Abstract
Breeding has been used successfully for many years in the fruit industry, giving rise to most of today’s commercial fruit cultivars. More recently, new molecular breeding techniques have addressed some of the constraints of conventional breeding. However, the development and commercial introduction of such novel fruits has been slow and limited with only five genetically engineered fruits currently produced as commercial varieties—virus-resistant papaya and squash were commercialized 25 years ago, whereas insect-resistant eggplant, non-browning apple, and pink-fleshed pineapple have been approved for commercialization within the last 6 years and production continues to increase every year. Advances in molecular genetics, particularly the new wave of genome editing technologies, provide opportunities to develop new fruit cultivars more rapidly. Our review, emphasizes the socioeconomic impact of current commercial fruit cultivars developed by genetic engineering and the potential impact of genome editing on the development of improved cultivars at an accelerated rate.
Subject terms: Metabolic engineering, Molecular engineering in plants
Introduction
The conventional breeding of fruit crops can take more than two decades due to the long juvenile period of woody species1. Genetic engineering allows improved varieties to be developed more quickly, and the vegetative propagation of fruit trees allows the engineered cultivars to achieve coverage of larger areas than crops that depend on sexual reproduction2. All genetically engineered fruit crops have been produced either by Agrobacterium-mediated transformation or direct DNA transfer. In each case, the efficiency of transformation is highly dependent on the species and even cultivar, requiring the development of bespoke optimized methods consisting of efficient gene delivery, selection, and regeneration from transformed explants2. Most fruit tree species are highly heterozygous, and to maintain the characteristics of the original variety the transgenic events should be derived from mature tissue (such as leaves) rather than embryogenic explants3.
The first genetically engineered fruit product (Flavr Savr™ tomato) was deregulated in 1992 and introduced into the market in 19944. A gene that triggers pectin solubilization was downregulated in the transgenic fruits, resulting in delayed fruit softening and an extended shelf-life5. Several additional fruit crops with traits improved by genetic engineering have received regulatory approval for commercialization in different parts of the world, and are intended for cultivation either as human food or animal feed. These are tomato (Solanum lycopersicum)6–9, papaya (Carica papaya L.)10,11, pepper (Capsicum annuum)12, plum (Prunus domestica)13, eggplant (Solanum melongena L.)14, apple (Malus domestica Borkh.)15, melon (Cucumis melo L.)16, and pineapple (Ananas comosus L. Merr.)17. Most of the transgenic fruits were developed to improve agronomic productivity by conferring pest or disease resistance, or delayed ripening. However, more recent products have addressed quality traits by eliminating fruit browning or adding new visual traits such as flesh color. Some engineered fruit crops have been withdrawn from the market because they were not commercially viable (Flavr Savr™ tomato4,18) or were never commercialized (Melon A and B16,19).
Advances in genetic engineering, particularly the development of genome editing technologies have provided new tools for the generation of improved fruit varieties. Many proof-of-concept examples involving fruit crops have been reported and the further development and marketing of such varieties could have a major socioeconomic impact. Here we discuss the history and current status of genetically engineered fruit crops and the promise offered by genome editing. In recent years, several countries have amended their current regulations or have developed new guidelines to regulate genome-edited plants and its products20. This may make it possible that genome-edited fruits, similarly to all other genome-edited crops, reach the market faster in countries with a genome editing friendly policy20,21. Here, we first discuss fruit varieties that have already been approved for commercialization, focusing on those that are on the market. We then consider fruit varieties developed more recently using genetic engineering or genome editing, and their potential socioeconomic impact.
Genetically engineered fruits approved for commercialization
Trait description and drivers
Genetically engineered fruits have been developed with unique agronomic characteristics that are often difficult to achieve by conventional breeding, and are designed to meet the specific needs of growers and/or customers. Fruits that have been developed by genetic engineering are shown in Fig. 1. Some varieties were approved but not ultimately commercialized, or were launched but subsequently removed from the market, and these are not considered in detail.
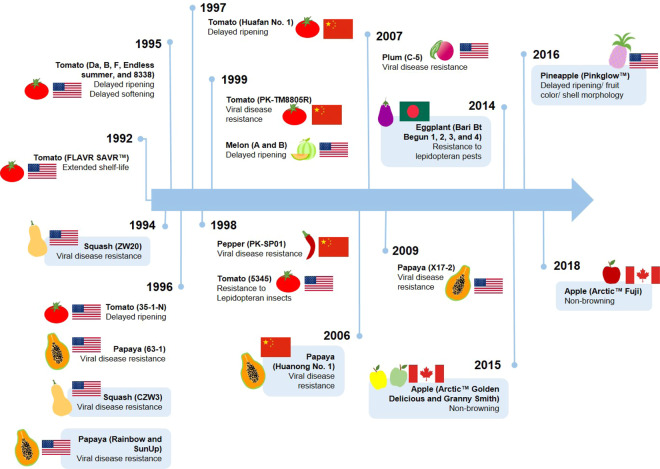
Fig. 1. Timeline of development of fruit crops with engineered traits.
Year indicates the year of first approval. Currently on the market indicated as light blue boxes
Papaya resistant to papaya ringspot virus
In 1992, papaya ringspot virus (PRSV) was detected in Puna, the major papaya-producing district in Hawaii. PRSV resistance was not found in papaya germplasm or in wild Carica species suitable as candidates for interspecific hybridization. Furthermore, insecticides failed to control the aphid vectors responsible for virus transmission22, and many orchards were therefore abandoned due to PRSV infestation10. The widely cultivated ‘Sunset’ papaya was transformed with a gene derived from a Hawaiian strain to produce the transgenic papaya ‘SunUp’, which is completely resistant to PRSV in Hawaii10. ‘SunUp’ papaya was crossed with ‘Kapoho’, a non-engineered cultivar, to obtain the yellow-flesh ‘Rainbow’ papaya, which is also resistant to PRSV23.
In China, PRSV has threatened the papaya industry for 50 years24. Similarly to the ‘SunUp’ variety, transgenic Huanong No. 1 papaya is resistant to the four predominant PRSV strains found in South China (Hainan, Guangdong, Guangxi, and Yunnan provinces), namely Ys, Vb, Sm and Lc24. Additionally, Huanong No. 1 produces bigger fruits with thicker flesh than the original cultivar24. In 2012, some Huanong No. 1 papayas grown in Hainan exhibited PRSV-like symptoms, suggesting that resistance is beginning to break. Phylogenetic analysis revealed the presence of a new virus lineage in Hainan and Guangdong papaya plantations, which may pose a threat to Huanong No. 1 papaya cultivation25.
Tomato and sweet pepper resistant to cucumber mosaic virus
In 1990, tomato crops in Fujian province (China) were affected by a virulent strain of cucumber mosaic virus (CMV) causing severe necrosis26. CMV is a major threat to tomato and sweet pepper and thus the tomato line PK-TM8805R and the sweet pepper line PK-SP01 were developed24. Both fruits express a CMV protein gene, conferring resistance to CMV, but data concerning the performance of these cultivars have not been published26.
Squash resistant to potyviruses
Like CMV, zucchini yellow mosaic virus (ZYMV) and watermelon mosaic virus 2 (WMV 2) are potyviruses transmitted by aphids. Together, these viruses can reduce the yields of squash by up to 80%27. Resistance to these viruses is not found in squash germplasm, and cannot be introduced by interspecific hybridization due to hybrid incompatibility and the concomitant transfer of undesirable traits28. In 1995, several transgenic inbred squash lines were developed by transformation with single or multiple viral protein genes from ZYMV, WMV2, and CMV. Transgenic lines ZW-20 and CZW-3 showed complete resistance to ZYMV and WMV2, line CZW-3 showed additional resistance to CMV28.
Eggplant resistant to eggplant fruit and shoot borer
In Bangladesh, eggplant is the second most important fruit crop and a major source of income for small, resource-poor farmers29. Eggplant fruits are unmarketable when infested with eggplant fruit and shoot borer (EFSB) larvae (Leucinodes orbonalis) but effective prevention requires the application of more than 100 sprays of insecticide each season. In addition to the detrimental impact on the environment, this accounts for more than a quarter of production costs, and there are still losses due to the prevalence of EFSB30. Resistant cultivars have not been developed by conventional breeding31, but a transgenic variety producing Bacillus thuringiensis (Bt) toxins is resistant to EFSB has been commercialized30. Infestations of the Bt variety occur at a frequency of 0.04–0.88% compared to 48–57% for the equivalent non-transgenic cultivar. In 2019, the average yield of Bt eggplant in Bangladesh was 19.8 t/ha, compared to 16.6 t/ha for the non-transgenic cultivar29.
Non-browning apple
Fruit quality is affected by the activity of polyphenoloxidases (PPOs), which oxidize phenolic compounds and cause gradual browning in fleshy fruits such as apple. PPOs are activated by exposure to oxygen, resulting in browning when fruits are damaged, peeled, or cut. Enzymatic browning can be prevented by storage in an air-free environment, the inactivation of PPOs by irradiation, or through the use of chemical inhibitors and natural antioxidants32. The Arctic® apple concept was developed by silencing of PPOs33,34. Currently, there are three commercial varieties of Arctic® apple: Arctic® Golden Delicious, Arctic® Granny Smith, and Arctic® Fuji. Commercial harvest of Arctic® Golden Delicious and Arctic® Granny Smith started in 2016, and Arctic® Fuji will be on the market in 202135.
Pink-fleshed pineapple
Fruits with different skin and flesh colors have been developed by conventional breeding36 and in proof-of-concept engineering experiments37. In 2005, the Pinkglow™ transgenic pineapple was developed, in which the pink flesh accumulates lycopene due to the modification of the carotenoid pathway17. The skin of the Pinkglow™ pineapple also has a combination of green, yellow, orange, and red colors, whereas conventional pineapple is green and yellow. In addition to the modulation of carotenoid accumulation, an endogenous ethylene biosynthesis gene was suppressed to control flowering, but this trait has yet to be evaluated17.
Development of commercial transgenic fruits (currently on the market)
In 1986, the coat protein of a Hawaiian PRSV isolate was cloned at Cornell University in collaboration with the Asgrow Seed Company. The USDA Section 406 grant program supported the development of transgenic PRSV-resistant papaya with the aim to control PRSV in Hawaii. In 1992, the first PRSV-resistant papayas were developed through a collaboration involving Cornell University, University of Hawaii and the Asgrow company10. The University of Hawaii established the protocol for papaya transformation by particle bombardment using zygotic embryos as the starting material10,38, whereas Huanong No. 1 papaya was generated using an Agrobacterium-mediated procedure established by an independent laboratory11. Transgenic papaya resistant to PRSV were developed using a pathogen-derived resistance approach, in which the resistance is mediated via RNA post-transcriptional gene silencing. The underpinning mechanism involves the expression of a partial or full pathogen gene sequence in the host to disrupt the pathogen’s replication39. ‘SunUp’ and ‘Rainbow’ papaya contain the coat protein gene from the mild PRSV HA 5-1 isolate10. The coat protein is required for virus survival outside the cell and for aphid transmission40. The required RNA specificity explains why PRSV-resistant transgenic papaya shows a narrow spectrum of resistance to particular PRSV isolates41. Huanong No.1 contains the replicase protein domain (NIb) from the PRSV Ys isolate, the most prevalent strain in China in 199424. The N1b and N1a proteins are needed for virus replication40.
Seminis Vegetable Seeds and Monsanto Company developed transgenic virus-resistant squashes in 199527. ZW-20 and CZW-2 virus-resistant squashes were generated using an Agrobacterium-mediated transformation protocol28 PTGS has been also used to produce ZW-20 and CZW-3 squash. Specifically, these lines contain the coat protein gene from FL isolates of ZYMV and WMV2, and line CZW-3 contains in addition the coat protein gene from CMV strain C28.
In 2000, the Maharashtra Hybrid Seeds Company (Mahyco) started to develop Bt eggplant with the collaboration of Monsanto, in India. In 2003, the Agricultural Biotechnology Support Project II (ABSPII) funded a partnership between Mahyco, Cornell University, the US Agency for International Development (USAID), and public-sector partners in India, Bangladesh, and the Philippines to develop and commercialize Bt eggplant. Under the ABSPII agreement, the EE-1 eggplant event, resistant to EFSB, was donated to the public Bangladesh Agricultural Research Institute (BARI) by Mahyco via a public–private partnership30. EFSB resistance was incorporated into nine local eggplant lines by BARI. The ASBPII project ended in 2014 and the distribution of Bt eggplant to farmers in Bangladesh was funded by the South Asia Eggplant Improvement Partnership (SAEIP), which comprises BARI, Cornell University, USAID, the University of the Pihilippines Los Banos, and Allience for Science14,30. Mahyco also set up its own eggplant transformation pipeline. Cotyledons from eggplant seedlings were used as explants for Agrobacterium-mediated transformation with the Bt cry1Ac gene, producing the EE-1 transgenic variety42.
Okanagan Specialty Fruits developed Arctic® Apple events GD743 (Golden Delicious), GS784 (Granny Smith)33 and GS784 (Fuji)35 using their patented method to limit quinone biosynthesis43. Quinones are produced from diphenols in a reaction catalyzed by PPO, and their condensation with amino acids and proteins generates lignin-like compounds that cause browning. Cell damage is needed for plastidial PPO to act on vacuolar substrates, which is why browning only occurs in cut or otherwise damaged fruit43. RNA interference (RNAi) technology was used to target four apple PPO genes by expressing a chimeric sense RNA containing partial coding sequences of PPO2, GPO3, APO5 and pSR7, leading to the generation of dsRNA and the suppression of homologous genes by post-transcriptional silencing32.
Del Monte started to develop the Pinkglow™ pineapple by modulating the carotenoid pathway44. ‘MD2’, also known as the Del Monte Gold pineapple, is a commercial variety developed by the company and was used as starting material. Ten years later, this transgenic pineapple was patented in the US17. Del Monte also patented the transformation method, which involved the cultivation of organogenic pineapple cells with A. tumefaciens. Conventional pineapple on the market has yellow flesh, reflecting the β-carotene content. The Pinkglow™ pineapple expresses the tangerine (Citrus reticulata) PSY gene, which is a rate-limiting enzyme in carotenoid biosynthesis during fruit development17. In addition, the endogenous lycopene β and ε cyclase genes (βLYC and εLYC) were suppressed by RNAi17. Ethylene promotes flowering in pineapple, and 1-aminocyclopropane-1 carboxylic acid (ACC) is the immediate ethylene precursor in plants45. A meristem-specific ACC synthase (ACS) was suppressed by RNAi in the Pinkglow™ pineapple to inhibit flowering17.
Regulatory approval and commercialization of improved fruit crops
The USA has issued the most approvals for transgenic fruit cultivation either for human consumption or as animal feed. Like other genetically engineered crops, three government agencies are responsible for the oversight of transgenic fruit cultivation and import: the US Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS), the US Environmental Protection Agency (EPA), and the US Food and Drug Administration (FDA), which is part of the Department of Health and Human Services. Depending on its characteristics, a genetically engineered fruit may fall under the jurisdiction of one or more of these agencies46. APHIS regulates the environmental release of genetically engineered organisms that may pose a risk to plant health, the EPA oversees pesticides, including genetically engineered plants expressing plant incorporated protectants (PIP), and the FDA ensures the safety of all human food and animal feed (also from plant origin).
In 2020, APHIS published a revision of its 1987 biotechnology regulations47. The new framework, known as the SECURE rule (Sustainable, Ecological, Consistent, Uniform, Responsible, and Efficient) differs from the previous regulatory framework by focusing on an organism’s properties and not on the production method47.
Flavr Savr™ tomato developed by Monsanto Company was the first genetically engineered fruit to gain non-regulated status from APHIS and approval by the FDA5,18. Flavr Savr™ was also approved for import into Mexico in 1995 by the Federal Commission for the Protection against Sanitary Risk (COFEPRIS), a decentralized organ of the Mexican Secretariat of Health that oversees the safe release and import of genetically engineered plants48. COFREPIS also permitted the import of the engineered tomato varieties Da, B, F, and Endless summer. Similarly, in 1995 Health Canada and Agriculture and Agri-food Canada determined that the Flavr Savr™ tomato was safe for human consumption and did not pose risks as a plant pest49. In Canada, the Flavr Savr™ tomato was marketed under the brand name MacGregor, allowing consumers to make an informed choice49. Flavr Savr™ was removed from the market in 1997 because the fruits were less firm than expected and the costs of production were uncompetitive18.
APHIS deregulated additional engineered tomato lines in the 1990s, namely Da, B, F developed by Zeneca and Petoseed Company; 35-1-N developed by Agritope, Inc; and 5345 and 8338 “Endless summer” developed by the Monsanto Company6–9,50. These lines were also approved as food and feed. The Da, B, and F lines were intended for processing4. Between 1996 and 1999, more than 1.8 million cans derived from hybrids of the F line were sold in the UK18, but from 1998 onwards were no longer used as food ingredients18. In 2000 Health Canada also approved line 5345, which was resistant to insect pests, but it has not been released onto the market51.
In 1999, Agritope was granted FDA approval of the Melon A and B lines for use as food16. The company also requested the deregulation of these lines, but withdrew the APHIS petition the same year19, and neither line has been commercialized.
The Pinkglow™ pineapple received FDA approval in 2016 and was marketed for the first time in October 2020 by Fresh del Monte52,53. This cultivar is grown on a single farm in Costa Rica. The C5 plum (HoneySweet) developed by the US Department of Agriculture, which is resistant to plum pox virus (PPV), has also been deregulated by APHIS, approved by the FDA and registered by the EPA54. It was patented in the US in 2004, but no trees have been planted thus far and it is therefore not on the market. On request, the Agricultural Research Service (the research branch of the USDA) can freely provide a limited number of heat-treated bud wood samples to be used as a genetic resource for the breeding of PPV-resistant varieties55.
Genetically engineered squash has been on the US market for 25 years. CZW3 squash is also approved for import as food by Health Canada56. The cultivation of genetically engineered papaya in the US began in 1996, and the current predominant variety is ‘Rainbow’ because it has yellow fruit flesh favored by consumers4. Canada and Japan are the major importers of genetically engineered papaya produced in the US, although it is also approved for cultivation in Japan57. Two additional papaya lines resistant to PRSV were approved for cultivation by APHIS: 63-1 developed by Cornell University and the University of Hawaii58, and X17-2 developed by the University of Florida, respectively59. Neither lines have been commercialized4.
Arctic® apples were developed by Okanagan Specialty Fruits Company in Canada, and the Golden Delicious, Granny Smith, and Fuji varieties have received approval for cultivation, human consumption and use as animal feed in both Canada and the US15,60–62. However, Arctic® apples are only grown in the US, and it is unclear if Artic varieties are among the 206,259 tons of apples (including dried apples) imported to Canada, most of which are grown in the US63,64.
In China, the commercialization of all genetically engineered crops is regulated by the Ministry of Agriculture (MOA)65, with safety advice provided mainly by the Biosafety Management Division of the Center for Science and Technology Development (CSTD) and the National Biosafety Committee (NBC). The NBC can recommend safety certification based on product testing and field trials, but only the MOA can formally provide regulatory clearance25. After registration, genetically engineered crops can be cultivated and commercialized but approval for commercialization is only granted at the province/region level and not nationwide.
Huafan No. 1 tomato developed by Huazhong Agricultural University was the first genetically engineered fruit to be approved for cultivation, human consumption and use as animal feed in China, followed by Da Dong No. 9 (Institute of Microbiology, CAS) and PK-TM8805R (Beijing University) tomatos26. Huafan No. 1 and Da Dong No. 9 are no longer cultivated in China, and the status of PK-TM8805R is unclear26. Similarly, the genetically engineered sweet pepper PK-SP01 developed by Beijing University was approved for cultivation and for human consumption, but the extent of its cultivation is unclear26. PRSV-resistant papaya Huanong No. 1 was approved for cultivation in 2006 and is commercially available in China.
In Bangladesh, the National Committee on Biosafety (NCB) grants regulatory approvals for all genetically engineered crops, assisted by a Biosafety Core Committee (BCC)66. The eggplant varieties Bari Bt Begun 1, 2, 3, and 4 were approved for cultivation and food use in Bangladesh, and in 2020 they are the only genetically engineered fruit commercialized in this country29,30.
Socioeconomic impact of commercialized fruits with improved traits
The socioeconomic impact of genetically engineered fruits is growing with the scale of cultivation, although less than 0.01% of the 185.43 million ha cultivated with genetically engineered crops in 2018 was represented by fruits67. Production and adoption rate details are provided in Table 1. PRSV-resistant papaya is the most widely cultivated genetically engineered fruit, followed by Bt eggplant, virus-resistant squash, Arctic® apples, and Pinkglow™ pineapple.
Table 1.
Production and adoption rates of genetically engineered fruits on the market. Adoption rate = ha of transgenic crop (dark orange)/total ha of crop (light orange)a
| Fruit | Modified trait | Trade or event name | Production (ha) | Adoption rate |
|---|---|---|---|---|
 |
Non-browning | Arctic™ Golden Delicious, Granny Smith, and Fuji Apples | 500 (2019, US) | 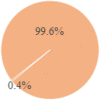 |
 |
Resistance to papaya ringspot virus | Rainbow, SunUp | 405 (2017, US) | 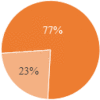 |
 |
Resistance to papaya ringspot virus | Huanong No. 1 | 7130 (2017, China) | 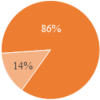 |
 |
Resistance to Eggplant fruit and shoot borer (Leucinodes orbonalis) | Bari Bt Begun 1, 2, 3 and 4 | 2400 (2017, Bangladesh) | 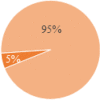 |
 |
Delayed ripening/senescence Fruit color Shell morphology |
Pinkglow™ | 25 (2017, Costa Rica) | 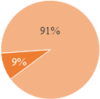 |
 |
Resistance to cucumber mosaic cucumovirus, zucchini yellow mosaic potyvirus and watermelon mosaic potyvirus 2 | CZW3 and ZW20 | 1000 (2017, US) | 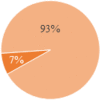 |
Virus-resistant fruits
China grew 9600 ha of PRSV-resistant papaya in 2018. Initial plantings took place in the southern Guangdong Province in 2006, but Hainan Island became the leading location for PRSV-resistant papaya production in 2017 (46%), followed by Guangdong (36%) and Guangxi (18%) provinces57. CMV-resistant sweet pepper and tomato have been cultivated in China since 1998 and 1999, respectively, in Beijing municipality and in Fujian and Yunnan provinces, but the scale of cultivation is unclear26. Data on the profitability of PRSV-resistant papaya have not been published by the Chinese authorities, so the socioeconomic impact is difficult to judge68.
In the US, PRSV-resistant papaya has been commercially grown in Hawaii since 1999 and it has prevented the collapse of the Hawaiian papaya industry due to the prevalence of PRSV in orchards of conventional varieties23. In 1992, when PRSV was first detected on Hawaii, the Puna district produced 95% of all Hawaiian papaya grown (~24,000 tons) but yields had fallen to ~12,000 tons in 1998. Two years after the introduction of the resistant variety, yields recovered to ~18,000 tons23. Although lower than 1992 levels, the lack of production was not caused by the virus but by the falling demand from Japan, resulting in the papaya cultivation area in Hawaii declining from more than 500 ha in 2015 to only 250 ha in 20184,67. The shrinking Japanese market partly reflected the reluctance of retailers to handle genetically engineered products and partly the increased competition from Philippine papaya growers4. Nevertheless, the yield of genetically engineered papaya in 2018 was 17% higher than conventional papaya, with a net farm income gain of $2623/ha. Overall, the accumulated farm income benefit between 1999 and 2018 was $38.4 million67. Cultivation of PRSV-resistant papaya in Hawaii has also reduced the threat of PRSV in the Puna district, allowing papaya growers to cultivate non-transgenic varieties alongside the genetically engineered crop23.
Virus-resistant squash has been commercially grown in the US since 2004, mainly in Florida and Georgia. In 2018, virus-resistant squash was planted on 1000 ha, representing 6% of total squash production in the US67. The genetically engineered varieties achieve higher yields than conventional squash, resulting in a net gain to farmers of $10.1 million. Overall, the cumulative farm income benefit between 2004 and 2018 was $310.9 million67.
Insect-resistant fruit crops
Bt eggplant was first grown commercially in Bangladesh in 2014, and was cultivated on 2975 ha in 201867. Eggplant is mostly grown by resource-poor farmers, who can obtain seed at no or minimal cost from three organizations: BARI, the Department of Agricultural Extension, and the Bangladesh Agricultural Corporation. Accordingly, the cost of this technology to the farmers is near zero29. The Bt eggplant was initially provided to 20 farmers, but by 2018, the variety had been adopted by 20,695 farmers29. Bt eggplant achieved 20% higher yields than conventional eggplant in 2018, and the enhanced quality resulted in a 10% increase in price. As a result, farm income has increased by $616–704/ha29,67.
As well as the direct income gains, Bt eggplant also helps to reduce pesticides. In 2016, farmers in 35 districts cultivating Bt eggplant spent 61% less on pesticides compared to farmers growing conventional varieties69. This difference solely represents the cost of pesticides to control EFSB because different chemicals are used to control other pests. However, the prevention of damage caused by EFSB also reduces infestations by secondary pests such as leaf-eating beetles, thrips, whitefly, mites, leaf wing bugs, and leaf roller, by 42–60%70.
Fruits with enhanced quality traits
Arctic® apples were first planted in 2016 (70,000 trees planted over 80 ha). This had grown to 300,000 trees over 101 ha by 2018 and in 2019 the cultivated area exceeded 500 ha71. Although the profitability of growing this variety has not been made public, Okanagan Specialty Fruits states that Arctic® apples are more suitable for mechanical harvesting and suffer less impact from finger bruising, bin rubs and other superficial damage, which results in higher packouts (an industry measure of fruit suitable for market) and therefore less waste, and similar benefits for retailers72. Furthermore, the Arctic® Golden variety does not require warm packing, reducing the cost of production. Del Monte commercialized the Pinkglow™ pineapple in October 2020 so the socioeconomic impact of this variety will not be known until market data are available.
Technological advances in gene functional analysis and genetic modification of fruits
Genetic engineering can be used to investigate the functions of genes and to exploit these functions for the improvement of traits such as biotic and abiotic stress tolerance, flowering time, ripening, fruit flavor, and nutrient content. In this section, we discuss genetic engineering and genome editing technologies that have been used for the enhancement of target traits in fruit crops, which may facilitate commercialization in the future (Table 2). Use of CRISPR and associated genome editing technologies for the development or enhancement of fruit crops may open the door to new commercial opportunities, potentially circumventing restrictions on GM crops in many parts of the world20. While marketability will vary by country, additional, transgene-free cultivars may be accessible to consumers in the near future20,73,74.
Table 2.
Current status of improving fruits through molecular tools (until mid-2020).
| Fruit | Trait | Modification strategy | G | F | Outcome |
|---|---|---|---|---|---|
 |
Flowering time | OE, GE | ✓ | Early flowering103 | |
| Fruit morphology | OE, GS | ✓ | Different color38 | ||
| Different shape138 | |||||
| Quality improvement | GS | ✓ | Increased firmness107 | ||
| Plant morphology | OE | ✓ | Smaller trees139 | ||
| Dwarf tree119 | |||||
| Disease resistance | OE, GE | ✓ | ✓ | Increased resistance to bacteria and fungi76,77,99 | |
| Tolerance to abiotic stress | OE | ✓ | Increased tolerance to drought and cold stress140 | ||
| Increased tolerance to salinity95 | |||||
 |
Plant morphology | GE | ✓ | Shorter trees141 | |
| Disease resistance | GE, GS, OE | ✓ | ✓ | Increased resistance to bacteria and virus79,89,142–144 | |
| Nutritional improvement | GE | ✓ | Increased carotenoid content145 | ||
 |
Flowering time | OE | ✓ | Early flowering146 | |
 |
Fruit morphology | GS | ✓ | Smaller fruits147 | |
| Disease resistance | GS | ✓ | Increased resistance to virus148 | ||
| Citrus rootstock species | Plant morphology | OE, GS | ✓ | Shorter trees149 | |
| Disease resistance | OE | ✓ | Increased resistance to bacteria150 | ||
| Tolerance to abiotic stress | OE | ✓ | Increased tolerance to drought stress98 | ||
| Flowering time | OE | ✓ | Early flowering104 | ||
 |
Disease resistance | GE, OE, DR | ✓ | Increased resistance to bacteria78,82,83,87,88 | |
| Nutritional improvement | GS | ✓ | Increased carotenoid content114 | ||
 |
Disease resistance | OE | ✓ | Increased resistance to fungi84,85 | |
 |
Disease resistance | OE | ✓ | Increased resistance to virus151 | |
 |
Disease resistance | GE | ✓a | Increased resistance to virus93 | |
 |
Disease resistance | OE | ✓ | Increased resistance to virus152 | |
| Fruit morphology | OE | ✓ | Reduce pathogen-induced mortality120 | ||
| Tolerance to abiotic stress | OE | ✓ | Different color112 | ||
| Increased tolerance to salinity153 | |||||
| Increased tolerance to cold stress100 | |||||
 |
Nutritional improvement | OE | ✓ | Increased carotenoid content154 | |
| Quality improvement | GS | ✓ | Ripening108 | ||
| Tolerance to abiotic stress | OE | ✓ | Increased tolerance to salinity96 | ||
 |
Disease resistance | GS | ✓ | Increased resistance to virus90 | |
 |
Quality improvement | GS | ✓ | Delayed fruit ripening109 | |
 |
Quality improvement | OE, GS | ✓ | Decreased ethylene production110 | |
| Disease resistance | OE | ✓ | Increased resistance to bacteria86 | ||
| Nutritional improvement | OE | ✓ | Increased tocopherol content155 | ||
 |
Disease resistance | OE | ✓ | Increased resistance to fungi80 | |
| Tolerance to abiotic stress | OE | ✓ | Increased tolerance to salinity97 | ||
 |
Flowering time | OE | ✓ | Early flowering105 | |
| Disease resistance | GS | ✓ | Increased resistance to virus156 | ||
 |
Flowering time | GE | ✓ | Early flowering157 | |
| Nutritional improvement | GS | ✓ | Decreased starch and increased soluble sugar content111 | ||
| Increased anthocyanin content113 | |||||
| Quality improvement | OE, GS | ✓ | Increased fruit firmness158 | ||
 |
Flowering time | GE | ✓ | ✓ | Early flowering102 |
| Quality improvement | GE | ✓ | ✓ | Increased shelf-life159 | |
| Fruit morphology | OE, GE | ✓ | Parthenocarpic fruits160 | ||
| Nutritional improvement | GE | ✓ | Increased lycopene content161 | ||
| Disease resistance | OE, GE | ✓ | Increased resistance to bacteria162 | ||
 |
Insect resistance | OE | ✓ | Increased resistance to insect94 | |
 |
Pest resistance | GE | ✓ | Increased herbicide resistance122 | |
| Disease resistance | GS | ✓ | Increased resistance to virus91 |
OE overexpression, GS gene silencing, GE genome editing, DR down-regulation.
Stage of development: G greenhouse, F field trials.
A detailed list of modified genes and outcomes is provided in Table S1.
aNet-house.
Pathogen and pest resistance
Pathogens and pests are severe constraints affecting the growth and development of fruit trees, the development and ripening of fruits, and the quality of fruit products. In 2017 up to 30% of the fruit and vegetables losses worldwide were pre-harvest, mainly caused by pests and pathogens75. In many cases, conventional breeding for resistance is not possible because strong resistance is not present in available germplasm and the introgression process would take too long2. One strategy to enhance disease resistance in fruit crops is the modification of receptors that directly interact with or perceive the presence of a specific pathogen. In apple, overexpression of the HcrVf2 gene encoding such a receptor resulted in near-complete resistance to fungal scab (Venturia inaequalis)76. Recently, CRISPR/Cas9-mediated inactivation of the susceptibility-associated gene DspA/E-interacting protein (DIPM4), also encoding a receptor, significantly reduced bacterial fire blight (Erwinia amylovora) symptoms by 50% in apple77.
Another strategy for the mitigation of pathogen symptoms is the targeting of response pathways (innate immunity) in the host. For example, the nonexpressor of pathogenesis-related 1 (NPR1) gene encodes a transcriptional regulator of pathogenesis-related (PR) protein genes as part of the salicylic acid-dependent systemically acquired resistance (SAR) pathway. Sweet orange trees (Citrus sinensus) overexpressing NPR1 under the control of the phloem-specific SUC2 promoter exhibited enhanced resistance to huánglóngbìng (citrus greening disease), and up to 46% of the engineered plants remained disease-free for 2 years78. These findings highlight the importance of promoter selection in overexpression studies and indicate that NPR1 possesses a conserved role among tree fruit species in the response to pathogens.
Other PR-associated proteins have been targeted for modification in banana, chili pepper, and citrus in order to mitigate the effect of bacterial and fungal pathogens. In banana, the induction of a hypersensitive response (HR) by the overexpression of genes encoding an HR-assisting protein and a plant ferredoxin-like protein conferred resistance to banana Xanthomonas wilt, with 50–60% of the transgenic plants displaying no disease symptoms following inoculation79. Overexpression of the pepper carboxylesterase gene in chili pepper reduced infections by anthracnose fungus from 70% in wild-type plants to 20%80. Similarly, expressing the J1-1 gene encoding an antifungal defensin reduced the frequency of anthracnose lesions by up to 90%80,81. CRISPR/Cas9 was used to inactivate the grapefruit lateral organ boundary domain family protein 1 and orange WRKY22 genes, which regulate immunity responses, improving resistance to canker caused by Xanthomonas citri subsp. citri (Xcc) in Duncan grapefruit (Citrus ✕ paradisi) and Wanjincheng orange (Citrus sinensis (L.) Osbeck)82–85. The CRISPR-induced mutation rate in grapefruit was 23–89%, and Xcc resistance was correlated with the mutation rate, as shown by the corresponding range of canker symptoms85. Similar findings were reported for orange plants with mutations in the WRKY22 gene83.
In addition to the knockout of host genes to improve pathogen and pest resistance, pathogen-derived transgenes (or other heterologous genes) serve as additional routes for the improvement of fruit traits. In pear, the expression of a bovine lactoferrin gene, which encodes a bactericidal glycoprotein, reduced fire blight symptoms by 78% compared to controls86. In sweet orange, expression of the E. amylovora hairpin protein triggered HR in the host plants and reduced susceptibility to citrus canker by up to 79%87. The expression of a synthetic insect antimicrobial peptide (cecropin B) in blood orange improved long-term resistance to huánglóngbìng by 85–100%88.
An important strategy in the fight against viral diseases is the expression of non-translatable pathogen genes to elicit a PR response or to silence viral components essential for replication, packaging, or systemic spreading. RNAi-mediated silencing of viral components has been achieved in banana, resulting in the complete absence of bunchy top virus disease symptoms in transgenic plants 6 months after challenge89. Similarly, transgenic melon and watermelon (Citrullus lanatus) lines displayed up to 100% resistance when challenged with several cucurbit viruses90,91, and grafted transgenic plum lines remained resistant to PPV for more than 9 years92. In cucumber, the CRISPR/Cas9 system was used to mutate the eukaryotic translation initiation factor 4E gene, which is associated with CMV susceptibility, resulting in 100% virus-free fruits in the T3 generation93. Bt cry genes have been expressed in kiwifruit (Actinidia chinensis) and walnut (Juglans regia) to protect them against insect pests, resulting in 75–100% insect pest mortality94.
Abiotic stress tolerance
Abiotic factors, such as drought, are also among the main factors causing pre-harvest losses of fruit and vegetables75. The engineering of abiotic stress tolerance in fruit trees allows them to be grown in environments where temperatures are sub-optimal, water is scarce, or high concentrations of salt and/or heavy metals in the soil are toxic and prevent the uptake of water and nutrients. Overexpression of the Na+/H+ cation antiporter gene NHX1 in apple and kiwifruit prolonged survival in saline conditions by allowing the accumulation of higher concentrations of antioxidant flavonoids (60% more than normal) as well as sodium and potassium (2x more than normal) thus delaying the stress response95,96. In chili pepper, the expression of a tobacco osmotin gene increased yields by 31% accompanied by higher levels of proline, chlorophyll and reactive oxygen species (ROS) scavengers, as well as a higher relative water content97. Transgenic citrumelo (Citrus paradise × Poncirus trifoliata) plants overexpressing the enzyme Δ1-pyrroline-5-carboxylate synthase, required for proline synthesis, showed a 2.5-fold increase in drought tolerance, as determined by turgor pressure maintenance, stomatal conductance, photosynthetic rate, and transpiration rate98.
Fruit crops are often threatened by cold temperatures, which affect plant growth as well as the quality of maturing and ripening fruits. Cold tolerance is therefore an important target in commercial fruit development programs. In apple, overexpression of the transcription factor MYB4, which regulates cold-induced dormancy and stress pathways, allowed the transgenic plants to tolerate cold temperatures for long periods while maintaining normal water content, reflecting the accumulation of glucose, fructose, and sucrose to levels 30–38% higher than normal99. Overexpression of the Arabidopsis dehydration response element-binding 1b protein in grapevine reduced cold-induced wilting by 73%100. Similarly, the expression of a Poncirus trifoliata basic helix-loop-helix protein in pumello (Citrus grandis) enhanced cold tolerance, reduced electrolyte leakage by 13% and increased proline levels by up to 67% compared to wild-type plants101.
Flowering time and dormancy release
Flowering time is a very important trait targeted for improvement in fruit crops because of its close association with the timing of fruit development. This trait is under strict genetic regulation and is dependent on environmental conditions, particularly temperature and day length, which limits the geographical regions in which crops can be cultivated102. Genetic engineering has been used to express floral activators or repressors, allowing the specification of floral transition and dormancy requirements in major fruit tree species. In transgenic apple, plum, and citrus trees, the overexpression of FT family floral activators needed to trigger bud breaking promoted early flowering (by up to 45 weeks in apple and 12 weeks in orange), and reduced dormancy requirements, eliminating them completely in plum103–105. Recently, CRISPR/Cas9 was used to inactivate the self-pruning 5G gene in tomato, which abolished sensitivity to day length and reduced the time to harvest by 2 weeks, translating to a greatly accelerated flowering stage and early fruit yield102. In kiwifruit, CRISPR/Cas9-mediated repression of the CEN-like genes also led to rapid and early terminal flowering106. These experiments provide insights into the genetic and environmental control of flowering time in different fruits and form the basis for additional engineering strategies to develop early or late-flowering cultivars adapted to specific growing regions.
Fruit ripening and sensory attributes
The modulation of fruit ripening is one of the major strategies by which flavor, aroma, and nutrient profiles can be adjusted, and by which the shelf-life can be extended to improve marketability and reduce waste. In climacteric fruits such as apple, banana, and tomato, the key targets are genes associated with ethylene biosynthesis and degradation. In apple, the silencing of ACS and ACC oxidase (ACO) by expressing antisense RNA generated fruit that produced 60% less ethylene, increasing firmness by 20% and allowing cold storage for up to 3 years107. Although the synthesis of volatile esters was suppressed, sugar and organic acid accumulation were unaffected. Co-suppression and knockdown of ethylene-biosynthetic genes achieved similar results in pear, kiwifruit, and papaya108–110.
Sugar and organic acid content can be modified to enhance fruit flavor. In strawberry, the suppression of ADP-glucose pyrophosphorylase by expressing antisense RNA under the control of a fruit-specific promoter inhibited the conversion of sugar to starch and reduced the starch content of transgenic fruits by up to 47% while increasing the soluble sugar content by up to 37%111. Plant pigments such as anthocyanins and carotenoids are also major targets for metabolic engineering in fruits because they provide health benefits and allow the production of fruits with unique colors. The overexpression of MYB family transcription factors in apple, grapevine, and strawberry enhanced the production and storage of anthocyanins, with transgenic fruits accumulating up to 50% more than normal36,112,113. The accumulation of carotenoids has been achieved by the RNAi-mediated silencing of β-carotene hydroxylase in sweet orange, preventing conversion of β-carotene to xanthophylls and thus increasing the β-carotene content in the fruit pulp by 26-fold. Caenorhabditis elegans adults fed with diets supplemented with β-carotene-enriched orange pulp were 20% more resistant to hydrogen peroxide-induced oxidative stress than those fed with control diet114. These studies demonstrate how genetic engineering and genome editing can be used to produce fruits with enhanced flavor, texture, and nutrient levels.
Trans-grafting
Grafting is widely used during the propagation of fruit trees to allow the selection of rootstock and scions with different favorable characteristics that may be difficult or laborious to combine in one cultivar (such as high fruit yields paired with resistance to root pests). The rootstock and scion still influence each other by exchanging soluble signals, but the two components maintain their genetic integrity115. Trans-grafting refers to grafting of a non-transgenic scion onto a transgenic rootstock. Some desirable characteristics of the rootstock, such as dwarfing or disease resistance, are conferred upon the scion by the vascular transport of RNA, hormones or signaling proteins, but the shoot, leaves, and fruits remain transgene-free116,117. Although the specific regulations vary by country, trans-grafting can be used to circumvent restrictions on the marketing of GM products in certain jurisdictions118. This technology has been used in apple, by grafting non-transgenic scions onto rootstock expressing the Agrobacterium rhizogenes rolB gene, which confers dwarfing characteristics on the scion119. In grapevine, non-transgenic scions were grafted onto rootstocks engineered to produce an antimicrobial peptide and a protein that inhibits cell wall degradation. These proteins were transported to the scion through the xylem, resulting in the enhanced mobilization of water and nutrients and a 30–95% reduction in pathogen-induced mortality120. Transgenic rootstocks can therefore improve the production of commercially important fruit trees but the fruits and seeds do not carry any exogenous DNA79.
Moving beyond transgenesis—genome editing technologies
Genome editing is perhaps the most important recent development in crop breeding, and protocols based on the versatile CRISPR/Cas9 system have been optimized for several fruit species to increase the editing efficiency. In apple, CRISPR/Cas9 produced transgene-free edits121. In cucumber, wild strawberry, and watermelon, CRISPR/Cas9 constructs were integrated as part of the T-DNA but segregation was then achieved through back-crossing122–125. A major challenge to the commercial development of edited varieties is the successful transmission of targeted mutations through the germline126. This is particularly difficult in woody species, including fruit trees, because they are propagated vegetatively. Back-crossing could take decades (depending on the species) and could result in the unintentional outcrossing of the edited gene. It is also difficult to achieve homozygosity at the edited locus within the desired genetic background because most fruit trees are self-incompatible and thus require obligate outcrossing. Such characteristics hinder the introduction of genome edits that are stable and heritable127–129. Several new derivatives of the original CRISPR/Cas9 editing platform have been proposed, including CRISPR/Cas9 ribonucleoprotein (RNP) technology, CRISPR cytidine and adenosine base editors (CBEs/ABEs), CRISPR flippase, and new CRISPR-associated nucleases such as Cas12a/Cpf1, which may help to address these challenges and accelerate the development and commercialization of genome-edited crops77,126,129–132.
CRISPR RNP technology
Transgene-free genome editing improves the commercialization potential of modified crops (including fruits) because the CRISPR/Cas9 cassette is not inserted into the genome and, in many jurisdictions, the resulting variety is regulated in the same manner as a conventional crop, with certain caveats21. CRISPR/Cas9 RNP technology avoids transgene integration by delivering purified RNPs containing the Cas9 protein and gRNA into plant protoplasts and the subsequent regeneration of plants133,134. This approach has already been used in apple and grapevine to introduce mutations that confer resistance to fire blight and powdery mildew, respectively129. In addition to Cas9 RNPs, CRISPR/Cpf1-RNPs have also been employed successfully for gene editing in protoplasts of soybean and tobacco, paving the way for future use in other crops, including fruits and vegetables134. Subsequent optimization experiments permitted plant regeneration from protoplasts and improved the transformation protocol for grape protoplasts, reducing the amount of time needed for RNP delivery and genome editing to less than 3 weeks131. It is likely that species- and even cultivar-specific protocol optimization will be necessary to achieve satisfactory editing efficiencies because the major hurdle is not the delivery of RNPs across the protoplast membrane, but the subsequent recovery and regeneration of fertile plants.
CRISPR base editing
Whereas conventional CRISPR/Cas9 editing tends to introduce short insertions or deletions at the target locus, cytidine and adenosine base editing facilitates the targeted introduction of single nucleotide replacements by direct C-to-T or A-to-G base conversion, respectively. Base editors have been used to introduce herbicide resistance traits in fruit crops in proof-of-concept experiments. For example, CBE in the watermelon ALS gene resulted in a single amino acid substitution that was sufficient to confer broad-spectrum and heritable resistance to commercial sulfonylurea herbicides122.
CRISPR flippase
Flp/FRT is a yeast site-specific recombinase system in which the recombinase Flp (flippase) catalyzes recombination between two copies of the 34-bp FRT site, resulting in the excision or inversion of the intervening DNA, depending on the relative orientation of the FRT sites. The Flp/FRT system has been used to remove selectable markers in T1 apple, apricot, citrus, and grapevine plants, leaving a single FRT site behind as a footprint2. These studies laid the foundations for more recent work in which the FLP/FRT system was placed under the control of a heat-shock promoter and incorporated into the CRISPR/Cas9 plasmid, allowing the editing of a disease susceptibility gene in apple and subsequent removal of the CRISPR/Cas9 components77. This technology has yet to be applied in other fruit crops, but it shows great promise given the efficiency of editing and T-DNA excision.
New CRISPR nucleases
Most CRISPR studies thus far have used the endonuclease Cas9 from Streptococcus pyrogenes (SpCas9). In its native form, SpCas9 requires a trans-activating CRISPR RNA (tracrRNA) and a CRISPR-RNA (crRNA) to induce blunt double-strand breaks in target DNA. These functions were combined into a single gRNA for the development of CRISPR/Cas9 as an engineering tool. But SpCas9 is only one of a large family of CRISPR-associated nucleases with diverse properties, some of which may be advantageous for genome editing in fruit crops by improving efficiency, specificity, or versatility, or by reducing costs135. For example, Cas9 from Staphylococcus aureus (SaCas9) differs from SpCas9 in terms of protospacer adjacent motif (PAM) specificity but has a similar editing efficiency. It has been used in several model plant species and also recently in citrus, and provides greater versatility by extending the range of potential genomic targets126.
Cas12a/Cpf1 from Prevotella and Francisella spp. recognizes a T-rich PAM and generates compatible cohesive ends with overhangs of 4–5 nt, differing from the blunt ends introduced by Cas9, and increasing the efficiency of DNA integration (knock-in)136. Cas12a/Cpf1 is also a smaller protein than Cas9, which improves the efficiency of multiplex editing. CsmI is also smaller than Cas9136, and recognizes AT-rich PAM sites thus improving the accuracy of genome editing in AT-rich regions135. This approach has been employed to edit the PDS gene in citrus, establishing the feasibility of Cpf1-mediated, DNA-free editing in fruit crops137.
Conclusions
Genetic engineering facilitates the development of fruits with useful agronomic or quality traits that are difficult or laborious to achieve by conventional breeding, either due to the lack of suitable germplasm or the long breeding cycles and need for multiple rounds of back-crossing. The same traits can be introduced by genetic engineering in one generation, often directly into elite varieties. Some genetically engineered fruits have been on the market for more than 25 years, and have achieved a remarkable positive socioeconomic impact by reducing pests and diseases and increasing the quality of the end product, both of which help to increase income for farmers. Further benefits to farmers, consumers, and the environment reflect the reduced use of pesticides. The development of new molecular breeding technologies such as trans-grafting and genome editing not only offer the promise of further commercial fruit varieties with resistance to biotic and abiotic stresses, improved flavor and nutrient content, and modified flowering and ripening times, but also help to address some of the regulatory constraints that limit the cultivation of first-generation transgenic crops. In particular, the development of transgene-free genome editing methods based on CRISPR/Cas9 and other nucleases offers a way to introduce precise changes at preselected genomic sites with no genetic footprints and no off-targets. In many jurisdictions, some varieties generated through genome editing are exempt from GMO regulations. These tools and techniques are available for the accelerated development of fruit crops with properties that satisfy the needs of producers, retailers, and consumers, in a sustainable and environmentally friendly manner.
Supplementary information
Acknowledgements
M.L.-G., P.S.G.-C., P.C., and T.C. would like to acknowledge funding from MINECO, Spain (PGC2018-097655-B-I00 to P Christou), Generalitat de Catalunya Grant 2017 SGR 828 to the Agricultural Biotechnology and Bioeconomy Unit (ABBU). P.S.G.-C. was supported through an Agrotecnio postdoctoral fellowship. A.D. acknowledges the support from Washington State University Agriculture Center Research Hatch grant WNP00011.
Author contributions
P.C and A.D. conceived the idea. M.L.-G. and P.S.G.-C. planned the outline. M.L.-G., S.L.H., and P.S.G.-C. collected the literature and wrote the paper. M.L.-G. and P.S.G.-C. prepared the figure and tables. P.C., T.C., and A.D. critically reviewed and improved the paper. All authors approved the final version.
Conflict of interest
The authors declare no competing interests.
Supplementary information
The online version contains supplementary material available at 10.1038/s41438-021-00601-3.
References
- 1.Janick, J. in Plant Breeding Reviews Ch. 8, Vol. 25 (ed Janick, J.) (Springer, 2005).
- 2.Song G, Prieto H, Orbovic V. Agrobacterium-mediated transformation of tree fruit crops: methods, progress, and challenges. Front. Plant Sci. 2019;10:226. doi: 10.3389/fpls.2019.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Limera C, Sabbadini S, Sweet JB, Mezzetti B, Kühn-institut J. New biotechnological tools for the genetic improvement of major woody fruit species. Front. Microbiol. 2017;8:1–16. doi: 10.3389/fpls.2017.01418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baranski R, Klimek-Chodacka M, Lukasiewicz A. Approved genetically modified (GM) horticultural plants: a 25-year perspective. Folia Hortic. 2019;31:3–49. doi: 10.2478/fhort-2019-0001. [DOI] [Google Scholar]
- 5.Kramer MG, Redenbaugh K. Commercialization of a tomato with an antisense polygalacturonase gene: The FLAVR SAVR™ tomato story. Euphytica. 1994;79:293–297. doi: 10.1007/BF00022530. [DOI] [Google Scholar]
- 6.APHIS. Interpretative ruling on DNA Plant Technology Corporation petition for determination of non-regulated status of delayed-ripening tomato line 1345-4. Docket No. 94-092-2 (1994).
- 7.APHIS. Interpretative ruling on Agritope, Inc. petition for determination of regulatory status of cherry tomatoes with a S - adenosylmethionine hydrolase gene. Docket No. 95-097-1 (1995).
- 8.APHIS. Interpretative ruling on Zeneca Plant Science and Petoseed Company Inc. petition for release from regulation for modified lines B, Da- and F derived from T7 varieties of processing tomatoes. Docket No. 95–016–1 (1995).
- 9.APHIS. Interpretive ruling on Monsanto petition of nonregulated status for insect resistant tomato line 5345. Docket number: 97-114-1 (1998).
- 10.Fitch M, Manshardt R, Gonsalves D, Slightom J, Sanford J. Virus resistant papaya plants derived from tissues bombarded with the coat protein gene of papaya ringspot virus. Nat. Biotechnol. 1992;10:1466–1472. doi: 10.1038/nbt1192-1466. [DOI] [Google Scholar]
- 11.Chen G, Ye CM, Huang JC, Yu M, Li BJ. Cloning of the papaya ringspot virus (PRSV) replicase gene and generation of PRSV-resistant papayas through the introduction of the PRSV replicase gene. Plant Cell Rep. 2001;20:272–277. doi: 10.1007/s002990000311. [DOI] [Google Scholar]
- 12.Chen ZL, et al. Safety assessment for genetically modified sweet pepper and tomato. Toxicology. 2003;188:297–307. doi: 10.1016/S0300-483X(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 13.Scorza R, et al. Transgenic plums (Prunus domestica L.) express the plum pox virus coat protein gene. Plant Cell Rep. 1994;14:18–22. doi: 10.1007/BF00233291. [DOI] [PubMed] [Google Scholar]
- 14.Shelton, A. M. et al. Bt Eggplant: a genetically engineered ‘minor’ crop comes of age in Bangladesh and the Philippines. ISB News Rep. (VTechWorks, 2017).
- 15.USDA. USDA announces deregulation of non-browning apples. (2015). Available at: https://www.aphis.usda.gov/stakeholders/downloads/2015/SA_arctic_apples.pdf [Accessed June 2020].
- 16.Biosafety Clearing-House. Melon A and B. Country’s decision or any other communication (1999). Available at: http://bch.cbd.int/database/record.shtml?documentid=6351 [Accessed Sept 2020]
- 17.Firoozbady, E. & Young, T. R. Pineapple plant named “Rose”. US Patent PP25,763 P3 (2015).
- 18.Bruening, G. & Lyons, M. J. The case of the FLAVR SAVR tomato. Calif. Agric. 54, 6–7 (2000).
- 19.APHIS. Petition for determination of nonregulated status—Melon. Petition number 98-350-01p (1999).
- 20.Menz J, Modrzejewski D, Hartung F, Wilhelm R, Sprink T. Genome edited crops touch the market: a view on the global development and regulatory environment. Front. Plant Sci. 2020;11:586027. doi: 10.3389/fpls.2020.586027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Álvarez D, et al. Fruit crops in the era of genome editing–closing the regulatory gap. Plant Cell Rep. 2021;40:915–930. doi: 10.1007/s00299-021-02664-x. [DOI] [PubMed] [Google Scholar]
- 22.Conover RA. Distortion ringspot, a severe virus disease of papaya in Florida. Proc. Fl. State Hortic. Soc. 1964;77:440–444. [Google Scholar]
- 23.Gonsalves D, Basin P, Ferreira S, Sciences EP, Resources H. Transgenic papaya: a case for managing risks of papaya ringspot virus in Hawaii plant health progress. Plant Manag. Netw. 2003;1:1–5. [Google Scholar]
- 24.Ye C, Li H. 20 years of transgenic research in china for resistance to papaya ringspot virus. Transgenic Plant J. 2010;4:58–63. [Google Scholar]
- 25.Wu, Z., Mo, C., Zhang, S. & Li, H. Characterization of papaya ringspot virus isolates infecting transgenic papaya ‘Huanong No.1’ in South China. Sci. Rep. 8, 8206 (2018). [DOI] [PMC free article] [PubMed]
- 26.ChinaAg. Market reports - The splice must grow: The bright and shady sides of GM agriculture in China. (2016). Available at: https://www.chinaag.org/markets/gm-agriculture-in-china/ [Accessed May 2020].
- 27.APHIS. Interpretive ruling on Asgrow Seed Company petition for determination of nonregulated status: Squash containing the coat protein genes from cucumber mosaic virus (CMV), watermelon mosaic virus 2 (WMV 2) and zucchini yellow mosaic virus (ZYMV). Docket No. 96-002-1 (1995).
- 28.Tricoli DM, et al. Field evaluation of transgenic squash containing single or multiple virus coat protein gene constructs for resistance to cucumber mosaic virus, watermelon mosaic virus 2, and zucchini yellow mosaic virus. Nat. Biotechnol. 1995;13:1458–1465. doi: 10.1038/nbt1295-1458. [DOI] [Google Scholar]
- 29.Shelton AM, Sarwer SH, Hossain MJ, Brookes G, Paranjape V. Impact of Bt Brinjal cultivation in the market value chain in five districts of Bangladesh. Front. Bioeng. Biotechnol. 2020;8:1–12. doi: 10.3389/fbioe.2020.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shelton AM, et al. Bt eggplant project in Bangladesh: History, present status, and future direction. Front. Bioeng. Biotechnol. 2018;6:1–6. doi: 10.3389/fbioe.2018.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alam, S. N. et al. Development of an integrated pest management strategy for eggplant fruit and shoot borer in South Asia. AVRDC3, (2003).
- 32.Moon, K. M., Kwon, E. Bin, Lee, B. & Kim, C. Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules25, 2754 (2020). [DOI] [PMC free article] [PubMed]
- 33.APHIS. Interpretative rule on Okanagan Speciality Fruits petition for determination of nonregulated status: Arctic™ Apple (Malus × domestica). Events GD743 and GS784. Docket No. APHIS-2012-0025 (2012).
- 34.Stowe E, Dhingra A. Development of the Arctic® Apple. Plant Breed. Rev. 2021;44:273–296. doi: 10.1002/9781119717003.ch8. [DOI] [Google Scholar]
- 35.Okanagan Specialty Fruits. Arctic apples: our apples. (2020). Available at: https://arcticapples.com/our-apples/ [Accessed May 2020].
- 36.Zhang J, et al. Decreased protein abundance of lycopene á-cyclase contributesto red flesh in domesticated watermelon. Plant Physiol. 2020;183:1171–1183. doi: 10.1104/pp.19.01409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espley RV, et al. Red colouration in apple fruit is due to the activity of theMYB transcription factor, MdMYB10. Plant J. 2007;49:414–427. doi: 10.1111/j.1365-313X.2006.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitch MMM, Manshardt RM, Gonsalves D, Slightom JL, Sanford JC. Stable transformation of papaya via microprojectile bombardment. Plant Cell Rep. 1990;9:189–194. doi: 10.1007/BF00232177. [DOI] [PubMed] [Google Scholar]
- 39.Tennant P, et al. Papaya ringspot virus resistance of transgenic Rainbow and SunUp is affected by gene dosage, plant development, and coat protein homology. Eur. Eur. J. Plant Pathol. 2001;107:645–653. doi: 10.1023/A:1017936226557. [DOI] [Google Scholar]
- 40.Ivanov KI, Eskelin K, Lõhmus A, Mäkinen K. Molecular and cellular mechanisms underlying potyvirus infection. J. Gen. Virol. 2014;95:1415–1429. doi: 10.1099/vir.0.064220-0. [DOI] [PubMed] [Google Scholar]
- 41.Tennant PF, et al. Differential protection against papaya ringspot virus isolates in coat protein gene transgenic papaya and classically cross-protected papaya. Phytopathology. 1994;84:1359–1366. doi: 10.1094/Phyto-84-1359. [DOI] [Google Scholar]
- 42.Bhagirath, C. & Kadambini, G. The development and regulation of Bt Brinjal in India. ISAAA Briefs38, xii + 102 (2009).
- 43.Armstrong, J. & Lane, W. D. Genetically modified reduced-browning fruit-producing plant and produced fruit thereof, and method of obtaining such. US Patent 8,563,805 B2 (2013).
- 44.Farré G, et al. Engineering complex metabolic pathways in plants. Annu. Rev. Plant Biol. 2014;65:187–223. doi: 10.1146/annurev-arplant-050213-035825. [DOI] [PubMed] [Google Scholar]
- 45.Adams DO, Yang SF. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc. Natl Acad. Sci. 1979;76:170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.USDA. Regulation of biotech plants. (2020). Available at: https://www.usda.gov/topics/biotechnology/how-federal-government-regulates-biotech-plants [Accessed Oct 2020].
- 47.APHIS. About the secure rule. (2020). Available at: https://www.aphis.usda.gov/aphis/ourfocus/biotechnology/biotech-rule-revision/secure-rule/secure-about/340_2017_perdue_biotechreg [Accessed Oct 2020].
- 48.COFEPRIS. Organismos Géneticamente Modificados. (2017). Available at: https://www.gob.mx/cofepris/acciones-y-programas/organismos-geneticamente-modificados [Accessed Aug 2020].
- 49.Health Canada. Safety Assessment of the Flavr Savr™ Tomato. (1997). Available at: https://www.canada.ca/en/health-canada/services/food-nutrition/genetically-modified-foods-other-novel-foods/approved-products/information-safety-assessment-flavr-savr-tomato.html [Accessed Feb, June 2020].
- 50.APHIS. Interpretative ruling on Monsanto Company petition for determination of regulatory status of tomatoes with delayed ripening gene. Docket No. 95-042-1 (1994).
- 51.ILSI. A review of the environmental safety of the Cry1Ac protein. Environ. Biosaf. Res. 2011;10:27–49. doi: 10.1051/ebr:2012002. [DOI] [PubMed] [Google Scholar]
- 52.FDA. New plant variety consultations, Pineapple event EF2-114. BNF No. 149 (2016).
- 53.Fresh del Monte. Pinkglow pineapple. (2020). Available at: https://www.pinkglowpineapple.com/ [Accessed Dec 2020].
- 54.Scorza R, et al. ‘HoneySweet’—a transgenic plum pox virus resistant plum—from laboratory and experimental field plots, to regulatory approval. Acta Hortic. 2013;974:57–64. doi: 10.17660/ActaHortic.2013.974.6. [DOI] [Google Scholar]
- 55.Scorza R, et al. Honeysweet (C5), the first genetically engineered plum pox virus-resistant plum (Prunus domestica L.) cultivar. HortScience. 2016;51:601–603. doi: 10.21273/HORTSCI.51.5.601. [DOI] [Google Scholar]
- 56.Health Canada. Novel food information—Virus resistant squash line CZW-3. (1999). Available at: https://www.canada.ca/en/health-canada/services/food-nutrition/genetically-modified-foods-other-novel-foods/approved-products/virus-resistant-squash-line-czw-3.html [Accessed May 2020].
- 57.ISAAA. Global status of commercialized biotech/GM Crops in 2017: Biotech crop adoption surges as economic benefits accumulate in 22 years. ISAAA Briefs. 2017;53:1–143. [Google Scholar]
- 58.APHIS. Interpretative ruling on Cornell University and the University of Hawaii petition for determination of nonregulated status for ‘Sunset’ papaya lines 55-1 and 63-1. Docket No. 96-024-1 (1996).
- 59.APHIS. Interpretative ruling on University of Florida petition for determination of nonregulated status for X17-2 papaya resistant to papaya ringspot virus. Docket No. APHIS-2008-0054 (2009).
- 60.Health Canada. Arctic® Apple Events GD743 and GS784. (2015). Available at: https://www.canada.ca/en/health-canada/services/food-nutrition/genetically-modified-foods-other-novel-foods/approved-products/novel-food-information-arctic-apple-events-gd743-gs784.html [Accessed June 2020].
- 61.APHIS. Extended determination of nonregulated status for Okanagan Specialty Fruits non-browning Arctic® apple (16-004-01p). Docket No. APHIS-2016-0043 (2016).
- 62.Health Canada. Novel food Information—Arctic Fuji Apple event NF872. (2018). Available at: https://www.canada.ca/en/health-canada/services/food-nutrition/genetically-modified-foods-other-novel-foods/approved-products/arctic-fuji-apple/information.html [Accessed June 2020].
- 63.Statistics Canada. Statistical Overview of the Canadian Fruit Industry 2019. Fruit export volume by commodity – metric tonnes. (2020). Available at: https://www.agr.gc.ca/eng/horticulture/horticulture-sector-reports/statistical-overview-of-the-canadian-fruit-industry-2019/?id=1564485377504 [Accessed Oct 2020].
- 64.USDA. List of bioengineered foods, BE Apple crop. (2020). Available at: https://www.ams.usda.gov/sites/default/files/media/BEAppleCropSummary.pdf [Accessed June 2020].
- 65.Huang J, Wang Q. Agricultural biotechnology development and policy in China. AgBioForum. 2002;5:122–135. [Google Scholar]
- 66.Bangladesh Biosafety. 2017 user’s guide to biosafety regulatory process for genetically engineered plants in Bangladesh. (2017). Available at: https://bangladeshbiosafety.org/wp-content/uploads/2017/11/Users_Guide_to_Biosafety_Regulatory_Process_Bangladesh_2017.pdf [Accessed Aug 2020].
- 67.Brookes, G. & Barfoot, P. GM crops: global socio-economic and environmental impacts 1996-2018. PG Econ. Ltd, UK 1–213 (2020).
- 68.Brookes G, Barfoot P. GM crop technology use 1996–2018: farm income and production impacts. GM Crop. Food. 2020;11:242–261. doi: 10.1080/21645698.2020.1779574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rashid M, Hasan M, Matin M. Socio-economic performance of Bt eggplant cultivation in Bangladesh. Bangladesh. J. Agric. Res. 2018;43:187–203. [Google Scholar]
- 70.Ahmed, A., Hoddinott, J. F., Abedin, N. & Hossain, N. Z. Economic and health impacts of genetically modified eggplant: Results from a randomized controlled trial of Bt brinjal in Bangladesh. IFPRI Discussion paper 01866 (2019).
- 71.Fresh Plaza. Non-browning fruit grower eyes big growth. (2019). Available at: https://www.freshplaza.com/article/9153190/non-browning-fruit-grower-eyes-big-growth/ [Accessed Sept 2020].
- 72.Okanagan Specialty Fruits. Supply chain benefits of Arctic® apples. (2020). Available at: https://www.okspecialtyfruits.com/apple-supply-chain/ [Accessed Oct 2020].
- 73.Wang T, Zhang H, Zhu H. CRISPR technology is revolutionizing the improvement of tomato and other fruit crops. Hortic. Res. 2019;6:77. doi: 10.1038/s41438-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Erpen-Dalla Corte L, et al. Development of Improved Fruit, Vegetable, and Ornamental Crops Using the CRISPR/Cas9 Genome Editing Technique. Plants. 2019;8:601. doi: 10.3390/plants8120601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.FAO. Food loss and waste database (2019). Available at: http://www.fao.org/platform-food-loss-waste/flw-data/en/ [Accessed Jan 2021].
- 76.Szankowski I, et al. Highly scab-resistant transgenic apple lines achieved by introgression of HcrVf2 controlled by different native promoter lengths. Tree Genet. Genomes. 2009;5:349–358. doi: 10.1007/s11295-008-0191-8. [DOI] [Google Scholar]
- 77.Pompili V, Dalla Costa L, Piazza S, Pindo M, Malnoy M. Reduced fire blight susceptibility in apple cultivars using a high-efficiency CRISPR/Cas9-FLP/FRT-based gene editing system. Plant Biotechnol. J. 2020;18:845–858. doi: 10.1111/pbi.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dutt M, Barthe G, Irey M, Grosser J. Transgenic citrus expressing an Arabidopsis NPR1 gene exhibit enhanced resistance against Huanglongbing (HLB; Citrus greening) PLoS ONE. 2015;10:1–17. doi: 10.1371/journal.pone.0137134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tripathi L, et al. Field trial of Xanthomonas wilt disease-resistant bananas in East Africa. Nat. Biotechnol. 2014;32:868–870. doi: 10.1038/nbt.3007. [DOI] [PubMed] [Google Scholar]
- 80.Seo HH, et al. Overexpression of a defensin enhances resistance to a fruit-specific anthracnose fungus in pepper. PLoS One. 2014;9:1–11. doi: 10.1371/journal.pone.0097936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ko M, et al. Constitutive expression of a fungus-inducible carboxylesterase improves disease resistance in transgenic pepper plants. Planta. 2016;244:379–392. doi: 10.1007/s00425-016-2514-6. [DOI] [PubMed] [Google Scholar]
- 82.Peng A, et al. Engineering canker‐resistant plants through CRISPR/Cas9‐targeted editing of the susceptibility gene Cs LOB 1 promoter in citrus. Plant Biotechnol. J. 2017;15:1509–1519. doi: 10.1111/pbi.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang, L. et al. CRISPR/Cas9-mediated editing of CsWRKY22 reduces susceptibility to Xanthomonas citri subsp. citri in Wanjincheng orange (Citrus sinensis (L.) Osbeck. Plant Biotechnol. Rep.13, 501–510 (2019).
- 84.Jia H, Orbovic V, Jones JB, Wang N. Modification of the PthA4 effector binding elements in Type I CsLOB1 promoter using Cas9/sgRNA to produce transgenic Duncan grapefruit alleviating XccΔpthA4: DCsLOB1.3 infection. Plant Biotechnol. J. 2016;14:1291–1301. doi: 10.1111/pbi.12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jia H, et al. Genome editing of the disease susceptibility gene CsLOB1 in citrus confers resistance to citrus canker. Plant Biotechnol. J. 2017;15:817–823. doi: 10.1111/pbi.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malnoy M, Venisse J-S, Brisset M-N, Chevreau E. Expression of bovine lactoferrin cDNA confers resistance to Erwinia amylovora in transgenic pear. Mol. Breed. 2003;12:231–244. doi: 10.1023/A:1026365311067. [DOI] [Google Scholar]
- 87.Barbosa-Mendes JM, et al. Genetic transformation of Citrus sinensis cv. Hamlin with hrpN gene from Erwinia amylovora and evaluation of the transgenic lines for resistance to citrus canker. Sci. Hortic. (Amst.). 2009;122:109–115. doi: 10.1016/j.scienta.2009.04.001. [DOI] [Google Scholar]
- 88.Zou X, et al. Transgenic citrus expressing synthesized cecropin B genes in the phloem exhibits decreased susceptibility to Huanglongbing. Plant Mol. Biol. 2017;93:341–353. doi: 10.1007/s11103-016-0565-5. [DOI] [PubMed] [Google Scholar]
- 89.Shekhawat UKS, Ganapathi TR, Hadapad AB. Transgenic banana plants expressing small interfering RNAs targeted against viral replication initiation gene display high-level resistance to banana bunchy top virus infection. J. Gen. Virol. 2012;93:1804–1813. doi: 10.1099/vir.0.041871-0. [DOI] [PubMed] [Google Scholar]
- 90.Emran AM, Tabei Y, Kobayashi K, Yamaoka N, Nishiguchi M. Molecular analysis of transgenic melon plants showing virus resistance conferred by direct repeat of movement gene of Cucumber green mottle mosaic virus. Plant Cell Rep. 2012;31:1371–1377. doi: 10.1007/s00299-012-1237-9. [DOI] [PubMed] [Google Scholar]
- 91.Lin C-Y, et al. Development of transgenic watermelon resistant to Cucumber mosaic virus and Watermelon mosaic virus by using a single chimeric transgene construct. Transgenic Res. 2012;21:983–993. doi: 10.1007/s11248-011-9585-8. [DOI] [PubMed] [Google Scholar]
- 92.Sidorova T, Mikhailov R, Pushin A, Miroshnichenko D, Dolgov S. Agrobacterium-mediated transformation of Russian commercial plum cv. “Startovaya” (Prunus domestica L.) with virus-derived hairpin RNA construct confers durable resistance to PPV infection in mature plants. Front. Plant Sci. 2019;10:1–15. doi: 10.3389/fpls.2019.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chandrasekaran J, et al. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 2016;17:1140–1153. doi: 10.1111/mpp.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dandekar AM, et al. High levels of expression of full-length cryIA(c) gene from Bacillus thuringiensis in transgenic somatic walnut embryos. Plant Sci. 1998;131:181–193. doi: 10.1016/S0168-9452(97)00256-2. [DOI] [Google Scholar]
- 95.Li Y, et al. Overexpression of a Malus vacuolar Na+/H+ antiporter gene (MdNHX1) in apple rootstock M.26 and its influence on salt tolerance. Plant Cell. Tissue Organ Cult. 2010;102:337–345. doi: 10.1007/s11240-010-9738-0. [DOI] [Google Scholar]
- 96.Tian N, Wang J, Xu ZQ. Overexpression of Na+/H+ antiporter gene AtNHX1 from Arabidopsis thaliana improves the salt tolerance of kiwifruit (Actinidia deliciosa) South Afr. J. Bot. 2011;77:160–169. doi: 10.1016/j.sajb.2010.07.010. [DOI] [Google Scholar]
- 97.Subramanyam K, Sailaja KV, Subramanyam K, Muralidhara Rao D, Lakshmidevi K. Ectopic expression of an osmotin gene leads to enhanced salt tolerance in transgenic chilli pepper (Capsicum annum L.) Plant Cell. Tissue Organ Cult. 2011;105:181–192. doi: 10.1007/s11240-010-9850-1. [DOI] [Google Scholar]
- 98.de Campos MKF, et al. Drought tolerance and antioxidant enzymatic activity in transgenic ‘Swingle’citrumelo plants over-accumulating proline. Environ. Exp. Bot. 2011;72:242–250. doi: 10.1016/j.envexpbot.2011.03.009. [DOI] [Google Scholar]
- 99.Malnoy M, Jin Q, Borejsza-Wysocka EE, He SY, Aldwinckle HS. Overexpression of the apple MpNPR1 gene confers increased disease resistance in Malus × domestica. Mol. Plant-Microbe Interact. 2007;20:1568–1580. doi: 10.1094/MPMI-20-12-1568. [DOI] [PubMed] [Google Scholar]
- 100.Jin W, et al. Improved cold-resistant performance in transgenic grape (Vitis vinifera L.) overexpressing cold-inducible transcription factors AtDREB1b. HortScience. 2009;44:35–39. doi: 10.21273/HORTSCI.44.1.35. [DOI] [Google Scholar]
- 101.Geng J, Wei T, Wang Y, Huang X, Liu JH. Overexpression of PtrbHLH, a basic helix-loop-helix transcription factor from Poncirus trifoliata, confers enhanced cold tolerance in pummelo (Citrus grandis) by modulation of H2O2 level via regulating a CAT gene. Tree Physiol. 2019;39:2045–2054. doi: 10.1093/treephys/tpz081. [DOI] [PubMed] [Google Scholar]
- 102.Soyk S, et al. Variation in the flowering gene SELF PRUNING 5G promotes day-neutrality and early yield in tomato. Nat. Genet. 2017;49:162–168. doi: 10.1038/ng.3733. [DOI] [PubMed] [Google Scholar]
- 103.Tränkner C, et al. Over-expression of an FT-homologous gene of apple induces early flowering in annual and perennial plants. Planta. 2010;232:1309–1324. doi: 10.1007/s00425-010-1254-2. [DOI] [PubMed] [Google Scholar]
- 104.Endo T, et al. Ectopic expression of an FT homolog from Citrus confers anearly flowering phenotype on trifoliate orange (Poncirus trifoliata L. Raf.) Transgenic Res. 2005;14:703–712. doi: 10.1007/s11248-005-6632-3. [DOI] [PubMed] [Google Scholar]
- 105.Srinivasan C, Dardick C, Callahan A, Scorza R. Plum (Prunus domestica) trees transformed with poplar FT1 result in altered architecture, dormancy requirement, and continuous flowering. PLoS ONE. 2012;7:1–11. doi: 10.1371/journal.pone.0040715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Varkonyi‐Gasic E, et al. Mutagenesis of kiwifruit CENTRORADIALIS‐like genes transforms a climbing woody perennial with long juvenility and axillary flowering into a compact plant with rapid terminal flowering. Plant Biotechnol. J. 2019;17:869–880. doi: 10.1111/pbi.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dandekar AM, et al. Effect of down-regulation of ethylene biosynthesis on fruit flavor complex in apple fruit. Transgenic Res. 2004;13:373–384. doi: 10.1023/B:TRAG.0000040037.90435.45. [DOI] [PubMed] [Google Scholar]
- 108.Atkinson RG, et al. Dissecting the role of climacteric ethylene in kiwifruit (Actinidia chinensis) ripening using a 1-aminocyclopropane-1-carboxylic acid oxidase knockdown line. J. Exp. Bot. 2011;62:3821–3835. doi: 10.1093/jxb/err063. [DOI] [PubMed] [Google Scholar]
- 109.López-Gómez R, et al. Ripening in papaya fruit is altered by ACC oxidase cosuppression. Transgenic Res. 2009;18:89–97. doi: 10.1007/s11248-008-9197-0. [DOI] [PubMed] [Google Scholar]
- 110.Gao M, et al. Gene expression and ethylene production in transgenic pear (Pyrus communis cv.‘La France’) with sense or antisense cDNA encoding ACC oxidase. Plant Sci. 2007;173:32–42. doi: 10.1016/j.plantsci.2007.03.014. [DOI] [Google Scholar]
- 111.Park J-I, et al. Modification of sugar composition in strawberry fruit by antisense suppression of an ADP-glucose pyrophosphorylase. Mol. Breed. 2006;17:269–279. doi: 10.1007/s11032-005-5682-9. [DOI] [Google Scholar]
- 112.Cutanda-Perez MC, et al. Ectopic expression of VlmybA1 in grapevine activates a narrow set of genes involved in anthocyanin synthesis and transport. Plant Mol. Biol. 2009;69:633–648. doi: 10.1007/s11103-008-9446-x. [DOI] [PubMed] [Google Scholar]
- 113.Lin-Wang K, et al. An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biol. 2010;10:50. doi: 10.1186/1471-2229-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pons E, et al. Metabolic engineering of β-carotene in orange fruit increases its in vivo antioxidant properties. Plant Biotechnol. J. 2014;12:17–27. doi: 10.1111/pbi.12112. [DOI] [PubMed] [Google Scholar]
- 115.Rugini, E., Bashir, M. A., Cristofori, V., Ruggiero, B. & Silvestri, C. A review of genetic improvement of main fruit trees through modern biotechnological tools and considerations of the cultivation and research of the engineered plant restrictions. Pakistan J. Agric. Sci. 57, 17–42 (2020).
- 116.Albacete A, et al. Unravelling rootstock×scion interactions to improve food security. J. Exp. Bot. 2015;66:2211–2226. doi: 10.1093/jxb/erv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Orbović, V. Editorial: new developments in Agrobacterium-Mediated transformation of tree fruit crops. Front. Plant Sci. 10.3389/fpls.2019.01253 (2019). [DOI] [PMC free article] [PubMed]
- 118.Mitter, N. & Gleeson, M. Acceptance of disease-resistant GM rootstocks for non-GM fruit. In: XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): III 1124. pp 91–96 (2014).
- 119.Smolka A, Li X-Y, Heikelt C, Welander M, Zhu L-H. Effects of transgenicrootstocks on growth and development of non-transgenic scion cultivars inapple. Transgenic Res. 2010;19:933–948. doi: 10.1007/s11248-010-9370-0. [DOI] [PubMed] [Google Scholar]
- 120.Dandekar AM, et al. Trans-graft protection against Pierce’s disease mediated by transgenic grapevine rootstocks. Front. Plant Sci. 2019;10:84. doi: 10.3389/fpls.2019.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen L, et al. A method for the production and expedient screening of CRISPR/Cas9-mediated non-transgenic mutant plants. Hortic. Res. 2018;5:1–12. doi: 10.1038/s41438-017-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tian S, et al. Engineering herbicide-resistant watermelon variety through CRISPR/Cas9-mediated base-editing. Plant Cell Rep. 2018;37:1353–1356. doi: 10.1007/s00299-018-2299-0. [DOI] [PubMed] [Google Scholar]
- 123.Hu B, et al. Engineering non-transgenic gynoecious cucumber using an improved transformation protocol and optimized CRISPR/Cas9 system. Mol. Plant. 2017;10:1575–1578. doi: 10.1016/j.molp.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 124.Zhou J, Wang G, Liu Z. Efficient genome editing of wild strawberry genes, vector development and validation. Plant Biotechnol. J. 2018;16:1868–1877. doi: 10.1111/pbi.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nekrasov V, et al. Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci. Rep. 2017;7:1–6. doi: 10.1038/s41598-017-00578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Veillet F, et al. Transgene-free genome editing in tomato and potato plants using Agrobacterium-mediated delivery of a CRISPR/Cas9 cytidine base editor. Int. J. Mol. Sci. 2019;20:1–10. doi: 10.3390/ijms20020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou J, et al. Application and future perspective of CRISPR/Cas9 genome editing in fruit crops. J. Integr. Plant Biol. 2020;62:269–286. doi: 10.1111/jipb.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sattar MN, et al. CRISPR/Cas9: a practical approach in date palm genome editing. Front. Plant Sci. 2017;8:1469. doi: 10.3389/fpls.2017.01469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nishitani C, et al. Efficient genome editing in apple using a CRISPR/Cas9 system. Sci. Rep. 2016;6:1–8. doi: 10.1038/srep31481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Malnoy M, et al. DNA-free genetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front. Plant Sci. 2016;7:1–9. doi: 10.3389/fpls.2016.01904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Osakabe Y, et al. CRISPR–Cas9-mediated genome editing in apple and grapevine. Nat. Protoc. 2018;13:2844–2863. doi: 10.1038/s41596-018-0067-9. [DOI] [PubMed] [Google Scholar]
- 132.Metje-Sprink J, Menz J, Modrzejewski D, Sprink T. DNA-free genome editing: past, present and future. Front Plant Sci. 2019;9:1957. doi: 10.3389/fpls.2018.01957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Woo JW, et al. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat. Biotechnol. 2015;33:1162–1164. doi: 10.1038/nbt.3389. [DOI] [PubMed] [Google Scholar]
- 134.Kim J, Chang C, Tucker ML. To grow old: regulatory role of ethylene and jasmonic acid in senescence. Front Plant Sci. 2015;6:20. doi: 10.3389/fpls.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ghogare R, Williamson-Benavides B, Ramírez-Torres F, Dhingra A. CRISPR-associated nucleases: the Dawn of a new age of efficient crop improvement. Transgenic Res. 2020;29:1–35. doi: 10.1007/s11248-019-00181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Begemann MB, et al. Precise insertion and guided editing of higher plant genomes using Cpf1 CRISPR nucleases. Sci. Rep. 2017;7:1–6. doi: 10.1038/s41598-017-11760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jia, H., Zou, X., Orbovic, V. & Wang, N. in Plant Genome Editing with CRISPR Systems (ed Qi, Y.) 235–241 (Springer, 2019).
- 138.Yao, J. L. et al. Ectopic expression of the PISTILLATA homologous MdPI inhibits fruit tissue growth and changes fruit shape in apple. Plant Direct2 (2018). [DOI] [PMC free article] [PubMed]
- 139.Jia D, et al. Overexpression of a novel apple NAC transcription factor gene, MdNAC1, confers the dwarf phenotype in transgenic apple (Malus domestica) Genes (Basel) 2018;9:229. doi: 10.3390/genes9050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pasquali G, Biricolti S, Locatelli F, Baldoni E, Mattana M. Osmyb4 expression improves adaptive responses to drought and cold stress in transgenic apples. Plant Cell Rep. 2008;27:1677–1686. doi: 10.1007/s00299-008-0587-9. [DOI] [PubMed] [Google Scholar]
- 141.Shao X, et al. Using CRISPR/Cas9 genome editing system to create MaGA20ox2 gene-modified semi-dwarf banana. Plant Biotechnol. J. 2020;18:17–19. doi: 10.1111/pbi.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tripathi JN, et al. CRISPR/Cas9 editing of endogenous banana streak virus in the B genome of Musa spp. overcomes a major challenge in banana breeding. Commun. Biol. 2019;2:1–11. doi: 10.1038/s42003-019-0288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tripathi L, Mwaka H, Tripathi JN, Tushemereirwe WK. Expression of sweet pepper Hrap gene in banana enhances resistance to Xanthomonas campestris pv. musacearum. Mol. Plant Pathol. 2010;11:721–731. doi: 10.1111/j.1364-3703.2010.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Namukwaya B, et al. Transgenic banana expressing Pflp gene confers enhanced resistance to Xanthomonas wilt disease. Transgenic Res. 2012;21:855–865. doi: 10.1007/s11248-011-9574-y. [DOI] [PubMed] [Google Scholar]
- 145.Kaur N, et al. CRISPR/Cas9 directed editing of lycopene epsilon-cyclase modulates metabolic flux for β-carotene biosynthesis in banana fruit. Metab. Eng. 2020;59:76–86. doi: 10.1016/j.ymben.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 146.Song Gqing, Chen Q. Overexpression of the MADS-box gene K-domain increases the yield potential of blueberry. Plant Sci. 2018;276:22–31. doi: 10.1016/j.plantsci.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 147.Qi X, Liu C, Song L, Li Y, Li M. PaCYP78A9, a cytochrome P450, regulates fruit size in sweet cherry (Prunus avium L.) Front. Plant Sci. 2017;8:1–14. doi: 10.3389/fpls.2017.02076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Song G, et al. Engineering cherry rootstocks with resistance to Prunus necrotic ring spot virus through RNA i‐mediated silencing. Plant Biotechnol. J. 2013;11:702–708. doi: 10.1111/pbi.12060. [DOI] [PubMed] [Google Scholar]
- 149.Fagoaga C, et al. Engineering of gibberellin levels in citrus by sense and antisense overexpression of a GA 20-oxidase gene modifies plant architecture. J. Exp. Bot. 2007;58:1407–1420. doi: 10.1093/jxb/erm004. [DOI] [PubMed] [Google Scholar]
- 150.Hao G, Stover E, Gupta G. Overexpression of a modified plant thionin enhances disease resistance to citrus canker and huanglongbing (HLB) Front. Plant Sci. 2016;7:1–11. doi: 10.3389/fpls.2016.01078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cervera M, et al. Transgenic expression in citrus of single-chain antibody fragments specific to Citrus tristeza virus confers virus resistance. Transgenic Res. 2010;19:1001–1015. doi: 10.1007/s11248-010-9378-5. [DOI] [PubMed] [Google Scholar]
- 152.Vigne E, Komar V, Fuchs M. Field safety assessment of recombination in transgenic grapevines expressing the coat protein gene of Grapevine fanleaf virus. Transgenic Res. 2004;13:165–179. doi: 10.1023/B:TRAG.0000026075.79097.c9. [DOI] [PubMed] [Google Scholar]
- 153.Zok A, et al. Effect of Medicago sativa ferritin gene on stress tolerance in transgenic grapevine. Plant Cell. Tissue Organ Cult. 2010;100:339–344. doi: 10.1007/s11240-009-9641-8. [DOI] [Google Scholar]
- 154.Kim M, et al. Transformation of carotenoid biosynthetic genes using a micro-cross section method in kiwifruit (Actinidia deliciosa cv. Hayward) Plant Cell Rep. 2010;29:1339–1349. doi: 10.1007/s00299-010-0920-y. [DOI] [PubMed] [Google Scholar]
- 155.Wang C, et al. Molecular cloning and heterologous expression analysis of JrVTE1 gene from walnut (Juglans regia) Mol. Breed. 2015;35:1–12. doi: 10.1007/s11032-015-0202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rubio J, et al. Silencing of one copy of the translation initiation factor eIFiso4G in Japanese plum (Prunus salicina) impacts susceptibility to Plum pox virus (PPV) and small RNA production. BMC Plant Biol. 2019;19:1–12. doi: 10.1186/s12870-019-2047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Martín-Pizarro C, Triviño JC, Posé D. Functional analysis of the TM6 MADS-box gene in the octoploid strawberry by CRISPR/Cas9-directed mutagenesis. J. Exp. Bot. 2019;70:885–895. doi: 10.1093/jxb/ery400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee YK, Kim IJ. Modulation of fruit softening by antisense suppression of endo-β-1,4-glucanase in strawberry. Mol. Breed. 2011;27:375–383. doi: 10.1007/s11032-010-9438-9. [DOI] [Google Scholar]
- 159.Yu QH, et al. CRISPR/Cas9-induced targeted mutagenesis and gene replacement to generate long-shelf life tomato lines. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Klap C, et al. Tomato facultative parthenocarpy results from Sl AGAMOUS-LIKE 6 loss of function. Plant Biotechnol. J. 2017;15:634–647. doi: 10.1111/pbi.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li X, et al. Lycopene is enriched in tomato fruit by CRISPR/Cas9-mediated multiplex genome editing. Front. Plant Sci. 2018;9:1–12. doi: 10.3389/fpls.2018.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tang X, et al. Overexpression of Pto activates defense responses and confers broad resistance. Plant Cell. 1999;11:15–29. doi: 10.1105/tpc.11.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.