Abstract
Idiopathic Parkinson’s disease (iPD) is a movement disorder characterized by degeneration of dopaminergic neurons and aggregation of the protein α-synuclein. Patients with iPD vary in age of symptom onset, rate of progression, severity of motor and non-motor symptoms, and extent of central and peripheral inflammation. Genetic and environmental factors are believed to act synergistically in iPD pathogenesis. We propose that environmental factors (pesticides and infections) increase risk for iPD via the immune system and that the role of PD risk genes in immune cells is worthy of investigation. This review highlights the major PD-relevant genes expressed in immune cells and key environmental factors which activate immune cells and, alone or in combination with other factors, may contribute to iPD pathogenesis. By reviewing these interactions, we seek to enable future development of immunomodulatory approaches to prevent or delay onset of iPD.
Introduction
Mutations in Parkinson’s disease- (PD-) linked genes account for 3-5% of PD occurrences, indicating that the majority of PD is the result of a multifactorial pathogenic process [1] . Within some loci, both rare (causative) and common (risk) variants associated with PD have been identified, including SNCA and LRRK2. Several of the genes associated with PD risk function in the immune system [2]. Peripheral immune activation as well as neuroinflammation in the brain have been documented in idiopathic PD (iPD) [3], and these are thought to contribute to neuropathology and ultimately neurodegeneration.
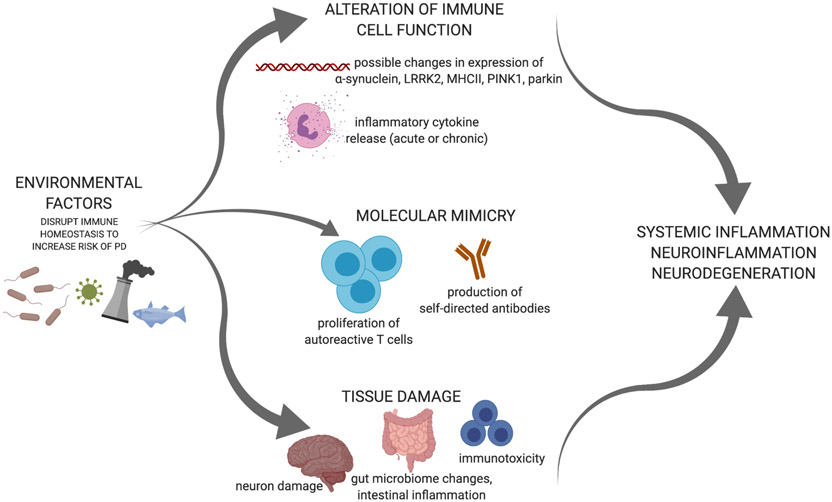
Sources of peripheral immune activation may include chemicals, viruses, and bacteria. Environmental factors may disrupt immune homeostasis via the process of molecular mimicry, by altering immune cell function, or by directly causing tissue damage that releases damage-associated molecular patterns (DAMPs) that are then recognized by immune cell pattern recognition receptors (Figure 1). Regardless of route, it is likely that the earliest pathology associated with iPD appears in the periphery, at least in some iPD patients. Specifically, a gut-driven model of iPD pathogenesis was first proposed by Braak and colleagues in 2003 where α-synuclein immunoreactivity was staged from the periphery to the brain [4]. This work has led to more in-depth hypotheses in which intestinal and systemic inflammation, alterations in the microbiota, gut and blood-brain barrier dysfunction, and impaired proteostasis may contribute to iPD pathogenesis. This highlights the importance of peripheral immune alterations in iPD due to environmental conditions [5].
Figure 1. Environmental factors disrupt immune homeostasis to increase risk for iPD.
Infections and pesticides are examples of environmental factors that can influence immune system function by altering immune cell gene expression and cytokine release. Environmental factors may drive autoreactive adaptive immune activation via molecular mimicry which could promote iPD pathogenesis. Environmental factors are also capable of directly damaging tissues. Created with BioRender.com.
Lack of appreciation for the complex mix of genetic variation and environmental exposures in each patient has resulted in the categorization of iPD patients as a uniform group, possibly contributing to the failure of all clinical interventions to modify disease progression to date. iPD patients are indeed a heterogenous group [6], and enriching trials for subjects with specific genetic risk factors and select environmental exposures could bolster the probability of identifying more successful disease-modifying therapies.
ALPHA-SYNUCLEIN
Intracellular aggregation of α-synuclein and loss of neuromelanin-containing neurons in the substantia nigra pars compacta are the defining histopathological hallmarks of PD. Duplications, triplications, and pathogenic missense variants of the gene for α-synuclein, SNCA, cause PD, while several common genetic variants in SNCA increase iPD risk [7].
ALPHA SYNUCLEIN IN IMMUNE CELLS AND INTESTINAL TISSUE
Although studies of α-synuclein in PD have largely focused on neurons, immune cells, specifically erythroid precursors and megakaryocytes in bone marrow and peripheral blood, express α-synuclein at high levels under basal conditions [8, 9]. While the precise function of α-synuclein in immune cells remains unclear, SNCA knockout mice have increased numbers of CD3+CD4−CD8− thymocytes and fewer single positive (CD4+ or CD8+) T cells [10]. Knockout of SNCA also affects IL-2 production by CD4+ T cells and the frequency of regulatory T cells (Tregs) [10]. These observations are consistent with the hypothesis that α-synuclein affects the affinity of T cell receptors (TCRs) for major histocompatibility complex (MHC) proteins that have been loaded with antigen, as Treg development results from high-affinity peptide-antigen binding. In the mechanism mediating the TCR:peptide-MHC connection, α-synuclein appears to function as it does in neurons. There, α-synuclein is a chaperone for the soluble N-ethylmalemide-sensitive factor attachment protein receptor (SNARE) complex and contributes to vesicle fusion with the plasma membrane [11, 12]. SNARE complexes are important for localizing TCRs, proper formation of the “immunological synapse” with peptide-MHC complexes, and the exocytic release of lytic granules in CD8+ T cells [13, 14]. Thus, disruptions in the SNARE-binding function of α-synuclein in T cells could conceivably have extensive effects on adaptive immune system response.
In addition to its presence within immune cells, α-synuclein protein is present in intestinal tissue, mucosa of the appendix, and cells of the enteric nervous system in non-PD and PD cases [15-17]. Multiple studies report that levels of α-synuclein in PD gut are higher than in the gut of non-PD controls [15, 18, 19]. In vitro and in vivo studies have suggested that microglia have the capacity to phagocytose certain species of α-synuclein as well as debris released by degenerating dopamine neurons [20-23]. Intraneuronal inclusions (Lewy bodies and neurites) are comprised of possible immunogenic antigens derived from α-synuclein or neuromelanin that become extracellular as neurons die, and, particularly if encountered by immune cells in the context of DAMPs released by the damaged neurons, these could serve as a stimulus for autoimmunity.
ALPHA SYNUCLEIN-INDUCED IMMUNE RESPONSES
Despite the fact that α-synuclein is expressed during development and is abundant in blood and serum, several forms of the protein or species derived from it have been observed to elicit an immune response featuring inflammation. Recent studies link chronic inflammation with failure to resolve early inflammation, a process performed by specialized pro-resolving mediators, including resolvins. Interestingly, rats overexpressing human α-synuclein display microglia activation and perturbations of inflammatory and pro-resolving mediators, namely IFN-γ and resolvin D1, in addition to neurodegeneration [24]. Innate immune cell activation and inflammation induced by α-synuclein could give rise to autoreactive T cells specific for α-synuclein or post-translationally modified or aggregated forms of this protein. In response to exposure to microglia activated by α-synuclein, murine nigra dopamine neurons up-regulate MHC class I, flagging themselves for cell killing mediated by antigen-specific CD8+ T cells [25]. If, as has been reported, human nigra neurons exprss MHC class I, it may be the case that α-synuclein can trigger a neuron auto-antigen presention [25].
In 2017, an initial report demonstrated that some T cells found in the peripheral blood mononuclear cell (PBMC) populations from iPD patients recognize α-synuclein-derived peptides [26]. Another study from the same group reported the presence of α-synuclein-specific T cells in PD patients and identified that T cell inflammatory cytokine release in response to α-synuclein can occur early in disease, around the time of PD diagnosis [27]. A major question for PD researchers in the future is to understand how such apparently autoreactive T cells develop in iPD. While α-synuclein is expressed beginning at least as early as embryonic day 9.5, it is possible that alterations in post-translational modifications with disease, such as phosphorylation, nitration, and/or aggregation may render the protein “foreign” to the immune system [28]. It has been hypothesized that loss of self-tolerance can be triggered by environmental factors such as injury or infection [29, 30] and loss of self-tolerance also occurs as a function of age [31]. α-Synuclein levels in humans have also been shown to increase in response to certain infections [32-34], and it is possible that, over time, repeated exposure of immune cells to α-synuclein in the context of damage and infection may support the development of autoimmune responses to it. Furthermore, increases in blood-brain barrier permeability produced by ongoing systemic inflammation [35, {Varatharaj, 2017 #282] may give immune cells increased access to epitopes shielded earlier in life. Subsequently, α-synuclein-derived epitopes would be loaded for antigen presentation into MHC proteins and presented to low affinity CD4+ T cells that evaded negative thymic selection, as has been hypothesized for other autoimmune disorders [36].
An individual’s propensity for autoimmune responses, expression level of α-synuclein, and alterations in the access of peripheral immune cells to synuclein-derived antigens will influence the extent to which α-synuclein contributes to iPD pathogenesis. Furthermore, it remains to be determined whether an autoreaction to α-synuclein plays a causal role in iPD pathogenesis or is a consequence of degeneration and protein aggregation initiated due to other factors. Regardless, it is likely that not all patients with iPD will benefit from therapeutic strategies that manipulate the response of the immune system to α-synuclein. Additionally, α-synuclein may interact with other genetic factors discussed below, such as leucine-rich repeat kinase 2 (LRRK2) and MHCII, as well as pathogens. The combination of abundant α-synuclein-derived antigen, increased capacity for antigen presentation via MHCII, as well as an environmental exposure that increases α-synuclein expression and/or inflammatory cytokine secretion (see below) could create a state in which dopaminergic neurons are vulnerable to immune-driven stress.
LEUCINE-RICH REPEAT KINASE 2
Mutations in LRRK2 cause the most common known monogenic form of PD and are autosomal dominantly inherited [37, 38]. LRRK2 functions in innate immune activation and could mediate T cell cytokine production [39-43]. Expression in leukocytes can be detected after stimulation with IFN-γ, and, to some extent, IFN-β, TNF, or IL-6 [43-45]. In response to inflammatory stimuli, LRRK2 expression is upregulated via a pathway mediated by JAK/STAT, ERK5, NFkB, and MAPK [46]. Peripheral monocytes and neutrophils express high levels of LRRK2 [47]. Recently, LRRK2 expression was shown to be strongly induced by immune signals, particularly IFN-γ, in human-derived induced pluripotent stem cell (iPSC) macrophages and microglia [48]. Importantly, LRRK2 is required for RAB8a and RAB10 recruitment to phagosomes, implying that LRRK2 operates at the intersection between phagosome maturation and recycling pathways in these phagocytes. Increased LRRK2 expression in peripheral blood immune cells of iPD subjects relative to controls has been reported [49], but the functional consequences of such increases are not known. B cells and T cells from iPD patients have higher expression of LRRK2 than healthy controls, independent of any known LRRK2 variation or mutation [50]. CD16+ non-classical monocytes, also called alternatively activated or patrolling monocytes, display higher LRRK2 expression in iPD patients than in controls as well [49]. Inflammatory cytokine secretion from monocytes is greater in iPD patients than in controls and correlates with LRRK2 expression in T cells in iPD patients (but not controls) [49]. These findings suggest that the function of LRRK2 in immune cells is altered in iPD and should be considered in research regarding the role of LRRK2 in iPD pathogenesis (reviewed in [51]). Furthermore, when assessing therapies to target LRRK2, it is important to consider that the function of LRRK2 is highly cell type-dependent and poorly understood.
LRRK2 MUTATION
The immunological impact of LRRK2 is evident in studies of the G2019S LRRK2 mutation. Patients who carry the G2019S mutation in LRRK2 can have increased inflammatory cytokines in sera, specifically IL-1β [52]. Inducing intestinal and systemic inflammation in G2019S rats dampens CD4+ T cell responses, especially Th17 response in the colon, brain, and blood [53]. This suggests that the increased kinase activity associated with the G2019S LRRK2 mutation may contribute to shifts in immune cell population frequencies and function.
LRRK2 AND ALPHA SYNUCLEIN
Additionally, recent studies have investigated the interaction between LRRK2 and α-synuclein, particularly in the context of clearance of α-synuclein by immune cells. LRRK2-deficient microglia and macrogphages have dampened cytokine release after exposure to α-synuclein fibrils, while LRRK2 knockout rats exhibit reduced activated microglia following viral vector-mediated α-synuclein expression in the substantia nigra [54, 55]. Furthermore, LRRK2-deficient cells more effectively internalize α-synuclein and in larger amounts relative to wildtype controls [56]. On the contrary, LRRK2 G2019S transgenic mice subjected to α-synuclein pre-formed fibril (PFF) injection in the striatum display increased α-synuclein aggregation, dopaminergic degeneration, and neuroinflammation relative to controls (non-transgenic mice injected with α-synuclein PFFs), indicating that the G2019S mutation has a functional impact distinct from LRRK2 deficiency [57]. G2019S knock-in mice expressing human A53T synuclein in the substantia nigra display significantly more dopaminergic loss and higher loads of pSer129 α-synuclein aggregates at 12 months of age relative to wildtype controls [56, 58]. It is hypothesized that LRRK2, via its kinase activity, alters the autophagy pathway, impairing degradation of α-synuclein and contributing to accumulation. More broadly, LRRK2 may shift phagocytic clearance associated with anti-inflammatory responses to damaging pro-inflammatory responses associated with IFNγ pathways. Future investigations need to explore this hypothesis in the context of immune cells particularly in the gut and periphery as the role of LRRK2 is highly likely to be cell- and context-specific.
LRRK2 IN IMMUNE CELL FUNCTION
LRRK2 has been linked to several infections by epidemiological studies and in animal and cellular models. Analysis of gene expression from tuberculosis infections identified LRRK2 as significantly enriched during active infections [59]. LRRK2 knockout mice show decreased Mycobacterium tuberculosis burden early in infection due to mitochondrial stress in macrophages [60, 61]. Phagosome maturation and bacterial control increases in human and mouse macrophages treated with LRRK2 kinase inhibitors, suggesting that dampened LRRK2 kinase activity is protective against MTB [60]. On the other hand, LRRK2 knockout mice infected with Salmonella typhimurium exhibit impaired clearance of pathogens due to inflammasome activation in macrophages [62]. A recent study suggested that wildtype LRRK2 is protective from S. typhimurium and reovirus infection in a sex-dependent manner with females exhibiting impaired ability to control infection [63]. These studies suggest LRRK2 expression changes in response to infection; however, future studies are needed to explore this relationship in humans as most of these studies have been conducted in rodent or cell models with sometimes conflicting results and interpretations. Activation of LRRK2 in the periphery, particularly in the gut, by infection or dysbiosis could contribute to PD pathogenesis.
LRRK2 AND SYSTEMIC INFLAMMATION
Inflammatory bowel disease (IBD) has been associated via several GWAS with the LRRK2 gene [64-69]. Specifically, the M2397T LRRK2 mutation has been associated with increased incidence of Crohn’s disease (CD) [70]. The newly identified N2081D variant in the LRRK2 gene has been shown to be associated with increased risk for both CD and PD while the N551K variant is associated with reduced risk for both diseases [64]. Studies in IBD mouse models based on dextran-sulfate administration have been difficult to interpret, with the LRRK2 knockout initially more susceptible to damage with involvement of the NFAT pathway [39], and in more recent studies, overexpression of LRRK2 was associated with more severe colitis [71]. Furthermore, LRRK2 mRNA is upregulated in inflamed CD tissue, in particular in the lamina propria, relative to uninflamed tissue from the same patient [43]. These associations and studies suggest that LRRK2 in immune cells in the gut may play a role in PD pathogenesis and need to be further investigated in iPD patients.
Given the hypothesis that iPD may begin in the periphery, the knowledge that LRRK2 expression is substantially higher in peripheral tissues than in the brain, and the observation that expression levels are increased in iPD patients, ongoing studies are currently targeting LRRK2 as a potential therapeutic agent. However, caution should be exercised in applying LRRK2 therapeutic interventions, as targeting LRRK2 may not be effective for all iPD patient subsets. Future investigations should examine LRRK2 expression in LRRK2 mutation carriers to identify whether expression is increased in PD patients independent of genotype. LRRK2 levels can also be assessed for correlation with disease progression, inflammatory perturbations (recent infections, etc.) and Unified Parkinson’s Disease Rating Scale scores to optimize potential therapeutic windows to delay or mitigate PD pathogenesis.
PINK1 and PARKIN
While LRRK2 is one of the major genetic contributors to autosomal dominant PD, PINK1 and Parkin contribute to autosomal recessive PD. PINK1 and Parkin protect cells against mitochondrial stress by regulating mitophagy. PINK1 is a kinase stabilized at the surface of damaged mitochondria where it phosphorylates both ubiquitin and Parkin, promoting the recruitment of mitophagy receptors to degrade dysfunctional mitochondria [72, 73]. Importantly, in the absence of Parkin or PINK1, high levels of mitochondrial antigens are presented on MHC class I molecules in both macrophages and dendritic cells by a vacuolar pathway distinct from mitophagy [74]. Mitochondrial antigen presentation (MitAP) is driven by the formation of mitochondria-derived vesicles (MDVs), a quality control mechanism allowing the shuttle of specific mitochondrial cargos to late endosomes [75]. PINK1 and Parkin actively inhibit MDV formation and MitAP, providing a link between mitochondrial dynamics and the potential engagement of autoimmune mechanisms in the etiology of PD.
Another study supports a role for PINK1- and parkin-mediated mitophagy in restraining innate immunity. Acute and chronic in-vivo mitochondrial stress were found to lead to a stimulator of interferon genes (STING)-meditated type I interferon response in mice in the absence of parkin or PINK1 [76]. Mitochondrial stress can lead to the release of DAMPs that can activate innate immunity, suggesting that mitophagy may mitigate inflammation. Loss of dopaminergic neurons and motor defects are rescued by loss of STING, suggesting that inflammation facilitates this phenotype [76]. Importantly, increased cytokine levels in serum of biallelic Parkin mutation carriers was found [76] and confirmed in both Parkin and PINK1 mutation carriers in larger patient cohorts (Borsche et al., Brain, in press). Therefore, it is hypothesized that parkin and PINK1 may protect against inflammation and neurodegeneration by clearing damaged mitochondria. Therapeutic interventions that enhance mitophagy, potentially via parkin and PINK1, may regulate inflammation in iPD. Although iPD has not yet been investigated in depth in terms of cellular immune mechanisms, recent findings through genome-wide association studies (GWAS) suggest PD-linked genetic targets implicated in immune response networks.
PD RISK GENES IMPLICATED IN IMMUNE FUNCTION
GWAS in PD have enhanced biological knowledge about the disease. In a recent meta-analysis of 17 datasets, a total of 90 independent genome-wide significant risk signals were identified across 78 genomic regions [7]. Implicating immune response in PD by GWAS, a previously reported association was found between PD and an MHCII haplotype present in 15% of the general population [78].
MAJOR HISTOCOMPATIBILITY COMPLEX CLASS II
Antigen presentation relies on MHC proteins class I and II. MHC proteins, encoded by human leukocyte antigen (HLA) genes, are expressed in a coordinated fashion following induction by cytokines or other immune activation signals. Several GWAS have identified single nucleotide polymorphisms (SNPs) in HLA genes as associated with incidence of PD, both variants protective from PD and those associated with susceptibility [78-84].
For example, it has been reported that, in some populations, the GG genotype at the SNP rs3129882 in the first intron of HLA-DRA is associated with increased risk of PD. It is not thought that this specific SNP directly drives the increase in MHCII expression and risk for PD, rather that the SNP is linked to a regulatory event or element(s) that controls MHCII expression at a long distance. The GG genotype at rs3129882 is associated with increased baseline expression of MHCII regardless of disease, as well as greater inducibility of MHCII on B cells and monocytes in PD [85]. On the basis of these findings and this interpretation, it was hypothesized that high expression of MHCII on brain myeloid cells is detrimental to dopamine neurons in the substantia nigra while diminished antigen presentation capacity should be neuroprotective. Consistent with this hypothesis, a study of globally MHCII-deficient mice reported that dopamine neurons are less susceptible to α-synuclein-induced neuroinflammation and degeneration [86]. In rats, increased numbers of MHCII+ cells in the substantia nigra following the overexpression of human α-synuclein is associated with greater dopamine neuron loss [2]. Thus, increased MHCII-dependent antigen presentation may promote PD pathology.
MHCII expression has been shown to synergize with certain environmental exposures, constituting a true gene-by-environment interaction that increases risk for PD. The rs3129882 GG genotype is associated with increased risk of PD in individuals who also have an exposure to pyrethroid pesticides [85]. Because both the GG SNP genotype and exposure to pyrethroids are common, these factors and their potential interactions may affect PD pathogenesis in a relatively large patient subpopulation. The mechanism by which pyrethroid exposure enhances risk for PD is not yet understood. Possible routes by which pyrethroid exposure may augment PD risk resulting from rs3129882 genotype include further increasing MHCII expression, increasing costimulatory molecule expression, driving sustained cytokine secretion, or increasing the proliferation rate of T cells, perhaps those activated by a PD-related antigen such as α-synuclein. Future work should explore the extent to which human immune cells from patients with or without PD and with or without the high risk MHCII SNP genotype respond to pyrethroid exposure.
Gene-environment interactions in PD have recently been reviewed elsewhere [87]. Genetic risk factors for PD may have different effects on the immune system depending on an individual’s environmental exposures. Figure 1 represents how the disruption of immune homeostasis through bacterial or viral infections or exposure to chemicals such as pyrethroid pesticides may change gene expression in immune cells and/or alter the type and amount of antigen in circulation. The effect of PD-related genes on immune system function has been reviewed above. We next discuss these environmental factors’ influence on the immune system.
ENVIRONMENTAL RISK FACTORS
Several environmental risk factors have been associated with PD. The environment can include, but certainly is not limited to, chemicals we encounter, nutrients, physical activities, psychological factors such as stress, infectious agents, the climate, and experiences emerging from our race, gender, or socioeconomic status. It is crucial to consider the biological processes downstream of an exposure when considering disease risk assessment. In response to an exposure or experience, physiological and epigenetic regulation can occur, and the resulting cell signaling cascades could influence disease pathogenesis [88]. Importantly, because neurons are long-lived, environmental exposures throughout the lifespan may influence their health. In the immune system, where there is ample cell turnover, early life exposures may still have long-lasting effects as the phenomenon of immunological memory forms a record of epitopes that have activated immune cells. It is also possible that the same factor encountered at different timepoints throughout the lifespan carries varying degrees of disease risk. Here we will specifically consider environmental exposures that elicit known processes within the immune system and are associated with PD, with the caveat that there surely exist many other factors yet to be identified.
PYRETHROID PESTICIDES
Epidemiological studies report a link between insecticide exposure and incidence of PD [89-91] . The pesticide cypermethrin is, globally, among the most commonly used agricultural and domestic insecticides, replacing insecticides such as organophosphates [92, 93]. Cypermethrin is a synthetic class II pyrethroid. Humans are primarily exposed to pyrethroids via skin contact, but ingestion through food and water consumption is also possible. This emphasizes the point that exposures are not only a hazard for those employed in pest control professions.
PYRETHROID TOXICITY
The mechanism of toxicity of cypermethrin and other pyrethroids in insects is to extend the opening of voltage-gated sodium channels, leading to depolarization of neurons and, thus, sustained excitation [94-96]. To what extent might cypermethrin and other pyrethroids act directly on immune cells via sodium ion channels? Immune cells express a variety of ion channels to maintain and regulate their negative membrane potential and the influx of divalent ions that act as second messengers (Ca2+, Mg2+). Human T cells are not widely thought to express voltage-gated sodium channels (Nav), though some cell line research suggests Nav1.5 expression by CD4+ T cells may play a role in positive selection [97-99]. Phagocytosis is regulated by Nav channels, specifically Nav1.5 and 1.6 in macrophages and 1.7 in human dendritic cells [100, 101].
Several studies have reported immunotoxic effects of pyrethroids on mouse splenocytes, thymic immune cells, and rat neutrophils [102-104]. Female mice given 1 dose of permethrin (1100 mg/kg, topically) exhibit a 32% decrease in spleen T cell and thymic cell proliferation relative to untreated mice [105]. Signs of apoptosis are detectable in CD8+ and CD4−CD8− thymocytes following permethrin treatment [105]. Oral administration of permethrin is associated with neutrophil abnormalities as well [104]. In mouse splenocytes or rat thymic cells, deltamethrin induces apoptosis [102, 106]. Cypermethrin has been shown to decrease plasma IL-2, IL-8, IL-12, and IFNγ [107]. Humans exposed to cypermethrin via their employment have a lower CD4/CD8 ratio, as well as changes in IL-12, p70, IL-2, IL-8, and IFNγ [108]. Thus, in thinking about the mechanism by which pyrethroids increase risk for PD, considerations should not be limited to neurons.
FUTURE DIRECTIONS IN PYRETHROID iPD STUDIES
Pyrethroid exposure has been shown to synergize with rs3129882 SNP genotype to increase risk for iPD, as discussed above. The extent to which pyrethroid exposure synergizes with other genetic risk factors remains to be investigated. Pyrethroids are rapidly metabolized, although there is evidence that exposure to pyrethroids during early development can create long-term neurological change [91, 109]. However, the mechanism by which they may produce long-lasting immune effects are yet to be fully elucidated, as are the consequences of exposures later in life.
VIRAL AND BACTERIAL INFECTIONS
Braak and colleagues proposed that infectious agents targeting the gut and olfactory bulbs may be the initial trigger for PD pathogenesis [110]. Although it is clear that a single organism is not consistently associated with PD, a PD-like combination of motor-symptoms known as parkinsonism has been observed in the aftermath of infections. Influenza is highlighted here as example case of environment-by-immune interactions that may affect risk of iPD and/or PD-like syndromes.
INFLUENZA AND PARKINSONISM
A member of the Orthomyxoviridae family, the influenza virus is a single-stranded, RNA virus with a genome encoding 11 proteins [111]. Highly pathogenic strains of influenza have been associated with subsequent neurological complications, including neurodegeneration and neuroinflammation [112]. Following the 1918 Spanish influenza pandemic, an outbreak of an encephalitic condition known as encephalitis lethargica (EL) occurred, causing high fever, mental confusion, lethargy, and vision problems in patients previously exposed to influenza [113]. No clear biological mechanism has been identified as a causal connection between influenza and EL.
In 80% of people who survived EL, secondary parkinsonism was later described [113], although, as with influenza and EL, no clear causal factor has been identified, and the association is contested by some [114]. Nonetheless, it has been reported that individuals who were young at the time of the Spanish flu pandemic and may have been exposed to highly pathogenic influenza had a 2-3-fold higher risk of developing parkinsonism [112]. Similarly, parkinsonism subsequent to neurotropic alpha virus infection has been reported and can be reproduced in mouse models [115]. How might viral infection give rise to dopamine depletion? One possibility is that some direct viral invasion of the central nervous system and/or a systemic cytokine storm caused by a virus predisposes death of midbrain dopamine neurons. Whether neurovirulence is strictly necessary for influenza virus exposure to increase risk for parkinsonism remains to be fully understood. Neuroinflammatory response is observed independent of neurotropism, at least in mouse models [116] suggesting that peripheral immune activation, an incredibly common environmental exposure, can be sufficient to disrupt dopamine neuron health. It is thus possible that antiviral interventions can mitigate virus-related neurodegeneration and act as neuroprotective agents [116]. It may be the case that a subpopulation of people could benefit from treatments during or after viral infection to prevent later development of parkinsonism or iPD, but it remains to be determined who would fall into such a group. Factors such as age at time of infection, severity of viral infection, repeat infection, cytokine storm, fever, etc. could all influence whether a viral infection increases risk for iPD.
INFLUENZA AS AUTOIMMUNE TRIGGER
Infection with the flu or other pathogens also has the potential to elicit an autoimmune reaction resulting in immune system-driven neurodegeneration. This could occur through the production of cryptic antigens (antigens normally invisible to the immune system that become exposed due to disease processes). Also, differential processing of self-proteins by infection-related protease action could produce neoantigens potentially recognized by existing T cells. As proposed in [117], various mechanisms for infection-induced autoimmunity exist and are not mutually exclusive: molecular mimicry and the adjuvant effects of infectious pathogens may function early in the development of an autoimmune reaction in the pathogenesis of PD, followed by bystander activation and epitope spreading as inflammation persists. Indeed, humoral cross-reactivity has been identified between α-synuclein and herpes simplex-1 [118]. Bystander activation occurs when there is proliferation of a previously small population of activated, autoreactive T cells due to chronic inflammation triggered by a pathogen or tissue damage. Epitope spreading is defined as the case in which a healthy, effective immune response, initially directed against some portion of an antigen, shifts to include a different portion of the same protein or a different protein. If this new antigen is similar to a self-protein, there can be cross recognition resulting in the destruction of self-tissue. It is possible that the immune response to certain influenza strains in certain human populations could produce this type of induced autoimmunity, putting substantia nigra dopamine neurons at risk.
BACTERIA AND iPD RISK
In addition to viral infection, exposre to certain bacteria may also influence iPD risk. As mentioned above, expression of the PD-related gene LRRK2 is enriched during an active tuberculosis infection [61]. In some populations, tuberculosis exposure itself increases risk for iPD [119]. Beyond infection, the activity of gastrointestinal microbes is increasingly being investigated in relation to PD pathology. Long before the compositional differences in the gut bacterial communities of PD patients were identified (recently reviewed by [120]), the involvement of species of Helicobacter, a bacterium that colonizes the stomach and can cause gastric ulceration and ultimately gastric cancer, in PD was proposed. In 1999, Charlett et al. reported that PD patients as well as their siblings exhibit greater frequency of Helicobacter pylori (H. pylori) seropositivity [121], and a 2013 study reported greater incidence of H. suis in gastric biopsies from iPD patients compared to healthy controls [122]. Another study in the same year, however, found no differences in frequencies of H. pylori infection in PD patients and controls [123]. Most studies report Helicobacter infection in roughly a third of PD patients [122-124], and while Helicobacter infection has been associated with a 23-45% enhanced risk for PD development [125], a meta-analysis found that Helicobacter infection frequency in PD did not differ significantly from the general population [126]. The mechanisms by which these bacterial exposures may alter the functioning of specific immune cells and create an iPD-permissive or -prone state remains to be elucidated.
Conclusion
Therapeutic strategies may involve both unspecific anti-inflammatory treatment as well as drugs interfering with specific known pathways, such as, for example, compounds affecting the STING pathway in the case of well-defined (genetic) subtypes of PD. Although many drugs are available and therapeutic targets continue to emerge, randomized, placebo-controlled clinical trials are urgently needed to evaluate the potential efficacy of these biologically plausible and readily available agents. As the field moves forward investigating immunomodulatory therapeutic options, it is crucial to consider the patient subpopulations created by combinations of genetic variations and environmental exposures. We have highlighted here α-synuclein, LRRK2, PINK1 and Parkin, and MHCII, as these proteins are all found in immune cells. Further work is needed to understand their function, interactions with one another, and how their expression is influenced by environmental exposures. The immune system’s response to pyrethroids, viruses, and bacteria may depend on SNCA, LRRK2, PINK1, and MHCII genetic variations or mutations. There is a gene-by-environment-by-immune-system triangle in PD pathogenesis, and failure to appreciate this triad in PD clinical trials could lead to findings of futility for treatments that could profoundly benefit a cluster of PD patients with a particular immunophenotype, infection history, degree of autoimmunity, etc. We must be mindful of the context produced by genetic and environmental factors contributing to each patient’s risk of PD. There may be protection and risk conferred by an individual’s genes and exposures, and this will likely influence immune system tone throughout the lifespan as well as responsiveness to treatment. It is likely that no single drug will be able to address the vast array of underlying pathologies involved in PD. It is also likely that it will be more feasible to prevent the degeneration of neurons rather than regenerate the substantia nigra and other affected nuclei. This requires a thorough understanding of the genetic and environmental factors converging on the immune system to increase risk for PD.
Acknowledgements
Funding for the authors was partially derived from The Michael J. Fox Foundation for Parkinson’s Research, The Parkinson’s Foundation, Emory University School of Medicine Movement Disorders Center, Udall Center for Excellence in Parkinson’s Research, and the National Institutes of Health 1R01NS092122, 1RF1AG051514, 1RF1AG057247 (MGT), T32-GM08605 (MKH and EMK), 1R01NS064934, P50NS108675 and R33NS097643 (ABW), and by the DFG (Cluster of Excellence ,Precision Medicine in Chronic Inflammation and FOR 2488 (PS and CK).
Footnotes
Conflicts of Interests
MGT is a current member of advisory boards for the W. Garfield Weston Foundation advisory board, the World Parkinson Coalition, the Quebec Parkinson’s Network, the Michael J. Fox Foundation for Parkinson’s Research, the Alzheimer’s Association Medical and Scientific Advisory Board, and has active collaborations/consults with/for INmune Bio, Denali, Merck, Biogen/Ionis, Lundbeck, Longevity Bio, Cerebral Therapeutics, and Prevail Therapeutics. ABW is a current member of the Michael J. Fox Foundation for Parkinson’s Research Executive Scientific Advisory Board and consults for EscapeBio Inc. and Neuro23 Inc. CK is a medical advisor to Centogene for genetic testing reports in the fields of movement disorders and dementia, excluding Parkinson’s disease.
References
- 1.Domingo A and Klein C, Genetics of Parkinson disease. Handb Clin Neurol, 2018. 147: p. 211–227. [DOI] [PubMed] [Google Scholar]
- 2.Jimenez-Ferrer I and Swanberg M, Immunogenetics of Parkinson's Disease, in Parkinson's Disease: Pathogenesis and Clinical Aspects, Stoker TB and Greenland JC, Editors. 2018: Brisbane (AU). [Google Scholar]
- 3.Hirsch EC and Standaert DG, Ten Unsolved Questions About Neuroinflammation in Parkinson's Disease. Mov Disord, 2020. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, et al. , Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging, 2003. 24(2): p. 197–211. [DOI] [PubMed] [Google Scholar]
- 5.Houser MC and Tansey MG, The gut-brain axis: is intestinal inflammation a silent driver of Parkinson's disease pathogenesis? NPJ Parkinsons Dis, 2017. 3: p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espay AJ and Lang AE, Parkinson Diseases in the 2020s and Beyond: Replacing Clinico-Pathologic Convergence With Systems Biology Divergence. J Parkinsons Dis, 2018. 8(s1): p. S59–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nalls MA, et al. , Identification of novel risk loci, causal insights, and heritable risk for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet Neurol, 2019. 18(12): p. 1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto M, et al. , NACP, a synaptic protein involved in Alzheimer's disease, is differentially regulated during megakaryocyte differentiation. Biochem Biophys Res Commun, 1997. 237(3): p. 611–6. [DOI] [PubMed] [Google Scholar]
- 9.Nakai M, et al. , Expression of alpha-synuclein, a presynaptic protein implicated in Parkinson's disease, in erythropoietic lineage. Biochem Biophys Res Commun, 2007. 358(1): p. 104–10. [DOI] [PubMed] [Google Scholar]
- 10.Shameli A, et al. , A critical role for alpha-synuclein in development and function of T lymphocytes. Immunobiology, 2016. 221(2): p. 333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendor JT, Logan TP, and Edwards RH, The function of alpha-synuclein. Neuron, 2013. 79(6): p. 1044–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diao J, et al. , Native alpha-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2. Elife, 2013. 2: p. e00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das V, et al. , Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity, 2004. 20(5): p. 577–88. [DOI] [PubMed] [Google Scholar]
- 14.Pattu V, et al. , SNARE protein expression and localization in human cytotoxic T lymphocytes. Eur J Immunol, 2012. 42(2): p. 470–5. [DOI] [PubMed] [Google Scholar]
- 15.Gold A, Turkalp ZT, and Munoz DG, Enteric alpha-synuclein expression is increased in Parkinson's disease but not Alzheimer's disease. Mov Disord, 2013. 28(2): p. 237–40. [DOI] [PubMed] [Google Scholar]
- 16.Gray MT, et al. , Alpha-synuclein in the appendiceal mucosa of neurologically intact subjects. Mov Disord, 2014. 29(8): p. 991–8. [DOI] [PubMed] [Google Scholar]
- 17.Bottner M, et al. , Expression pattern and localization of alpha-synuclein in the human enteric nervous system. Neurobiol Dis, 2012. 48(3): p. 474–80. [DOI] [PubMed] [Google Scholar]
- 18.Shannon KM, et al. , Is alpha-synuclein in the colon a biomarker for premotor Parkinson's disease? Evidence from 3 cases. Mov Disord, 2012. 27(6): p. 716–9. [DOI] [PubMed] [Google Scholar]
- 19.Forsyth CB, et al. , Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS One, 2011. 6(12): p. e28032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, et al. , Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J, 2005. 19(6): p. 533–42. [DOI] [PubMed] [Google Scholar]
- 21.Park JY, et al. , Microglial phagocytosis is enhanced by monomeric alpha-synuclein, not aggregated alpha-synuclein: implications for Parkinson's disease. Glia, 2008. 56(11): p. 1215–23. [DOI] [PubMed] [Google Scholar]
- 22.Marinova-Mutafchieva L, et al. , Relationship between microglial activation and dopaminergic neuronal loss in the substantia nigra: a time course study in a 6-hydroxydopamine model of Parkinson's disease. J Neurochem, 2009. 110(3): p. 966–75. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, et al. , Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: implications for progression of Parkinson's disease. Neurotox Res, 2011. 19(1): p. 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krashia P, et al. , Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson's disease. Nat Commun, 2019. 10(1): p. 3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cebrian C, et al. , MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat Commun, 2014. 5: p. 3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sulzer D, et al. , T cells from patients with Parkinson's disease recognize alpha-synuclein peptides. Nature, 2017. 546(7660): p. 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindestam Arlehamn CS, et al. , alpha-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson's disease. Nat Commun, 2020. 11(1): p. 1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong SC, et al. , Expression and subcellular location of alpha-synuclein during mouse-embryonic development. Cell Mol Neurobiol, 2010. 30(3): p. 469–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croisier E, et al. , Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation, 2005. 2: p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chervonsky AV, Influence of microbial environment on autoimmunity. Nat Immunol, 2010. 11(1): p. 28–35. [DOI] [PubMed] [Google Scholar]
- 31.Goronzy JJ, et al. , The janus head of T cell aging - autoimmunity and immunodeficiency. Front Immunol, 2013. 4: p. 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stolzenberg E, et al. , A Role for Neuronal Alpha-Synuclein in Gastrointestinal Immunity. J Innate Immun, 2017. 9(5): p. 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beatman EL, et al. , Alpha-Synuclein Expression Restricts RNA Viral Infections in the Brain. J Virol, 2015. 90(6): p. 2767–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bu XL, et al. , The association between infectious burden and Parkinson's disease: A case-control study. Parkinsonism Relat Disord, 2015. 21(8): p. 877–81. [DOI] [PubMed] [Google Scholar]
- 35.Haruwaka K, et al. , Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun, 2019. 10(1): p. 5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marrack P and Kappler JW, Do MHCII-presented neoantigens drive type 1 diabetes and other autoimmune diseases? Cold Spring Harb Perspect Med, 2012. 2(9): p. a007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paisan-Ruiz C, et al. , Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron, 2004. 44(4): p. 595–600. [DOI] [PubMed] [Google Scholar]
- 38.Zimprich A, et al. , Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron, 2004. 44(4): p. 601–7. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z, et al. , The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat Immunol, 2011. 12(11): p. 1063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graef IA, et al. , Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell, 2003. 113(5): p. 657–70. [DOI] [PubMed] [Google Scholar]
- 41.Greenblatt MB, et al. , Calcineurin regulates innate antifungal immunity in neutrophils. J Exp Med, 2010. 207(5): p. 923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao A, Luo C, and Hogan PG, Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol, 1997. 15: p. 707–47. [DOI] [PubMed] [Google Scholar]
- 43.Gardet A, et al. , LRRK2 is involved in the IFN-gamma response and host response to pathogens. J Immunol, 2010. 185(9): p. 5577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thevenet J, et al. , Regulation of LRRK2 expression points to a functional role in human monocyte maturation. PLoS One, 2011. 6(6): p. e21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hakimi M, et al. , Parkinson's disease-linked LRRK2 is expressed in circulating and tissue immune cells and upregulated following recognition of microbial structures. J Neural Transm (Vienna), 2011. 118(5): p. 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuss M, Adamopoulou E, and Kahle PJ, Interferon-gamma induces leucine-rich repeat kinase LRRK2 via extracellular signal-regulated kinase ERK5 in macrophages. J Neurochem, 2014. 129(6): p. 980–7. [DOI] [PubMed] [Google Scholar]
- 47.Fan Y, et al. , Interrogating Parkinson's disease LRRK2 kinase pathway activity by assessing Rab10 phosphorylation in human neutrophils. Biochem J, 2018. 475(1): p. 23–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee H, et al. , LRRK2 Is Recruited to Phagosomes and Co-recruits RAB8 and RAB10 in Human Pluripotent Stem Cell-Derived Macrophages. Stem Cell Reports, 2020. 14(5): p. 940–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cook DA and Tansey MG, LRRK2, in Neuroimmune Pharmacology, Gendelman HE and Ikezu T, Editors. 2017, Springer, Cham. [Google Scholar]
- 50.Cook DA, et al. , LRRK2 levels in immune cells are increased in Parkinson's disease. NPJ Parkinsons Dis, 2017. 3: p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallings RL, Herrick MK, and Tansey MG, LRRK2 at the Interface Between Peripheral and Central Immune Function in Parkinson's. Front Neurosci, 2020. 14: p. 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dzamko N, Rowe DB, and Halliday GM, Increased peripheral inflammation in asymptomatic leucine-rich repeat kinase 2 mutation carriers. Mov Disord, 2016. 31(6): p. 889–97. [DOI] [PubMed] [Google Scholar]
- 53.Park J, et al. , Parkinson disease-associated LRRK2 G2019S transgene disrupts marrow myelopoiesis and peripheral Th17 response. J Leukoc Biol, 2017. 102(4): p. 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daher JP, et al. , Abrogation of alpha-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc Natl Acad Sci U S A, 2014. 111(25): p. 9289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Russo I, et al. , Leucine-rich repeat kinase 2 positively regulates inflammation and down-regulates NF-kappaB p50 signaling in cultured microglia cells. J Neuroinflammation, 2015. 12: p. 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maekawa T, et al. , Leucine-rich repeat kinase 2 (LRRK2) regulates alpha-synuclein clearance in microglia. BMC Neurosci, 2016. 17(1): p. 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bieri G, et al. , LRRK2 modifies alpha-syn pathology and spread in mouse models and human neurons. Acta Neuropathol, 2019. 137(6): p. 961–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Novello S, et al. , G2019S LRRK2 mutation facilitates alpha-synuclein neuropathology in aged mice. Neurobiol Dis, 2018. 120: p. 21–33. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, et al. , Meta-analysis of human gene expression in response to Mycobacterium tuberculosis infection reveals potential therapeutic targets. BMC Syst Biol, 2018. 12(1): p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hartlova A, et al. , LRRK2 is a negative regulator of Mycobacterium tuberculosis phagosome maturation in macrophages. EMBO J, 2018. 37(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weindel CG, et al. , LRRK2 maintains mitochondrial homeostasis and regulates innate immune responses to Mycobacterium tuberculosis. Elife, 2020. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu W, et al. , LRRK2 promotes the activation of NLRC4 inflammasome during Salmonella Typhimurium infection. J Exp Med, 2017. 214(10): p. 3051–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shutinoski B, et al. , Lrrk2 alleles modulate inflammation during microbial infection of mice in a sex-dependent manner. Sci Transl Med, 2019. 11(511). [DOI] [PubMed] [Google Scholar]
- 64.Hui KY, et al. , Functional variants in the LRRK2 gene confer shared effects on risk for Crohn's disease and Parkinson's disease. Sci Transl Med, 2018. 10(423). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michail S, Bultron G, and Depaolo RW, Genetic variants associated with Crohn's disease. Appl Clin Genet, 2013. 6: p. 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Umeno J, et al. , Meta-analysis of published studies identified eight additional common susceptibility loci for Crohn's disease and ulcerative colitis. Inflamm Bowel Dis, 2011. 17(12): p. 2407–15. [DOI] [PubMed] [Google Scholar]
- 67.Franke A, et al. , Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet, 2010. 42(12): p. 1118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hugot JP, et al. , Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature, 2001. 411(6837): p. 599–603. [DOI] [PubMed] [Google Scholar]
- 69.Barrett JC, et al. , Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet, 2008. 40(8): p. 955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fava VM, et al. , A Missense LRRK2 Variant Is a Risk Factor for Excessive Inflammatory Responses in Leprosy. PLoS Negl Trop Dis, 2016. 10(2): p. e0004412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takagawa T, et al. , An increase in LRRK2 suppresses autophagy and enhances Dectin-1-induced immunity in a mouse model of colitis. Sci Transl Med, 2018. 10(444). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heo JM, et al. , The PINK1-PARKIN Mitochondrial Ubiquitylation Pathway Drives a Program of OPTN/NDP52 Recruitment and TBK1 Activation to Promote Mitophagy. Mol Cell, 2015. 60(1): p. 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lazarou M, et al. , The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature, 2015. 524(7565): p. 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matheoud D, et al. , Parkinson's Disease-Related Proteins PINK1 and Parkin Repress Mitochondrial Antigen Presentation. Cell, 2016. 166(2): p. 314–327. [DOI] [PubMed] [Google Scholar]
- 75.Sugiura A, et al. , A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J, 2014. 33(19): p. 2142–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sliter DA, et al. , Parkin and PINK1 mitigate STING-induced inflammation. Nature, 2018. 561(7722): p. 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Panicker N, et al. , Fyn Kinase Regulates Microglial Neuroinflammatory Responses in Cell Culture and Animal Models of Parkinson's Disease. J Neurosci, 2015. 35(27): p. 10058–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wissemann WT, et al. , Association of Parkinson disease with structural and regulatory variants in the HLA region. Am J Hum Genet, 2013. 93(5): p. 984–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamza TH, et al. , Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson's disease. Nat Genet, 2010. 42(9): p. 781–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo Y, et al. , HLA rs3129882 variant in Chinese Han patients with late-onset sporadic Parkinson disease. Neurosci Lett, 2011. 501(3): p. 185–7. [DOI] [PubMed] [Google Scholar]
- 81.Ahmed I, et al. , Association between Parkinson's disease and the HLA-DRB1 locus. Mov Disord, 2012. 27(9): p. 1104–10. [DOI] [PubMed] [Google Scholar]
- 82.Hill-Burns EM, et al. , Evidence for more than one Parkinson's disease-associated variant within the HLA region. PLoS One, 2011. 6(11): p. e27109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun C, et al. , HLA-DRB1 alleles are associated with the susceptibility to sporadic Parkinson's disease in Chinese Han population. PLoS One, 2012. 7(11): p. e48594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.International Parkinson Disease Genomics, C., et al. , Imputation of sequence variants for identification of genetic risks for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet, 2011. 377(9766): p. 641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kannarkat GT, et al. , Common Genetic Variant Association with Altered HLA Expression, Synergy with Pyrethroid Exposure, and Risk for Parkinson's Disease: An Observational and Case-Control Study. NPJ Parkinsons Dis, 2015. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harms AS, et al. , MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci, 2013. 33(23): p. 9592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marras C, Canning CG, and Goldman SM, Environment, lifestyle, and Parkinson's disease: Implications for prevention in the next decade. Mov Disord, 2019. 34(6): p. 801–811. [DOI] [PubMed] [Google Scholar]
- 88.Niedzwiecki MM, et al. , The Exposome: Molecules to Populations. Annu Rev Pharmacol Toxicol, 2019. 59: p. 107–127. [DOI] [PubMed] [Google Scholar]
- 89.Breckenridge CB, et al. , Association between Parkinson's Disease and Cigarette Smoking, Rural Living, Well-Water Consumption, Farming and Pesticide Use: Systematic Review and Meta-Analysis. PLoS One, 2016. 11(4): p. e0151841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noyce AJ, et al. , Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol, 2012. 72(6): p. 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yan D, et al. , Pesticide exposure and risk of Parkinson's disease: Dose-response meta-analysis of observational studies. Regul Toxicol Pharmacol, 2018. 96: p. 57–63. [DOI] [PubMed] [Google Scholar]
- 92.Crawford MJ, Croucher A, and Hutson DH, Metabolism of cis- and transcypermethrin in rats. Balance and tissue retention study. J Agric Food Chem, 1981. 29(1): p. 130–5. [DOI] [PubMed] [Google Scholar]
- 93.EPA), E.P.A.U.S. Pyrethroid Cumulative Risk Assessment. 2011. [cited 2011; Available from: http://www.epa.gov/oppsrrd1/reevaluation/pyrethroids-pyrethrins.html#epa.
- 94.Eells JT and Dubocovich ML, Pyrethroid insecticides evoke neurotransmitter release from rabbit striatal slices. J Pharmacol Exp Ther, 1988. 246(2): p. 514–21. [PubMed] [Google Scholar]
- 95.Brown LD and Narahashi T, Modulation of nerve membrane sodium channel activation by deltamethrin. Brain Res, 1992. 584(1-2): p. 71–6. [DOI] [PubMed] [Google Scholar]
- 96.Singh AK, et al. , A current review of cypermethrin-induced neurotoxicity and nigrostriatal dopaminergic neurodegeneration. Curr Neuropharmacol, 2012. 10(1): p. 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Verheugen JA, Oortgiesen M, and Vijverberg HP, Veratridine blocks voltage-gated potassium current in human T lymphocytes and in mouse neuroblastoma cells. J Membr Biol, 1994. 137(3): p. 205–14. [DOI] [PubMed] [Google Scholar]
- 98.Lo WL, Donermeyer DL, and Allen PM, A voltage-gated sodium channel is essential for the positive selection of CD4(+) T cells. Nat Immunol, 2012. 13(9): p. 880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feske S, Wulff H, and Skolnik EY, Ion channels in innate and adaptive immunity. Annu Rev Immunol, 2015. 33: p. 291–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Craner MJ, et al. , Sodium channels contribute to microglia/macrophage activation and function in EAE and MS. Glia, 2005. 49(2): p. 220–9. [DOI] [PubMed] [Google Scholar]
- 101.Zsiros E, et al. , Developmental switch of the expression of ion channels in human dendritic cells. J Immunol, 2009. 183(7): p. 4483–92. [DOI] [PubMed] [Google Scholar]
- 102.Kumar A, Sasmal D, and Sharma N, Immunomodulatory role of piperine in deltamethrin induced thymic apoptosis and altered immune functions. Environ Toxicol Pharmacol, 2015. 39(2): p. 504–14. [DOI] [PubMed] [Google Scholar]
- 103.Kumaran R and Cookson MR, Pathways to Parkinsonism Redux: convergent pathobiological mechanisms in genetics of Parkinson's disease. Hum Mol Genet, 2015. 24(R1): p. R32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gabbianelli R, et al. , Permethrin induces lymphocyte DNA lesions at both Endo III and Fpg sites and changes in monocyte respiratory burst in rats. J Appl Toxicol, 2009. 29(4): p. 317–22. [DOI] [PubMed] [Google Scholar]
- 105.Chrustek A, et al. , Current Research on the Safety of Pyrethroids Used as Insecticides. Medicina (Kaunas), 2018. 54(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumar A, et al. , Deltamethrin-induced oxidative stress and mitochondrial caspase-dependent signaling pathways in murine splenocytes. Environ Toxicol, 2016. 31(7): p. 808–19. [DOI] [PubMed] [Google Scholar]
- 107.Costa C, et al. , Cytokine patterns in greenhouse workers occupationally exposed to alpha-cypermethrin: an observational study. Environ Toxicol Pharmacol, 2013. 36(3): p. 796–800. [DOI] [PubMed] [Google Scholar]
- 108.El Okda ES, Abdel-Hamid MA, and Hamdy AM, Immunological and genotoxic effects of occupational exposure to alpha-cypermethrin pesticide. Int J Occup Med Environ Health, 2017. 30(4): p. 603–615. [DOI] [PubMed] [Google Scholar]
- 109.Magby JP and Richardson JR, Developmental pyrethroid exposure causes long-term decreases of neuronal sodium channel expression. Neurotoxicology, 2017. 60: p. 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hawkes CH, Del Tredici K, and Braak H, Parkinson's disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol, 2007. 33(6): p. 599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bouvier NM and Palese P, The biology of influenza viruses. Vaccine, 2008. 26 Suppl 4: p. D49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jang H, et al. , Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci U S A, 2009. 106(33): p. 14063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Henry J, et al. , Parkinsonism and neurological manifestations of influenza throughout the 20th and 21st centuries. Parkinsonism Relat Disord, 2010. 16(9): p. 566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vilensky JA, Gilman S, and McCall S, A historical analysis of the relationship between encephalitis lethargica and postencephalitic parkinsonism: a complex rather than a direct relationship. Mov Disord, 2010. 25(9): p. 1116–23. [DOI] [PubMed] [Google Scholar]
- 115.Bantle CM, et al. , Infection with mosquito-borne alphavirus induces selective loss of dopaminergic neurons, neuroinflammation and widespread protein aggregation. NPJ Parkinsons Dis, 2019. 5: p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sadasivan S, et al. , Synergistic effects of influenza and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) can be eliminated by the use of influenza therapeutics: experimental evidence for the multi-hit hypothesis. NPJ Parkinsons Dis, 2017. 3: p. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Delogu LG, et al. , Infectious diseases and autoimmunity. J Infect Dev Ctries, 2011. 5(10): p. 679–87. [DOI] [PubMed] [Google Scholar]
- 118.Caggiu E, et al. , Humoral cross reactivity between alpha-synuclein and herpes simplex-1 epitope in Parkinson's disease, a triggering role in the disease? J Neuroimmunol, 2016. 291: p. 110–4. [DOI] [PubMed] [Google Scholar]
- 119.Shen CH, et al. , Association Between Tuberculosis and Parkinson Disease: A Nationwide, Population-Based Cohort Study. Medicine (Baltimore), 2016. 95(8): p. e2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nuzum ND, et al. , Gut microbiota differences between healthy older adults and individuals with Parkinson's disease: A systematic review. Neurosci Biobehav Rev, 2020. 112: p. 227–241. [DOI] [PubMed] [Google Scholar]
- 121.Charlett A, et al. , Parkinsonism: siblings share Helicobacter pylori seropositivity and facets of syndrome. Acta Neurol Scand, 1999. 99(1): p. 26–35. [DOI] [PubMed] [Google Scholar]
- 122.Blaecher C, et al. , Significantly higher frequency of Helicobacter suis in patients with idiopathic parkinsonism than in control patients. Aliment Pharmacol Ther, 2013. 38(11-12): p. 1347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fasano A, et al. , The role of small intestinal bacterial overgrowth in Parkinson's disease. Mov Disord, 2013. 28(9): p. 1241–9. [DOI] [PubMed] [Google Scholar]
- 124.Tan AH, et al. , Helicobacter pylori infection is associated with worse severity of Parkinson's disease. Parkinsonism Relat Disord, 2015. 21(3): p. 221–5. [DOI] [PubMed] [Google Scholar]
- 125.Nielsen HH, et al. , Treatment for Helicobacter pylori infection and risk of Parkinson's disease in Denmark. Eur J Neurol, 2012. 19(6): p. 864–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rees K, et al. , Helicobacter pylori eradication for Parkinson's disease. Cochrane Database Syst Rev, 2011(11): p. CD008453. [DOI] [PubMed] [Google Scholar]