Significance
One of biology’s most pressing goals is to understand how organisms adapt to their climates. Researchers have greatly clarified the ways that organisms evolve to improve their survival in warmer climates, yet a major gap in our knowledge remains. Despite >95% of eukaryotic organisms engaging in sexual reproduction, little is known about whether or not climatic adaptation entails optimizing the traits that organisms use to coordinate mating and reproduction. Here, we show that mating-related traits in male but not female dragonflies evolve in a highly predictable way as they adapt to climatic conditions. Failing to account for adaptive evolution of mating-related traits may therefore limit our ability to forecast how organisms will respond to climate change.
Keywords: citizen science, global warming, parallel evolution, sexual selection, temperature
Abstract
Adaptation to different climates fuels the origins and maintenance of biodiversity. Detailing how organisms optimize fitness for their local climates is therefore an essential goal in biology. Although we increasingly understand how survival-related traits evolve as organisms adapt to climatic conditions, it is unclear whether organisms also optimize traits that coordinate mating between the sexes. Here, we show that dragonflies consistently adapt to warmer climates across space and time by evolving less male melanin ornamentation—a mating-related trait that also absorbs solar radiation and heats individuals above ambient temperatures. Continent-wide macroevolutionary analyses reveal that species inhabiting warmer climates evolve less male ornamentation. Community-science observations across 10 species indicate that populations adapt to warmer parts of species’ ranges through microevolution of smaller male ornaments. Observations from 2005 to 2019 detail that contemporary selective pressures oppose male ornaments in warmer years; and our climate-warming projections predict further decreases by 2070. Conversely, our analyses show that female ornamentation responds idiosyncratically to temperature across space and time, indicating the sexes evolve in different ways to meet the demands of the local climate. Overall, these macro- and microevolutionary findings demonstrate that organisms predictably optimize their mating-related traits for the climate just as they do their survival-related traits.
Dating back to Darwin (1) and Wallace (2), biologists have long hypothesized that much of the Earth’s biodiversity was forged by adaptation to different climates. Characterizing how organisms respond to climatic factors, like temperature, is therefore an enduring goal in biology, which has become even more crucial due to the ongoing climate crisis (3). To date, researchers have uncovered many ways that organisms improve survival in their local climates through the evolution of traits such as physiological tolerance (4), life cycle timing (5), and body size (6). However, recent work reveals that climatic adaptation can also involve optimizing mating and reproduction in addition to survival (7). The evolution of sexual traits that coordinate mating could therefore be an important way that plants and animals improve fitness in their local climate from one generation to the next. Nevertheless, despite >95% of eukaryotic taxa engaging in sexual reproduction, it is unclear if sexual characters are a dimension of the phenotype that organisms typically optimize for the climate (3, 8–10).
One type of sexual trait that could often be involved in climatic adaptation is ornamental coloration, which many animals use to attract mates and intimidate rivals. As the dark and/or saturated colors used in many ornaments absorb solar radiation and lead to heating, the demands of warmer climates could force animals to evolve smaller or less saturated ornaments (9, 11, 12). Alternatively, because tropical species are frequently more ornately colored than their temperate relatives, some researchers have suggested that adaptation to warmer climates may instead favor more exaggerated ornamentation (13). By understanding how ornamental coloration responds to selective pressures in different climates, we can begin to resolve if the evolution of sexual traits is indeed a major feature of how organisms adapt to the climate (3, 10).
Testing if ornaments respond predictably to climatic factors across multiple lineages and/or timescales is one approach to assessing ornament evolution’s role in climatic adaptation (14, 15). If, for example, selective pressures in warmer climates require the evolution of less exaggerated ornamentation, then we should observe that animals inhabiting hotter environments consistently evolve less ornamental color regardless of timescale or historical contingencies (e.g., differing genetic backgrounds, or genetic drift) (14). Dragonflies and damselflies are well suited for such tests because they possess ornamental wing melanization that varies within and among species (16). Males with greater wing melanization typically attract more mates and ward off territorial rivals, and both sexes use these ornaments to signal their species’ identity to con- and heterospecifics (16). Though these advantages in courtship and rival intimidation often favor greater ornamentation, wing melanization can also heat individuals >2 °C (11, 12, 17). Such heating may provide modest locomotor benefits under cool conditions (11), but it can damage wing tissue, reduce male fighting ability and territorial defense, and even cause death under warm conditions (11, 17). In contrast, because females mainly spend their time foraging in cooler and/or more shaded microhabitats to maximize fecundity (16), their wing melanization may rarely cause overheating. These sex-specific thermal consequences for both reproduction and survival suggest that dragonflies should adapt to their local climates across space and time through the evolution of ornamental wing melanization in males but not necessarily in females (11, 12). We tested this hypothesis by exploring how male and female ornaments have responded to climatic differences across the macroevolutionary, microevolutionary, and contemporary history of Nearctic dragonflies.
To first evaluate if selective pressures in different climates have favored ornament evolution across macroevolutionary timescales, we tested if Nearctic dragonfly species inhabiting warmer ranges are less likely to have evolved wing melanization than those inhabiting cooler ranges. Using field guides, community-science observations, and >387,900 occurrence records from the Global Biodiversity Information Facility (https://www.gbif.org), we compiled phenotypic and climatic data for 319 Nearctic species (Fig. 1 A and B). After controlling for shared evolutionary history, we found sex-specific patterns of ornament evolution among climates. Species with warmer ranges are indeed less likely to have male wing melanization than species with cooler ranges ( ± SE = −0.078 ± 0.024, 95% CIs = −0.162 to −0.035; Fig. 1C). Species with the darkest patches of male wing melanization also tend to have the coolest ranges ( ± SE = −0.010 ± 0.004, 95% CIs = −0.017 to −0.003). However, interspecific patterns for female wing melanization contrasted starkly with these patterns for males. Species with warmer ranges have a somewhat higher probability of female wing melanization, though the trend is not different from zero ( ± SE = 0.027 ± 0.016, 95% CIs = −0.008 to 0.068; Fig. 1D). There is also no relationship between the temperature of a species’ range and the darkness of its female wing melanization ( ± SE = −0.006 ± 0.004, 95% CIs = −0.012 to 0.001). Thus, the evolution of male, but not female, wing melanization is a component of how dragonflies respond to climatic differences over long timescales.
Fig. 1.
Macroevolution of dragonfly wing melanization in relation to temperature. (A) Nearctic dragonfly phylogeny. Filled tips indicate the presence of male (green) and female (purple) wing melanization. (B) Dragonfly species across the Nearctic. (C and D) Probability of males (C) and females (D) possessing wing melanization. Tick marks are species (n = 319), and lines are from phylogenetic logistic regressions.
These macroevolutionary findings indicate that selective pressures in warmer climates have favored less male, but not female, ornamentation among Nearctic dragonfly species. However, most dragonfly species are much older than their current geographic distributions (16). Thus, as is true in many studies of ancient lineages, these biogeographic patterns probably stem from both ecological filtering and adaptation. For instance, following the Last Glacial Maximum, species may have recolonized regions where the climatic conditions did not make male ornamentation too costly (i.e., ecological filtering) (12). Additionally, because ornamentation is quite evolutionarily labile (18), these macroevolutionary patterns likely also arise from adaptation to local climates. The relative contributions of colonization and adaptation to interspecific ornament variation cannot yet be disentangled for this group or for many other ancient clades. Nevertheless, if adaptation to local climatic conditions has led species to evolve differing ornamentation over long timescales, then it should also entail ornament evolution across shorter timescales—such as those separating populations within the same species.
To evaluate if populations consistently adapt to their local climates via ornament evolution, we next tested for parallel shifts in ornament size across the ranges of 10 widely distributed Nearctic dragonfly species (14) (Fig. 2). Here, we measured the proportion of melanized wing area on >2,700 dragonfly observations from the community-science platform iNaturalist (https://www.inaturalist.org) (19). Despite some of these species being separated by >100 My of evolution, we found that their constituent populations exhibit remarkably parallel responses in their male, but not female, ornamentation. Within 7 of the 10 species, males in warmer regions had significantly less wing melanization than their counterparts in cooler areas (Fig. 2A and SI Appendix, Table S1). Consequently, when averaging across all 10 species’ responses, male wing melanization tended to decrease as local temperatures increased ( ± SE = −0.064 ± 0.031 SD per 1 °C; 95% CIs = −0.127 to −0.001). Because developing at warmer temperatures does not induce male dragonflies to express less ornamentation (20), genetic differences among populations are more likely to underlie these parallel responses than phenotypic plasticity alone. In contrast to the patterns in males, females possessed significantly less wing melanization in warmer climates for only 3 out of 10 species (Fig. 2A and SI Appendix, Table S1). As a result, the average female response to temperature across the 10 species was indistinguishable from 0 ( ± SE = −0.006 ± 0.024 SD per 1 °C; 95% CIs = −0.054 to 0.042). Thus, mirroring macroevolutionary patterns among species, the differing selective pressures among climates also favor consistent patterns of sex-specific ornament evolution within species (Fig. 2B). In particular, these sex-specific responses within species result in male ornaments being 25.8 ± 2.0% larger than female ornaments in the coolest parts of North America, on average, but only 2.0 ± 3.3% larger in the warmest areas.
Fig. 2.
Parallel evolution of wing melanization in response to mean annual temperature (MAT) within dragonfly species. (A) Graphs show species’ relationships for males (green) and females (purple). Points are individuals (n = 2,718), and lines are fitted from linear mixed-effects models. Asterisks indicate significant declines. (B) Average within-species SD change wing melanization (± SE) for 1 °C increase.
Across timescales ranging from >150 My to only dozens of millennia, our results show that dragonflies consistently adapt to their climate via sex-specific evolution of wing melanization. However, climatic projections indicate North America could warm >4.5 °C by 2070 (21). The ornament evolution that previously facilitated adaptation over thousands of years may therefore need to occur over fewer than 100 generations unless alternative responses can be employed. Two such alternatives to rapid ornament evolution are shifts in species’ distributions and phenologies (22). For instance, more-ornamented species could lessen the threat of overheating by tracking northward shifts of cooler temperatures. When we incorporated each species’ ornamentation into a recently published analysis of range shifts among 65 European dragonflies (23), however, we found that species with male ornamentation have not moved further northward than species without it (difference in northward range shifts ± SE = 10.50 ± 24.33 km, 95% CIs = −37.19 to 58.18). More-ornamented species could also alleviate the risk of overheating by shifting phenology to defend territories in cooler times of day or to reproduce in cooler times of year (22). Though we cannot rule out this possibility, it is notable that such phenological shifts, if they occur, have not enabled males to possess greater ornamentation in warmer climates over the previous >150 My. Rapid ornament evolution may therefore be necessary to avoid overheating as our planet’s climate changes (24).
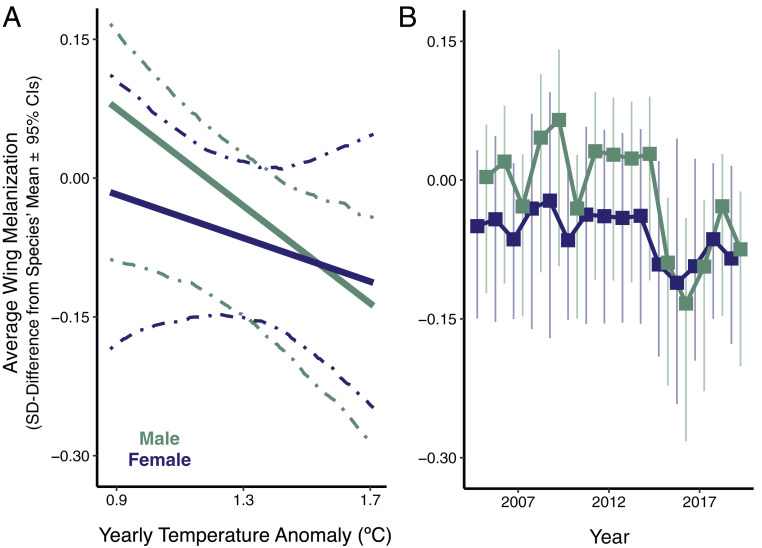
To evaluate how natural and sexual selection might alter ornamentation as the Earth warms, we tested if the 10 widely distributed dragonfly species (Fig. 2) possessed less wing melanization in years that were warmer than the Northern Hemisphere’s long-term average (mean temperature anomaly). Our analyses show that, from 2005 to 2019, species averaged less wing melanization in warmer years for males but not females (males: ± SE = −0.263 ± 0.103 SD per 1 °C, 95% CIs = −0.513 to −0.005; females: ± SE = −0.118 ± 0.146 SD per 1 °C, 95% CIs = −0.418 to 0.181; Fig. 3). However, since males and females responded more similarly to each other across annual variation than geographic variation, the estimated extent of sexual dimorphism was only modestly more male biased in cold years (16.0 ± 1.5%) than in warm years (14.5 ± 1.9%). Nonetheless, these results collectively reveal that male ornaments were smallest in this century’s warmest years. By contrast, our analyses show that the extent of wing melanization did not exhibit a net decrease across the 15-y timespan for either sex nor was it related to the previous year’s temperature (SI Appendix, Table S8). The temporal patterns in ornament size therefore likely emerge from processes operating within generations rather than across generations. As dragonflies do not develop less wing melanization when reared under warmer conditions (20), a probable explanation for this within-generation effect is that selection in warmer years consistently reduces the number of highly ornamented individuals in breeding populations.
Fig. 3.
Wing melanization shifts with interannual temperature variation. (A) Lines show fitted relationship (with 95% CIs) between wing melanization (SD relative to mean) and the Northern Hemisphere’s yearly temperature anomaly from 2005 to 2019 (n = 2,620). (B) Estimated-marginal mean wing melanization (with 95% CIs) for 2005 to 2019. The sexes’ points are offset horizontally to reduce overlap.
Since selective pressures in warmer years appear to favor less ornamented males, we estimated how wing melanization might shift as North America warms over the next several decades. Based on the best- and worst-case scenarios for climatic warming (21), we used the current geographic relationship between ornamentation and temperature to forecast the amount of wing melanization each species should possess in 2070 for the coolest third, thermal midpoint, and warmest third of its range (21, 24) (SI Appendix, Table S3). Our projections indicate that, on average, species’ male wing melanization will decline 0.205 to 0.328 SD by 2070 (Table 1 and SI Appendix, Table S3)—a modest loss of only up to 0.007 SD per generation. In contrast, species’ female wing melanization will not need to change significantly (Table 1 and SI Appendix, Table S3). The breeder’s equation can illuminate the plausibility of dragonflies losing this much male wing melanization each generation to match yearly warming of 0.09 °C (4.5 °C/50 y) (24). Assuming that phenotypic selection underlies the interannual ornament variation we observed (Fig. 3B), selection in each generation will favor, on average, 0.024 SD less male wing melanization than it did in the previous generation (−0.263 SD ornamentation °C−1 × 0.09 °C Y−1). For male ornamentation to keep pace with this intensity of selection each generation, heritability would need to average 0.277 ± 0.111. This h2 is similar to the estimated mean for all adult traits in animals [h2 = 0.247 ± 0.032 (25)] and smaller than the estimated mean for melanin-based traits in insects (h2 = 0.463 ± 0.114; see SI Appendix). Because the capacity for rapid responses to climatic warming is often limited along other phenotypic axes [e.g., physiological tolerance (4, 26, 27)], the modest projected responses and moderate requisite heritability of male wing melanization suggest that ornament evolution could be an important component of climatic adaptation in the coming years.
Table 1.
Average forecasted shifts (± SE), and 95% prediction intervals, that will be necessary for dragonflies to optimize their wing melanization to the climatic conditions of 2070 across North America
| Sex | Climatic zone | Global warming scenario | Total projected response ± SE* | 95% prediction intervals |
| Male | Coolest third | RCP 4.5 | −0.233 ± 0.028 | −0.289 to −0.178 |
| RCP 8.5 | −0.328 ± 0.033 | −0.393 to −0.263 | ||
| Thermal midpoint | RCP 4.5 | −0.219 ± 0.035 | −0.287 to −0.151 | |
| RCP 8.5 | −0.311 ± 0.043 | −0.395 to −0.226 | ||
| Warmest third | RCP 4.5 | −0.205 ± 0.051 | −0.306 to −0.105 | |
| RCP 8.5 | −0.293 ± 0.059 | −0.409 to −0.178 | ||
| Female | Coolest third | RCP 4.5 | −0.020 ± 0.035 | −0.089 to 0.049 |
| RCP 8.5 | −0.025 ± 0.037 | −0.098 to 0.048 | ||
| Thermal midpoint | RCP 4.5 | −0.020 ± 0.037 | −0.093 to 0.052 | |
| RCP 8.5 | −0.026 ± 0.042 | −0.108 to 0.056 | ||
| Warmest third | RCP 4.5 | −0.021 ± 0.051 | −0.122 to 0.080 | |
| RCP 8.5 | −0.026 ± 0.057 | −0.138 to 0.085 |
*Forecasts represent the average expected within-species change (number of SD) relative to current levels (SI Appendix, Table S3 shows each species’ projections).
Collectively, our analyses indicate that male, but not female, ornament evolution is a predictable feature of climatic adaptation in dragonflies. Males experience different thermal conditions from females primarily while defending sunlit territories, and selection in this reproductive context therefore seems likely to underlie the male-specific patterns of divergence. However, studying how male ornaments jointly affect survival, territorial success, and courtship success in warm versus cool regions will be necessary to identify which selective mechanisms are directly responsible for the parallel patterns of ornament evolution. For example, male ornamentation could improve territorial and/or courtship success across all climates (16, 28) but increase the risk of lethal overheating during territorial defense in only the warmest areas (11, 12). Alternatively, the potential advantages of ornament-induced heating for success at fighting rivals or courting mates in cool climates could facilitate male ornament exaggeration in those regions (11). We also cannot rule out that other factors contribute to male ornament evolution among climates. For instance, highly ornamented males may incur disproportionate metabolic costs in warmer environments if they are challenged more frequently by rivals (28). Regardless of the precise mechanism(s), our results show that climatic adaptation in dragonflies entails some of the most predictable responses ever observed for a sexual trait (8).
In contrast to parallel responses among males, our study reveals that females adapt more idiosyncratically to the climate across space and time. In particular, female ornaments show no consistent relationship with climatic conditions within or among species, suggesting that ornaments have different thermal consequences for males and females (29). Such a pattern was somewhat expected: females’ typically cooler microhabitats likely shield them from the threat of ornament-induced overheating in warm climates (16). However, future research should include investigations of: 1) why females do not take greater advantage of the potential benefits of ornament-induced heating in cool climates, and 2) what other selective pressures shape female ornament evolution [e.g., crypsis (30), competition during foraging (31)]. Nevertheless, though we often assume that climatic adaptation requires similar evolution between the sexes (3), our findings indicate that the climate should be considered alongside the many other environmental factors for which adaptive evolution is known to be sex specific (29).
Our projections further indicate that sex-specific ornament evolution will be a plausible response to future global warming. Evolutionary shifts in a species’ ornamentation may be faster or more likely in some regions than others, however, and unraveling species’ histories of sex-specific selection will be valuable to future forecasting and management efforts (32). For species that began the current interglacial period with little sexual dimorphism, selection would have favored male-biased ornamentation during expansion into northern climates (12). Male ornaments may then have less standing genetic variation in northern regions for such taxa, which could greatly constrain the forecasted evolutionary reductions for those populations (Table 1). Alternatively, for species that had considerable sexual dimorphism at the end of the last glaciation, selection in previously unglaciated areas would have favored low levels of ornamentation in both sexes as the planet gradually warmed (12). In this scenario, historical selection in southern populations would have winnowed genetic variation in male ornaments and promoted strong between-sex genetic correlations (33). Because the optimal response should differ between the sexes in the coming years (Table 1), any strong between-sex genetic correlations could impede climatic adaptation for these populations. Thus, while our findings suggest that rapid ornament evolution could be a valuable component of future adaptation, studying species’ histories of sex-specific selection may add insight into which populations are likely to achieve such shifts.
Overall, we have shown that climatic adaptation requires optimizing mating-related traits across space and time. Because melanin coloration is a taxonomically widespread character that animals and plants use to coordinate reproduction (9, 34), the patterns of ornament evolution that we observed here may be a major feature of climatic adaptation in many organisms. However, the tree of life contains a remarkable array of ornaments, weapons, and vocalizations, and much remains unknown about the breadth of ways that organisms optimize their sexual traits for reproduction in different climates. Though the direction of evolution may not be the same for every sexual trait or every organism, our findings demonstrate that mating-related traits are a dimension of the phenotype that must be optimized for the local climate just like survival-related traits.
Methods
Interspecific Variation.
We created a database of species’ wing melanization, as well as the climatic conditions each species experiences across its range (see SI Appendix for full details). We assessed the presence/absence and darkness (dark/light/none) of a species’ melanization from comprehensive field guides or iNaturalist observations (SI Appendix, Fig. S1) (11, 12, 35−36). A species’ average climatic conditions were calculated by averaging the mean annual temperature across its range (37, 38; see SI Appendix for estimation and rationale).
After pruning a recently published odonate phylogeny to the 319 focal species (39, 40), we first used a phylogenetic logistic regression [phylolm (41)] to assess how climatic temperature influences the likelihood of a species possessing male and female wing melanization. We fit separate models for each sex. Terms were considered significant if their 95% CIs of 1,000 bootstrap replicates did not overlap 0 (42). Supplemental analyses revealed precipitation does not underlie the interspecific patterns of ornamentation (SI Appendix). We further considered whether ornament darkness (dark = 2, light = 1, and none = 0) was related to mean temperature of species’ ranges using phylogenetic generalized least squares. Results were similar when comparing mean range temperature among species with ornaments of differing darkness (SI Appendix).
Intraspecific Variation.
We examined ornament variation within 10 species that each possess wing melanization, wide latitudinal ranges, and a large number of iNaturalist observations (Fig. 2). For each species, we downloaded 271.8 ± 22.0 images (mean ± SD; SI Appendix, Table S1), encompassing the temporal and geographic breadth of iNaturalist observations. For each observation, we recorded the date, latitude, longitude, state/province, and county (or its municipal equivalent). We then measured the proportion of each individual’s wing area that was melanized (following ref. 19; see SI Appendix for details). After measuring individuals, we downloaded the mean annual temperature for each observation’s location (38; see SI Appendix for rationale). Similar approaches have been validated for measuring spatiotemporal variation in animal coloration (19, 43).
Using the 2,718 observations, we tested whether males and females within each species have smaller ornaments in warmer regions. We first used separate linear mixed-effects models to quantify each species’ relationship between climatic temperatures and male and female wing melanization (lme4) (44). Each individual’s proportion of melanized wing area was fit as the response, with sex, mean annual temperature at the individual’s location, and their interaction as fixed effects. To account for the nonindependence of individuals from the same or nearby populations, we included a random effect of county nested within state/province. Analogous statistical approaches are common (11, 19, 45) and provide similar results to those that directly incorporate spatial distance among observations (11). Quadratic effects of mean annual temperature were significant for two species (Fig. 2) and were removed from the other models. Significance of terms was determined by 95% CIs that did not overlap 0 (SI Appendix, Table S1).
To assess if these 10 species were responding in a consistent fashion, we fit a single model for each sex that included the observations of all 10 species as the response and the linear effect of temperature as a fixed effect. For random effects in these models, we included county nested within state/province, as well as species with a random slope of mean annual temperature. Because few species exhibit a quadratic effect of temperature, we considered only the linear effect. We z-transformed each sex’s wing melanization within each species to improve model convergence and interpretability (46). A predictable effect of temperature on wing melanization was indicated by 95% CIs that did not overlap 0. As there was no evidence for phylogenetic signal, we did not incorporate a phylogeny into these analyses (SI Appendix, Table S4) (47). Supplemental analyses indicate that precipitation does not underlie the intraspecific patterns of ornamentation (SI Appendix). Finally, we calculated how the extent of ornament dimorphism between the sexes changed with temperature by estimating and comparing each sex’s average wing melanization in cool (1 °C) and warm (23 °C) climates.
Interspecific Range Shifts.
We tested if ornamentation is related to range shifts by updating a dataset of 65 European dragonflies (23). Leveraging iNaturalist observations, we classified if each species possessed wing melanization (11, 12). We then used phylogenetic generalized least squares (48) to test if species with male wing melanization had moved further northward from 1988 to 2006 than species without it. As the phylogeny from our main analyses included few of these European species, we employed a digitized version of the phylogeny in their article (23). However, model comparison indicated that the best-supported model did not include phylogeny (SI Appendix, Table S5) (47). Observed patterns were similar when comparing range shifts to a species’ possession of 1) female wing melanization or 2) wing melanization in either sex (SI Appendix, Table S6).
Contemporary Wing Color Shifts.
Using the observations in our intraspecific dataset (Fig. 2), we evaluated the relationship between each year’s mean temperature and mean wing melanization from 2005 to 2019. We focused on this time period because each sex had at least 15 observations in each of those years. To estimate yearly temperatures, we averaged the monthly temperature anomalies for the Northern Hemisphere’s land surface for each year (49).
Pooling across species, we fit separate linear mixed-effects models (44) for each sex’s wing melanization. Each individual’s wing melanization [z-transformed within each species’ sex (46); Intraspecific Variation] was fit as the response variable and the temperature anomaly of the observation’s year as a fixed effect. To account for the nonindependence of individuals sampled in the same or nearby populations, we included a random intercept of county nested within state/province. To ensure any overall relationship between annual temperature and ornamentation was not biased by species-specific responses to geographic variation in temperature (Fig. 2), or to annual variation in temperature itself, we included a random intercept of species with random slopes for mean annual temperature and annual temperature anomaly (SI Appendix, Table S2). Similar approaches are commonly employed with museum specimens (50, 51) and have been validated for iNaturalist observations (19). We did not include a phylogeny because phylogenetic signal was not observed in species’ responses (SI Appendix, Table S7) (47). Supplemental analyses indicate that neither temporal nor geographic changes in iNaturalist usage underlie the observed pattern (SI Appendix). We further assessed how the extent of sexual dimorphism changed across the focal timespan by estimating the relative size difference in male and female wing melanization in the coolest (0.94 °C) and warmest years (1.69 °C). Lastly, we fit models where the previous year’s temperature anomaly, or the year of the observation, were included instead of the current year’s temperature anomaly (SI Appendix, Table S8).
Projections for Future Wing Color Shifts.
We estimated how much wing melanization should change in order to track temperatures through 2070. For each of the 10 species in our intraspecific dataset, we downloaded forecasted temperatures for the coldest third, thermal midpoint, and the hottest third of their current range (38). To include a realistic range of possible temperatures in each location, we chose the Representative Concentration Pathways (RCPs) 4.5 and 8.5 from the Intergovernmental Panel on Climate Change as best- and worst-case scenarios (21). Using the regression-estimated relationship between male wing melanization and temperature across the 10 species (Intraspecific Variation), we then projected each species’ wing melanization for the current and future temperatures at each location (Table 1 and SI Appendix, Table S3). We used parametric bootstrapping to generate prediction intervals for each species’ forecast (SI Appendix, Table S3). To estimate how each species by sex combination will respond, we calculated the difference between the future and current levels of wing melanization and divided by the SD of the current wing melanization. Finally, we estimated the mean expected change by averaging across the species’ projections for the best- and worst-case scenarios at each temperature zone.
We then used the breeder’s equation (Eq. 1) to estimate how much additive genetic variance in male wing melanization would be necessary for an average species to track the worst-case global warming scenario:
| [1] |
where R is the per-generation response to selection, h2 is the narrow-sense heritability, and S is intensity of selection. Here, the per-generation response to selection was the shift that would be necessary if the Northern Hemisphere warms annually by 0.09 °C for the next 50 generations (4.5 °C/50 y; Table 1). The intensity of selection for such yearly warming was estimated from the relationship between male wing melanization and mean temperature anomaly (Fig. 3) (−0.263 SD ornamentation °C−1 × 0.09 °C Y−1). The requisite heritability is then equal to the per-generation response to selection divided by the per-generation intensity of selection.
Supplementary Material
Acknowledgments
This work would not have been possible without the thousands of iNaturalist users who photograph dragonflies. Conversations and feedback from C. Donihue, M. Ohmer, H. Rollins, B. Seymoure, the K.D.F.-F. laboratory, E. Svensson, and an anonymous referee improved the study. M. Piper provided technical assistance. Support was generously provided by G. Kornblum and the Living Earth Collaborative (M.P.M.), the Tyson Research Center (K.A.M.), and the Saint Louis University Research Institute (K.D.F.-F.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2101458118/-/DCSupplemental.
Data Availability
Data and code have been deposited in the Dryad Digital Repository (DOI: 10.5061/dryad.dv41ns1z2) (52).
References
- 1.Darwin C., On the Origin of Species (John Murray, 1859). [Google Scholar]
- 2.Wallace A. R., Tropical Nature, and Other Essays (MacMillan and Company, 1878). [Google Scholar]
- 3.Keller I., Seehausen O., Thermal adaptation and ecological speciation. Mol. Ecol. 21, 782–799 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Kellermann V., et al., Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proc. Natl. Acad. Sci. U.S.A. 109, 16228–16233 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franks S. J., Sim S., Weis A. E., Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc. Natl. Acad. Sci. U.S.A. 104, 1278–1282 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huey R. B., Gilchrist G. W., Carlson M. L., Berrigan D., Serra L., Rapid evolution of a geographic cline in size in an introduced fly. Science 287, 308–309 (2000). [DOI] [PubMed] [Google Scholar]
- 7.García-Roa R., Garcia-Gonzalez F., Noble D. W. A., Carazo P., Temperature as a modulator of sexual selection. Biol. Rev. Camb. Philos. Soc. 95, 1607–1629 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Maan M. E., Seehausen O., Ecology, sexual selection and speciation. Ecol. Lett. 14, 591–602 (2011). [DOI] [PubMed] [Google Scholar]
- 9.West P. M., Packer C., Sexual selection, temperature, and the lion’s mane. Science 297, 1339–1343 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Qvarnström A., Ålund M., McFarlane S. E., Sirkiä P. M., Climate adaptation and speciation: Particular focus on reproductive barriers in Ficedula flycatchers. Evol. Appl. 9, 119–134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore M. P., Lis C., Gherghel I., Martin R. A., Temperature shapes the costs, benefits and geographic diversification of sexual coloration in a dragonfly. Ecol. Lett. 22, 437–446 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Svensson E. I., Waller J. T., Ecology and sexual selection: Evolution of wing pigmentation in calopterygid damselflies in relation to latitude, sexual dimorphism, and speciation. Am. Nat. 182, E174–E195 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Dale J., Dey C. J., Delhey K., Kempenaers B., Valcu M., The effects of life history and sexual selection on male and female plumage colouration. Nature 527, 367–370 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Langerhans R. B., DeWitt T. J., Shared and unique features of evolutionary diversification. Am. Nat. 164, 335–349 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Losos J. B., Convergence, adaptation, and constraint. Evolution 65, 1827–1840 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Corbet P. S., Dragonflies (Cornell Univ. Press, 1999). [Google Scholar]
- 17.Svensson E. I., Gomez-Llano M., Waller J. T., Selection on phenotypic plasticity favors thermal canalization. Proc. Natl. Acad. Sci. U.S.A. 117, 29767–29774 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svensson E. I., Eco-evolutionary dynamics of sexual selection and sexual conflict. Funct. Ecol. 33, 60–72 (2019). [Google Scholar]
- 19.Drury J. P., Barnes M., Finneran A. E., Harris M., Grether G. F., Continent-scale phenotype mapping using citizen scientists’ photographs. Ecography 42, 1436–1445 (2019). [Google Scholar]
- 20.Lis C., Moore M. P., Martin R. A., Warm developmental temperatures induce non-adaptive plasticity in the intrasexually selected colouration of a dragonfly. Ecol. Entomol. 45, 663–670 (2020). [Google Scholar]
- 21.Collins M., et al., “Long-term climate change: Projections, commitments and irreversibility” in Climate Change 2013: The Physical Basis, Stocker T. F., et al., Eds. (Cambridge Uni. Press, 2013), pp. 1029–1136. [Google Scholar]
- 22.Parmesan C., Ecological and evolutionary responses to climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 (2006). [Google Scholar]
- 23.Grewe Y., Hof C., Dehling D. M., Brandl R., Brändle M., Recent range shifts of European dragonflies provide support for an inverse relationship between habitat predictability and dispersal. Glob. Ecol. Biogeogr. 22, 403–409 (2013). [Google Scholar]
- 24.Hoffmann A. A., Sgrò C. M., Climate change and evolutionary adaptation. Nature 470, 479–485 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Moore M. P., Whiteman H. H., Martin R. A., A mother’s legacy: The strength of maternal effects in animal populations. Ecol. Lett. 22, 1620–1628 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Bennett J. M., et al., The evolution of critical thermal limits of life on Earth. Nat. Commun. 12, 1198 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan R., Finnøen M. H., Jensen H., Pélabon C., Jutfelt F., Low potential for evolutionary rescue from climate change in a tropical fish. Proc. Natl. Acad. Sci. U.S.A. 117, 33365–33372 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tynkkynen K., Kotiaho J. S., Luojumäki M., Suhonen J., Interspecific aggression causes negative selection on sexual characters. Evolution 59, 1838–1843 (2005). [PubMed] [Google Scholar]
- 29.Svensson E. I., et al., Sex differences in local adaptation: What can we learn from reciprocal transplant experiments? Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 2017420 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuchta S. R., Svensson E. I., Predator-mediated natural selection on the wings of the damselfly Calopteryx splendens: Differences in selection among trait types. Am. Nat. 184, 91–109 (2014). [DOI] [PubMed] [Google Scholar]
- 31.West-Eberhard M. J., Sexual Selection, social competition, and speciation. Q. Rev. Biol. 58, 155–183 (1983). [Google Scholar]
- 32.Razgour O., et al., Considering adaptive genetic variation in climate change vulnerability assessment reduces species range loss projections. Proc. Natl. Acad. Sci. U.S.A. 116, 10418–10423 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gosden T. P., Chenoweth S. F., The evolutionary stability of cross-sex, cross-trait genetic covariances. Evolution 68, 1687–1697 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Cuthill I. C., et al., The biology of color. Science 357, eaan0021 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Paulson D. R., Dragonflies of the West (Princeton Univ. Press, 2009). [Google Scholar]
- 36.Paulson D. R., Dragonflies and Damselflies of the East (Princeton Univ. Press, 2012). [Google Scholar]
- 37.Vilela B., Villalobos F., letsR: A new package for data handling and analysis in macroecology. Methods Ecol. Evol. 6, 1229–1234 (2015). [Google Scholar]
- 38.Hijmans R.J., raster: Geographic Data Analysis and Modeling, Version 2.9-23, R package. https://cran.r-project.org/web/packages/raster/index.html. Accessed 15 June 2021.
- 39.Waller J. T., Svensson E. I., Body size evolution in an old insect order: No evidence for Cope’s rule in spite of fitness benefits of large size. Evolution 71, 2178–2193 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Rocha-Ortega M., Rodríguez P., Bried J., Abbott J., Córdoba-Aguilar A., Why do bugs perish? Range size and local vulnerability traits as surrogates of Odonata extinction risk. Proc. Biol. Sci. 287, 20192645 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho Ls., Ané C., A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst. Biol. 63, 397–408 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Ives A. R., Garland T. Jr, Phylogenetic logistic regression for binary dependent variables. Syst. Biol. 59, 9–26 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Leighton G. R. M., et al., Just Google it: Assessing the use of Google images to describe geographical variation in visible traits of organisms. Methods Ecol. Evol. 7, 1060–1070 (2016). [Google Scholar]
- 44.Bates D., Machler M., Bolker B., Walker S., Fitting linear mixed-effects models using ‘lme4’. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 45.Outomuro D., Ocharan F. J., Wing pigmentation in Calopteryx damselflies: A role in thermoregulation? Biol. J. Linn. Soc. Lond. 103, 36–44 (2011). [Google Scholar]
- 46.Schielzeth H., Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103–113 (2010). [Google Scholar]
- 47.Revell L. J., Phylogenetic signal and linear regression on species data. Methods Ecol. Evol. 1, 319–329 (2010). [Google Scholar]
- 48.Revell L. J., phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012). [Google Scholar]
- 49.National Centers for Environmental Information , Global surface temperature anomalies. https://www.ncdc.noaa.gov/monitoring-references/faq/anomalies.php#anomalies. Accessed 10 November 2020.
- 50.Kiat Y., Vortman Y., Sapir N., Feather moult and bird appearance are correlated with global warming over the last 200 years. Nat. Commun. 10, 2540 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koski M. H., MacQueen D., Ashman T.-L., Floral pigmentation has responded rapidly to global change in ozone and temperature. Curr. Biol. 30, 4425–4431.e3 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Moore M. P., Data for: Sex-specific ornament evolution is a consistent feature of climatic adaptation across space and time in dragonflies. Dryad Digital Repository. 10.5061/dryad.dv41ns1z2. Deposited 29 April 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code have been deposited in the Dryad Digital Repository (DOI: 10.5061/dryad.dv41ns1z2) (52).