Abstract
Purpose:
Several immune checkpoint inhibitors (ICIs) are FDA approved for treatment of genitourinary (GU) malignancies. We aim to determine demographic and clinicopathologic characteristics that significantly affect clinical outcomes in patients with advanced stage GU malignancies treated with ICIs.
Materials and Methods:
We performed a single-center, consecutive, retrospective cohort analysis on patients with metastatic or unresectable GU malignancies who were treated with ICIs at the University of Michigan. Immune-related adverse events (irAEs), putative immune-mediated allergies, and overall response rates (ORR) were assessed. Comorbidity index scores were calculated. Survival analysis was performed to evaluate progression-free survival (PFS) and overall survival (OS), stratifying and controlling for a variety of clinicopathologic baseline factors including site of metastases.
Results:
160 patients were identified with advanced renal cell carcinoma (RCC) or urothelial carcinoma. Median PFS and OS were 5.0 and 23.6 months for RCC, and 2.8 and 9.6 months for urothelial carcinoma, respectively. Patients who experienced increased frequency and higher grade irAEs had better ICI treatment response (p<0.0001). Presence of liver metastases was associated with poor response to ICI therapy (p=0.001). Multivariable modeling demonstrates that patients with urothelial carcinoma and liver metastases had statistically worse PFS and OS compared to patients with RCC or other sites of metastases, respectively.
Conclusion:
Greater frequency and higher grades of irAEs are associated with better treatment response in patients with RCC and urothelial malignancy receiving ICI therapy. The presence of liver metastases denotes a negative predictive marker for immunotherapy efficacy.
Keywords: Genitourinary malignancy, Immune checkpoint inhibitors, Renal cell carcinoma, Urothelial carcinoma, Liver metastases, Immune-related adverse events
Summary
Immune checkpoint inhibitors (ICI) are increasingly used to treat genitourinary (GU) malignancies. However, clinical data regarding patients with advanced-stage GU malignancies treated with ICI is lacking. Thus, we performed a single-center, retrospective cohort study on patients with metastatic and unresectable renal cell carcinoma (RCC) and urothelial carcinoma who were treated with ICIs at the University of Michigan to provide demographic and clinicopathologic data regarding this population. We specifically focused on immune-related adverse events (irAEs), immune-mediated allergies, and the associated overall response rates (ORR). To better assess performance status, we calculated comorbidity scores for all patients. Finally, survival analyses for progression-free survival (PFS) and overall survival (OS) were performed using Kaplan-Meier analysis and Cox proportional hazards modeling, stratifying and controlling for clinicopathologic baseline factors, including sites of metastases, in our multivariable analysis.
A total of 160 patients were identified with advanced RCC or urothelial carcinoma. We found decreased PFS (2.8 vs 5.0 months) and decreased OS (9.8 vs 23.6 months) for urothelial carcinoma compared to RCC patients. We noted that patients who experienced increased frequency and higher grades of irAEs had better treatment ORR with ICI therapy (p=<0.0001). The presence of liver metastases was associated with worse ORR (p=0.001), PFS (p=0.0014), and OS (p=0.0028) compared to other sites of metastases including lymph node, lung, and CNS/bone. The poor PFS and OS associated with urothelial carcinoma and liver metastases were preserved in our multivariable modeling after controlling for pertinent clinical factors.
We conclude that greater frequency and higher grades of irAEs are associated with better treatment response in GU malignancy patients receiving ICI, a finding that is consistent with published studies in other cancers. The presence of liver metastases represents a significantly poor predictive marker in GU malignancy treated with ICI. Our findings contribute to the growing body of literature that seeks to understand the clinicopathologic variables and outcomes associated with ICI therapy.
1.1. Introduction
Genitourinary (GU) malignancies represent a heterogenous population of cancers that is specific to an anatomical and physiological function [1,2]. The most common histological subtypes include prostate adenocarcinoma, renal cell carcinoma (RCC), and urothelial carcinoma of bladder, ureter, and renal pelvis. An estimated 67,000 Americans are expected to die from GU malignancies in 2020 [3].
As our understanding of tumor immunogenicity has grown, immune checkpoint inhibitors (ICIs) have entered the therapeutic landscape for effective management of patients with GU malignancies, particularly for RCC and urothelial carcinoma. Several ICIs are approved for management of advanced staged GU malignancies [4]. FDA approved ICIs for RCC include anti-PD-1 inhibitors (pembrolizumab, nivolumab) and combination anti-CTLA-4 and anti-PD-1 inhibitor (ipilimumab/nivolumab). ICIs approved for urothelial carcinoma include anti-PD-1 inhibitors (pembrolizumab, nivolumab) and anti-PD-L1 inhibitors (atezolizumab, durvalumab, avelumab). Due to disappointing outcomes of ICIs in prostate cancer [5], such therapies are currently available through clinical trials.
There remains an unmet need to identify factors that better predict therapy efficacy with ICIs. With the advent of ICIs, previous studies considered possible connections between autoimmunity and other biomarkers that may be used to predict ICI therapy response. As immune-related adverse events (irAEs) are thought to be the result of ICI-mediated autoimmune responses against nonmalignant tissue, ICI-treated patients with autoimmune diseases comprise a unique study population. Although these patients were mostly excluded from clinical trials due to confounding and concern for increased ICI-mediated toxicities, retrospective studies demonstrated that irAEs are not an immediate contraindication to ICI in patients with autoimmune conditions [6]. While these patients often experienced more irAEs, there does not appear to be a significant difference in treatment response compared against those without baseline autoimmune disease [7,8]. This data does raise questions about whether other forms of immune activation, including pre-existing drug immune-mediated allergies, may affect ICI treatment response and development of irAEs. Thus, irAEs has been proposed as a possible clinical biomarker of ICI treatment response, along with tumor burden [9].
In this study, we conducted a retrospective analysis on outcomes of patients with advanced stage RCC and urothelial carcinoma patients treated with ICIs. We specifically evaluated comorbidity indices, pre-existing immune-mediated drug allergies, sites of disease metastases, and frequency and grade irAEs that may lead to differential treatment responses and/or survival outcomes.
2.1. Materials and Methods
2.1.1. Study Population
We performed a single-institution, retrospective cohort analysis on consecutive patients diagnosed with advanced stage RCC and urothelial carcinoma at the University of Michigan between 2011 to 2018 treated with any FDA approved ICI including nivolumab, pembrolizumab, atezolizumab, or combination ipilimumab/nivolumab. The cohort included patients who were treatment naïve or previously treated with other systemic agents before receiving the ICI. Patients had histologically proven unresectable stage III or stage IV disease following American Joint Committee on Cancer (AJCC) 8th edition criteria [10].
Eligible patients were identified by pharmacy administration records and data was collected via electronic medical record (EMR) system review and stored in a REDCap database hosted by the University of Michigan. Institutional review board approval was obtained (HUM00139259).
2.1.2. Study Design
Patients were stratified based on GU malignancy subtype. We characterized baseline patient demographics including age, gender, and race (Table 1). Comorbidity assessments of each patient using the Charlson Comorbidity Index [11] and National Cancer Institute (NCI) Comorbidity Index [12,13] were documented before examination of therapy options. Other variables assessed included patient immune-mediated allergies, duration of treatment with ICI, and sites of metastases. Endpoints of each treatment included best overall response rate (ORR), treatment-related toxicities, progression free survival (PFS), and overall survival (OS).
Table 1.
Patient Characteristics
| RCC (n = 88) | Urothelial (n = 72) | Overall (n = 160) | |
|---|---|---|---|
| Gender | |||
| Female | 25 (28.4%) | 22 (30.6%) | 47 (29.4%) |
| Male | 63 (71.6%) | 50 (69.4%) | 113 (70.6%) |
| Age | |||
| Mean (SD) | 61.3 (8.57) | 70.9 (11.0) | 65.6 (10.8) |
| Primary Race | |||
| Caucasian | 83 (94.3%) | 67 (93.1%) | 150 (93.8%) |
| African American | 3 (3.4%) | 2 (2.8%) | 5 (3.1%) |
| Other/Unknown | 2 (2.3%) | 3 (4.2%) | 5 (3.0%) |
| Ethnicity | |||
| Non-Hispanic | 86 (97.7%) | 70 (97.2%) | 156 (97.5%) |
| Hispanic or Latino | 1 (1.1%) | 0 (0%) | 1 (0.6%) |
| Unknown | 1 (1.1%) | 2 (2.8%) | 3 (1.9%) |
| Charlson Comorbidity Index | |||
| 2 – 4 | 38 (43.2%) | 37 (51.4%) | 75 (46.9%) |
| 5 – 7 | 28 (31.8%) | 16 (22.2%) | 44 (27.5%) |
| ≥8 | 22 (25.0%) | 19 (26.4%) | 41 (25.6%) |
| NCI Comorbidity Index | |||
| 0 | 47 (53.4%) | 33 (45.8%) | 80 (50.0%) |
| 0.01–2 | 22 (25.0%) | 24 (33.3%) | 46 (28.8%) |
| >2 | 19 (21.6%) | 15 (20.8%) | 34 (21.3%) |
| Site of Metastasis | |||
| Lymph node only1 | 33 (37.5%) | 34 (47.2%) | 67 (41.9%) |
| Lung/other2 | 19 (21.6%) | 11 (15.3%) | 30 (18.8%) |
| CNS/Bone3 | 20 (22.7%) | 18 (25.0%) | 38 (23.8%) |
| Liver4 | 16 (18.2%) | 9 (12.5%) | 25 (15.6%) |
| First Line Treatment with ICI Agent | |||
| No | 69 (78.4%) | 64 (88.9%) | 133 (83.1%) |
| Yes | 19 (21.6%) | 8 (11.1%) | 27 (16.9%) |
| Number of ICI Regimens | |||
| 1 | 77 (87.5%) | 72 (100%) | 149 (93.1%) |
| 2 | 11 (12.5%) | 0 (0%) | 11 (6.9%) |
| First ICI Agent | |||
| Nivolumab | 70 (79.5%) | 0 (0%) | 70 (43.8%) |
| Pembrolizumab | 5 (5.7%) | 48 (66.7%) | 53 (33.1%) |
| Atezolizumab | 0 (0%) | 24 (33.3%) | 24 (15.0%) |
| Ipilimumab/N ivolumab | 13 (14.8%) | 0 (0%) | 13 (8.1%) |
| Median (25, 75) duration of therapy (months) | 2.3 (1.4, 5.5) | 2.1 (0.8, 4.6) | 2.2 (0.9, 5.1) |
| Second ICI Agent if applicable | |||
| Nivolumab | 10 (11.4%) | 0 (0%) | 10 (6.2%) |
| Pembrolizumab | 1 (1.1%) | 0 (0%) | 1 (0.6%) |
| Median (25, 75) duration of therapy (months) | 2.1 (0.9, 2.5) | N/A | 2.1 (0.9, 2.5) |
Lymph node metastases only
Lung metastases without bone, CNS, or liver metastases
CNS and/or bone metastases without liver metastases
Liver metastases with any other sites of metastases
RCC = renal cell carcinoma; SD = standard deviation; CNS = central nervous system; ICI = immune checkpoint inhibitor
2.1.3. Definition of Variables
We assessed best ORR by utilizing the revised RECIST guideline (version 1.1) as measured by complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) [14]. Immune-related adverse events (irAEs) were defined as any new autoimmune toxicity involving one or more organ systems attributable to ICI. The number of irAEs was recorded in association with the designated ICI treatment. Toxicities associated with the irAEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0 [15].
Sites of metastatic disease were categorized into four groups: (1) lymph node disease involvement only; (2) lung metastases with or without other sites of metastases, excluding bone, central nervous system (CNS), or liver metastases; (3) bone and/or CNS metastases, excluding liver metastases; (4) liver metastases with or without other sites of metastases.
Pre-existing reported drug immune-mediated allergies were obtained via EMR. Immune-mediated allergies were distinguished from adverse drug reactions by definition of an abnormal immunologic response to a medication with one or more of the following reported symptoms: hives, itching or pruritis, dyspnea or shortness of breath, wheezing, watery eyes, rhinorrhea or runny nose, throat swelling, or anaphylaxis. Food and topical allergies were excluded.
Comorbidities were extracted from EMR up to time of ICI initiation. The NCI Comorbidity Index and Charlson Comorbidity Index were calculated from review of pertinent medical history. The Charlson Comorbidity Index and the NCI Comorbidity Index values were grouped into three categories: 2–4, 5–7, ≥8, and 0, 0.01–2, and >2, respectively.
2.1.4. Statistical Methods
Demographic and clinical variables were summarized using means, standard deviations, ranges, and frequencies. OS and PFS was measured from the date of ICI initiation. OS was determined based on EMR review. PFS was defined as time to clinical progression on imaging by RECIST v1.1 criteria or date of death. Patients alive at the time of the analysis were censored at last known follow up. Kaplan-Meier plots were used to evaluate OS and PFS. Cox proportional hazards models were used to further assess survival outcomes. Multivariable Cox regression models were performed to compare the effects of immunotherapy on survival from the initiation of therapy adjusted by GU malignancy subtype age, gender, NCI comorbidity index, number of immune-mediated allergies, and site of metastases. The association between all variables, except GU malignancy subtype and gender, with best ORR (scored as PD=0, SD=1, PR=2, CR=3), number of irAEs, and grade irAEs were assessed using Spearman’s correlation coefficient. The association between best ORR and irAEs controlling for duration of therapy, comorbidity index, and ICI therapy type was assessed using a stratified Spearman’s correlation coefficient [16]. GU malignancy and gender association was assessed using Mann-Whitney U test. All analyses were performed in R (version 3.6.0).
3.1. Results
160 patients with advanced stage GU malignancies were identified in our study (Table 1). 88 patients had RCC and 72 patients had urothelial carcinoma. 21.6% of RCC and 11.1% of urothelial carcinoma patients received ICI therapy in the first-line treatment setting. 70 patients received nivolumab, 53 patients received pembrolizumab, 24 patients received atezolizumab, and 13 patients received combination ipilimumab/nivolumab. Median duration of therapy for patients treated with ICI was 2.3 months and 2.1 months for RCC and urothelial carcinoma respectively.
Median follow-up was 10.8 months. Overall median age was 65.6 years old. Median patient age in the RCC and urothelial carcinoma cohort was 61.3 and 70.9 years, respectively. The majority of the patients were Caucasian and non-Hispanic, with most having low comorbidity scores. Sites of metastatic disease were documented prior to initiation of ICI. Lymph node only disease was present in 37.5% and 47.2% in the RCC and urothelial carcinoma cohort, respectively. Presence of lung metastases were seen in 21.6% and 15.3% in the RCC and urothelial carcinoma cohort, respectively. 22.7% in the RCC cohort and 25% of the urothelial carcinoma cohort had sites of metastases that included CNS and/or bone, but without liver metastases. The presence of liver metastases with any other sites of disease involvement was present in 18.2% and 12.5% in the RCC and urothelial carcinoma cohort, respectively.
Univariate analysis demonstrates site of metastatic disease correlated significantly with ORR to immunotherapy (p=0.001, Table 2a). There was a positive trend seen with ICI therapy and improved ORR in RCC patients (compared to urothelial carcinoma, p=0.33) and 1 or ≥2 (compared to 0, p=0.35) immune-mediated allergies. Patients with higher grade irAEs had better ORR (rho=0.37, p<0.0001, Table 2b). The strength of the association was still positive when controlling for duration of therapy (rho=0.21, p=0.01), comorbidity index (rho=0.37, p<0.0001), and ICI therapy type (rho=0.38, p<0.0001). The association between number of irAE’s and ORR was stronger (rho=0.48, p<0.0001). The strength of the association was still positive when controlling for duration of therapy (rho=0.35, p<0.0001), comorbidity index (rho=0.48, p<0.0001), and ICI therapy type (rho=0.50, p<0.0001). There was no statistical difference in ORR among comparative groups by age, gender, NCI Comorbidity Index, Charlson Comorbidity Index, and number of irAEs.
Table 2a.
Overall response rates to immune checkpoint inhibitor (ICI) therapy stratified by the following baseline characteristics: GU malignancy subtype; age; gender; NCI Comorbidity Index; Charlson Comorbidity Index; number of immune-mediated allergies; and sites of metastases. n = 132.
| Variable | Description | p-value | Responsea | |||
|---|---|---|---|---|---|---|
| Complete Response | Partial Response | Stable Disease | Progressive Disease | |||
| GU Malignancy Subtype | RCC | 0.33b | 7 (9.3%) | 21 (28.0%) | 13 (17.3%) | 34 (45.3%) |
| Urothelial | 4 (7.0%) | 11 (19.3%) | 13 (22.8%) | 29 (50.9%) | ||
| Age | <65 | 0.87c | 6 (9.4%) | 13 (20.3%) | 9 (14.1%) | 36 (56.2%) |
| ≥65 | 5 (7.4%) | 19 (27.9%) | 17 (25.0%) | 27 (39.7%) | ||
| Gender | Male | 0.81b | 10 (11.2%) | 21 (23.6%) | 14 (15.7%) | 44 (49.4%) |
| Female | 1 (2.3%) | 11 (25.6%) | 12 (27.9%) | 19 (44.2%) | ||
| NCI Comorbidity Index | 0 | 0.79c | 6 (9.4%) | 16 (25.0%) | 9 (14.1%) | 33 (51.6%) |
| 0.01–2 | 3 (7.5%) | 9 (22.5%) | 11 (27.5%) | 17 (32.5%) | ||
| >2 | 2 (7.1%) | 7 (25.0%) | 6 (21.4%) | 13 (46.4%) | ||
| Charlson Comorbidity Index | 2–4 | 0.71c | 7 (10.8%) | 16 (24.6%) | 12 (18.5%) | 30 (46.2%) |
| 5–7 | 2 (6.1%) | 8 (24.2%) | 4 (12.1%) | 19 (57.6%) | ||
| ≥8 | 2 (5.9%) | 8 (23.5%) | 10 (29.4%) | 14 (41.2%) | ||
| Number of Allergies | 0 | 0.35c | 5 (9.8%) | 11 (21.6%) | 7 (13.7%) | 28 (54.9%) |
| 1 | 3 (7.3%) | 10 (24.4%) | 9 (22.0%) | 19 (46.3%) | ||
| ≥2 | 3 (7.5%) | 11 (27.5%) | 10 (25.0%) | 16 (40.0%) | ||
| Site of Metastases | Lymph node | 0.001c* | 3 (5.3%) | 20 (35.1%) | 16 (28.1%) | 18 (31.6%) |
| Lung/other | 5 (19.2%) | 5 (19.2%) | 3 (11.5%) | 13 (50.0%) | ||
| CNS/Bone | 3 (9.4%) | 5 (15.6%) | 6 (18.8%) | 18 (56.2%) | ||
| Liver | 0 (0%) | 2 (11.8%) | 1 (5.9%) | 14 (82.4%) | ||
| Overall | 11 (8.3%) | 32 (24.2%) | 26 (19.7%) | 63 (47.7%) | ||
Statistically significant
Tumor response to immunotherapy per RECIST (Response Evaluation Criteria in Solid Tumors) version 1.1
Mann-Whitney U test
Spearman’s rank correlation test
GU = genitourinary; RCC = renal cell carcinoma; Urothelial Cell = urothelial cell carcinoma
Table 2b.
Overall response rates to immune checkpoint inhibitor (ICI) therapy stratified by the following outcomes: number of irAEs and grade toxicity of irAEs. n = 132.
| Variable | Description | rho p-valueb |
Responsea | |||
|---|---|---|---|---|---|---|
| Complete Response | Partial Response | Stable Disease | Progressive Disease | |||
| Number of irAEs | 0 | 0.48 <0.0001* |
3 (5.2%) | 7 (12.1%) | 6 (10.3%) | 42 (72.4%) |
| 1 | 2 (4.8%) | 10 (23.8%) | 13 (31.0%) | 17 (40.5%) | ||
| ≥2 | 6 (18.8%) | 15 (46.9%) | 7 (21.9%) | 4 (12.5%) | ||
| Grade Toxicity of irAEsc | 0 | 0.37 <0.0001* |
3 (5.2%) | 7 (12.1%) | 6 (10.3%) | 42 (72.4%) |
| 1 | 4 (13.8%) | 11 (37.9%) | 5 (17.2%) | 9 (31.0%) | ||
| 2 | 1 (3.4%) | 8 (27.6%) | 12 (41.4%) | 8 (27.6%) | ||
| ≥3 | 3 (18.8%) | 6 (37.5%) | 3 (18.8%) | 4 (25.0%) | ||
| Overall | 11 (8.3%) | 32 (24.2%) | 26 (19.7%) | 63 (47.7%) | ||
Statistically significant
Tumor response to immunotherapy per the RECIST (Response Evaluation Criteria in Solid Tumors) version 1.1
Spearman’s rank correlation test
CTCAE (Common Terminology Criteria for Adverse Events) version 5
irAEs = immune related adverse events
Median PFS were 5.0 months and 2.8 months for RCC and urothelial carcinoma, respectively (Figure 1). Median OS was 23.6 months and 9.6 months for RCC and urothelial carcinoma respectively (Figure 2). Multivariable Cox modeling demonstrates that patients with liver metastases had worse PFS compared to nodal only metastases [HR 3.06, 95% CI: 1.75–5.35, p=0.0001]; lung metastases [HR 2.50, 95% CI: 1.33–4.71, p=0.0044]; and CNS and/or bone metastases [HR 2.49, 95% CI: 1.30–4.41, p=0.0053] (Table 3a). Hazard for PFS was statistically worse for urothelial carcinoma compared to RCC [HR 1.91, 95% CI: 1.23–2.95, p=0.0037]. In regards to OS, patients with liver metastases also had worse OS compared to nodal only metastases [HR 3.12, 95% CI: 1.66–5.87, p=0.0004]; lung metastases [HR 3.77, 95% CI: 1.76–8.08, p=0.0007]; and CNS and/or bone metastases [HR 2.06, 95% CI: 1.03–4.15, p=0.0420] (Table 3b). Hazard for OS was also statistically worse for urothelial carcinoma compared to RCC [HR 2.61, 95% CI: 1.55–4.39, p=0.0003].
Figure 1:
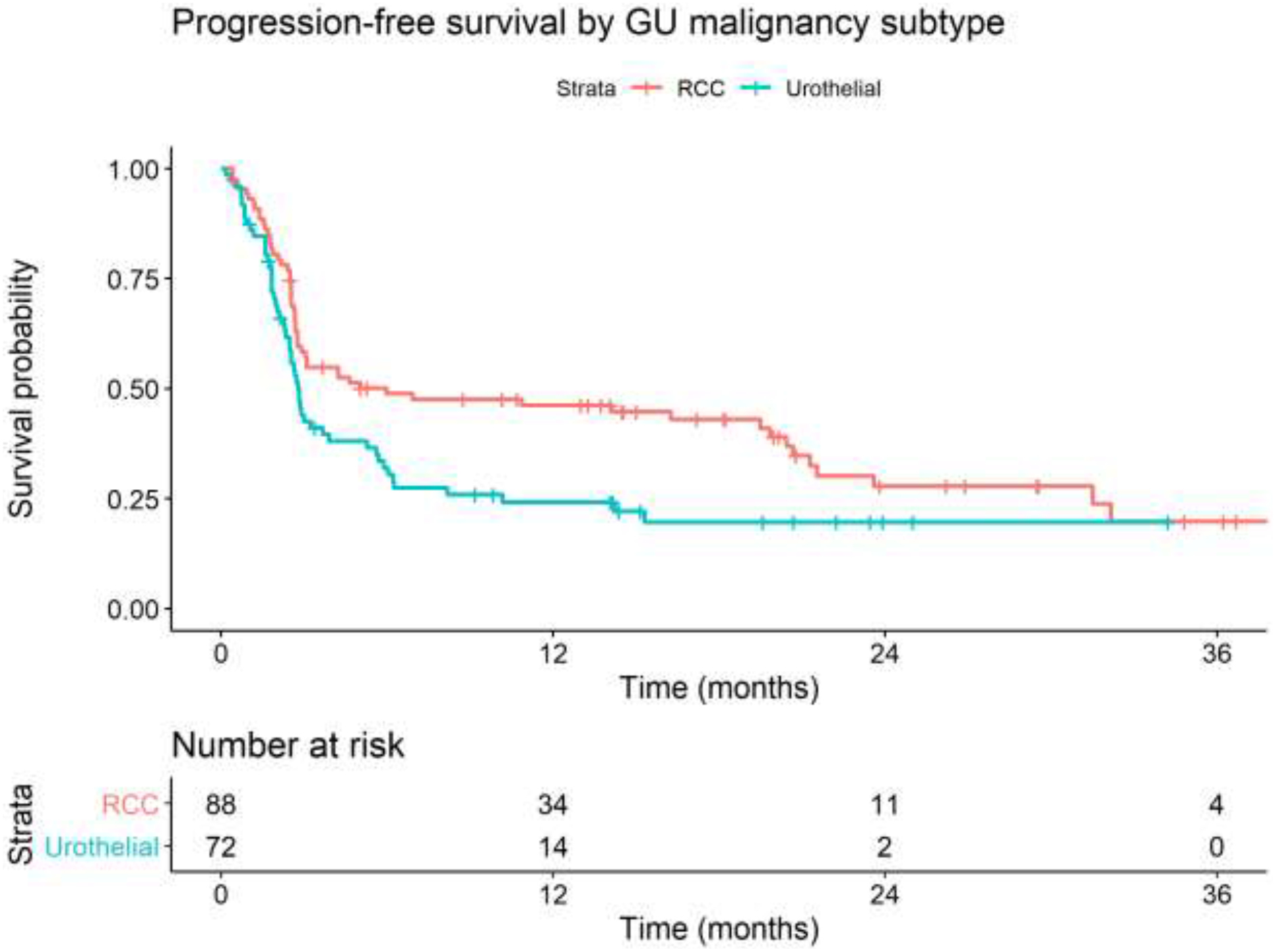
Kaplan-Meier curves of progression free survival stratified by GU malignancy subtype. GU = genitourinary; RCC = renal cell carcinoma
Figure 2:

Kaplan-Meier curves of overall survival stratified by GU malignancy subtype. GU = genitourinary; RCC = renal cell carcinoma
Table 3a.
Univariate and multivariable Cox models for progression-free survival by GU malignancy subtype, age, gender, comorbidity index, number of immune-mediated allergies, and sites of metastases.
| Variable | Description | Univariate Hazard Ratio (95% CI) | p-value | Multivariable Hazard Ratio (95% CI) | p-value |
|---|---|---|---|---|---|
| GU Malignancy Subtype | Urothelial vs RCC | 1.55 (1.07, 2.26) | 0.022* | 1.91 (1.23, 2.95) | 0.0037* |
| Age | per 10 years | 1.07 (0.90, 1.27) | 0.44 | 0.92 (0.76, 1.12) | 0.42 |
| Gender | Male vs Female | 0.89 (0.59, 1.33) | 0.56 | 0.87 (0.58, 1.33) | 0.53 |
| NCI Comorbidity Index | 0.01–2 vs 0 | 1.16 (0.75, 1.79) | 0.52 | 1.25 (0.79, 2.00) | 0.34 |
| >2 vs 0 | 1.18 (0.73, 1.89) | 0.50 | 1.38 (0.83, 2.29) | 0.19 | |
| Number of Allergies | 1 vs 0 | 1.17 (0.75, 1.83) | 0.48 | 1.24 (0.77, 1.97) | 0.41 |
| ≥2 vs 0 | 1.04 (0.66, 1.66) | 0.86 | 1.07 (0.64, 1.78) | 0.89 | |
| Site of Metastases | Liver vs LN only | 2.34 (1.39, 3.94) | 0.0014* | 3.06 (1.75, 5.35) | 0.0001* |
| Liver vs Lung | 2.15 (1.17, 3.95) | 0.0140* | 2.50 (1.33, 4.71) | 0.0044* | |
| Liver vs CNS/Bone | 2.05 (1.14, 3.68) | 0.0170* | 2.49 (1.30, 4.41) | 0.0053* |
Statistically significant
GU = genitourinary; RCC = renal cell carcinoma; CNS = central nervous system; LN = lymph node
Table 3b.
Univariate and multivariable Cox models for overall survival by GU malignancy subtype, age, gender, comorbidity index, number of immune-mediated allergies, and sites of metastases.
| Variable | Description | Univariate Hazard Ratio (95% CI) | p-value | Multivariable Hazard Ratio (95% CI) | p-value |
|---|---|---|---|---|---|
| GU Malignancy Subtype | Urothelial vs RCC | 2.08 (1.32, 3.26) | 0.0015* | 2.61 (1.55, 4.39) | 0.0003* |
| Age | per 10 years | 1.18 (0.96, 1.45) | 0.12 | 0.99 (0.79, 1.26) | 0.95 |
| Gender | Male vs Female | 0.81 (0.51, 1.28) | 0.36 | 0.77 (0.48, 1.25) | 0.29 |
| NCI Comorbidity Index | 0.01–2 vs 0 | 0.69 (0.39, 1.23) | 0.21 | 0.70 (0.38, 1.27) | 0.24 |
| >2 vs 0 | 1.36 (0.81, 2.28) | 0.25 | 1.73 (0.99, 3.02) | 0.054 | |
| Number of Allergies | 1 vs 0 | 0.92 (0.55, 1.55) | 0.76 | 0.95 (0.55, 1.64) | 0.85 |
| ≥2 vs 0 | 0.91 (0.53, 1.57) | 0.73 | 0.88 (0.48, 1.62) | 0.69 | |
| Site of Metastases | Liver vs LN only | 2.48 (1.37, 4.50) | 0.0028* | 3.12 (1.66, 5.87) | 0.0004* |
| Liver vs Lung | 2.97 (1.43, 6.16) | 0.0034* | 3.77 (1.76, 8.08) | 0.0007* | |
| Liver vs CNS/Bone | 2.06 (1.06, 4.00) | 0.0340* | 2.06 (1.03, 4.15) | 0.0420* |
Statistically significant
GU = genitourinary; RCC = renal cell carcinoma; CNS = central nervous system; LN = lymph node
Amongst clinically relevant baseline characteristics, patients with RCC were statistically more likely than urothelial carcinoma patients to have 2 or more irAEs attributed to ICI therapy (p=0.005, Supplemental Table 1).
4.1. Discussion
In our retrospective analysis, we assessed various demographic and clinicopathologic characteristics that may impact clinical outcomes in patients with metastatic RCC and urothelial carcinoma treated with ICI therapy. In our study, a greater frequency of irAEs and higher grade irAEs were associated with better ICI treatment response even after controlling for duration of therapy, comorbidity index, and therapy type. GU malignancy subtype and site of metastatic disease were found to be significant factors impacting survival outcomes. Among the examined sites, liver metastases were associated with worse ORR, PFS, and OS. These findings were persistent after accounting for several variables including GU malignancy subtype, age, gender, comorbidity index, and number of immune-mediated allergies.
Recent literature has emerged that site of metastases in solid malignancies may impact clinical outcomes associated with ICI therapy [17]. Liver metastases have been associated with poor outcomes in response to immunotherapy in patients with melanoma [18], non-small cell lung cancer [18,19], and hepatocellular carcinoma [20]. Based on our findings, we echo previous studies that liver metastases portend a comparatively worse ORR, PFS, and OS than other metastatic sites. Our finding was persistent after accounting for other pertinent clinical characteristics. Many biochemical and cellular mechanisms are involved in maintaining the tolerogenic environment in the liver [20]. Several studies have examined the hepatic immune tolerance as playing a major role in liver-induced systemic immune tolerance in conditions like autoimmune diseases and organ transplantation [21,22,23]. Multiple mechanisms have been proposed including hepatic trapping and deletion of activated cytotoxic T cells [22,24], poor CD4+ T-cell activation, inhibition of regulatory T-cell expansion [23], and elimination of autoreactive T cells [21,25]. However, the role of the liver immune tolerance in the context of malignancy and immunotherapy remains poorly understood and stands as an unmet need for ongoing investigation. Furthermore, the heterogeneous immune microenvironment of various tumors of different patients may influence tumor growth and response to ICI [26].
Patients who experienced irAEs after immunotherapy were found to have improved PFS and OS compared to those who did not, in both cancer type-agnostic meta-analysis [27] and single-center studies [28] published in advanced urothelial carcinoma patients. Our study supports a growing body of literature suggesting the presence of irAEs as a good correlative marker of anti-tumor response to ICI across a variety of cancer types [29,30]. The exact mechanisms remain unclear, but possible contributing factors include the bystander effect from activated T-cells due to shared antigens between tumor and organ involved site [31] and the gut microbiome diversity regulating T-cell activation [32]. However, many questions remain unanswered about the relationship between ICI treatment response and the severity of irAEs. Potential confounding variables include baseline comorbidities, length of time on therapy, and ICI therapy choice (single vs dual agent ICI) [33]. Our study suggests a positive correlation after accounting for these covariates, but further prospective studies are warranted to further validate our findings.
While the association between immune-mediated allergies and response to immunotherapy in our study failed to achieve significance, it did demonstrate a positive trend between number of allergies and treatment response (Table 2a). The immune-mediated mechanism is concordant with the finding that patients who experienced higher grade irAEs were likely to have better treatment responses. Additionally, we found that patients with increased number of allergies were more likely to have more irAEs (Supplemental Table 1), but statistical significance was not reached. To our knowledge, this relationship has not been previously examined or reported in literature and would benefit from directed investigation, although it appears to be concordant with results from ICI-treated patients with autoimmune diseases [7].
As anticipated in our findings, metastatic RCC treated with ICI had better survival outcomes than urothelial carcinoma in our study. Beyond histological differences, plausible factors that favor RCC include the availability of dual-agent ICI (ipilimumab/nivolumab) and the first-line ICI option in treatment naïve-patients. Because of the availability of ipilimumab/nivolumab in RCC, this may conversely account for higher frequency of irAEs compared to urothelial carcinoma patients. Baseline assessment of PD-L1 and TMB status pre-initiation of ICI was not collected in our study due to their dynamic measurements, variable assays, and their historically uninformative markers of response in RCC and urothelial carcinoma [34]. To assess for comorbidities as a factor impacting treatment response and survival, we opted for comorbidity assessment with two validated indices instead due to inter-rater subjectivity with ECOG scoring. To our knowledge, the utility of the Charlson and NCI Comorbidity indices have not been formally assessed in cancer patients treated with ICIs [11,12]. Although our study found no correlation with outcomes to ICI therapy, ongoing efforts are merited to develop comorbidity indices that better predict outcomes in the era of immunotherapy.
There were several limitations to our retrospective analysis. Our cohort included patients with prior lines of therapy who elected to be treated at a tertiary referral medical center, which also had numerous therapeutic options available. As combination therapy with ipilimumab and nivolumab is approved for advanced stage RCC, we were unable to account for the differences among patients that could have driven the selection of ipilimumab/nivolumab or single-agent PD-1 inhibitor for treatment. Following the time frame in which our cohort was analyzed, several ICIs were approved including nivolumab, avelumab, and durvalumab for urothelial cancer. Additionally, combination pembrolizumab/axitinib and avelumab/axitinib was recently approved for metastatic RCC which have promising implications for improved survival than either agent alone. Thus, we are unable to account for modern day use of other ICIs or their combination with targeted agents. Although the majority of the patients (primarily RCC) were treated with ICI in the front-line setting, we were unable to account for the heterogeneous prior lines of systemic therapy which could have impacted response to immunotherapy. It was similarly difficult to account for various subtypes of RCC (clear cell and non-clear cell) and urothelial carcinoma (adenocarcinoma, squamous, and small cell) due to small subsets. Finally, the small sample size limited statistical analysis, and a multi-center collaboration would strengthen the associations reported in this study.
5.1. Conclusion
In advanced stage RCC and urothelial carcinoma, the presence of liver metastases is a negative predictive marker for ORR, PFS, and OS in patients treated with ICI therapy. Patients who experienced greater frequency and higher grade irAEs were likely to have better treatment response rates, concordant with other emerging studies. A positive correlation was present between the number of pre-existing immune-mediated allergies and treatment response rates, but the association was not significant. These findings may help to guide immunotherapy decisions in patients with advanced stage GU malignancies, although larger cohort studies are warranted to confirm our reported associations.
Supplementary Material
Highlights.
Among various sites of metastases, liver metastases portend a worse survival outcome for genitourinary (GU) malignancy patients treated with immune checkpoint inhibitor (ICI) therapy.
Patients who experienced increased frequency and higher grade immune-related adverse events are associated with better ICI treatment response.
Pre-existing immune-mediated allergies may be associated with better ICI treatment response rates, but this finding warrants a larger cohort assessment.
Comorbidity indices do not appear to be reliable markers for survival in GU malignancy patients treated with ICI.
Acknowledgements
The ability to conduct this retrospective analysis was made possible by the Michigan Institute for Clinical & Health Research (MICHR) and Michigan Center for Translational Pathology (MCTP). We would also like to thank Xavier Owens and Marcin Cieslik for their contribution to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest/Disclosure
Dr. Ajjai Alva serves as a consultant for Merck, AstraZeneca, Bristol-Myers Squibb, and Pfizer/EMD Serono. Dr. Ajjai Alva receives research funding through the University of Michigan from Merck, Genentech, Prometheus Laboratories, Mirati Therapeutics, Roche, Bayer, Progenics, Astellas Pharma, Arcus Biosciences, AstraZeneca, Bristol-Myers Squibb, and Clovis Oncology. Dr. Jeremy Taylor serves as a consultant for Sarepta. Dr. Vincent Ma, Dr. Christopher Su, Miriam Hu, Stephanie Daignault-Newton, Olesia Kellezi, Megan Dal, Miloni Sha, Stephanie Erickson, Jessica Lora, Reema Hamasha, Alicia Ali, Sabrina Yancey, Leah Kiros, Hannah Balicki, Daniel Winfield, and Dr. Michael Green have no conflict of interest to declare.
References
- 1.Mei M and Twardowski P. Metastatic Genitourinary Malignancies. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000–2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK153873/. [Google Scholar]
- 2.Zarrabi K, Paroya A, Wu S. Emerging therapeutic agents for genitourinary cancers. J Hematol Oncol. 2019. September; 12(1):89. Doi: 10.1186/s13045-019-0780-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020. January;70(1):7–30. Doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 4.Corbaux P, Maillet D, Boespflug A, et al. Older and younger patients treated with immune checkpoint inhibitors have similar outcomes in real-life setting. Eur J Cancer. 2019. November; 121:192–201. Doi: 10.1016/j.ejca.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Madan RA, Gulley JL. Finding an Immunologic Beachhead in the Prostate Cacner Microenvironment. J Natl Cancer Inst. 2019. March 1;111(3):219–220. Doi: 10.1093.jnci/djy145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy LC, Bhatia S, Thompson JA, Grivas P. Preexisting Autoimmune Disease: Implications for Immune Checkpoint Inhibitor Therapy in Solid Tumors. J Natl Compr Canc Netw. 2019. June 1;17(6):750–757. Doi: 10.6004/jnccn.2019.7310. [DOI] [PubMed] [Google Scholar]
- 7.Leonardi GC, Gainor JF, Altan M, et al. Safety of Programmed Death-1 Pathway Inhibitors Among Patients with Non-Small-Cell Lung Cancer and Preexisting Autoimmune Disorders. J Clin Oncol. 2018. July 1;36(19):1905–1912. Doi: 10.1200/JCO.2017.77.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danlos FX, Voisin AL, Dyevre V, et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer. 2018. March;91:21–29. Doi: 10.1016/j.ejca.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Buder-Bakhaya K, Hassel JC. Biomarkers for Clinical Benefit of Immune Checkpoint Inhibitor Treatment - A Review From the Melanoma Perspective and Beyond. Front Immunol. 2018. June 28;9:1474. Doi: 10.3389/fimmu.2018.01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornejo KM, Rice-Stitt T, Wu C. Updates in Staging and Reporting of Genitourinary Malignancies. Arch Pathol Lab Med. 2020. March; 144(3):305–319. Doi: 10.5858/arpa.2019-0544-RA. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J Chronic Dis. 1987; 40(5):373–383. Doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000. December; 53(12):1258–1267. Doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 13.Stedman MR, Doria-Rose P, Warren JL, Klabunde CN, Mariotto A. The Impact of Different SEER-Medicare Claims-based Comorbidity Indexes on Predicting Non-cancer Mortality for Cancer Patients. NCI Division of Cancer Control & Population Sciences. Comorbidity Technical Report [Internet]. Accessed on March 2020. Available from: https://healthcaredelivery.cancer.gov/seermedicare/considerations/comorbidity-report.html.
- 14.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009. January;45(2):228–247. Doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 2014; 106. Doi: 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor JMG. Kendall’s and Spearman’s correlation coefficients in the presence of a blocking variable. Biometrics, 43, 409–416, 1987. [PubMed] [Google Scholar]
- 17.Bilen MA, Shabto JM, Martini DJ, et al. Sites of metastasis and association with clinical outcome in advanced stage cancer patients treated with immunotherapy. BMC Cancer. 2019. August 29; 19(1):857. Doi: 10.1186/s12885-019-6073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tumeh PC, Hellmann MD, Hamid O, et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol Res. 2017. May;5(5):417–424. Doi: 10.1158/2326-6066.CIR-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitadai R, Okuma Y, Hakozaki T, Hosomi Y. The efficacy of immune checkpoint inhibitors in advanced non-small-cell lung cancer with liver metastases. J Cancer Res Clin Oncol. 2020. March; 146(3):777–785. Doi: 10.1007/s00432-019-03104-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu L, Hsu C, Shao Y, et al. Differential Organ-Specific Tumor Response to Immune Checkpoint Inhibitors in Hepatocellular Carcinoma. Liver Cancer. 2019. November; 8(6):480–490. Doi: 10.1159/000501275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000; 6:1348–1354. Doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- 22.Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000; 174: 47–62. Doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 23.Li F, Tian Z. The liver works as a school to educate regulatory immune cells. Cell Mol Immunol. 2013;10: 292–302. Doi: 10.1038/cmi.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian S, Lu L, Fu F, Li Y, Li W, Starzl TE, et al. Apoptosis within spontaneously accepted mouse liver allografts: evidence for deletion of cytotoxic T cells and implications for tolerance induction. J Immunol. 1997; 158:4654–61. [PMC free article] [PubMed] [Google Scholar]
- 25.Jenne CN and Kubes P. Immune surveillance by the liver. Nat Immunol. 2013; 14:1996–1006. Doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- 26.Salmon H, Remark R, Gnjatic S, Merad M. Host tissue determinants of tumor immunity. Nat Rev Cancer. 2019; 19:215–27. Doi: 10.1038/s41568-019-0125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are Immune-Related Adverse Events Associated With the Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer? A Systematic Review and Meta-Analysis. BMC Med. 2020. April; 18(1):87. Doi: 10.1186/s12916-020-01549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi K, Suzuki K, Makoto H, et al. Association of Immune-Related Adverse Events With Pembrolizumab Efficacy in the Treatment of Advanced Urothelial Carcinoma. Oncology. 2020; 98(4):237–242. Doi: 10.1159/000505340. [DOI] [PubMed] [Google Scholar]
- 29.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019; 7(306). Doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riudavets M, Barba A, Maorto P, et al. Correlation between immune-related adverse events (irAEs) and efficacy in patients with solid tumors treated with immune-checkpoints inhibitors (ICIs). J Clin Oncol. 2018; 36(15_suppl):3064.30188784 [Google Scholar]
- 31.Passat T, Touchefeu Y, Gervois N, et al. Physiopathological mechanisms of immune-related adverse events induced by anti-CTLA-4, anti-PD-1, and anti-PD-L1 antibodies in cancer treatment. Bull Cancer. 2018; 105(11):1033–41. Doi: 10.1016/j.bulcan.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018. January; 359(6371):97–103. Doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xing P, Zhang F, Wang G, et al. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumors treated with NIVO or NIVO+IPI: a systematic review and meta-analysis. J Immunother Cancer. 2019. December; 7(1):341. Doi: 10.1186/s40425-019-0779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu J, Armstrong AJ, Friedlander TW, et al. Biomarkers of immunotherapy in urothelial and renal cell carcinoma: PD-L1, tumor mutational burden, and beyond. J Imunother Cancer. 2018. January; 6(4). Doi: 10.1186/s40425-018-0314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


