Abstract
Objectives
COVID-19 is a multisystemic disease. Ophthalmological abnormalities are relatively rare among COVID-19-infected patients. The aim of our study was to report orbital and visual pathways MRI findings in a nationwide multicenter cohort of patients with severe COVID-19.
Methods
This IRB-approved retrospective multi-center study included participants presenting with severe COVID-19, who underwent brain MRI from March 4th to May 1st 2020. Two neuroradiologists (“blinded”), blinded to all data, individually analyzed morphological MRIs focusing on the orbits and the visual pathways. A second consensus reading session was performed in the case of disagreement between both readers. Clinical and ophthalmological data were compared to MRI findings. Descriptive statistical analysis and interobserver agreement for MRI reading using non-weighted Cohen kappa statistics were performed.
Results
129 participants (43 [33%] women and 86 [67%] men, mean age 63 ± 14 years) were included in the study. 17/129 (13%) patients had abnormal MRI findings of the orbit or visual pathways. 11/17 (65%) patients had a FLAIR-WI hyperintense optic disc. 6/17 (35%) patients had abnormal signal of at least one of the visual pathway structures: 6/6 (100%) of the optic nerve, 1/6 (17%) of the optic chiasm, 2/6 (33%) of the optic tract and 1/6 (17%) of the optic radiations.
Conclusions
Our study showed that a substantial number of patients with severe COVID-19 presented with abnormal MRI findings of the orbit or visual pathways, which might lead to potentially severe visual impairment.
Keywords: MRI, Orbit, COVID-19, SARS-CoV-2
Abbreviations and Acronyms: MRI, Magnetic Resonance Imaging; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; OCT, Optical Coherence Tomography; FLAIR, Fluid-attenuated inversion recovery; RT-PCR, Reverse transcriptase-polymerase chain reaction
Keypoints.
Abnormal MRI findings of the orbit or visual pathways were detected in 13% of patients with severe COVID-19.
Abnormal signal on FLAIR-WI of at least one of the visual pathway structures was detected in 35% of patients.
FLAIR-WI hyperintense optic disc was visible in 65% of patients.
Alt-text: Unlabelled box
Introduction
COVID-19 is a pandemic infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). When symptomatic, COVID-19 is typically known to present with systemic and respiratory manifestations.1 , 2 Increasing data suggest multiorgan involvement with various organs negatively affected, such as the central nervous system.3, 4, 5, 6, 7, 8
Although the main mode of SARS-CoV-2 transmission is through the respiratory route, several studies have shown that ocular tissues and fluids might be an alternative mode of contamination. Exposure of unprotected eyes to the virus may also lead to infection.9 , 10
COVID-19 has been reported to be associated with ophthalmological abnormalities, such as conjunctivitis, chemosis, retinopathy or optic neuritis.11, 12, 13, 14, 15 These findings are relatively rare among COVID-19-infected patients, ranging from 2 to 32%.14, 15, 16, 17, 18 Only a few MRI studies reported orbital and/or visual pathways abnormalities in patients with severe COVID-19.19, 20, 21, 22, 23, 24 Among them, the French Society of Neuroradiology (SFNR) reported ocular (i.e. involving the eyeball only) MRI abnormalities from patients with severe COVID-19 from 16 hospitals including 11 university hospitals and 5 general hospitals.24
The aim of our study was to complete this analysis by reporting orbital (excluding ocular findings) and visual pathways MRI findings in the same nationwide multicenter cohort of patients with severe COVID-19.
Material and methods
Study design
This retrospective observational multicenter study was initiated by the French Society of Neuroradiology (SFNR). It included clinical and imaging data of patients from 16 hospitals including 11 university hospitals and 5 general hospitals. The study was approved by the ethical committee of Strasbourg University Hospital (CE-2020-37) and was in accordance with the 1964 Helsinki Declaration and its later amendments. Due to the emergency in the context of the COVID-19 pandemic, signed informed consent was waived. This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Population
From March 4th to May 1st 2020, consecutive patients presenting with severe COVID-19 who underwent brain MRI were included. Inclusion criteria were: (i) diagnosis of COVID-19 based on possible exposure history or symptomatic presentation, validated with a detection of SARS-CoV-2 by reverse transcriptase-polymerase chain reaction (RT-PCR) assays on the nasopharyngeal, throat or lower respiratory tract swabs; (ii) severe COVID-19 infection defined as requiring hospitalization and oxygen therapy; (iii) the completion of a brain MRI within the context of COVID-19 treatment.
Exclusion criteria were: (i) patients with missing or non-contributory data regarding orbital or brain MRI, such as a lack of sequences or too numerous artifacts.
Clinical and ophthalmological data
Clinical and ophthalmological data, such as that from visual acuity testing, fundoscopy, visual field, fluorescein angiography, and optical coherence tomography (OCT) were extracted from the patients’ electronic medical records.
Virological assessment
Quantitative real-time RT-PCR tests for SARS-CoV-2 nucleic acid were performed on nasopharyngeal or lower respiratory tract swabs and cerebrospinal fluid. Primer and probe sequences targeted two regions on the RdRp gene and were specific to SARS-CoV-2. Assay sensitivity was around 10 copies/reaction (in house-method, Institut Pasteur, Paris, France).
MRI protocols
Imaging studies were conducted either on 1.5- or 3-Tesla MRI. The multicenter nature of the study and the various clinical setups did not allow standardization of sequences. The most frequent sequences performed were 3D T1 weighted spin-echo MRI with and without contrast enhancement, diffusion-weighted imaging, gradient-echo T2 or Susceptibility-weighted imaging, and 2D or 3D FLAIR-WI after administration of gadolinium-based contrast agent.
Morphological imaging analysis
MRI examinations were anonymized and sent to the GE Picture Archiving and Communication System (General Electric, Milwaukee, WI, USA) of a single core center.
Two neuroradiologists with 9 years (“blinded”) and 30 years (“blinded”) of experience in ophthalmological imaging, blinded to all data, individually read anonymized MR images on a dedicated workstation. A second consensus reading session was performed in case of disagreement between both readers.
The readers assessed the presence of abnormal morphology, signal or enhancement of the following structures for each patient:
-
•
The orbit including the extraocular muscles, the orbital fat, the lacrimal gland.
-
•
The visual pathways including the optic disc and optic nerve, the optic chiasm, the optic tract and the optic radiations. Normal-appearing white matter was used as a reference to assess abnormal signal changes.
The analysis of ocular (i.e. eyeball) structures have already been reported in a previous publication.24 Therefore, they were not analyzed for this study.
Statistical analysis
Quantitative variables are presented as mean, standard deviation or median, interquartile range (IQR), and categorical variables as percentages. Interobserver agreement for the MRI reading was assessed using non-weighted Cohen kappa statistics and interpreted as follows: 0.0 to 0.2, poor correlation; 0.21 to 0.4, fair correlation; 0.41 to 0.6, moderate correlation; 0.61 to 0.8, good correlation; and 0.81 to 1, almost perfect correlation. Data were analyzed using the R software package version 3.6.1.
Results
Study population
147 consecutive patients with COVID-19 infection underwent a brain MRI from March 4th to May 1st 2020. Among them, 18 patients with too many artifacts to allow interpretation of the MRI examinations were excluded. A total of 129 patients was included for analysis (43 [33%] women and 86 [67%] men, mean age 63 ± 14 years). Among them, 17/129 (13%) patients (5/17 [29%] women and 12/17 [71%] men, mean age 65 ± 9 years) had abnormal MRI findings of the orbit or visual pathways (Fig. 1 ). 4/17 (24%) patients underwent specific ophthalmological examination and tests. Characteristics of the patients are provided in Table 1 .
Fig. 1.
flowchart. MRI=Magnetic Resonance Imaging.
Table 1.
Clinical characteristics, comorbidities and outcomes of patients presenting with abnormal MRI findings of the orbit or visual pathways. (NA= Not applicable).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | M | M | M | F | M | M | F | M | F | F | M | M | M | M | M | M | F |
| Age (years) | 46 | 65 | 67 | 64 | 75 | 54 | 52 | 73 | 79 | 67 | 67 | 67 | 58 | 57 | 55 | 71 | 63 |
| Diabetes | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | NA | NA | NA | NA | NA |
| Obesity | 29 | 30 | 34 | 35 | 27 | 28 | 41 | NR | NR | 27 | NR | 30 | NA | NA | NA | NA | NA |
| Hypertension | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | NA | NA | NA | NA | NA |
| Asthma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Hospitalization in Intensive Care Unit (ICU) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Simplified Acute Physiology Score (SAPS II) | 32 | 51 | 53 | 61 | 68 | 30 | 36 | 29 | 51 | 63 | 37 | 57 | NA | NA | NA | NA | NA |
| Acute respiratory distress syndrome (ARDS) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| Intubation | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | NA | NA | NA | NA |
| Prone position | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | NA | NA | NA | NA | NA |
| Curarization | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | NA | NA | NA | NA | NA |
| Extracorporeal membrane oxygenation (ECMO) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Dialysis | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | NA | NA | NA | NA | NA |
| Severity of pulmonary involvement on CT [0, 1 (<10%), 2 (10–25%), 3 (25–50%), 4 (50–75%), 5 (> 75%)] | 5 | 2 | 4 | 5 | 1 | 3 | 3 | 5 | 5 | 4 | 4 | 3 | NA | NA | NA | NA | NA |
| Number of days in ICU | 16 | 32 | 23 | 8 | 32 | 18 | 83 | 66 | 17 | 39 | 37 | 47 | NA | NA | NA | NA | NA |
| Death | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Orbital MRI findings
No patient had signal abnormality of the extraocular muscles, the orbital fat or the lacrimal glands.
Visual pathways MRI findings
11/17 (65%) patients had a FLAIR-WI hyperintense optic disc (Fig. 2 ). 6/17 (35%) patients had abnormal signal on FLAIR-WI of at least one of the visual pathway structures: 6/6 (100%) of the optic nerve, 1/6 (17%) of the optic chiasm, 2/6 (33%) of the optic tract and 1/6 (17%) of the optic radiations (Fig. 3, Fig. 4 ). Detailed imaging characteristics are displayed in Table 2 .
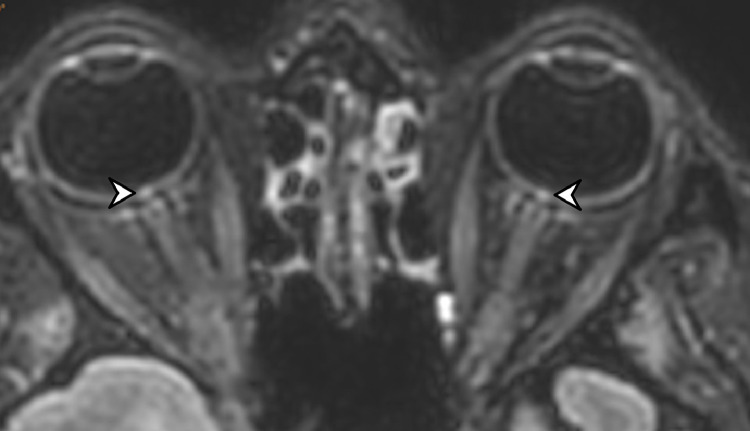
Fig. 2.
67 year-old woman. 3D FLAIR-weighted MRI reformatted in the axial plane showing bilateral high signal intensity of the optic discs (arrowhead).
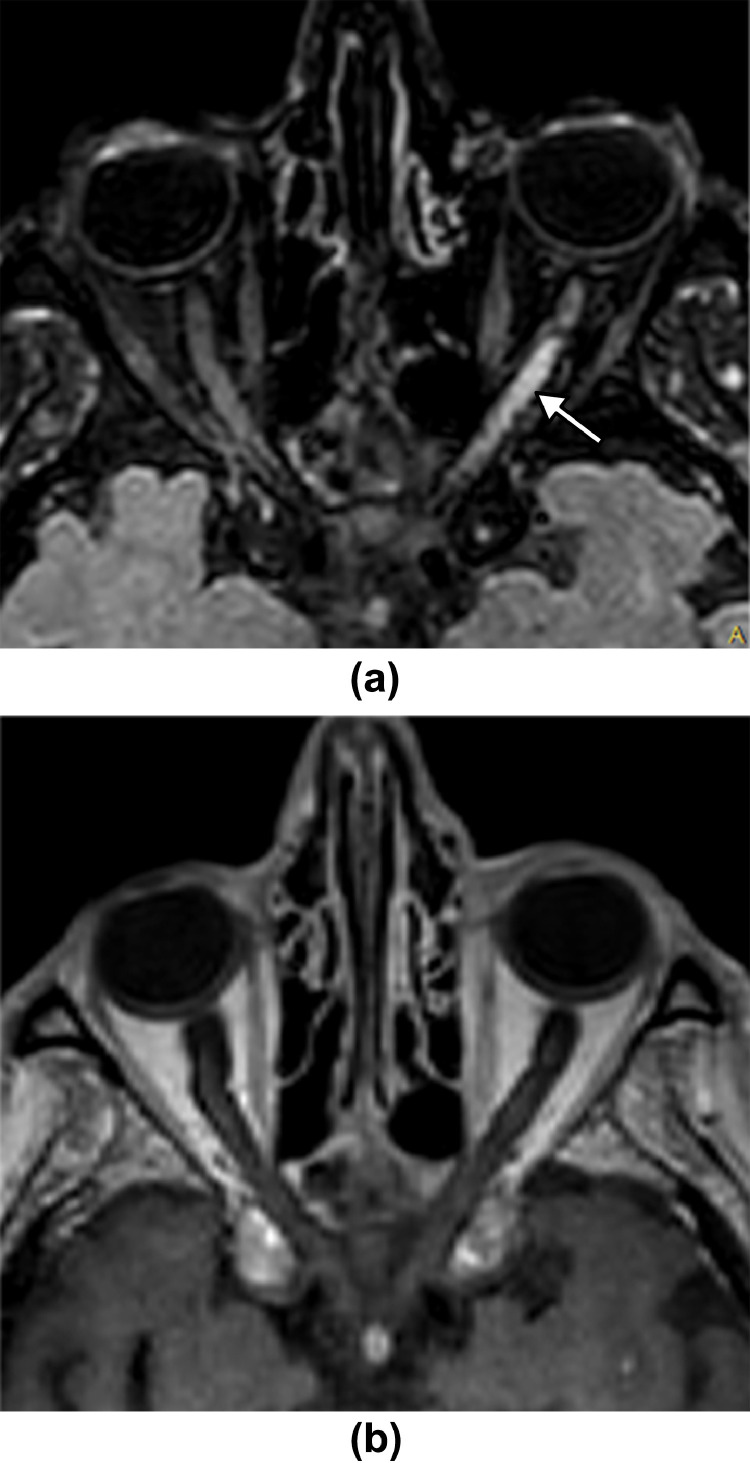
Fig. 3.
73 year-old man. 3D FLAIR-weighted MRI reformatted in the axial plane (a,b) showing extensive bilateral high signal intensity of the optic nerves, optic chiasm and optic tracts (arrows). Post-contrast 3D T1-weighted MRI reformatted in the axial plane (c) showing no enhancement. 3D FLAIR-weighted MRI reformatted in the coronal plane (d) showing bilateral high signal intensity of the optic nerves (arrows) and diffuse extensive white matter hyperintense lesions (arrowheads).
Fig. 4.
75 year-old man. 3D FLAIR-weighted MRI reformatted in the axial plane (a) showing high signal intensity of the left optic nerve (arrow). Post-contrast 3D T1-weighted MRI reformatted in the axial plane (b) showing no enhancement.
Table 2.
Clinical and imaging characteristics of patients presenting with abnormal MRI findings of the orbit or visual pathways.
| Number of patients (n = 17) | % | |||||
|---|---|---|---|---|---|---|
| Demographics | Gender | Male | 12/17 | 71 | ||
| Female | 5/17 | 29 | ||||
| Age (mean ± standard deviation) | 65 ± 9 | |||||
| MRI Orbital findings | Signal abnormality or enhancement of the extraocular muscles | 0/17 | 0 | |||
| Signal abnormality or enhancement of the orbital fat | 0/17 | 0 | ||||
| Signal abnormality or enhancement of the lacrimal glands | 0/17 | 0 | ||||
| MRI Visual pathways findings | FLAIR-WI hyperintense optic disc | Total | 11/17 | 65 | ||
| Bilateral | 9/11 | 82 | ||||
| Unilateral | 2/11 | 18 | ||||
| T1-WI | Hypersignal | 0/11 | 0 | |||
| Isosignal | 11/11 | 100 | ||||
| Hyposignal | 0/11 | 0 | ||||
| Post-contrast enhancement | 0/11 | 0 | ||||
| Abnormal signal on FLAIR-WI | Any of the visual pathway structures | 6/17 | 35 | |||
| Optic nerve | Total | 6/6 | 100 | |||
| Left | 4/6 | 67 | ||||
| Right | 1/6 | 17 | ||||
| Bilateral | 1/6 | 17 | ||||
| Orbital portion | 6/6 | 100 | ||||
| Canalicular portion | 3/6 | 50 | ||||
| Cisternal portion | 1/6 | 17 | ||||
| Post-contrast enhancement | 0/6 | 0 | ||||
| Optic chiasm | Total | 1/6 | 0 | |||
| Left | 0/1 | 0 | ||||
| Right | 0/1 | 0 | ||||
| Bilateral | 1/1 | 100 | ||||
| Post-contrast enhancement | 0/1 | 0 | ||||
| Optic tract | Total | 2/6 | 33 | |||
| Left | 0/2 | 0 | ||||
| Right | 0/2 | 0 | ||||
| Bilateral | 2/2 | 100 | ||||
| Post-contrast enhancement | 0/2 | 0 | ||||
| Optic radiation | Total | 1/6 | 17 | |||
| Left | 0/1 | 0 | ||||
| Right | 0/1 | 0 | ||||
| Bilateral | 1/1 | 100 | ||||
| Post-contrast enhancement | 0/1 | 0 | ||||
| Atrophy | Optic nerve | 4/17 | 24 | |||
| Optic chiasm | 2/17 | 12 | ||||
| Optic tract | 0/17 | 0 | ||||
| Optic radiations | 0/17 | 0 | ||||
Correlations between MRI and ophthalmological findings
None of the patients with FLAIR-WI hyperintense optic disc underwent fundoscopy. 1/6 (17%) patients with abnormal FLAIR-WI signal of at least one of the visual pathway structures had an ophthalmological examination, which was unremarkable.
Inter-reader agreement
Inter-reader agreement was almost perfect for assessing abnormalities of the orbit or the visual pathways: κ = 0.95 [0.87–1], 0.92 [0.84–1] and 0.96 [0.91–1], respectively.
Discussion
Our study showed that a substantial number of patients with severe COVID-19 presented with abnormal MRI findings of the orbit or visual pathways. To the best of our knowledge, our study is the first large multicenter series to date to report these findings.
We showed that 13% of our patients presented with at least one MRI signal abnormality of the orbit or the visual pathways. This rate is substantially higher than the prevalence of 5.5% of ocular manifestations among COVID-19 patients reported in a recent meta-analysis.14 It might be explained by the inclusion of patients with severe COVID-19 in our series, who are more at-risk to develop ocular manifestations related to COVID-19,15 , 25 , 26 and by the analysis of retro-ocular structures on MRI which were not analyzed in previous ophthalmological series.
65% of our patients had FLAIR-WI hyperintense optic discs, almost always bilateral, with no enhancement after gadolinium injection. Only one patient underwent fundoscopy, showing no optic disc abnormality.
35% of our patients presented with FLAIR-WI high signal abnormalities of at least one of the visual pathways structures, mostly a unilateral high signal intensity of the optic nerve. One patient had striking bilateral extensive involvement of the optic nerves, optic chiasm, optic tracts and optic radiations. There was no enhancement after contrast injection. Only one of these patients underwent fundoscopy showing no ocular abnormality. The prevalence of visual pathway structures abnormalities is surprisingly high in our series, given that no increase in the incidence of ischemic or inflammatory optic neuropathies cases related to COVID-19 has been reported in the literature yet. Only a few cases of optic neuritis in association with a COVID-19 infection have been reported in the literature so far, probably due to an immune response triggered by viral antigens rather than being a direct consequence of the infection.11, 12, 13 , 23 As opposed to cases reported in the literature, none of our patients had brain MRI abnormalities suggestive of associated demyelinating disease.
The origin of the various MRI abnormalities that we described in our series remains unknown. Human and animal coronaviruses were reported to cause inflammation of varying ocular segments, causing retinitis, choroiditis, retinal detachment or optic neuritis in the literature.27, 28, 29 To explain retinal or optic nerve damage a wide variety of mechanisms has been described, such as direct infiltration of the retina and retina pigment epithelium by the virus, vasculitis, or autoimmune process, the latter mechanism being most frequent.27 , 28
Our study advocates for a dedicated exploration of the orbit and the visual pathways in all patients presenting with severe COVID-19. Severe eye problems might go largely unnoticed as these patients are hospitalized in intensive care units under sedation. Our data support the need for an ophthalmological evaluation and follow-up of these patients to improve the management of potentially severe, negative ophthalmological outcomes and to provide appropriate treatment.
Our study suffers from several limitations. Firstly, it is a retrospective study with a limited number of patients and non-optimized MRI protocols. Secondly, ophthalmological data were not available for most of our patients, due to the severity of their disease. Thirdly, we lacked follow-up MRI and clinical evaluation. Fourthly, we did not have a control group of critically ill patients hospitalized in intensive care unit without COVID-19 infection. Therefore, we cannot be certain that the MRI abnormalities we reported in our study were related to COVID-19 infection.
Conclusion
Our study showed that a substantial number of patients with severe COVID-19 infection presented with abnormal MRI findings of the orbit or visual pathways, which might lead to potentially severe visual impairments.
Declaration of Competing Interest
We have no conflict of interest.
Acknowledgments
Laura McMaster provided professional English-language medical editing of this article.
Footnotes
Financial Support: None
References
- 1.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ai T., Yang Z., Hou H., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kremer S., Lersy F., Anheim M., et al. Neurologic and neuroimaging findings in COVID-19 patients: a retrospective multicenter study. Neurology. 2020 doi: 10.1212/WNL.0000000000010112. [DOI] [PubMed] [Google Scholar]
- 4.Kremer S., Lersy F., de Sèze J., et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020 doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chougar L., Shor N., Weiss N., et al. Retrospective observational study of brain MRI findings in patients with acute SARS-CoV-2 infection and neurologic manifestations. Radiology. 2020;297:E313–E323. doi: 10.1148/radiol.2020202422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poillon G., Obadia M., Perrin M., Savatovsky J., Lecler A. Cerebral venous thrombosis associated with COVID-19 infection: causality or coincidence? J Neuroradiol J Neuroradiol. 2020 doi: 10.1016/j.neurad.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu C., Liu X., Jia Z. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395:e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng W., Bao L., Gao H., et al. Ocular conjunctival inoculation of SARS-CoV-2 can cause mild COVID-19 in rhesus macaques. Nat Commun. 2020;11:4400. doi: 10.1038/s41467-020-18149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou S., Jones-Lopez E.C., Soneji D.J., Azevedo C.J., Patel V.R. Myelin oligodendrocyte glycoprotein antibody–associated optic neuritis and myelitis in COVID-19. J Neuroophthalmol. 2020 doi: 10.1097/WNO.0000000000001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Ruijter N.S., Kramer G., Gons R.A.R., Hengstman G.J.D. Neuromyelitis optica spectrum disorder after presumed coronavirus (COVID-19) infection: a case report. Mult Scler Relat Disord. 2020;46 doi: 10.1016/j.msard.2020.102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palao M., Fernández-Díaz E., Gracia-Gil J., Romero-Sánchez C.M., Díaz-Maroto I., Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord. 2020;45 doi: 10.1016/j.msard.2020.102377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulhaq Z.S., Soraya G.V. The prevalence of ophthalmic manifestations in COVID-19 and the diagnostic value of ocular tissue/fluid. Graefes Arch Clin Exp Ophthalmol. 2020;258:1351–1352. doi: 10.1007/s00417-020-04695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu P., Duan F., Luo C., et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei province, China. JAMA Ophthalmol. 2020;138:575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L., Deng C., Chen X., et al. Ocular manifestations and clinical characteristics of 534 cases of COVID-19 in China: a cross-sectional study. MedRxiv. 2020 doi: 10.1101/2020.03.12.20034678. 2020.03.12.20034678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling X.C., Kang E.Y.-.C., Lin J.-.Y., et al. Ocular manifestation, comorbidities, and detection of severe acute respiratory syndrome-coronavirus 2 from conjunctiva in coronavirus disease 2019: a systematic review and meta-analysis. Taiwan J Ophthalmol. 2020;10:153–166. doi: 10.4103/tjo.tjo_53_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavalleri M., Brambati M., Starace V., et al. Ocular features and associated systemic findings in SARS-CoV-2 infection. Ocul Immunol Inflamm. 2020;28:916–921. doi: 10.1080/09273948.2020.1781198. [DOI] [PubMed] [Google Scholar]
- 19.Turbin R.E., Wawrzusin P.J., Sakla N.M., et al. Orbital cellulitis, sinusitis and intracranial abnormalities in two adolescents with COVID-19. Orbit Amst Neth. 2020;39:305–310. doi: 10.1080/01676830.2020.1768560. [DOI] [PubMed] [Google Scholar]
- 20.Mehta S., Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12:e10726. doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moonis G., Filippi C.G., Kirsch C.F.E., et al. The spectrum of neuroimaging findings on CT and MRI in adults with coronavirus disease (COVID-19) AJR Am J Roentgenol. 2020 doi: 10.2214/AJR.20.24839. [DOI] [PubMed] [Google Scholar]
- 22.Stevens D.V., Tran A.Q., Kim E. Complications of orbital emphysema in a COVID-19 patient. Ophthalmology. 2020;127:990. doi: 10.1016/j.ophtha.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawalha K., Adeodokun S., Kamoga G.-.R. COVID-19-induced acute bilateral optic neuritis. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620976018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lecler A., Cotton F., Lersy F., Kremer S., Héran F., SFNR's COVID Study Group Ocular MRI findings in patients with severe COVID-19: a retrospective multicenter observational study. Radiology. 2021 doi: 10.1148/radiol.2021204394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrishami M., Tohidinezhad F., Daneshvar R., et al. Ocular manifestations of hospitalized patients with COVID-19 in northeast of Iran. Ocul Immunol Inflamm. 2020;28:739–744. doi: 10.1080/09273948.2020.1773868. [DOI] [PubMed] [Google Scholar]
- 26.Loffredo L., Pacella F., Pacella E., Tiscione G., Oliva A., Violi F. Conjunctivitis and COVID-19: a meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seah I., Agrawal R. Ocul Immunol Inflamm. 2020. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals; pp. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hooks J.J., Percopo C., Wang Y., Detrick B. Retina and retinal pigment epithelial cell autoantibodies are produced during murine coronavirus retinopathy. J Immunol Baltim Md 1950. 1993;151:3381–3389. [PubMed] [Google Scholar]
- 29.Abdul-Kadir M.-.A., Lim L.T. Human coronaviruses: ophthalmic manifestations. BMJ Open Ophthalmol. 2020;5 doi: 10.1136/bmjophth-2020-000630. [DOI] [PMC free article] [PubMed] [Google Scholar]