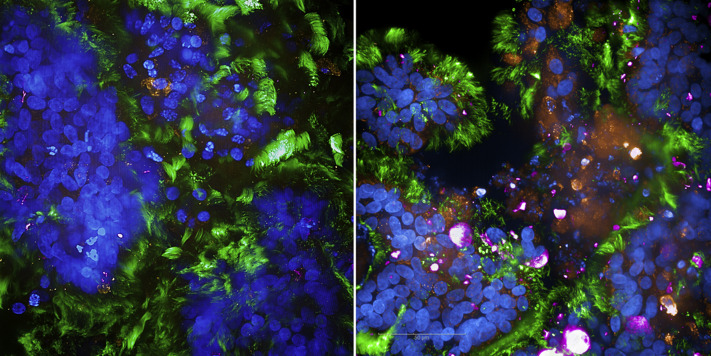
To monitor first SARS-CoV-2 interactions with primary, fully differentiated, ciliated, and mucus-producing epithelial tissue models, infection with clinical isolates derived from patients with SARS-CoV-2 was performed and monitored after 2 days by using confocal microscopy (Fig 1 [right]). Mock-treated, uninfected (UI) cultures served as negative controls (left). Immunofluorescence analyses revealed a significant infection of the tissue model with SARS-CoV-2 (pink [right]) and significant destruction, as recently described by us1 and as observed by high fragmentation of nuclei (blue) in particular within mucous areas (orange [right]). Infection with SARS-CoV-2 of both ciliated (green [right]) and mucus-producing cells (orange [right]) was detected. These analyses, which were performed by using the MUC5AC antibody for goblet cell staining in SARS-CoV-2–infected and UI tissues, revealed that infected human airway tissues showed an exaceberated mucus hypersecretion and mucus plugs (orange [right]), whereas in UI tissues no such plugs were monitored (left). UI tissues illustrated goblet cell staining (orange [left]) and intact, multilayered epithelia (blue [left]). Mucus plug formation was described when critically ill COVID-19 patients with airway obstruction and respiratory failure were analyzed (reviewed in Khan et al2), and mucus hypersecretion in combination with proinflammatory cytokine activation, which we recewntly illustrated in our model,1 and reduced mucociliary clearance3 shape a vicious circle resulting in airway tissue destruction. Of note, these clinical manifestations were not only reported in COVID-19 but also during other viral infections, colonization by pathogenic opportunistic bacteria, or asthma (reviewed in Khan et al2).
Fig 1.
SARS-CoV-2 causes exaceberated mucus hypersecretion and plug formation. Visualization of mock-treated, uninfected or SARS-CoV-2 infected human airway tissues were analyzed after 2 days by immunofluorescence analyses (nuclei [blue], ciliated cells [green], mucous-producing cells [orange], SARS-CoV-2 [pink]).
Acknowledgments
We thank Viktoria Zaderer, MSc, and Professor Rosa Bellmann-Weiler from the Internal Medicine II for their valuable support and for providing SARS-CoV-2 isolates. Written informed consent was obtained from all donors of leftover nasopharyngeal and oropharyngeal specimens and EDTA-treated blood by the participating clinics. The ethics committee of the Medical University of Innsbruck (ECS1166/2020) approved the use of anonymized leftover specimens from patients with COVID-19 for scientific purposes.
Footnotes
Supported by the Austrian Science Fund (grants P 34070-B [to W.P.] and P33510-B [to D.W.]), the Anniversary Fund of the Austrian National Bank (grants P 17614 [to W.P.] and 17633 [to D.W.]), and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant UM1AI068618 [to D.W.]).
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Posch W., Vosper J., Zaderer V., Lass-Florl C., Wilflingseder D. C5aR inhibition of nonimmune cells suppresses inflammation and maintains epithelial integrity in SARS-CoV-2-infected airway epithelia. J Allergy Clin Immunol. 2021;147:2083–2097. doi: 10.1016/j.jaci.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan M.A., Khan Z.A., Charles M., Pratap P., Naeem A., Siddiqui Z. Cytokine storm and mucus hypersecretion in COVID-19: review of mechanisms. J Inflamm Res. 2021;14:175–189. doi: 10.2147/JIR.S271292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busse P.J., Zhang T.F., Srivastava K., Lin B.P., Schofield B., Sealfon S.C. Chronic exposure to TNF-alpha increases airway mucus gene expression in vivo. J Allergy Clin Immunol. 2005;116:1256–1263. doi: 10.1016/j.jaci.2005.08.059. [DOI] [PubMed] [Google Scholar]