Abstract
Background:
Reflectance confocal microscopy (RCM) allows accurate, noninvasive, in vivo diagnosis for skin cancer. However, its impact on physicians’ diagnostic confidence and management is unknown.
Objectives:
We sought to assess the physicians’ diagnostic confidence and management before and after RCM of equivocal skin lesions.
Methods:
Prospective, 2-center, observational study. During clinical practice, 7 dermatologists recorded their diagnostic confidence level (measured in a scale from 0 to 10), diagnosis, and management before and after RCM of clinically/dermoscopically equivocal lesions that raised concern for skin cancer. We also evaluated the diagnostic accuracy before and after RCM.
Results:
We included 272 consecutive lesions from 226 individuals (mean age, 53.5 years). Diagnostic confidence increased from 6.2 to 8.1 after RCM (P < .001) when RCM confirmed or changed the diagnosis. Lesion management changed in 33.5% cases after RCM (to observation in 51 cases and to biopsy/excision in 31 cases). After RCM, the number needed to excise was 1.2. Sensitivity for malignancy before and after RCM was 78.2% and 85.1%, respectively. Specificity before and after RCM was 78.8% and 80%, respectively.
Limitations:
Small sample size, real-life environment, and different levels of expertise among RCM users.
Conclusion:
Physicians’ diagnostic confidence and accuracy increased after RCM when evaluating equivocal tumors, frequently resulting in management changes while maintaining high diagnostic accuracy.
Keywords: basal cell carcinoma, diagnostic accuracy, diagnostic confidence level, management, melanoma, reflectance confocal microscopy, skin cancer
Meta-analyses have shown that dermoscopy, a noninvasive, handheld skin-imaging technique, improves diagnostic accuracy for skin cancer detection,1–4 and reflectance confocal microscopy (RCM) allows noninvasive, real-time skin examination with cellular resolution. RCM has high diagnostic accuracy for skin cancer and considerable clinical utility, saving unnecessary excisions when used as a complementary tool in sequential digital dermoscopy surveillance for patients with high number of nevi.5,6 Both techniques are useful for the identification of early, featureless, and/or amelanotic skin cancers and allow for improved diagnostic accuracy compared with naked-eye examination alone.5,7–11 Although the ability to differentiate benign lesions from skin cancer with RCM has been shown in both retrospective and prospective cohort studies,5,7,8 the impact in real life of combining dermoscopy and RCM on diagnostic physicians’ confidence and the management of equivocal lesions has not been assessed.
The aim of this study was to quantify the effect of RCM imaging on physicians’ confidence levels in the diagnosis of clinically and dermoscopically equivocal skin lesions with a differential diagnosis that includes malignancy. In addition, we also assessed the impact on final diagnosis and management after RCM when evaluating such challenging lesions in daily practice in 2 skin cancer referral centers in the United States and Spain.
METHODS
Study design and inclusion/exclusion criteria
After institutional review board approval (Memorial Sloan Kettering Cancer Center protocol X15–041), from December 2015 through August 2017 we conducted a prospective observational study in 2 centers: Memorial Sloan Kettering Cancer Center (New York, NY) and Hospital Clínic de Barcelona, Barcelona, Spain. The main aim was to measure the impact of RCM on physicians’ diagnostic confidence levels and management decisions when assessing clinically/dermoscopically equivocal skin lesions in real life.
In these centers and during clinical practice, 7 dermatologists with varying experience with RCM (3 with ≤5 years of experience with RCM [Drs Marghoob, Rossi, and Marchetti] and 4 with >5 years of experience with RCM [Drs Halpern, Carrera, Puig, and Malvehy]) participated in the study by using RCM to assess skin lesions that were clinically and/or dermoscopically equivocal. We considered lesions to be equivocal when it was not possible for the physician to render or exclude a diagnosis of skin cancer clinically and/or dermoscopically with absolute confidence. We included consecutive lesions that fulfilled this inclusion criterion, were evaluated with RCM, and had a follow-up of at least 12 months. Unequivocal benign or malignant lesions, lesions imaged for academic purposes, or lesions imaged to monitor treatment were not included in this study. We also excluded cases that were considered benign but were excised because of the patient’s preference, lesions that were considered malignant but for which the patient refused excision/biopsy, and cases that were lost to follow-up.
For eligible lesions, the participating physicians ordered an RCM examination, which was obtained at the participating centers and read at the same time by the same dermatologist. In addition, physicians were required to fill in a survey for each case before and after RCM evaluation, on which they recorded their (1) primary diagnosis, (2) confidence level (numerical scale from 0 to 10, with 10 being 100% sure and 0 being 100% unsure), and (3) proposed management. The RCM evaluation was performed by using either the wide-probe Vivascope 1500 microscope (Caliber ID, Rochester, NY) or the handheld Vivascope 3000 microscope (Caliber ID, Rochester, NY) according to clinical practice. The handheld microscope was used in concave-convex areas, and the wide-probe microscope was used in flat areas. After RCM evaluation, the physician decided to biopsy or treat the lesion (biopsy/excision, cryotherapy, topical treatment) or to monitor it, integrating the clinical, dermoscopic, and RCM information, as per clinical practice.
Data analysis
The data recorded on the forms was later transferred to a deidentified Access 2013 database (Microsoft, Redmond, WA), which was completed with demographic information and status after 1 year of follow-up. Later, we grouped the lesions as benign or malignant and calculated the diagnostic accuracy for malignancy before and after RCM. We considered a lesion malignant if the histopathologic analysis rendered the diagnosis of a skin cancer or if a lesion diagnosed as superficial basal cell carcinoma (BCC) or squamous cell carcinoma (SCC) in situ by using in vivo RCM was treated nonsurgically and did not show recurrence after 1 year. We considered a lesion benign if the histopathologic analysis rendered a benign diagnosis or if the lesion remained stable after at least 12 months. Actinic keratoses were considered to be benign in this study.
Statistical analysis
Descriptive statistics, such as means, standard deviations, medians, minimum and maximum values, and relative frequencies, were used to describe the patient and lesion characteristics. RCM levels of confidence were categorized as low when scored 0 to 3, medium when scored 4 to 6, and high when scored 7 to 10. Comparisons between pre- and post-RCM data were performed by using t tests for paired data. Diagnostic accuracy for malignancy was calculated based on the histologic results when lesions were biopsied/excised or based on the absence of malignancy (evaluated clinically, dermoscopically, and confocally) in lesions followed up for at least 12 months. After plotting sensitivity and 1-specificity for malignancy before and after RCM in receiver operating characteristic curves, diagnostic accuracy was compared using the DeLong test by comparing the area under the curve before and after RCM examination. Analyses were performed using PASW Statistics 18 (SPSS Corp, Chicago, IL) and MedCalc 18.2.1 (MedCalc Software, Ostend, Belgium).
RESULTS
A total of 288 lesions were imaged, 58.8% in Barcelona and 41.2% in New York. We excluded 16 lesions (3 malignant lesions for which patients refused excision, 3 benign lesions excised at the patient’s request, 4 lesions with incomplete data, and 6 cases lost to follow-up). Ultimately, 272 cases from 226 patients were included (mean age, 53.5 years; standard deviation, 17.0; 121 women).
Impact of RCM on diagnostic confidence
The diagnostic confidence level increased from 6.2 (standard deviation, 1.6) before RCM to 8.1 (SD, 1.7) after RCM (P < .001). Overall, the confidence level increased in 222 cases (81.6%), decreased in 15 (5.5%), and remained the same from before to after RCM imaging in 35 (12.9%). When categorizing the confidence level as low, medium, or high, before RCM the confidence level was medium or low (score, ≤6) in 147 lesions (54%), whereas after RCM only 41 lesions (15%) had a low or medium confidence level (Supplemental Table I; available at https://data.mendeley.com/datasets/9637ykct38/1#file-3431f5dd-7cb7-42b4-854b-399081d1dd9a2a0).
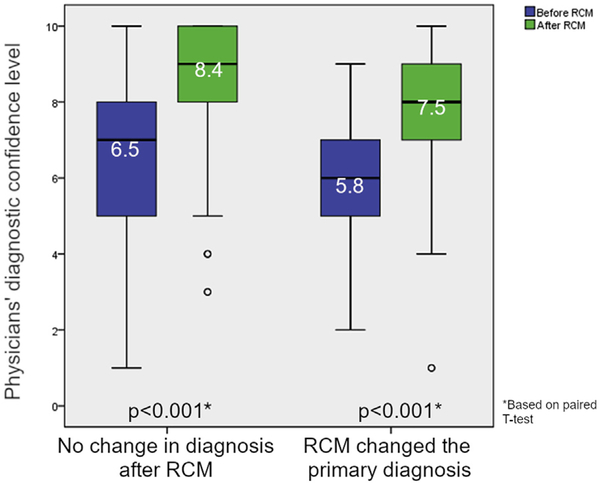
In 87 of 272 cases (32%) the primary clinical diagnosis changed after RCM imaging. However, confidence level increased both for lesions for which RCM confirmed the same diagnosis (mean confidence level before RCM, 6.5 points [SD, 1.7]; mean confidence level after RCM, 8.4 points [SD, 1.7]; P < .0001) and in lesions for which the diagnosis changed after RCM (mean before RCM, 5.8 points [SD, 1.4]; mean after RCM, 7.5 points [SD, 1.7]; P < .0001) (Fig 1).
Fig 1.
Boxplot of physicians’ confidence levels in lesion diagnosis before and after RCM evaluation. The mean diagnostic confidence level significantly increased for both lesions in which RCM confirmed the diagnosis (increase of 1.9 points) and for lesions in which the diagnosis changed after RCM (increase of 1.7 points). RCM, reflectance confocal microscopy.
Impact of RCM on management decisions
Management changed in 91 of 272 (33.5%) lesions after RCM (Table I). Management changed from biopsy/surgery to observation in 56% (n = 51) of these cases, from observation to biopsy/surgery in 34% (n = 31) (Fig 2), and from biopsy/surgery to nonsurgical treatments (cryotherapy, laser, or topical imiquimod) in 9.9% (n = 9).
Table I.
Cross-tabulation comparing management before and after reflectance confocal microscopy
| Management planned before RCM* | Management after RCM |
||
|---|---|---|---|
| Observation | Treatment† | Total | |
| Observation | |||
| Count | 49 | 31 | 80 |
| % of total | 18 | 11.4 | 29.4 |
| Treatment | |||
| Count | 51 | 141 | 192 |
| % of total | 18.8 | 51.8 | 70.6 |
| Total | |||
| Count | 100 | 172 | 272 |
| % of total | 36.8 | 63.2 | 100 |
RCM, Reflectance confocal microscopy.
Treatment before RCM included surgery/biopsy in 190 cases, imiquimod in 1 case, and cryotherapy in 1 case.
Treatment after RCM included surgery/biopsy in 162 cases, imiquimod in 9 cases, and cryotherapy in 1 case.
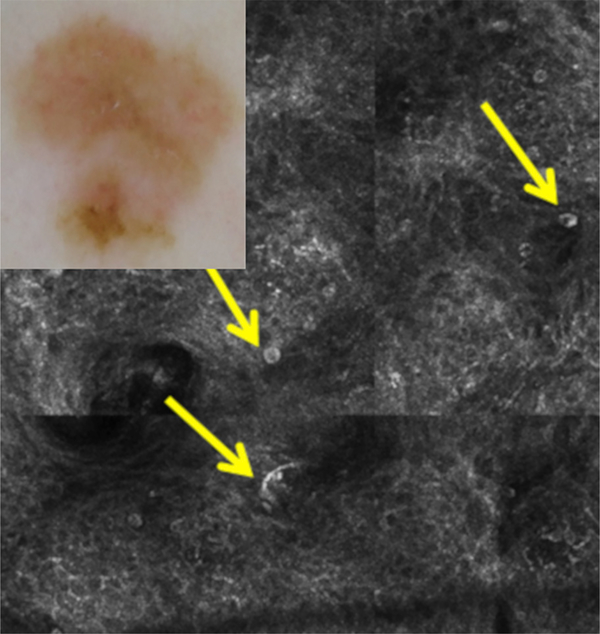
Fig 2.
A case for which RCM increased the diagnostic confidence level from 6 to 9 and changed management from observation to excision (improved diagnosis and management). Diagnosis and management before RCM: dermoscopy (inset) shows asymmetry and structureless light brown areas, nonspecific for melanoma. The clinical/dermoscopic diagnosis was atypical nevus with a confidence level of 6 (medium), and observation would have been chosen with dermoscopy. Diagnosis and management after RCM: RCM shows multiple large, round, and dendritic nucleated cells (arrows) in the superficial epidermis in a pagetoid fashion, compatible with melanoma (confidence level, 9 [high]). Excision was performed with the histopathologic diagnosis of melanoma in situ.
RCM led to the biopsy/surgery of 31 of 272 lesions (11.4%) that would have been longitudinally monitored with dermoscopy. The histopathologic diagnoses of these 31 lesions included moderately dysplastic nevus (n = 13), melanoma (n = 3), severely dysplastic nevus (n = 3), BCC (n = 2), SCC (n = 2), actinic keratosis (n = 2), atypical spitzoid tumor (n = 1), intradermal nevus (n = 1), inflamed lentigo (n = 1), lichenoid keratosis (n = 1), inflammation (n = 1), and clear cell acanthoma (n = 1).
Impact on diagnostic accuracy and number needed to excise index
A total of 162 of 272 (59.6%) lesions were biopsied immediately after RCM, and 75 of 272 (27%) were malignant (Table II): there were 39 BCCs, 30 melanomas (1 metastatic, 22 in situ, 7 invasive [median Breslow thickness, 0.35 mm; range, 0.21–0.95 mm], and 6 SCCs). Among the benign lesions biopsied, 7 lesions were severely dysplastic nevi, and 9 were neoplasms with spitzoid features. Additionally, 10 of 272 (3.7%) lesions were empirically treated nonsurgically, and 100 of 272 (36.8%) lesions were monitored for at least 12 months. Among the monitored, nontreated lesions, 94 of 100 (94%) did not show changes or additional signs suggestive of malignancy, but 6 of 100 (6%) showed worrisome changes on digital dermoscopy and RCM evaluation, which then prompted a biopsy. Histologic analysis of these lesions showed 1 BCC, 1 lichenoid keratosis, 2 dysplastic nevi, and 2 melanomas (1 melanoma in situ associated with a nevus and 1 melanoma with 0.2-mm Breslow thickness).
Table II.
Diagnosis of lesions excised or empirically treated included in the study
| Excised lesions | Excised just after RCM | Nonsurgically treated after RCM | Excised during follow-up |
|---|---|---|---|
| Valid | |||
| Basal cell carcinomas | 39 | 10 | 1 |
| Melanomas* | 30 | — | 2 |
| Severely dysplastic nevi | 7 | — | — |
| Dysplastic/inflamed nevi | 34 | — | 2 |
| Spitzoid neoplasms | 9 | — | — |
| Squamous cell carcinomas | 6 | — | — |
| Actinic keratoses | 11 | — | — |
| Melanocytic nevi | 7 | — | — |
| Lentigines | 2 | — | — |
| Seborrheic keratoses | 3 | — | — |
| Lichenoid keratosis | 4 | — | 1 |
| Angiofibroma | 2 | — | — |
| Inflammation | 3 | — | — |
| Clear cell acanthomas | 2 | — | — |
| Scar | 1 | — | — |
| Ruptured cyst | 1 | — | — |
| Epidermolytic acanthoma | 1 | — | — |
| Subtotal | 162 | 10 | 6 |
| Total | 178 | ||
RCM, Reflectance confocal microscopy.
Melanoma characteristics: 1 metastatic, 23 in situ, 8 invasive (median Breslow thickness, 0.35 mm; range, 0.2–0.95 mm).
After 12 months of follow-up, a total of 168 lesions were biopsied/excised (90 benign, 78 malignant), 10 BCCs were empirically treated and did not show recurrence, and 94 were observed and remained stable (Fig 3 and Table II). Therefore, the number needed to excise (NNE) to identify a skin cancer (also known as the benign-to-malignant ratio) was 1.2 after integrating clinical, dermoscopic, and RCM information. Additionally, overall sensitivities for malignancy before and after RCM were 78.2% and 85.1%, respectively. Specificities for malignancy before and after RCM were 78.8% and 80%, respectively. Overall, the area under the curve rose from 0.785 (95% CI, 0.732–0.833) to 0.825 (95% CI, 0.775–0.868) after RCM, although it did not reach statistical significance (P = .14).
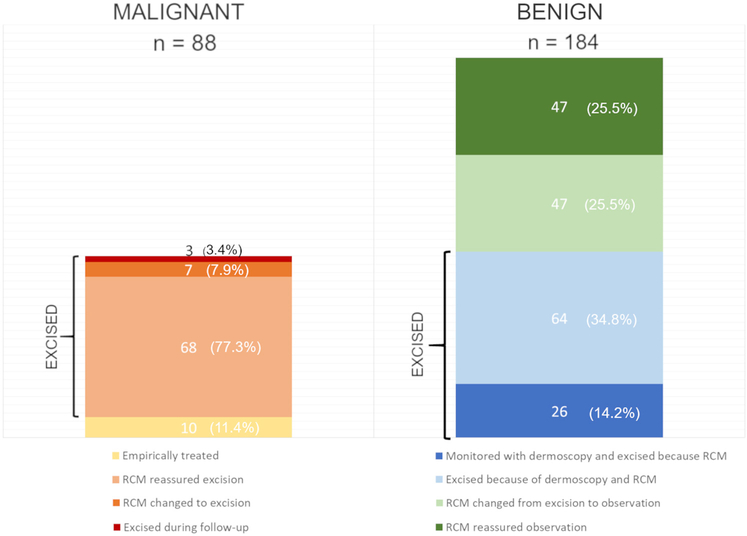
Fig 3.
Bar graph comparing the final outcome at the end of the study (malignant vs benign) and how reflectance confocal microscopy affected their management. Among the malignant lesions, RCM helped identify 7 skin cancers that would have been missed with dermoscopy alone (3 melanomas, 2 BCCs, and 2 SCC)s. However, 3 skin cancers (1 BCC, 2 melanomas) were not diagnosed initially with dermoscopy or RCM and were identified during sequential monitoring. Among the benign lesions, RCM prompted the excision of 26 benign lesions that would have been monitored with dermoscopy. Conversely, thanks to RCM, 47 lesions that would have been excised with dermoscopy were monitored and remained stable.
DISCUSSION
Our results show that RCM strengthens physicians’ diagnostic confidence under real conditions in the clinical setting. This frequently results in management changes of equivocal skin lesions. Physicians’ confidence levels increased after RCM, both when the diagnosis changed and when RCM helped confirm the diagnosis. In fact, only 15% of cases remained with medium or low levels of diagnostic certainty (confidence level, ≤6). This is important because physicians may be subject to considerable stress when evaluating complex conditions, such as in patients with atypical mole syndrome or patients with extensive sun damage. In these situations, patients may have multiple challenging skin lesions, making excision not feasible or desirable. Because RCM increases diagnostic confidence, RCM reassures the physician when making a diagnosis and complements clinical and dermoscopic evaluation. This potentially improves the management of equivocal skin lesions, thus reducing the number of unnecessary biopsies needed to render a correct diagnosis.
In fact, in our study, RCM saved immediate biopsy/surgery in 51 (18.8%) cases and prompted the use of nonsurgical therapies in an additional 10 (3.7%) cases. Conversely, RCM led to biopsy/surgery of 31 (11.4%) lesions that would have been monitored based only on dermoscopy. After 12 months of monitoring 100 lesions, only 6 additional biopsies were needed for changes not previously identified. One may argue that RCM saves biopsies at the expense of missing skin cancers. However, in our study, RCM helped identify 7 skin cancers that would have been missed with dermoscopy alone (3 melanomas, 2 BCCs, and 2 SCCs) (Fig 3). In addition, RCM led to the excision of 7 severely dysplastic nevi and 9 spitzoid neoplasms, lesions that are generally not possible to be distinguished from malignancy in vivo and for which excision is recommended.12
Overall, after 1 year of follow-up, the NNE to identify a skin cancer in our study was low (NNE, 1.2). In addition, sensitivity improved approximately 9% after using RCM, although the increase in sensitivity and specificity did not reach statistical significance. In other words, although the lesions included in this study were challenging and none of them were unequivocal malignant lesions, the integration of RCM with sequential digital monitoring and close surveillance maximized the performance of the different diagnostic tools in patients treated in skin cancer units. Our results support those previously published regarding patients with high-risk melanoma with multiple nevi especially under sequential digital dermoscopy monitoring, which suggests that RCM improves the diagnostic accuracy for skin cancer and lowers the NNE.5–8,13
However, it is true that clinical examination, dermoscopy, and RCM obtain a static picture of a lesion that may, in fact, change over time. In these situations, close monitoring is crucial. In our study, among the 6 lesions for which monitoring was decided and changes were detected, 2 were noteworthy because they were melanomas. One was a melanoma arising in a nevus and was identified because of a change in the dermoscopic and RCM features that occurred after the baseline visit. The other melanoma occurred in a patient who had received laser epilation treatment on her legs, and despite the presence of atypical features on dermoscopy and RCM, short-term monitoring was chosen to exclude inflammation secondary to the laser treatment. However, after persistence of the atypical features on dermoscopy and RCM, the lesion was excised, and a microinvasive melanoma was diagnosed. This highlights the importance of integrating the entire clinical scenario for each patient and underscores the importance of continued active surveillance of lesions that undergo RCM examination.
Limitations
Our study has several limitations, which mainly are due to the fact that it was performed to assess RCM impact on real-life conditions. These include a follow-up period of 1 year for the monitored lesions, the nonhistologic confirmation of empirically treated lesions, the use of both reflectance confocal microscopes (handheld and wide probe), potential different levels of expertise in RCM among the different physicians, and lesion inclusion differences between the 2 participating centers (ie, in the United States there was a predominance of pink lesions because the most common phototypes were I and II, whereas in Spain the majority of lesions were pigmented because the majority of phototypes were III and IV). However, we did not identify significant differences between the 2 centers or among the different participating physicians regarding changes in diagnostic confidence and management.
CONCLUSION
RCM increases physicians’ diagnostic confidence, reducing uncertainty when facing challenging lesions and improving the management of difficult lesions. RCM helps narrow the diagnostic gray zone of equivocal lesions and allows the identification of skin cancers that would be otherwise missed based on clinical or dermoscopic examination alone. This results in increased diagnostic sensitivity for malignancy and a reduction in unnecessary biopsy of benign skin lesions. However, thin, subtle skin cancers may be unrecognized after clinical, dermoscopic, and RCM examination alone, emphasizing that long-term sequential monitoring of equivocal lesions is very important in patients at high risk.
Supplementary Material
CAPSULE SUMMARY.
Reflectance confocal microscopy allows noninvasive in vivo diagnosis for skin cancer with high accuracy, but its impact on physicians’ diagnostic confidence and management is unknown.
Reflectance confocal microscopy evaluation of 272 dermoscopically equivocal lesions increased malignant tumor detection with higher diagnostic confidence and led to a management change in 33.5% of cases.
Acknowledgments
Funding sources: Supported in part by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748 and personal grants to Dr Yélamos (by Beca Fundación Piel Sana) and Dr Carrera (by Hospital Clínic Barcelona for foreign research stay). Research and confocal imaging in the Melanoma Unit in Barcelona is partly funded by the Centro de Investigación Biomédica en Red (CIBER) de Enfermedades Raras of the Instituto de Salud Carlos III, Spain, cofinanced by European Development Regional Fund "A way to achieve Europe" European Regional Development Fund (ERDF) and by the European Commission under the 7th Framework Programme (Diagnoptics).
Abbreviations used:
- BCC
basal cell carcinoma
- NNE
number needed to excise
- RCM
reflectance confocal microscopy
- SCC
squamous cell carcinoma
- SD
standard deviation
Footnotes
Disclosure: Dr Rajadhyaksha is a former employee of and owns equity in Caliber Imaging and Diagnostics (formerly Lucid Inc), the company that manufactures and sells the Vivascope confocal microscope. The Vivascope is the commercial version of an original laboratory prototype that he developed at Massachusetts General Hospital, Harvard Medical School. Drs Yélamos, Manubens, Jain, Chavez-Bourgeois, Pulijal, Dusza, Marchetti, Barreiro, Marino, Malvehy, Cordova, Rossi, Halpern, Puig, Marghoob, and Carrera have no conflicts of interest to declare.
We are grateful to Abel Caño, Beatriz Alejo, Mireia Dominguez, Pablo Iglesias, and all the fellows involved in the project, as well as all the patients who kindly agreed to allow us to image and sample their tumors to use them for scientific purposes.
Reprints not available from the authors.
REFERENCES
- 1.Bafounta ML, Beauchet A, Aegerter P, Saiag P. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137:1343–1350. [DOI] [PubMed] [Google Scholar]
- 2.Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669–676. [DOI] [PubMed] [Google Scholar]
- 3.Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159–165. [DOI] [PubMed] [Google Scholar]
- 4.Xiong YQ, Ma SJ, Mo Y, Huo ST, Wen YQ, Chen Q. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alarcon I, Carrera C, Palou J, Alos L, Malvehy J, Puig S. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014;170:802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanganelli I, Longo C, Mazzoni L, et al. Integration of reflectance confocal microscopy in sequential dermoscopy follow-up improves melanoma detection accuracy. Br J Dermatol. 2015;172:365–371. [DOI] [PubMed] [Google Scholar]
- 7.Pellacani G, Pepe P, Casari A, Longo C. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014; 171:1044–1051. [DOI] [PubMed] [Google Scholar]
- 8.Lovatto L, Carrera C, Salerni G, Alos L, Malvehy J, Puig S. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatol Venereol. 2015;29:1918–1925. [DOI] [PubMed] [Google Scholar]
- 9.Longo C, Moscarella E, Argenziano G, et al. Reflectance confocal microscopy in the diagnosis of solitary pink skin tumours: review of diagnostic clues. Br J Dermatol. 2015;173: 31–41. [DOI] [PubMed] [Google Scholar]
- 10.Castro RP, Stephens A, Fraga-Braghiroli NA, et al. Accuracy of in vivo confocal microscopy for diagnosis of basal cell carcinoma: a comparative study between handheld and wide-probe confocal imaging. J Eur Acad Dermatol Venereol. 2015;29:1164–1169. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson AD, Mickan S, Mallett S, Ayya M. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lallas A, Apalla Z, Ioannides D, et al. Update on dermoscopy of Spitz/Reed naevi and management guidelines by the International Dermoscopy Society. Br J Dermatol. 2017;177:645–655. [DOI] [PubMed] [Google Scholar]
- 13.Edwards SJ, Mavranezouli I, Osei-Assibey G, Marceniuk G, Wakefield V, Karner C. VivaScope® 1500 and 3000 systems for detecting and monitoring skin lesions: a systematic review and economic evaluation. Health Technol Assess. 2016;20:1–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.