Abstract
Purpose
The diagnosis and management of liver cirrhosis remain challenging due to its diverse clinical manifestations and elusive severity evaluation. Pyroptosis, an identified inflammatory form of cell death, has recently been reported to participate in cirrhosis development. Nonetheless, the clinical significance of pyroptosis in liver cirrhosis remains largely unexplored.
Patients and Methods
One hundred and fifty-one liver cirrhosis patients either alone or in combination with various complications and twenty-nine gender- and age-matched healthy controls (HCs) were enrolled in this study. Pyroptosis-related indicators gasdermin D (GSDMD), IL-1ß and IL-18 were measured by IHC in tissue section and by ELISA in serum, respectively, and correlations of their circulating levels with disease severity as well as their potential as biomarkers for monitoring cirrhosis progression were evaluated.
Results
Increased levels of the circulating pyroptosis-related indicators GSDMD, IL-1ß and IL-18 were observed in liver cirrhosis patients, especially those with an etiology of viral infection. In addition, all three indicators were positively correlated with disease severity parameters, including Child-Pugh classification, APRI scores and compensated status. Furthermore, receiver operating characteristic (ROC) analysis showed that circulating IL-1ß exerted potential discriminating power for SBP occurrence in liver cirrhosis, but GSDMD possessed differentiating power for SBP in liver cirrhosis with ascites, which yielded area under the ROC curve (AUC) of 0.81 and 0.80, respectively.
Conclusion
Liver cirrhosis patients exhibited increased levels of circulating GSDMD, IL-1ß and IL-18, all of which were positively correlated with disease severity. More importantly, the identified circulating IL-1ß and GSDMD exhibited potentials as novel biomarkers for liver cirrhosis patients presenting with SBP.
Keywords: GSDMD, IL-1ß, IL-18, liver cirrhosis, severity, complication
Introduction
Liver cirrhosis represents a type of end-stage liver disease due to long-term damage and inflammation characterized by the replacement of the functional liver tissue with nonfunctional scar tissue.1 Progression towards cirrhosis is a heterogeneous process influenced by many etiological factors, including viral infection, alcohol consumption, autoimmunity, non-alcoholic fatty liver disease (NAFLD) and other rare inherited liver diseases. The natural history of cirrhosis is highly variable or involves processes from a compensated stage to a decompensated stage, defined as the presence of ascites, bleeding esophageal varices, spontaneous bacterial peritonitis and hepatic encephalopathy.2 Currently, many noninvasive tests and imaging techniques have been developed as diagnostic and evaluable approaches for cirrhosis. Nonetheless, several deficiencies still need to be overcome, including the sensitivity and specificity of these methods for severity diagnosis and evaluation, which may lead to misdiagnosis, inaccurate disease assessment and missing the treatable stage.3,4 Therefore, developing new quantifiable biomarkers that closely reflect pathogenesis as well as disease severity is urgently needed.
Accumulation of fibrous tissue composed of a complex assembly of different extracellular matrix (ECM) molecules in and around damaged or inflamed tissue is a pathological feature that appears during the progressive process from fibrosis to cirrhosis.5 A key cellular mediator of liver fibrosis and cirrhosis is activation of ECM-producing myofibroblasts driven by fibrogenic cytokines and growth factors that are induced by liver necroinflammation.6 Proinflammatory signals activation and accumulation of cytokines, chemokines and reactive oxygen species can be emitted by liver-resident cells and inflammatory-immune cells, facilitating the necrotic demise of hepatocytes.7 In turn, the release of cellular constituents from necrotic hepatocytes also elicits a highly hepatotoxic feedforward cycle of inflammation and cell death, resulting in further tissue injury and nonfunctional fibrotic scarring.8 Therefore, there is a crucial role for the inflammatory-immune response elicited by mediators secreted from damaged hepatocyte in fibrosis and even cirrhosis. Nonetheless, it is still not entirely clear how inflammatory immune responses participate in this process and whether key mediators serve as useful indicators for cirrhosis progression.
Pyroptosis, an identified inflammatory form of cell death, is triggered by certain inflammasomes and leads to caspase-1 activation and gasdermin D (GSDMD)-mediated formation of membrane pores with subsequent cellular lysis, which further induces inflammatory responses via activation and release of pro-inflammatory IL-1β and IL-18.9 Pyroptosis is reportedly involved in multiple human diseases, including metabolic and inflammatory diseases, infection, and cancer.10,11 Recent studies also demonstrated that pyroptosis may participate in liver diseases progression, which is associated with liver-resident cell death caused by infection, alcohol, drugs, toxins or immune system attacks.12 Specifically, induction of pyroptosis can boost disease severity by increasing pro-fibrogenic factor expression and collagen deposition in liver tissues during fibrosis or cirrhosis in mouse experiment.13 Mechanistically, the pyroptosis-related effectors IL-1β and IL-18 were reported to modulate proliferation and activation of HSCs, inducing fiber protein expression in vitro.14,15 Several in vivo studies have demonstrated that inhibition of pyroptosis by targeting caspase-1 or NLRP3 significantly inhibits liver fibrosis or cirrhosis.16,17 Therefore, pyroptosis may play a pivotal role in liver diseases, especially cirrhosis. However, the relationship between pyroptosis-related indicators and disease severity in liver cirrhosis patients remains elusive.
In the present study, we examined circulating pyroptosis-related indicators (GSDMD, IL-1ß and IL-18) and explored their correlation with clinical parameters in a well-defined cohort of liver cirrhosis patients either alone or in combination with various complications, aiming to explore whether these indicators can be used as potential biomarkers during liver cirrhosis progression.
Patients and Methods
Patients
A total of one hundred and fifty-one patients with liver cirrhosis were enrolled in the present study between March 2019 and December 2020 at the Second Affiliated Hospital of Chongqing Medical University. Diagnosis was primarily established by histology, appearance on ultrasound or radiological imaging and medical history. Disease etiologies, such as viral infection, alcohol consumption and autoimmunity, were determined according to clinical, serological and histological findings. All liver cirrhosis patients with etiology of viral infection belong to the chronic phase and have not been previously treated. Liver cirrhosis patients with an etiology of alcohol consumption have drunk heavily with one or more alcoholic drinks per week (males: alcohol consumption > 40 g/day; female: alcohol consumption > 20 g/day) for at least eight years. Child-Pugh classification and AST-to-Platelet Ratio Index (APRI) score were calculated to determine disease severity.18,19 Exclusion criteria included patients with chest wall trauma, a history of pituitary or hypothalamic disease, tuberculous peritonitis, chronic renal failure, the presence of hepatocellular carcinoma or other active malignancies or electrolyte disorders. Additionally, twenty-nine gender-matched healthy volunteers who did not have evidence of liver disease were enrolled as healthy controls (HCs). Clinical characteristics were recorded. Blood samples of as much as 3 mL were drawn from the antecubital vein and inserted into the vacutainer tube at the time of diagnosis. The blood is centrifuged for 10 minutes to obtain serum. The obtained serum is stored at −80 °C until further processing. Biopsy specimens from diagnosed liver cirrhosis patients and five normal liver tissue samples form HCs that underwent liver biopsy to exclude liver disease were also performed and stored until use. This study was approved by the Institutional Ethics Committee for human studies at the Second Affiliated Hospital of Chongqing Medical University (no. 2019–295). All procedures followed the Declaration of Helsinki. Patient characteristics are summarized in Table 1.
Table 1.
The Clinical Characteristics of Enrolled Individuals
| Serum Samples | Tissue Specimens | |||
|---|---|---|---|---|
| Liver Cirrhosis (n=151) | HCs (n=62) | Liver Cirrhosis (n=12) | HCs (n=5) | |
| Age year (IQR) | 53 (18) | 54 (16) | 50 (16) | 53 (14) |
| Gender | ||||
| Male n (%) | 97 (64.23) | 36 (58.06) | 7 (58.33) | 3 (60) |
| Fale n (%) | 54 (35.76) | 26 (41.94) | 5 (41.67) | 2 (40) |
| Aetiology | ||||
| Viral hepatitis | NA | 7 (58.33) | NA | |
| HBV n (%) | 87 (73.73) | |||
| HCV n (%) | 31 (26.27) | |||
| Alcohol n (%) | 15 (9.93) | NA | 2 (16.67) | NA |
| Cholestatic/Autoimmune n (%) | 18 (11.92) | NA | 3 (25) | NA |
| Child-Pugh Classification. | ||||
| A n (%) | 60 (39.73) | NA | 2 (16.66) | NA |
| B n (%) | 46 (30.46) | NA | 5 (41.67) | NA |
| C n (%) | 45 (29.81) | NA | 5 (41.67) | NA |
| Phase | ||||
| Compensated stage n (%) | 48 (31.79) | NA | 4 (33.33) | NA |
| Decompensated stage n (%) | 103 (68.21) | NA | 8 (66.67) | NA |
| APRI score | 2.11 (2.52) | 0.29 (0.11) | 2.1 (2.84) | 0.26 (0.1) |
| AST (U/L) | 63 (89) | 24 (6) | 52 (68.55) | 23 (4.5) |
| PLT | 92 (78) | 204 (76) | 67 (45) | 207 (72) |
Abbreviations: IQR, interquartile range; HCs, healthy controls; APRI, AST to Platelet Ratio Index; NA, not applicable.
Immunohistochemical (IHC)
The expression of GSDMD, IL-1ß and IL-18 in liver specimens was examined by IHC. The sections from the formalin fixed, paraffin-embedded tissues were deparaffinized and dehydrated. Then, the sections were boiled for 10 min in a 0.01 M citrate buffer and incubated with 0.3% hydrogen peroxide in methanol for 15 min to block endogenous peroxidase. The sections were then incubated with the anti-GSDMD (20770-1-AP; Proteintech Group, USA), IL-1ß (16806-1-AP; Proteintech Group, USA) and IL-18 (10663-1-AP; Proteintech Group, USA) overnight at 4 °C, following incubation with secondary antibody tagged with the peroxidase enzyme (SP-9000, Zhongshan Golden Bridge, China) for 30 min at room temperature and were visualized with 0.05% 3,3-diamino-benzidine tetrachloride till the desired brown reaction product was obtained. The sections were finally counter-stained with hematoxylin. All the slides were observed under a Nikon E400 Light Microscope, and representative photographs were taken.
Enzyme-Linked Immunosorbent Assay
Serum samples were analyzed by commercial human GSDMD (MBE1217; Mengbio, China), IL-1ß (MBE0058; Mengbio, China) and IL-18 (MBE0066; Mengbio, China) enzyme-linked immunosorbent assay (ELISA) kits. All serum samples were diluted 10 times in standard buffer diluent before testing according to the manufacturer’s protocols. The assay for human GSDMD ELISA kit has a linear range between 0.625 ng/mL and 20 ng/mL and a sensitivity of < 0.1 ng/mL. The assay for human IL-1ß ELISA kit has a linear range between 1 pg/mL and 80 pg/mL and a sensitivity of < 1 pg/mL. The assay for human IL-18 ELISA kit has a linear range between 1 pg/mL and 120 pg/mL and a sensitivity of < 1 pg/mL. The coefficients of variation for these assays were less than 15%. Samples were run in duplicate.
Statistical Analysis
Data were analyzed using SPSS 17.0 with Mann–Whitney or Kruskal–Wallis test to determine the significance of GSDMD, IL-1ß and IL-18 in liver cirrhosis patients with various clinical features. Correlation coefficients (r) were calculated using Spearman correlation. Receiver operating characteristic (ROC) curves were generated to classify patients in different groups, as well as for evaluation of predictive power of serum GSDMD, IL-1ß and IL-18 levels via calculation of the area under the ROC curve (AUC), with sensitivity and specificity according to standard formulas. Multiple linear regression analysis was performed to determine independent determinants for levels of GSDMD, IL-1ß and IL-18. All data are expressed as the median and interquartile range (IQR). A p value < 0.05 was considered to be statistically significant.
Results
Levels of GSDMD, IL-1ß and IL-18 in Patients with Liver Cirrhosis
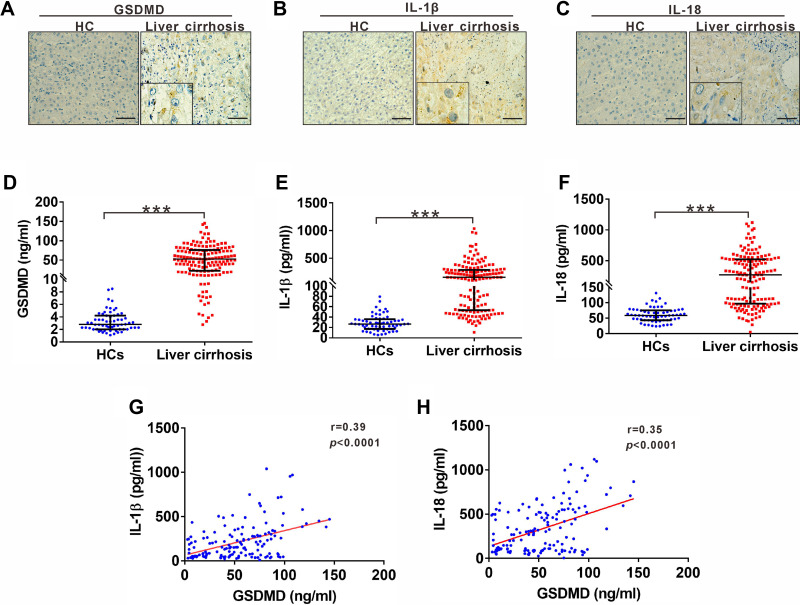
Pyroptosis occurrence was verified by detecting and analyzing its markers GSDMD, IL-1ß and IL-18 in fresh tissue sections from patients with liver cirrhosis. We observed increased levels of GSDMD, IL-1ß and IL-18 in cirrhosis tissues compared with normal tissues (Figure 1A–C). To explore whether serum levels of pyroptosis-related indicators GSDMD, IL-1ß and IL-18 are abnormally altered in liver cirrhosis, we assessed their levels in liver cirrhosis patients and compared them to healthy controls (HCs). Serum levels of GSDMD were significantly higher in liver cirrhosis patients [51.91 (50.34) ng/mL] than in the HC group [2.79 (2.19) ng/mL] (Figure 1D). Similarly, serum levels of IL-1ß and IL-18 were also prominently higher in liver cirrhosis patients [154.5 (235.89) pg/mL; 270.3 (425.87) pg/mL], respectively, compared to HC [26.8 (18.69) pg/mL; 59.04 (31.77) pg/mL] (Figure 1E and F). Since GSDMD can trigger the formation of membrane pores to facilitate IL-1ß and IL-18 release, we next determined the relationship between GSDMD and IL-1ß or IL-18. Both IL-1ß and IL-18 were positively correlated with GSDMD (Figure 1G and H).
Figure 1.
Expression of GSDMD, IL-1ß and IL-18 in patients with liver cirrhosis. (A-C) Representative IHC staining for GSDMD, IL-1ß and IL-18 in liver cirrhosis patients. Black scale bars, 100µm. (D) ELISA analysis of serum GSDMD levels in HC and liver cirrhosis patients. (E) ELISA analysis of serum IL-1ß levels in HC and liver cirrhosis patients. (F) ELISA analysis of serum IL-18 levels in HC and liver cirrhosis patients. (G) Correlation between serum IL-1ß and GSDMD in liver cirrhosis patients. (H) Correlation between serum IL-18 and GSDMD in liver cirrhosis patients. ***p<0.001.
Abbreviations: HC, health control; IQR, data represents the median.
Distribution of GSDMD, IL-1ß and IL-18 in Liver Cirrhosis with Various Etiologies
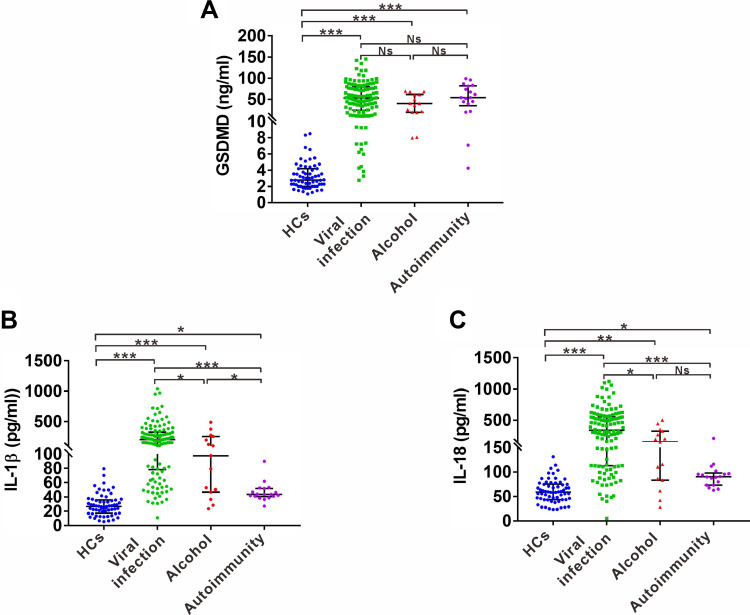
Liver cirrhosis can arise due to viral infection, autoimmunity, alcohol consumption, NAFLD and other rare inherited liver diseases. We next examined the distribution of serum pyroptosis-related indicators in liver cirrhosis patients of three main etiologies, including viral infection, alcohol consumption and autoimmunity. Serum levels of GSDMD were significantly higher in all etiological subgroups of liver cirrhosis patients [53.2 (55.62) ng/mL; 39.9 (42.32) ng/mL; 54.12 (46.94) ng/mL] than in the HC group [2.79 (2.19) ng/mL]. However, GSDMD showed no statistically significant difference among the three etiological subgroups (Figure 2A). IL-1ß levels were also significantly higher in various etiological subgroups [205.3 (246.92) pg/mL, 97.55 (208.63) pg/mL, 43.72 (11.21) pg/mL] compared to the HC group [26.8 (18.69) pg/mL]. In particular, higher IL-1ß levels were found in the viral infection-derived subgroups compared to the alcohol or autoimmunity subgroups (Figure 2B). A similar tendency was also observed in IL-18 analysis, showing that the three subgroups of liver cirrhosis patients had higher IL-18 levels [341.4 (444.9) pg/mL, 151.4 (244.2) pg/mL, 90.29 (25) pg/mL] than that of HCs [59.04 (31.77) pg/mL], and the viral infection-derived subgroup exhibited higher IL-18 levels than alcohol subgroup (Figure 2C), while no significant difference was observed between alcohol and autoimmunity subgroups (Figure 2C).
Figure 2.
Serum levels of GSDMD, IL-1ß and IL-18 in patients with various etiologies. (A) ELISA analysis of serum GSDMD levels in liver cirrhosis patients with three main etiologies, including viral infection, alcohol consumption and autoimmunity. (B) ELISA analysis of serum IL-1ß levels in liver cirrhosis patients with three main etiologies, including viral infection, alcohol consumption and autoimmunity. (C) ELISA analysis of serum IL-18 levels in liver cirrhosis patients with three main etiologies, including viral infection, alcohol consumption and autoimmunity. Data represents the median (IQR). *p<0.05; ***p<0.001.
Determinants Associated with GSDMD, IL-1ß and IL-18 in Liver Cirrhosis Patients
Univariate analysis was performed for the determinants of serum levels of GSDMD, IL-1ß and IL-18. Among the studied variables, etiologies, Child-Pugh classification and compensated status were significantly linked to GSDMD, and etiologies, AST, Child-Pugh classification and compensated status were significantly linked to IL-1ß and IL-18 (Table 2). To determine the independent determinants of GSDMD, IL-1ß and IL-18, multiple linear regression analysis was performed. Child-Pugh classification was independently related to serum levels of GSDMD (Table 2). Etiologies and Child-Pugh classification were independently related to serum levels of IL-1ß or IL-18 (Table 2).
Table 2.
Significant Factors Associated with GSDMD, IL-1ß and IL-18 by Step Forward Multiple Linear Regression Analysis in Liver Cirrhosis Patients
| Variables | GSDMD | IL-1ß | IL-18 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate* | Multivariate+ | Univariate* | Multivariate+ | Univariate* | Multivariate+ | |||||||
| ß | p value | ß | p value | ß | p value | ß | p value | ß | p value | ß | p value | |
| Age (Years) | −0.492 | 0.059 | −1.049 | 0.283 | −1.852 | 0.32 | ||||||
| Gender | ||||||||||||
| Malea | ||||||||||||
| Female | −1.649 | 0.768 | −22.072 | 0.517 | −40.429 | 0.375 | ||||||
| Aetiology | ||||||||||||
| Viral hepatitisa | ||||||||||||
| Alcohol | 15.151 | 0.084 | −95.263 | 0.069 | −189.248 | 0.006 | 65.041 | 0.001 | ||||
| Autoimmune | −20.27 | 0.013 | 0.069 | 0.191 | −157.97 | < 0.001 | −49.842 | 0.008 | −292.305 | < 0.001 | −240.457 | 0.002 |
| AST (U/L) | 0.042 | 0.003 | 0.008 | 0.883 | 0.231 | 0.006 | 0.146 | 0.066 | 0.288 | 0.008 | 0.096 | 0.205 |
| PLT | −0.019 | 0.672 | 0.158 | 0.561 | 0.155 | 0.67 | ||||||
| APRI score | 3.048 | < 0.001 | −0.016 | 0.774 | 14.225 | 0.003 | 0.117 | 0.16 | 16.228 | 0.012 | 0.020 | 0.800 |
| Child-Pugh classification | 31.33 | < 0.001 | 31.343 | <0.001 | 78.197 | < 0.001 | 59.919 | 0.003 | 118.197 | < 0.001 | 101.793 | < 0.001 |
| Compensated status | 7.707 | 0.178 | 132.87 | < 0.001 | 0.116 | 0.197 | 191.081 | < 0.001 | 0.133 | 0.13 | ||
Notes: a, reference group; ß, regression coefficient; *, univariate coefficients; +, a stepwise multivariate regression analysis was performed; APRI: AST to Platelet Ratio Index. p value < 0.05 was considered statistically significant.
Correlation of Serum GSDMD, IL-1ß and IL-18 Levels with Disease Severity in Cirrhosis Patients
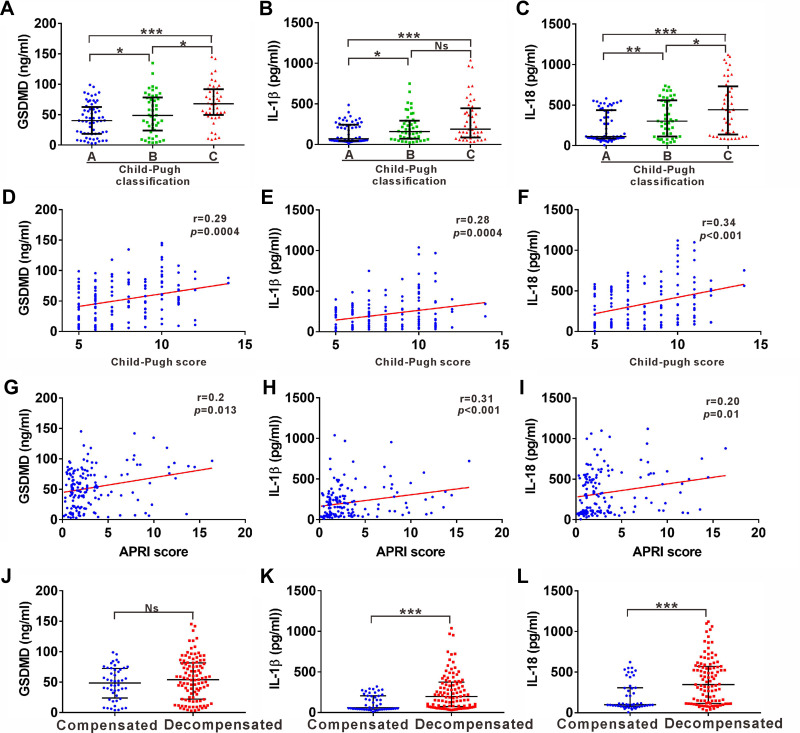
The Child-Pugh classification represents a widely used means of assessing liver cirrhosis severity. We next analyzed the relationship between pyroptosis-related indicators and Child-Pugh classification in liver cirrhosis. The increased levels of all three indexes were nearly proportional to the Child-Pugh classification. Liver cirrhosis patients with Child-Pugh classification C showed higher serum levels of GSDMD and IL-18 than patients with Child-Pugh classification A or B (Figure 3A and C). Furthermore, patients with Child-Pugh classification A showed the lowest levels of the three indexes (Figure 3A–C). All three indexes were also positively correlated with Child-Pugh scores (Figure 3D–F). In addition, APRI score has also been widely used for predicting the severity of hepatic cirrhosis.19 The relationship between pyroptosis-related indicators and APRI score was also assessed. All three indexes were positively correlated with APRI scores (Figure 3G–I). Liver cirrhosis development is also characterized by a long asymptomatic phase (compensated cirrhosis) which then develops into a much shorter phase (decompensated cirrhosis). All three indexes were prominently higher in the decompensated phase than that in the compensated phase (Figure 3J–L).
Figure 3.
Correlation of serum GSDMD, IL-1ß and IL-18 levels with disease severity in cirrhosis patients. (A-C) ELISA analysis of serum levels of GSDMD (A), IL-1ß (B) and IL-18 (C) in liver cirrhosis patients with Child-Pugh classification A, B and C. (D-F) Correlation between serum GSDMD (D), IL-1ß (E) and IL-18 (F) with Child-Pugh score in liver cirrhosis patients. (G-I) Correlation between serum GSDMD (G), IL-1ß (H) and IL-18 (I) with APRI score in liver cirrhosis. (J-L) Distribution of GSDMD (J), IL-1ß (K) and IL-18 (L) in compensated and decompensated cirrhosis. *p<0.05; **p<0.01; ***p<0.001.
Abbreviation: Ns, no statistical significance.
Serum GSDMD, IL-1ß and IL-18 Levels in Liver Cirrhosis Patients Presenting with Various Complications
Cirrhosis can lead to portal hypertension and/or hepatic dysfunction, which induces a variety of complications, including ascites, bleeding esophageal varices (BEV), spontaneous bacterial peritonitis (SBP) and hepatic encephalopathy (HE).20 We next monitored and analyzed the pyroptosis-related indicators GSDMD, IL-1ß and IL-18 in liver cirrhosis patients with diagnosed various complications. For liver cirrhosis patients with diagnosed ascites or BEV, serum levels of IL-1ß and IL-18, but not GSDMD, were significantly increased (Table 3). All the three indexes showed no statistically significant change in patients with diagnosed HE (Table 3). In particular, all three indexes were prominently increased in patients with diagnosed SBP (Table 3). Since SBP only occurs in the setting of ascites, the levels of these three indexes in SBP group were compared to that in ascites group. Serum levels of GSDMD, but not IL-1ß and IL-18 were significantly increased in SBP group (Table 3).
Table 3.
Serum Levels of GSDMD, IL-1ß and IL-18 in Patients with Liver Cirrhosis with Complications
| GSDMD | p value | IL-1ß | p value | IL-18 | p value | |
|---|---|---|---|---|---|---|
| LC (Ascites) | ||||||
| - (n=48) | 48.5 (48.47) | p=0.32 | 59.98 (163.58) | p =0.0111 | 99.63 (227.55) | p=0.0104 |
| + (n=16) | 44.29 (31.53) | 159 (268.26) | 294.7 (429.3) | |||
| LC (BEV) | ||||||
| - (n=48) | 48.5 (48.47) | p=0.1299 | 59.98 (163.58) | p =0.0250 | 99.63 (227.55) | p=0.0008 |
| + (n=29) | 38.88 (49.59) | 143.4 (218.81) | 327.5 (425.57) | |||
| LC (SBP) | ||||||
| - (n=48) | 48.5 (48.47) | p=0.0006 | 59.98 (163.58) | p<0.0001 | 99.63 (227.55) | p<0.0001 |
| + (n=42) | 74.13 (47.45) | 282.1 (308.7) | 516.9 (526.6) | |||
| LC (HE) | ||||||
| - (n=48) | 48.5 (48.47) | p=0.0658 | 59.98 (163.58) | p=0.2129 | 99.63 (227.55) | p=0.4357 |
| +(n=16) | 78.88 (58.78) | 80.36 (238.68) | 175.2 (460.28) | |||
| LC (Ascites vs SBP) | ||||||
| Ascites (n=16) | 44.29 (31.53) | p=0.0001 | 159 (268.26) | p=0.09 | 294.7 (429.3) | p=0.18 |
| SBP (n=42) | 74.13 (47.45) | 282.1 (308.7) | 516.9 (526.6) |
Notes: Data are presented as median (interquartile range).
Abbreviations: BEV, bleeding esophageal varices; SBP, spontaneous bacterial peritonitis; HE, hepatic encephalopathy.
The Clinical Predictive Power of GSDMD, IL-1ß and IL-18 in Liver Cirrhosis Patients Presenting with Various Complications
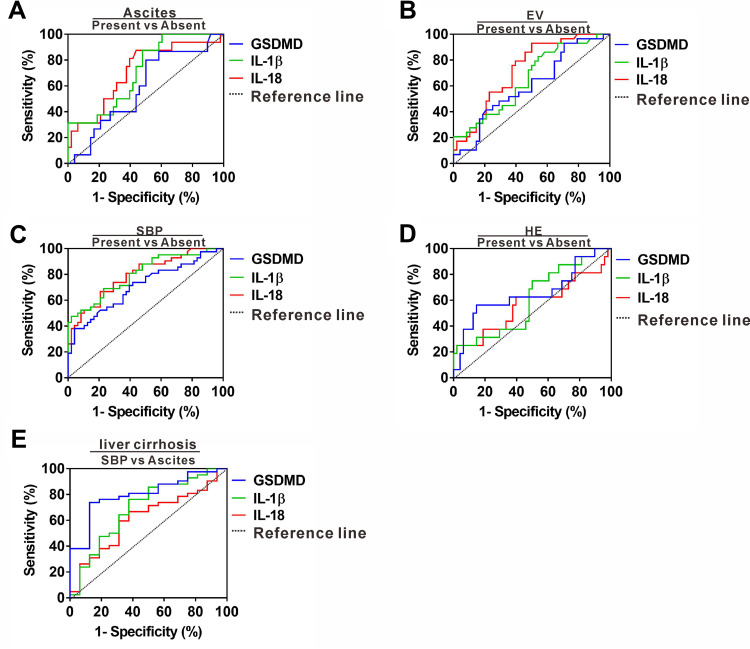
A sizable proportion of liver cirrhosis is frequently asymptomatic or unsuspected until various complications emerge, leading to misdiagnosis, inaccurate disease assessment and missing the treatable stage.21 We next evaluated the predictive power of GSDMD, IL-1ß and IL-18 for liver cirrhosis presenting with complications. ROC analysis revealed that GSDMD, IL-1ß and IL-18 yielded AUC of 0.59 (95% CI, 0.42–0.74), 0.71 (95% CI, 0.57–0.84) and 0.71 (95% CI, 0.56–0.85) for predicting liver cirrhosis presenting with ascites (Figure 4A), indicating that all three indexes had no better diagnostic efficacy for patients with ascites occurrence. In addition, the three indexes yielded AUCs of 0.61 (95% CI, 0.47–0.73), 0.65 (95% CI, 0.52–0.77) and 0.72 (95% CI, 0.61–0.83) for predicting liver cirrhosis presenting with BEV (Figure 4B), suggesting that the three indexes did not exhibit effective differential abilities in liver patients with BEV occurrence. No better diagnostic efficacy was also found by using these three indexes for distinguishing patients presenting with HE occurrence, showing AUCs of 0.65 (95% CI, 0.48–0.82), 0.60 (95% CI, 0.44–0.76) and 0.56 (95% CI, 0.38–0.75) (Figure 4C). We further evaluated their predictive power in patients presenting with SBP occurrence. GSDMD, IL-1ß and IL-18 yielded AUCs of 0.70 (95% CI, 0.60–0.81), 0.81 (95% CI, 0.71–0.89) and 0.79 (95% CI, 0.70–0.88), in which IL-1ß had the highest AUC with 70% sensitivity and 77.1% specificity with a cutoff value 208.7 pg/mL, suggesting that IL-1ß efficiently distinguishs liver cirrhosis patients in SBP occurrence (Figure 4D). Since SBP clinically occurs in the setting of ascites, we further analyzed whether these three indexes can distinguish SBP from ascites. GSDMD, IL-1ß and IL-18 yielded AUCs of 0.80 (95% CI, 0.68–0.92), 0.69 (95% CI, 0.53–0.85) and 0.61 (95% CI, 0.45–0.77), in which GSDMD had the highest AUC with 73.81% sensitivity and 87.5% specificity with a cutoff value 53.55 ng/mL, suggesting that GSDMD efficiently differentiates patients with ascites in SBP occurrence (Figure 4E).
Figure 4.
Differentiating power of serum GSDMD, IL-1ß and IL-18 for liver cirrhosis with various complications. (A) ROC curves of serum GSDMD, IL-1ß and IL-18 for predicting liver cirrhosis with ascites. (B) ROC curves of serum GSDMD, IL-1ß and IL-18 for predicting liver cirrhosis with EV. (C) ROC curves of serum GSDMD, IL-1ß and IL-18 for predicting liver cirrhosis with HE. (D) ROC curves of serum GSDMD, IL-1ß and IL-18 for predicting liver cirrhosis with SBP. (E) ROC curves of serum GSDMD, IL-1ß and IL-18 for predicting SBP occurrence in the setting of ascites for liver cirrhosis patient.
Discussion
Liver cirrhosis is characterized by the replacement of functional liver tissue with nonfunctional scar tissue due to long-term damage and inflammation. A sizable proportion of liver cirrhosis patients never come to clinical attention, leading to misdiagnosis and missing the treatable stage. Although several noninvasive serological tests have achieved great progress for cirrhosis diagnosis and evaluation, none meets the criteria for an ideal surrogate fibrosis or cirrhosis marker. Given that a pivotal role of pyroptosis has been recently identified in liver cirrhosis, we focused on pyroptosis-related indicators and assessed their relationship with cirrhosis severity, aiming to determine whether they represent useful biomarkers for monitoring cirrhosis progression. Herein, liver cirrhosis patients rendered increased levels of the pyroptosis-related indicators GSDMD, IL-1ß and IL-18, all of which were associated with cirrhosis severity, specifically IL-1ß, which efficiently predicted liver cirrhosis in patients presenting with SBP.
Growing evidence indicates that pyroptosis-mediated necroinflammation plays an important role in infection, inflammatory diseases and cancer.22,23 The importance of pyroptosis in liver cirrhosis has also been confirmed in previous mouse model studies.13,16 However, the clinical significance of pyroptosis, such as its predictive power for liver cirrhosis progression, has not been well understood. Here, elevated levels of the pyroptosis-related indicators GSDMD, IL-1ß and IL-18 were confirmed from a well-defined cohort, further supporting the significance of pyroptosis in liver cirrhosis. Recently, pyroptosis-mediated necroinflammation has also been reported in other fibrosis-associated diseases, including cardiovascular fibrosis,24 pulmonary fibrosis,25 and kidney fibrosis.26 Consistent with these fibrosis-related studies, pyroptosis-mediated necroinflammation may represent a common feature for mediating fibrogenesis following tissue injury, providing a new intervention target for liver cirrhosis therapy.
Cirrhosis can arise due to viral infection, autoimmunity, alcohol consumption, NAFLD and other rare inherited liver diseases. Our present study demonstrated increased levels of pyroptosis-related indicators in liver cirrhosis patients with three different etiologies, including viral infection, autoimmunity, and alcohol consumption. These findings may be explained by previous reports that pyroptosis in liver diseases can be activated by multiple stimulants, including pathogen-associated molecular patterns (PAMPs) (eg, viral DNA/RNA and protein),12,27,28 alcohol-induced ROS29 and danger-associated molecular patterns (DAMPs) derived from damaged hepatocytes.12 More and more evidences showed that hepatitis virus (PAMPs) together with DAMPs derived from damaged hepatocytes synergically contributed to the exaggeration of inflammation.30 Here, liver cirrhosis with an etiology of viral infection exhibited higher levels of pyroptosis-related proinflammatory factors IL-1ß and IL-18 than the other two etiologies, suggesting that hepatitis viral-derived PAMPs may exert the strongest stimulatory effect on pyroptosis-related inflammation by synergy with undetermined DAMPs derived from damaged hepatocytes during liver cirrhosis progress. Together, further studies are required to identify and confirm these undetermined DAMPs released from damaged hepatocytes during liver cirrhosis progress.
As the final stage of chronic liver disease, cirrhosis is increasing in importance as a public health problem due to its high morbidity and mortality. Score systems such as Child-Pugh classification and APRI have been widely used for assessing disease severity in liver cirrhosis.3 A positive correlation between pyroptosis-related indicators with these score systems was confirmed in the present data, suggesting that pyroptosis-induced necroinflammation participates in cirrhosis progression. It has been generally accepted that the presence of complications represents a crucial symbolic phase for the elevation of portal hypertension and/or hepatic dysfunction. Here, we demonstrated that the three pyroptosis-related indicators were all significantly increased in complication of SBP. Cirrhosis patients with SBP generally have common symptoms of fever, abdominal pain or tenderness, and altered mental status.30 Laboratory diagnosis primarily depends on ascitic fluid analysis, including ascitic fluid culture and polymorphonuclear leukocyte (PMN) count. Nonetheless, several limitations are still in need of improvement, including diagnosis of asymptomatic patients with subtle clinical symptoms and lower detection rate of ascitic fluid culture method,31–33 leading to inaccurate disease assessment and missing the treatable stage. Herein, we observed better predictive power from circulating IL-1ß with an AUC of 0.81 that generated 70% sensitivity and 77.1% specificity, suggesting that circulating IL-1ß may represent a potential candidate for predicting cirrhosis in patients presenting with SBP. Since SBP only occurs in the setting of ascites, we then evaluate whether these indexes can distinguish SBP from ascites. We observed a better power for GSDMD in differentiating SBP occurrence with an AUC of 0.80 that generated 73.81% sensitivity and 87.5% specificity.
Multiple factors such as gastrointestinal stasis, intestinal bacterial overgrowth, delayed intestinal transport, altered intestinal permeability and immune dysfunction, are involved in the pathogenesis of SBP.34 Among these factors, bacterial translocation and infection represent the major cause of SBP occurrence.35 It is widely appreciated that intestinal bacterial infection can elicit pyroptosis and mediate necroinflammation.36 Therefore, we speculate that pyroptosis in SBP might be elicited not only by liver-resident cells but also by intestinal epithelial cells or circulating peripheral inflammatory cells, which requires verification by future pathogenic studies.
Conclusion
In conclusion, the present study demonstrated that liver cirrhosis patients exhibited increased levels of the pyroptosis-related indicators GSDMD, IL-1ß and IL-18, all of which were positively correlated with disease severity. More importantly, circulating IL-1ß and GSDMD levels exerted valuable differentiating power in liver cirrhosis patients presenting with SBP, suggesting that monitoring circulating IL-1ß and GSDMD may facilitate decision-making regarding prophylaxis and treatment ultimately leading to a lower incidence of SBP in liver cirrhosis.
Funding Statement
Funding for this study was provided by the National Natural Science Foundation of China (Grant No. 82072364), Natural Science Foundation of Chongqing (Grant No. cstc2019jcyj-msxmX0859), Chongqing Health Commission (no. 2019QNXM028) and Kuanren Talents Program of the second affiliated hospital of Chongqing Medical University.
Abbreviations
GSDMD, gasdermin D; SBP, spontaneous bacterial peritonitis; ROC, receiver operating characteristic; AUC, area under the ROC curve; ECM, extracellular matrix; APRI, AST-to-Platelet Ratio Index; HCs, healthy controls; IQR, interquartile range; BEV, bleeding esophageal varices; HE, hepatic encephalopathy; PMN, polymorphonuclear leukocyte.
Ethics Approval Statement
Patients consented to serum acquisition per institutional review board (IRB)-approved protocol. All participants were informed as to the purpose of the study. This study was approved by the Institutional Ethics Committee for human studies at the Second Affiliated Hospital of Chongqing Medical University (no.2019-295). All procedures followed the Declaration of Helsinki.
Authorship Contributions
LD and WC contributed to the conception and design of the study. RW, YY and LD coordinated the investigation of subjects’ sample analysis. DW and XZ performed the experiments. LD drafted the manuscript. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval for the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mazumder NR, Celaj S, Atiemo K, et al. Liver-related mortality is similar among men and women with cirrhosis. J Hepatol. 2020. doi: 10.1016/j.jhep.2020.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crismale JF, Friedman SL. Acute Liver Injury and Decompensated Cirrhosis. Med Clin North Am. 2020;104(4):647–662. doi: 10.1016/j.mcna.2020.02.010 [DOI] [PubMed] [Google Scholar]
- 3.Haj M, Rockey DC. Predictors of clinical outcomes in cirrhosis patients. Curr Opin Gastroenterol. 2018;34(4):266–271. doi: 10.1097/MOG.0000000000000450 [DOI] [PubMed] [Google Scholar]
- 4.Lurie Y, Webb M, Cytter-Kuint R, Shteingart S, Lederkremer GZ. Non-invasive diagnosis of liver fibrosis and cirrhosis. World j Gastroenterol. 2015;21(41):11567–11583. doi: 10.3748/wjg.v21.i41.11567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iredale JP, Thompson A, Henderson NC. Extracellular matrix degradation in liver fibrosis: biochemistry and regulation. Biochim Biophys Acta. 2013;1832(7):876–883. doi: 10.1016/j.bbadis.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 6.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. doi: 10.1002/path.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017;127(1):55–64. doi: 10.1172/JCI88881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59(3):583–594. doi: 10.1016/j.jhep.2013.03.033 [DOI] [PubMed] [Google Scholar]
- 9.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7(2):99–109. doi: 10.1038/nrmicro2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuk JM, Silwal P, Jo EK. Inflammasome and Mitophagy Connection in Health and Disease. Int J Mol Sci. 2020;21(13). doi: 10.3390/ijms21134714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong W, Shi Y, Ren J. Research progresses of molecular mechanism of pyroptosis and its related diseases. Immunobiology. 2020;225(2):151884. doi: 10.1016/j.imbio.2019.11.019 [DOI] [PubMed] [Google Scholar]
- 12.Al Mamun A, Wu Y, Jia C, et al. Role of pyroptosis in liver diseases. Int Immunopharmacol. 2020;84:106489. doi: 10.1016/j.intimp.2020.106489 [DOI] [PubMed] [Google Scholar]
- 13.Wree A, Eguchi A, McGeough MD, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59(3):898–910. doi: 10.1002/hep.26592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaping Z, Ying W, Luqin D, Ning T, Xuemei A, Xixian Y. Mechanism of interleukin-1beta-induced proliferation in rat hepatic stellate cells from different levels of signal transduction. APMIS. 2014;122(5):392–398. doi: 10.1111/apm.12155 [DOI] [PubMed] [Google Scholar]
- 15.Khan F, Peltekian KM, Peterson TC. Effect of interferon-alpha, ribavirin, pentoxifylline, and interleukin-18 antibody on hepatitis C sera-stimulated hepatic stellate cell proliferation. J Interferon Cytokine Res. 2008;28(11):643–651. doi: 10.1089/jir.2007.0123 [DOI] [PubMed] [Google Scholar]
- 16.Mridha AR, Wree A, Robertson AAB, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol. 2017;66(5):1037–1046. doi: 10.1016/j.jhep.2017.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon LJ, Berk M, Thapaliya S, Papouchado BG, Feldstein AE. Caspase-1-mediated regulation of fibrogenesis in diet-induced steatohepatitis. Lab Invest. 2012;92(5):713–723. doi: 10.1038/labinvest.2012.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durand F, Valla D. Assessment of the prognosis of cirrhosis: child-Pugh versus MELD. J Hepatol. 2005;42 Suppl(1):S100–7. doi: 10.1016/j.jhep.2004.11.015 [DOI] [PubMed] [Google Scholar]
- 19.Loaeza-del-Castillo A, Paz-Pineda F, Oviedo-Cardenas E, Sanchez-Avila F, Vargas-Vorackova F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann Hepatol. 2008;7(4):350–357. [PubMed] [Google Scholar]
- 20.Snyder SR, KivIehan SM, Collopy KT. Cirrhosis and Its Complications. Catch this liver scarring problem early, because its effects can be life-threatening. EMS World. 2015;44(10):32–37. [PubMed] [Google Scholar]
- 21.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371(9615):838–851. doi: 10.1016/S0140-6736(08)60383-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia X, Wang X, Zheng Y, Jiang J, Hu J. What role does pyroptosis play in microbial infection? J Cell Physiol. 2019;234(6):7885–7892. doi: 10.1002/jcp.27909 [DOI] [PubMed] [Google Scholar]
- 23.Xia X, Wang X, Cheng Z, et al. The role of pyroptosis in cancer: pro-cancer or pro-”host”? Cell Death Dis. 2019;10(9):650. doi: 10.1038/s41419-019-1883-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinar AA, Scott TE, Huuskes BM, Tapia Caceres FE, Kemp-Harper BK, Samuel CS. Targeting the NLRP3 inflammasome to treat cardiovascular fibrosis. Pharmacol Ther. 2020;209:107511. doi: 10.1016/j.pharmthera.2020.107511 [DOI] [PubMed] [Google Scholar]
- 25.Sayan M, Mossman BT. The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part Fibre Toxicol. 2016;13(1):51. doi: 10.1186/s12989-016-0162-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorenz G, Darisipudi MN, Anders HJ. Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrology Dialysis Transplantation. 2014;29(1):41–48. doi: 10.1093/ndt/gft332 [DOI] [PubMed] [Google Scholar]
- 27.Negash AA, Olson RM, Griffin S, Gale M. Modulation of calcium signaling pathway by hepatitis C virus core protein stimulates NLRP3 inflammasome activation. PLoS Pathog. 2019;15(2):e1007593. doi: 10.1371/journal.ppat.1007593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie WH, Ding J, Xie XX, et al. Hepatitis B virus X protein promotes liver cell pyroptosis under oxidative stress through NLRP3 inflammasome activation. Inflammation Res. 2020;69(7):683–696. doi: 10.1007/s00011-020-01351-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khanova E, Wu R, Wang W, et al. Pyroptosis by caspase 11/4-gasdermin-D pathway in alcoholic hepatitis in mice and patients. Hepatology. 2018;67(5):1737–1753. doi: 10.1002/hep.29645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Q, Shi Y, Yang Y, Lou G, Chen Z. The sterile inflammation in the exacerbation of HBV-associated liver injury. Mediators Inflamm. 2015;2015:508681. doi: 10.1155/2015/508681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lata J, Stiburek O, Kopacova M. Spontaneous bacterial peritonitis: a severe complication of liver cirrhosis. World j Gastroenterol. 2009;15(44):5505–5510. doi: 10.3748/wjg.15.5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribeiro TC, Chebli JM, Kondo M, Gaburri PD, Chebli LA, Feldner AC. Spontaneous bacterial peritonitis: how to deal with this life-threatening cirrhosis complication? Ther Clin Risk Manag. 2008;4(5):919–925. doi: 10.2147/tcrm.s2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riggio O, Angeloni S. Ascitic fluid analysis for diagnosis and monitoring of spontaneous bacterial peritonitis. World j Gastroenterol. 2009;15(31):3845–3850. doi: 10.3748/wjg.15.3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li YT, Huang JR, Peng ML. Current Status and Prospects of Spontaneous Peritonitis in Patients with Cirrhosis. Biomed Res Int. 2020;2020:3743962. doi: 10.1155/2020/3743962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellot P, Frances R, Such J. Pathological bacterial translocation in cirrhosis: pathophysiology, diagnosis and clinical implications. Liver Int. 2013;33(1):31–39. doi: 10.1111/liv.12021 [DOI] [PubMed] [Google Scholar]
- 36.Zhou CB, Fang JY. The role of pyroptosis in gastrointestinal cancer and immune responses to intestinal microbial infection. Biochimica Et Biophysica Acta Rev Cancer. 2019;1872(1):1–10. doi: 10.1016/j.bbcan.2019.05.001 [DOI] [PubMed] [Google Scholar]