Abstract
Accumulating evidence showed that berberine possessed the anti-inflammatory action in various diseases caused by inflammation. However, it was still unclear whether both inhalation and injection with berberine produced pulmonary protective role in acute respiratory distress syndrome (ARDS). This study was aimed to evaluate the effects of both administration routes including inhalation and injection with berberine in ARDS induced by lipopolysaccharide (LPS) inhalation. Histopathological examination and weight of lung were evaluated. Phosphorylation of NF-κB, JAK2 and STAT3 were measured to assess the activity of inflammation related signaling pathways. Proinflammatory cytokines including interleukin (IL)-1β and tumor necrosis factor (TNF)-α in the bronchoalveolar lavage fluid (BALF) and serum were also detected. The results showed that LPS caused the lung injury, while both administration routes with berberine attenuated the injury and improved the pulmonary morphology. In addition, the primary TLR4/NF-κB and secondary JAK2/STAT3 signaling pathways which were activated by LPS in lung were totally inhibited by berberine administration. Moreover, proinflammatory cytokines in both BALF and serum were decreased by berberine. Considering that molecular docking simulation indicated that berberine could bind with TLR4, the present suggested that the inhibition of the inflammation related TLR4/NF-κB and JAK2/STAT3 signaling pathways might be involved in the pulmonary protective effect of berberine in LPS-induced ARDS.
Keywords: Berberine, Acute respiratory distress syndrome (ARDS), TLR4, Molecular docking, STAT3, JAK2
Graphical abstract
1. Introduction
Acute lung injury and its more severe form of acute respiratory distress syndrome (ARDS) refer to the damage of pulmonary capillary endothelial cells and alveolar epithelial cells caused by various internal and external factors other than cardiogenic, which in turn cause the alveolar capillary barrier lesions, infiltration of inflammatory cells in the lungs, diffuse alveolar and pulmonary interstitial edema, and ultimately lead to clinical syndromes characterized by respiratory distress and progressive hypoxemia (Reilly et al., 2019). Although some progress has been made in the diagnosis, treatment and pathogenesis of ARDS, there is still no effective treatment due to the multiple pathogenic factors and the complicated pathogenesis of ARDS (Abraham and Krasnodembskaya, 2020). As a consequence, the rehabilitation of patients with ARDS is affected and the mortality rate of the ARDS is still as high as 40% (van Wessem and Leenen, 2018). Therefore, the treatment of ARDS and acute lung injury has always been a hot and difficult point in Critical care medicine research.
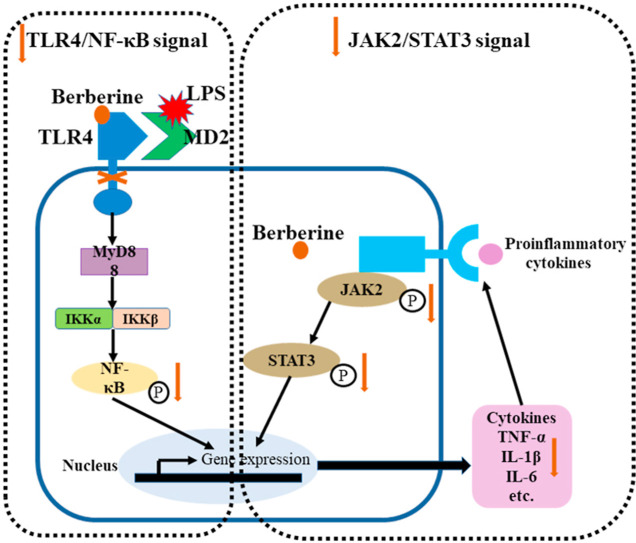
A variety of clinical diseases can induce the occurrence and development of acute respiratory distress syndrome. The most common predisposing factor is primary pneumonia caused by bacterial, viral or fungal infections (Cilloniz et al., 2018; Matthay et al., 2020; Prohaska et al., 2020). The endotoxin, lipopolysaccharide (LPS) of bacterial can stimulate inflammatory cells to release pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α, and result in an increased pulmonary capillary permeability, pulmonary interstitial edema, neutrophil exudation and other inflammatory injuries (Cao et al., 2020; Domscheit et al., 2020). Acute systemic Gram-negative bacterial infections are accompanied by release of LPS endotoxins into the bloodstream and an innate immune host response via the well-known toll like receptor 4 (TLR4) pathway, and then LPS associates non-covalently with TLR4 to form an activated heterodimer (LPS/MD2/TLR4)2 complex in vivo (Jagtap et al., 2020; Miller et al., 2005; Wang et al., 2015). In addition, during the development of experimental acute lung injury, the TLR4/NF-κB signaling pathway was activated by LPS (Gao et al., 2020; Zhao et al., 2020), and the inflammatory effect was stimulated, which subsequently triggered the activation of other inflammation-related signaling pathways such as the JAK2/STAT3 pathway (Ding et al., 2019). Therefore, anti-inflammation is one of the strategies for the treatment of ARDS.
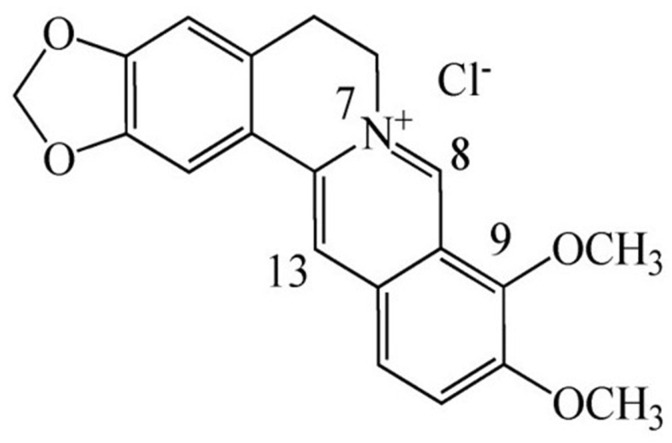
Berberine is a very famous isoquinoline quaternary alkaloid (C20H19NO5, 5,6-dihydro-dibenzoquinolizinium derivative, chemical structure see Fig. 1 .) extracted from the herb of Coptis chinensis because of possessing a variety of important biological functions (Song et al., 2020). Previous studies have shown the anti-inflammatory activity of berberine. For example, berberine inhibits LPS-induced inflammation via modulating ER stress response in macrophages and hepatocytes (Wang et al., 2020b). Cartilage was repaired by berberine administration through upregulating surfactant protein D via the inhibition of TLR4/NF-ĸB signaling. (Zhou et al., 2020). There was evidence that berberine inhibited NLRP3 inflammasome pathway in MDA-MB-231 cell (Yao et al., 2019). In recent years, many studies have also focused on the efficacy of berberine in lung injury. Berberine ameliorated cigarette smoke-induced acute lung injury through its anti-inflammatory activity (Lin et al., 2013). In addition, Zhang et., demonstrated that berberine reversed lung injury by inhibiting proinflammatory cytokines and cytosolic phospholipase A2 activation induced by LPS (Zhang et al., 2008). Moreover, berberine prevented influenza virus-induced NLRP3 activation by promoting mitophagy and reducing mitochondrial oxidative stress (Liu et al., 2020a). However, the effects of berberine on lung injury is still not fully understood. In particular, it is unclear whether inhalation of berberine could be an intervention for lung injury in ARDS caused by LPS inhalation. In this study, we confirmed the effect of berberine in attenuating LPS-induced lung injury through intraperitoneal administration and inhalation administration. Subsequently, based on molecular docking simulations, we further studied the mechanism of berberine involved in the regulation of TLR4/NF-κB and JAK2/STAT3 signaling pathways during lung injury of ARDS.
Fig. 1.
Chemical structure of berberine.
2. Materials and methods
2.1. Drugs and reagents
Berberine (purity>99% verified by HPLC) was purchased from Aladdin (Shanghai, China). Dexamethasone sodium phosphate injection (5 mg/ml, Guangzhou Baiyunshan Tianxi Pharmaceutical Co., Ltd., China) was used as the positive drug in this research. Elisa kits of mouse TNF-α and IL-1β were obtained from Nanjing Jiancheng Institute of Biotechnology (Nanjing, China). Tissue protein extraction kit and BCA protein assay kit were purchased from KeyGen Biotech. CO., Ltd. (Nanjing, China). The primary antibodies rabbit mAb NF-κB, mouse mAb phospho-NF-κB, rabbit mAb STAT3, rabbit mAb phospho-STAT3, rabbit polyclone β-Actin antibody, rabbit mAb JAK2 and rabbit mAb phosphor-JAK2 (Tyr 1007/1008) were purchased from Cell Signaling Technology.
2.2. Animals and model
Male C57BL/6 J mice (Jiangsu Jicui Experimental Animal Co., Ltd.) with a body weight of 30 ± 2 g were randomly divided into 8 groups (n = 8): Normal control group (NC), Model control group (MC, induced by LPS), Positive control group (PC, dexamethasone sodium phosphate injection, 2 mg/kg bodyweight, i.p.)(Lin et al., 2019) and Positive inhalation control group (PIC, 0.2 mg/ml of dexamethasone sodium phosphate solution, nebulized inhalation for 20 min), Berberine intraperitoneal injection at low dose group (BL, 1 mg/kg bodyweight), berberine intraperitoneal injection at high dose group (BH, 2 mg/kg bodyweight), berberine inhalation at low dose group (BIL, 0.1 mg/ml, nebulized inhalation for 20 min) as well as berberine inhalation at high dose group (BIH, 0.2 mg/ml, nebulized inhalation for 20 min). After three consecutive days of administration, the mice excepted control group were anesthetized with sodium pentobarbital (45 mg/kg body weight) by intraperitoneal injection. At the time of the positive reflex disappeared, 50 μl of LPS solution (5 mg/kg body weight) was injected into the oral cavity against the posterior pharyngeal wall, then the nose of mouse was pinched quickly for 30 s (Qin et al., 2020; Zhang et al., 2015). The slight tracheal rales from mice indicates successful ALI model. 12 h later, all mice were killed.
This study was approved by Laboratory Animal Management Committee of Xiamen Medicine Research Center (YKSLL-A20200211) and was arranged to minimize suffering and to reduce the number of animals used. All the animals received humane care in compliance with Guide for the Care and Use of Laboratory Animals. All mice were anesthetized with isoflurane and killed for the collection of lung tissue samples.
2.3. Nebulized inhalation method of drug
In order to compare the effect of the two administration routes of berberine, the low and high doses were set to 1 mg/kg and 2 mg/kg respectively in both the routes. Firstly, Berberine was dissolved into the liquid of 0.1 mg/ml and 0.2 mg/ml with distilled water. The dexamethasone sodium phosphate injection was diluted to inhalation solution of 0.2 mg/ml with 5% glucose injection. Nebulized inhalation of berberine and dexamethasone was administrated by a Yuwell 403C Air compressing nebulizer (Yuwell Technology Co., Ltd., China). All the parameters of nebulized inhalation refer to the relevant details of the Inhalation Preparation for a Nebulizer in Part 0111 of Volume Ⅳ of Chinese Pharmacopoeia 2015 edition (Zhang et al., 2019b). During the inhaled administration, 1 ml of berberine or dexamethasone solution will be atomized within 20 min through adjusting the frequency of atomizer. The delivery dose of berberine was calculated according to the previous described method (Robichaud et al., 2015).
2.4. Lung tissue exudation detection (wet lung/dry lung weight ratio)
The whole lung tissue was dissected and weighed. Meanwhile, appropriate amount left lung was washed with Hank's solution and weighted (Zhou et al., 2019). Then the tissue was dehydrated in 72 °C ovens for 72 h until the weight with no change and weighed again. The wet/dry weight ratio of lung (wet/dry) was calculated to evaluate the degree of pulmonary edema (Yang et al., 2019).
2.5. Inflammatory cell count of bronchoalveolar lavage fluid (BALF)
A sterile plastic tube was inserted and fixed in trachea, and the lung lavage was performed 20 times and 1.2 ml of normal saline each time (Hu et al., 2020). All BALF was filtered with double-layer sterile gauze to remove the mucus, then centrifuged 1200 × g at 4 °C for 10 min. The supernatant was stored at −20 °C for cytokine detection. The precipitated cell components were quantitatively diluted and prepared cell suspension. Finally, cell smears were made via cell centrifugal smear device, and the cells were counted by Wright-Giemsa stain (Li et al., 2019).
2.6. Histopathological examination
The lung tissues were fixed with 4% paraformaldehyde solution for 72 h and then embedded in conventional paraffin. The tissue was cut into slices of 4 μm, following by dewaxing, hydration, hematoxylin and eosin (H&E) staining (Matute-Bello et al., 2011). Subsequently, the tissue slides were dealed with gradient alcohol and xylene respectively. Finally, the slices were sealed with neutral gum and examined under a microscope.
2.7. Molecular docking
Crystal structure of the TLR4-MD2 complex were derived from the RCSB Protein Data Bank (http://www.rcsb.org/). TLR4 complex (PDB code, 3FXI) and the structure of berberine were imported into Dock6 and simulated by the software.
2.8. Enzyme-linked immunosorbent assay (ELISA) measurement
The levels of IL-1β and TNF-α concentrations in serum and BALF samples were measured by ELISA kit according to the instruction of manufacturer. The total protein concentration of BALF samples were detected with BCA protein assay method for normalizing cytokines in BALF.
2.9. Western blot
Appropriate amount of pulmonary tissues of mice was cut and stored in liquid nitrogen. Protein was extracted by RIPA buffer supplemented with protease inhibitors as well as phosphatase inhibitors. The homogenates were then centrifuged 15000 × g at 4 °C for 15 min. The concentration of protein in supernatants was determined with the BCA method. Samples containing 20 μg of protein were loaded and subjected to 8% SDS-PAGE gel electrophoresis. Then the protein in gel was transferred onto polyvinylidene difluoride membrane with constant pressure of 50 V for 2 h. Before incubated with primary antibody (1:1000) at 4 °C overnight, the membranes were blocked with 5% BSA for 2 h at room temperature. Immunoreactivity was visualized by incubation with horseradish peroxidase-labeled goat anti-rabbit or anti-mouse IgG (1:5000) for 2 h at room temperature. ECL chemiluminescence reagent was used for exposure. Finally, Bio-Rad gel imaging system was used to analyze the bands. Three parallel holes were set for each sample, and β-actin was used as an internal reference.
2.10. Statistical analysis
Data were represented as mean ± S.E.M., and analyzed by one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls test for multiple comparisons. Differences with P < 0.05 were considered statistically significant.
3. Results
3.1. Berberine alleviated LPS inhalation-induced pulmonary edema
The lung coefficient and w/d ratio of lung were used for evaluation of pulmonary edema and hydration. As shown in Fig. 2 , there was a significant difference in lung coefficient (P < 0.01) and w/d ratio of lung (P < 0.01) between Control and LPS animals, indicating the swollen and hyperhydration caused by ARDS. In contrast, both injection (P < 0.01) and inhalation (P < 0.01) with dexamethasone at two administration routes significantly decreased the lung coefficient. Meanwhile, both injection (P < 0.01, P < 0.01) and inhalation (P < 0.01, P < 0.01) with berberine at two doses significantly decreased the lung coefficient. In addition, berberine also reduced the w/d ratio of lung by injection (P < 0.01, P < 0.01) and inhalation (P < 0.01, P < 0.01) administration.
Fig. 2.
The weight and leakage of lung was decreased by berberine in LPS induced ARDS mice. (A) Organ coefficients of lung of mice in each group; (B) Wet/dry lung weight ratio of mice in each group. NC, Normal control mice; MC, LPS mice; PC, Positive control mice (injection with dexamethasone, 2 mg/kg), BL, injection with berberine at low dose (1 mg/kg) of berberine; BH, injection with berberine at high dose (2 mg/kg); PIC, inhalation with dexamethasone liquid (0.2 mg/ml); BIL, inhalation with berberine at low concentration (0.1 mg/ml); BIH, inhalation with berberine at high concentration (0.2 mg/ml). All data are expressed as mean ± S.E.M. (n = 8). **P < 0.01 vs NC; #P < 0.05 and ##P < 0.01 vs MC.
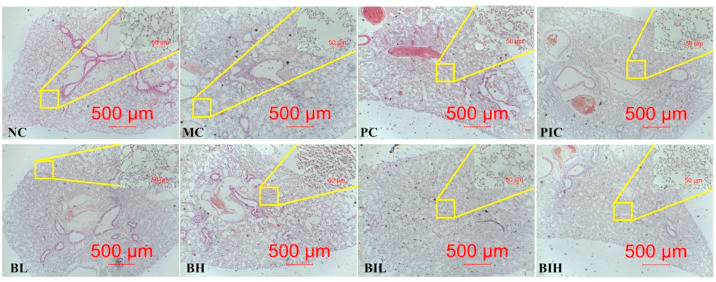
3.2. Berberine alleviated LPS inhalation-induced impaired lung morphology
Post-mortem visual examination of the lungs was provided in Fig. 3 . The Control animals exhibited a pale pink lung with no signs of pathology. On the contrary, after LPS inhalation, the lungs changed to be cherry red with numerous spotty hemorrhages. There was foam liquid in the bronchi. The abnormal lung morphology was improved by dexamethasone and berberine as interstitial edema and inflammatory cells infiltration were attenuated. Importantly, treatment with berberine attenuated the severity of lung injuries induced by LPS challenge. As shown in Fig. 3, the lung histopathology observation of various groups was demonstrated. There was no abnormality in lung tissue section of the Control animals. In contrast to the sections from the Control animals, where the alveolar structure was incomplete, the arrangements of alveolar space was disordered, the edema, cell necrosis and inflammatory cell infiltration were observed in the LPS animals. The result indicates that acute lung injury of mice was induced successfully by LPS inhalation. In the groups treated with dexamethasone and berberine via different routes of administration, the situations of interstitial edema, inflammatory cells infiltration and the disordered arrangements of alveolar were alleviated than LPS animals, especially in the BH groups. Among the groups of berberine treatment with different concentration and routes of administration, the therapeutic effects of BH group on ARDS were the best compared with the PC, PIC, BL, BIL and BIH groups.
Fig. 3.
The histopathological injury of lung was ameliorated by berberine in ARDS mice induced by LPS inhalation. After three consecutive days of pretreatment with berberine or dexamethasone, C57BL/6 J mice were intratracheally administered LPS. Lungs from each experimental group were processed for histological evaluation at 12 h after the LPS inhalation. Representative photomicrographs of pulmonary histology (H&E staining, × 40, scale bar 500 μm) are shown as low power full image, the upper-right corner of each picture (H&E staining, × 400, scale bar 50 μm) is high magnification image of the area within the yellow rectangle. NC, Normal control mice; MC, LPS mice; PC, Positive control mice (injection with dexamethasone, 2 mg/kg), BL, injection with berberine at low dose (1 mg/kg) of berberine; BH, injection with berberine at high dose (2 mg/kg); PIC, inhalation with dexamethasone liquid (0.2 mg/ml); BIL, inhalation with berberine at low concentration (0.1 mg/ml); BIH, inhalation with berberine at high concentration (0.2 mg/ml).
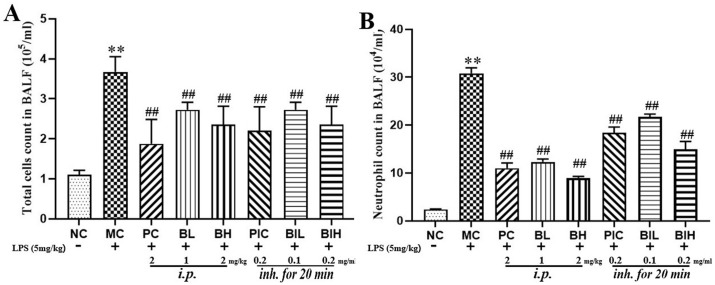
3.3. Berberine attenuated LPS inhalation-induced the elevation of cells and neutrophils in BALF
During the progression of ARDS, neutrophils gather in the lung and mediate lung injury. Thus, the effect of dexamethasone and berberine on the total number of cells as well as neutrophils in the BALF was investigated (Fig. 4 ). It was observed that the number of total cells (P < 0.01) and neutrophils (P < 0.01) in BALF was significantly increased after LPS inhalation. These abnormal elevations of cells (PC: P < 0.01; PIC: P < 0.01; BL: P < 0.01; BH: P < 0.01; BIL: P < 0.01; BIH: P < 0.01) and neutrophils (PC: P < 0.01; PIC: P < 0.01; BL: P < 0.01; BH: P < 0.01; BIL: P < 0.01; BIH: P < 0.01) in BALF were partly reversed by dexamethasone and berberine administrated with injection and inhalation, indicating that dexamethasone and berberine could inhibit the inflammatory cell infiltration in ARDS.
Fig. 4.
The inflammatory cells count of BALF. (A) Total cells count in BALF; (B) Neutrophils count in BALF. NC, Normal control mice; MC, LPS mice; PC, Positive control mice (injection with dexamethasone, 2 mg/kg), BL, injection with berberine at low dose (1 mg/kg) of berberine; BH, injection with berberine at high dose (2 mg/kg); PIC, inhalation with dexamethasone liquid (0.2 mg/ml); BIL, inhalation with berberine at low concentration (0.1 mg/ml); BIH, inhalation with berberine at high concentration (0.2 mg/ml). All data are expressed as mean ± S.E.M. (n = 8). **P < 0.01 vs NC; ##P < 0.01 vs MC.
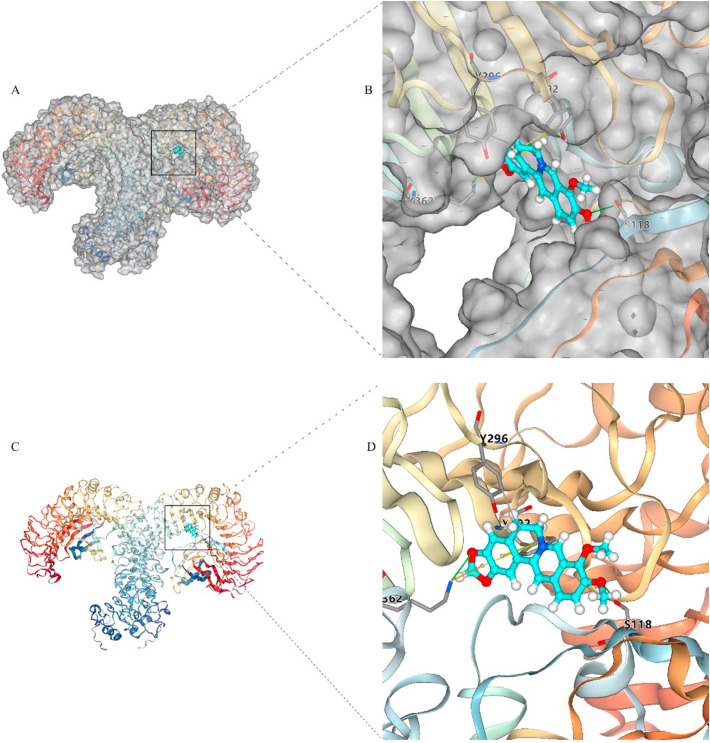
3.4. Molecular docking analysis of berberine with TLR4
Molecular docking simulation demonstrated berberine was in the pocket of the TLR4-MD2 complex (Fig. 5 ). The interaction analysis indicated that berberine could interact with TYR296 by hydrophobic interactions, interact with SER118 and LYS362 by hydrogen bonding interactions, interact with TYR292 by π-π stacking interactions, and interact with LYS362 by π-cation interactions, respectively.
Fig. 5.
The molecular docking indicating the complex between TLR4-MD2 and berberine. (A) Whole view of TLR4-MD2 showing the surface of binding pocket with berberine. (B) Enlarged view showing the surface of the binding pocket with berberine. (C) Berberine is depicted in the TLR4-MD2 without protein surface. Enlarged view showing the binding between docking pocket and berberine without protein surface.
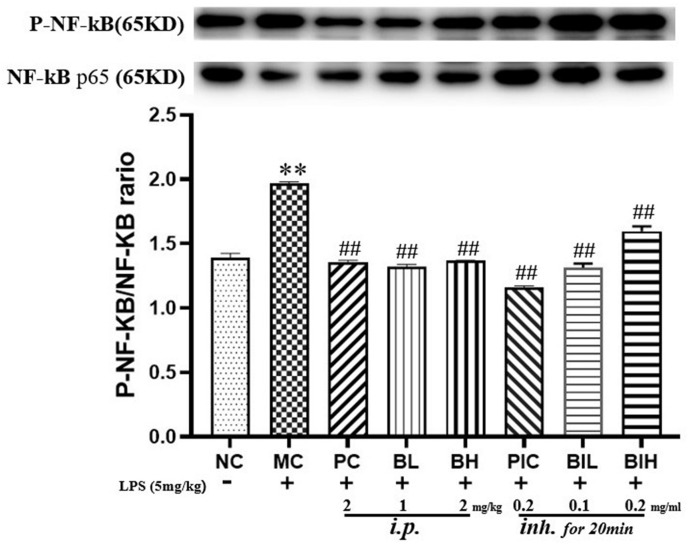
3.5. Berberine attenuated LPS inhalation-induced TLR4/NF-κB signaling
TLR4/NF-κB signaling pathways were activated by LPS inhalation, as the phosphorylation of NF-κB was markedly increased (P < 0.01) according to Fig. 6 . The post hoc test revealed that both dexamethasone and berberine administration by injection (PC: P < 0.01; BL: P < 0.01; BH: P < 0.01) and inhalation (PIC: P < 0.01; BIL: P < 0.01; BIH: P < 0.01) significantly inhibited the expression of phosphorylated NF-κB p-65 in the pulmonary tissues induced by LPS.
Fig. 6.
The TLR4/NF-κB signaling was inhibited by administration with berberine in LPS induced ARDS mice. NC, Normal control mice; MC, LPS mice; PC, Positive control mice (injection with dexamethasone, 2 mg/kg), BL, injection with berberine at low dose (1 mg/kg) of berberine; BH, injection with berberine at high dose (2 mg/kg); PIC, inhalation with dexamethasone liquid (0.2 mg/ml); BIL, inhalation with berberine at low concentration (0.1 mg/ml); BIH, inhalation with berberine at high concentration (0.2 mg/ml). All data are expressed as mean ± S.E.M. (n = 8). **P < 0.01 vs NC; ##P < 0.01 vs MC.
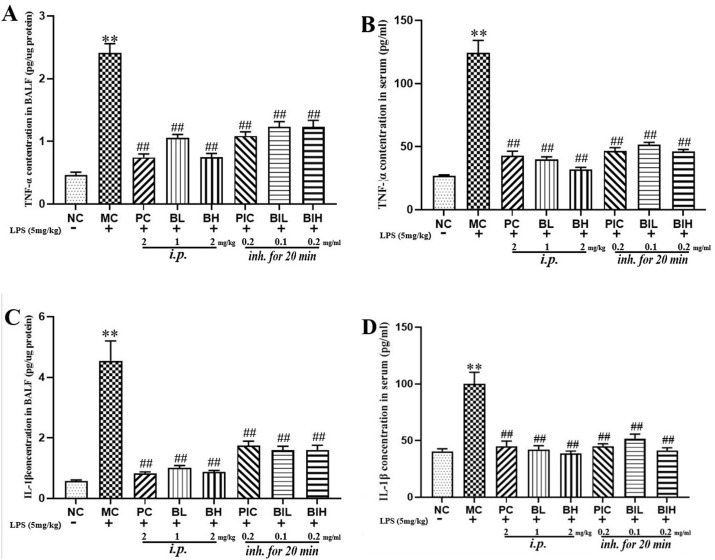
3.6. Berberine attenuated LPS inhalation-induced overexpression of proinflammatory cytokines in BALF and serum
As shown in Fig. 7 , LPS inhalation increased the levels of TNF-α (P < 0.01) and IL-1β (P < 0.01) in both BALF and serum, while dexamethasone and berberine attenuated the release of TNF-α and IL-1β.
Fig. 7.
The levels of inflammatory cytokines (TNF-α, IL-1β) in the serum and BALF were decreased by berberine administration in LPS induced ARDS mice. NC, Normal control mice; MC, LPS mice; PC, Positive control mice (injection with dexamethasone, 2 mg/kg), BL, injection with berberine at low dose (1 mg/kg) of berberine; BH, injection with berberine at high dose (2 mg/kg); PIC, inhalation with dexamethasone liquid (0.2 mg/ml); BIL, inhalation with berberine at low concentration (0.1 mg/ml); BIH, inhalation with berberine at high concentration (0.2 mg/ml). All data are expressed as mean ± S.E.M. (n = 8). **P < 0.01 vs NC; ##P < 0.01 vs MC.
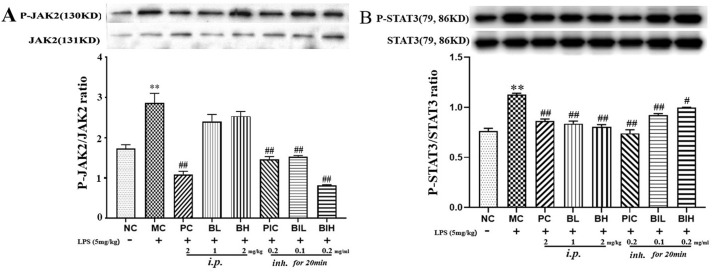
3.7. Berberine inhibited LPS inhalation-induced JAK2/STAT3 signaling
Besides TLR4/NF-κB signaling, it is believed that activation of JAK2/STAT3 involved in the inflammation and the production of proinflammatory cytokines. The phosphorylation and total of JAK2 and STAT3 were determined using western blotting (Fig. 8 ). The results showed that LPS significantly induced the phosphorylation of JAK2 (P < 0.01) and STAT3 (P < 0.01). Both injection and inhalation with dexamethasone and berberine significantly inhibited JAK2 (PC: P < 0.01; PIC: P < 0.01; BIL: P < 0.01; BIH: P < 0.01) and STAT3 (PC: P < 0.01; BL: P < 0.01; BH: P < 0.01; PIC: P < 0.01; BIL: P < 0.01; BIH: P < 0.05) phosphorylation in the lung, suggesting the anti-inflammatory activity of berberine.
Fig. 8.
The JAK2/STAT3 signaling was inhibited by administration with berberine in LPS induced ARDS mice. NC, Normal control mice; MC, LPS mice; PC, Positive control mice (injection with dexamethasone, 2 mg/kg), BL, injection with berberine at low dose (1 mg/kg) of berberine; BH, injection with berberine at high dose (2 mg/kg); PIC, inhalation with dexamethasone liquid (0.2 mg/ml); BIL, inhalation with berberine at low concentration (0.1 mg/ml); BIH, inhalation with berberine at high concentration (0.2 mg/ml). All data are expressed as mean ± S.E.M. (n = 8). **P < 0.01 vs NC; #P < 0.05 and ##P < 0.01 vs MC.
4. Discussion
In the present study, two different administration routes with berberine were evaluated in inhaled LPS-induced ARDS. Both injection and inhalation with berberine attenuated the lung injury and inflammatory activity. Moreover, there was no significant difference between the two administration routes, indicating that injection with berberine can reach lesion and take action against ARDS.
Injection of LPS was widely used for induce lung injury in animals (Li et al., 2020; Liu et al., 2020b). However, injection with LPS causes the systemic inflammation and thus lacks tissue specificity (Wang et al., 2020a; Weng et al., 2019). In the present study, LPS inhalation which mimicked the actual route of infection was used for animal ARDS establishment (Yang et al., 2020). The cytopathology of ARDS includes the destruction of the integrity of alveolar capillary membranes, excessive neutrophil migration, and promotion of the production and secretion of inflammatory cytokines (Chang, 2019). Consistent with the pathological features in clinical investigation, the present study indicated that lung exhibited massive apoptosis of alveolar epithelial cells, increased permeability of alveolar capillaries and fibrosis, accompanied by severe acute inflammation with LPS inhalation. Similarly, the organ coefficient and wet/dry ratio of lung were elevated in LPS-induced mice, indicating the pulmonary edema and hyperhydration induced by LPS inhalation. On the contrary, both injection and inhalation of berberine alleviated the injured lung, berberine treatment not only reduced edema of lung, inflammatory cell in BALF and serum, but also improved lung histology injury significantly.
In the present study, nebulization successfully delivered berberine to the injured lungs. According to the histopathological examination, berberine decreased pulmonary inflammatory edema and apoptosis in LPS inhaled mice. In addition, neutrophil chemotaxis and inflammation were reduced by berberine administration, as the total number of neutrophil and levels of pro-inflammatory cytokines in the BAFL were reduced. However, it should be noted that the efficacy of inhaled administration was less than that of injected administration. This might be due to the characteristics of inhaled particles. It is well-known that the inhaled nanoparticles can reach the deep part of the lung tissue and directly contact the alveoli, which can greatly increase drug dissolution and absorption. However, in the present study, berberine is not prepared into nanoparticles and thus the large size berberine particles could not entirely reach the deep part of lung tissue and could not maintain a high blood concentration. In this context, further study is required to prepare and optimize berberine nanoparticle, and evaluate its efficacy against acute lung injury.
In addition to the present study, several previous studies also showed that injection with berberine protected pulmonary morphology in LPS injection induced lung injury (Liang et al., 2019; Zhang et al., 2008). In parallel to histopathological evaluation, berberine also reduced the organ coefficient and wet/dry ratio of lung. This observation indicated that berberine may attenuate the core symptom, acute lung injury in inhaled LPS-induced ARDS.
After entering the body, LPS is combined with lipopolysaccharide binding protein, and then combines with CD14 to form a complex, which activates TLR4 and its downstream signaling pathway. After being activated, TLR4 forms a complex with the junction protein myeloid differentiation factor 88 (MyD88) located in the cytoplasm. MyD88 then activates downstream IKK kinase and regulates NF-κB activation (Takeuchi and Akira, 2001). During the translocation of NF-κB from cytoplasm to nucleus, NF-κB is involved in the production of multiple inflammatory factors and initiates a series of inflammation-related reactions. Therefore, inhibition of TLR4/NF-κB related targets provides a potential therapeutic approach to treat inflammatory diseases (Pan et al., 2018; Roy et al., 2016). More importantly, previous studies showed that berberine could reversed the activation of TLR4/NF-κB signaling in LPS-induced inflammation in brain, liver and kidney (Liu et al., 2018; Sadraie et al., 2019; Zhang et al., 2019a). Therefore, we speculated that berberine could inhibited TLR4/NF-κB signaling in the lung as well. Firstly, we used molecular docking to elucidate whether berberine possesses an affinity for TLR4. The docking simulation showed that berberine competitively bound to TLR4-MD2 complex, preventing their binding to LPS. The formation of hydrophobic interaction with TYR296, hydrogen bonding with SER118 and LYS362, and π-π stacking with TYR292 supported this progress. The distances between berberine and the residues of SER118, LYS362 and TYR292 were only 3.9 Å, 3.2 Å and 4.9 Å.
In parallel to the results from molecular docking, Western blot showed that LPS increased the phosphorylation of NF-κB, indicating the activation of TLR4/NF-κB signaling. On the contrary, treatment of berberine markedly reversed the upregulation of NF-κB phosphorylation, which was in accordance to a previous study showing that berberine alleviated acute lung inflammation induced by cigarette smoking (Lin et al., 2013). In addition, consistent with other reports showing that injection with LPS caused the overexpression of proinflammatory cytokines (Ko et al., 2020; Sun et al., 2020), this study also indicated that LPS inhalation increased proinflammatory cytokines such as IL-1β and TNF-α levels in BALF and serum. In contrast, both injection and inhalation of berberine attenuated the increase of IL-1β and TNF-α levels, which was similar to a previous study showing that berberine attenuated LPS injection induced lung injury by inhibiting TNF-α production (Zhang et al., 2008). In this way, these results showed the pulmonary protective effects of berberine in ARDS were mediated at least by the inhibition of TLR4/NF-κB signaling and cytokine production.
After the activation of primary pathway TLR4/NF-κB signaling, inflammation was amplified by other signaling pathways. The JAK2/STAT3 signaling pathway plays a key role in immune and inflammatory responses (Kong et al., 2020). The binding of proinflammatory cytokines to their receptors causes the dimerization of the receptors, making JAK2 coupled to the receptors and activated by tyrosine phosphorylation (Teng et al., 2014). Activated JAK2 forms corresponding docking sites for STAT3 and leads the activation of STAT3. STAT3 then enters the nucleus and further regulates the expression of inflammation related genes (Leonard, 2001). In the present study, phosphorylation of JAK2 and STAT3 were upregulated by LPS inhalation, while berberine could inhibited JAK2 and STAT3 phosphorylation in the lung, suggesting besides primary TLR4/NF-κB signaling, the secondary inflammatory signaling pathways were also inhibited. It has been reported that berberine inhibited colorectal cancer invasion and metastasis via inhibition of JAK2/STAT3 signaling pathway (Liu et al., 2015). A recent study also showed that the antiproliferation effect of berberine was mediated by the repression of JAK2/STAT3 signaling in keratinocytes (Sun et al., 2019). In this case, the observation suggested that the inactivation of JAK2/STAT3 was involved in the pulmonary protective effects of berberine in ARDS.
In conclusion, the present study demonstrated that berberine takes its efficacy against LPS-induced ARDS in either injection or inhalation administration. Combined with the molecular docking and biochemical analysis, the present study indicated that berberine could bind with TLR4 and inhibited the primary inflammatory TLR4/NF-κB signaling but also the secondary inflammatory JAK2/STAT3 signaling pathway, the inhibition of the inflammation related signaling pathway by berberine attenuated the lung injury and decreased the release of proinflammatory cytokines. The finding suggested the potential role of berberine in the treatment of ARDS in clinical application.
Author contributions
Guanghui Xu and Litao Yi designed this experiment, Guanghui Xu, Youhua Luo, Wen Chen, Yiqi Huang and Huiqi Wan performed all the animal experiment and molecular biological experiments, Litao Yi conducted the molecular docking analysis. Guanghui Xu, Litao Yi and Huiqi Wan drafted the manuscript. All the authors reviewed and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research was supported by the General Program of National Natural Science Foundation of China (No. 81773790), Statistical Information Center of National Health Commission of the People's Republic of China (No. 09202003), Natural Science Foundation of Fujian Province (No.2019D018) and Xiamen Municipal Bureau of Science and Technology (No. 3502Z20184053).
References
- Abraham A., Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl Med. 2020;9(1):28–38. doi: 10.1002/sctm.19-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F., Tian X., Li Z., Lv Y., Han J., Zhuang R., Cheng B., Gong Y., Ying B., Jin S., Gao Y. Suppression of NLRP3 inflammasome by erythropoietin via the EPOR/JAK2/STAT3 pathway contributes to attenuation of acute lung injury in mice. Front. Pharmacol. 2020;11:306. doi: 10.3389/fphar.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J.C. Acute respiratory distress syndrome as an organ phenotype of vascular microthrombotic disease: based on hemostatic theory and endothelial molecular pathogenesis. Clin. Appl. Thromb. Hemost. 2019;25 doi: 10.1177/1076029619887437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilloniz C., Ferrer M., Liapikou A., Garcia-Vidal C., Gabarrus A., Ceccato A., Puig de La Bellacasa J., Blasi F., Torres A. Acute respiratory distress syndrome in mechanically ventilated patients with community-acquired pneumonia. Eur. Respir. J. 2018;51(3) doi: 10.1183/13993003.02215-2017. [DOI] [PubMed] [Google Scholar]
- Ding Q., Sun J., Xie W., Zhang M., Zhang C., Xu X. Stemona alkaloids suppress the positive feedback loop between M2 polarization and fibroblast differentiation by inhibiting JAK2/STAT3 pathway in fibroblasts and CXCR4/PI3K/AKT1 pathway in macrophages. Int. Immunopharm. 2019;72:385–394. doi: 10.1016/j.intimp.2019.04.030. [DOI] [PubMed] [Google Scholar]
- Domscheit H., Hegeman M.A., Carvalho N., Spieth P.M. Molecular dynamics of lipopolysaccharide-induced lung injury in rodents. Front. Physiol. 2020;11:36. doi: 10.3389/fphys.2020.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Wang X., Qu X., Zhai J., Tao L., Zhang Y., Song Y., Zhang W. Omeprazole attenuates cisplatin-induced kidney injury through suppression of the TLR4/NF-kappaB/NLRP3 signaling pathway. Toxicology. 2020;440:152487. doi: 10.1016/j.tox.2020.152487. [DOI] [PubMed] [Google Scholar]
- Hu X., Qin H., Li Y., Li J., Fu L., Li M., Jiang C., Yun J., Liu Z., Feng Y., Yao Y., Yin B. Biochanin A protect against lipopolysaccharide-induced acute lung injury in mice by regulating TLR4/NF-kappaB and PPAR-gamma pathway. Microb. Pathog. 2020;138:103846. doi: 10.1016/j.micpath.2019.103846. [DOI] [PubMed] [Google Scholar]
- Jagtap P., Prasad P., Pateria A., Deshmukh S.D., Gupta S. A single step in vitro bioassay mimicking TLR4-LPS pathway and the role of MD2 and CD14 coreceptors. Front. Immunol. 2020;11:5. doi: 10.3389/fimmu.2020.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko I.G., Hwang J.J., Chang B.S., Kim S.H., Jin J.J., Hwang L., Kim C.J., Choi C.W. Polydeoxyribonucleotide ameliorates lipopolysaccharide-induced acute lung injury via modulation of the MAPK/NF-kappaB signaling pathway in rats. Int. Immunopharm. 2020;83:106444. doi: 10.1016/j.intimp.2020.106444. [DOI] [PubMed] [Google Scholar]
- Kong F., Sun Y., Song W., Zhou Y., Zhu S. MiR-216a alleviates LPS-induced acute lung injury via regulating JAK2/STAT3 and NF-kappaB signaling. Hum. Cell. 2020;33(1):67–78. doi: 10.1007/s13577-019-00289-7. [DOI] [PubMed] [Google Scholar]
- Leonard W.J. Role of Jak kinases and STATs in cytokine signal transduction. Int. J. Hematol. 2001;73(3):271–277. doi: 10.1007/BF02981951. [DOI] [PubMed] [Google Scholar]
- Li M., Zhao Y., He J., Deng W., Cheng L., Jiang Z., Wang D. Protein kinase C theta inhibition attenuates lipopolysaccharide-induced acute lung injury through Notch signaling pathway via suppressing Th17 cell response in mice. Inflammation. 2019;42(6):1980–1989. doi: 10.1007/s10753-019-01058-2. [DOI] [PubMed] [Google Scholar]
- Li X., Ye C., Mulati M., Sun L., Qian F. Ellipticine blocks synergistic effects of IL-17A and TNF-alpha in epithelial cells and alleviates severe acute pancreatitis-associated acute lung injury. Biochem. Pharmacol. 2020;177:113992. doi: 10.1016/j.bcp.2020.113992. [DOI] [PubMed] [Google Scholar]
- Liang Y., Fan C., Yan X., Lu X., Jiang H., Di S., Ma Z., Feng Y., Zhang Z., Feng P., Feng X., Feng J., Jin F. Berberine ameliorates lipopolysaccharide-induced acute lung injury via the PERK-mediated Nrf2/HO-1 signaling axis. Phytother Res. 2019;33(1):130–148. doi: 10.1002/ptr.6206. [DOI] [PubMed] [Google Scholar]
- Lin K., Liu S., Shen Y., Li Q. Berberine attenuates cigarette smoke-induced acute lung inflammation. Inflammation. 2013;36(5):1079–1086. doi: 10.1007/s10753-013-9640-0. [DOI] [PubMed] [Google Scholar]
- Lin Y., Zhang M., Lu Q., Xie J., Wu J., Chen C. A novel chalcone derivative exerts anti-inflammatory and anti-oxidant effects after acute lung injury. Aging (N Y) 2019;11(18):7805–7816. doi: 10.18632/aging.102288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Zhang Y., Liu Y., Hou L., Li S., Tian H., Zhao T. Berberine modulates gut microbiota and reduces insulin resistance via the TLR4 signaling pathway. Exp. Clin. Endocrinol. Diabetes. 2018;126(8):513–520. doi: 10.1055/s-0043-125066. [DOI] [PubMed] [Google Scholar]
- Liu H., You L., Wu J., Zhao M., Guo R., Zhang H., Su R., Mao Q., Deng D., Hao Y. Berberine suppresses influenza virus-triggered NLRP3 inflammasome activation in macrophages by inducing mitophagy and decreasing mitochondrial ROS. J. Leukoc. Biol. 2020;108(1):253–266. doi: 10.1002/JLB.3MA0320-358RR. [DOI] [PubMed] [Google Scholar]
- Liu X., Ji Q., Ye N., Sui H., Zhou L., Zhu H., Fan Z., Cai J., Li Q. Berberine inhibits invasion and metastasis of colorectal cancer cells via COX-2/PGE2 mediated JAK2/STAT3 signaling pathway. PloS One. 2015;10(5) doi: 10.1371/journal.pone.0123478. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liu Z., Wang P., Lu S., Guo R., Gao W., Tong H., Yin Y., Han X., Liu T., Chen X., Zhu M.X., Yang Z. Liquiritin, a novel inhibitor of TRPV1 and TRPA1, protects against LPS-induced acute lung injury. Cell Calcium. 2020;88:102198. doi: 10.1016/j.ceca.2020.102198. [DOI] [PubMed] [Google Scholar]
- Matthay M.A., Aldrich J.M., Gotts J.E. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med. 2020;8(5):433–434. doi: 10.1016/S2213-2600(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute-Bello G., Downey G., Moore B.B., Groshong S.D., Matthay M.A., Slutsky A.S., Kuebler W.M., Acute Lung Injury in Animals Study, G. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011;44(5):725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.I., Ernst R.K., Bader M.W. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 2005;3(1):36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- Pan W.Z., Du J., Zhang L.Y., Ma J.H. The roles of NF-kB in the development of lung injury after one-lung ventilation. Eur. Rev. Med. Pharmacol. Sci. 2018;22(21):7414–7422. doi: 10.26355/eurrev_201811_16281. [DOI] [PubMed] [Google Scholar]
- Prohaska S., Henn P., Wenz S., Frauenfeld L., Rosenberger P., Haeberle H.A. A case report of fatal disseminated fungal sepsis in a patient with ARDS and extracorporeal membrane oxygenation. BMC Anesthesiol. 2020;20(1):107. doi: 10.1186/s12871-020-01031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L., Li M., Tan H.L., Yang H.X., Li S.D., Luan Z.X., Chen Y.F., Yang M.H. Mechanistic target of rapamycin-mediated autophagy is involved in the alleviation of lipopolysaccharide-induced acute lung injury in rats. Int. Immunopharm. 2020;78:105790. doi: 10.1016/j.intimp.2019.105790. [DOI] [PubMed] [Google Scholar]
- Reilly J.P., Calfee C.S., Christie J.D. Acute respiratory distress syndrome phenotypes. Semin. Respir. Crit. Care Med. 2019;40(1):19–30. doi: 10.1055/s-0039-1684049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud A., Fereydoonzad L., Schuessler T.F. Delivered dose estimate to standardize airway hyperresponsiveness assessment in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;308(8):L837–L846. doi: 10.1152/ajplung.00343.2014. [DOI] [PubMed] [Google Scholar]
- Roy A., Srivastava M., Saqib U., Liu D., Faisal S.M., Sugathan S., Bishnoi S., Baig M.S. Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int. Immunopharm. 2016;40:79–89. doi: 10.1016/j.intimp.2016.08.026. [DOI] [PubMed] [Google Scholar]
- Sadraie S., Kiasalari Z., Razavian M., Azimi S., Sedighnejad L., Afshin-Majd S., Baluchnejadmojarad T., Roghani M. Berberine ameliorates lipopolysaccharide-induced learning and memory deficit in the rat: insights into underlying molecular mechanisms. Metab. Brain Dis. 2019;34(1):245–255. doi: 10.1007/s11011-018-0349-5. [DOI] [PubMed] [Google Scholar]
- Song D., Hao J., Fan D. Biological properties and clinical applications of berberine. Front. Med. 2020 doi: 10.1007/s11684-019-0724-6. [DOI] [PubMed] [Google Scholar]
- Sun S., Zhang X., Xu M., Zhang F., Tian F., Cui J., Xia Y., Liang C., Zhou S., Wei H., Zhao H., Wu G., Xu B., Liu X., Yang G., Wang Q., Zhang L., Gong Y., Shao C., Zou Y. Berberine downregulates CDC6 and inhibits proliferation via targeting JAK-STAT3 signaling in keratinocytes. Cell Death Dis. 2019;10(4):274. doi: 10.1038/s41419-019-1510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Cheng H., Liu B., Du Y., Dong J., Huang J. Icariin reduces LPS-induced acute lung injury in mice undergoing bilateral adrenalectomy by regulating GRalpha. Eur. J. Pharmacol. 2020;876 doi: 10.1016/j.ejphar.2020.173032. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Toll-like receptors; their physiological role and signal transduction system. Int. Immunopharm. 2001;1(4):625–635. doi: 10.1016/s1567-5769(01)00010-8. [DOI] [PubMed] [Google Scholar]
- Teng Y., Ross J.L., Cowell J.K. The involvement of JAK-STAT3 in cell motility, invasion, and metastasis. JAK-STAT. 2014;3(1) doi: 10.4161/jkst.28086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wessem K.J.P., Leenen L.P.H. Incidence of acute respiratory distress syndrome and associated mortality in a polytrauma population. Trauma Surg Acute Care Open. 2018;3(1) doi: 10.1136/tsaco-2018-000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Wei X., Wei X., Sun X., Huang X., Liang Y., Xu W., Zhu X., Lin X., Lin J. 4-hydroxybenzo[d]oxazol-2(3H)-one ameliorates LPS/D-GalN-induced acute liver injury by inhibiting TLR4/NF-kappaB and MAPK signaling pathways in mice. Int. Immunopharm. 2020;83 doi: 10.1016/j.intimp.2020.106445. [DOI] [PubMed] [Google Scholar]
- Wang Y., Shan X., Chen G., Jiang L., Wang Z., Fang Q., Liu X., Wang J., Zhang Y., Wu W., Liang G. MD-2 as the target of a novel small molecule, L6H21, in the attenuation of LPS-induced inflammatory response and sepsis. Br. J. Pharmacol. 2015;172(17):4391–4405. doi: 10.1111/bph.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhou X., Zhao D., Wang X., Gurley E.C., Liu R., Li X., Hylemon P.B., Chen W., Zhou H. Berberine inhibits free fatty acid and LPS-induced inflammation via modulating ER stress response in macrophages and hepatocytes. PloS One. 2020;15(5) doi: 10.1371/journal.pone.0232630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng L., Dong S., Wang S., Yi L., Geng D. Macranthol attenuates lipopolysaccharide-induced depressive-like behaviors by inhibiting neuroinflammation in prefrontal cortex. Physiol. Behav. 2019;204:33–40. doi: 10.1016/j.physbeh.2019.02.010. [DOI] [PubMed] [Google Scholar]
- Yang J., Kim E.K., Park H.J., McDowell A., Kim Y.K. The impact of bacteria-derived ultrafine dust particles on pulmonary diseases. Exp. Mol. Med. 2020;52(3):338–347. doi: 10.1038/s12276-019-0367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Ji W., Li M., Qi Z., Huang R., Qu J., Wang H., Wang H. Protective effect of nimesulide on acute lung injury in mice with severe acute pancreatitis. Am J Transl Res. 2019;11(9):6024–6031. [PMC free article] [PubMed] [Google Scholar]
- Yao M., Fan X., Yuan B., Takagi N., Liu S., Han X., Ren J., Liu J. Berberine inhibits NLRP3 Inflammasome pathway in human triple-negative breast cancer MDA-MB-231 cell. BMC Compl. Alternative Med. 2019;19(1):216. doi: 10.1186/s12906-019-2615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Zeng M., Li M., Kan Y., Li B., Xu R., Wu Y., Wang S., Zheng X., Feng W. Protopine protects mice against LPS-induced acute kidney injury by inhibiting apoptosis and inflammation via the TLR4 signaling pathway. Molecules. 2019;25(1) doi: 10.3390/molecules25010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Li Y., Chen T., Gao Y., Sun J., Yang W., Song L., Su P., Ma M., Zhang Z., Zhang H., Yang Y., Li H., Ye Z., Hou H. Comparative study of the efficacy and pharmacokinetics of reducing injection and atomization inhalation. Biomed. Pharmacother. 2019;118:109226. doi: 10.1016/j.biopha.2019.109226. [DOI] [PubMed] [Google Scholar]
- Zhang H.Q., Wang H.D., Lu D.X., Qi R.B., Wang Y.P., Yan Y.X., Fu Y.M. Berberine inhibits cytosolic phospholipase A2 and protects against LPS-induced lung injury and lethality independent of the alpha2-adrenergic receptor in mice. Shock. 2008;29(5):617–622. doi: 10.1097/SHK.0b013e318157ea14. [DOI] [PubMed] [Google Scholar]
- Zhang X., Li C., Li J., Xu Y., Guan S., Zhao M. Protective effects of protocatechuic acid on acute lung injury induced by lipopolysaccharide in mice via p38MAPK and NF-kappaB signal pathways. Int. Immunopharm. 2015;26(1):229–236. doi: 10.1016/j.intimp.2015.03.031. [DOI] [PubMed] [Google Scholar]
- Zhao J., Bi W., Zhang J., Xiao S., Zhou R., Tsang C.K., Lu D., Zhu L. USP8 Protects against Lipopolysaccharide-Induced Cognitive and Motor Deficits by Modulating Microglia Phenotypes through TLR4/MyD88/NF-kappaB Signaling Pathway in Mice. Brain, behavior, and immunity. 2020 doi: 10.1016/j.bbi.2020.04.052. [DOI] [PubMed] [Google Scholar]
- Zhou J., Fu Y., Liu K., Hou L., Zhang W. miR-206 regulates alveolar type II epithelial cell Cx43 expression in sepsis-induced acute lung injury. Experimental and therapeutic medicine. 2019;18(1):296–304. doi: 10.3892/etm.2019.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Ming J., Deng M., Li Y., Li B., Li J., Ma Y., Chen Z., Liu S. Berberine-mediated up-regulation of surfactant protein D facilitates cartilage repair by modulating immune responses via the inhibition of TLR4/NF-kB signaling. Pharmacol. Res. 2020;155:104690. doi: 10.1016/j.phrs.2020.104690. [DOI] [PubMed] [Google Scholar]