To the Editor:
We read with interest the article “SARS-CoV-2 infection in patients with autoimmune hepatitis” by Marjot, Buescher and Sebode et al. 1 recently published in the Journal of hepatology. While immunosuppressive therapy for autoimmune hepatitis (AIH) had no negative impact on the immediate outcome of COVID-19,[1], [2], [3] the question remained, whether COVID-19 convalescents immunosuppressed for AIH (AIH-Con) have the same level of protection against SARS-CoV-2 reinfection as non-immunosuppressed convalescents (non-IS-Con).
To address this question, we prospectively quantified anti-SARS-CoV-2 antibodies against various SARS-CoV-2 antigens (Antigen Panel 1 IgG, IgM, IgA assays Millipore HC19SERM1-85K-04, HC19SERA1-85K-04, HC19SERG1-85K-04) and IFN-γ responses to anti-SARS-CoV-2 antigen pools, as previously described,4 in patients with AIH at their first appointment at our center following SARS-CoV-2 infection. We recruited 6 AIH-Con receiving ongoing immunosuppression (prednisolone 5-80 mg/day in 4/6 patients; mycophenolate 1,000 mg/day in 2/6 patients; azathioprine 50 and 75 mg in 2/6 patients). AIH-Con were compared to a matched cohort of 24 non-IS-Con (AIH-Con vs. non-IS-Con (Table S1): female sex: 50% vs. 46% (Fisher exact test: p = 1.0); age (median): 47 vs. 51 years (Mann-Whitney U test p = 0.705); time after COVID-19 (median): 48 vs. 52 days (p = 0.631); WHO COVID-19 severity: 100% mild-moderate vs. 91% mild-moderate; 9% severe-critical (p = 1.0). Two of the AIH-Con had concomitant primary sclerosing cholangitis, 3/6 had cirrhosis, 1/6 AIH-Con acquired COVID-19 during the diagnostic work-up of AIH and COVID-19 was diagnosed in 1 patient with AIH 4 days after the first mRNA vaccination.
Quantification of anti-SARS-CoV-2 antibodies was available in 4/6 AIH-Con and for 2 of these patients we had cryo-conserved pre-pandemic samples from our biorepository. Quantification of cellular immune response was available in 5/6 AIH-Con.
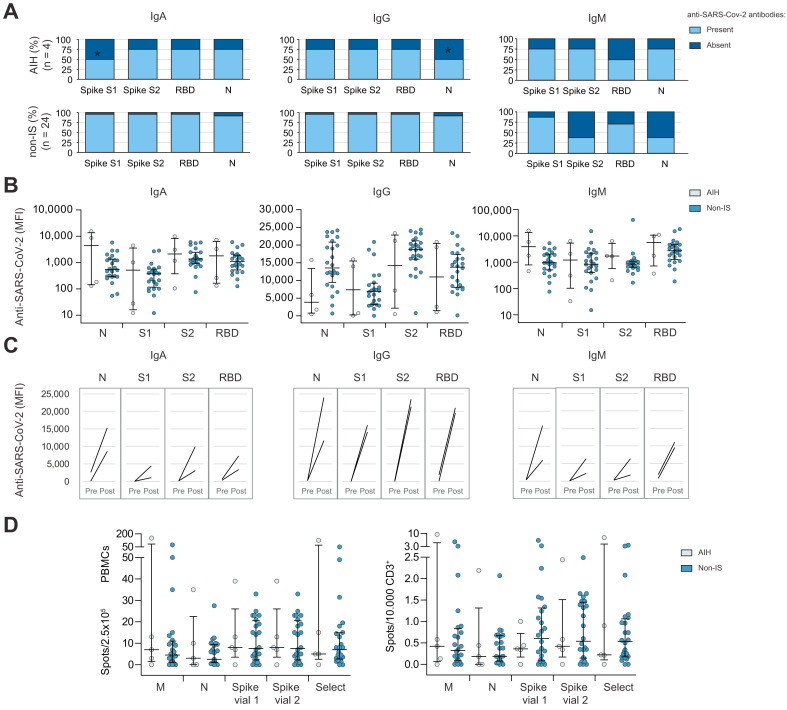
Apart from lower frequencies of IgA against spike S1 peptides and IgG against the nucleocapsid, the presence of all other anti-SARS-CoV-2 IgA, IgG and IgM specificities was comparable in AIH-Con and non-IS-Con (Fig. 1 A). The actual antibody concentrations, quantified by the mean fluorescence intensity (MFI) in the assays, were not significantly different between AIH-Con and non-IS-Con (Fig. 1B). IgA cross-reacting with nucleocapsid peptides (1/2 patients), IgA and IgM cross-reacting with the receptor binding domain (RBD) of the spike protein (2/2 and 1/2 patients) and IgA cross-reacting with the spike S1 peptides (1/2 patients) were found in 2 AIH-Con in pre-pandemic samples. However, the concentration of anti-SARS-CoV-2 antibodies relevantly increased during COVID-19 in AIH-Con irrespective of whether preformed cross-reactive antibodies were present or not (Fig. 1C). AIH-Con produced similar amounts of IFN-γ normalized to numbers of peripheral blood mononuclear cells (PBMCs) and T cells like non-IS-Con (Fig. 1D). Similarly, the interferon-γ response against other respiratory viruses (endemic corona viruses (HCoV-OC43; HCoV-229E), RSV, influenza) were not different between AIH-Con and non-IS-Con (Fig. S1).
Fig. 1.
SARS-CoV-2-specific immunity in immunosuppressed COVID-19 convalescence with autoimmune hepatitis.
(A) IgM, IgA and IgG with reactivity against various SARS-CoV-2 antigens (spike protein, RBD, N) were measured in COVID-19 convalescents with AIH and in a local cohort of non-immunosuppressed convalescents (non-IS) (∗p <0.05 in Fisher’s exact test compared to IS-Con). (B) Concentration of anti-SARS-CoV-2 antibodies (measured as MFI) in AIH and non-IS convalescents. Non-significant differences in Mann-Whitney U tests were not outlined. (C) Anti-SARS-CoV-2 antibody concentrations measured longitudinally before and after COVID-19 in 2 patients with AIH with available pre-pandemic blood samples. (D) IFN-γ production upon stimulation with various SARS-CoV-2 antigen sets in ELISPOT assays normalized to numbers of circulating PBMCs as well as CD3+ T cells in immunosuppressed COVID-19 convalescents with AIH and non-IS. Non-significant differences in Mann-Whitney U tests were not outlined. AIH, autoimmune hepatitis; non-IS, non-immunosuppressed convalescents; MFI median fluorescence intensity; N, nucleocapsid; PBMCs, peripheral blood mononuclear cell; RBD, receptor binding domain.
With all the limitations inherent to a statistical analysis of such a small study we found no evidence for a relevant reduction in humoral or cellular immunity against SARS-CoV-2 in AIH-Con compared to matched non-IS-Con. Although the frequency of some antibody specificities (anti-spike S1 IgA; anti-nucleocapsid IgG) was significantly lower in AIH-Con, the actual antibody concentrations were not different between AIH-Con and non-IS-Con. Similar findings of slightly reduced humoral but otherwise robust cellular immunity against SARS-CoV-2 have been reported for liver transplant recipients (LTRs), who usually receive much stronger immunosuppression.[5], [6], [7] As for patients with AIH, COVID-19 mortality did not seem to be higher in LTRs,[1], [2], [3] , 8 , 9 while the association of COVID-19 with the intake of mycophenolate and tacrolimus was ambiguous in 2 LTR studies.8 , 9 In light of these recent studies from more immunosuppressed LTRs, a comparable immunity against SARS-CoV-2 in AIH-Con is not surprising but is reassuring. However, the development of an immunity against SARS-CoV-2 as strong as in non-IS-Con is remarkable especially with respect to the high cirrhosis rate of 50% in AIH-Con. Similar to LTRs, AIH-Con developed robust immunity even after mild COVID-19.6 , 7 Unfortunately, the AIH-Con cohort is too small to allow for subgroup analyses, e.g. strength of immunosuppression or COVID-19 severity.
This study has many limitations beyond the small sample number. The cross-sectional approach at a single time point cannot describe longitudinal changes over time, like declining anti-SARS-CoV-2 antibody in LTRs within 3-6 months after COVID-19.10 Furthermore, we cannot exclude a bias towards false high IgG antibody concentrations in AIH-Con with persistent hypergammaglobulinemia (Table S1). However, the parallel quantification of IgA antibodies, that are usually not elevated in AIH and which confer mucosal immunity, did not suggest a relevant bias by a hypergammaglobulinemia in AIH-Con. Fortunately, the success of the vaccination programs prevented a further recruitment of non-immunized patients with AIH, because nearly all patients with ongoing immunosuppression and/or advanced liver disease are already vaccinated at our center.
In summary, patients with AIH develop immunity against SARS-CoV-2 as robust as in matched non-IS-Con despite ongoing immunosuppression. This finding might explain in part the missing negative impact of immunosuppression on COVID-19 outcomes in patients with AIH.
Financial support
The work was supported by grants from the German Research Foundation (KFO250 project 7), the state of Lower Saxony (14 -76103-184 CORONA-12/20), the COVID-19 Research Network of the State of Lower Saxony (COFONI) with funding from the Ministry of Science and Culture of Lower Saxony, Germany (14-76403-184; project 1FT21), the Federal Ministry of Health (ZMVI1-2520COR804). R.T. was supported by the Core 100 advanced clinician scientist program from the Hannover Medical School. Collection and analysis of samples of non-immunosuppressed patients were funded by Förderstiftung MHHPlus Hannover.
Authors’ contributions
TK acquired, handled and evaluated the patient data. CSF and BEV performed measurements of humoral and cellular immunity. EJ, CSF, BEV, RT acquired funding. All authors evaluated the data and drafted the manuscript. RT designed and supervised the study.
Conflict of interest
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgement
We thank Konstantinos Iordanidis, Agnes Bonifacius, Hagen Sauer, Sophia Heinrich, Milena Stietzel, Dörthe Rokitta, Nicole Neumann, Juliane Ebersold, Daniel Gussarow, Leonie Cousido, Kerstin Beushausen and Jana Keil for assistance in performing this study. We are grateful to all study participants for providing samples and to the MVZ Laboratory Limbach Hannover GbR for RT-PCR analysis.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.07.012.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Marjot T., Buescher G., Sebode M., Barnes E., Barritt ASt, Armstrong M.J., et al. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74:1335–1343. doi: 10.1016/j.jhep.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Efe C., Dhanasekaran R., Lammert C., Ebi B., Higuera-de la Tijera F., Aloman C., et al. Outcome of COVID-19 in patients with autoimmune hepatitis: an international multi-centre study. Hepatology. 2021 doi: 10.1002/hep.31797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neurath M.F. COVID-19: biologic and immunosuppressive therapy in gastroenterology and hepatology. Nat Rev Gastroenterol Hepatol. 2021 doi: 10.1038/s41575-021-00480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonifacius A., Tischer-Zimmermann S., Dragon A.C., Gussarow D., Vogel A., Krettek U., et al. COVID-19 immune signatures reveal stable antiviral T cell function despite declining humoral responses. Immunity. 2021;54 doi: 10.1016/j.immuni.2021.01.008. 340-354 e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fava A., Donadeu L., Sabe N., Pernin V., Gonzalez-Costello J., Llado L., et al. SARS-CoV-2-specific serological and functional T cell immune responses during acute and early COVID-19 convalescence in solid organ transplant patients. Am J Transplant. 2021 doi: 10.1111/ajt.16570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Ruiz M., Olea B., Almendro-Vazquez P., Gimenez E., Marcacuzco A., San Juan R., et al. T cell-mediated response to SARS-CoV-2 in liver transplant recipients with prior COVID-19. Am J Transplant. 2021 doi: 10.1111/ajt.16708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirchner T., Sauer H., Bonifacius A., Ruhl L., Pink I., Engel B., et al. SARS-CoV-2-specific cellular and humoral immunity in COVID-19 convalescence after liver transplantation. J Hepatol. 2021;75:S473–S474. [Google Scholar]
- 8.Belli L.S., Fondevila C., Cortesi P.A., Conti S., Karam V., Adam R., et al. Protective role of tacrolimus, deleterious role of age and comorbidities in liver transplant recipients with covid-19: results from the ELITA/ELTR multi-center European study. Gastroenterology. 2021;160 doi: 10.1053/j.gastro.2020.11.045. 1151-1163 e1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colmenero J., Rodriguez-Peralvarez M., Salcedo M., Arias-Milla A., Munoz-Serrano A., Graus J., et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caballero-Marcos A., Salcedo M., Alonso-Fernandez R., Rodriguez-Peralvarez M., Olmedo M., Graus Morales J., et al. Changes in humoral immune response after SARS-CoV-2 infection in liver transplant recipients compared to immunocompetent patients. Am J Transplant. 2021 doi: 10.1111/ajt.16599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.