref. 17 for further details and SI Appendix, Dataset S1 for a comprehensive list of all GPCR-Gα strains.
Mammalian.
HEK293T (HEK293T/17) cells were purchased from American Type Culture Collections and maintained in Dulbecco’s Modified Eagle Medium (DMEM; Thermo Fisher; 11995–065) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher; 10082–147) and 1% Penicillin-Streptomycin (Thermo Fisher; 15140–122).
Intracellular pH Measurements.
pHluorin purification.
Ratiometric pHluorin (14) was overexpressed in E. coli using autoinduction. First, 25 ng pLIC-His-pHluorin was transformed into competent BL21(DE3) RIPL cells. Transformants were grown overnight in 5 mL Luria broth + carbenicillin (100 μg/mL; Sigma-Aldrich; C1389). The next morning, the overnight culture was transferred into 800 mL autoinduction media (Media), grown at 37 °C with shaking (200 rpm) for 8 h, and then grown overnight at 18 °C with shaking (200 rpm). The 800-mL autoinduction culture was split over two 500-mL centrifuge bottles and cells were harvested by centrifugation at 4,500 rpm for 30 min. The supernatant was removed, and the pellet was resuspended in 35 mL PBS, transferred to 50-mL tubes, and stored at −20 °C. To purify overexpressed ratiometric pHluorin, cell pellets were thawed on ice and treated with TCEP, MgCl2, EDTA (all to 1 mM), PMSF (to 0.15 mM), Triton (to 0.1%), lysozyme, and 10 μL DNase and then lysed using a NanoDeBEE homogenizer (BEE International) at 30,000 psi. The cell lysate was transferred to a 50-mL tube and centrifuged at 15,000 rpm for 30 min. The supernatant was then transferred to a 15-mL tube and incubated with 1 mL His bead slurry (Sigma-Aldrich; P6611) for 20 min at 4 °C. The beads were harvested by centrifugation at 1,000 rpm for 1 min and then washed five times with PBS-TCEP (Media). The beads were then transferred to a 2-mL spin column (Thermo Scientific; 89896) and eluted with PBS-TCEP + 200 mM imidazole. The eluted protein was collected in a 15-mL tube by centrifugation at 1,000 rpm for 1 min, transferred to a slide-a-lyzer dialysis cassette (Thermo Scientific; 87730), and dialyzed overnight in 4 L PBS-TCEP at 4 °C. Purified pHluorin stocks were stored at −80 °C.
pHluorin calibration.
A pHluorin standard curve was calculated using purified ratiometric pHluorin. Briefly, 2 μL ratiometric pHluorin (100 μM) was resuspended in 198 μL PBS buffer titrated to each pH (Media), and 50-μL aliquots were moved into a 96-well microplate (CytoOne; CC7626-7596) in technical triplicate. Excitation spectra were collected using a ClarioStar microplate reader (BMG LabTech) with the following parameters: top read, 40 flashes/well, excitation start: 340 to 10 nm, excitation end: 495 to 10 nm, 1 nm steps; emission: 520 to 10 nm; instrument gain: 1,700. Raw fluorescence values at 385 and 475 nm were used to calculate the pHluorin ratio (385/475 nm) for each technical replicate. A standard curve was built by plotting the pHluorin ratio as a function of pH and fit (sigmoidal, 4PL, X is log[concentration]) using GraphPad Prism. Experimental error was calculated in GraphPad Prism as the SD of the mean for the n = 3 technical replicates.
Measuring yeast intracellular pH.
Yeast intracellular pH measurements were collected using the pH biosensor ratiometric pHluorin expressed in the cytosol from a high-copy yeast 2μ plasmid. A total of 150 ng plasmid pYEplac181-pHluorin was transformed into the wild-type yeast BY4741 using standard methods. Transformed cells were selected for synthetic defined plates lacking leucine (Media). Eight individual colonies expressing cytosolic pHluorin were grown to midlog phase in synthetic defined media lacking leucine. Cells were harvested and washed three times in SCD media titrated to various pH values (5.0, 5.5, 6.0, 6.5, 7.0, 7.5, and 8.0) and then transferred to a 384-well plate (Greiner; 781096). At 10 min post-pH treatment, spectral scans were collected using a ClarioStar microplate reader with the following parameters: top read, 40 flashes/well, excitation start: 340 to 10 nm, excitation end: 495 to 10 nm, 5 nm steps; dichroic: 430 nm; emission filter: 520 to 10 nm. pHluorin spectral scans were generated by subtracting the background fluorescence of media at each pH. pHluorin ratios were calculated from pHluorin spectral scans for each replicate by dividing the fluorescence at 385 nm by the fluorescence at 475 nm. Intracellular pH was quantified using the pHluorin ratios as previously described (12). Experimental error was calculated in GraphPad Prism as the SD of the mean for the n = 8 experimental replicates.
Measuring HEK293T intracellular pH.
HEK293T intracellular pH measurements were collected using the pH biosensor ratiometric pHluorin expressed in the cytosol from a transiently transfected pcDNA3.1(+) plasmid. Prior to transfection, HEK293T cells were seeded into 6-well plates (CytoOne; CC7682-7506) at a density of 700,000 to 800,000 cells per well. After 4 h, cells were transfected with 2 μg plasmid DNA (pcDNA3.1(+) empty vector; n = 1 well, or pcDNA3.1(+)-pHluorin; n = 8 wells) complexed with 6 μL TransIT-2020 transfection reagent (Mirus Bio; MIR 5404) at a final DNA concentration of 8 ng/uL in Advanced DMEM (Thermo Fisher; 12491–015). After 72 h, cells were detached using TrypLE (Thermo Fisher; 12604–013), resuspended in 1 mL media (DMEM; 10% FBS, 1% PenStrep), and transferred to a 1.5-mL tube. Cells were harvested by centrifugation (600 rpm, 3 min) and resuspended in 600 μL 1× Hanks’ Balanced Salt Solution (HBSS; 20 mM MES and 20 mM Hepes). A total of 100 μL cell suspension was transferred to six 1.5-mL tubes and harvested by centrifugation (600 rpm, 3 min). Cells were washed with 1× HBSS (20 mM MES and 20 mM Hepes) titrated to each pH (5.0, 5.5, 6.0, 6.5, 7.0, and 7.5) and transferred into a 384-well plate. At 10 min post-pH treatment, a spectral scan of each well was collected using a ClarioStar microplate reader using the settings described above in Measuring yeast intracellular pH. pHluorin spectral scans were generated by subtracting the background fluorescence of cells transfected with pcDNA3.1(+) from those transfected with pcDNA3.1(+)-pHluorin. pHluorin ratios were calculated from pHluorin spectral scans for each replicate by dividing the fluorescence at 385 nm by the fluorescence at 475 nm. Intracellular pH was quantified using the pHluorin ratios as previously described (12). Experimental error was calculated in GraphPad Prism as the SD of the mean for the n = 8 experimental replicates.
DCyFIR Profiling.
Production strain growths.
Each GPCR-Gα strain was struck from glycerol stock onto YPD plates and incubated for 2 d at 30 °C. A single colony was selected and used to inoculate 1 mL SCD media in a 96-well deep well plate. Cells were grown overnight without shaking at 30 °C to saturation. For each production run growth, cells were then back diluted to an optical density (OD)600 of 0.05 (pH 5.0) and 0.1 (pH 7.0), grown to midlog phase during the day, and back diluted into a fresh 96-well deep well block to an OD600 that would produce an OD600 value of 1.0 at a desired time the following day, which typically corresponded to an overnight growth at 30 °C for 16 to 18 h without shaking. The difference in starting OD600 between pH 7.0 and 5.0 is necessary because yeast grow slightly slower at pH 7.0 (doubling time 4 h) than pH 5.0 (doubling time 1.8 h).
Experimental setup, measurements, and replicates.
Following the production strain growth procedure described above, cells were harvested and normalized to a starting OD600 of 0.1. These normalized cultures were then distributed into a 384-well microplate in the following manner: per condition (vehicle, agonist, pH), 36 μL of each normalized GPCR-Gα culture was distributed to four wells of a 384-well plate to establish n = 4 independent experimental replicates and a total of 16 data points (four for each vehicle and each agonist treatment at pH 5.0 and 7.0). Each well was brought to a total volume of 40 μL by adding 4 μL vehicle or 10× agonist and grown overnight for 18 (pH 5.0) and 24 (pH 7.0) h until reaching an OD600 between 3 and 4. As mentioned above, this time difference is necessary because yeast grow slightly slower at pH 7.0. For a limited subset of receptors exhibiting high constitutive activity, it was necessary to grow the treated cultures for an additional 24 h to reach an OD600 between 3 and 4. As such, all DCyFIR profiling data reported in Figs. 2, 4, and 5 were obtained from OD600-matched cultures. GPCR signaling was quantified via the mTq2 transcriptional reporter by measuring the fluorescence of the OD-matched cultures using a ClarioStar microplate reader (bottom read, 10 flashes/well, excitation filter: 430 to 10 nm; dichroic filter: low pass (LP) 458 nm; emission filter: 482 to 16 nm; gain = 1,300).
Data collection, data processing, and experimental error.
We next calculated the log2 fold change (log2FC) between treated and untreated at each pH for all 10 possible GPCR-Gα coupling combinations per receptor. However, not every GPCR-Gα coupling combination was signaling competent. We removed this subset of GPCR-Gα strains from our analysis using a minimal relative florescence unit (RFU) cutoff of 25,000 for treated samples and a minimal log2FC cutoff of 0.5 at either pH tested. This process gave the subset of 131 agonist-responsive GPCR-Gα strains reported in Fig. 2. To stringently identify cases of constitutive activity, we used a minimal log2FC cutoff of 1.0 between untreated GPCR-Gα strains and their corresponding control DCyFIR strains lacking integrated receptors. This process identified the 48 constitutively active GPCR-Gα strains reported in Fig. 5. The values for each GPCR-Gα strain in the log2FC bar plots reported in Figs. 2 and 5 represent the mean log2FC values between pH 7.0 and 5.0 calculated in the following way: 1) log2FC values between pH 7.0 and 5.0 were calculated separately for each of the four paired experimental replicates, 2) the four resultant log2FC values were then averaged to give a single mean log2FC value, and 3) the error bars were calculated in GraphPad Prism as the SD of the mean log2FC value. This calculation is described by the equation
| [1] |
where F is mTq2 fluorescence and subscript rep,n corresponds to each independent experimental replicate (n = 4).
Classifying agonist-dependent Boolean-like behaviors.
A subset of agonist-treated GPCR-Gα strains exhibited Boolean-like behavior by signaling only at pH 7.0 or 5.0. In these cases, log2FC values could not be calculated between pH 7.0 and 5.0 in the manner described above. Instead, the log2FC calculation was restricted to the single pH value at which the GPCR-Gα strain signaled. As such, the process for calculating the log2FC values for Boolean-like behaviors in Figs. 2 and 5 were modified accordingly: 1) the log2FC values between treated and untreated at pH 7.0 or 5.0 were calculated separately for each of the four paired experimental replicates, 2) the four resultant log2FC values were then averaged to give a single mean log2FC value, and 3) the error bars were calculated in GraphPad Prism as the SD of the mean log2FC value. In the case of a GPCR-Gα strain that signaled only at pH X (5 or 7), this calculation is described by the equation
| [2] |
where F is mTq2 fluorescence and subscript treated,rep,n and untreated,rep,n corresponds to each independent experimental replicate at the single pH X (n = 4).
Classifying agonist-independent Boolean-like behaviors.
A subset of GPCR-Gα strains exhibited Boolean-like constitutive activity by signaling only at pH 7.0 or 5.0. As such, the process for calculating the log2FC values for constitutively active Boolean-like behaviors in Figs. 2 and 5 were modified accordingly: 1) log2FC values between pH 7.0 and 5.0 were calculated separately for each of the four paired experimental replicates, 2) the four resultant log2FC values were then averaged to give a single mean log2FC value, and 3) the error bars were calculated in GraphPad Prism as the SD of the mean log2FC value. In the case of a GPCR-Gα strain that exhibited constitutive activity only at pH 5 or 7, this calculation is described by the equation
| [3] |
where F is mTq2 fluorescence and subscript untreated,rep,n corresponds to each independent experimental replicate at each pH (n = 4).
Calculating net GPCR-Gα responses.
For a given GPCR, the net agonist responses (Fig. 2C) or constitutive activity (Fig. 5C) were calculated by summing the log2FC values for those GPCR-Gα strains that signaled.
pH Titrations.
Production strain growths.
Each GPCR-Gα strain was struck from glycerol stock onto YPD plates and incubated for 2 d at 30 °C. For agonist-treated pH titrations, a single colony was selected and used to inoculate 5 mL SCD media in 50-mL conical tubes at pH 5.0, 5.5, 6.0, 6.5, and 7.0. For pH titrations of constitutive activity, a single colony was selected and used to inoculate 5 mL SCD media in 50-mL conical tubes at pH 5.0 or 7.0, depending upon whether the receptor was activated by high or low pH, respectively. For each production run growth, cells were then back diluted to an OD600 of 0.05 (for pH 5.0, 5.5, and 6.0 growths) or 0.1 (for pH 6.5 and 7.0 growths) in 5 mL each pH media in a 50-mL conical, grown with shaking at 30 °C to midlog phase during the day, and back diluted into 10 mL fresh pH media in a new 50-mL conical to produce an OD600 value of 1.0 at the desired time after overnight growth with shaking at 30 °C. The following day, cells were harvested by centrifugation, and stock cultures were prepared with OD600 values normalized in each pH media to 0.1 (for agonist-treated GPCR-Gα strains) or 0.5 (for constitutively active GPCR-Gα strains).
Experimental setup and replicates.
The pH stock cultures described above were distributed into a 384-well microplate in the following manner: per pH condition (agonist or no agonist in the case of constitutively active GPCR-Gα strains), 36 μL cells were distributed to four wells of a 384-well plate to establish n = 4 independent experimental replicates. Each well was brought to a total volume of 40 μL by adding 4 μL 10× agonist or media (for pH titrations of constitutively active GPCR-Gα strains). The microplates were covered with a breathable adhesive seal (Diversified Biotech; BERM-2000) and incubated at 30 °C without shaking. Incubation times are discussed below in Data collection, data processing, and experimental error. As such, each family of pH titrations for a given agonist- or inhibitor-treated GPCR-Gα strain occupied 320 microplate wells: 5 pH values × 16 agonist/inhibitor concentrations × 4 independent experimental replicates. Similarly, each family of pH titrations for a given constitutively active GPCR-Gα strain occupied 40 microplate wells: 10 pH values × 4 independent experimental replicates.
Data collection, data processing, and experimental error.
Using our previously described slope method protocol (8), fluorescence of the mTq2 transcriptional reporter was measured across the wells of each microplate at 0, 2, 4, 6, 8, 10, and 24 h using a ClarioStar microplate reader (bottom read, 10 flashes/well, excitation filter: 430 to10 nm; dichroic filter: LP 458 nm; emission filter: 482 to 16 nm; gain = 1,300). We have shown that the slope method approach enables us to 1) circumvent matching OD600 values at the different pH values over the course of an experiment and 2) robustly measure agonist or inhibitor responses in GPCR-Gα strains with constitutive activity (demonstrated both here and in ref. 8). The process of measuring mTq2 fluorescence at regular intervals over time generated thousands of data points. For each agonist or inhibitor-treated GPCR-Gα strain titration experiment, this process generated 2,240 data points: 320 microplate wells × 7 time points. For titrations of constitutively active GPCR-Gα strain titration experiments, this process generated 440 data points: 40 microplate wells × 11 time points. We processed this large amount of data by writing custom Python code that extracted and consolidated the mTq2 fluorescence values for a given microplate well over time. The linear kinetic mTq2 transcriptional reporter data for each well was then fit in GraphPad Prism to obtain a slope value corresponding to mTq2 RFU over OD600 at each time. For a given set of n = 4 replicates, these slope values were summed and averaged using the equation
| [4] |
where m is the slope, superscript well,n is the microplate well, and subscript rep,n corresponds to each independent experimental replicate at each pH (n = 4). The reported experimental error was calculated in GraphPad Prism as the SD of the mean for the four slope values.
For normalized data in Figs. 4 and 5, the mean of the four slope values were then normalized and fit using GraphPad Prism (log(agonist) versus response − variable slope [four parameters] with constraints: bottom = 0 and top = 100).
Confirming ADORA2A-Gαs signaling.
DCyFIR profiling and pH titrations of GPCR-Gα strains overexpressing ADORA2A were done using the same approaches described in DCyFIR Profiling and pH Titrations with the exception of the following modifications: 50 ng of the pYEplac181-ADORA2A vector was transformed into 1) the Gi and Gs base DCyFIR strains and 2) Gi and Gs ADORA2A DCyFIR strains (SI Appendix, Dataset S1). Transformed cells were plated and maintained in selective synthetic defined media lacking leucine (refer to Media for details).
TRUPATH assay.
Prior to transfection, HEK293T cells were seeded into 6-well plates (CytoOne; CC7682-7506) at a density of 700,000 to 800,000 cells per well. After 24 h,, cells were transfected with 2 μg plasmid DNA [donor control: 1.5 μg pcDNA3.1(+)/0.5 μg plasmid containing Gα-Rluc8; experimental condition: 0.5 μg of each plasmid containing the receptor, Gα-Rluc8, Gβ, and Gγ-GFP2 (28)] complexed with 6 μL TransIT-2020 transfection reagent (Mirus Bio; MIR 5404) at a final DNA concentration of 8 ng/uL in Advanced DMEM (Thermo Fisher; 12491–015). After 24 h, cells were washed with 1 mL warm Dulbecco’s PBS, detached using trituration, and transferred into 1.5-mL Eppendorf tubes. Cells were harvested by centrifugation at 600 rpm for 2 min and resuspended in 1.3 mL 1× HBSS (20 mM MES and 20 mM Hepes, pH = 7.4). For each titration replicate, 80 μL cells were transferred to eight wells of a black 96-well plate (Greiner; 655209) followed by 10 μL 50 μM coelenterazine 400a (NanoLight; 340–5) and 10 μL 10× ligand. After 5 min of equilibration, plates were read six times using a ClarioStar microplate reader with the following settings: top read, 1 s integration time, emission filters: 400 to 10 nm (Rluc8) and 520 to 10 nm (GFP2); gain = 3,500. BRET2 ratios were determined by calculating the ratio between GFP2 (520 to 10 nm) and Rluc8 (400 to 10 nm) emission for each independent replicate (n = 6 for both control and experimental conditions). netBRET2 values were then calculated as the difference between the mean experimental and mean control BRET2 ratios. Experimental error was reported as the SEM for n = 6 experimental replicates.
Confocal Microscopy.
Yeast.
Wild-type yeast transformed with pYEplac181-pHluorin were grown to midlog phase in media lacking leucine. Cells were normalized to an OD600 of 1.0 in low-fluorescence media (Media), transferred to a 1-cm square agar “pad” on a glass microscope slide (VWR; 16004–422), and covered with a glass coverslip (VWR; 48366–227). Fluorescence images were collected using an LSM800 confocal microscope (Zeiss) with the following parameters: pinhole 1.00 Airy unit (AU)/44 μm; laser wavelength 488 nm, 1.0% intensity; 495 nm excitation; 520 nm emission.
HEK293T.
Cells transfected with pcDNA3.1(+)-pHluorin were detached with TrypLE (Thermo Fisher; 12604–013) and transferred to confocal dishes (VWR; 75856–742) at a density of 300,000 cells/dish in a total of 3 mL media (DMEM; 10% FBS and 1% PenStrep). After 48 h, fluorescence images were collected using an LSM800 confocal microscope (Zeiss) with the following parameters: pinhole 0.67 AU/32 μm; laser wavelength 488 nm, 3.0% intensity; 495 nm excitation; 520 nm emission.
Supplementary Material
Acknowledgments
This work was supported by the NIH through the National Institute of General Medical Sciences (Grant R35GM119518) to D.G.I. We thank the Bryan Roth laboratory at the University of North Carolina, Chapel Hill, for providing the plasmids for the TRUPATH assay.
Footnotes
Competing interest statement: N.J.K., J.B.R., G.J.T., W.M.M., and D.G.I. have filed a patent application with the US Patent and Trademark Office related to this work.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2100171118/-/DCSupplemental.
Data Availability
All relevant data, protocols, results, and analyses are available in the main text and/or SI Appendix. All yeast strains associated with this work are available upon request.
Change History
July 9, 2021: The article text has been updated.
References
- 1.Strotmann R., et al., Evolution of GPCR: Change and continuity. Mol. Cell. Endocrinol. 331, 170–178 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Ludwig M. G., et al., Proton-sensing G-protein-coupled receptors. Nature 425, 93–98 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Wang J. Q., et al., TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J. Biol. Chem. 279, 45626–45633 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Carlton J. G., Cullen P. J., Coincidence detection in phosphoinositide signaling. Trends Cell Biol. 15, 540–547 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyashita T., et al., Mg(2+) block of Drosophila NMDA receptors is required for long-term memory formation and CREB-dependent gene expression. Neuron 74, 887–898 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schönichen A., Webb B. A., Jacobson M. P., Barber D. L., Considering protonation as a posttranslational modification regulating protein structure and function. Annu. Rev. Biophys. 42, 289–314 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frantz C., et al., Cofilin is a pH sensor for actin free barbed end formation: Role of phosphoinositide binding. J. Cell Biol. 183, 865–879 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowe J. B., Kapolka N. J., Taghon G. J., Morgan W. M., Isom D. G., The evolution and mechanism of GPCR proton sensing. J. Biol. Chem. 296, 100167 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei W. C., et al., Coincidence detection of membrane stretch and extracellular pH by the proton-sensing receptor OGR1 (GPR68). Curr. Biol. 28, 3815–3823.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Yeung J., et al., GPR56/ADGRG1 is a platelet collagen-responsive GPCR and hemostatic sensor of shear force. Proc. Natl. Acad. Sci. U.S.A. 117, 28275–28286 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sando R., Jiang X., Südhof T. C., Latrophilin GPCRs direct synapse specificity by coincident binding of FLRTs and teneurins. Science 363, eaav7969 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isom D. G., et al., Protons as second messenger regulators of G protein signaling. Mol. Cell 51, 531–538 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isom D. G., Sridharan V., Dohlman H. G., Regulation of Ras paralog thermostability by networks of buried ionizable groups. Biochemistry 55, 534–542 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miesenböck G., De Angelis D. A., Rothman J. E., Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195 (1998). [DOI] [PubMed] [Google Scholar]
- 15.Dowell S. J., Brown A. J., Yeast assays for G-protein-coupled receptors. Recept. Channels 8, 343–352 (2002). [PubMed] [Google Scholar]
- 16.Dowell S. J., Brown A. J., Yeast assays for G protein-coupled receptors. Methods Mol. Biol. 552, 213–229 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Kapolka N. J., et al., DCyFIR: A high-throughput CRISPR platform for multiplexed G protein-coupled receptor profiling and ligand discovery. Proc. Natl. Acad. Sci. U.S.A. 117, 13117–13126 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe J. B., Taghon G. J., Kapolka N. J., Morgan W. M., Isom D. G., CRISPR-addressable yeast strains with applications in human G protein-coupled receptor profiling and synthetic biology. J. Biol. Chem. 295, 8262–8271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bardwell L., A walk-through of the yeast mating pheromone response pathway. Peptides 26, 339–350 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Dohlman H. G., Pheromone signaling mechanisms in yeast: A prototypical sex machine. Science 306, 1508–1509 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Kapolka N. J., Isom D. G., HCAR3: An underexplored metabolite sensor. Nat. Rev. Drug Discov. 19, 745 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feinstein T. N., et al., Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J. Biol. Chem. 288, 27849–27860 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves P. M., Kang Y. L., Kirchhausen T., Endocytosis of ligand-activated sphingosine 1-phosphate receptor 1 mediated by the clathrin-pathway. Traffic 17, 40–52 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomsen A. R. B., et al., GPCR-G protein-β-arrestin super-complex mediates sustained G protein signaling. Cell 166, 907–919 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C. Y., Ferrell J. E. Jr, Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. U.S.A. 93, 10078–10083 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotowski S. J., Hopf F. W., Seif T., Bonci A., von Zastrow M., Endocytosis promotes rapid dopaminergic signaling. Neuron 71, 278–290 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y., et al., Identification of two functionally distinct endosomal recycling pathways for dopamine D2 receptor. J. Neurosci. 32, 7178–7190 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsen R. H. J., et al., TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome. Nat. Chem. Biol. 16, 841–849 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data, protocols, results, and analyses are available in the main text and/or SI Appendix. All yeast strains associated with this work are available upon request.