Significance
Bioisoteric replacements for aromatic rings to improve medicinal properties are in high demand. This work presents a comprehensive analysis of heretofore-inaccessible 1,2-difunctionalized bicyclo[1.1.1]pentanes from both chemical and biological vantage points. This analysis will aid those in drug discovery regarding when the application of such systems makes sense and how to access them in such circumstances.
Keywords: synthesis, medicinal chemistry, bioisosteres
Abstract
The development of a versatile platform for the synthesis of 1,2-difunctionalized bicyclo[1.1.1]pentanes to potentially mimic ortho/meta-substituted arenes is described. The syntheses of useful building blocks bearing alcohol, amine, and carboxylic acid functional handles have been achieved from a simple common intermediate. Several ortho- and meta-substituted benzene analogs, as well as simple molecular matched pairs, have also been prepared using this platform. The results of in-depth ADME (absorption, distribution, metabolism, and excretion) investigations of these systems are presented, as well as computational studies which validate the ortho- or meta-character of these bioisosteres.
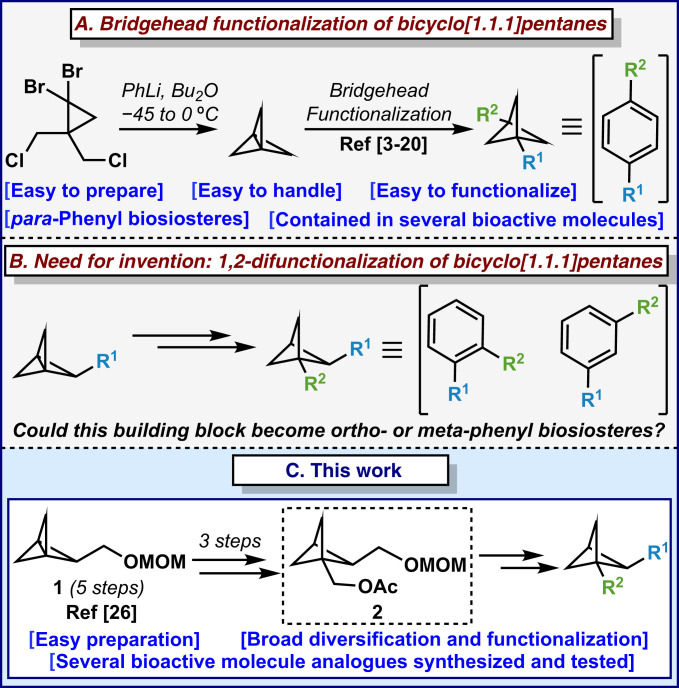
Over the past decade, medicinal chemists’ mission to “escape from flatland” has altered the way synthesis in drug discovery is conducted (1, 2). Specifically, C(sp3)-rich structures, where the venerable benzene ring is replaced with saturated bioisosteres, have started to rapidly populate the patent literature, compound libraries, and the toolbox of medicinal chemistry tactics (1, 2). These three-dimensional moieties often exhibit improved biological activities, physicochemical properties and metabolic profiles compared to the parent benzene ring (3–8). These singular characteristics have encouraged several academic and industrial groups, including the Pfizer/Scripps team, to achieve the functionalization of these small structures (for some recent examples, see refs. 9–20). As a subset of these bioisosteres, bicyclo[1.1.1]pentane (BCP) cores have been extensively studied and proven valuable alternatives to benzene rings (for some recent examples, see refs. 9–20). However, searching the literature highlights that bridgehead functionalizations, mimicking para-substitution of the benzene ring, have been the most accessible thus far (Fig. 1A). Only recently have reports emerged delineating creative approaches to fill this gap [an initial disclosure of this study was deposited on ChemRxiv (21); subsequently, two additional reports (22, 23) have appeared describing different and orthogonal strategies]. This is certainly due to the absence of synthetic methods to achieve 1,2-difunctionalization of BCPs rather than the lack of desire to replace ortho- and meta-disubstituted benzenes in bioactive molecules, which has been regularly mentioned over the years (3–8). [According to incomplete statistics on “Drug Bank” (24), there are about 100 approved drugs that contain ortho-disubstituted benzene system and 50 approved drugs that contain meta-disubstituted benzene systems, respectively.] Recently, Mykhailiuk and coworkers (25) reported the synthesis of bicyclo[2.1.1]hexanes as the first example of saturated bioisosteres of ortho-disubstituted benzenes. Conversely, there is still a gap in the literature for the synthesis of 1,2-difunctionalized BCP skeletons that would act as ortho- or meta-disubstituted benzenes (Fig. 1B). Our foray into the synthesis of such compounds started with a directed C–H activation strategy where a bridgehead amide directing group was installed to activate the side position of the BCP. Unfortunately, all efforts toward this directed approach proved unsuccessful. While trying other tactics, we came across a paper published by the group of Schlüter in 1995 (26). In their report, the authors synthesized the [1.1.1]propellane 1 bearing a methoxymethyl (MOM) protected hydroxyl group in the side chain (26). Inspired by this unexplored precedent and capitalizing on newly emerging methods for the cross-coupling of previously unreactive carboxylic acids (via decarboxylative functionalization), we report herein the development of a platform to synthesize 1,2-difunctionalized bicyclo[1.1.1]pentanes from compound 2 (Fig. 1C), synthesized from 1 in three steps.
Fig. 1.
Introduction to bicyclo[1.1.1]pentanes as arene bioisosteres. Bridgehead functionalization of bicyclo[1.1.1]pentanes (A), need for invention: 1,2-difunctionalization of bicyclo[1.1.1]pentanes (B), and this work (C).
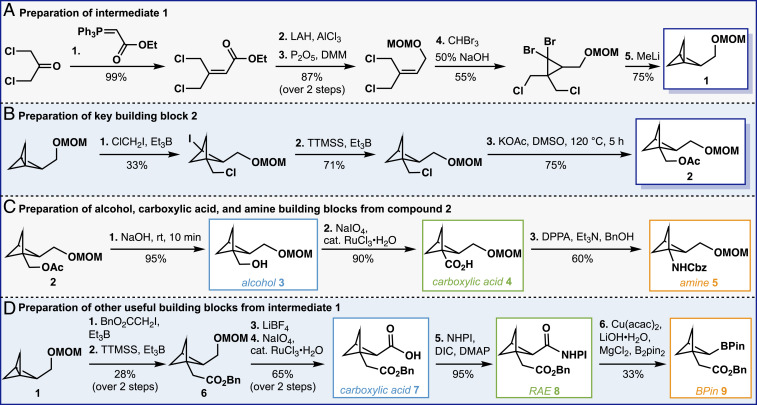
Fig. 2 highlights the preparation of several key building blocks that allow for the development of various 1,2-difunctionalized BCP skeletons. Toward this end, our studies commenced by optimizing Schlüter’s synthetic sequence leading to compound 1, which was obtained in 36% yield over five steps (Fig. 2A). With compound 1 in hand, building block 2 was easily prepared in three steps through radical difunctionalization (12), deiodination (12), and chlorine–acetate exchange (18% overall yield; Fig. 2B). Compound 2 was chosen for the synthetic versatility it offers. Indeed, alcohol 3, carboxylic acid 4, and amine 5 were easily obtained from 2 in one, two, and three steps, respectively (Fig. 2C) via simple functional group manipulation. Finally, functionalization of the [1.1.1]propellane 1 side chain was undertaken (Fig. 2D). Radical ring opening and dehalogenation of 1 afforded compound 6, which was directly deprotected and oxidized to afford carboxylic acid 7 in 18% yield over four steps. The carboxylic acid moiety was the adequate handle to prepare redox-active ester 8 and test its ability to undergo decarboxylative transformations. Pleasingly, compound 8 delivered borylated compound 9 in satisfactory yield under our reported copper-catalyzed decarboxylative borylation conditions (27). It is worth noting that compounds 8 and 9 offer limitless possibilities regarding the preparation of medicinal chemistry-relevant structures. The synthesis of these 1,2-difunctionalized bicyclo[1.1.1]pentane building blocks encouraged us to examine the practicality of the platform in the context of preparing drug bioisosteres.
Fig. 2.
Preparation of diverse 1,2-difunctionalized bicyclo[1.1.1]pentane building blocks. Preparation of intermediate 1 (A), preparation of key building block 2 (B), preparation of alcohol, carboxylic acid, and amine building blocks (C), and preparation of other useful building blocks (D).
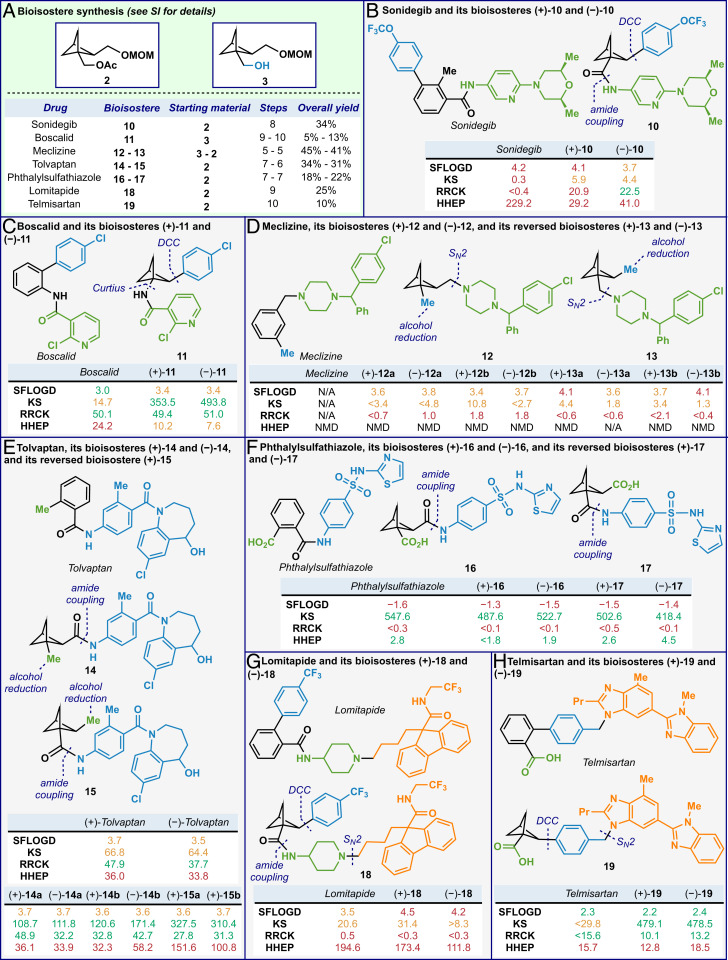
As summarized in Fig. 3A, seven drugs were selected by the Pfizer team and a total of 10 different bioisosteres were synthesized. The complete routes for synthesis are outlined in SI Appendix and key disconnections are highlighted in the figure. Essential to generating the aryl-BCP linkage was the use of decarboxylative cross-coupling methods. (For selected decarboxylative cross-coupling works from our laboratory, see refs. 28–38.) All the compounds were obtained starting from either building blocks 2 or 3 in 5- to 10-step sequences with overall yields ranging from 10 to 45%. The versatility of the platform was demonstrated by the synthesis of several bioisosteres and their reversed analogs using similar synthetic sequences but in a different order of operation (Fig. 3 D–F). Supercritical fluid chromatography (SFC) was used to separate enantiomers, and when the drugs contained additional chiral centers, diastereoisomers were also separated. All the prepared bioisosteres were individually tested using four different biological assays affording data regarding the lipophilicity (shake flask log D [SFLOGD]), kinetic aqueous solubility (KS) (39), permeability (Ralph Russ canine kidney [RRCK]) (40), and metabolic stability (human hepatocyte stability [HHEP]) (descriptions of methods for the SFLOGD and HHEP assays can be found in SI Appendix). The results, summarized in Fig. 3, provide some indication as to the role that the BCP moiety can play in a drug development program. For the majority of the assay data obtained, it seemed that the BCP isosteres performed similarly to the known drug compounds; however, the BCP imparts a level of three-dimensionality not observed in the known drug compounds, which could have a large effect on the aqueous solubility of these compounds (1). For the compounds that remain neutral at physiological pH (7.4), the solubility of the bioisosteres was increased when compared to the known drug compounds [see data for sonidegib (41), boscalid (42), tolvaptan (43), telmisartan (44), axitinib (45, 46), and their bioisosteres]. However, for bioisosteres that would be charged at physiological pH, not much advantage in solubility was noted [see data for meclizine (47), lomitapide (48), and their isosteres]. Interestingly, phthalylsulfathiazole (49), which is not charged at physiological pH, displayed no solubility improvement for the bioisosteres, possibly because phthalylsulfathiazole already possesses a high solubility.
Fig. 3.
Bioisosteres synthesis (A, details can be found in SI Appendix), ADME (absorption, distribution, metabolism, and excretion) data of selected drugs and their bioisosteres: sonidegib (B), boscalid (C), meclizine (D), tolvaptan (E), phthalysulfathiazole (F), lomitapide (G), and telmisartan (H). SFLOGD, shake flask log D (good range: 1 to 3) (descriptions of methods for the SFLOGD assay can be found in SI Appendix); KS, kinetic solubility (good range: >200 μM) (39); RRCK, Ralph Russ canine kidney (good range, >10) (40); HHEP, human hepatocyte stability (good range: cline <7 μL/min) (descriptions of methods for the HHEP assay can be found in SI Appendix). NMD, nonmetabolic decline. Low risk, green; moderate risk, orange; high risk, red. Note: The data for (−)-15a and (−)-15b were not provided as they are a pair of inseparable diastereomers.
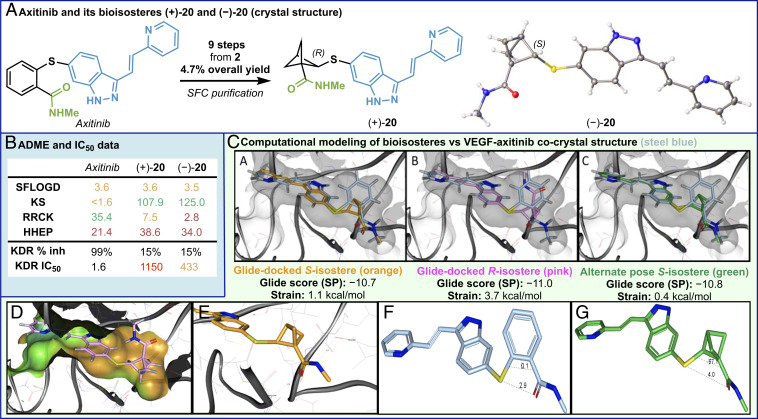
Efforts then turned to the synthesis of axitinib bioisostere 20, a VEGF kinase inhibitor, which bears a sulfur at the bridgehead position (Fig. 4A). Starting from acetate 2, a nine-step sequence afforded the desired bioisostere in 4.7% yield. After SFC separation, the IC50 values of both enantiopure BCP bioisosteres (+)-20 and (−)-20 were obtained and compared to axitinib (Fig. 4 A and B).
Fig. 4.
Crystal structure (A), bioassay data (B), and computational modeling (C) of axitinib bioisosteres. KDR, kinase insert domain receptor; KDR %inh, at 0.1 μM; KDR IC50 unit, nM. Similar Glide scores for (+)-20 and (−)-20 (Top, subpanels A–C); the R-isostere [pink, (+)-20] amide is docked in a hydrophobic pocket (subpanel D); the S-isostere (orange, (−)-20) amide is docked near polar groups such as ASP-1046 (2.9 Å) and GLU-885 (2.6 Å) (subpanel E); 250-fold difference in potency (KDR IC50) between axitinib and (−)-20; the torsion angle between the adjacent substituents on the ring: axitinib (0°, subpanel F); the S-isostere (−)-20 (67°, subpanel G); the distance between S and O: axitinib (2.9 Å, subpanel F); and (−)-20 (4.0 Å, subpanel G).
Unfortunately, the most potent axitinib BCP isostere, (−)-20, was 250-fold less active than axitinib (Fig. 4B). A crystal structure of (−)-20 (confirmed to be the S-isomer) and molecular modeling (50, 51) were used to gain a better insight into the binding modes of the enantiomers of the axitinib BCP isosteres (+)-20 and (−)-20. [Molecular modeling used Glide (50) for docking and Jaguar (51) for strain calculations (52). See SI Appendix for method details.] The two stereoisomers of the axitinib BCP isostere were modeled into the crystal structure of VEGFR2 bound to axitinib [N-methyl-2-(3-((E)-2-pyridin-2-yl-vinyl)-1H-indazol-6-ylsulfanyl)-benzamide; Protein Data Bank ID 4AGC]. Glide docking provided poses where the S-isomer more closely resembled the axitinib orientation of the amide moiety (see Fig. 4C, compare subpanels A and B). The Glide poses consistently flipped the orientation of the vinyl group compared to the 4AGC ligand for both the R- and S-isomers. An alternate pose where the vinyl group resembled the crystal structure conformation was generated outside of Glide, and when scored with Glide SP, it remained a good scoring pose (Fig. 4C, subpanel C). Despite the similar Glide scores, the R-isostere amide was docked in the hydrophobic pocket while the S-isostere amide was docked near the polar groups such as ASP-1046 and GLU-885 (Fig. 4). The potential for hydrogen bonding between the amide of the S-isomer and polar groups of the enzyme may explain why the S-isomer was more potent than the R-isomer. Compared to the small molecule crystal structure, the Glide-docked S-isostere had about 1.1 kcal/mol of conformation strain, while the Glide-docked R-isostere had about 3.7 kcal/mol of strain. The alternate pose S-isostere had about 0.4 kcal/mol of strain. The 250-fold difference in potency between axitinib and the S-isostere was not unexpected due to the conformational differences between the ortho-substituted benzamide in axitinib and the α-substituted BCP amide of the isostere. One of the main differences between the phenyl group in axitinib and the BCP isostere is the torsion angle between the adjacent substituents on the rings. In axitinib, the angle is 0°, while the angle in the isostere is 67° (Fig. 4C, subpanels F and G). Although the efficacy of this series dropped, a significant increase in kinetic solubility was observed (consistent with observations from Fig. 3). Overall, the in-depth study of this axitinib bioisostere further confirms that such a substitution can be quite useful from the standpoint of dramatically improving the solubility of a lead series but would require retuning of peripheral substituents to regain high efficacy.
Conclusion
For years, chemists in both industry and academia have been focusing on the incorporation of the bicyclo[1.1.1]pentane skeletons that mimic para-substituted benzenes. Herein, we disclose the development of a useful platform that answers the long-standing challenge of accessing 1,2-difunctionalized bicyclo[1.1.1]pentane building blocks that could serve as ortho- and meta-substituted benzene analogs. Through the systematic analysis of the key properties of several medicines and their bioisosteric analogs, this work will serve as a useful guide to practicing medicinal chemists seeking to employ such a strategy. Specifically, increased solubility can often be observed by performing this replacement. While the approach is admittedly lengthy, the key intermediates from which divergent synthesis can take place are easily accessed on scale, amenable to outsourcing, and will likely be of high interest to those working in the fields of organic synthesis, agrochemistry, and medicinal chemistry.
Materials and Methods
All reagents were commercially available and used as supplied without further purification. The details of the materials, methods including synthesis and characterization of compounds, bioassay methods, and computational modeling methods are described in SI Appendix.
Supplementary Material
Acknowledgments
Financial support for this work was provided by Pfizer and NIH grant (GM-118176). We are grateful to Dr. Dee-Hua Huang and Dr. Laura Pasternack (Scripps Research) for assistance with NMR spectroscopy, to Dr. Jason Chen, Brittany Sanchez, and Emily Sturgell (Scripps Automated Synthesis Facility) for assistance with high-performance liquid chromatography, high-resolution mass spectrometry, and liquid chromatography–mass spectrometry, and to Prof. Arnold L. Rheingold, Dr. Milan Gembicky, and Dr. John B. Bailey (University of California San Diego) for X-ray crystallographic analysis.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2108881118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Lovering F., Bikker J., Humblet C., Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Lovering F., Escape from flatland 2: Complexity and promiscuity. MedChemComm 4, 515–519 (2013). [Google Scholar]
- 3.Blakemore D. C., et al., Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Kirichok A. A., Shton I., Kliachyna M., Pishel I., Mykhailiuk P. K., 1-substituted 2-azaspiro[3.3]heptanes: Overlooked motifs for drug discovery. Angew. Chem. Int. Ed. Engl. 56, 8865–8869 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Kirichok A. A., et al., Synthesis of multifunctional spirocyclic azetidines and their application in drug discovery. Chemistry 24, 5444–5449 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Chalyk B. A., et al., Synthesis of spirocyclic pyrrolidines: Advanced building blocks for drug discovery. Chemistry 23, 16782–16786 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Mykhailiuk P. K., Saturated bioisosteres of benzene: Where to go next? Org. Biomol. Chem. 17, 2839–2849 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Locke G. M., Bernhard S. S. R., Senge M. O., Nonconjugated hydrocarbons as rigid-linear motifs: Isosteres for material sciences and bioorganic and medicinal chemistry. Chemistry 25, 4590–4647 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Gianatassio R., et al., Organic chemistry. Strain-release amination. Science 351, 241–246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kokhan S. O., et al., Design, synthesis, and application of an optimized monofluorinated aliphatic label for peptide studies by solid-state 19F NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 55, 14788–14792 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Kanazawa J., Maeda K., Uchiyama M., Radical multicomponent carboamination of [1.1. 1] propellane. J. Am. Chem. Soc. 139, 17791–17794 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Caputo D. F. J., et al., Synthesis and applications of highly functionalized 1-halo-3-substituted bicyclo[1.1.1]pentanes. Chem. Sci. (Camb.) 9, 5295–5300 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shelp R. A., Walsh P. J., Synthesis of BCP benzylamines from 2-azaallyl anions and [1.1.1]propellane. Angew. Chem. Int. Ed. Engl. 57, 15857–15861 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Makarov I. S., Brocklehurst C. E., Karaghiosoff K., Koch G., Knochel P., Synthesis of bicyclo[1.1.1]pentane bioisosteres of internal alkynes and para-disubstituted benzenes from [1.1.1]propellane. Angew. Chem. Int. Ed. Engl. 56, 12774–12777 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Bychek R. M., et al., Difluoro-substituted bicyclo[1.1.1]pentanes for medicinal chemistry: Design, synthesis, and characterization. J. Org. Chem. 84, 15106–15117 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Yu S., Jing C., Noble A., Aggarwal V. K., 1,3-Difunctionalizations of [1.1.1]propellane via 1,2-metallate rearrangements of boronate complexes. Angew. Chem. Int. Ed. Engl. 59, 3917–3921 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Garlets Z. J., et al., Enantioselective C–H functionalization of bicyclo [1.1.1] pentanes. Nat. Catal. 3, 351–357 (2020). [Google Scholar]
- 18.Kim J. H., Ruffoni A., Al-Faiyz Y. S. S., Sheikh N. S., Leonori D., Divergent strain-release amino-functionalization of [1.1.1]propellane with electrophilic nitrogen-radicals. Angew. Chem. Int. Ed. Engl. 59, 8225–8231 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwärzer K., Zipse H., Karaghiosoff K., Knochel P., Highly regioselective addition of allylic zinc halides and various zinc enolates to [1.1.1]propellane. Angew. Chem. Int. Ed. Engl. 59, 20235–20241 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X., et al., Copper-mediated synthesis of drug-like bicyclopentanes. Nature 580, 220–226 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J.-X., et al., 1,2-Difunctionalized bicyclo[1.1.1]pentanes: Long sought after bioisosteres for ortho/meta-substituted arenes. ChemRxiv [Preprint] (2020). https://chemrxiv.org/engage/chemrxiv/article-details/60c7511fbb8c1a389e3dbc11 (Accessed 25 June 2021).
- 22.Ma X., Han Y., Bennett D. J., Selective synthesis of 1-dialkylamino-2-alkylbicyclo-[1.1.1] pentanes. Org. Lett. 22, 9133–9138 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Yang Y., et al., Synthesis of multi-substituted bicycloalkyl boronates: An intramolecular coupling approach to alkyl bioisosteres. ChemRxiv [Preprint] (2021). https://chemrxiv.org/engage/chemrxiv/article-details/60c754cc9abda2e4b5f8e231 (Accessed 25 June 2021). [DOI] [PMC free article] [PubMed]
- 24.DrugBank , DrugBank online (2021). https://go.drugbank.com/. Accessed 25 June 2021.
- 25.Denisenko A., Garbuz P., Shishkina S. V., Voloshchuk N. M., Mykhailiuk P. K., Saturated bioisosteres of ortho-substituted benzenes. Angew. Chem. Int. Ed. Engl. 59, 20515–20521 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Klopsch R., Schlüter A.-D., A [1.1.1] propellane with an unprotected hydroxy group in the side chain. Tetrahedron 51, 10491–10496 (1995). [Google Scholar]
- 27.Wang J., et al., Cu-catalyzed decarboxylative borylation. ACS Catal. 8, 9537–9542 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornella J., et al., Practical Ni-catalyzed aryl–alkyl cross-coupling of secondary redox-active esters. J. Am. Chem. Soc. 138, 2174–2177 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin T., et al., A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science 352, 801–805 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J., et al., Nickel-catalyzed cross-coupling of redox-active esters with boronic acids. Angew. Chem. Int. Ed. Engl. 55, 9676–9679 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin T., et al., Nickel-catalyzed Barton decarboxylation and Giese reactions: A practical take on classic transforms. Angew. Chem. Int. Ed. Engl. 56, 260–265 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C., et al., Decarboxylative borylation. Science 356, eaam7355 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards J. T., et al., Decarboxylative alkenylation. Nature 545, 213–218 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith J. M., et al., Decarboxylative alkynylation. Angew. Chem. Int. Ed. Engl. 56, 11906–11910 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni S., et al., A general amino acid synthesis enabled by innate radical cross-coupling. Angew. Chem. Int. Ed. Engl. 57, 14560–14565 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kingston C., et al., Direct carbon isotope exchange through decarboxylative carboxylation. J. Am. Chem. Soc. 141, 774–779 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen T.-G., et al., Quaternary centers by nickel-catalyzed cross-coupling of tertiary carboxylic acids and (hetero)aryl zinc reagents. Angew. Chem. Int. Ed. Engl. 58, 2454–2458 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ni S., et al., A radical approach to anionic chemistry: Synthesis of ketones, alcohols, and amines. J. Am. Chem. Soc. 141, 6726–6739 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saal C., Petereit A. C., Optimizing solubility: Kinetic versus thermodynamic solubility temptations and risks. Eur. J. Pharm. Sci. 47, 589–595 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Di L., et al., Development of a new permeability assay using low-efflux MDCKII cells. J. Pharm. Sci. 100, 4974–4985 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Bajwa J. S., De La Cruz M., Dodd S. K., Waykole L. M., Wu R., “Salts of N-[6-cis-2,6-dimethylmorpholin-4yl)pyridine-3yl]-2-methyl-4′-(trifluoromethoxy)[1,1′-biphenyl]-3-carboxamide” (Novartis AG). Patent WO 2010033481 A1 20100325 (2010).
- 42.Mayer H., Golsch D., Isak H., Schröder J., “Method for producing 2-halogen-pyridine-carboxylic acid amides” (BASF Aktiengesellschaf). Patent WO 2003037868 A1 20030508 (2003).
- 43.Ogawa H., et al., “Vasopressin antagonistic agent” (Otsuka Pharmaceutical Co., Ltd.). Patent JP 04321669 A 19921111 (1992).
- 44.Reddy K. S., et al., An efficient and impurity-free process for telmisartan: An antihypertensive drug. Org. Process Res. Dev. 11, 81–85 (2007). [Google Scholar]
- 45.Kania R. S., et al., “Indazole compounds and pharmaceutical compositions for inhibiting protein kinases, and methods for their use” (Agouron Pharmaceuticals, Inc.), US 6534524 B1 (2003).
- 46.Kania R. S., “Structure-based design and characterization of axitinib” in Kinase Inhibitor Drugs, Li R., Stafford J. A., Eds. (Wiley, Hoboken, NJ, 2009), pp. 167–201. [Google Scholar]
- 47.Lal B., et al., “Novel water based process for the preparation of substituted diphenylmethyl piperazines” (Calyx Chemicals and Pharmaceuticals Pvt. Ltd.), Patent WO 2010046908 A2 (2010).
- 48.Vadali L. R., Yerva E. R., Vemavarapu G. P. S., Rao P. B., “Process for making lomitapide mesylate” (Mylan Laboratories Ltd.). Patent WO 2016012934 A1 (2016).
- 49.Moore M. L., Miller C. S., Dicarboxylic acid derivatives of sulfonamides. J. Am. Chem. Soc. 64, 1572–1576 (1942). [Google Scholar]
- 50.Friesner R. A., et al., Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 47, 1739–1749 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Bochevarov A. D., et al., Jaguar: A high-performance quantum chemistry software program with strengths in life and materials sciences. Int. J. Quantum Chem. 113, 2110–2142 (2013). [Google Scholar]
- 52.Schrödinger, LLC , Schrödinger Release 2019-1: Glide and Jaguar (Schrödinger, LLC, New York, 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.