Abstract
Objective
To evaluate the efficacy and safety of Hua Shi Bai Du Granule (Q-14) plus standard care compared with standard care alone in adults with coronavirus disease (COVID-19).
Study Design
A single-center, open-label, randomized controlled trial.
Setting
Wuhan Jinyintan Hospital, Wuhan, China, February 27 to March 27, 2020.
Participants
A total of 204 patients with laboratory-confirmed COVID-19 were randomized into the treatment group and control group, consisting of 102 patients in each group.
Interventions
In the treatment group, Q-14 was administered at 10 g (granules) twice daily for 14 days, plus standard care. In the control group, patients were provided standard care alone for 14 days.
Main Outcome Measure
The primary outcome was the conversion time for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral assay. Adverse events were analyzed in the safety population.
Results
Among the 204 patients, 195 were analyzed according to the intention-to-treat principle. A total of 149 patients (71 vs. 78 in the treatment and control groups, respectively) tested negative via the SARS-CoV-2 viral assay. There was no statistical significance in the conversion time between the treatment group and control group (Full analysis set: Median [interquartile range]: 10.00 [9.00-11.00] vs. 10.00 [9.00-11.00]; Mean rank: 67.92 vs. 81.44; P = 0.051). The recovery time for fever was shorter in the treatment group than in the control group. The disappearance rate of symptoms like cough, fatigue, and chest discomfort was significantly higher in the treatment group. In chest computed tomography (CT) examinations, the overall evaluation of chest CT examination after treatment compared with baseline showed that more patients improved in the treatment group. There were no significant differences in the other outcomes.
Conclusion
The combination of Q-14 and standard care for COVID-19 was useful for the improvement of symptoms (such as fever, cough, fatigue, and chest discomfort), but did not result in a significantly higher probability of negative conversion in the SARS-CoV-2 viral assay. No serious adverse events were observed.
Trial Registration
ChiCTR2000030288
Keywords: COVID-19, Chinese medicine, efficacy, safety, Randomized Controlled Trial, Hua Shi Bai Du Granule
Introduction
The coronavirus disease (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in considerable morbidity and mortality in more than 200 countries. It has become a major public health crisis on a global scale (Wang et al., 2020). Information regarding the epidemiology and clinical features of COVID-19 (Wu et al., 2020; Chen et al., 2020) provides good support for its clinical prevention and treatment, but many of the drugs previously hailed as star drugs, such as remdesivir, favipiravir, lopinavir-ritonavir, and chloroquine or hydroxychloroquine have not shown ideal results in clinical trials, and some of them have been found to have obvious toxic side effects (Wang et al., 2020; Tang et al., 2020). Scientists around the world are actively seeking potentially effective drugs or developing vaccines to treat COVID-19. Widespread use of the vaccine has been important in halting the spread of COVID-19, but the number of cases worldwide continues to rise due to a lack of vaccine capacity and the mutation of the virus. There is still a long way to go to develop effective drugs for COVID-19.
China has quickly halted the spread of COVID-19 with strong containment measures (Li et al., 2020). The strategy of integrated Traditional Chinese Medicine (TCM) with western medicine plays an important role in the prevention and control of COVID-19 in China, with more than 90% of confirmed cases receiving TCM therapy (SCIO-China, 2020). At the beginning of the COVID-19 pandemic, the China National Medical Team of Traditional Chinese Medicine of China Academy of Chinese Medical Sciences (CNMTCM-CACMS Team) went to Wuhan to carry out integrated TCM with Western medicine treatment for COVID-19 in Wuhan Jinyintan Hospital, which is one of the hospitals receiving most patients with COVID-19. In the absence of specific drugs for an emerging infectious disease, based on the theory of TCM with thousands of years of experience in the prevention and treatment of infectious diseases, in particular, accumulated clinical experience of TCM in the SARS in 2003, Chinese medicine (Q-14) was developed for clinical application in the treatment of COVID-19 in Wuhan, China. Q-14, also known as Huashi Baidu granule, is a compound granule composed of 14 Chinese herbs, including Mahuang (Herba Ephedrae), Kuxingren (Armeniacae Semen Amarum), Shigao (Gypsum Fibrosum), Gancao (Glycyrrhizae Radix et Rhizoma), Huoxiang (PogostemonisHerba), Houpu (Magnoliae Officinalis Cortex), Cangzhu (Atractylodis Rhizoma), Caoguo (TsaokoFructus), Fabanxia (Pinelliae Rhizoma Praeparatum), Fuling (Poria), Shengdahuang (Rhei Radix et Rhizoma), Shenghuangqi (Astragali radix), Tinglizi (Descurainiae Semen Lepidii Semen), Chishao (Paeoniae Radix Rubra). The National Health Commission of the People's Republic of China (NHC-China) and National Administration of Traditional Chinese Medicine of the People's Republic of China developed the “National clinical practice guideline for COVID-19 in China” (NHC-NATCM-China guidelines) (NHC-China, 2020). Q-14 is one of the main Chinese herbal preparations for the treatment of COVID-19 in NHC-NATCM-China guidelines, which was widely used in clinical practice during the outbreak of COVID-19 in Wuhan, China. One of the important ways to improve the international recognition of Chinese herbal medicine is to evaluate the clinical efficacy and safety of Chinese herbal medicine with evidence-based medicine (Tang, 2006).
At the critical moment of the COVID-19 outbreak in Wuhan, China, we conducted a single-center, open-label, randomized controlled trial to assess the efficacy and safety of Q-14 in hospitalized adults with laboratory-confirmed COVID-19.
Methods
Trial Oversight
In this open-label randomized trial, we recruited patients with COVID-19 from the Wuhan Jinyintan Hospital, Wuhan, China. The protocol was designed in compliance with the Declaration of Helsinki, Good Clinical Practice guidelines, and local regulatory requirements. The study protocol was approved by the Ethics Review Committee of the China Academy of Chinese Medical Sciences. The protocol was registered on the China Clinical Trial Registry website (www.chictr.org/cn/, ChiCTR2000030288). Written informed consent was obtained from all patients.
The Chinese Academy of Chinese Medical Sciences research group provided the Chinese Medicine composition, and Huayi Pharmaceutical Co., Ltd. produced the compound granules. The results of this trial are reported in accordance with the Consolidated Standards of Reporting Trials guidelines.
Trial design, randomization, and procedures
This study was a single-center, randomized, parallel, open-label trial of Q-14 in patients with laboratory-confirmed COVID-19. Participants were enrolled by investigators working at the Wuhan Jinyintan Hospital. No placebo was used, and the compound granules were not masked. SAS software (version 9.4) was used to generate random sequences in a 1:1 ratio by an independent statistician from the China Center for Evidence-Based Traditional Chinese Medicine who was not aware of the trial protocol. Two research nurses used the mobile software program “CLINICALCRS” to apply for randomization number and assigned participants to interventions. All Participants received standard care in accordance with the NHC-NATCM-China guidelines (version 6.0, published on February 18, 2020). Patients in the treatment group were given Huashi Baidu granule (Q-14) the day after randomization, with a dose of 10 g (granules), twice daily for 14 days. The day of randomization was defined as day 0, and the following days were defined as days 1 to 14. Investigators, patients, and statisticians were not masked to the group assignment. Routine care givers and laboratory staff were unaware of treatment. Datawere recorded using the Medroad Cloud Electronic Data Capture System (Jiangsu Famous Medicine Science and Technology Ltd.), and double data entry was carried out by two different recorders. Study quality control was executed at three levels:the first level by a clinical research associate, the second level by an independent Good Clinical Practice staff who did not participate in the trial, and the third level by a clinical research management department of China Academy of Chinese Medical Sciences.
Patients
We recruited 204 patients with laboratory-confirmed COVID-19 at the Wuhan Jinyintan Hospital between February 27 and March 27, 2020.
The inclusion criteria were as follows: 1) compliance with the diagnostic criteria for general type COVID-19 in the NHC-NATCM-China guidelines (version 6.0); 2) 18 ≤ aged ≤ 75 years; 3) Acceptance to participate in the trial by the patient, the legal guardian, or the person in charge of the medical situation signed the informed consent through a paper signature.
The exclusion criteria included: 1) critical patients; 2) patients who could not guarantee compliance of using Q-14 during the treatment period, or patients who had difficulty taking drugs via the oral or nasal route; 3) patients with severe primary respiratory disease or other pathogenic microbial pneumonia with similar symptoms as COVID-19; 4) pregnant or parturient women, including patients who had plans of getting pregnant or a positive urine pregnancy test; 5) patients with other systemic malignant diseases such as malignant tumors, mental illnesses, etc., which the researchers considered unsuitable for participation in the trial; 6) patients with allergy or intolerance to taking Chinese medicine herbs.
The definition of critical patients was in accordance with the disease severity of COVID-19 in the National Clinical Practice Guidelines for COVID-19 in China (version 6.0).
Outcome and Assessment
The primary outcome of this trial was the conversion time of the SARS-CoV-2 viral assay. The secondary outcomes were: 1) the change in the 7-point scale; 2) the rate of critical aggravation; 3) blood routine test outcomes; 4) blood biochemical test outcomes; 5) recovery time for fever; 6) symptom improvement, and 7) evaluation of chest CT examination after treatment compared with baseline. Adverse events were reviewed daily to ensure patient safety.
SARS-CoV-2 viral assays for specimens from the upper respiratory tract were performed on days 0, and 7 to 15. If the test result was negative, the SARS-CoV-2 viral assay was conducted at least 24 h later to ensure that there were two consecutive reports of negative results. When there were two consecutive negative results, the patient was defined as a conversion-of-negative ending, and the day of the first negative report was determined as the conversion day. The time from day 0 to the day of conversion was defined as the conversion time.
The 7-point scale was a seven-category ordinal scale to test the condition and prognosis of severity during treatment. It consisted of the following categories: 1) not hospitalized with the resumption of normal activities; 2) not hospitalized,but unable to resume normal activities; 3) hospitalized, not requiring supplemental oxygen; 4) hospitalized, requiring supplemental oxygen; 5) hospitalized, requiring nasal high-flow oxygentherapy, noninvasive mechanical ventilation, orboth; 6) hospitalized, requiring extracorporeal membrane oxygenation, invasivemechanical ventilation, or both; and 7) death. The 7-point scale was recorded from day 1 to 14. The change in the 7-point scale was the score difference after treatment compared with baseline.
The classification of COVID-19 was recorded on days 0, 1, and 14. The classification included four categories: mild, moderate, severe, and critical, in increasing severity. If the classification changed to critical during days 1 to 14, the patient was defined as having critical aggravation. The rate of critical aggravation was assessed after treatment.
Routine blood tests and blood biochemical test outcomes included: complete blood cell count with differential, blood chemistry, creative protein, hypersensitive C-reactive protein, amyloid protein, myohemoglobin, hypersensitive troponin, erythrocyte sedimentation rate, ferroprotein, D-dimer, and interleukin 6, which were tested on days 0, 7, and 14.
Vital signs were recorded on days 0, 1, and 14. If the body temperature was > 37.3 °C, the patient was defined having a fever. We recorded the day when it began to remain stable (below 37.3 °C) in order to determinethe fever recovery time.
Symptoms including cough, fatigue, headache, chest discomfort, and dry throat were recorded on days 0 and 14. The disappearance of symptoms was recorded after treatment. Symptom improvement was evaluated based on the disappearance rate of the five symptoms.
Chest computed tomography (CT) examinations were performed on days 0 and 14. Two radiologists with at least 20 years of working experience reviewed the CT reports and images, who were unaware of the randomization. The judgment of chest CT included: 1) score of ground glass area; 2) score of consolidation area; 3) density change of ground glass imaging manifestation; 4) density change of consolidation imaging manifestation; 5) overall evaluation of chest CT examination after treatment compared with baseline. To count the score of the lesion area, each lung was divided into 10 regions according to anatomy, with 20 regions for both sides. If the lesion area was over 50% (contained) in one region, it was judged as 2 points, and if it was below 50%, it was judged as 1 point. The range of scores for the area ranged from 0 to 40 points. Density change was recorded after treatment as decreased, no change, or increased. The overall evaluation of chest CT examination was the case and proportion of improved, no-change, and aggravated patients in each group.
In this trial, we recorded the timing, duration, severity, management, and consequences of adverse events, and determined their association with the use of study medications (Annex 1, Annex 2, Annex 3).
Annex 1.
Research Period
Annex 2.
Symptom improvement-FAS
Annex 3.
Symptom improvement-PPS
Statistical analysis
We took the occurrence of nucleic acid negative conversion and its duration after diagnosis as the certain event, calculate sample size and power by Cox PH (proportional hazards) 1-Sided superiority of time-to-event analysis. The sample size was calculated based on the alternative hypothesis that the hazard ratio of SARS-CoV-2 viral assay negative conversion rate between the treatment and control groups during the 14-day trial period was . It was assumed that the SARS-CoV-2 viral assay-negative conversion time followed an exponential distribution. We assumed that the SARS-CoV-2 viral assay negative conversion rate was 0.6, in all patients within 14 days, and the hazard ratio based on the experience of treatment at the time. The sample size distribution between the treatment and control groups was 1:1, the one-sided superiority type I error was 0.05, and the type II error was 0.2, that is, the power was 80%. We estimated a total sample size of 204 patients with adropout rate of 15%.
For the primary outcome, the SARS-CoV-2 viral assay negative conversion time, we first used the Wilcoxon rank-sum test to test the difference in SARS-CoV-2 viral assay negative conversion time between the treatment group and control group and presented the medians and interquartile range (IQR). The Kaplan-Meier method was used to estimate the cumulative negative conversion rate and to draw the survival curve. The negative conversion rate was compared among groups using the log-rank test, and the median time and 95% confidence interval (CI) were calculated. The hazard ratios were estimated using the Cox regression model. Hazard ratios greater than 1 indicated that the negative conversion rate of the treatment group was higher than that of the control group.
For secondary outcomes, the change inthe 7-point scale was tested using the Wilcoxon rank-sum test. The rate of critical aggravation was tested using Pearson's chi-square test. Both blood routine and blood biochemistry outcomes were numerical data, which were tested by the t-test for normal distribution data and Wilcoxon rank-sum test for non-normal distribution data. Fever recovery time was tested using the Wilcoxon rank-sum test. For symptom improvement, which indicated the disappearance rate of cough, chest tightness, headache, fatigue, and pharynx, we used the Pearson chi-square test. For the chest CT outcome, the score of area and density for both ground glass and consolidation imaging manifestations, and overall evaluation of chest CT examination, were tested using the Wilcoxon rank-sum test.
Safety analyses were based on patients’ actual exposure to the treatment. In this study, statistical packages base, Stats, Rcompanion, and survival in R version-3.6.2, and SPSS 20.0, were used for statistical analyses and verifications.
Patient and public involvement
No patients were involved in the research design or implementation plan. There is no plan to disseminate the results of this trial to the study participants.
Result
Patients
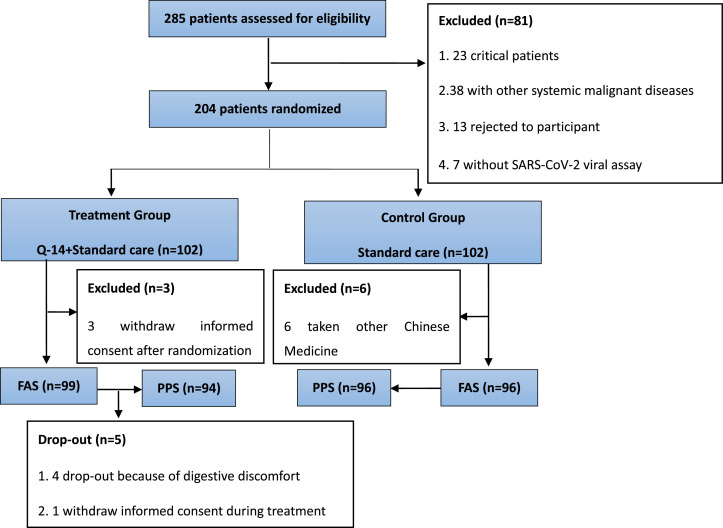
Of the 285 patients assessed from February 27 to March 27, 81 patients did not meet the eligibility criteria. The remaining 204 patients were randomized at a ratio of 1:1 to the treatment and control groups. A total of 195 patients were included in the full analysisset (FAS) (99 in the treatment group and 96 in the control group) (Fig 1 ).The mean age was 54 years, and 36.4% were men. Table 1 shows the baseline demographic and clinical characteristics of the patients in the treatment and control groups.
Fig. 1.
Research flow chart
Table 1.
Baseline demographic and clinical characteristics of patients in FAS
| Characteristics | Treatment group (n=99) | Control Group (n=96) | Total (n=195) | P |
|---|---|---|---|---|
| Median (IQR)age, years | 56.00 (48.50-62.00) | 56.50 (48.75-62.25) | 56.00 (48.50- 62.00) | 0.695 |
| SEX | ||||
| Male | 36 (36.4%) | 37 (38.5%) | 73 (37.4%) | 0.753 |
| Disease severity | ||||
| Mild and Moderate | 74 (74.7%) | 89(92.7%) | 163 (83.6%) | 0.001 |
| Severe | 25 (25.3%) | 7 (7.3%) | 32 (16.4%) | |
| Coexisting condition | ||||
| Hypertension | 63 (63.6%) | 54 (56.2%) | 117 (60.0%) | 0.293 |
| Hyperlipemia | 6 (6.1%) | 10 (10.4%) | 16 (8.2%) | 0.268 |
| Coronary heart disease | 7 (7.1%) | 3 (3.1%) | 10 (5.1%) | 0.331 |
| Thyroid disorder | 1 (1.0%) | 1 (1.0%) | 2 (1.0%) | > 0.999 |
| Cerebral lacunar infarction | 1 (1.0%) | 3 (3.1%) | 4 (2.1%) | 0.363 |
| Diabetes | 18 (18.2%) | 9 (9.4%) | 27 (13.8%) | 0.075 |
| Rheumatoid disease | 2 (2.0%) | 1 (1.0%) | 3 (1.5%) | > 0.999 |
| Chronic bronchitis | 1 (1.0%) | 1 (1.0%) | 2 (1.0%) | > 0.999 |
| Symptoms | ||||
| Fever | 85 (85.9%) | 90 (93.8%) | 175 (89.7%) | -- |
| Cough | 86 (86.9%) | 73 (76.0%) | 159 (81.5%) | 0.051 |
| Fatigue | 84 (84.8%) | 87 (90.6%) | 171 (87.7%) | 0.220 |
| Headache | 14 (14.1%) | 12 (12.5%) | 26 (13.3%) | 0.736 |
| Chest discomfort | 54 (54.5%) | 59 (61.5%) | 113 (57.9%) | 0.328 |
| Dry throat | 87 (87.9%) | 87 (90.6%) | 174 (89.2%) | 0.536 |
| Laboratory parameters—Median(IQR)/mean (SD) | ||||
| White blood cell count, × 109/l | 5.84 (4.90-7.29) | 5.68 (4.78-6.75) | 5.77 (4.82-6.89) | 0.174 |
| Red blood cell count, × 1012/l | 4.13 (3.84-4.44) | 4.04 (3.67-4.35) | 4.06 (3.76-4.39) | 0.049 |
| Platelet count, × 109/l | 229.00 (172.00-267.50) | 212.00 (184.75-261.75) | 222.00 (179.50-265.50) | 0.889 |
| Hemoglobin, g/l | 125.00 (118.00-135.50) | 122.50 (111.00-133.50) | 124.00 (116.00-135.00) | 0.149 |
| Absolute Neutrophil count, × 109/l | 3.66 (2.69-4.43) | 3.28 (2.61-4.19) | 3.42 (2.67-4.35) | 0.215 |
| Absolute Lymphocyte count, × 109/l | 1.73 (1.48-2.02) | 1.65 (1.38-2.07) | 1.68 (1.42-2.05) | 0.145 |
| Monocyte count, × 109/l | 0.41 (0.35-0.49) | 0.38 (0.32-0.46) | 0.40 (0.34-0.48) | 0.077 |
| Neutrophil percentage, % | 59.30 (54.35-65.55) | 58.85 (50.53-64.10) | 59.10 (52.60-65.20) | 0.387 |
| Lymphocyte percentage, % | 30.17 (8.41) | 30.27 (9.54) | 30.22 (8.96) | 0.940 |
| Monocyte percentage, % | 7.20 (6.10-7.80) | 6.80 (5.88-7.85) | 7.00 (6.00-7.80) | 0.621 |
| Total bilirubin, μmol/l | 12.20 (10.00-15.55) | 11.90 (9.80-14.88) | 12.10 (9.85-15.25) | 0.700 |
| Alanine transaminase, U/l | 25.00 (19.00-37.50) | 27.50 (19.00-35.00) | 26.00 (19.00-35.00) | 0.601 |
| Glutamic oxalacetic transaminase, U/l | 25.00 (22.00-31.00) | 29.00 (23.00-34.00) | 26.00 (22.00-33.00) | 0.133 |
| Albumin, g/l | 39.40 (37.25-40.85) | 39.10 (37.20-40.50) | 39.20 (37.20-40.70) | 0.751 |
| Urea, mmol/l | 4.50 (3.65-5.23) | 4.57 (3.60-5.50) | 4.54 (3.60-5.37) | 0.737 |
| Creatinine, μmol/l | 62.40 (53.50-73.90) | 64.60 (56.50-73.10) | 63.00 (54.65-73.90) | 0.319 |
| Blood glucose, mmol/l | 5.20 (4.80-6.05) | 5.10 (4.80-6.12) | 5.20 (4.80-6.10) | 0.776 |
| Creatine kinase, U/l | 63.00 (45.25-85.75) | 65.50 (50.00-95.00) | 63.50 (48.00-87.00) | 0.380 |
| Creatine kinase isoenzyme, U/l | 10.00 (8.00-12.00) | 11.00 (8.00-12.25) | 10.00 (8.00-12.00) | 0.778 |
| Blood potassium, mmol/l | 4.20 (4.00-4.50) | 4.30 (4.00-4.50) | 4.30 (4.00-4.50) | 0.613 |
| Blood sodium, mmol/l | 140.00 (139.00-141.00) | 140.00 (138.00-142.00) | 140.00 (138.00-142.00) | 0.498 |
| Blood chlorine, mmol/l | 106.00 (104.00-108.00) | 106.00 (104.00-107.00) | 106.00 (104.00-107.00) | 0.217 |
| C-reactive protein, mg/l | 1.00 (0.50-2.90) | 1.00 (0.60-1.70) | 1.00 (0.50-2.38) | 0.647 |
| Hypersensitive C-reactive protein, mg/l | 1.10 (0.50-2.00) | 1.10 (0.50-2.80) | 1.10 (0.50-2.35) | 0.673 |
| Amyloid protein, mg/l | 3.70 (3.70-6.45) | 3.70 (3.70-5.75) | 3.70 (3.70-6.15) | 0.699 |
| Myoglobin, ng/ml | 30.60 (24.70-42.40) | 32.40 (24.95-41.85) | 31.00 (24.80-41.92) | 0.585 |
| Hypersensitive troponin, pg/ml | 1.50 (0.70-3.40) | 1.70 (0.70-3.20) | 1.70 (0.70-3.40) | 0.704 |
| Blood sedimentation, mm/h | 15.00 (7.00-23.00) | 16.00 (11.00-25.00) | 16.00 (9.00-25.00) | 0.333 |
| Ferritin, ng/ml | 196.43 (128.24-309.99) | 195.99 (114.96-300.11) | 195.99 (119.33-309.75) | 0.648 |
| D-dimer, μg/ml | 0.38 (0.23-0.65) | 0.44 (0.24-0.84) | 0.40 (0.24-0.76) | 0.323 |
| Interleukin 6, pg/ml | 7.02 (5.46-8.59) | 6.78 (5.73-8.43) | 6.94 (5.54-8.54) | 0.804 |
| Procalcitonin, ng/ml | 0.05 (0.05-0.05) | 0.05 (0.05-0.05) | 0.05 (0.05-0.05) | 0.554 |
| Chest CT parameters—Median(IQR) | ||||
| score of ground glass area | 16.50 (10.25-21.75) | 17.00 (8.00-27.50) | 16.75 (9.00-24.12) | 0.701 |
| Score of consolidation area | 0.00 (0.00-0.00) | 0.00 (0.00-0.00) | 0.00 (0.00-0.00) | 0.901 |
Primary outcome
Among 195 patients with FAS, 149 (76.4%) patients (71 treatment group, 78 control group) converted to negative before the cut-off day of analysis, and the remaining 46 patients (28 treatment group, 18 control group) did not arrive at a conversion-to-negative result.
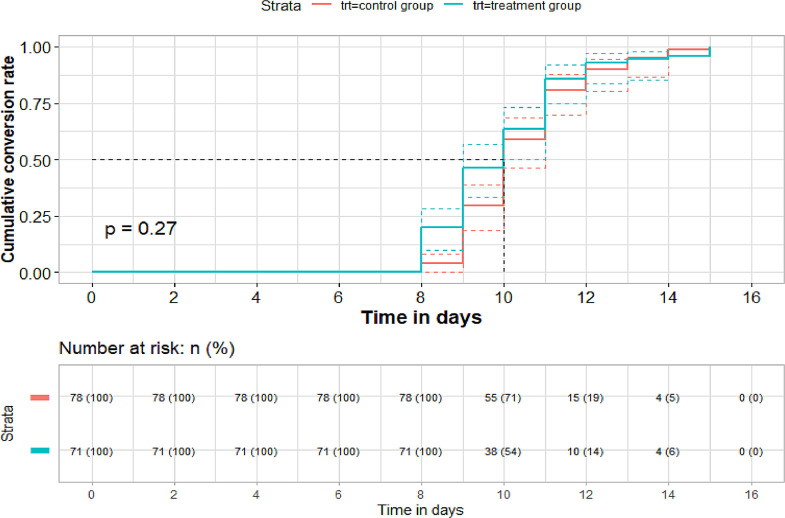
For the conversion time of SARS-CoV-2 viral assay, there was no significant difference between the treatment and control groups in both FAS and PPS (per-protocolset) (FAS: n = 149. Median [IQR]: 10.00 [9.00-11.00] vs. 10.00 [9.00-11.00]; Mean rank: 67.92 vs. 81.44; P = 0.051. PPS: n = 148. Median [IQR]: 10.00 [9.00-11.00] vs. 10.00 [9.00-11.00]; Mean rank: 67.86 vs. 80.46; P = 0.068). The average time of conversion to negative showed some advantage in the treatment group (Mean [SD]: FAS: 10.01 [1.74] vs. 10.44 [1.49] days). PPS: 10.04 [1.73] vs. 10.44[1.49] days). The median time to conversion between the treatment group and control group showed no significant difference as well (FAS: 10.00 [9.00-10.00] vs. 10.00 [10.00-11.00] days, HR (95%CI): 1.165 (0.84-1.61), P = 0.27. PPS: 10.00 [9.00-11.00] vs. 10.00 [10.00-11.00] days, HR (95%CI): 1.15 (0.83-1.59) P = 0.31, by log rank test; Fig. 2, Fig. 3 ).
Fig. 2.
Time to conversion analysis of SARS-CoV-2 viral assay and conversion ending (n = 149, FAS)
Fig. 3.
Time to conversion analysis of SARS-CoV-2 viral assay and conversion ending (n = 148, PPS)
Among the 116 participants with cardiovascular disease who reached conversion-to-negative, the conversion time of SARS-CoV-2 viral assay was significantly shorter in the treatment group than in the control group. (Median [IQR]: 10.00 [9.00-11.00] vs. 10.00 [9.00-11.00]; Mean rank: 51.63 vs. 65.61; P = 0.022.). The average time of conversion to negative showed some advantage in the treatment group (Mean [SD]: FAS: 9.85 [1.42] vs. 10.54 [1.56] days). The median time to conversion between the treatment and control groups was significantly different (Median [IQR]: 10.00 [9.00-10.00] vs. 10.00 [10.00- 11.00] days, HR (95%CI): 1.484 (1.02-2.15), P = 0.023 by log-rank test. Fig 4 ).
Fig. 4.
Time to conversion analysis of SARS-CoV-2 viral assay and conversion ending, in participants with cardiovascular disease who reached negative conversion (n = 116)
Secondary outcome
The change in the 7-point scale was similar between the two groups (Median [IQR]: FAS: -2.00 [-3.00-0.00] vs. -2.00 [-3.00-0.00], P = 0.109). PPS: -2.00 [-3.00-0.00] vs. -2.00 [-3.00-0.00], P = 0.232).
No patient turned to the critical category during the observation.
There was no significant difference in blood routine test and blood biochemical test.
Fever recovery time was shorter in the treatment group than in the control group (Median [IQR]: FAS: 2.00 [2.00-3.00] vs. 3.00 [1.00-4.00] days, P = 0.029. PPS: 2.00 [2.00-3.00] vs. 3.00 [1.00-4.00], P = 0.049).
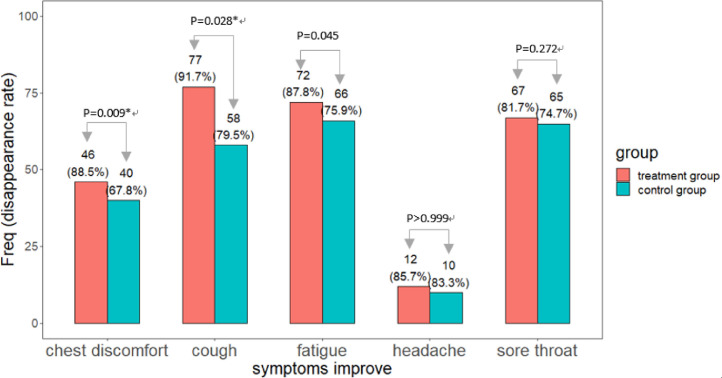
The symptom disappearance rate of cough (FAS: 90.7% vs. 79.5%, P = 0.045). PPS: 91.7% vs. 79.5%, P = 0.028), fatigue (FAS: 88.1% vs. 75.9%, P = 0.038. PPS: 87.8% vs. 75.9%, P = 0.045), chest discomfort (FAS: 87.0% vs. 67.8%, P = 0.015. PPS: 88.5% vs. 67.8%, P = 0.009) was significantly higher in the treatment group. The symptom disappearance rate of headache (FAS and PPS: 85.7% vs. 83.3%, P > 0.999) and dry throat (FAS: 82.8% vs. 74.7%, P = 0.195. PPS: 81.7% vs. 74.7%, P = 0.272) were similar between the two groups.
In chest CT examinations, the score of the ground glass area was significantly lower in the treatment group than in the control group after treatment (Median [IQR]: FAS: 8.50 [5.00-15.25] vs. 14.00 [7.00-21.75], P = 0.031. PPS: 9.00 [4.62-16.00] vs. 14.00 [7.00-21.75], P = 0.042) with a larger decrease (Median [IQR]: FAS: -5.75 [-11.25- -2.00] vs. -0.75 [-2.12- 2.12], P < 0.001. PPS: -6.50 [-11.75- -2.00] vs. -0.75 [-2.12- 2.12], P < 0.001). The density of the ground glass area decreased significantly in the treatment group (FAS: decreased 36 [80.0%], no-change 9 [20.0%], increased 0 [0%] vs. decreased 17 [51.5%], no change 15 [45.5%], increased 1 [3.0%], P = 0.007). PPS: decreased 33 [78.6%], no change 9 [21.4%], increased 0 [0%] vs. decreased 17 [51.5%], no change 15 [45.5%], increased 1 [3.0%], P = 0.013). The overall evaluation of chest CT examination after treatment compared with baseline showed that more patients showed improvement in the treatment group (FAS: improved 69 [85.2%], nochange 12 [14.8%], aggravated 0 [0%] vs. improved 55 [67.9%], nochange 23 [28.4%], aggravated 3 [3.7%], P = 0.008). PPS: improved 65 [85.5%], no change 11 [14.5%], aggravated 0 [0%] vs. improved 55 [67.9%], no-change 23 [28.4%], aggravated 3 [3.7%], P = 0.008). There was no significant difference in the scores of area and density of consolidation manifestation.
Safety
Among the 204 patients, a total of 202 patients were analyzed as the safety population (100 treatment group, 102 control groups), as two patients inthe treatment group withdrew their informed consent on the day of randomization and had no baseline information. A comparison of recorded adverse events showed that there were 14 patients (16 adverse events) in the treatment group and 15 patients (23 adverse events) in the control group (Table 2 ). No serious adverse events were observed. Diarrhea (n = 15) was the most common adverse event in this study. For patients who experienced digestive discomfort, the Q-14 dosage in the treatment group was adjusted to half the dosage daily until the symptoms improved. Other adverse events were transient, with a duration of one to two days.
Table 2.
Summary of adverse events in safety population*
| Adverse events | Treatment group (n=100) | Control Group (n=102) | Total (n=202) |
|---|---|---|---|
| All adverse event | 16 | 23 | 35 |
| Diarrhea | 8 (8.0%) | 7 (6.9%) | 15 (7.4%) |
| Abdominal discomfort | 2 (2.0%) | 3 (2.9%) | 5 (2.5%) |
| Decreased appetite | 3 (3.0%) | 0 (0) | 3 (1.5%) |
| Anxiety | 0 (0) | 2 (2.0%) | 2 (1.0%) |
| Oral ulcer | 1 (1.0%) | 1 (1.0%) | 2 (1.0%) |
| short breath | 0 (0) | 2 (2.0%) | 2 (1.0%) |
| Constipation | 0 (0) | 1 (1.0%) | 1 (0.5%) |
| Vomiting | 1 (1.0%) | 0 (0) | 1 (0.5%) |
| Itchy skin | 0 (0) | 1 (1.0%) | 1 (0.5%) |
| Lower extremity edema | 0 (0) | 1 (1.0%) | 1 (0.5%) |
| Dry eye | 1 (1.0%) | 0 (0) | 1 (0.5%) |
| Limb pain | 0 (0) | 1 (1.0%) | 1 (0.5%) |
*Values stand for numbers (percentages of patient happened adverse events)
Discussion
This clinical trial (conducted during the outbreak of the COVID-19 pandemic in Wuhan, China) is the first registered randomized controlled trial to evaluate the administration of Huashi Baidu granule (Q-14) in hospitalized adult patients with laboratory-confirmed COVID-19 in Wuhan, China. These findings provide evidence to support a notable improvement in typical uncomfortable symptoms caused by COVID-19, such as fever, cough, fatigue, and chest discomfort. Additionally, a significant improvement in chest CT by a 14-day course of Q-14 administration to standard care in hospitalized adult patients with COVID-19.
At present, there is no definite antiviral drug to accelerate the probability of negativeconversion for SARS-CoV-2 (Parvathaneni and Gupta, 2020; Yadav(Yadav et al., 2020), and the development of a vaccine (Torreele, 2020; Wang et al., 2020) appears to be the last straw for the prevention and control of COVID-19. The development of new antivirals requires considerable time and effort for drug design and validation. Clearly, not only will this take time, but everything is unknown. Administration of Huashi Baidu granule (Q-14) did not result in a significantly higher probability of negative conversion than the standard of care alone in patients admitted to hospital with COVID-19. However, it is beneficial for the improvement of typical symptoms in COVID-19 patients. Improvement in clinical symptoms is important to reduce the level of discomfort in hospitalized adult patients with COVID-19. Thus, relieving depression or anxiety caused by typical symptoms of COVID-19 patients or medical workers (Elhadi et al., 2020; Wang et al., 2020) such as fever, cough, fatigue, and chest discomfort is beneficial. Why Q14 can relieve some symptoms but not others is also one of the research directions that we need to focus on in the future. According to the theory of traditional Chinese medicine, the compatibility of the constituent drugs of Q14 plays a wonderful and important role, which may be one of the important basis for the optimization of prescription in the future.
In our study, the safety of Huashi Baidu granule (Q-14) was investigated. No serious adverse events were reported, except for diarrhea (8 cases in the treatment group) or other adverse reactions in some patients, which resolved within a few days. The overall clinical safety of a 14-day course of Huashi Baidu granule (Q-14) administration is documented.
On the other hand, we performed a subgroup analysis, an increase in the probability of negative conversion of SARS-CoV-2 viral assay conferred by the addition of a 14-day course of Huashi Baidu granule (Q-14) administration to the standard care in hospitalized adult patients with COVID-19 and cardiovascular diseases. Although the results were statistically significant, they were not particularly significant in terms of time (days). This is not within our expectations, which is an interesting finding. Although we cannot rule out the possibility of a chance finding, we think it may be related to the following factor. The majority of COVID-19 patients with cardiovascular diseases are hypertensive; Huashi Baidu granule (Q-14) may have a certain targeted improvement effect on the pathological status of hypertension in addition to standard care. Molecular docking showed that baicalein and quercetin were the top two compounds of Huashi Baidu granule (Q-14), baicalein had a strong affinity for SARS-CoV-2, and quercetin had a strong affinity for ACE2 3CL, indicating that baicalein and quercetin might play an important role in the treatment of SARS-CoV-2 infection (Tao et al., 2020). Future clinical studies with larger sample sizes are needed to confirm this effect of Huashi Baidu granule (Q-14). COVID-19 patients with pre-existing cardiovascular diseases experience disproportionately worse mortality outcomes (Peng et al., 2020; GolemiMinga et al., 2020), and the findings of this clinical study may provide an effective drug for the prevention and treatment of COVID-19 patients with hypertension. More rigorous clinical and basic research is needed to validate this speculation.
Chest CT is used for the diagnosis of COVID-19, as an important complement to the reverse-transcription polymerase chain reaction (RT-PCR) tests (Ai et al., 2020; Wong et al., 2020). In the early stages of the COVID-19 disease, chest CT may havea greater diagnostic value than RT-PCR tests. At the same time, it will be a very important observational indicator to evaluate the curative effect ofthe therapeutic schedule. Inaddition, scientists have proposed a non-invasive and quantitative prognostic tool for predicting poor outcomes in patients with COVID-19 based on CT (Wu et al.,2020). Our study found that after 14 days of treatment, Huashi Baidu granule (Q-14) with standard care can significantly improve abnormal chest CT changes in COVID-19 patients and significantlydecrease the score of the ground glass area (characteristic chest CT change of COVID-19). This result suggests that Huashi Baidu granule (Q-14) has an important therapeutic role in alleviating the condition of COVID-19 patients.
Clinical evidence has shownthat TCM plays a significant role in the prevention and control of COVID-19 (Hu et al., 2020; Xiao et al., 2020; Xiong et al., 2020). Although these studies showed that TCM does not have a direct antiviral effect in clinical practice, its ability to quickly relieve clinical symptoms may be closely related to the enhancement of the immune ability of the body.
Limitations of Study
The present study had some limitations. First, we did not set up a placebo as a Huashi Baidu granule (Q-14) control. In the context of the COVID-19 early outbreak in Wuhan, China, many clinical trials related to COVID-19 are yet to be prepared because of Wuhan quarantine, especially for the Huashi Baidu granule (Q-14) placebo. As we did not adopt stratification randomization according to disease severity, the cases of severe patients showed significant differences between the two groups. This may have greatly influenced the conclusions of this trial, and the placebo effect of Huashi Baidu granule (Q-14) cannot be ruled out. Second, the open-label rather than double-blind design may have resulted in the possibility of biased assessments. Third, we began to determine SARS-CoV-2 RNA conversion from the 7th day of the intervention, which may have overlooked some patients who hada negative conversion of SARS-CoV-2 within 7 days. Finally, the specimens collected in our trial for SARS-CoV-2 RNA determination were mostly from the upper respiratory tract rather than bronchoalveolar lavage fluid, which could result in false-negative results.
Conclusion and Policy Implications
The global introduction of TCM is a demand forthetimes, which is anurgent worldwide demand for TCM. This trial provides evidence to support a notable improvement in typical uncomfortable symptoms caused by COVID-19 such as fever, cough, fatigue, and chest discomfort. Additionally, there was a significant improvement inchest CT by the addition of a 14-day course of Huashi Baidu granule (Q-14) administration to standard care in hospitalized adult patients with COVID-19. In the current situation, Huashi Baidu granule (Q-14) may provide a safe and effective option for controlling the spread of COVID-19, or a solution with Chinese characteristics. China's great success in the prevention and control of COVID-19 has demonstrated the feasibility of the Chinese approach.
Funding
The study was funded by National Key Research and Development Plan for the Emergency Management of Novel Coronavirus Pneumonia(No.2020YFC0841500)
Credit Author Statement
JL contributed to the writing on this paper and in charge of the trial management during this study. WY contributed to the data cleaning and statistical analysis. YL contributed to the result analysis and modified the study report. CL contributed to the data collection organization on-site in Wuhan Jinyint Hospital and carried out the first level quality control. LR contributed to the organization in Wuhan Jinyintan Hospital and communicated with the patients. CZ designed the protocol and finished the trial registration. RH contributed to the data cleaning and assisted the trial management. XS led the data collection and data recording. QM, WL, HL contributed as clinical expert on-site in Wuhan Jinyintan Hospital from protocol designing to implementation. HS contributed to the trial drug coordinating on-site in Wuhan. YC,ZZ contributed to randomisation number application. LH, ZY, LZ, BW, GD, YX, BL, CX, YC, YB, JG, JY, JW, WQ, SC, BY, WW, JL, XX, MX, JJ, GW, XC contributed to theimplementation of intervention during this trial.HZ, JS, LL, DL contributed to collect data on-site in Wuhan. LG contributed to finalize the structure of this paper. LH organized the research group and contributed to the overall designing and management of this trialfor the corresponding authorship. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Exclusive Licence
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, a worldwide licence to the Publishers and its licensees in perpetuity, in all forms, formats and media according to the requirement on exclusive licence of the publication.
Data Availability Statement
The data of this article could be shared within six months after the trial complete via the Medroad Cloud Electronic Data Capture System (cloud.medroad.cn/).
Funding source
The study was funded by National Key Research and Development Plan for the Emergency Management of Novel Coronavirus Pneumonia (No. 2020YFC0841500)
Declaration of Competing Interest
None
Acknowledgments
We thank China National Medical Team of Traditional Chinese Medicine of China Academy of Chinese Medical Sciences (CNMTCM-CACMS Team), who went to Wuhan in the most critical moment for medical aid and with their hard working, we finished this trial. The member of CNMTCM-CACMS Team includingLuqi Huang, Cheng Lu, Qing Miao, Wenliang Lv, Hao Li, Huaxin Shi, Lijie Hu, Zhixu Yang, Li Zhang, Bing Wang, Guoju Dong, Yongyue Xian, Bin Li, Zhenqi Zhou, Chunyan Xu, Yingying Chen, YongjunBian, Jing Guo, Jinliang Yang, Jian Wang, Wensheng Qi, Suping Chen, Yang Chen, Bei Yan, Wei Wang, Jing Li, XiaoleiXie, Ming Xu, Jianxin Jiang, Gang Wang, Xiaodong Cong, Haoning Zhu, Jiaheng Shi, LuxingLeng, Dongxu Li. Xinghua Xiang and Yunfei Xing assisted the statistical work. Mingjiang Yao, Ping He, Xiao Liang, Ruiyan Cao, Li Li, Benliang Zou, Ning Wang, Shihuan Tang, Min Li, Xunlu Yin, Hao Wang, Chuanzhou Yang, Chunyu Gao, Liying Wang, Yongli Dong, Ming Chen, Lu Zhang, Xu Wei contributed to the data recording. Yang Zhao, Qiaoning Yang, Rui Li, Yingjue Jia, Yu Dong carried out the second level quality control. Hongjun Yang carried out the third level quality control and Yanping Wang contributed to the management of the trial. Xuedong Yang and Chunzhi Li contributed to the CT reports and images review. Meng Wu contributed to the CT review data verifying. Hui Li contributed to the active ingredients and fingerprint analysis.
References
- Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q., 2020. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ368, m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed]
- Elhadi M, Msherghi A, Elgzairi M, Alhashimi A, Bouhuwaish A, Biala M, Abuelmeda S, Khel S, Khaled A, Alsoufi A, Elmabrouk A, Alshiteewi FB, Alhadi B, Alhaddad S, Gaffaz R, Elmabrouk O, Hamed T.B., Alameen H, Zaid A, Elhadi A, Albakoush A. Psychological status of healthcare workers during the civil war and COVID-19 pandemic: A cross-sectional study. J. Psychosom. Res. 2020;137 doi: 10.1016/j.jpsychores.2020.110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GolemiMinga I, Golemi L, Tafur A, Pursnani A. The Novel Coronavirus Disease (COVID-19) and Its Impact on Cardiovascular Disease. Cardiol. Rev. 2020;28:163–176. doi: 10.1097/CRD.0000000000000317. [DOI] [PubMed] [Google Scholar]
- Hu K, Guan W.J., Bi Y, Zhang W, Li L, Zhang B, Liu Q, Song Y, Li X, Duan Z, Zheng Q, Yang Z, Liang J, Han M, Ruan L, Wu C, Zhang Y, Jia ZH, Zhong NS. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chen Q, Feng L, Rodewald L, Xia Y, Yu H, Zhang R, An Z, Yin W, Chen W, Qin Y, Peng Z, Zhang T, Ni D, Cui J, Wang Q, Yang X, Zhang M, Ren X, Wu D, Sun X, Li Y, Zhou L, Qi X, Song T, Gao GF, Feng Z. Active case finding with case management: the key to tackling the COVID-19 pandemic. Lancet. 2020;396:63–70. doi: 10.1016/S0140-6736(20)31278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Commission of the People's Republic of China (NHC-China) and National Administration of Traditional Chinese Medicine of the People's Republic of China, published on February 18, 2020. National clinical practice guideline for COVID-19 in China (Tentative 6th edition). February 19, 2020. www.gov.cn/zhengce/zhengceku/2020-02/19/content_5480948.htm.
- Parvathaneni V, Gupta V. Utilizing drug repurposing against COVID-19 - Efficacy, limitations, and challenges. Life Sci. 2020;259 doi: 10.1016/j.lfs.2020.118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Meng K, He M, Zhu R, Guan H, Ke Z, Leng L, Wang X, Liu B, Hu C, Ji Q, Keerman M, Cheng L, Wu T, Huang K, Zeng Q. Clinical Characteristics and Prognosis of 244 Cardiovascular Patients Suffering from Coronavirus Disease in Wuhan. China.J Am Heart Assoc9. 2020 doi: 10.1161/JAHA.120.016796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, Wu Y, Xiao W, Liu S, Chen E, Chen W, Wang X, Yang J, Lin J, Zhao Q, Yan Y, Xie Z, Li D, Yang Y, Liu L, Qu J, Ning G, Shi G, Xie Q. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ369. 2020 doi: 10.1136/bmj.m1849. m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Q, Du J, Li X, Zeng J, Tan B, Xu J, Lin W, Chen X.L. Network pharmacology and molecular docking analysis on molecular targets and mechanisms of Huashi Baidu formula in the treatment of COVID-19. Drug Dev. Ind. Pharm. 2020;46:1345–1353. doi: 10.1080/03639045.2020.1788070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E.A., Zenilman J, Brinkley-Rubinstein L. Ethical Considerations for COVID-19 Vaccine Trials in Correctional Facilities. JAMA. 2020;324:1031–1032. doi: 10.1001/jama.2020.15589. [DOI] [PubMed] [Google Scholar]
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H.Y.F., Lam H.Y.S., Fong A.H., Leung S.T., Chin T.W., Lo C.S.Y, Lui M.M., Lee J.C.Y., Chiu K.W., Chung T.W., Lee E.Y.P., Wan E.Y.F., Hung I.F.N., Lam T.P.W., Kuo M.D., Ng M.Y. Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology. 2020;296:E72–E78. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Wang S, Li L, Wu Q, Qian W, Hu Y, Li L, Zhou X, Ma H, Li H, Wang M, Qiu X, Zha Y, Tian J. Radiomics Analysis of Computed Tomography helps predict poor prognostic outcome in COVID-19. Theranostics. 2020;10:7231–7244. doi: 10.7150/thno.46428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M, Tian J, Zhou Y, Xu X, Min X, Lv Y, Peng M, Zhang Y, Yan D, Lang S, Zhang Q, Fan A, Ke J, Li X, Liu B, Jiang M, Liu Q, Zhu J, Yang L, Zhu Z, Zeng K, Li C, Zheng Y, Wu H, Lin J, Lian F, Li X, Tong X. Efficacy of HuoxiangZhengqi dropping pills and LianhuaQingwen granules in treatment of COVID-19: A randomized controlled trial. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Wang P, Su K, Cho W.C., Xing Y. Chinese herbal medicine for coronavirus disease 2019: A systematic review and meta-analysis. Pharmacol. Res. 2020;160 doi: 10.1016/j.phrs.2020.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Dhagat S, Eswari J.S. Emerging strategies on in silico drug development against COVID-19: challenges and opportunities. Eur J Pharm Sci1. 2020;55 doi: 10.1016/j.ejps.2020.105522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this article could be shared within six months after the trial complete via the Medroad Cloud Electronic Data Capture System (cloud.medroad.cn/).