Abstract
Background
Randomized clinical trials support deescalation of axillary surgery in breast cancer patients with low-volume axillary disease treated with a surgery-first approach. However, few data exist to guide axillary surgery following neoadjuvant endocrine therapy (NET). Therefore, we evaluated the extent and outcomes of axillary surgery in a contemporary cohort of NET patients, a treatment approach that has become particularly relevant during the coronavirus disease-19 (COVID-19) pandemic.
Patients and Methods
We identified invasive breast cancer patients treated with NET between October 2008 and November 2019. Patients presenting with stage IV disease or recurrent disease were excluded. Statistical analyses were performed using chi-square, Fisher’s exact, and Wilcoxon rank-sum tests.
Results
194 invasive breast cancers in 186 patients (median age 66 years) were evaluated; 81 patients had breast-conserving surgery (BCS), while 113 underwent mastectomy. Eighty-four patients (43.3%) were biopsy-proven cN+ with 4/84 (4.8%) ypN0 following NET. Among cN+ patients, 14 (16.7%) had sentinel lymph node biopsy (SLNB) only, 27 (32.1%) had SLNB + axillary lymph node dissection (ALND), and 43 (51.2%) had ALND. Among 110 cN0 patients, 99 had axillary surgery with 28/99 (28.3%) ypN+: SLNB in 83 (75.5%), SLNB+ALND in 14 (12.7%), and ALND in 2 (1.8%). Among all ypN+ patients, 23/108 (21.3%) had SLNB alone: 18/43 (41.9%) of BCS and 5/65 (7.7%) mastectomy patients (p < 0.001). After median follow-up of 35 months, no regional recurrences were observed.
Conclusions
Among biopsy-proven cN+ NET patients, we observed deescalation of axillary surgery in selected patients, despite a low nodal pathologic complete response (pCR) rate, without nodal recurrences. These data suggest that patients with low-volume axillary disease treated with NET may be managed similarly to patients treated with a surgery-first approach.
Supplementary Information
The online version contains supplementary material available at 10.1245/s10434-021-10385-4.
Hormone-receptor-positive disease is the most common type of breast cancer, accounting for > 80% of newly diagnosed cases.1 For patients with hormone-receptor-positive disease, neoadjuvant endocrine therapy (NET) has been shown to improve breast conservation rates and reduce tumor size, and may downstage axillary disease.2–5 More recently, NET is being tested in clinical trials as a means of assessing short- and longer-term response to endocrine therapy as a potential way to identify which patients might avoid cytotoxic chemotherapy.6–10
For patients with estrogen-receptor-positive (ER+) breast cancer who proceed directly to operation, axillary surgery has evolved considerably following publication of the results of the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial. The ACOSOG Z0011 trial found no clinically significant differences in survival or recurrence for clinically node-negative breast cancer patients treated with sentinel lymph node (SLN) surgery versus axillary lymph node dissection (ALND) with low-volume disease in one or two SLNs and managed with breast-conserving surgery followed by whole-breast irradiation.11,12 Patients in the Z0011 trial were treated with a surgery-first approach, not neoadjuvant therapy, although the majority were prescribed adjuvant endocrine therapy. This approach is supported by similar data from other clinical trials that enrolled patients with early-stage ER+ breast cancer and low axillary disease burden whose first course of treatment was operation.13–15
In an effort to decrease long-term toxicities, especially for patients with favorable disease, there has been a concerted effort to deescalate axillary surgery over the past decade in the context of multidisciplinary care.16 In parallel, efforts to base decisions for chemotherapy in breast cancer patients on biologic tumor features have led to a decrease in administration of cytotoxic chemotherapy for ER+ breast cancer patients, including those with node-positive disease.17 Another approach has been to test the efficacy of endocrine therapy (versus chemotherapy) by administering endocrine therapy prior to surgery, NET. This has been pursued increasingly on and off clinical trials.6,9,10
During the current COVID-19 pandemic, NET has been used as the initial approach to treat ER+ breast cancer when surgical access is restricted due to resource reallocation. As healthcare institutions ramp up operations, surgical demand may again exceed capacity as both newly diagnosed patients and those whose surgery was canceled or deferred present for surgical treatment. In March and April 2020, multiple national organizations rapidly created guidelines to help manage this workflow. An overall consensus emerged suggesting that it was safe for patients with early ER+ operable breast cancer to be treated with NET for 6–12 months prior to definitive operation.18–20 Thus, in this past year, we are seeing a rapid uptick in the number of newly diagnosed ER+ breast cancer patients treated with NET.
Prior studies of NET addressing surgical management have focused most on efficacy of this approach in downstaging disease in the breast to permit breast conservation; however, few data exist on axillary lymph node status and treatment following NET. For patients with low-volume axillary disease, in whom a surgery-first approach is employed, axillary dissection may be avoided if operation identifies two or fewer positive SLNs. Thus, it is important that initiating treatment with NET does not result in more extensive axillary surgery. The aim of this study is to evaluate the impact of NET on axillary surgical treatment, pathologic nodal stage, and cancer outcomes.
Patients and Methods
With institutional review board (IRB) approval, we identified all patients with ER+ breast cancer treated with NET for a minimum of 30 days prior to surgery between October 2008 and November 2019 from our prospective breast surgery registry. Patients presenting with stage IV disease or recurrence, or treated with radiation or any axillary surgery prior to NET were excluded. All patients with invasive breast cancer undergo axillary ultrasound; if abnormal lymph nodes (LNs) are identified, percutaneous biopsy of the most abnormal node is performed. Data were verified by review of the electronic medical record and imaging studies. Data collected included patient characteristics and demographics, clinical and imaging data, duration and agents for NET, details of surgical treatment of the breast and axilla, pre- and posttreatment pathology, adjuvant therapies, and follow-up information regarding disease status, including any local, regional, or distant recurrence. Pathologic nodal positivity was defined as metastasis size > 0.2 mm; patients with either ypN0 or ypN0i+ disease were classified as node negative.
Statistical Analysis
Descriptive statistics for the cohort overall and by clinical nodal (cN) status as reported as median (range) for continuous variables and frequency (percentage) for categorical variables. Comparisons between groups were performed using chi-square or Fisher’s exact tests for nominal variables and Wilcoxon rank-sum tests for ordinal or continuous variables. Staging shift between clinical (pre-NET) and pathologic (post-NET) stage was compared using McNemar’s test for paired proportions, and tumor size estimate from pre-NET imaging was compared with invasive tumor size at surgery using Wilcoxon signed-rank test. Recurrence-free survival (RFS) was defined as time from diagnosis to last follow-up or to an event of either disease recurrence or death. RFS was estimated using the Kaplan–Meier method and is reported with 95% confidence interval (CI), with comparisons between groups performed using log-rank tests. p values < 0.05 were considered statistically significant. Analysis was performed using SAS (version 9.4, SAS Institute, Inc., Cary, NC).
Results
We identified 194 breast cancers (8 bilateral) in 186 patients who were treated with NET followed by surgical resection. Median (range) patient age was 66 (29–96) years. After nodal staging with axillary ultrasound, and fine needle aspiration (FNA) if suspicious findings were identified, 110/194 cancers (56.7%) were cN0 and 84 (43.3%) biopsy-verified cN+ at presentation. Patients most commonly presented with a palpable mass (110/194, 57.6%). Of 195 tumors, 185 were strongly ER+ (> 75% staining by immunohistochemistry), and the majority (192/194, 99.0%) were human epidermal growth factor receptor 2 (HER2) negative. Patient and tumor variables are summarized in Table 1.
Table 1.
Baseline and clinical characteristics of NET patients by clinical N status
| cN0 (N = 110) | cN+ (N = 84) | Total (N = 194) | p value | |
|---|---|---|---|---|
| Age (years) | 0.22 | |||
| Median (range) | 67 (34–96) | 64 (29–84) | 66 (29–96) | |
| Race, n (%) | 0.17 | |||
| White | 97 (88.2%) | 74 (88.1%) | 171 (88.1%) | |
| Black or African American | 1 (0.9%) | 4 (4.8%) | 5 (2.6%) | |
| Asian | 2 (1.8%) | 0 | 2 (1.0%) | |
| American Indian or Alaska Native | 0 | 1 (1.2%) | 1 (0.5%) | |
| Unknown/unreported | 10 (9.1%) | 5 (6.0%) | 15 (7.7%) | |
| Ethnicity, n (%) | ||||
| Not Hispanic or Latino | 99 (90.0%) | 80 (95.2%) | 179 (92.3%) | 0.18 |
| Unknown/unreported | 11 (10.0%) | 4 (4.8%) | 15 (7.7%) | |
| Presentation, n (%) | 0.68 | |||
| Palpable mass | 61 (55.5%) | 49 (58.3%) | 110 (56.7%) | |
| Abnormal imaging | 31 (28.2%) | 25 (29.8%) | 56 (28.9%) | |
| Other | 18 (16.4%) | 10 (11.9%) | 28 (14.4%) | |
| Histology | 0.48 | |||
| IDC | 58 (52.7%) | 51 (60.7%) | 109 (56.2%) | |
| ILC | 38 (34.5%) | 21 (25.0%) | 59 (30.4%) | |
| IMC | 9 (8.2%) | 9 (10.7%) | 18 (9.3%) | |
| Other | 5 (4.5%) | 3 (3.6%) | 8 (4.1%) | |
| Clinical T category, n (%) | 0.21 | |||
| cT1 | 28 (25.5%) | 16 (19.0%) | 44 (22.7%) | |
| cT2 | 52 (47.3%) | 41 (48.8%) | 93 (47.9%) | |
| cT3 | 24 (21.8%) | 19 (22.6%) | 43 (22.2%) | |
| cT4 | 6 (5.5%) | 8 (9.5%) | 14 (7.2%) | |
| Suitable for BCS at presentation, n (%) | 0.85 | |||
| No | 50 (45.5%) | 37 (44.0%) | 87 (44.8%) | |
| Yes | 60 (54.5%) | 47 (56.0%) | 107 (55.2%) | |
| Number of abnormal LNs on axillary ultrasound, n (%) | <0.001 | |||
| 0 | 65 (62.5%) | 1 (1.2%) | 66 (35.1%) | |
| 1 | 26 (25.0%) | 49 (58.3%) | 75 (39.9%) | |
| 2 | 8 (7.7%) | 10 (11.9%) | 18 (9.6%) | |
| > 2 | 5 (4.8%) | 24 (28.6%) | 29 (15.4%) | |
| Missing | 6 | 0 | 6 | |
| Tumor size on imaging (cm) | 0.42 | |||
| Median (range) | 2.6 (0.6–11) | 2.6 (0.9–10) | 2.6 (0.6–11) | |
| N missing | 2 | 1 | 3 | |
| Grade from core biopsy, n (%) | 0.28 | |||
| I | 30 (27.8%) | 21 (25.0%) | 51 (26.6%) | |
| I–II | 6 (5.6%) | 7 (8.3%) | 13 (6.8%) | |
| II | 66 (61.1%) | 45 (53.6%) | 111 (57.8%) | |
| II–III | 2 (1.9%) | 2 (2.4%) | 4 (2.1%) | |
| III | 4 (3.7%) | 9 (10.7%) | 13 (6.8%) | |
| Missing | 2 | 0 | 2 | |
| Ki-67 (%) | 0.11 | |||
| Median | 13 (1–59) | 15 (1–70) | 14 (1–70) | |
| N missing | 36 | 19 | 55 | |
| Ki-67 category | 0.01 | |||
| ≤ 10% | 33 (44.6%) | 16 (24.6%) | 49 (35.3%) | |
| > 10% | 41 (55.4%) | 49 (75.4%) | 90 (67.4%) | |
| Missing | 36 | 19 | 55 | |
| Duration of NET, n (%) | 0.43 | |||
| 4–12 weeks | 16 (14.5%) | 9 (10.7%) | 25 (12.9%) | |
| > 12 weeks | 94 (85.5%) | 75 (89.3%) | 169 (87.1%) | |
| Breast operation, n (%) | 0.54 | |||
| BCS | 48 (43.6%) | 33 (39.3%) | 81 (41.8%) | |
| Mastectomy | 62 (56.4%) | 51 (60.7%) | 113 (58.2%) | |
| Axillary operation, n (%) | < 0.001 | |||
| None | 11 (10.0%) | 0 (0.0%) | 11 (5.7%) | |
| SLN only | 82 (74.5%) | 14 (16.7%) | 96 (49.5%) | |
| SLN + cALND | 15 (13.6%) | 27 (32.1%) | 42 (21.6%) | |
| ALND only | 2 (1.8%) | 43 (51.2%) | 45 (23.2%) | |
| Pathologic node status | < 0.001 | |||
| ypNX | 11 (10.0%) | 0 | 11 (5.7%) | |
| ypN0 | 71 (64.5%) | 4 (4.8%) | 75 (38.7%) | |
| ypN+ | 28 (25.5%) | 80 (95.2%) | 108 (55.7%) | |
| Largest LN metastasis size (mm) among ypN+ subset | <0.001 | |||
| Median (range) | 5 (0.5–30) | 11 (0.3–51) | 10 (0.3–51) | |
| N missing | 2 | 1 | 3 | |
| Number of positive lymph nodes among ypN+ subset | 0.001 | |||
| Median (range) | 1.5 (1–39) | 3.5 (1–35) | 3 (1–39) | |
IDC Invasive ductal carcinoma, ILC Invasive lobular carcinoma, IMC Invasive mammary carcinoma
NET consisted of an aromatase inhibitor (±Zoladex) in 168/186 (90.3%), tamoxifen only in 15 (8.1%), and tamoxifen + Zoladex in 3 (1.6%). Most patients (164/186, 88.2%) underwent long-course (> 12 weeks) NET; the median duration of NET was 25 weeks (interquartile range 21–37 weeks).
Surgical Treatment of Breast
At presentation, 107 patients (55.2%) were considered suitable candidates for breast-conserving surgery (BCS), which was similar among the cN0 and cN+ patients (54.5% versus 56.0%, p = 0.85). After NET, breast operation was BCS in 81/194 (41.8%) and mastectomy in 113 (58.2%). Among the 87 (44.8%) cancers not suitable for BCS at presentation, 19 (21.8%) were ultimately treated with BCS after NET, including 14/50 (28.0%) cN0 patients and 5/37 (13.5%) cN+ patients (p = 0.11). Across all patients, the breast pCR rate was 3.6% (7/194). There was no significant difference between estimated clinical tumor size based on imaging at diagnosis (median 2.6 cm, range 0.6–11 cm) and pathologic invasive tumor size (median 2.4 cm, range 0–13 cm) following NET (p = 0.55). However, tumors were downstaged in a subset of patients: 77.3% (150/194) had clinical T category > cT1 prior to NET compared with 59.3% (115/194) with pathologic T category > ypT1 at operation (p < 0.001).
Surgical Treatment of Axilla
Details of pathology and treatment data by cN category are provided in Tables 2 and 3 and by ypN category in Supplementary Tables 1 and 2. Of patients treated with BCS, 8 (9.9%) had no axillary operation, while 47 (58.0%) had sentinel lymph node biopsy (SLNB) surgery only, 11 (13.6%) SLNB and ALND, and 15 patients (18.5%) ALND. Among patients treated with mastectomy, 3 patients (2.7%) had no axillary operation, 49 (43.4%) had SLNB only, 31 (27.4%) had SLNB and ALND, and 30 (26.5%) had ALND.
Table 2.
Treatment and pathologic characteristics for cN+ patients treated with NET
| BCS (N = 33) | Mastectomy (N = 51) | Total (N = 84) | p value | |
|---|---|---|---|---|
| Axillary operation, n (%) | 0.003 | |||
| SLN only | 11 (33.3%) | 3 (5.9%) | 14 (16.7%) | |
| SLN + cALND | 7 (21.2%) | 20 (39.2%) | 27 (32.1%) | |
| ALND only | 15 (45.5%) | 28 (54.9%) | 43 (51.2%) | |
| Grade, n (%) | 0.23 | |||
| I (well differentiated) | 9 (27.3%) | 19 (37.3%) | 28 (33.3%) | |
| II (moderately differentiated) | 21 (63.6%) | 30 (58.8%) | 51 (60.7%) | |
| III (poorly differentiated) | 3 (9.1%) | 2 (3.9%) | 5 (6.0%) | |
| Histology, n (%) | 0.03 | |||
| IDC | 24 (72.7%) | 27 (52.9%) | 51 (60.7%) | |
| ILC | 3 (9.1%) | 18 (35.3%) | 21 (25.0%) | |
| IMC | 4 (12.1%) | 5 (9.8%) | 9 (10.7%) | |
| Other | 2 (6.1%) | 1 (2.0%) | 3 (3.6%) | |
| Pathologic T category, n (%) | 0.002 | |||
| ypT0/Tis | 1 (3.0%) | 1 (2.0%) | 2 (2.4%) | |
| ypT1 | 18 (54.5%) | 14 (27.5%) | 32 (38.1%) | |
| ypT2 | 12 (36.4%) | 21 (41.2%) | 33 (39.3%) | |
| ypT3 | 2 (6.1%) | 11 (21.6%) | 13 (15.5%) | |
| ypT4 | 0 (0.0%) | 4 (7.8%) | 4 (4.8%) | |
| Primary tumor LVI, n (%) | 0.47 | |||
| Not present | 22 (66.7%) | 37 (74.0%) | 59 (71.1%) | |
| Present | 11 (33.3%) | 13 (26.0%) | 24 (33.3%) | |
| Missing | 0 | 1 | 1 | |
| Ki-67 (%) | 0.01 | |||
| Median (range) | 22.6 (3–56) | 13.9 (1–70) | 15 (1–70) | |
| N missing | 12 | 7 | 19 | |
| Pathologic N category, n (%) | 0.05 | |||
| ypN0 | 1 (3.0%) | 1 (2.0%) | 2 (2.4%) | |
| ypN0(i+) | 1 (3.0%) | 1 (2.0%) | 2 (2.4%) | |
| ypN1mi | 1 (3.0%) | 1 (2.0%) | 2 (2.4%) | |
| ypN1 | 21 (63.6%) | 21 (41.2%) | 42 (50.0%) | |
| ypN2 | 6 (18.2%) | 16 (31.4%) | 22 (26.2%) | |
| ypN3 | 3 (9.1%) | 11 (21.6%) | 14 (16.7%) | |
| Largest LN metastasis size (mm) among ypN+ subset | 0.63 | |||
| Median (range) | 11 (2–37) | 11 (0.3–51) | 11 (0.3–51) | |
| N missing | 0 | 1 | 1 | |
| Extranodal extension among ypN+ subset, n (%) | < 0.001 | |||
| No | 18 (58.1%) | 9 (19.1%) | 27 (34.6%) | |
| Yes | 13 (41.9%) | 38 (80.9%) | 51 (65.4%) | |
| Missing | 0 | 2 | 2 | |
| Number of positive lymph nodes among ypN+ subset | 0.01 | |||
| Median (range) | 2 (1–27) | 4 (1–35) | 3.5 (1–35) | |
| Adjuvant radiation therapy, n (%) | 0.37 | |||
| No | 4 (12.1%) | 10 (19.6%) | 14 (16.7%) | |
| Yes | 29 (87.9%) | 41 (80.4%) | 70 (83.3%) |
Table 3.
Treatment and pathologic characteristics for cN0 patients treated with NET
| BCS (N = 48) | Mastectomy (N = 62) | Total (N = 110) | p value | |
|---|---|---|---|---|
| Axillary operation, n (%) | 0.07 | |||
| None | 8 (16.7%) | 3 (4.8%) | 11 (10.0%) | |
| SLN only | 36 (75.0%) | 46 (74.2%) | 82 (74.5%) | |
| SLN + cALND | 4 (8.3%) | 11 (17.7%) | 15 (13.6%) | |
| ALND only | 0 (0.0%) | 2 (3.2%) | 2 (1.8%) | |
| Grade, n (%) | 0.76 | |||
| I (well differentiated) | 18 (37.5%) | 23 (37.1%) | 41 (37.3%) | |
| II (moderately differentiated) | 25 (52.1%) | 35 (56.5%) | 60 (54.5%) | |
| III (poorly differentiated) | 5 (10.4%) | 4 (6.5%) | 9 (8.2%) | |
| Histology, n (%) | 0.002 | |||
| IDC | 30 (62.5%) | 28 (45.2%) | 58 (52.7%) | |
| ILC | 8 (16.7%) | 30 (48.4%) | 38 (34.5%) | |
| IMC | 6 (12.5%) | 3 (4.8%) | 9 (8.2%) | |
| Other | 4 (8.3%) | 1 (1.6%) | 5 (4.5%) | |
| Pathologic T category, n (%) | 0.02 | |||
| ypT0/Tis | 3 (6.3%) | 2 (3.2%) | 5 (4.5%) | |
| ypT1 | 22 (45.8%) | 19 (30.6%) | 41 (37.3%) | |
| ypT2 | 18 (37.5%) | 20 (32.3%) | 38 (34.5%) | |
| ypT3 | 2 (4.2%) | 19 (30.6%) | 21 (19.1%) | |
| ypT4 | 3 (6.3%) | 2 (3.2%) | 5 (4.5%) | |
| Primary tumor LVI | 0.19 | |||
| Not present | 45 (95.7%) | 55 (88.7%) | 100 (91.7%) | |
| Present | 2 (4.3%) | 7 (11.3%) | 9 (8.3%) | |
| Missing | 1 | 0 | 1 | |
| Ki-67 (%) | 0.22 | |||
| Median (range) | 15 (3–50) | 12 (1–59) | 13 (1–59) | |
| N missing | 15 | 21 | 36 | |
| Pathologic N category, n (%) | 0.13 | |||
| ypNX | 8 (16.7%) | 3 (4.8%) | 11 (10.0%) | |
| ypN0 | 27 (56.3%) | 38 (61.3%) | 65 (59.1%) | |
| ypN0(i+) | 1 (2.1%) | 5 (8.1%) | 6 (5.5%) | |
| ypN1mi | 3 (6.3%) | 3 (4.8%) | 6 (5.5%) | |
| ypN1 | 8 (16.7%) | 11 (17.7%) | 19 (17.3%) | |
| ypN2 | 0 | 1 (1.6%) | 1 (0.9%) | |
| ypN3 | 1 (2.1%) | 1 (1.6%) | 2 (1.8%) | |
| Largest LN metastasis size (mm) among ypN+ subset | 0.26 | |||
| Median (range) | 3.3 (0.5–15) | 5.0 (1.5–30) | 5.0 (0.5–30) | |
| N missing | 0 | 1 | 1 | |
| Extranodal extension among ypN+ subset | 0.25 | |||
| No | 9 (75.0%) | 8 (53.3%) | 17 (63.0%) | |
| Yes | 3 (25.0%) | 7 (46.7%) | 10 (37.0%) | |
| Missing | 0 | 1 | 1 | |
| Number of positive lymph nodes among ypN+ subset | 0.48 | |||
| Median (range) | 1 (1–39) | 2 (1–16) | 1.5 (1–39) | |
| Adjuvant radiation therapy, n (%) | < 0.001 | |||
| No | 8 (16.7%) | 44 (71.0%) | 52 (47.3%) | |
| Yes | 40 (83.3%) | 18 (29.0%) | 58 (52.7%) |
Among 110 cN0 patients, 11 (10.0%) were ypNX (no axillary surgery), 71 (64.5%) were ypN0 at operation, and 28 (25.5%) were ypN+. All ypN0 patients underwent SLN only, while the 28 with positive nodes at surgery were treated with SLN surgery alone in 11 (39.3%), with SLN surgery and ALND in 15 (53.6%), and with ALND in 2 (7.1%). Among the 11 ypN+ patients who had SLNB only, 10/11 (90.9%) had one or two positive SLNs (8 with one positive SLN, 2 with two positive SLNs) and 1/11 had three positive SLNs; 9/11 (81.8%) had no extranodal extension, 4/11 (36.4%) had ypN1mi disease, and none had more than two abnormal LNs identified on pretreatment axillary ultrasound.
Of the 84 cN+ patients, 4 (4.8%) were ypN0 at operation while the remainder were node positive (Fig. 1a). Among the ypN+ patients, 12 (15.0%) were treated with SLN surgery alone, 27 (33.8%) with SLNB and ALND, and 41 (51.3%) with ALND. There were 12 cN+ ypN+ patients treated with SLN surgery alone. The majority (9/12, 75.0%) had one or two positive SLNs, with a minimum of two SLNs examined in all cases and more than two SLNs examined in 9/12 (75.0%). The four biopsy-proven cN+ patients with nodal pCR received NET with aromatase inhibitors (anastrozole in two, letrozole in two) for a median of 222 days (individual durations of 151, 202, 241, and 268 days). Three had cN1 disease, whereas one patient had cN2 disease. On axillary ultrasound at presentation, one patient had one abnormal axillary node, two had two abnormal axillary nodes, and the remaining patient had more than two sonographically abnormal nodes. Three of the four patients had concomitant T category downstaging.
Fig. 1.
a Distribution of ypN category post-NET by clinical nodal status at presentation. b Temporal trends in percentage of patients undergoing SLN surgery only comparing 2008–2013 versus 2014–2020 stratified by clinical node status and breast operation. The change in use of SLNB alone for cN0 BCS patients from the first (2008–2013) to latter time period (2014–2020) was statistically significant: an increase from 72.7% (8/11) to 96.6% (28/29), respectively (p = 0.04)
Among 108 ypN+ patients (combining the cN0 and cN+ cohorts), SLNB alone was performed more frequently for patients treated with BCS than mastectomy (41.9% versus 7.7%, p < 0.001). Trends over time stratified by cN status and breast operation are illustrated in Fig. 1b. A significant change in use of SLNB alone was seen for cN0 BCS patients comparing the first half of the time period (2008–2013) with the latter half (2014–2020), with an increase from 72.7% (8/11) to 96.6% (28/29), respectively (p = 0.04).
Adjuvant Therapy and Outcomes
Adjuvant radiation was administered to 69/81 (85.2%) of BCS patients and 59/113 (52.2%) of mastectomy patients. Endocrine therapy was continued in the adjuvant setting in 180/194 patients (92.8%), while 47/194 (24.2%) received adjuvant chemotherapy.
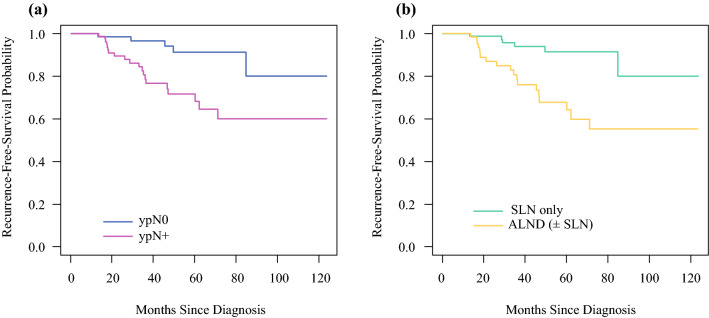
With median follow-up of 35 months, no regional nodal recurrences were observed across the entire patient cohort. Fourteen patients did have a breast cancer recurrence, including four patients with both local and distant recurrence, eight patients with distant recurrence only, and two patients with local recurrence only. A total of 23 patients died during follow-up, of whom 8 died due to breast cancer and 15 due to other causes. For the entire group, RFS was 100% at 1 year, 93% (95% CI: 89–97%) at 2 years, 87% (95% CI: 82–93%) at 3 years, 81% (95% CI: 74–88%) at 4 years, and 80% (95% CI: 72–88%) at 5 years. RFS was substantially better for ypN0 patients than for ypN+ patients (p = 0.003), with 5-year RFS of 91% (95% CI: 83–100%) and 72% (95% CI: 61–85%), respectively (Fig. 2a). Assessed by axillary surgery type, the best RFS outcomes were for patients in the SLN surgery-only group, who had 5-year RFS of 91% (95% CI: 84–99%) (Fig. 2b).
Fig. 2.
Recurrence-free survival by pathologic nodal status post-NET (a) and by axillary surgery type among those undergoing axillary surgery (b). Five-year RFS was substantially better for ypN0 patients (91%, 95% CI: 83–100%) than for ypN+ patients (72%, 95% CI: 61–85%) (p = 0.003) (a). Similarly, five-year RFS was better for patients treated with SLN surgery alone (91%, 95% CI: 84–99%), versus treatment that included ALND (68%: 95% CI 56–83%), (p < 0.001) (b)
Discussion
We report that, among breast cancer patients treated with NET, selective deescalation of axillary surgery can be done safely in a manner that parallels the current approach to patients with similar breast cancers who are treated first with surgery. Among clinically node-negative patients, although 25.5% were ypN+ at operation, only 14.5% were treated with axillary dissection. Among 84 biopsy-proven cN+ patients, NET resulted in nodal pCR in only 4 patients (4.8%), yet 14 (16.7%) were managed without axillary dissection. With this risk-adapted approach to treatment, using clinical judgment and extrapolating from studies addressing management of low-volume axillary disease in breast cancer patients with similar tumor biology whose first course of treatment was surgery, we observed no nodal recurrences after median follow-up of 35 months.
This report, which assesses pathologic response to NET in 84 patients with biopsy-proven nodal disease at presentation, is, to the best of our knowledge, the largest such series to date including postmenopausal women. One clinical trial (NCT01622361) randomized premenopausal women with ER+ histologically proven node-positive breast cancer to neoadjuvant chemotherapy (NAC) versus NET.21 Among 87 women treated with 24 weeks of NET consisting of ovarian function suppression with goserelin + tamoxifen, nodal pCR was noted in 4 patients (4.9%), paralleling the findings of our study. Details of ypN stage and axillary operation were not provided in that publication. Three smaller series have reported on pathologic response to NET for patients with biopsy-proven cN+ disease. Dixon et al. reported in 2011 the results of a trial of neoadjuvant letrozole for patients with invasive lobular carcinoma in which patients were screened with axillary ultrasound and FNA of suspicious nodes.22 There were 28 cN+ patients included, and at operation there were no nodal pCRs. Hammond and colleagues studied 39 biopsy-proven cN+ patients treated with NET between 2012 and 2019, the majority (94%) with cN1 disease.23 After a minimum of 12 weeks (median 18 weeks) of NET, despite a clinical nodal response rate of 77%, only one patient (2.6%) had nodal pCR. The third study evaluating axillary response to NET evaluated 38 patients with biopsy-proven nodal disease at presentation from among 127 cancers treated with NET.24 This study included patients treated on clinical trials testing enzalutamide and taselisib with preoperative endocrine therapy and reported nodal pCR in 4 of 38 cN+ patients (10.5%). The median duration of NET was 6.4 months for the four patients with nodal pCR versus 3.7 months for the cN+ patients without nodal pCR, but this difference did not reach statistical significance (p = 0.10). The sum of these findings is in line with our nodal pCR rate of 4.8% after a median duration of NET of 25 weeks.
While most studies of NET have focused on downstaging the breast tumor to permit BCS or, more recently, to assess response to NET in an effort to determine whether chemotherapy might be avoided, some recent larger-scale reports also have examined the effect of NET on axillary nodal disease and treatment. However, these studies were based on clinical assessment of the regional nodes without cytologic or histologic confirmation of axillary disease. Axillary ultrasound and FNA or core-needle biopsy (CNB) of suspicious nodes is well established as the most accurate approach to axillary staging at presentation, while physical examination noting palpable ipsilateral axillary adenopathy has accuracy of approximately 60%.15,25 Weiss and colleagues evaluated axillary treatment in stage II and III patients treated with NET, neoadjuvant chemotherapy, or a surgery-first approach from the National Cancer Database (NCDB) during 2012–2015.26 They reported nodal pCR in 76 of 571 cN+ patients (13.1%) treated with NET. Unfortunately, the NCDB does not provide information on cytologic or histologic nodal staging prior to treatment, thus this study likely overestimates the frequency of axillary nodal pCR following NET. Similarly, Stafford et al. studied patients from the NCDB during 2010–2016 with ER+/HER2– breast cancers treated with NET for > 30 days and reported a slightly higher axillary nodal pCR rate of 14.48% among 4580 patients staged as cN+.27 They observed a higher likelihood of nodal pCR among patients with invasive ductal carcinoma, cN1 disease (75.9% of the study population), and grade II tumors. In that study, the duration of NET was unknown and patients with metastatic disease were included. Again, as the NCDB does not provide information on how clinical nodal status was determined, it is not surprising that nodal pCR rates from these two studies evaluating similar patient populations from the same observational database are higher than reported herein and the three NET studies discussed above in which clinical node positivity was determined by percutaneous biopsy at time of diagnosis. Further, we provide data on regional disease control, which is lacking from these two larger studies. A third study, also evaluating NCDB data, found no difference in overall survival in NET patients versus a matched upfront-surgery patient cohort stratified by ypN stage.28 The authors suggested that those data supported a similar surgical approach to axillary operation for NET patients as for patients whose first course of treatment is operation.
In the current study, the breast pCR rate across all patients was 3.6%, but among the 87 cancers not suitable for BCS at presentation (44.8%), 19 (21.8%) were ultimately treated with BCS following NET. Similarly, while we found no difference in tumor size estimates at presentation (considering the largest linear diameter across both clinical and imaging examinations) versus pathologic tumor size at operation, we did see a shift from > cT1 disease at presentation to ypT1 disease in 23% of patients. Prior studies of NET report widely varying conversion rates from unsuitable to suitable for BCS, ranging up to 80%.5,22,24 This metric is determined by a number of variables, including study patient inclusion criteria such as T category at diagnosis, tumor location within the breast, presence of multifocal or multicentric disease, ratio of tumor to breast size, and the inherently subjective assessment of acceptable cosmesis. Duration of NET also influences breast tumor downstaging rates. This is illustrated in a study of NET with exemestane in which the BCS rate increased by ~ 10% when the duration of preoperative treatment increased from 3–6 months in responding patients,29 and another testing letrozole, in which the median duration of treatment needed to downstage tumors sufficiently to permit BCS was 7.5 months.30 A similar approach to extending therapy in responders might be explored as a future strategy for downstaging axillary nodal disease.
Historically, NET was reserved for patients unsuitable for surgical treatment of their hormone-receptor-positive breast cancer. More recently, greater attention has been focused on NET as a strategy to downstage the breast tumor to permit breast-conserving surgery and to define the optimal approach to systemic therapy, particularly in postmenopausal women. Clinical trials, as well as retrospective series, have demonstrated the safety of this approach as well as the utility of NET in facilitating breast-conserving surgery in patients with larger tumors.5,22 Currently, trials such as Alliance A011106 ALTERNATE are expanding upon prior efforts to test a biomarker-driven strategy using NET to identify breast cancer patients unlikely to benefit from chemotherapy.6,9 While prior studies have shown low, albeit slowly increasing, uptake of NET for patients with ER+ breast cancer, interest in this approach has been greatly accelerated by the COVID-19 pandemic and the rapid creation and adoption of guidelines for use of NET when surgery must be postponed.4,18,27
Limitations of this study include its retrospective design without uniform selection criteria for NET, although patients were prospectively accrued. Additionally, longer-term follow-up will be important to further characterize oncologic outcomes given that ER+ breast cancers generally recur later than other biologic subtypes. Strengths of this work include that patients were all triaged with axillary ultrasound with FNA of suspicious nodes, providing an accurate cN stage that is absent from most other studies evaluating NET, as well as the granular data we provide on imaging, pathology, and clinical course among this contemporary patient cohort.
Anticipating a considerable uptick in the number of NET patients this year than previously because of the pandemic, our study shows the safety of this approach, as well as potential benefits in terms of improving suitability and feasibility of BCS and deescalating axillary surgery without the toxicities of chemotherapy in appropriately selected patients. We propose that, just as breast pCR is not required for deescalating breast surgery, axillary nodal pCR is not necessarily required to deescalate axillary surgery following NET in appropriately selected patients with ER+ breast cancer. Data from the ACOSOG Z0011 and IBCSG 23-01 trials showing a low risk of locoregional recurrence suggest that these patients are adequately managed without axillary dissection or axillary-directed radiation therapy, whereas the AMAROS trial suggests axillary radiation can be substituted for axillary dissection for patients with one or two positive sentinel nodes.11,13,14 Thus, we suggest that NET patients might be managed appropriately by adopting an approach similar to the one we currently take for patients with similar biology tumors treated with a surgery-first approach. For patients with one or two positive sentinel nodes at operation, whether staged as cN1 disease based on axillary ultrasound findings at diagnosis or not revealed until operation following NET, we propose a selective approach to completion axillary dissection based on multidisciplinary input and consideration of patient and tumor features. Future efforts that focus on refining patient selection for NET, augmenting the response to endocrine therapy, and further tailoring multidisciplinary treatment of ER+ breast cancer patients are warranted both to improve oncologic outcomes and to minimize the morbidities of treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Disclosures
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li Y, Yang D, Yin X, et al. Clinicopathological characteristics and breast cancer-specific survival of patients with single hormone receptor-positive breast cancer. JAMA Netw Open. 2020;3(1):e1918160. doi: 10.1001/jamanetworkopen.2019.18160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aihara T, Munakata S, Morino H, Takatsuka Y. Feasibility of sentinel node biopsy for breast cancer after neoadjuvant endocrine therapy: a pilot study. J Surg Oncol. 2004;85(2):77–81. doi: 10.1002/jso.20010. [DOI] [PubMed] [Google Scholar]
- 3.Charehbili A, Fontein DB, Kroep JR, et al. Neoadjuvant hormonal therapy for endocrine sensitive breast cancer: a systematic review. Cancer Treat Rev. 2014;40(1):86–92. doi: 10.1016/j.ctrv.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Chiba A, Hoskin TL, Heins CN, et al. Trends in neoadjuvant endocrine therapy use and impact on rates of breast conservation in hormone receptor-positive breast cancer: a National Cancer Data Base study. Ann Surg Oncol. 2017;24(2):418–424. doi: 10.1245/s10434-016-5585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol. 2011;29(17):2342–2349. doi: 10.1200/JCO.2010.31.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis MJ, Suman V, Leitch AM, et al. Abstract PD2-10: validation of a predictive model for potential response to neoadjuvant endocrine therapy (NET) in postmenopausal women with clinical stage II or III estrogen receptor positive (ER+) and HER2 negative (HER2-) breast cancer (BC): an ALTERNATE trial analysis (Alliance A011106) Cancer Res. 2021;81(Suppl 4):PD2–PD10. [Google Scholar]
- 7.Ma CX, Gao F, Luo J, et al. NeoPalAna: Neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin Cancer Res. 2017;23(15):4055–4065. doi: 10.1158/1078-0432.CCR-16-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma CX, Suman V, Goetz MP, et al. A Phase II Trial of Neoadjuvant MK-2206, an AKT inhibitor, with anastrozole in clinical stage II or III PIK3CA-mutant ER-positive and HER2-negative breast cancer. Clin Cancer Res. 2017;23(22):6823–6832. doi: 10.1158/1078-0432.CCR-17-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma CX, Suman VJ, Leitch AM, et al. ALTERNATE: Neoadjuvant endocrine treatment (NET) approaches for clinical stage II or III estrogen receptor-positive HER2-negative breast cancer (ER+ HER2− BC) in postmenopausal (PM) women: alliance A011106. J Clin Oncol. 2020;38(Suppl 15):504–504. doi: 10.1200/JCO.2020.38.15_suppl.504. [DOI] [Google Scholar]
- 10.Reinert T, Gonçalves R, Ellis MJ. Current status of neoadjuvant endocrine therapy in early stage breast cancer. Curr Treat Options Oncol. 2018;19(5):23. doi: 10.1007/s11864-018-0538-9. [DOI] [PubMed] [Google Scholar]
- 11.Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318(10):918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23–01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14(4):297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hieken TJ, Trull BC, Boughey JC, et al. Preoperative axillary imaging with percutaneous lymph node biopsy is valuable in the contemporary management of patients with breast cancer. Surgery. 2013;154(4):831–840. doi: 10.1016/j.surg.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Haffty BG, McCall LM, Ballman KV, et al. Impact of radiation on locoregional control in women with node-positive breast cancer treated with neoadjuvant chemotherapy and axillary lymph node dissection: results from ACOSOG Z1071 clinical trial. Int J Radiat Oncol Biol Phys. 2019;105(1):174–182. doi: 10.1016/j.ijrobp.2019.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurian AW, Bondarenko I, Jagsi R, et al. Recent trends in chemotherapy use and oncologists’ treatment recommendations for early-stage breast cancer. J Natl Cancer Inst. 2018;110(5):493–500. doi: 10.1093/jnci/djx239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartlett DL, Howe JR, Chang G, et al. Management of cancer surgery cases during the COVID-19 pandemic: considerations. Ann Surg Oncol. 2020;27(6):1717–1720. doi: 10.1245/s10434-020-08461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Society of Surgical Oncology. Resource for Management Options of Breast Cancer During COVID-19. 2020. https://www.surgonc.org/wp-content/uploads/2020/03/Breast-Resource-during-COVID-19-3.30.20.pdf. Accessed 2 March 2021.
- 20.The American Society of Breast Surgeon. ASBrS Resource Guide to Endocrine Therapy in the COVID-19 Pandemic. 2020. https://www.breastsurgeons.org/docs/statements/ASBrS-Resource-Guide-on-Endocrine-Therapy-in-the-COVID-19-Pandemic.pdf.v1. Accessed 2 March 2021.
- 21.Kim HJ, Noh WC, Lee ES, et al. Efficacy of neoadjuvant endocrine therapy compared with neoadjuvant chemotherapy in pre-menopausal patients with oestrogen receptor-positive and HER2-negative, lymph node-positive breast cancer. Breast Cancer Res. 2020;22(1):54. doi: 10.1186/s13058-020-01288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixon JM, Renshaw L, Dixon J, Thomas J. Invasive lobular carcinoma: response to neoadjuvant letrozole therapy. Breast Cancer Res Treat. 2011;130(3):871–877. doi: 10.1007/s10549-011-1735-4. [DOI] [PubMed] [Google Scholar]
- 23.Hammond JB, Parnall TH, Scott DW, et al. Gauging the efficacy of neoadjuvant endocrine therapy in breast cancer patients with known axillary disease. J Surg Oncol. 2020 doi: 10.1002/jso.26047. [DOI] [PubMed] [Google Scholar]
- 24.Montagna G, Sevilimedu V, Fornier M, et al. How effective is neoadjuvant endocrine therapy (NET) in downstaging the axilla and achieving breast-conserving surgery? Ann Surg Oncol. 2020;27(12):4702–4710. doi: 10.1245/s10434-020-08888-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hieken TJ. The promise of axillary imaging in individualized surgical management of breast cancer patients: another step forward. Ann Surg Oncol. 2014;21(11):3369–3371. doi: 10.1245/s10434-014-3853-9. [DOI] [PubMed] [Google Scholar]
- 26.Weiss A, Wong S, Golshan M, et al. Patterns of axillary management in stages 2 and 3 hormone receptor-positive breast cancer by initial treatment approach. Ann Surg Oncol. 2019;26(13):4326–4336. doi: 10.1245/s10434-019-07785-y. [DOI] [PubMed] [Google Scholar]
- 27.Stafford A, Williams A, Edmiston K, et al. Axillary response in patients undergoing neoadjuvant endocrine treatment for node-positive breast cancer: systematic literature review and NCDB analysis. Ann Surg Oncol. 2020;27(12):4669–4677. doi: 10.1245/s10434-020-08905-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kantor O, Wong S, Weiss A, Metzger O, Mittendorf EA, King TA. Prognostic significance of residual nodal disease after neoadjuvant endocrine therapy for hormone receptor-positive breast cancer. npj Breast Cancer. 2020;6:35. doi: 10.1038/s41523-020-00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fontein DB, Charehbili A, Nortier JW, et al. Efficacy of six month neoadjuvant endocrine therapy in postmenopausal, hormone receptor-positive breast cancer patients–a phase II trial. Eur J Cancer. 2014;50(13):2190–2200. doi: 10.1016/j.ejca.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Carpenter R, Doughty JC, Cordiner C, et al. Optimum duration of neoadjuvant letrozole to permit breast conserving surgery. Breast Cancer Res Treat. 2014;144(3):569–576. doi: 10.1007/s10549-014-2835-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.