Significance
Numerous studies have demonstrated that single-stranded DNA phages of the family Microviridae can dominate the human virome, yet shockingly little is known about these diminutive viruses. By synthesizing and propagating microviruses in an experimental system, we demonstrate the existence of a widespread mechanism of phage defense by which these viruses antagonize one another. This mechanism functions through the repurposing of an essential viral structural component instead of through the acquisition of accessory genes, a necessity of the size constraints imposed by the minimal microviral genome. This study thus not only sheds much needed light on the biology of the Microviridae but also emphasizes the importance of virus–virus interaction in the evolution of viral genomes.
Keywords: prophage defense, virus–host interactions, lysogeny, Gokushovirinae, Microviridae
Abstract
Single-stranded DNA phages of the family Microviridae have fundamentally different evolutionary origins and dynamics than the more frequently studied double-stranded DNA phages. Despite their small size (around 5 kb), which imposes extreme constraints on genomic innovation, they have adapted to become prominent members of viromes in numerous ecosystems and hold a dominant position among viruses in the human gut. We show that multiple, divergent lineages in the family Microviridae have independently become capable of lysogenizing hosts and have convergently developed hypervariable regions in their DNA pilot protein, which is responsible for injecting the phage genome into the host. By creating microviruses with combinations of genomic segments from different phages and infecting Escherichia coli as a model system, we demonstrate that this hypervariable region confers the ability of temperate Microviridae to prevent DNA injection and infection by other microviruses. The DNA pilot protein is present in most microviruses, but has been recruited repeatedly into this additional role as microviruses altered their lifestyle by evolving the ability to integrate in bacterial genomes, which linked their survival to that of their hosts. Our results emphasize that competition between viruses is a considerable and often overlooked source of selective pressure, and by producing similar evolutionary outcomes in distinct lineages, it underlies the prevalence of hypervariable regions in the genomes of microviruses and perhaps beyond.
Numerous studies have found members of the viral family Microviridae as a, if not the, dominating force in the human gut virome (e.g., refs. 1–3). Many human viromes are composed almost entirely of these small, single-stranded DNA (ssDNA) phages, which have fundamentally different evolutionary origins than the more commonly studied double-stranded DNA (dsDNA) phages (4). To date, few aspects of their ecology and evolution as a whole have been studied because most microviruses are known only from metagenomic data rather than from physical isolates (5).
Several microviral taxa have been detected as prophages of the Bacteroidetes, Firmicutes, and Proteobacteria (6–9). As prophages, they link their fate to the survival and replication of their bacterial host (10)—“piggybacking-the-winner” rather than “killing-the-winner” by preying on abundant bacteria (11, 12). Therefore, the transition from a lytic to a temperate lifestyle [which, in microviruses, occurred through the acquisition of a short integration motif recognized by bacterial integrase/recombinase systems (9)] imposes new selective pressures on viral populations (13). For example, the lytic microviruses of the subfamily Bullavirinae, exemplified by the venerable phiX174, and the recently discovered temperate members of the subfamily Gokushovirinae both infect Escherichia coli but display very different survival strategies: the former excels at rapid replication and lysis of its bacterial host (14), whereas the latter are slow replicators that can reside in host cultures for long periods of time, with no apparent effect on host fitness (9).
Temperate Gokushovirinae, like other prophages, render their bacterial hosts immune to subsequent infection by related viruses (9) via a process referred to as superinfection exclusion or immunity (SiEx) (15–17). SiEx not only serves to defend hosts against further phage infection but also offers an offensive strategy to bacteria. By providing a way to differentiate infected self- from uninfected nonself cells (18), the excision of prophages produces viral particles that infect and kill nonself strains (19, 20). Given the role of SiEx in competition between bacteria harboring different phage types, as well as between the phages themselves, numerous mechanisms of SiEx exist. SiEx has been studied for decades (21, 22) but primarily in double-stranded DNA viruses such as Lambda, P1, T4, and Mu. In these phages, SiEx is typically conferred by accessory proteins that are not essential to viral function and often lost or gained through recombination (e.g., refs. 23–25). However, the small size of microviral genomes and the broadly overlapping gene sets of lytic and temperate microviruses render the evolution of SiEx through the horizontal acquisition of genes unlikely.
In this study, we link the evolution of a hypervariable region (HVR) in the DNA pilot protein of microviral capsids to the viral ability to both mediate and overcome SiEx. We demonstrate that multiple divergent variants of this region, resulting from competition between otherwise identical phages, are present in populations of lysogens. By synthesizing hybrid phages composed of segments derived from different gokushoviruses and conducting superinfection experiments, we pinpoint the genomic region responsible for SiEx—a region that is almost uniform in lytic viruses but highly variable in temperate viruses. Our results show that divergent microviruses, as a result of (pro)phage arms races, have converged on the identical defensive strategy based on the evolution of HVRs in the same ancestral, structural gene. These findings advance our understanding of the biology and evolution of the ubiquitous microviruses, whose small genomes lack space to evolve or acquire accessory genes to confer new traits. Rather, already existing genes are repurposed for novel functions while preserving compact genome sizes.
Results
HVRs in the DNA Pilot Proteins of Phylogenetically Distinct Microviruses.
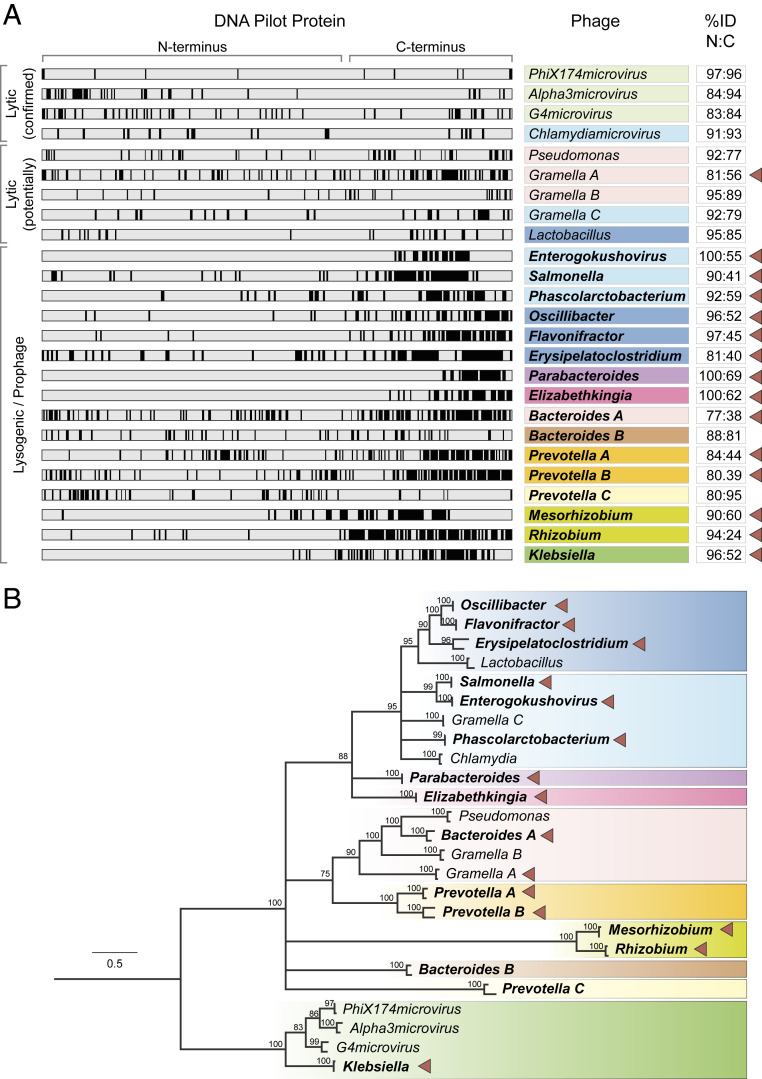
We previously discovered an HVR spanning ∼50 amino acids near the C-terminal end of the DNA pilot protein VP2 of temperate gokushoviruses Enterogokushovirus (SI Appendix, Fig. S1A) (9)]. This region is characterized by a strong decrease in sequence identity between VP2 orthologs of closely related prophages, but interestingly, similar regions are absent in all three genera of the phiX-like microvirus taxon Bullavirinae as well as in lytic Gokushovirinae infecting Chlamydia (Fig. 1A).
Fig. 1.
Variability in DNA pilot proteins is associated with phage lifestyle. (A) Comparisons of pilot proteins from closely related pairs of microviruses. Black vertical lines within each protein (horizontal gray bars) represent positions of amino acid differences between the DNA pilot proteins of closely related phages within the bacterial taxon or phage-type (colored boxes). Protein sizes were normalized and alignment gaps removed. Bolded taxon names indicate pilot protein pairs from prophages, and numbers indicate percent pairwise identities of the N and C terminus (first two-thirds and last one-third of the protein, respectively) of the corresponding taxon or phage-type, with carets indicating presence of an HVR. (B) Phylogeny of microviral taxa based on major capsid protein VP1. Clade colors and notations correspond to those in A. Branch support was estimated using 100 bootstrap replicates, with nodes with <70% support collapsed. Scale bar represents amino acid substitutions per site. Sequence accession numbers are listed in Dataset S1.
To investigate the prevalence and extent of hypervariability in DNA pilot proteins, we searched available microvirus genomes and prophages for pairs of closely related phages that were of at least 75% identical in the first two-thirds of their DNA pilot proteins and at least 25% lower identity in the terminal third. Whereas many phage genomes (including those of the lytic gokushoviruses of Spiroplasma and Bdellovibrio) are not sufficiently similar for comparative purposes, we detected suitably related pairs of microviruses infecting phylogenetically diverse groups of Alpha- and Gammaproteobacteria, Bacteroides, and Firmicutes. Additionally, we detected distantly related microviruses that were recovered from the same host genus (e.g., Bacteroides, Escherichia, Gramella, and Prevotella; Fig. 1B). With only two exceptions (Bacteroides B and Prevotella C), all prophages display HVRs in their DNA pilot protein, with most proceeding to the C-terminal end of the protein (Fig. 1A), and there is only a single case of putatively lytic genome pair (Gramella A) exhibiting an HVR (Fig. 1A).
The difference between temperate and lytic phages with respect to the variability in their DNA pilot proteins is illustrated in the prophage genomes hosted in the genus Klebsiella (NCBI accession nos. LAAO01000012 and LVUT01000012). Both are closely related to the exclusively lytic phiX-like microviruses of Bullavirinae yet are clearly integrated into host genome and represent the first instance of lysogeny in this group of phages (SI Appendix, Fig. S2A). These two Klebsiella prophages average over 90% nucleotide identity across their genomes, which are syntenic to lytic phiX-like microviruses but lack homologs to the small, nonessential gene K and lysis gene E. Most notably, their DNA pilot protein contains a C-terminal HVR that is not present in their lytic relatives (Fig. 1A and SI Appendix, Fig. S2B).
Several metagenomically assembled microvirus genomes also form pairs suitable for comparison and many contain HVRs (SI Appendix, Fig. S3). Those genomes with, and those without, HVRs do not separate into phylogenetically distinct clades (SI Appendix, Fig. S4). Whereas closely related groups of phages often exhibit similar patterns of HVR presence or absence, the presence of HVRs is not conserved within larger clades, an indication that the DNA pilot proteins of microviruses have evolved (and/or lost) these HVR regions multiple times.
Multiple HVRs Circulate in a Prophage Population.
Since HVRs in the pilot protein VP2, as well as in the major capsid protein VP1, are widespread among Microviridae (6, 26), we wanted to investigate their prevalence within a bacterial population (defined here as consisting of multiple strains assigned to a single bacterial species at a single geographic location). A dataset that meets this criterion consists of 125 sequenced strains of E. coli isolated from the guts of marmots (Marmota himalayana) living on the Qinghai-Tibet plateau (27). Of these, more than 25% contain Enterogokushovirus prophage sequences, which, upon phylogenetic analysis, assort into three divergent clades (I, II, and III, colored blue, green, and violet in Fig. 2A) that share only about 75% average nucleotide identity (ANI). Within each clade, members have >90% ANI; however, sequence variation in the HVRs of VP1 and VP2 accounts for most of the within-clade differences. Notably, HVRs can be sorted into “types,” such that within each type, HVRs are nearly 100% identical, but between different types, there is only 30 to 40% amino acid identity (Fig. 2B).
Fig. 2.
Enterogokushovirus prophages within an E. coli population. (A) Whole-genome phylogeny of 125 strains of E. coli, highlighting those strains harboring Enterogokushovirus prophages. Colors of innermost wedges indicate prophage clade assignment (Clade I, blue; Clade II, green; and Clade III, violet), with colors in middle and outer rings denoting HVR1 and HVR2 types, respectively. Those strains shown to harbor Enterogokushovirus prophages but lacking HVR1 and/or HVR2 notations represent cases in which prophages are incomplete or disrupted by insertions. Asterisks indicate HVR types with 80 to 90% identity to another HVR type, see alignments below the tree, where dark letters indicate differences and light gray letters indicate agreements to the consensus amino acid sequence. Prophages labeled a through d are the Clade I representatives included in B. (B) Prophage genome alignments showing sequence variation in four Clade I representatives. Organization and orientation of open reading frames are colored gray, the VP2 gene is colored blue, and the locations of HVR1 and HVR2, as well as predicted promoters P1 and P2, are denoted. Locations of single nucleotide polymorphisms relative to the consensus sequence are plotted in the horizontal bar above each genome. Matrices on the Right show average pairwise nucleotide identities of whole-prophage genomes and average pairwise amino acid identities of HVR1 and HVR2, with cells shaded according to % identity. Genome sequence accession numbers are listed in Dataset S2.
Different HVR types, both for VP1 and VP2, can be observed in prophages that are otherwise almost identical, as exemplified by prophage Clade I (Fig. 2B). Clade I prophages encode two types in the HVRs in VP1 (HVR1 for short) and three types in VP2 (HVR2), Clade II encodes a single HVR1 type and two HVR2 types, and Clade III, represented by only a single member in this population, encodes one HVR1 and HVR2 type (Fig. 2 A and B). Notably, this single HVR2 type found in the Clade III prophage is 82% identical to an HVR2 type in Clade I, and another HVR2 type in Clade II is 88% identical to the major HVR type found in Clade I. Overall, the sharing of a number of HVR types within and between microvirus clades occurring in a single population indicates that phages with different HVRs come into contact with one another, which suggests that they arose as an outcome of phage–phage interactions.
HVRs Determine Prophage Protection.
To experimentally test the role of HVRs in phage–phage interactions, we created a set of phages that mimicked the variation observed in nature—namely, a collection of prophages from different clades encoding different VP1 and VP2 HVR types, all from a single host species (i.e., E. coli). After previously showing that the transformation of a circular double-stranded Enterogokushovirus EC6098 genome into E. coli K12 leads to the production of infective phage particles capable of lysogenizing their hosts (9), we developed an in vitro genome-construction scheme to synthesize circular phage genomes consisting of various combinations of different genomic segments and HVR types (Fig. 3 A and B).
Fig. 3.
Superinfection immunity conferred by synthetic hybrid phages. (A) Sequence relationships of phages used to construct hybrid genomes. Colored circles represent the four wild-type phage genomes whose segments were rearranged for the assembly of synthetic hybrid genomes. Matrices show average pairwise nucleotide identities of whole genomes and average pairwise amino acid identities of their HVR1 and HVR2, with cells shaded according to % identity. Genome accession numbers are presented in Dataset S2. (B) Diagram of experimental protocol. Subdivided segments of the phage genome (labeled L, H, R, and P in outer black circle) and their constituent genes (inner circle) (I) are subcloned into plasmids (II) and assembled in vitro into hybrid phages and transformed into E. coli hosts (III). Primers used to amplify individual fragments are listed in Dataset S3. (C) Patterns of SiEx between hybrid phages. Matrix shows the ability of infecting phages, whose genome components for L-, H-, and R-segments (P-segments being uniform across phages) are color coded as in A and shown horizontally along the x-axis, to superinfect E. coli BW25113ΔfhuA containing different prophages, whose compositions are similarly color coded and shown vertically along the y-axis.
Hybrid phage genomes were assembled from segments of distinct Enterogokushovirus genomes derived from four Escherichia host strains (MOD1-EC6098, MOD1-EC5150, MOD1-EC2703, and MOD1-EC6163; colored green, blue, yellow, and violet, respectively, in Fig. 3A). Each prophage, hereafter denoted by their host strain number, belongs to a different clade sharing <90% ANI and encodes different HVR1 and HVR2 types (Fig. 3A, note that EC2703 and EC6163 share 92% amino acid identity in their HVR2 but have much lower sequence identity elsewhere in the genome). Transformation of these hybrid genomes into host cells generally results in functional phage; however, not all possible combinations of phage segments result in phage production. Notably, those containing HVR1 from the major capsid proteins of EC5150 or EC6163 prophages fail to produce infective phage particles. Furthermore, phage genomes containing an R-segment derived from EC6163 produce viable lytic phages but do not stably integrate into host genomes.
E. coli BW25113 hosts were individually lysogenized with wild-type or hybrid phages and then subjected to superinfection by each of the other phages (Fig. 3C). The results of these infections were binary: either there is unimpaired lysis compared to a nonlysogenized strain or there is complete failure to form any plaques or zones of lysis. While H- and L-segments had no effect on infectivity, there is a clear pattern of SiEx based on the particular R-segment (which contains the 3′-end of VP1 and the complete pilot protein VP2, including its HVR2) of the resident prophage and infecting phage—identical R-segments led to SiEx, whereas divergent R-segments resulted in lysis. The only exceptions are the R-segments of EC2703 and EC6163: prophages with R-segment derived from EC2703 can also prevent superinfection of phages with R-segments from EC6163 (SI Appendix, Fig. S5). R-segments of EC2703 and EC6163 differ considerably from one another in all but the HVR2 of the VP2 gene, where they differ at only six nucleotide positions (Figs. 3A and 4A) and are classified to the same HVR2 type. To further confirm the role of HVR2 in SiEx, we replaced only the HVR2 region of phage EC6098 with that of EC6163. Infection with this phage followed the pattern of phages with the EC6163 (and EC2703) R-segment, further indicating that HVR2 is the sole determinant of SiEx (Fig. 4B).
Fig. 4.
HVR in microviral DNA pilot protein VP2 confers SiEx. (A) Nucleotide alignment of R-segments for phages used in this study. Colored vertical notches indicate position of single nulceotide polymorphisms (A = red, T = green, G = yellow, and C = blue) relative to consensus. Arrows indicate position of open reading frames, with the VP2 gene colored blue. (B) Phage infectivity as a consequence of the pairing of R-segments of the infecting phage (R-segment indicated by colored bars) and the prophage in E. coli BW25113ΔfhuA (R-segment indicated on x-axis). R-segments are color coded as in A. (Note that EC6098:6163HVR is striped green and purple to denote its hybrid structure.) Plaque-forming units on lysogens per µL of phage lysate are shown in comparison to nonlysogen control. Error bars represent SD calculated from three independent replicates. These results are the same as those obtained with plasmid-borne R-segments (SI Appendix, Fig. S5). (C) Phage transduction as a consequence of the pairing of VP2 protein of transducing particles (VP2 indicated by colored bars) and the R-segment of prophage in E. coli BW25113ΔfhuA (labeled on x-axis). Percent reduction in transductant numbers are shown relative to nonlysogenized cells. Error bars represent SD calculated from three independent replicates. (D) Transformation of noninfective phage genomes with different R-segments into E. coli DH5α cells harboring pYTK001 plasmids carrying either similar (i.e., SiEx-inducing) or different Enterogokushovirus R-segments (see A). Percent reduction of transformants is shown relative to transformation of a pYTK001 plasmid-borne H segment from EC6098. Box-and-whiskers plots show median, 25th, and 75th percentiles (Upper and Lower hinges) and 1.5 interquartile range (whiskers), with individual experiments represented by dots.
Replicate experiments using plasmids encoding only the R-segment yield similar results: plasmids containing only the R-segment confer complete protection against infection by phages having the same R-segment. As in the experiments with prophages, the R-segment of EC2703 also blocked phages with the R-segment of EC6163 and vice versa (SI Appendix, Fig. S5). In contrast to experiments using only prophages (Fig. 4B), we also observe reductions in infectivity with different R-segments, perhaps indicating a dosage-dependent effect of SiEx. The magnitude of this effect differs between HVR2 types; however (other than the complete lack of infection when paired with an identical/similar HVR2), there is no obvious association between the efficiency of SiEx and the overall similarity in HVRs, or the presence or absence of particular amino acid substitutions in these regions. Additionally, this effect is observed in lieu of a plasmid-encoded promoter—a predicted second promoter upstream of the VP2 region is likely responsible for expression of the VP2 gene both in a plasmid and in linearized prophages, whose main promoter is located in the P-segment and faces outwards toward the bacterial genome (arrows in Fig. 2B). Defense occurs regardless of the actual integration status of the phage: both integrated prophage and nonintegrated plasmid can block superinfecting phages due to the presence of the putative VP2 promoter upstream of the VP2 gene in both circular and linear genomes.
Microviral SiEx Prevents Successful Phage Infection at the Stage of DNA Injection.
To further investigate the nature of our observation, we next tested whether phages are either unable to attach to host cells protected by prophages (due to loss or blockage of cellular receptors) or to attach but unable to infect and/or replicate inside host cells. Incubation of phages with hosts either protected or unprotected by prophages show a considerable reduction (two-tailed t test, P < 0.03) in free viral particles over time (SI Appendix, Fig. S6). Therefore, we conclude that the observed mechanism does not prevent attachment to lysogenized cells.
The observed protection could potentially occur at the nucleotide or protein level. It was not possible to directly test the actions of VP2 proteins since their expression from plasmids with inducible promoters had a toxic effect on cells. However, since VP2 proteins are an essential part of microvirus capsids (14), transduction of plasmid genomes lacking the VP2 gene can be used to rule out CRISPR or RNA-interference-like nucleotide–nucleotide interactions and assess the involvement of proteins. Indeed, transduction through microvirus virions that carried no VP2 DNA but contained VP2 proteins exhibited the same pattern that we observed with phage infection—HVR2 type determined whether DNA was transduced or not (Fig. 4C). Note that in contrast to phage infection experiments where no plaques were observed for bacteria protected by the appropriate HVR2, we did observe a small number of transductants in some pairs with identical HVR2. On further analysis, these colonies had been cured of prophages and were therefore amenable to transduction. Overall, our experiments show a dependency of VP2 protein on SiEx. As transduced plasmids do not rely on microviral replication machinery, this experiment also provides evidence against an involvement of replication in the HVR2-specific defense.
An alternative to the SiEx mechanism operating at the stage of replication (once DNA has entered the cell) is prevention of the DNA from successfully entering the cell in the first place. To further differentiate these two options, we replaced the H-segment of microviral genomes with an antibiotic resistance cassette. This disruption of the major capsid protein gene creates a virus genome capable of replication inside of the host but incapable of producing infective virions. If phage protection acts at the level of replication, electroporation of these genomes (thereby skipping the infection step) is still expected to result in a reduction in the number of transformants dependent on the resident and transformed R-segment/HVR2 type. Whereas overall transformation rates decrease by about 50% compared to a control with no R-segment (indicating an additional, more general effect of the R-segment), there are, however, no reductions in the number of transformants specific to the HVR2 type, markedly different from what was observed in the superinfection experiments (two-tailed t test, P = 0.98) (Fig. 4D).
In sum, our results indicate that the HVR in the pilot protein of microvirus prophages is responsible for specific protection against microviral infection at the stage of DNA injection.
Discussion
Among microviruses, SiEx has previously been studied exclusively in lytic phages—phiX174 and its close relatives. These phages establish SiEx shortly after (within 10 min) infection (28, 29), and paralleling our results, SiEx is conferred by the C-terminal region of the DNA pilot protein (30). Yet, in contrast to the phages characterized in the present study, phiX-like microviruses have not previously been observed to contain an HVR in their DNA pilot proteins. PhiX-like phages are distantly related to other microviruses, to the point where most cases of gene homology can be deduced only from similarities in protein structure or function. However, the phiX-like prophages that we discovered in Klebsiella genomes possess conspicuous HVRs but are otherwise very similar in genomic structure and sequence to the lytic phiX174.
The presence of such HVRs in phiX-like prophages, as well as in a number of other prophages present in Bacteroidetes, Firmicutes, and diverse Proteobacteria, indicates that such regions repeatedly arise and coevolve with the ability to lysogenize hosts. Moreover, the reliance on the same protein region in viruses that differ broadly in genome organization, ecology, and host specificity suggests that the mechanism underlying SiEx is ancestral to microviruses. The VP2 protein serves as a DNA conduit for infection across microviruses (31–34), and its role in SiEx appears to be an auxiliary function, increased in its importance by shifts in viral survival strategy from lytic (kill-the-winner) to temperate (piggyback-the-winner).
The genes that underlie SiEx are often subject to diversifying selection, which can lead to the formation of HVRs (15). For example, HVRs exist in the sequences specifying both the repressor protein and operator region involved in SiEx in the genomes of dsDNA prophages of mycobacteria (35). As with other prophages that exhibit SiEx, microviruses produced by lysogenized hosts are unable to infect other bacterial cells harboring their own protective prophage, and therefore, genetic changes that overcome this defense are beneficial. This selective pressure shapes the sequence variation in the C terminus of the DNA pilot protein and the eventual evolution of an HVR. In contrast, purely lytic phages gain little from SiEx, which would only prove useful during short periods immediately between infection and subsequent lysis. Therefore, there is no benefit to altering the pilot protein, as evident by the lack of HVRs in phiX174 and other lytic microviruses.
This arms-race selection for HVRs need not be limited to prophages embedded in the host chromosome; in fact, a number of complete microvirus genomes with HVRs, lacking corresponding prophages, can be assembled from metagenomes. We previously showed that gokushoviruses with partial deletions in the genomic dif-motifs required for lysogeny are retained in the host cytoplasm and confer SiEx (9). We hypothesized that this situation represents a transition state in the evolution from lytic and temperate lifestyles, suggesting that some microviral lineages could have evolved SiEx-associated HVRs before the ability to integrate into hosts.
SiEx can operate at three stages of phage infection (36, 37). First, at the level of host attachment through blockage or alteration of phage receptors on the cell surface, as observed in tailed E. coli phage T5 or in many phages of Pseudomonas (38, 39). Second, at the level of injection of genetic material, after phage particles have attached to the cell, as exemplified by the T4-encoded Sp and Imm (40). Third, after genetic material has been injected into the cell. Defense at this stage can occur through the aforementioned binding and silencing of infecting viral operator regions via repressor proteins expressed by prophages or a number of other mechanisms inhibiting phage replication or virion assembly (36, 37).
Our results so far indicate that microviral SiEx is acting at the stage of DNA injection through interaction between the prophage and phage copies of the DNA pilot protein. As homologs of the SiEx-conferring DNA pilot protein are found exclusively in the Microviridae, the observed mechanism of phage defense is exclusive to microviruses. In phiX174, the DNA pilot protein has been shown to play crucial roles in both DNA injection and, subsequently, in genome replication. Multiple copies of the pilot protein are packaged into the phage capsid (31), and upon phage attachment to a target cell, these proteins oligomerize and form a tunnel via transmembrane alpha helices located in the N terminus of the protein (32, 33). The formation of this complex mediates transfer of both the pilot proteins and the viral ssDNA into the host cell (34), in which the pilot proteins trigger production of dsDNA, the replicative intermediate of microvirus genomes (41). In support of the view that microviral SiEx acts during DNA injection, we found the presence of VP2 in the host cell diminishes both injection of DNA and replication, but HVR-specific effects are only observed at the level of the former. It is possible that microviral SiEx, as suggested for phiX-like phages specifically, results from either the unproductive oligomerization between the pilot proteins of cytosolic and superinfecting phage (30) and/or the sequestration or inactivation of incoming pilot proteins through interactions with VP2 prophage DNA (28–30).
Microviruses are among the smallest known bacteriophages (42), and the size constraints imposed by their small capsid volume prevents the accumulation of accessory genes that usually confer novel functions to phages (43). Instead, they rely on repurposing resident sequences through overprinting and hypervariability, whose evolution is fostered by their high mutation rates (14). HVRs are a common feature of viruses and have been cataloged from both comparative genomic and metagenomic studies. The presence of such regions is usually ascribed to antagonism and coevolution between viruses and hosts, e.g. surface attachment or the avoidance of the host immune response (44–47). In our study, we provide evidence that the ubiquitous HVRs of Microviridae have evolved numerous times for a different reason—competition between (pro)phages, as mediated by SiEx. Due to their pathogenic properties, studies of viral adaptation have largely focused on the selective pressures imposed by hosts; however, increasing evidence for competitive interactions between viruses (e.g., refs. 48–50) should lead to reconsideration of the set of challenges that viruses endure.
Materials and Methods
Detecting Microviral Prophages.
To extract the sequences of the microviruses present in sequenced bacterial genomes, we performed iterative Hidden Markov model searches using jackhmmr as implemented in hmmer 3.2.1 (51) on the 597,712 bacterial genomes available in the GenBank database of NCBI (accessed April 13, 2020) using the major capsid proteins of phages phiX174 (Bullavirinae, NC_001422), EC6098 (Gokushovirinae, MT185428), and WZ-2015a (divergent microvirus, KT264812) as queries, with an e-value cutoff of 0.05. After removing contaminating sequences identical to phiX174 and sequences from individual prophage hosts without relatives in at least one closely related bacterium, we manually extracted prophage genomes from bacterial contigs as follows: proceeding from the assumptions that microviral prophages are 1) less than 10 kb in length, 2) that all genes were oriented in same direction as its predicted VP1 gene, and 3) that prophages are flanked either by recognizable bacterial genes or by large genes facing in the opposite orientation. We then reannotated all prophages using PHANOTATE 1.5.0 (52) and manually curated the resulting annotations to remove predicted genes oriented in the opposite direction as the majority of genes. Promoter regions in E. coli prophages were predicted using BPROM (53).
Sequence Alignment and Phylogenetic Analysis.
To find closely related phages suitable for comparison of their VP2 homologs, we screened available microvirus genomes from NCBI, the aforementioned prophages, and phage genomes from other sources (6, 9, 54). We performed all-versus-all searches via DIAMOND 0.9.32 (55) on all phage proteins using an e-value cutoff of 0.001 and then clustered these proteins into groups of close relatives with an identity cutoff of 35% and coverage cutoff of 80% using SiLiX 1.2.9 (56). Based on the presence and absence of protein groups, phage genomes were subsequently clustered into groups of close relatives using the Map equation (57). Alignments of closely related phage genomes were performed with ClustalO 1.2.4, using standard settings (58). Average pairwise nucleotide and amino acid identities of genomes or genomic regions were calculated and visualized using Geneious R9 (https://www.geneious.com). Maximum likelihood phylogenetic trees of prophages were generated with RAxML 8.0.26 (59) using the GTR+GAMMA substitution model, with branch support estimated using 100 fast-bootstrap replicates. Accession numbers of all phage and prophage pairs are listed in Dataset S1.
Core-genome alignments of members of an E. coli population (accession numbers listed in Dataset S2) were constructed based on protein families having minimum of 30% amino acid identity (USEARCH 11) (60), which were aligned in MUSCLE 3.8.31 (61) as implemented in the BPGA 1.3 pipeline (62). The maximum likelihood phylogenetic tree of core-genome alignments was built in RAxML 8.0.26 (59) using the GTR+PROTGAMMAWAG substitution model, with branch support estimated using 100 fast-bootstrap replicates.
Construction of Hybrid Phages Using Golden Gate Assembly.
To enable Golden Gate assembly (63) of complete phage genomes, we conceptually divided the conserved circular genome of Enterogokushoviruses into four segments termed: L (encompassing genes VP4, VP5, VP3, and the 5′ end of VP1 up until HVR1), H (HVR1 domain of VP1), R (3′ end of VP1 and VP2 including HVR2), and P (VP8 and the predicted main promoter region of the phage) (Fig. 2B). Conserved primers containing selected restriction-site overhangs were designed to allow the amplification of the segments and subsequently enabling circular reassembly of prophages in order L-H-R-P-L. A slightly modified primer set was used for the amplification of segments facilitating the exchange of just HVR2 (Dataset S3). Additionally, we designed primers to insert a kanamycin-resistance cassette of plasmid pBTK622 (64) instead of the H segment, facing the same orientation as the other phage genes (Dataset S3).
To obtain circular templates of phage genomes for PCR, we grew cultures of Escherichia spp. MOD1-EC2703, MOD1-EC5150, MOD1-EC6098, and MOD1-EC6163 (Dataset S2) overnight in 5 mL Lysogeny Broth (LB) medium at 37 °C with mild shaking (200 rpm). Phage particles were separated from bacteria by centrifugation at 5,000 g for 5 min, and filtration of supernatants through 0.22-µm filters. The individual phage segments were amplified from 1 µL filtrate using Phusion High-Fidelity DNA polymerase (New England Biolabs). PCR was conducted under the following conditions: 98 °C for 3 min; 30 cycles of 98 °C for 15 s, 50 °C for 15 s, and 72 °C for 2.5 min; followed by 72 °C for 5 min. Amplified segments were isolated and purified from agarose gels using Monarch DNA Gel Extraction Kit (New England Biolabs) and eluted in 20 µL ddH2O. Sample concentrations were determined by ThermoFisher Qubit dsDNA assays.
Phage/kanamycin-resistance cassette segments were cloned into plasmids by Golden Gate assembly in 20 µL reaction volumes using 1 µL each of T4 DNA Ligase (Promega) and BsmBI (New England Biolabs) to integrate 20 fmol of each segment into 10 fmol of pYTK001 plasmids (65) under the following conditions: 25 cycles of 42 °C for 1.5 min and 16 °C for 3 min; 50 °C for 5 min; followed by 80 °C for 10 min. A total of 1 μL circularized construct was electroporated into 100 µL electrocompetent E. coli DH5α, and after immediate addition of 1 mL Super Optimal Growth with Catabolite Repression (SOC) medium, incubated for 1 h at 37 °C. Cultures were plated on LB plates supplemented with 25 µg/mL chloramphenicol and incubated overnight at 37 °C. GFP-negative colonies (indicating the replacement of the gfp gene with the target segment) were restreaked for purity and grown in 5 mL LB supplemented with chloramphenicol at 37 °C with mild shaking (200 rpm). Plasmids were extracted using the Monarch Plasmid DNA Miniprep Kit (New England Biolabs) and eluted in 30 µL ddH2O. Construct identities were confirmed by Sanger sequencing.
We assembled complete phage genomes from 20 fmol of each of the plasmids containing separate L, H (or kanamycin-resistance cassette), R, and P segments in 20 µL total reaction volumes including 1 µL each of T4 DNA Ligase (Promega) and BsaI-HFv2 (New England Biolabs) under the following conditions: 25 cycles of 37 °C for 1.5 min and 16 °C for 3 min, followed by 37 °C for 5 min and 80 °C for 10 min. Reactions were desalted by dialysis on 0.045-µm filters for 1 h and then transformed into electrocompetent E. coli DH5α and incubated at 37 °C in 1 mL SOC with mild shaking (200 rpm). After 1 h, 4 mL LB was added, and incubation proceeded overnight under identical conditions. Supernatants containing phage were extracted as described above when growing cultures of E. coli for obtaining templates for phage genes, and concentrated to a volume of 100 µL using Millipore Amicon Ultra-2 Centrifugal Filter Unit with Ultracel-30 membranes. For infection-deficient phages containing a kanamycin-resistance cassette, electroporated cells were plated onto LB agar containing 50 ug/mL kanamycin. Colonies were subsequently restreaked and then grown overnight at 37 °C in LB agar supplemented with kanamycin, constructs extracted using a New England Biolabs Monarch Plasmid Miniprep Kit, and identity confirmed using Sanger sequencing.
Prophage Detection, Phage Growth, and Spot Assays.
To test for phage production and increase phage concentrations, we collected the bacteria from 100 µL overnight culture of BW25113ΔfhuA (which is immune to common lambdoid phage contaminants) by centrifugation, resuspended bacteria in Tris-Magnesium-Glycerol buffer, added 100 µL phage supernatants, and incubated the mixture at room temperature for 5 min prior to addition 3 mL 0.6% LB agarose before plating onto LB agar. To increase phage concentrations, we harvested phage lysates from plates exhibiting confluent lysis after overnight incubation at 37 °C. Phage titers were determined by spotting dilutions of lysates with 100 µL appropriate overnight cultures of host strains (BW25113ΔfhuA with or without pYTK001 plasmids containing cloned phage segments or the same bacterial strain with integrated hybrid prophages) onto 0.6% agar-overlay plates and incubating plates overnight at 37 °C. Putative lysogens were identified from surviving colonies on plates that had undergone confluent lysis, and prophage integration was confirmed via PCR using primers MG1655_fw and MG1655_rev, which flank the bacterial integration motif (9).
Transformation Assays.
E. coli DH5α cells carrying pYTK001 plasmids with R-segments from EC2703, EC5150, EC6098, and EC6163 and H-segment of EC6098 as control were grown to OD600 = 0.7 and made electrocompetent. A total of 30 µL electrocompetent cells were mixed with 1 µL phage-genomes with a kanamycin-resistance cassette instead of H-segments but retaining R-segments from EC2703, EC5150, EC6098, and EC6163, electroporated, and grown in SOC medium at 37 °C for 1 h. Numbers of transformants were determined by spread plating on LB agar supplemented with 50 µg/mL kanamycin and 25 µg/mL chloramphenicol and incubated overnight at 37 °C.
Transduction Assays.
We previously observed that pYTK001 plasmids carrying the ∼2,350 base pair (bp) L-segment could be successfully packaged into Enterogokushovirus capsids. These ∼4,300-bp constructs (about 200-bp smaller than a full phage genome) contain a 70-bp 5′-truncated copy of VP4, the full VP5 and VP3 genes, the 5′ end of VP1 lacking HVR1, as well as the full plasmid backbone including an antibiotic resistance marker and plasmid replication machinery. Absent are HVR1, the 3′ end of VP1, and the entire VP2 gene as well as VP8 and the phage integration motif. We produced lysates containing transducing particles by infection of bacterial cultures at OD600 = 0.7, carrying the pYTK001 plasmid with the L-segment of EC6098, with phages at multiplicity of infection of ∼1:1, subsequent agar overlay and harvesting as described in the section Prophage Detection, Phage Growth, and Spot Assays. For transduction, 100 µL lysates were mixed with 100 µL lysogenized/nonlysogenized BW25113ΔfhuA (OD600 = 2.5, resuspended in fresh LB), incubated for 5 min at room temperature before the addition of 800 µL LB medium, and further incubated at 37 °C for 1 h. Cells were subsequently spread on LB-agar plates supplemented with 25 µL/mL chloramphenicol to grow bacteria with transduced plasmids.
Phage Adsorption Assays.
As Enterogokushoviruses form faint and difficult to count plaques in agar-overlay assays, adsorption was calculated using transducing particles. Overnight cultures of bacterial strains E. coli BW25113ΔfhuA and BW25113ΔfhuA lysogenized by EC6098 were grown overnight at 37 °C, diluted to OD600 = 2.5, and infected with 100 µL EC6098 lysates containing transducing particles (produced as described in Transduction Assays). After incubation for various amounts of time, cells with attached particles were separated from supernatant with free particles by centrifugation at 14,000 rpm for 1 min. Supernatants were then used for transduction as described in Transduction Assays.
Supplementary Material
Acknowledgments
We thank Kim Hammond for assistance in figure design. This study was funded by NIH award R35GM118038 to H.O.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2102786118/-/DCSupplemental.
Data Availability
Alignment data have been deposited in Data Dryad (https://doi.org/10.5061/dryad.9zw3r22d4) (66). All other study data are included in the article and/or supporting information.
References
- 1.Zuo T., et al., Human-gut-DNA virome variations across geography, ethnicity, and urbanization. Cell Host Microbe 28, 741–751 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Shkoporov A. N., et al., The human gut virome is highly diverse, stable, and individual specific. Cell Host Microbe 26, 527–541 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Moreno-Gallego J. L., et al., Virome diversity correlates with intestinal microbiome diversity in adult monozygotic twins. Cell Host Microbe 25, 261–272 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kazlauskas D., Varsani A., Koonin E. V., Krupovic M., Multiple origins of prokaryotic and eukaryotic single-stranded DNA viruses from bacterial and archaeal plasmids. Nat. Commun. 10, 3425 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tisza M. J., et al., Discovery of several thousand highly diverse circular DNA viruses. eLife 9, e51971 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roux S., Krupovic M., Poulet A., Debroas D., Enault F., Evolution and diversity of the Microviridae viral family through a collection of 81 new complete genomes assembled from virome reads. PLoS One 7, e40418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krupovic M., Forterre P., Microviridae goes temperate: Microvirus-related proviruses reside in the genomes of Bacteroidetes. PLoS One 6, e19893 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhan Y., Chen F., The smallest ssDNA phage infecting a marine bacterium. Environ. Microbiol. 21, 1916–1928 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Kirchberger P. C., Ochman H., Resurrection of a global, metagenomically defined gokushovirus. eLife 9, e51599 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowles B., et al., Lytic to temperate switching of viral communities. Nature 531, 466–470 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Thingstad T. F., Elements of a theory for the mechanisms controlling abundance, diversity, and biogeochemical role of lytic bacterial viruses in aquatic systems. Limnol. Oceanogr. 45, 1320–1328 (2000). [Google Scholar]
- 12.Silveira C. B., Rohwer F. L., Piggyback-the-Winner in host-associated microbial communities. NPJ Biofilms Microbiomes 2, 16010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hampton H. G., Watson B. N. J., Fineran P. C., The arms race between bacteria and their phage foes. Nature 577, 327–336 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Doore S. M., Fane B. A., The Microviridae: Diversity, assembly, and experimental evolution. Virology 491, 45–55 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Berngruber T. W., Weissing F. J., Gandon S., Inhibition of superinfection and the evolution of viral latency. J. Virol. 84, 10200–10208 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abedon S. T., Look who’s talking: T-even phage lysis inhibition, the granddaddy of virus-virus intercellular communication research. Viruses 11, 951 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broecker F., Moelling K., Evolution of immune systems from viruses and transposable elements. Front. Microbiol. 10, 51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song S., Guo Y., Kim J. S., Wang X., Wood T. K., Phages mediate bacterial self–recognition. Cell Rep. 27, 737–749.e4 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Basso J. T. R., et al., Genetically similar temperate phages form coalitions with their shared host that lead to niche-specific fitness effects. ISME J. 14, 1688–1700 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeWerff S. J., Bautista M. A., Pauly M., Zhang C., Whitaker R. J., Killer Archaea: Virus-mediated antagonism to CRISPR-immune populations results in emergent virus-host mutualism. MBio 11, e00404–e00420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delbrück M., Interference between bacterial viruses: III. The mutual exclusion effect and the depressor effect. J. Bacteriol. 50, 151–170 (1945). [DOI] [PubMed] [Google Scholar]
- 22.Dulbecco R., Mutual exclusion between related phages. J. Bacteriol. 63, 209–217 (1952). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gentile G. M., et al., More evidence of collusion: A new prophage-mediated viral defense system encoded by mycobacteriophage sbash. MBio 10, e00196-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery M. T., Guerrero Bustamante C. A., Dedrick R. M., Jacobs-Sera D., Hatfull G. F., Yet more evidence of collusion: A new viral defense system encoded by Gordonia phage CarolAnn. MBio 10, 02417–02418 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koonin E. V., Makarova K. S., Wolf Y. I., Krupovic M., Evolutionary entanglement of mobile genetic elements and host defence systems: Guns for hire. Nat. Rev. Genet. 21, 119–131 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Hopkins M., et al., Diversity of environmental single-stranded DNA phages revealed by PCR amplification of the partial major capsid protein. ISME J. 8, 2093–2103 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu S., et al., Insights into the evolution of pathogenicity of Escherichia coli from genomic analysis of intestinal E. coli of Marmota himalayana in Qinghai-Tibet plateau of China. Emerg. Microbes Infect. 5, e122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchison C. A. III, Sinsheimer R. L., Requirement of protein synthesis for bacteriophage phi X174 superinfection exclusion. J. Virol. 8, 121–124 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tessman E. S., Borrás M. T., Sun I. L., Superinfection in bacteriophage S13 and determination of the number of bacteriophage particles which can function in an infected cell. J. Virol. 8, 111–120 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruboyianes M. V., Chen M., Dubrava M. S., Cherwa J. E. Jr, Fane B. A., The expression of N-terminal deletion DNA pilot proteins inhibits the early stages of phiX174 replication. J. Virol. 83, 9952–9956 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun L., Rossmann M. G., Fane B. A., High-resolution structure of a virally encoded DNA-translocating conduit and the mechanism of DNA penetration. J. Virol. 88, 10276–10279 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roznowski A. P., Fisher J. M., Fane B. A., Mutagenic analysis of a DNA translocating tube’s interior surface. Viruses 12, 670 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun L., et al., Icosahedral bacteriophage ΦX174 forms a tail for DNA transport during infection. Nature 505, 432–435 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Sun Y., et al., Structural changes of tailless bacteriophage ΦX174 during penetration of bacterial cell walls. Proc. Natl. Acad. Sci. U.S.A. 114, 13708–13713 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mavrich T. N., Hatfull G. F., Evolution of superinfection immunity in Cluster A mycobacteriophages. MBio 10, e00971-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rostøl J. T., Marraffini L., (Ph)ighting phages: How bacteria resist their parasites. Cell Host Microbe 25, 184–194 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labrie S. J., Samson J. E., Moineau S., Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8, 317–327 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Braun V., Killmann H., Herrmann C., Inactivation of FhuA at the cell surface of Escherichia coli K-12 by a phage T5 lipoprotein at the periplasmic face of the outer membrane. J. Bacteriol. 176, 4710–4717 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bondy-Denomy J., et al., Prophages mediate defense against phage infection through diverse mechanisms. ISME J. 10, 2854–2866 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu M. J., Henning U., Superinfection exclusion by T-even-type coliphages. Trends Microbiol. 2, 137–139 (1994). [DOI] [PubMed] [Google Scholar]
- 41.Jazwinski S. M., Marco R., Kornberg A., The gene H spike protein of bacteriophages phiX174 and S13. II. Relation to synthesis of the parenteral replicative form. Virology 66, 294–305 (1975). [DOI] [PubMed] [Google Scholar]
- 42.Dion M. B., Oechslin F., Moineau S., Phage diversity, genomics and phylogeny. Nat. Rev. Microbiol. 18, 125–138 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Taylor V. L., Fitzpatrick A. D., Islam Z., Maxwell K. L., The diverse impacts of phage morons on bacterial fitness and virulence. Adv. Virus Res. 103, 1–31 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Minot S., Grunberg S., Wu G. D., Lewis J. D., Bushman F. D., Hypervariable loci in the human gut virome. Proc. Natl. Acad. Sci. U.S.A. 109, 3962–3966 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosario K., Schenck R. O., Harbeitner R. C., Lawler S. N., Breitbart M., Novel circular single-stranded DNA viruses identified in marine invertebrates reveal high sequence diversity and consistent predicted intrinsic disorder patterns within putative structural proteins. Front. Microbiol. 6, 696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hannigan G. D., et al., Evolutionary and functional implications of hypervariable loci within the skin virome. PeerJ 5, e2959 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roux S., et al., Ecology and molecular targets of hypermutation in the global microbiome. Nat. Commun. 12, 3076 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dedrick R. M., et al., Prophage-mediated defence against viral attack and viral counter-defence. Nat. Microbiol. 2, 16251 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Díaz-Muñoz S. L., Sanjuán R., West S., Sociovirology: Conflict, cooperation, and communication among viruses. Cell Host Microbe 22, 437–441 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roux S., et al., Cryptic inoviruses revealed as pervasive in bacteria and archaea across Earth’s biomes. Nat. Microbiol. 4, 1895–1906 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wheeler T. J., Eddy S. R., nhmmer: DNA homology search with profile HMMs. Bioinformatics 29, 2487–2489 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNair K., Zhou C., Dinsdale E. A., Souza B., Edwards R. A., PHANOTATE: A novel approach to gene identification in phage genomes. Bioinformatics 35, 4537–4542 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solovyev V., Salamov A., BPROM—prediction of bacterial promoters. http://www.softberry.com/berry.phtml?topic=bpromandgroup=programsandsubgroup=gfindb. Accessed 28 June 2021.
- 54.Gregory A. C., et al., The Gut Virome Database Reveals Age-Dependent Patterns of Virome Diversity in the Human Gut. Cell Host Microbe 28, 724–740 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buchfink B., Xie C., Huson D. H., Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59–60 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Miele V., Penel S., Duret L., Ultra-fast sequence clustering from similarity networks with SiLiX. BMC Bioinformatics 12, 116 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edler D., Eriksson A., Rosvall A. M., The MapEquation software package. https://www.mapequation.org. Accessed 28 June 2021.
- 58.Sievers F., et al., Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stamatakis A., RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Edgar R. C., Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010). [DOI] [PubMed] [Google Scholar]
- 61.Edgar R. C., MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaudhari N. M., Gupta V. K., Dutta C., BPGA—an ultra-fast pan-genome analysis pipeline. Sci. Rep. 6, 24373 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engler C., Kandzia R., Marillonnet S., A one pot, one step, precision cloning method with high throughput capability. PLoS One 3, e3647 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suárez G. A., et al., Rapid and assured genetic engineering methods applied to Acinetobacter baylyi ADP1 genome streamlining. Nucleic Acids Res. 48, 4585–4600 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee M. E., DeLoache W. C., Cervantes B., Dueber J. E., A highly characterized yeast toolkit for modular, multipart assembly. ACS Synth. Biol. 4, 975–986 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Kirchberger P. C., Defensive hypervariable regions confer superinfection exclusion in microviruses. Dryad. 10.5061/dryad.9zw3r22d4. Deposited 2 March 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Kirchberger P. C., Defensive hypervariable regions confer superinfection exclusion in microviruses. Dryad. 10.5061/dryad.9zw3r22d4. Deposited 2 March 2021. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Alignment data have been deposited in Data Dryad (https://doi.org/10.5061/dryad.9zw3r22d4) (66). All other study data are included in the article and/or supporting information.