Abstract
A retrospective analysis was performed of epidemiological data assessing the survival of patients who had received radium-223 for castrate-resistant metastatic prostate cancer treated at a regional tertiary referral center over a 5-year period. The patients' age, date of first treatment, and the number of cycles of radium-223 given were obtained from the patients' electronic patient record (EPR). Data on the date of death were provided by national death registrations which update the EPR via a unique national health service number. A total of 187 patients (mean age on the date of first treatment: 73 years; range: 56–93) were treated from April 1, 2014, to June 30, 2019. The median overall survival of the 119 patients (71%) who had died by December 31, 2019, was 15 months. There was no significant age difference between those who had died and survivors (72 vs. 74 years). On a further analysis, it was found that the median overall survival of the 107 patients who had received all the six cycles of radium-223 was 31 months, significantly longer than the median overall survival of only 6 months for those eighty patients who had received less than the full course of six cycles of radium-223 (P = 0.001). Of those who received all the six cycles of treatment, 58 patients had died (58%) and the 1-year survival was 87%. This was compared to the group of patients receiving <6 cycles of radium-223 where 61 patients (76%) had died and the 1-year survival was 30%. Therefore, the hazard ratio of dying before 1 year if the patient did not receive all the six cycles of treatment was 2.9. Where the reason for stopping treatment was recorded on the EPR the most common cause for the cessation of treatment was because of the side effects caused by the treatment itself. Other causes were hospitalization with comorbidities, disease progression, or patient choice. Given the survival advantage of receiving the full course of all the six cycles of treatment, this should be administered if possible and the patients should be managed in such a way as to allow the complete treatment course to be given.
Keywords: Carcinoma of the prostate, median overall survival, radium-223
INTRODUCTION
Radionuclides have been used to treat pain from bone metastases due to metastatic prostate cancer for at least four decades initially using strontium-89 (Sr-89) and then Samarium-153 ethylenediaminetetranethylenephosphonic acid (Sm-153 EDTMP).[1,2,3,4,5,6,7] There has been a suggestion from a Phase II trial that Sm-153 EDTMP may improve survival. A more recent meta-analysis has failed to show any survival benefit from the use of beta-emitting radionuclides such as Sr-89 and Sm-153 in hormone-resistant and chemotherapy-refractory metastatic prostate cancer.[8,9]
One solution that was proposed was the use of alpha emitters such as radium-223, which like calcium is a Group-2 metal. It complexes into the bone matrix like calcium and has an 11-day half-life. It has a complex decay system involving isotopes of radon, thallium, polonium, and bismuth. Most of these daughters have very short half-lives measured in seconds or a few minutes.[10]
Phase II trials showed good anti-tumor affect, but the pivotal trial was the ALSYMPCA Phase III trial which compared six cycles of 50kBq/kg radium-223 every 4 weeks with a placebo. Over 1000 patients were recruited into the trial, and the results showed that the median overall survival of those patients treated with radium-223 was 15 months compared to 11 months in the control group.[11,12]
Since 2014, our center has offered radium-223 treatment. This treatment was offered on a regional basis to a mainly rural area with a population base of around 3.5 million spread over an area 130 km × 100 km. We soon started to observe much better survival in those patients who managed to receive the full course of all the six cycles of radium-223. Therefore, we performed an epidemiological retrospective review of the data of patients treated at our center to determine if those patients who were able to complete the full treatment course had a greater overall survival than those patients who were unable to receive the full six cycles of treatment.
MATERIALS AND METHODS
This was an epidemiological retrospective review of the demographic data to determine the effectiveness of radium-223 in patients with castrate-resistant prostate cancer where at least 95% of the known metastases were within the bone. This work was performed as part of our institution's clinical governance review of the effectiveness of new treatments. As such, it complies with the Declaration of Helsinki. No additional institutional ethics approval was therefore required. All patients gave their written informed consent to treatment. After their final cycle of treatment, there was no further contact with the patient. All patients who started their treatment after April 1, 2014, and completed their treatment by June 30, 2019, were included in the study. This allowed a minimum 6-month follow-up period up to December 31, 2019, when the data set was sealed.
All patients were treated in compliance with the guidelines provided by the United Kingdom National Institute for Healthcare and Clinical Excellence.[13] The indication for initiating radium-223 therapy was the evidence of progressive bone metastases despite 2nd-line chemical castration and, where indicated, taxane chemotherapy. Patients could not be treated if they had any evidence for liver, lung, or bone metastases on computed tomography or magnetic resonance imaging and having taken enzalutamide or abiraterone for 6 weeks. Patients taking pain relief and oral, intravenous, or depot intramuscular bisphosphonates could continue these medications. Each patient had to have reasonable bone marrow reserve (hemoglobin >100 g/L, >100,000 platelets/mL, and >1.5 × 109 neutrophils/L). There were no exclusion criteria for performance status, but patients had to be able to attend nuclear medicine on six occasions every 4 weeks. They also had to have a prognosis of >24 weeks at the date of the first injection of radium-223.
Treatment could be stopped due to a significant unfixable skeletal event, sustained severe hematological toxicity, nontolerable side effects, or patient choice.
The radium-223 service was set up in April 2014. It was designed as a regional service, so patients would be sent to our center for treatment. The primary urological oncology team would be responsible for selecting patients, ensuring that they were eligible and all attend the follow-up. This pattern of service was set up following regional discussions with the referring urological oncologists during 2013 and early 2014 with 6-monthly updates after the service commenced.
Demographic data including patient age at the time of first cycle, date of the first and last cycles, and the number of cycles of radium-223 were collected from the electronic patient record (EPR) (EPIC Health Care, Milwaukee, USA). The date of death was provided by the United Kingdom Office for National Statistics (ONS, Newport, UK). The EPR was automatically updated with the date of death by the ONS. Where possible, data concerning why a patient may have stopped treatment before all the six cycles were sought from the EPR. These were not normally available for patients seen by urological oncologists outside our institution. As so many patients were referred from institutions other than our own, it was decided not to collect data on prostate-specific antigen and alkaline phosphatase levels.
Data were collated using Excel (Microsoft, Redmond, USA) and analyzed using the standard “add-in” statistical package (Excel statistics add-in, Microsoft). The mean survival times were calculated using an unpaired Students' “t”-test. The median overall survival was calculated via a Kaplan–Meier curve. Death rates at 1 year were compared by a Chi-squared test. P < 0.05 was considered statistically significant.
RESULTS
A total of 187 patients with a mean age of 73 years (range: 56–93 years) were treated [Figure 1]. Of these patients, 119 (71%) had died by December 31, 2019. There were 107 patients who had received the full course of six cycles of radium-223 treatments, of whom 58 (54%) had died. In the remaining eighty patients who received less than the full course of six cycles of treatment, 61 (77%) had died [Table 1].
Figure 1.
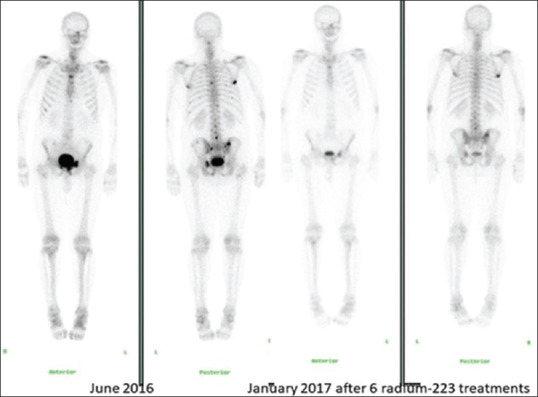
Patients' bone scintigraphy before and 6 weeks after treatment with the six cycles of radium-223. There has been a partial response in both the number and activity of bone metastases seen
Table 1.
Characteristics of patients receiving all the six cycles of radium-223 and those receiving less than six cycles
| Factor | All the six cycles | <6 cycles | P |
|---|---|---|---|
| Number of patients treated | 107 | 80 | - |
| Mean age | 73 | 73 | NS |
| Number of patients died (%) | 58 (54) | 61 (76) | <0.05 |
| Median overall survival (months) | 31 | 6 | <0.01 |
| 1-year survival (%) | 94 (87) | 24 (30) | <0.05 |
NS: Not significant
The median overall survival of all the 187 patients was 15 months [Figure 2], with four patients dying within 1 month of the first cycle of treatment and the longest survivor being alive 59 months after their first cycle of treatment. There was no significant difference between the ages of those who survived (74 years) and those who died (72 years). In those patients who received <6 months of treatment, the median overall survival was 6 months [Figure 3] (with four patients dying within 1 month of the first cycle of treatment and the longest survivors being two patients alive 51 months after their first cycle of radium-223). This was statistically significantly less (P = 0.00) than the median overall survival of 31 months for those patients receiving all the six cycles of radium-223 (three patients dying within 7 months of the first cycle of treatment and the longest survivor being alive 59 months after his/her first cycle of radium-223).
Figure 2.
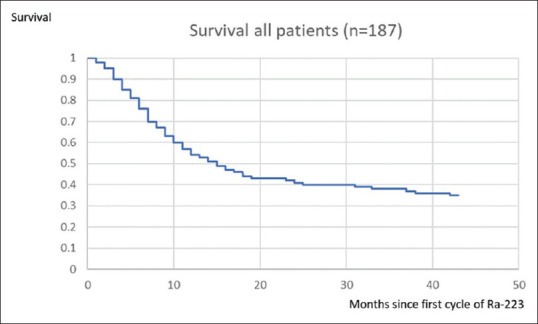
Kaplan–Meier plot of the survival of all the patients treated with radium-223
Figure 3.
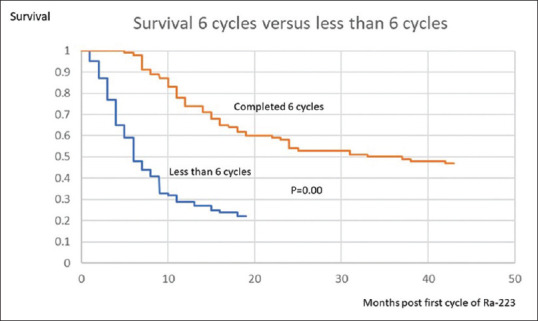
Kaplan–Meier curve of patients completing all the six cycles of treatment (orange line) and <6 cycles of treatment (blue line)
A total of 94 patients (87%) survived for the first 12 months after treatment, will all the six cycles of radium-223 compared to 24 (30%) who survived 1 year in the group of patients who did not receive the full course of the six cycles of radium-223. The death rate at 1 year was significantly greater for those who received <6 cycles of treatments (0.05 > 0 >0.01, χ2). The hazard ratio of dying before 12 months of receiving <6 cycles of treatments was thus 2.9.
The mean time of death after the first cycle of radium-223 in those 119 patients who had died by December 31, 2019 and who had received <6 cycles of treatments was 8 months (± 6 months). For those patients who received all the six cycles of treatments, the mean time of death was statistically significantly longer at 15 months (±7 months) [Figure 4] (P = 0.001 unpaired “t”-test).
Figure 4.
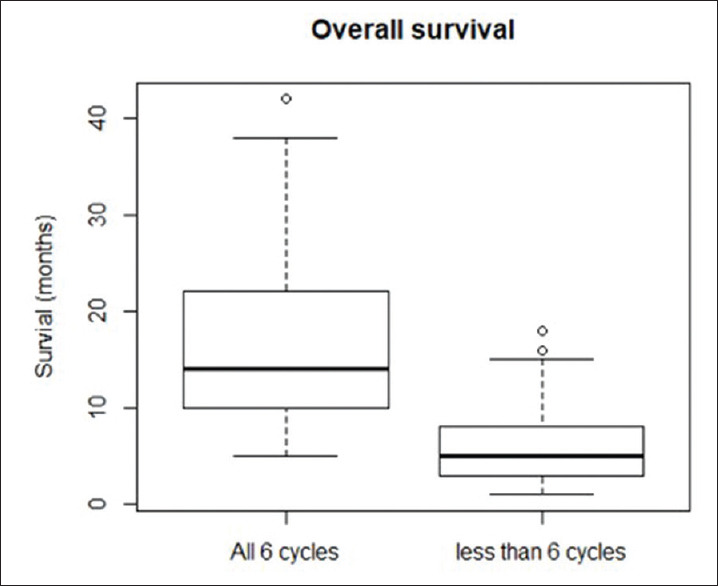
Box and whisker plot (showing mean, quartiles, and total range) showing mean time to death (and standard deviation) of the 119 patients who have died after receiving all 6 cycles of treatment and have died having received less than 6 cycles of treatment
Of the eighty patients who stopped treatment before all six cycles could be given, the reason for stopping treatment was only stated in the EPR of 32 these patients. Some patients recorded more than one reason. In twenty patients, they could not tolerate the disease-related diarrhea or nausea. In 15 patients, there was increased bone pain 7–10 days post treatment. The other main causes included admission to hospital with comorbidities commonly due to co-existent circulatory or respiratory disease. Patient choice was also a factor particularly in those patients who had to travel 3–4 h each way to receive their radium-223 injections. In 48 patients, no reason for stopping treatment was provided on the EPR. All these patients were referred in from other regional hospitals, and we had no access to their local medical notes. Anemia was not a cause of stopping treatment though 15 patients did have blood transfusions during their course of radium-223 treatment.
DISCUSSION
The results of this study are similar to those predicted from the ALSYMPCA trial with an identical median overall survival of 15 months.[12] This demonstrates that that the trial was similar to our “real-world” patient experience within the UK. This is not surprising as reimbursement for radium-223 treatment was dependent on adhering to the advice published by the National Institute for Clinical and Healthcare Excellence (NICE), which was based on the treatment criteria used in the ALSYMPCA trial.[13]
There are some other single-site and multicenter trials which also looked at survival in the first few years following treatment with radium-223 [Table 2]. Our series compares well with the other single-site trials published. The results are similar to the results of a multicentric trial of radium-223 conducted in the Netherlands,[20] where a similar median overall survival was recorded. Two UK and a single Canadian studies looked at the effect of completing at least five cycles of treatment and similar to our results, there was a survival benefit but at 5-9 months was less than the 25 months we found in our patient group.[16,17,18] The reason for this difference is unclear but maybe related to difference in patient selection at different treatment centers. For example, it was a policy in our region to refer patients for radium-223, if suitable, as soon as they failed chemical castration or chemotherapy.
Table 2.
Postregistration reports of median survival in patients receiving radium-223
| First author | Country of study | Number of patients | References | Notes |
|---|---|---|---|---|
| Fosbol M et al. | Denmark | 88 | [14] | Med OS>BSI 8 months Med OS 5 or less BSI 15 months |
| Parimi S et al. | Canada | 48 | [15] | Med OS overall 11 months<5 cycles Med OS 6 months 5 or 6 cycles Med OS 16 months |
| Parikh S et al. | The UK | 189 | [16] | Med OS overall 10 months 5–6 cycles Med OS 19 months |
| Dadhnia et al. | The UK | 113 | [17] | Med OS, 6 cycles 12 months Med OS<6 cycles 4 months |
| Uemura et al. | Japan | 49 | [18] | Med OS 19months |
| Badrising et al. | The Netherlands | 305 | [19] | Med OS 15 months multisite trial |
| Frantelizzi et al. | Italy | 173 | [20] | Med OS 8 and 16 months Depends on baseline quality of life |
Med OS: Median overall survival; BSI: Bone scintigraphy index
Several studies have tried to identify factors which could lead to a poor prognosis in treating bone metastases of castrate-resistant prostate cancer. One study looked at a semi-quantitative measure of bone involvement derived from the bone scintigraphy pre-radium-223, as the more extensive the bone involvement, the worse the prognosis.[15,22] The general well-being of the patient has also been identified as an issue with those patients having a poorer performance status tending not to complete all the six cycles of radium-223, and this results in a poorer prognosis.[21] A further recent study has shown a link between the poor performance status before the first cycle of radium-223, the onset of significant adverse events, and not receiving all the six cycles of treatment.[23] The authors clearly state that this led to a poorer prognosis as receiving <6 cycles of radium-223 is not an effective treatment for bone metastases from prostate cancer. It would appear that patient selection may be the key to success. There must be some concern so many patients died in the month after treatment despite the fact the NICE guidelines stated patients should be expected to live for 6 months after treatment is started.[13]
A significant weakness of our study is that we were unable to obtain a full data set including the pretreatment and posttreatment performance status of each patient and tumor marker such as prostate-specific antigen and alkaline phosphatase. However, this was not possible as we could only validate and use data which were held by our institution's EPR.
From the ALSYMPCA trial, it would be thought that haemotological toxicity would be a treatment-limiting issue as it is with the beta emitting radionuclides Sr-89 and Sm-153 (1-6,12) because the bone marrow is the critical organ. In this cohort of patients anaemia was not a significant issue as patients would be transfused if this was required and radium-223 treatment continued. A recent follow-up study shows that even 3 years post radium-223 treatment, there are no long-term bone marrow sequelae such as hematological malignancies or myelodysplasia.[24]
A further problem identified with poor prognosis has been skeletal fractures. Both abiraterone and enzalutamide have been implicated in increasing the probability of skeletal fractures, and a recent study was stopped because of the increased fracture rate in patients co-treated with abiraterone and radium-223.[25] The increased rate of skeletal fractures with these drugs in not unexpected as both are known to reduce bone density and increase fracture risk.[26] However, we developed a policy of rapid orthopedic fixing of fractures and early mobilization of patients so that any cycle of radium-223 was not delayed by >2 weeks.
Another main reason for which patients chose to stop the treatment was unacceptable gastrointestinal side effects such as diarrhea and nausea. Even though patients were counseled concerning the adverse effects during the consent process and supporting medications were offered, some patients were unable to continue the treatment. For patients within our own institution, this was less of an issue as they were able to access our symptom control teams.
The most likely cause of the poor prognosis in those patients who received <6 cycles of treatment was the advanced stage of their disease. This could mean that patient selection was not ideal, but the prognosis of patients with advanced metastatic cancer is not an exact science and it may be considered the best practice to give patients a least a trial of treatment. However, it is true that the effective dose of radium-223 is only achieved by giving all the six cycles and anything less is undertreatment. The need to give all the six cycles of treatment, if at all possible, is underlined in the latest advice from North America.[27] In this way, our patients will receive the best chance of success and can extend their lives.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank the staff and patients of the Nuclear Medicine Department at Cambridge University Hospitals.
REFERENCES
- 1.Sharifi N, Dahut WL. Figg WD Secondary hormonal therapy for prostate cancer: What lies on the horizon? BJU Int. 2008;101:271–4. doi: 10.1111/j.1464-410X.2007.07236.x. [DOI] [PubMed] [Google Scholar]
- 2.Harzstark AL, Ryan CJ. Therapies in development for castrate-resistant prostate cancer. Expert Rev Anticancer Ther. 2008;8:259–68. doi: 10.1586/14737140.8.2.259. [DOI] [PubMed] [Google Scholar]
- 3.Abouassaly R, Paciorek A, Ryan CJ, Carroll PR, Klein EA. Predictors of clinical metastasis in prostate cancer patients receiving androgen deprivation therapy: Results from CaPSURE. Cancer. 2009;115:4470–6. doi: 10.1002/cncr.24526. [DOI] [PubMed] [Google Scholar]
- 4.Nightengale B, Brune M, Blizzard SP, Ashley-Johnson M, Slan S. Strontium chloride Sr 89 for treating pain from metastatic bone disease. J Health Syst Pharm. 1995;52:2189–95. doi: 10.1093/ajhp/52.20.2189. [DOI] [PubMed] [Google Scholar]
- 5.Cowan RJ, Chilton HM, Cooper MR, Ferree CR, Watson EE, Robinson RG. Hematologic depression following therapy with strontium-89 chloride. Clin Nucl Med. 1986;11:845–6. doi: 10.1097/00003072-198612000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Collins C, Eary JF, Donaldson G, Vernon C, Bush NE, Petersdorf S, et al. Samarium-153-EDTMP in bone metastases of hormone refractory prostate carcinoma: A phase I/II trial. J Nucl Med. 1993;34:1839–44. [PubMed] [Google Scholar]
- 7.Sartor O, Reid RH, Hoskin PJ, Quick DP, Ell PJ, Coleman RE, et al. Quadramet 424Sm10/11 Study Group.Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology. 2004;63:940–5. doi: 10.1016/j.urology.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 8.Sartor O, Reid RH, Bushnell DL, Quick DP, Ell PJ. Safety and efficacy of repeat administration of samarium Sm-153 lexidronam to patients with metastatic bone pain.Sartor. Cancer. 2007;109:637–43. doi: 10.1002/cncr.22431. [DOI] [PubMed] [Google Scholar]
- 9.Terrisse S, Karamouza E, Parker CC, Sartor AO, James ND, Pirrie S, et al. Survival in men with bone metastases from castration-resistant prostate cancer treated with bone-targeting radioisotopes: A meta-analysis of individual patient data from randomized clinical trials. JAMA Oncol. 2019;6:206–16. doi: 10.1001/jamaoncol.2019.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheetham PJ, Petrylak DP. Alpha particles as radiopharmaceuticals in the treatment of bone metastases: Mechanism of action of radium-223 chloride (Alpharadin) and radiation protection. Oncology (Williston Park) 2012;26:330–7, 341. [PubMed] [Google Scholar]
- 11.Nilsson S, Franzén L, Parker C, Tyrrell C, Blom R, Tennvall J, et al. Two-year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin Genitourin Cancer. 2013;11:20–6. doi: 10.1016/j.clgc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, et al. ALSYMPCA investigators alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 13.Radium-223 dichloride for treating hormone relapsed prostate cancer with bone metastases. National Institute for Health Care and Excellence. 2016. [Last accessed on 2020 Oct 05]. Available from: https://www.nice.org.uk/guidance/ta412 .
- 14.Fosbøl MØ, Petersen PM, Kjaer A, Mortensen J. 223Ra therapy of advanced metastatic castration-resistant prostate cancer: Quantitative assessment of skeletal tumor burden for prognostication of clinical outcome and hematologic toxicity. J Nucl Med. 2018;59:596–602. doi: 10.2967/jnumed.117.195677. [DOI] [PubMed] [Google Scholar]
- 15.Parimi S, Tsang E, Alexander A, Mckenzie M, Bachand F, Sunderland K, et al. Population-based study of the use of radium 223 in metastatic castration-resistant prostate cancer: Factors associated with treatment completion. Can Urol Assoc J. 2017;11:350–5. doi: 10.5489/cuaj.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parikh S, Murray L, Kenning L, Bottomley D, Din O, Dixit S, et al. Real-world outcomes and factors predicting survival and completion of radium 223 in Metastatic castrate-resistant prostate cancer. Clin Oncol (R Coll Radiol) 2018;3:548–55. doi: 10.1016/j.clon.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Dadhania S, Alonzi R, Douglas S, Gogbashian A, Hughes R, Dalili D, et al. Single-centre experience of use of radium 223 with clinical outcomes based on number of cycles and bone marrow toxicity. Anticancer Res. 2018;38:5423–7. doi: 10.21873/anticanres.12873. [DOI] [PubMed] [Google Scholar]
- 18.Uemura H, Uemura H, Nagamori S, Wakumoto Y, Kimura G, Kikukawa H, et al. Three-year follow-up of a phase II study of radium-223 dichloride in Japanese patients with symptomatic castration-resistant prostate cancer and bone metastases. Int J Clin Oncol. 2019;24:557–66. doi: 10.1007/s10147-018-01389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badrising SK, Louhanepessy RD, van der Noort V, Coenen JLLM, Hamberg P, Beeker A, et al. A prospective observational registry evaluating clinical outcomes of Radium-223 treatment in a nonstudy population. Int J Cancer. 2020;147:1143–51. doi: 10.1002/ijc.32851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frantellizzi V, De Feo MS, Di Rocco A, Pontico M, Pani A, Farcomeni A, et al. Baseline quality of life predicts overall survival in patients with mCRPC treated with 223Ra-dichloride. Hell J Nucl Med. 2020;23:12–20. doi: 10.1967/s002449912001. [DOI] [PubMed] [Google Scholar]
- 21.Richmond PJ, Doeman AM, Busocmbe JR, Hilson AJ, Kaisary AV. Extent of disease in initial bone scan: An indicator of survival in prostate cancer patients. In: Taylor A, Nally JV, Thomeson H, editors. Radionuclides and Nephrourology. Reston, VA, USA: Society of Nuclear Medicine; 2000. pp. 211–15. [Google Scholar]
- 22.Parker CC, Coleman RE, Sartor O, Vogelzang NJ, Bottomley D, Heinrich D, et al. Three-year safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases from phase 3 randomized alpharadin in symptomatic prostate cancer trial. Eur Urol. 2018;73:427–35. doi: 10.1016/j.eururo.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Huynh-Le MP, Shults RC, Connor MJ, Hattangadi-Gluth JA. Adverse events associated with radium-223 in metastatic prostate cancer: Disproportionality analysis of fda data reflecting worldwide utilization. Clin Genitourin Cancer. 2020;18:192–20000. doi: 10.1016/j.clgc.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith M, Parker C, Saad F, Miller K, Tombal B, Ng QS, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:408–19. doi: 10.1016/S1470-2045(18)30860-X. [DOI] [PubMed] [Google Scholar]
- 25.Sternberg CN, Castellano D, Daugaard G, Géczi L, Hotte SJ, Mainwaring PN, et al. Abiraterone acetate for patients with metastatic castration-resistant prostate cancer progressing after chemotherapy: Final analysis of a multicentre, open-label, early-access protocol trial. Lancet Oncol. 2014;15:1263–8. doi: 10.1016/S1470-2045(14)70417-6. [DOI] [PubMed] [Google Scholar]
- 26.Todenhöfer T, Stenzl A, Hofbauer LC, Rachner TD. Targeting bone metabolism in patients with advanced prostate cancer: Current options and controversies. Int J Endocrinol. 2015;2015:838202. doi: 10.1155/2015/838202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurwitz M, Buscombe JR, Jacene HA, Klitzke AK, Lamonica D, Lu Y, et al. ACR-ACNM-ASTRO-SNMMI practice parameter for the performance of therapy with radium-223. Am J Clin Oncol. 2020;43:539–44. doi: 10.1097/COC.0000000000000702. [DOI] [PubMed] [Google Scholar]


